Introduction
Breast cancer is the most common cancer in female
adults worldwide (1) and the
second leading cause of cancer death (2). It has become the greatest threat to
women in our country. Triple-negative breast cancer (TNBC), which
is negative for estrogen, progesterone and HER-2/neu receptors, has
recently been identified in certain sub-groups of patients, and has
the highest recurrence rate, fast growth and poorest prognosis
(3). It represents up to 20% of
all breast cancers and currently has no standard treatment
(4). In the past five years,
evidence has emerged indicating that TNBC is associated with the
inactivation of BRCA1 and overexpression of epidermal growth factor
receptor (EGFR), which makes it sensitive to anti-EGFR therapies
(5). Additionally, novel
molecular-targeted treatments focusing on tyrosine kinase
inhibitors (TKI) (6),
anti-angiogenesis [vascular endothelial growth factor (VEGF)
antibody] (7), and the key enzymes
of cellular DNA repair such as poly-ADP ribose polymerase 1 (PARP1)
(8,9), have been developed but are still
undergoing trials. Therefore, the development of new, highly
effective and targeted carrier systems to transfer bioactive
substances directly into tumor cells is one feasible method for
tumor targeting therapy.
Over the past decade, some peptides with lengths
less than 30 amino acids have been found, which are named ‘cell
penetration peptides’ (CPP) (10).
They have the ability to penetrate the cell membrane or nuclear
membrane and become localized in the cytoplasm or nucleus after
internalization. They can carry a variety of materials without
limited cell types, including hydrophilic proteins, polypeptides,
DNA and even particulate matter, such as spaces between cells or
intracellular delivery (11). In a
previous study, we found an 11 amino acid peptide named ‘PI’, which
was selected from the pC89 phage display library. In the sequence
of PI, non-polar hydrophobic amino acids constitute the majority,
with a samller proportion of basic amino acids. The characteristics
of this peptide are different from those of most classical cell
penetrating peptides. We also found that PI is especially targeting
breast cancer, and not other cancer cells or non-cancer cells
(12). This finding suggests that
PI could be a potential discovery useful in breast cancer cell
therapy.
In this study, we demonstrate the value of PI as a
targeting vector to deliver therapeutic molecules including
chemotherapy drugs, proteins or polynucleotides in breast cancer
therapy, especially in triple-negative breast cancer therapy.
Materials and methods
Peptide synthesis and radiolabelling of
HYNIC-PI
PI (CASPSGALRSC) was synthesized with a cysteine
terminal. For biodistribution, radiolabeling of HYNIC-PI was
performed by mixing ~20 μg of HYNIC with 15 μl of tricine (100
mg/ml in citrate buffer, 20 mM citrate, 100 mM NaCl, pH 5.2), 100
μl of TPPTS and ~1.85 MBq of TcO4−, mixed in
nitrogen, reaction at 100°C for 25 min, then cooled down. The
resulting reaction mixture was purified by HPLC using a BioSep 2000
column eluted with PBS (0.01 mM Na2HPO4, 100
mM NaCl, pH 7.4) and the radiochemical purity of the final product
was >95%.
Cell culture
Approximately 25,000–30,000 adherent MDA-MB-231
cells were cultivated in 24-well tissue plate for 12 h in Lei
Ovitz's (L-15), supplemented with HEPES (25 mM),
penicillin/streptomycin (1%), fetal serum (10%), and L-glutamine in
a 5% CO2 humidified atmosphere at 37°C. Approximately
25,000–30,000 adherent MDA-MB-435 cells were cultivated in a
24-well tissue plate for 12 h in PRMI-1640 supplemented with HEPES
(25 mM), penicillin/streptomycin (1%), fetal serum (10%), and
L-glutamine in a 5% CO2 humidified atmosphere at 37°C.
All products were purchased from Gibco (CA, USA).
Construction and expression of
vectors
Oligonucleotide PI encoding region (sequence: 5′
TGCGCATCCCCATCTGG CGCCCTTCGTTGTTGC 3′) was synthesized by
Invitrogen Ltd. (Shanghai, China). Then, the duplexes were cloned
into the BamHI/EcoRI sites of pGEX-2T.
Similar to the above process, we constructed the
pET-28a (+)-PI. We used the primers to amplify TK gene fragments
from the HSV1-TK plasmid as a template. The PCR products were
digested with EcoRI/SalI, and subcloned into pET-28a
(+)-PI and pET-28a (+) to generate the plasmids pET-28a (+)-PI-TK
and pET-28a (+)-TK, respectively. All vectors were purchased from
Invivogen (CA, USA), and all enzymes were purchased from Takara
(Dalian, China). Non-recombinant pGEX-2T was used to produce GST as
a control protein.
Recombinant plasmids were identified by single
restriction enzyme digestion and analyzed by SDS-PAGE
electrophoresis. E. coli strain BL21 (DE3) was transformed
with the recombinant plasmids or vector alone, and then grown in LB
broth containing 100 μg/ml ampicillin at 37°C.
Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final
concentration of 1 mM, and incubation was continued at 37°C for 1–6
h. After IPTG induction, bacteria pellets were obtained by
centrifugation at 3500 rpm. The supernatant was collected and
stored at −80°C for use with western blotting.
Purification of the fusion proteins
PI-GST fused protein products in the supernatant
fraction were purified on a GST-sepharose columns. Purification was
carried out essentially as described by the manufacturer. Briefly,
100 ml of sample was centrifuged to remove any undissolved
membranes and cellular debris before being applied to the column.
Triton X-100 was then added to the collected supernatants. The
column was washed with 5–10 volumes of PBS to remove azide and
equilibrated with 3–5 volumes of PBS containing 1% Triton X-100.
Then, the sample was applied to the prepared column. The
flowthrough was collected as a control. The column was washed with
10 volumes of PBS until eluted and no protein could be detected.
The PI-TK fusion proteins were purified using a His TALON™
Cartridge Purification kit (Clontech, USA). The purified proteins
were run on 12% SDS-polyacrylamide gels.
Immunofluorescence
MDA-MB-231 and MDA-MB-435 (1×105) cells
were incubated with 200 ng/ml of PI-GST in culture medium for 8–12
h at 37°C, and washed six times with PBS. Then, the cultured cells
were fixed in 10% Triton X-100 for 10 min, and detected by 30-min
incubation with FITC-conjugated mouse monoclonal antibody
(anti-GST-FITC) (Santa Cruz). The cells were then washed twice with
PBS and immediately observed by fluorescence microscopy. The cells
were incubated with GST alone as a control. Non-recombinant
pGEX4T-1 was used to produce GST as a control protein.
