Introduction
Colorectal cancer, one of the three most common
malignancies in the world, remains a major public health problem
(1). In 2014, an estimated 71,830
men and 65,000 women were diagnosed with colorectal cancer and
26,270 men and 24,040 women will die of the disease (2). In recent years, remarkable advances
have been made in colorectal cancer therapeutic approaches by using
chemotherapy, radiotherapy, monoclonal antibodies and
small-molecule inhibitors. However, the clinical outcome was far
from expected as the effects of these improvements never continued
for a long duration (3,4). Therefore, new and better therapies
will be the key to reducing the incidence of colorectal cancer.
A new emerging concept implicates that treatment
failure occurs due to the existence of cancer stem cells (CSCs)
that evade the treatment regimen (5,6).
These cells had been characterized by pluripotency, self-renewal as
well as tumorigenicity (7–9). CSCs were resistant to traditional
therapies, leading to tumor recurrence and unpleasant prognosis for
cancer patients (10,11). Conventional treatments mainly
targeted the tumor cells, but not the CSCs. Thus, therapeutic
strategies specifically targeting CSCs could eradicate colorectal
cancer more effectively.
CSCs were firstly identified in hematopoietic tumors
by using cell surface and intracellular molecules (12). Also other types of tumors have been
examined for CSCs (13–16). Many studies had chosen specific
cell surface markers such as CD19, CD20, CD24, CD44 and CD133 to
characterize the subpopulation of CSCs. Transcription factor Oct4
was also typically an intracellular marker (7,17–19).
Oct4 (also known as OCT3, OCT3/4) belongs to the POU (Pit-Oct-Unc)
family of transcription factors. It can form a complex binding to
target genes in a sequence-specific manner and is involved in the
signal regulation of stem cells (20,21).
CD147 (also called EMMPRIN or basigin) is a
transmembrane glycoprotein which was initially discovered on the
surface of human cancer cells (22). Hao et al found both CD147
and CD44 were involved in prostate cancer invasion and played
significant roles in drug resistance (23). Upregulation of CD147 was shown to
accelerate invasion in vitro and tumorigenicity in nude mice
(24). In addition, CD147
silencing in lung cancer epithelial cells inhibited Wnt/β-catenin
signaling and cell anchorage-independent growth (25). Our research group previously found
CD147 was associated with colorectal tumor development in
vitro and in vivo. Therefore we sought to explore the
biological functions of CD147 in colorectal CSCs. To determine the
potential roles of CD147 in CSCs, the study was conducted by
measuring the expression pattern of CD147 in CSC-like HT-29 cells
and investigating its functions by assessment of proliferation,
invasion and chemosensitivity of CSCs with CD147 knockdown.
Materials and methods
Cell culture
Human colorectal cancer cells HT-29 were obtained
from Shanghai Cell Collection, the Chinese Academy of Science. The
cells were maintained in DMEM (Hyclone, UT, USA) containing 10%
fetal bovine serum (FBS, Hyclone) and cultured at 37°C in a
humidified atmosphere with 5% CO2.
Construction of Oct4-green fluorescent
protein (GFP) vector
The human Oct4 promoter (from 67,539 to 71,490 in
human DNA sequence with accession number BA000025) was cloned into
TOPO vectors and bi-directional sequencing was used to confirm the
fidelity of the DNA sequence as previously described (26). Finally, the correct Oct4 promoter
was cloned into the HindIII and AglI sites of pEGFP-1
vector. The vector of pEGFP-1 included the GFP gene sequence, so
the GFP expression can reflect the Oct4 promoter expression.
Transfection of cells and generation of
stable cell clones
HT-29 cells were plated in 6-well plates at a
density of 3×105 cells per well. When the cells reached
70–80% confluence, they were transfected with Oct4-GFP vector using
Lipofectamine 2000 (Invitrogen Life Technologies, CA, USA)
according to the manufacturer's instructions. Forty-eight hours
after transfection, cells were routinely passaged at a 1:10
dilution and neomycin resistance clones were selected in the medium
containing 0.8 mg/ml G418 (Gibco, NY, USA). The survived clones
were picked individually to 24-well plates and expanded to
establish cell lines with 0.5 mg/ml G418.
Isolation of GFP+ cells
According to Rothermund et al (27), G418-resistant cells obtained from
transfection were pooled and sorted with a Becton-Dickinson FACS
Vantage SE flow cytometer equipped with the FACS DiVa Option and
CellQuest software. The cytometer was equipped with an Innova
Enterprise Laser (Coherent, CA, USA). Cells were excited at 488 nm
and GFP signals were collected on the FITC detector with a 530/30
bandpass filter. Sorting gates were first drawn around FSC and SSC
populations to remove dead cells and debris. A subsequent gate was
set on GFP-positive cells. GFP+ and GFP−
cells were then sorted and analyzed with the flow cytometry.
Subsequently, GFP+ cells were expanded in the growth
medium containing 0.5 mg/ml G418.
CD44 and CD147 staining
Conjugated antibodies were carefully chosen to
accommodate laser and detector configurations and to avoid the need
to troubleshoot color compensation for antibody combinations. The
following antibodies were used for a Becton-Dickinson FACS Canto™
II flow cytometer (BD Bioscience, CA, USA): allophycocyanin labeled
monoclonal antibody against CD44 (CD44-APC, mouse anti-human IgG1,
Biolegend, CA, USA) and fluorescein isothiocyanate labeled
monoclonal antibody against CD147 (CD147-FITC, mouse anti-human
IgG1, Biolegend). Monolayer cells were detached from culture plates
with 0.25% trypsin (Gibco). Cells were then added into EP tubes and
staining was performed using conditions recommended by the
supplier. Quantitative evaluation of staining was performed with
the filter settings of 520/550-FITC and 670/830-APC.