Western blot analysis
MDA-MB-231 and MDA-MB-435 were seeded onto 6-well
plates at a density of 2×105 cells/well, and treated
with recombinant protein. Twenty-four hours later, the cells were
trypsinized with trypsin-EDTA (Gibco BRL, Life Technologies, UK)
and washed once with phosphate-buffered saline (PBS). Total cell
lysis buffer (Beyotime, China) was added to cell pellets and the
cells were incubated on ice for 30 min. Proteins were extracted and
30 μg of total protein was loaded on a 12% SDS-polyacrylamide gel,
followed by electrophoretic separation of the proteins and transfer
to PVDF membrane (Millipore, USA). The membrane was blocked for 1 h
in 10% skim milk, and reacted with 1:1,000 diluted anti-His or
1:1,000 anti-GST for 1 h at 37°C. The membrane was then incubated
with goat anti-mouse IgG (H+L), HRP conjugated secondary antibody
(1:2,000, Santa Cruz). Chemiluminescence detection was carried out
using an ECL Plus Western Blotting Detection system (Amersham
Biosciences) according to the manufacturer's instructions.
Cell proliferation assay
Cell viability was determined using the Cell
Counting kit-8 (CCK-8) cell proliferation assays according to the
manufacturer's instructions (Beyotime). Briefly, MDA-MB-231 or
MDA-MB-435 cells were plated in three 96-well microplates in 200 μl
of medium, and co-cultured with PI-TK fusion protein (0, 40, 80,
120, 160 and 200 μg/ml), and then supplemented with 10 mg/l
ganciclovir (GCV) the following day. After 48 h, 20 μl of CCK8
substrate was added into each well, and the palates were returned
to standard tissue incubator conditions for an additional 1 h. The
medium was then removed, the cells were solubilized in 150 μl of
dimethyl sulfoxide, and colorimetric analysis was performed
(wavelength, 450 nm). The inhibition rate was calculated as [1− (OD
value of the transfection/OD value of untreated cells)] × 100%.
Each experiment was performed in triplicate. TK protein was added
into the cells as a control under the same experiment conditions
and the procedure was repeated twice.
Cytotoxicity assay
Human breast carcinoma MDA-MB-231 cells were grown
in Lei Ovitz's (L-15) supplemented with 10% fetal born serum (Life
Technologies, Inc.) containing penicillin (100 U/ml) and
streptomycin (100 μg/ml). All of the cultures were maintained in a
37°C incubator with 5% CO2 in air. The cells were plated
in Costar 24-well culture plates at a density of 2×103
cells/well. The cells were treated with different amounts of
purified recombinant proteins for 6 days, and the cytotoxicity was
determined using the CCK8 method, as described above.
Persistent killing effect assay
MDA-MB-231 cells were treated with 200 μg/ml of
PI-TK, or TK as a control for 4 h, and then washed with fresh
medium and cultured in regular medium. The cells were subcultured
every 3 days, and samples were exposed to 10 μg/ml GCV at
intervals. The viability was assessed for 15 days, and determined
using the CCK8 method, as described above.
Biodistribution and SPECT imaging in
mice
The biodis-tribution analysis studies were carried
out in 30 BALB/c mice (4–6-week-old, weighing 20–22 g, purchased
from the Third Military Medical University, Chongqing, China).
99mTc-HYNIC-PI was injected into 30 mice via the tail
vein. Mice were divided into 6 groups and samples were obtained 30
min, 1, 2, 4 and 6 h after injection of 100 μl of radiotracer. All
the groups were dynamically imaged using Millennium™ MPR SPECT. At
each time-point, a 200-μl blood sample was taken from the
suborbital sinus of non-anaesthetized mice. Immediately after
obtaining this blood sample the animals were euthanized by cervical
dislocation and the main organs were dissected and counted in a
gamma counter. The injection dose rate (% of ID/g) and tumor/non
tumor tissue radioactivity (T/NT) ratio were calculated. All the
animal studies were conducted according to the international
standard and the ethics approval was granted by Ethics Committee of
Kunming Medical University, P.R. China.
Statistical analyses
The efficacy of the two treatment groups was
compared by Student's t-test. In cases of more than two variable
groups, two-way ANOVA and Bonferroni's post hoc test were used
(GraphPad Prism 6.0, GraphPad Software Inc., San Diego, CA,
USA).
Results
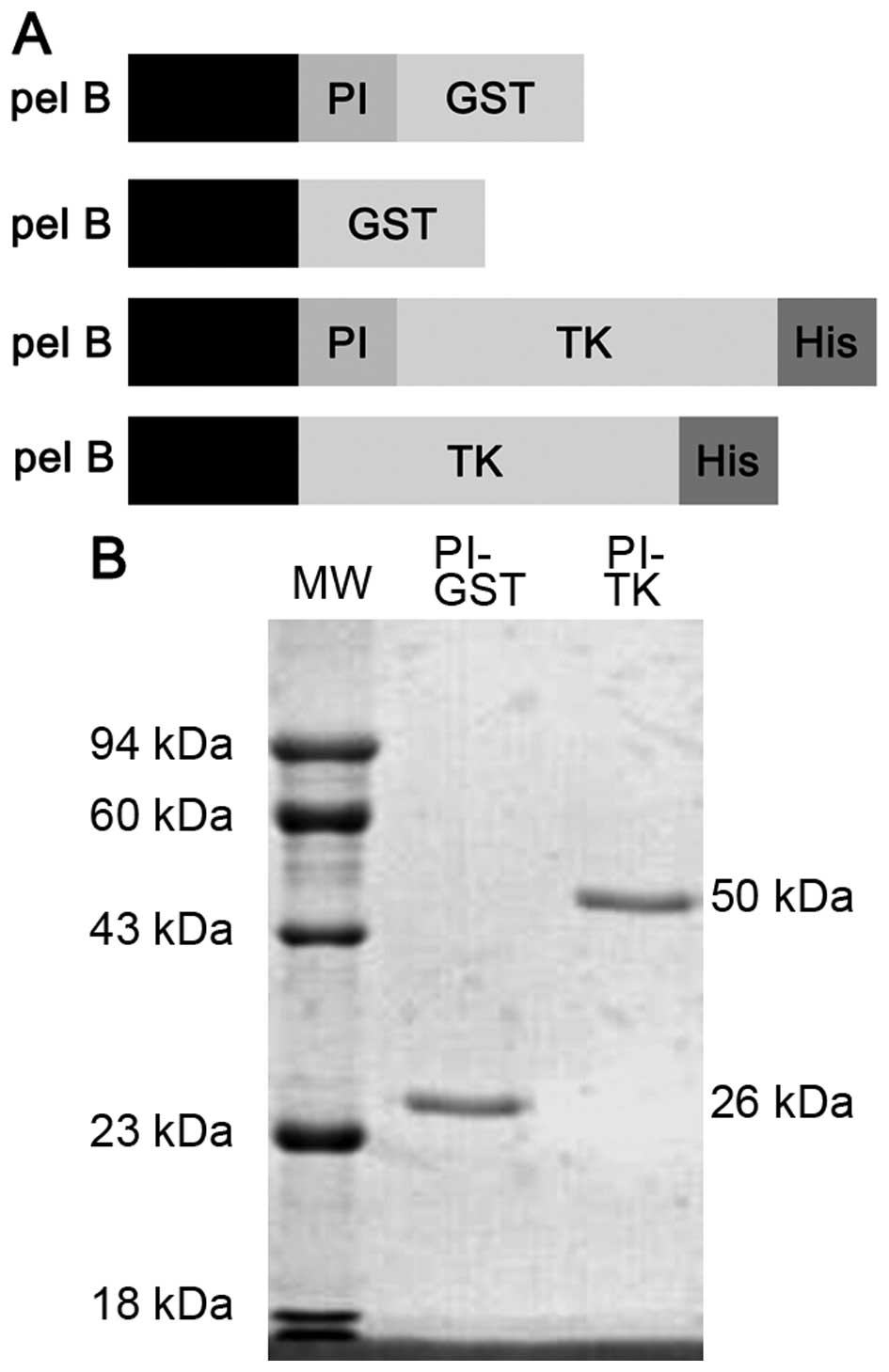
Expression and identification of fusion
proteins in vitro
The recombinant prokaryotic expression plasmids
pGEX-2T, pGEX-2T-PI, pET-28a (+)-PI-TK and pET-28a (+)-TK, were
first characterized by EcoRI and BamHI, EcoRI
and SalI, restriction enzyme digestion, respectively, in
accordance with the size of the target DNA, with no shift and no
mutation (Fig. 1A). Then, PI-GST
and PI-TK purified proteins were analyzed on a 12%
SDS-polyacrylamide gel. Single bands corresponding to molecular
masses of 26 and 50 kDa were observed, which were inconsistent with
the theoretical values (Fig.
1B).
Delivery location and efficiency of PI in
target cells
At the optimal peptide concentration of 200 mg/ml,
PI-GST was efficiently uptaken by MDA-MB-231 cells. In the initial
12 h of culturing, PI-GST gave strong staining mainly on the cell
membrane, but also showed substantial intracellular staining. After
24 h, the intracellular PI/GST signal was observed in the nucleus.
Forty-eight hours later, the signal of fusion proteins in cells
weakened and disappeared. Compared to MDA-MB-231 cells, the
intracellular fluorescence of PI-GST was not detected in MDA-MB-435
cells under the microscope (Fig.
2A).
To determine the PI delivery efficiency, we
incubated PI-GST or PI-TK, with breast cancer cells, and performed
immunoblotting with anti-GST and anti-His antibody (Fig. 2B). The results showed that PI
fusion proteins were expressed at detectable levels, but the
expression of non-PI fusion proteins was not observed.
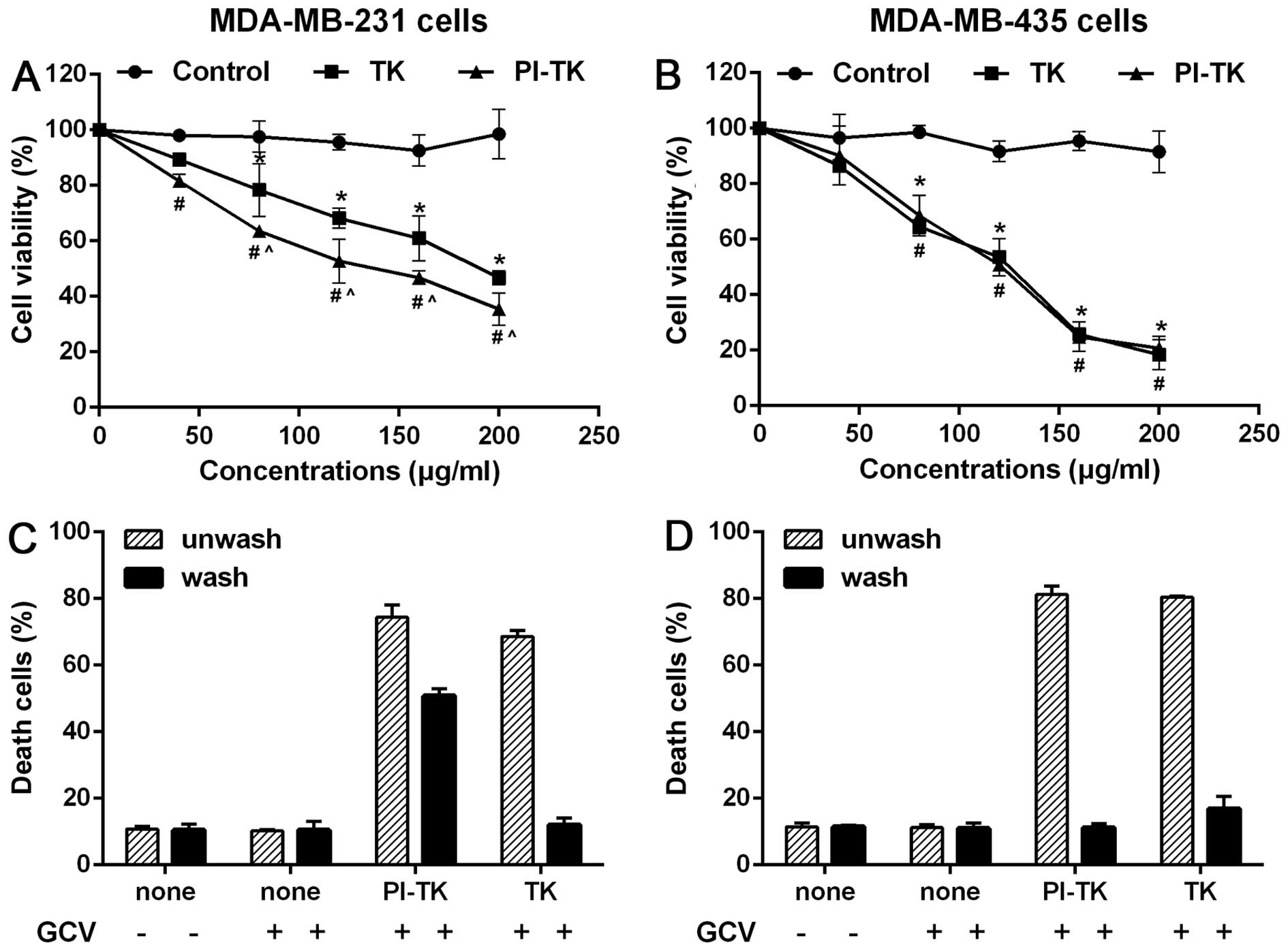
The cell type-specific killing effect of
PI-TK in the presence of GCV
To evaluate the cell-killing efficiency in
vitro, we co-cultured MDA-MB-231 or MDA-MB-435 cells with PI-TK
fusion protein with for 48 h at various concentrations and then
treated the cells with GCV. The cell viability results showed that
the proliferation rate of attached cells in the PI-TK and TK groups
gradually increased and that the cell growth inhibition rate in the
PI-TK group was higher than that in the TK group (Fig. 3A). As a control, the growth of
MDA-MB-435 cells treated with PI-TK or TK was significantly