Construction of shRNA expression vectors
specific for CD147 and incorporation into adenovirus
The vector pYr-mir30-shRNA was used to generate
short hairpin RNA (shRNA) specific for CD147 by selecting the
808–828 fragment as RNA interference (RNAi) target sites, and the
oligonucleotides encoding a non-specific shRNA are shown in
Table I. These oligonucleotides
were annealed and subcloned into the BsaI sites of the
vector according to the manufacturer's instructions. These
recombinant vectors were designated as pYr-mir30-shRNA and
pYr-mir30-shRNA-control, respectively. They were confirmed by DNA
sequencing for correct ligation. Then plasmids carrying the target
gene were transfected into HEK-293 cells together with adenoviral
vector using Lipofectamine 2000 (Invitrogen Life Technologies). The
growth medium was changed after 6 h and the cytopathic effect was
observed periodically. When majority of the pathologically abnormal
cells came off the bottom of the culture flask, the cells and
supernatant were collected, frozen and thawed at −80/37°C three
times and cells were centrifuged at 1,220 × g for 15 min. Then the
supernatant collected. With these procedures, two sets of
adenoviruses were obtained: Ad-shRNA and Ad-shRNA-control.
 | Table ISequences of the CD147-specific
shRNAs. |
Table I
Sequences of the CD147-specific
shRNAs.
| shRNAs | Sequence |
|---|
| shRNA |
5′-GATCCGTGACAAAGGCAAGAACGTCTTCAAGA-3′
5′-GAGACGTTCTTGCCTTTGTCATTTTTTGGAAA-3′ |
| shRNA |
5′-AGCTTTTCCAAAAAATGACAAAGGCAAGAACG-3′
5′-TCTCTCTTGAAGACGTTCTTGCCTTTGTCACG-3′ |
| shRNA-control |
5′-GATCCACTACCGTTGTTATAGGTGTTCAAGAGA-3′
5′-CACCTATAACAACGGTAGTTTTTTTGGAAA-3′ |
| shRNA-control |
5′-AGCTTTTCCAAAAAAACTACCGTTGTTATAGGT-3′
5′-GTCTCTTGAACACCTATAACAACGGTAGTG-3′ |
Virus infection to inhibit CD147 gene
expression
Isolated colorectal CSCs were seeded in 6-well
plates at a density of 3×105 cells per well. When the
cells reached 70–80% confluence, they were incubated with Ad-shRNA
and Ad-shRNA-control [10 plaque forming units (10 PFU)/cell] in
serum-free DMEM at 37°C for 4 h, respectively. After incubation,
serum-free DMEM was replaced with growth medium containing 0.5
mg/ml G418. The infected cells were maintained at 37°C for another
48 h.
RNA extraction, RT-PCR and reverse
transcription-quantitative PCR
Gene expression was analyzed after extraction of
cellular RNA from CSCs and infected cells with TRIzol reagent
(Invitrogen) according to the manufacturer's instructions. Total
RNA was reverse transcribed into cDNA using the PrimeScript RT
reagent kit with gDNA Eraser (Takara, Osaka, Japan). CD147 and
β-actin mRNA levels were analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) with
SYBR Premix Ex Tag™ II (Takara) under conditions described by the
supplier. cDNA (2 μl) was in a final volume of 20 μl. The primer
sequences were listed below. CD147 forward primer:
5′-CCATGCTGGTCTGCAAGTCAG-3′, and reverse primer:
5′-CCGTTCATGAGGGCCTTGTC-3′; β-actin forward primer:
5′-CTGGAACGGTGAAGGTGACA-3′, and reverse primer:
5′-AAGGGACTTCCTGTAACAACGCA-3′. The cycling program was 95°C for 30
sec, then followed by 40 cycles at 95°C for 5 sec, 60°C for 30 sec,
finally 95°C for 15 sec, 60°C for 1 min. Calculated Ct values for
CD147 mRNA were normalized to those obtained for the internal gene
β-actin, ΔCt=CtCD147 − Ctβ-actin and the
2−ΔΔCt methods were used to evaluate CD147 expression
change. RT-qPCR was performed by ABI 7500 Real-Time PCR system
(Applied Biosystems). Each sample was conducted in triplicate and
all reactions were repeated three times.
Western blot analysis
Cells in logarithmic phase were harvested in lysis
buffer (50 mmol/l Tris-HCl, pH 7.4, 150 mmol/l NaCl, 1 mmol/l
MgCl2, 100 mg/ml phenylmethanesulphonyl fluoride and 1%
Triton X-100) for 30 min on ice. Equal amounts (30 μg) of lysate
proteins were separated on 10% SDS-PAGE gels and transblotted onto
polyvinylidene difluoride (PVDF) membrane (Pall Corp., NY, USA).
Non-specific-binding sites were blocked by washing blots in 5%
non-fat dry milk in TBST buffer (10 mM Tris-HCl, pH 7.5, 150 mM
NaCl, and 1% Tween-20) for 2 h at room temperature with shaking.
Then the membranes were immunoblotted with either mouse anti-human
CD147 primary antibodies (1:500, Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) or anti-human β-actin primary antibodies
(1:500, Santa Cruz Biotechnology Inc.) overnight at 4°C, washed in
TBST and incubated with horseradish peroxidase conjugated secondary
antibody (goat anti-mouse, 1:2,000, Santa Cruz Biotechnology Inc.)
for 2 h at room temperature. Protein bands were detected using ECL
detection system (Boster, Wuhan, China). Each analysis was
performed at least three times.