inhibited under the same experimental conditions. However, no
difference in MDA-MB-435 cell proliferation was observed between
the PI-TK and TK groups (Fig.
3B).
Second, we performed the following experiments using
MDA-MB-231 and MDA-MB-435 lines. First, both cell types were
treated with recombinant proteins PI-TK, PI, and TK for 4 h. Then,
each set of treated cells was divided into two parts: one part was
extensively washed with the regular medium to remove the
recombinant proteins, and the other part was not. The cells in both
parts were then treated with GCV. As expected, unwashed MDA-MB-231
cells treated with PI-TK or TK alone were sensitive to the
subsequent GCV treatments (Fig.
3C, hatched bars). In the washed cultures, only
PI-TK-pretreated cells were sensitive to the GCV treatment
(Fig. 3C, solid bars). In similar
experiments with MDA-MB-435 (Fig.
3D), the removal of PI-TK or TK from the treated cells failed
to induce cell death upon the subsequent addition of GCV.
These results suggested that PI-TK could be taken up
specially by MDA-MB-231 cells and exerted cell-killing
activity.
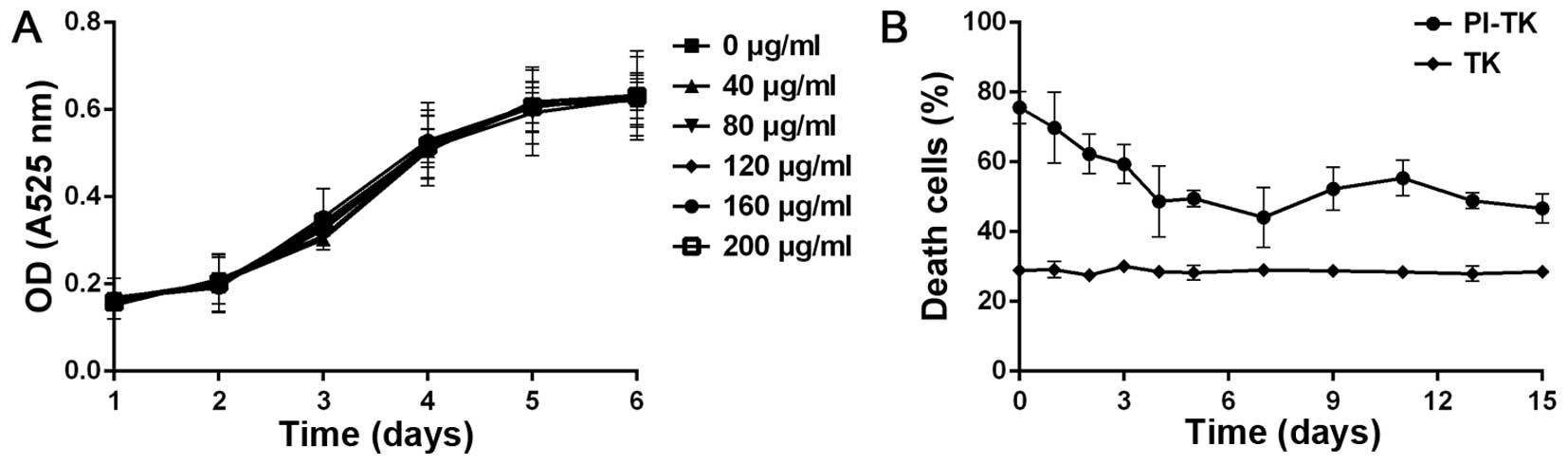
Cytotoxicity and stability of
internalized PI-TK
Herpes simplex virus thymidine kinase (HSV-TK)/GCV
system was delivered into tumor cells to test its killing effect.
To exclude the cytotoxicity and confirm adherence of the fusion
protein, PI-TK, we incubated the fusion protein with MDA-MB-231
alone. The results of the CCK-8 methods revealed that this system
was non-cytotoxic to cells, as shown in Fig. 4A.
To determine the stability of PI-TK, we carried out
cell-killing experiments. MDA-MB-231 cells were treated with 200
μg/ml of PI-TK or TK for 4 h then the recombinant proteins were
removed, and the cells were cultured in fresh medium. The
proliferation was detected at different times after treating with
GCV for 15 days. The sustained cell-killing was observed in the
PI-TK-treated cells, even at 15 days, and only the basal level of
cell death (t<10%) was observed in TK-treated cells (Fig. 4B). This observation supports the
sustained stability of the recombinant PI fusion protein.
Biodistribution and SPECT imaging of
99mTc-HYNIC-PI
The biodistribution and metabolization in normal
mice and nude mice bearing human breast cancer cells MDA-MB-231
were investigated in this study. The biodistribution of PI in
normal mice indicated that the blood radioactivity decreases
[(1.45±0.44)% ID/g] at 30 min and that 99mTc-HYNIC-PI
was cleared rapidly thereafter. The physiological uptakes of the
kidney [(14.83±1.48)% ID/g] was the highest at 4 h and revealed
that the radio-pharmaceutical was primarily excreted by the kidney.
The in vivo distribution of tumor-bearing mice was similar
to that of the normal mice. The tumor T/NT was higher and reached
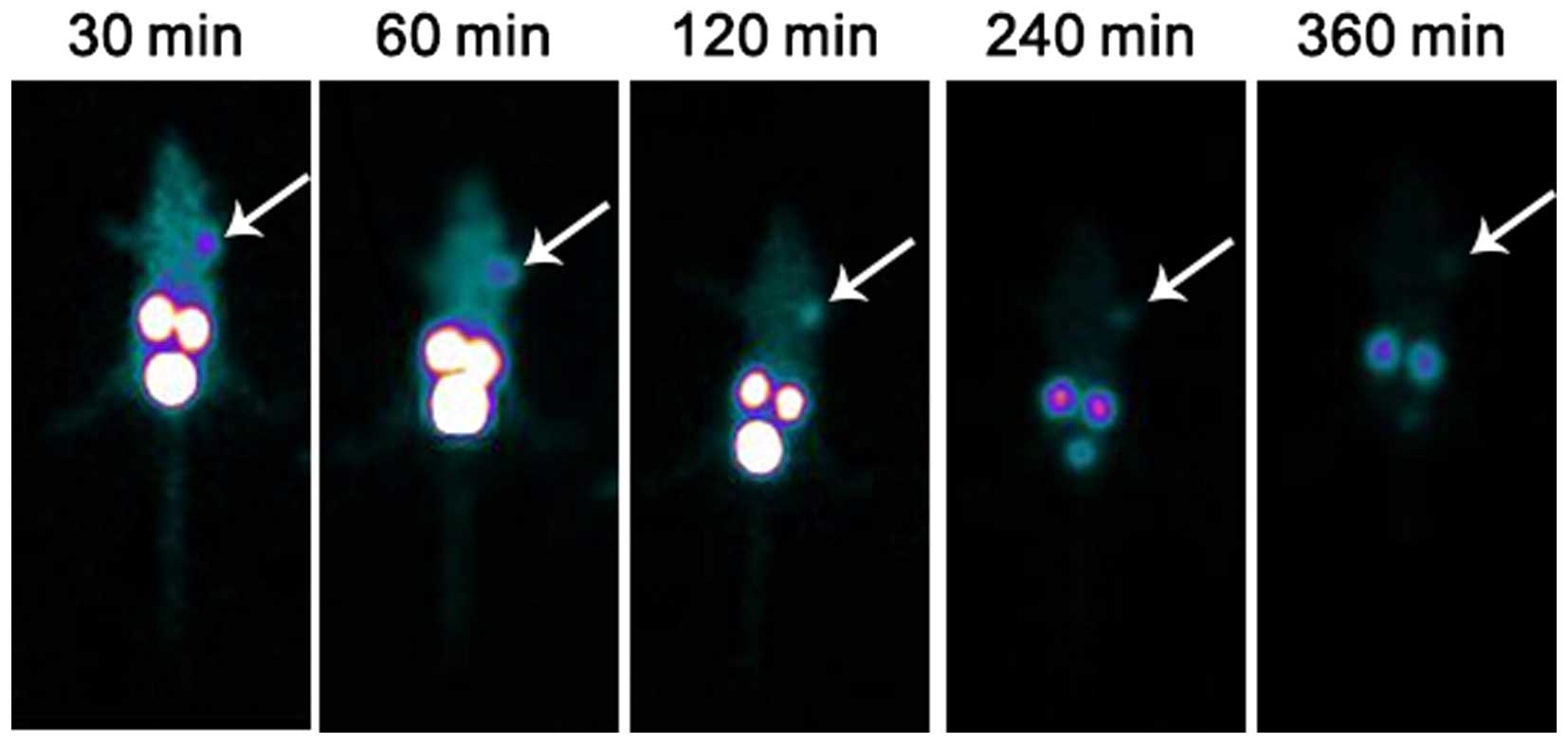
(9.67±2.88) at 4 h. All the data are shown in Tables I, II and III. SPECT showed that the imaging of
the mouse outline was clearly observed at 30 min. The radioactivity
uptake in the kidneys and bladder was also high. Tumor sites showed
rapid and intense tracer accumulation. The tumor imaging quality
was high at 1 h and then gradually decreased. By 6 h, the imaging
was blurred (Fig. 5).
 | Table IRadioactivity uptake of
99Tcm-(HYNIC-PI) (tricine) (TPPTS) in normal
mouse at each time point (mean ± SD, n=5, % ID/g). |
Table I
Radioactivity uptake of
99Tcm-(HYNIC-PI) (tricine) (TPPTS) in normal
mouse at each time point (mean ± SD, n=5, % ID/g).