Cell proliferation assay
The 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay was used to assess the
proliferation of infected cells. After infection for 48 h,
5×103 cells per well of each group were plated in
96-well plates. After 24, 48, 72 and 96 h of culture, respectively,
20 μl MTT (5 mg/ml) was added to each well and the plates were
returned to incubator for another 4 h. Then the medium was removed
and 150 μl of dimethylsulfoxide was added to each well. Ten minutes
later, spectrometric absorbance at 490 nm was measured with a
mircoplate reader. Each group was done in triplicate and repeated
three times.
Invasion assay
The upper chamber of transwell plates (8 μm
diameters, Corning Costar, USA) was coated with basement membrane
Matrigel (20 mg/ml, Becton-Dickinson). After the Matrigel
solidified at 37°C, each group of cells (1×105) in 200
μl serum-free DMEM were added to the upper chambers and the lower
chambers were filled with 500 μl DMEM containing 10% FBS. After
incubation at 37°C for 24 h, cells were fixed with 100% methanol
and then stained with 0.1% crystal violet for 10 min. Cells that
invaded the Matrigel and reached the lower surface of the chambers
were counted in five random fields at ×200 magnification under a
light microscope, and the results were expressed as mean of five
fields. The assay was performed in triplicate and repeated three
times.
Drug sensitivity assay
To assess the chemosensivity after infection, cells
were seeded in 96-well plates at a density of 1×104
cells per well and incubated for 24 h. The medium was then removed
and added with 200 μl medium containing gemcitabine, cisplatin,
docetaxel and paclitaxel, respectively, with varying
concentrations: 0.1, 1 and 10 μM. After 48 h, cells were treated
with MTT as described earlier. Spectrometric absorbance at 490 nm
was measured with a microplate reader. Each group was plated in
three wells and repeated three times.
Statistical analysis
SPSS 17.0 software was employed. Data were
represented as mean ± standard deviation (SD). One-way analysis of
variance (ANOVA) was used for comparing significance of differences
among groups. For all statistical analyses, the level of
significance was set as P<0.05.
Results
Selection of Oct4-GFP stable expression
transfectants
In our study, the human Oct4 promoter PCR fragment
was confirmed by DNA sequencing and was then inserted into the
pEGFP-1 reporter construct upstream of GFP to test the
applicability of the expression system. The constructed Oct4-GFP
was transfected into human colorectal cancer HT-29 cells. After
transfection, GFP was clearly observed in HT-29 cells as shown in
Fig. 1A.
 | Figure 1Characterization of Oct4-GFP
transfected HT-29 cells. (A) At 6 weeks, the expression of GFP is
shown by UV microscopy with the magnification of ×200. (B) In the
presence of 0.5 mg/ml G418 for 6 weeks, the expression levels of
GFP were measured with a flow cytometry. (C) Following isolation
for 1 week, 1 month and 4 months, the expression of CD44 was
measured by flow cytometry. (D) Four months after GFP+
cells isolated from HT-29 cell line, they were observed with light
microscopy and UV microscopy (with the magnification of ×200,
respectively), suggesting nearly 100% positivity. SSC, side
scatter; GFP, green fluorescent protein; APC, allophycocyanin. |
Isolation of GFP+ cells from
the HT-29 cell line
To confirm the above results, the GFP expression
levels of selected cells were measured by flow cytometry. As shown
in Fig. 1B, the percentage of
GFP+ cells in HT-29 cell line was ~47.0%. As described
previously (27), several thousand
GFP+ cells arising from HT-29 cells were sorted by flow
cytometry. These isolated HT-29 cells might possess some
characteristics of CSCs as the GFP expression could reflect the
Oct4 promoter expression. Thus, the CSC-like HT-29 cells were
chosen for measuring the expression of stem cell marker CD44.
Differentiation of the CSC-like cells can
be blocked
Following isolation for 1 week, 1 month and 4
months, we measured the expression levels of CD44 in CSC-like HT-29
cells (Fig. 1C). The stable
incorporation of the Oct4-GFP vector seemed to block the
differentiation of CSCs. Based on the results shown in Fig. 1D, we found the expression levels of
CD44 in isolated CSC-like HT-29 cells were similar and nearly 100%
GFP- positivite. To further explore the relationship between CD147
and CSC-like HT-29 cells, the expression levels of selected
biomarkers were measured by flow cytometry. For mono-color
fluorescent staining, the expression levels of CD44 and CD147 in
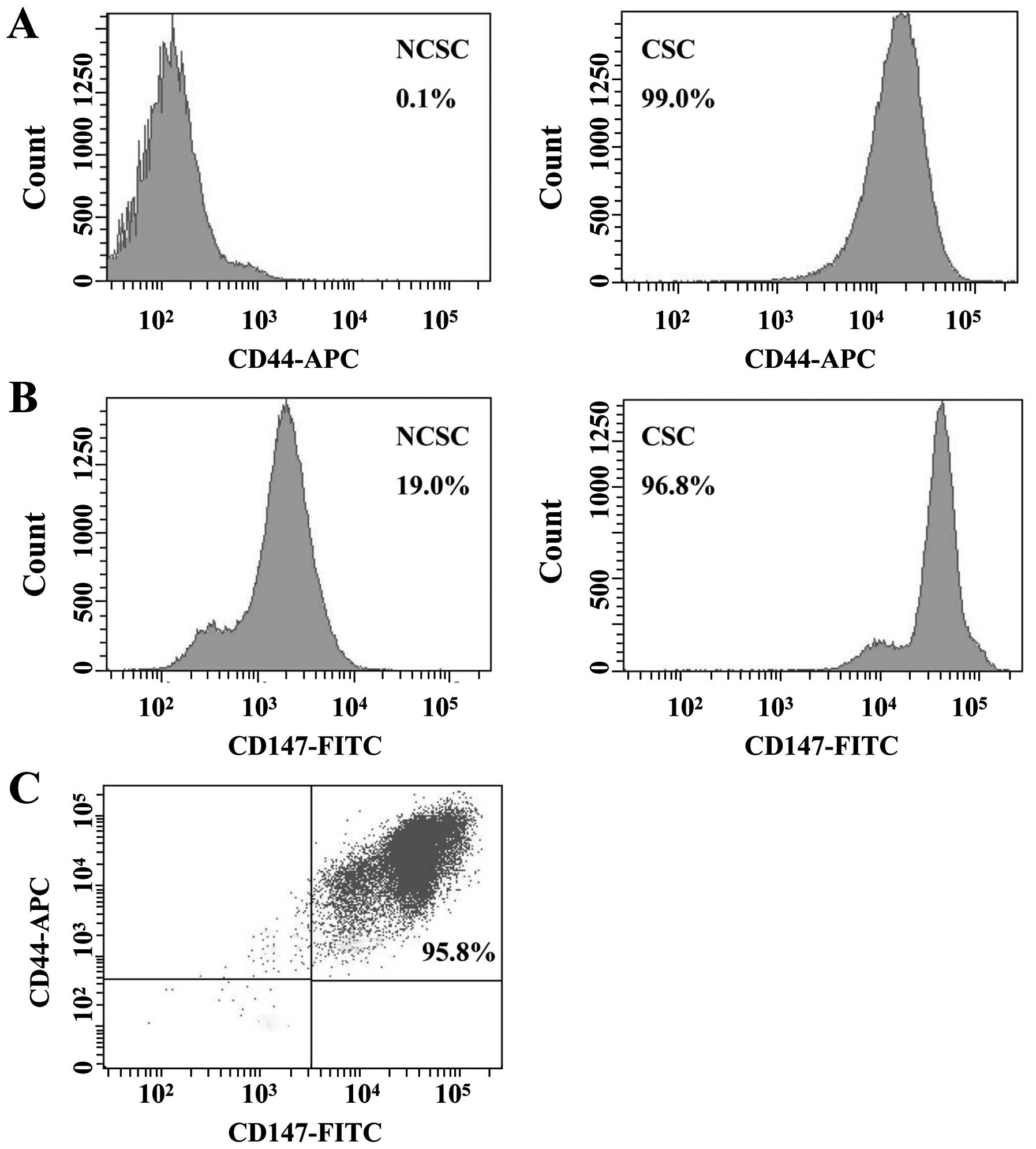
CSC-like cells were 99.0 and 96.8%, whereas the expression levels
of non-CSCs (NCSCs) were 0.1 and 19.0%, respectively (Fig. 2A and B). For double-color
fluorescent staining, the expression level of CD44/CD147 was 95.8%
(Fig. 2C). These results reflected
that CD147 might play a significant role in CSC-like cells.
Specific siRNA inhibits the expression of
CD147 in CSC-like HT-29 cells
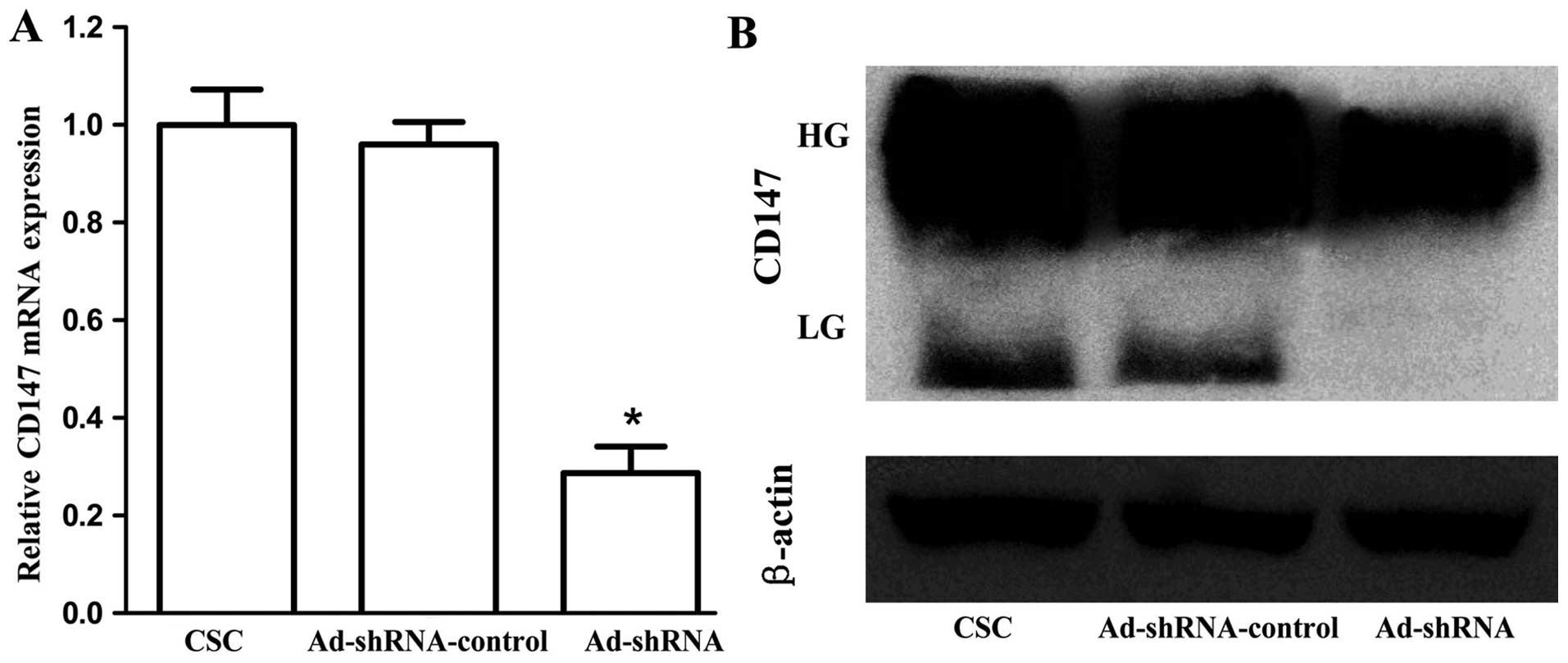
We used RT-qPCR and western blot analysis to
evaluate the knockdown efficiency of CD147 in CSC-like HT-29 cells.