| Time |
|---|
|
|
|---|
| Organ | 1 min | 5 min | 10 min | 30 min | 1 h | 2 h | 4 h | 6 h |
|---|
| Heart | 2.20±0.23 | 1.31±0.17 | 0.96±0.35 | 0.42±0.15 | 0.41±0.14 | 0.22±0.02 | 0.18±0.03 | 0.12±0.08 |
| Liver | 1.21±0.72 | 0.83±0.08 | 0.69±0.24 | 0.31±0.10 | 0.25±0.03 | 0.19±0.03 | 0.11±0.02 | 0.08±0.03 |
| Lung | 4.6±0.84 | 2.63±0.37 | 2.41±0.58 | 0.82±0.24 | 0.69±0.16 | 0.37±0.01 | 0.30±0,02 | 0.22±0.09 |
| Kidney | 7.72±0.94 | 9.33±0.33 | 10.31±4.00 | 10.75±2.24 | 13.53±2.47 | 13.57±1.79 | 14.83±1.48 | 11.23±1.34 |
| Spleen | 1.22±0.36 | 1.05±0.19 | 0.85±0.10 | 0.47±0.14 | 0.41±0.08 | 0.29±0.04 | 0.17±0.06 | 0.14±0.13 |
| Stomach | 1.00±0.45 | 0.51±0.22 | 0.48±0.30 | 0.16±0.04 | 0.14±0.03 | 0.10±0.04 | 0.11±0.04 | 0.09±0.05 |
| Bone | 1.34±0.13 | 1.21±0.60 | 1.08±12.10 | 0.69±0.16 | 0.44±0.30 | 0.21±0.02 | 0.23±0.07 | 0.20±0.14 |
| Muscle | 1.11±0.16 | 1.15±0.12 | 0.80±0.41 | 0.35±0.10 | 0.24±0.11 | 0.16±0.04 | 0.15±0.05 | 0.11±0.12 |
| Gastric | 1.49±0.24 | 1.46±0.28 | 1.14±0.44 | 0.37±0.12 | 0.35±0.23 | 0.17±0.03 | 0.12±0.02 | 0.09±0.06 |
| Blood | 2.53±0.61 | 2.27±0.13 | 1.75±0.35 | 1.45±0.44 | 0.46±0.17 | 0.23±0.03 | 0.17±0.03 | 0.13±0.07 |
 | Table IIRadioactivity uptake of
99Tcm-(HYNIC-PI) (tricine) (TPPTS) in tumor
bearing mice at each time point (mean ± SD, n=5, % ID/g). |
Table II
Radioactivity uptake of
99Tcm-(HYNIC-PI) (tricine) (TPPTS) in tumor
bearing mice at each time point (mean ± SD, n=5, % ID/g).
| Time |
|---|
|
|
|---|
| Organ | 30 min | 60 min | 120 min | 240 min | 360 min |
|---|
| Heart | 0.43±0.07 | 0.49±0.04 | 0.32±0.06 | 0.21±0.08 | 0.15±0.04 |
| Liver | 0.63±0.14 | 0.67±0.21 | 0.61±0.26 | 0.29±0.10 | 0.28±0.13 |
| Lung | 0.91±0.13 | 0.82±0.05 | 0.69±0.26 | 0.36±0.11 | 0.19±0.06 |
| Kidney | 11.66±1.01 | 12.08±0.98 | 13.96±4.68 | 26.98±9.31 | 22.72±6.84 |
| Spleen | 0.33±0.04 | 0.35±0.13 | 0.30±0.09 | 0.20±0.08 | 0.13±0.10 |
| Stomach | 0.24±0.06 | 0.36±0.04 | 0.31±0.10 | 0.28±0.03 | 0.26±0.08 |
| Bone | 0.44±0.06 | 0.61±0.21 | 0.56±0.06 | 0.42±0.18 | 0.33±0.06 |
| Muscle | 0.34±0.06 | 0.36±0.04 | 0.27±0.09 | 0.15±0.04 | 0.09±0.02 |
| Gastric | 0.44±0.19 | 0.51±0.13 | 0.42±0.08 | 0.28±0.08 | 0.30±0.05 |
| Tumor | 2.48±0.24 | 1.46±0.02 | 1.40±0.20 | 1.38±0.29 | 0.67±0.24 |
| Blood | 0.88±0.15 | 0.81±0.06 | 0.48±0.04 | 0.25±0.07 | 0.13±0.04 |
 | Table IIIT/NT ratio of
99Tcm-(HYNIC-PI) (tricine) (TPPTS) in
tumor-bearing mice. |
Table III
T/NT ratio of
99Tcm-(HYNIC-PI) (tricine) (TPPTS) in
tumor-bearing mice.
| Time |
|---|
|
|
|---|
| Ratio | 30 min | 60 min | 120 min | 240 min | 360 min |
|---|
| Tumor/muscle
(T/NT) | 7.49±1.83 | 4.14±0.69 | 5.54±1.40 | 9.67±2.88 | 5.75±1.60 |
| Tumor/blood
(T/NT) | 2.85±0.41 | 1.80±0.16 | 2.91±0.36 | 5.85±1.81 | 4.00±0.73 |
Discussion
Current approaches, including surgery, chemotherapy
and radiotherapy, are still insufficiently effective in treating
tumors because of their invasive, aggressive growth profile, as
well as the complex mechanisms involved in cancer development. New
anticancer strategies are thus urgently required. Targeted cancer
gene therapy is of unquestionable importance for improving
therapeutic efficacy and minimizing adverse effects (13,14).
Although current gene therapy is mainly limited by the lack of
efficient gene delivery vehicles, the recent development of cell
penetrating peptides (CPPs) has overcome these barriers, leading to
novel tumor-specific molecular therapeutics. The protein
transduction domains (PTD) (15,16),
can also facilitate cytoplasmic and nuclear delivery of a
conjugated cargo and have attracted much interest.
Importantly, the non-toxic mechanism of cell
penetration allows for the safe and effective systemic delivery of
cancer therapeutics, whereas when the PTDs are applied in antitumor
therapy, they transfer the exogenous genes or drugs not only into
cancer cells but also into normal cells, thus damaging these cells.
Therefore, it is desirable to find a tumor cell-specific targeted
vector to solve this problem. Recently, several laboratories have
selected peptides as a novel vehicle to target the specific cells
or tissues (17–19). This strategy offers an approach to
treat cancer in a specific manner by delivering optimized protein
or therapeutic cargo to achieve anticancer therapies. During the
past decade, numerous reports and patents of peptides have been
published in the field of cancer therapy, demonstrating their
potential for the treatment of leukemia and breast, lung,
pancreatic, ovarian and colon cancers (20–27).
However, there has been no peptide vehicle for breast cancer, so
our team was devoted to selecting a breast cancer-specific peptide
and evaluating its potential as a specific delivery system for
tumor targeting therapy. We co-cultured the pC89 phage display
library with MDA-MB-231 TNBC cells for the selection of a peptide
with tumor cellular specificity. In our results, we found four
peptides but CASPSGALRSC-presenting phage, named PI, was the best
candidate clone with the highest specificity for MDA-MB-231 cells
and was thus selected for the following cellular binding assay
(12). For comparison,
RGD-integrin binding phage was introduced as a control, because it
is known to be recognized and internalized by many types of human
cells (28,29).