β-actin was regarded as an normalization control. As shown in
Fig. 3A, cells infected with
Ad-shRNA effectively inhibited CD147 mRNA level (P<0.001), and
there was no significant difference between CSC and
Ad-shRNA-control (P=0.647). In addition, western blot analysis
confirmed the downregulation of CD147 protein by Ad-shRNA (Fig. 3B). The lowly glycosylated CD147
(LG-CD147, ~33 kDa) completely disappeared, and the level of highly
glycosylated (HG-CD147, ~40–60 kDa) was diminished.
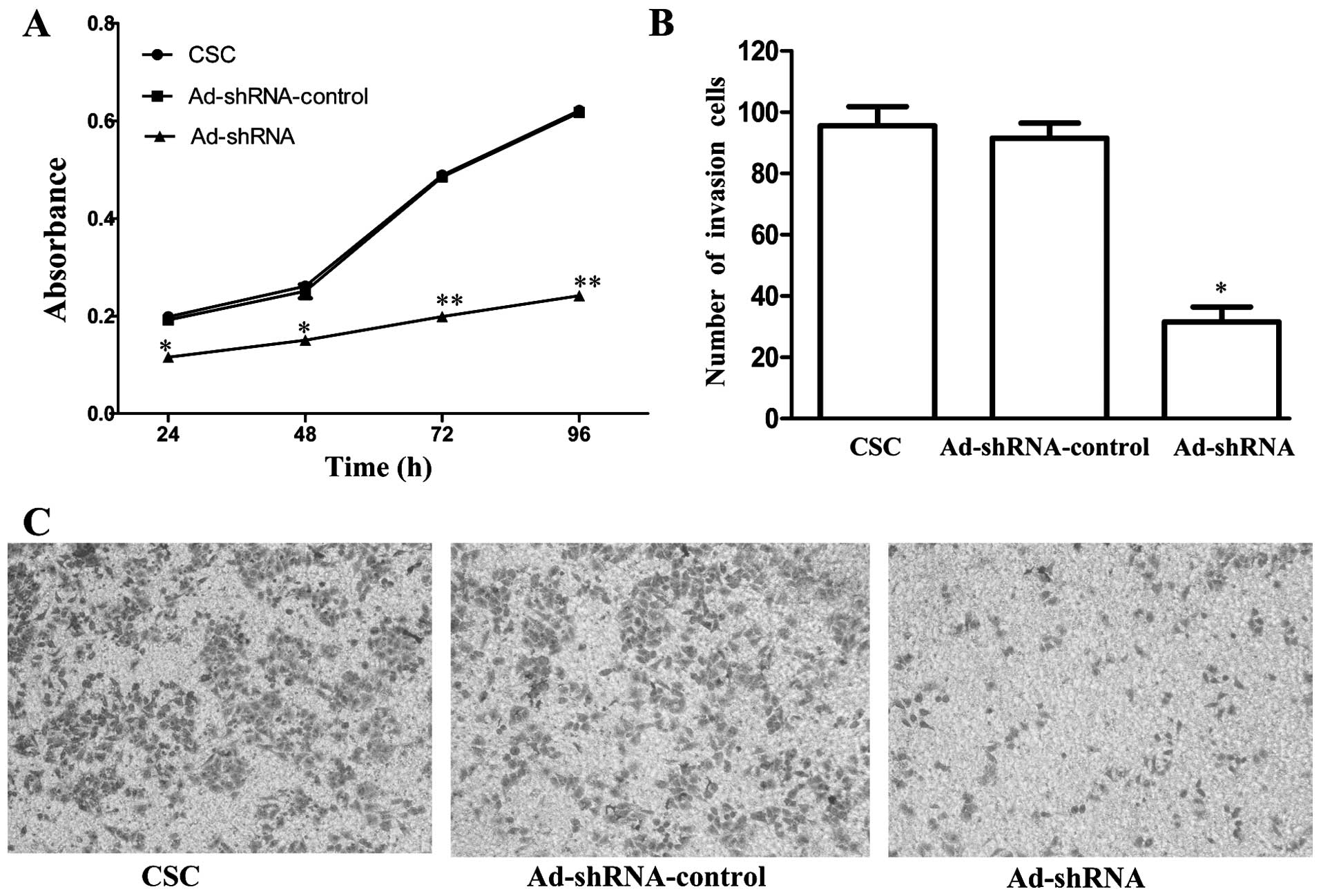
CD147 silencing reduces the proliferation
and invasion of CSC-like HT-29 cells
To examine whether CD147 silencing affects CSCs
proliferation, we used MTT assay to determine the proliferation of
CSCs, Ad-shRNA-control and Ad-shRNA, respectively. The data,
compared with CSCs, showed that the proliferation of Ad-shRNA was
inhibited to 42.4% (P=0.002), 43.7% (P=0.002), 59.3% (P<0.001),
61.1% (P<0.001) at 24, 48, 72 and 96 h, respectively (Fig. 4A). There was no significant
difference between CSC and Ad-shRNA-control (P=0.502, 0.387, 0.624
and 0.631, respectively). Furthermore, Matrigel Transwell analysis
was used to further assess this effect of CD147 knockdown on
invasive ability of CSCs. The number of Ad-shRNA cells passing
through the Matrigel was markedly lower than the number of CSCs and
Ad-shRNA-control cells, suggesting that the silencing of CD147
significantly inhibited invasion compared with CSC (P=0.001,
Fig. 4B). There was no significant
difference between CSC and Ad-shRNA-control (P=0.517).
CD147 silencing sensitizes CSC-like HT-29
cells to chemotherapeutic drugs
CSCs had been proposed to be more chemoresistant
than NCSCs. It had been reported that CD147 expression was
overexpressed in multidrug-resistant cells and could confer
resistance to some chemotherapeutic drugs. We then investigated
whether the downregulation of CD147 in the CSC-like cells, with an
RNAi method, could affect the sensitivity to chemotherapeutic
drugs. Using MTT assay, we tested the sensitivity of CSC-like HT-29
cells to four drugs. As shown in Fig.
5, shRNA mediated CD147 silencing increased the sensitivity of
CSC-like cells to gemcitabine, docetaxel and cisplatin at 0.1, 1
and 10 μM (Fig. 5A–C, P<0.05).
The results show that there was no significant difference among the
three groups to paclitaxel at 10 μM (Fig. 5D, P=0.19).
Discussion
CSCs play an important role in cancer recurrence and
metastasis. These cells are a specific small population that can
initiate tumor growth and sustain self-renewal. Previous studies
demonstrated that CSCs could resist traditional therapies and were
associated with tumor recurrence (10,11,28).
CSC-enriched population isolated from colorectal cancer cells may
provide new opportunities for targeted identification. As an
adhesion molecule, CD147 is widely expressed in a variety of cancer
types. Recently, Higashi et al (29) demonstrated that CD147 may be an
undifferentiated marker of human embryonic stem cells. Despite
substantial progress of CD147 in cancer pathogenesis, the
biological roles of CD147 are not clear in CSCs. To the best of our
knowledge, this is the first study performed to investigate the
biological functions of CD147 in CSC-like HT-29 cells. In the
present study, we demonstrated that blocking CD147 expression via
RNAi could effectively inhibit CSC-like HT-29 cell proliferation
and invasion activity, but increased their chemosensitivity.
Current purification methods generally only enrich
CSCs. Ginestier et al and Diehn et al indicated that
efficient tumori-genesis requires at least several hundred cells
even in highly immunocompromised hosts (30,31).
We successfully isolated several thousand GPF+ cells
from HT-29 cell line as shown in Fig.
1B. The currently used promising detection methods for CSC are
based on stem cell-specific biomarkers. Cell surface markers, such
as CD24, CD34, CD44, CD90, CD133, aldehyde dehydrogenase (ALDH) and
c-Met are generally used to identify CSCs by flow cytometry
(18,19,32,33).
Of many CSCs markers identified thus far, CD44 is of particular
biologic importance (34). Studies
have shown that nuclear CD44 directly reprograms stem cell
properties in colorectal cancer cells (35). Here, we chose CD44 as a surface
marker for GFP+ cells. Previously, Sajithlal et
al and Kang et al demonstrated that CD44 expression
level was high in CSC-like cells (36). We also observed upregulation of
CD44 in the CSC-like HT-29 cells. Our flow cytometry results showed
much higher expression level of CD147 in CSC-like cells than in
NCSCs. Hao et al and Slomiany et al studied CD147 and
CD44 expression levels in breast CSCs and found both CD147 and CD44
enriched in CSCs (37–39). Similar results were observed in our
studies. Interestingly we found that the expression levels of CD44
in isolated CSC-like HT-29 cells ranged from 1 week to 4 months
were similar. However, the specific molecular mechanism of how the
Oct4-GFP vector blocks the differentiation of CSC-like cells is
still unclear and we will explore it in the future.
Recently, the CSC theory was proposed and attracted
much attention of oncologists in the world. It provided a new angle
in the research of malignancy and gradually gained significance.
Thus, identification of pure colorectal CSCs is the key for
targeted therapies. However, enrichment of CSCs remains the
challenge in this process (40).
In this study, we isolated CSC-like cells from HT-29 cell line to
explore possible relevant therapeutic strategies against cancer.
Targeting of CSCs might shed light on cancer therapy, however, this
goal is challenging as CSCs are resistant to conventional
treatments (10,41). Therefore, new therapies based on
increased knowledge of CSCs are urgently needed.
shRNA that were processed to form small interfering
RNA (siRNA) enabled persistent inhibition of endogenous gene
expression (24). Here, we
silenced CD147 expression in CSC-like HT-29 cells by CD147-specific
siRNA. Using RNAi technology, Yang et al revealed that the
inhibition of CD147 expression reduced tumor cell invasion in
salivary adenoid cystic carcinoma cell lines (42). It was shown that high level of
Cyclophilin A could promote cell proliferation through interactions
with CD147 involving activation of ERK1/2 and p38 MAPKs in human
pancreatic cancer cells (43). In
our study, we detected the proliferation ability changes by MTT
assay. The results revealed that CD147 silencing reduced the
proliferation in CSC-like HT-29 cells. Matrigel invasion assay
suggested that invasion of Ad-shRNA cells was significantly
inhibited. These results indicated that CD147 might play a
potential role in promoting proliferation and invasion of CSCs.
Multidrug resistance remains the main cause of
treatment failure and mortality in colorectal cancer patients.