The affinity experiment suggested that PI is a new
type of transmembrane peptide with MDA-MB-231 cell specificity. The
notable cellular specificity of PI, the combination of it as a
vector of therapeutic protein, may be a practical way to improve
targeting efficiency in tumor therapy. In this report, with the aim
of investigating the delivery potency of PI, GST was introduced as
an exogenous protein which was fused with PI and acted as a marker
to assess the protein transduction ability of PI in vitro.
PI can successfully allow GST to penetrate into the cytoplasm and
maintain the non-degradation of exogenous proteins for ≥72 h.
Significant immunofluorescence signals of PI/GST were observed in
MDA-MB-231 cytoplasm, but there were no signals of GST in
MDA-MB-231 cells, indicating that PI can deliver exogenous protein
of ≥26 kDa size into MDA-MB-231 cells. Moreover, this transduction
procedure is cell-specific, as confirmed by co-culturing PI/GST
with different human breast cancer cell lines. No signal of PI/GST
was observed in MDA-MB-435 cells. This result revealed that PI
facilitated the delivery of exogenous molecules across the cell
membrane. Furthermore, the presence of intact PI/GST fusion protein
was detected around the cell nucleus, which suggested the
nucleophilicity of PI as a delivery system. This conjecture was
confirmed by observing the transportation of PI/GST into the cell
nucleus during the terminal 24 h of incubation. The
nucleus-localized nature of PI thus provides a promising method to
develop a vehicle to deliver therapeutic proteins or
polynucleotides to the target site and be effective. Therefore, we
supposed that PI can deliver drug proteins for cancer targeting
therapy in a cell-specific manner. However, whether it localizes
tumors and how it metabolizes in vivo remain unknown. To
determine its biodistribution in vivo, we marked PI with
99mTc as a tracer. As expected, 99mTc-
HYNIC-PI was mainly eliminated through the kidneys with some
residual activity. Radioactivity was reduced to near background
levels at 6 h after injection. In MDA-MB-231 cell-bearing nude
mice, tumors showed moderately increased activity compared to the
background, allowing the detection of PI by imaging and revealing
tumor-targeting localization. Free
99mTc-HYNIC-expression of the target in normal organs
was less intense. When imaging with 99mTc-HYNIC, these
tissues took up the radionuclide because of its negative charge.
99mTc-HYNIC-PI had good bioavailability with adequate
blood activity and minor tissue activity in normal organs. Target
mediated drug delivery may be further studied with PI due to its
tumor-specific enrichment. Studies have validated the potential of
PI as a vehicle for safe delivery, effectively killing proteins in
the target cells. In many studies, herpes simplex virus thymidine
kinase/ganciclovir suicide gene system (HSV-TK/GCV) has shown an
effective role in cancer therapy (30). However, using the HSV-TK/GCV system
alone has limitations, including the division of cells limiting the
killing effect, and the inability of targeting tumors with distant
metastases. Therefore, in our experiment, we used the specific
peptide PI delivering HSV-TK suicide gene transfer, combined with
systemically administered ganciclovir (GCV), as therapeutic
proteins delivered into target cells. When co-cultured with
MDA-MB-231 cells, PI-HSV-TK itself did not present any cytotoxic
effects, but when cultured with GCV, PI-TK/GCV was able to
obviously inhibit the proliferation of MDA-MB-231 cells and
exhibited a dose-dependent efficacy and an IC50 value of
152.64 μg/ml (data not shown). Our study also showed that death
increased when the ‘suicide gene’ product TK was fused with PI.
This result suggests that PI delivered the HSV-TK in a way that
allowed it to localize in the nucleus and exhibit its
target-killing effect and may be an efficient drug delivery vector
for MDA-MB-231 TNBC breast cancer targeting therapy. However, the
mechanism involved remains unclear.
In a previous study, we confirmed that the
specificity of PI combined with 231 cells was not related to the
expression of ER and P53 or MHC-I. In other preliminary research
using our phage library, the lipid rafts/caveolin pathway
inhibitors nystatin and MDA-MB-231 breast cancer cells were
co-cultured and then added to the PI. Fluorescence microscopy and
flow cytometry analysis showed lower and weaker fluorescent signal
than that with cells that were not treated with nystatin. This
finding suggests that nystatin has certain inhibitory effects for
the combination of PI and MDA-MB-231 breast cancer cells. We
preliminarily speculated that mechanism of PI transducing into
MDA-MB-231 cells was mediated by nest protein, which depended on
the Caveolae endocytosis mechanism, however, further research is
still necessary.
In conclusion, this study supports that the capacity
of PI to deliver molecules into target cells and indicate its
therapeutic potential in individual therapy. PI may be an efficient
drug delivery vectors for TNBC cancer targeting therapy. However,
our knowledge on this interesting phenomenon is limited, and the
exact transduction mechanism is unknown. Further work is needed to
identify the target molecule.
Acknowledgements
We thank Mr. Bo Yang for reviewing the manuscirpt.
This study was supported by grants (nos. 30860330 and 30460142) of
National Natural Science Foundation of China, ‘The applied basic
research projects in Yunnan Province’ (no. 2009CC023) and was a
major scientific research project of Yunnan Provincial Education
Fund (ZD2013005).
References
|
1
|
Aguas F, Martins A, Gomes TP, de Sousa M
and Silva DP; Portuguese Menopause Society and Portuguese
Gynaecology Society. Prophylaxis approach to a-symptomatic
post-menopausal women: Breast cancer. Maturitas. 52(Suppl 1):
S23–S31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dumitrescu RG and Cotarla I: Understanding
breast cancer risk - where do we stand in 2005? J Cell Mol Med.
9:208–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Toole SA, Beith JM, Millar EK, West R,
McLean A, Cazet A, Swarbrick A and Oakes SR: Therapeutic targets in
triple negative breast cancer. J Clin Pathol. 66:530–542. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hurley J, Reis IM, Rodgers SE,
Gomez-Fernandez C, Wright J, Leone JP, Larrieu R and Pegram MD: The
use of neoadjuvant platinum-based chemotherapy in locally advanced
breast cancer that is triple negative: Retrospective analysis of
144 patients. Breast Cancer Res Treat. 138:783–794. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Ejeh F, Shi W, Miranda M, Simpson PT,
Vargas AC, Song S, Wiegmans AP, Swarbrick A, Welm AL, Brown MP, et
al: Treatment of triple-negative breast cancer using
anti-EGFR-directed radioimmunotherapy combined with
radiosensitizing chemotherapy and PARP inhibitor. J Nucl Med.