Traditional therapies aimed at the bulk population of cancer cells
in the proliferation and mitotic phases, leading cancer cells into
interphase, the main cause of cancer recurrence. Our data
demonstrated that CD147 silence increased chemosensitivity to
gemcitabine, cisplatin, and docetaxel, but not paclitaxel at 10 μM,
indicating that the expression of CD147 is closely related to drug
resistance in CSCs. The results also suggested that the
concentration of panclitaxel must be higher than 10 μM when used in
treatment of colorectal cancer. Studies revealed that CD147 was
associated with drug resistance to some other anticancer agents in
breast CSCs (36,37). Xu et al found that CD147
promoted 5-FU resistance in colorectal cancer (44). The
CD44+/CD147+ phenotype is a unique property
of tumor cells associated with drug resistance in prostate cancer
(23). CD44 is a primary receptor
for hyaluronan (HA). CD147 was highly expressed in multidrug
resistance (MDR) cells and stimulated the production of HA in
mammary carcinoma cells leading to induction of MDR in a
HA-dependent manner (45,46). These studies suggested that CD147
might play an important role in drug resistance on various types of
cancer. Therefore, it might be possible for CD147 to regulate
drug-sensitivity of cancer cells.
In conclusion, our results provide some information
on colorectal CSCs regarding characteristic features of stem cells
and the biological functions of CD147 involved in proliferation,
invasion and multidrug resistance properties. By understanding the
accurate role of CD147 in the CSCs, we should consider developing
treatment based on the CSC model. Based on inhibition of CD147
expression, a novel and promising therapeutic approach may be
developed.
Acknowledgements
This study was supported by grants from The National
Natural Science Foundation of China (no. 81472027), Nanjing Science
and Technology Committee Project (no. 201108025), Nanjing Medical
Science and Technique Development Foundation to Y.Q.P. (nos.
QRX11255 and YKK13107) and B.S.H. (no. QRX11254).
References
|
1
|
Wasserman M, Baxter NN, Rosen B, Burnstein
M and Halverson AL: Systematic review of internet patient
information on colorectal cancer surgery. Dis Colon Rectum.
57:64–69. 2014. View Article : Google Scholar
|
|
2
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
George S, Wang Q, Heinrich MC, Corless CL,
Zhu M, Butrynski JE, Morgan JA, Wagner AJ, Choy E, Tap WD, et al:
Efficacy and safety of regorafenib in patients with metastatic
and/or unresectable GI stromal tumor after failure of imatinib and
sunitinib: A multicenter phase II trial. J Clin Oncol.
30:2401–2407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vacchelli E, Eggermont A, Galon J,
Sautès-Fridman C, Zitvogel L, Kroemer G and Galluzzi L: Trial
watch: Monoclonal antibodies in cancer therapy. OncoImmunology.
2:e227892013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Challen GA and Little MH: A side order of
stem cells: The SP phenotype. Stem Cells. 24:3–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang W, Peng J, Zhang Y, Cho WC and Jin
K: The implications of cancer stem cells for cancer therapy. Int J
Mol Sci. 13:16636–16657. 2012. View Article : Google Scholar
|
|
7
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dick JE: Stem cell concepts renew cancer
research. Blood. 112:4793–4807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar
|
|
10
|
Maugeri-Sacca M, Vigneri P and De Maria R:
Cancer stem cells and chemosensitivity. Clin Cancer Res.
17:4942–4947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Irvin DK, Jouanneau E, Duvall G, Zhang XX,
Zhai Y, Sarayba D, Seksenyan A, Panwar A, Black KL and Wheeler CJ:
T cells enhance stem-like properties and conditional malignancy in
gliomas. PLoS One. 5:e109742010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Awad O, Yustein JT, Shah P, Gul N, Katuri
V, O'Neill A, Kong Y, Brown ML, Toretsky JA and Loeb DM: High ALDH
activity identifies chemotherapy-resistant Ewing's sarcoma stem
cells that retain sensitivity to EWS-FLI1 inhibition. PLoS One.
5:e139432010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cameron SR, Dahler AL, Endo-Munoz LB,
Jabbar I, Thomas GP, Leo PJ, Poth K, Rickwood D, Guminski A and
Saunders NA: Tumor-initiating activity and tumor morphology of
HNSCC is modulated by interactions between clonal variants within
the tumor. Lab Invest. 90:1594–1603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michishita M, Akiyoshi R, Yoshimura H,
Katsumoto T, Ichikawa H, Ohkusu-Tsukada K, Nakagawa T, Sasaki N and
Takahashi K: Characterization of spheres derived from canine
mammary gland adenocarcinoma cell lines. Res Vet Sci. 91:254–260.
2011. View Article : Google Scholar
|
|
16
|
Smith BH, Gazda LS, Conn BL, Jain K, Asina
S, Levine DM, Parker TS, Laramore MA, Martis PC, Vinerean HV, et
al: Three-dimensional culture of mouse renal carcinoma cells in
agarose macrobeads selects for a subpopulation of cells with cancer
stem cell or cancer progenitor properties. Cancer Res. 71:716–724.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Overdevest JB, Thomas S, Kristiansen G,
Hansel DE, Smith SC and Theodorescu D: CD24 offers a therapeutic
target for control of bladder cancer metastasis based on a
requirement for lung colonization. Cancer Res. 71:3802–3811. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang KH, Dai YD, Tong M, Chan YP, Kwan PS,
Fu L, Qin YR, Tsao SW, Lung HL, Lung ML, et al: A CD90(+)
tumor-initiating cell population with an aggressive signature and
metastatic capacity in esophageal cancer. Cancer Res. 73:2322–2332.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blacking TM, Waterfall M and Argyle DJ:
CD44 is associated with proliferation, rather than a specific
cancer stem cell population, in cultured canine cancer cells. Vet
Immunol Immunopathol. 141:46–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schöler HR, Ruppert S, Suzuki N, Chowdhury
K and Gruss P: New type of POU domain in germ line-specific protein
Oct-4. Nature. 344:435–439. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okumura-Nakanishi S, Saito M, Niwa H and
Ishikawa F: Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic
stem cells. J Biol Chem. 280:5307–5317. 2005. View Article : Google Scholar
|
|
22
|
Biswas C: Tumor cell stimulation of
collagenase production by fibroblasts. Biochem Biophys Res Commun.
109:1026–1034. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hao JL, Cozzi PJ, Khatri A, Power CA and
Li Y: CD147/EMMPRIN and CD44 are potential therapeutic targets for
metastatic prostate cancer. Curr Cancer Drug Targets. 10:287–306.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou W, Yang H, Hou X, Zhang W, Chen B and
Xin X: Inhibition of CD147 gene expression via RNA interference
reduces tumor cell invasion, tumorigenicity and increases
chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett.
248:211–218. 2007. View Article : Google Scholar
|
|
25
|
Sidhu SS, Nawroth R, Retz M,
Lemjabbar-Alaoui H, Dasari V and Basbaum C: EMMPRIN regulates the
canonical Wnt/beta-catenin signaling pathway, a potential role in
accelerating lung tumorigenesis. Oncogene. 29:4145–4156. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gerrard L, Zhao D, Clark AJ and Cui W:
Stably transfected human embryonic stem cell clones express
OCT4-specific green fluorescent protein and maintain self-renewal
and pluripotency. Stem Cells. 23:124–133. 2005. View Article : Google Scholar
|
|
27
|
Rothermund K, Rogulski K, Fernandes E,
Whiting A, Sedivy J, Pu L and Prochownik EV: C-Myc-independent
restoration of multiple phenotypes by two C-Myc target genes with
overlapping functions. Cancer Res. 65:2097–2107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsai KS, Yang SH, Lei YP, Tsai CC, Chen
HW, Hsu CY, Chen LL, Wang HW, Miller SA, Chiou SH, et al:
Mesenchymal stem cells promote formation of colorectal tumors in
mice. Gastroenterology. 141:1046–1056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Higashi K, Yagi M, Arakawa T, et al: A
novel marker for undifferentiated human embryonic stem cells.
Monoclon Antib Immunodiagn Immunother. 34:7–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
31
|
Diehn M, Cho RW and Clarke MF: Therapeutic
implications of the cancer stem cell hypothesis. Semin Radiat
Oncol. 19:78–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leal JA and Lleonart ME: MicroRNAs and
cancer stem cells: Therapeutic approaches and future perspectives.
Cancer Lett. 338:174–183. 2013. View Article : Google Scholar
|
|
33
|
Medema JP: Cancer stem cells: The
challenges ahead. Nat Cell Biol. 15:338–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Su YJ, Lai HM, Chang YW, Chen GY and Lee
JL: Direct reprogramming of stem cell properties in colon cancer
cells by CD44. EMBO J. 30:3186–3199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sajithlal GB, Rothermund K, Zhang F, Dabbs
DJ, Latimer JJ, Grant SG and Prochownik EV: Permanently blocked
stem cells derived from breast cancer cell lines. Stem Cells.
28:1008–1018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang MJ, Kim HP, Lee KS, Yoo YD, Kwon YT,
Kim KM, Kim TY and Yi EC: Proteomic analysis reveals that
CD147/EMMPRIN confers chemoresistance in cancer stem cell-like
cells. Proteomics. 13:1714–1725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hao J, Chen H, Madigan MC, Cozzi PJ,
Beretov J, Xiao W, Delprado WJ, Russell PJ and Li Y: Co-expression
of CD147 (EMMPRIN), CD44v3-10, MDR1 and monocarboxylate
transporters is associated with prostate cancer drug resistance and
progression. Br J Cancer. 103:1008–1018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Slomiany MG, Grass GD, Robertson AD, Yang
XY, Maria BL, Beeson C and Toole BP: Hyaluronan, CD44, and emmprin
regulate lactate efflux and membrane localization of
monocarboxylate transporters in human breast carcinoma cells.
Cancer Res. 69:1293–1301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim KM and Yi EC: CD147 is critical for
cancer stem cell chemoresistance: what does this mean for the
clinic? Expert Rev Proteomics. 10:313–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bartucci M, Svensson S, Romania P, Dattilo
R, Patrizii M, Signore M, Navarra S, Lotti F, Biffoni M, Pilozzi E,
et al: Therapeutic targeting of Chk1 in NSCLC stem cells during
chemotherapy. Cell Death Differ. 19:768–778. 2012. View Article : Google Scholar :
|
|
42
|
Yang X, Zhang P, Ma Q, Kong L, Li Y, Liu B
and Lei D: EMMPRIN contributes to the in vitro invasion of human
salivary adenoid cystic carcinoma cells. Oncol Rep. 27:1123–1127.
2012.
|
|
43
|
Li M, Zhai Q, Bharadwaj U, Wang H, Li F,
Fisher WE, Chen C and Yao Q: Cyclophilin A is overexpressed in
human pancreatic cancer cells and stimulates cell proliferation
through CD147. Cancer. 106:2284–2294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu T, Zhou M, Peng L, Kong S, Miao R, Shi
Y, Sheng H and Li L: Upregulation of CD147 promotes cell invasion,
epithelial-to-mesenchymal transition and activates MAPK/ERK
signaling pathway in colorectal cancer. Int J Clin Exp Pathol.
7:7432–7441. 2014.
|
|
45
|
Rau KM, Kang HY, Cha TL, Miller SA and
Hung MC: The mechanisms and managements of hormone-therapy
resistance in breast and prostate cancers. Endocr Relat Cancer.
12:511–532. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Misra S, Ghatak S, Zoltan-Jones A and
Toole BP: Regulation of multidrug resistance in cancer cells by
hyaluronan. J Biol Chem. 278:25285–25288. 2003. View Article : Google Scholar : PubMed/NCBI
|