54:913–921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodler E, Korde L and Gralow J: Current
treatment options in triple negative breast cancer. Breast Dis.
32:99–122. 2010.
|
|
7
|
De Laurentiis M, Cianniello D, Caputo R,
Stanzione B, Arpino G, Cinieri S, Lorusso V and De Placido S:
Treatment of triple negative breast cancer (TNBC): Current options
and future perspectives. Cancer Treat Rev. 36(Suppl 3): S80–S86.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kummar S, Kinders R, Gutierrez ME,
Rubinstein L, Parchment RE, Phillips LR, Ji J, Monks A, Low JA,
Chen A, et al: Phase 0 clinical trial of the poly (ADP-ribose)
polymerase inhibitor ABT-888 in patients with advanced
malignancies. J Clin Oncol. 27:2705–2711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Helleday T, Bryant HE and Schultz N: Poly
(ADP-ribose) polymerase (PARP-1) in homologous recombination and as
a target for cancer therapy. Cell Cycle. 4:1176–1178. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koren E and Torchilin VP: Cell-penetrating
peptides: Breaking through to the other side. Trends Mol Med.
18:385–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryu JS, Kuna M and Raucher D: Penetrating
the cell membrane, thermal targeting and novel anticancer drugs:
The development of thermally targeted, elastin-like polypeptide
cancer therapeutics. Ther Deliv. 5:429–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong J, Liu W, Jiang A, Zhang K and Chen
M: A novel peptide, selected from phage display library of random
peptides, can efficiently target into human breast cancer cell.
Chin Sci Bull. 53:860–867. 2008. View Article : Google Scholar
|
|
13
|
Zadeh G, Qian B, Okhowat A, Sabha N,
Kontos CD and Guha A: Targeting the Tie2/Tek receptor in
astrocytomas. Am J Pathol. 164:467–476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Palma M, Venneri MA and Naldini L: In
vivo targeting of tumor endothelial cells by systemic delivery of
lentiviral vectors. Hum Gene Ther. 14:1193–1206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Green M and Loewenstein PM: Autonomous
functional domains of chemically synthesized human immunodeficiency
virus tat trans-activator protein. Cell. 55:1179–1188. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frankel AD and Pabo CO: Cellular uptake of
the tat protein from human immunodeficiency virus. Cell.
55:1189–1193. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du B, Han H, Wang Z, Kuang L, Wang L, Yu
L, Wu M, Zhou Z and Qian M: targeted drug delivery to
hepatocarcinoma in vivo by phage-displayed specific binding
peptide. Mol Cancer Res. 8:135–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li ZJ, Wu WKK, Ng SSM, Yu L, Li HT, Wong
CC, Wu YC, Zhang L, Ren SX, Sun XG, et al: A novel peptide
specifically targeting the vasculature of orthotopic colorectal
cancer for imaging detection and drug delivery. J Control Release.
148:292–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Yin G, Yan D, Wei Y, Ma C, Huang
Z, Liao X, Yao Y, Chen X and Hao B: In vitro screening of ovarian
tumor specific peptides from a phage display peptide library.
Biotechnol Lett. 33:1729–1735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu Z, Wu J, Wu S, Jia P, Tong Y, Wu X and
Wang Y: A recombinant cell-permeable p53 fusion protein is
selectively stabilized under hypoxia and inhibits tumor cell
growth. Cancer Lett. 279:101–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu M, Wang J, Chen P and Reilly RM: HIV-1
Tat peptide immunoconjugates differentially sensitize breast cancer
cells to selected antiproliferative agents that induce the
cyclin-dependent kinase inhibitor p21WAF-1/CIP-1. Bioconjug Chem.
17:1280–1287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bitler BG, Menzl I, Huerta CL, Sands B,
Knowlton W, Chang A and Schroeder JA: Intracellular MUC1 peptides
inhibit cancer progression. Clin Cancer Res. 15:100–109. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Massodi I, Bidwell GL III, Davis A,
Tausend A, Credit K, Flessner M and Raucher D: Inhibition of
ovarian cancer cell metastasis by a fusion polypeptide Tat-ELP.
Clin Exp Metastasis. 26:251–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bidwell GL III, Davis AN and Raucher D:
Targeting a c-Myc inhibitory polypeptide to specific intracellular
compartments using cell penetrating peptides. J Control Release.
135:2–10. 2009. View Article : Google Scholar
|
|
25
|
Harada H, Hiraoka M and Kizaka-Kondoh S:
Antitumor effect of TAT-oxygen-dependent degradation-caspase-3
fusion protein specifically stabilized and activated in hypoxic
tumor cells. Cancer Res. 62:2013–2018. 2002.PubMed/NCBI
|
|
26
|
Takada Y, Singh S and Aggarwal BB:
Identification of a p65 peptide that selectively inhibits NF-kappa
B activation induced by various inflammatory stimuli and its role
in down-regulation of NF-kappaB-mediated gene expression and
up-regulation of apoptosis. J Biol Chem. 279:15096–15104. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan M, Lan K-H, Yao J, Lu CH, Sun M, Neal
CL, Lu J and Yu D: Selective inhibition of ErbB2-overexpressing
breast cancer in vivo by a novel TAT-based ErbB2-targeting signal
transducers and activators of transcription 3-blocking peptide.
Cancer Res. 66:3764–3772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miller WH, Alberts DP, Bhatnagar PK,
Bondinell WE, Callahan JF, Calvo RR, Cousins RD, Erhard KF,
Heerding DA, Keenan RM, et al: Discovery of orally active
nonpeptide vitro-nectin receptor antagonists based on a
2-benzazepine Gly-Asp mimetic. J Med Chem. 43:22–26. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao H, Wang J-C, Sun Q-S, Luo C-L and
Zhang Q: RGD-based strategies for improving antitumor activity of
paclitaxel-loaded liposomes in nude mice xenografted with human
ovarian cancer. J Drug Target. 17:10–18. 2009. View Article : Google Scholar
|
|
30
|
Burrows FJ, Gore M, Smiley WR, Kanemitsu
MY, Jolly DJ, Read SB, Nicholas T and Kruse CA: Purified herpes
simplex virus thymidine kinase retroviral particles: III.
Characterization of bystander killing mechanisms in transfected
tumor cells. Cancer Gene Ther. 9:87–95. 2002. View Article : Google Scholar : PubMed/NCBI
|