Introduction
Lung cancer is a very common malignant tumor with
incidence and mortality rates the first worldwide, almost one
million patients are diagnosed with lung cancer each year all over
the world. NSCLC accounts for 75–85% among the total number of lung
cancer, and elderly patients aged >65 years accounted for
>50% (1). It is always at the
late stage when patients were discovered (stage III or IV) and the
5-year survival rate is only 12–15% (2,3),
thus, these patients cannot receive surgical treatment (4,5).
For elderly advanced NSCLC patients, chemotherapy is
still the first choice of treatment (6,7), it
is a relatively effective treatment for elderly NSCLC patients who
cannot tolerant surgical operation. It can effectively reduce the
progress of lung cancer and the recurrence, enhance the effect of
clinical treatment, prolong patient survival rate and improve their
quality of life (8).
There are some chemotherapy regimens and drugs for
elderly NSCLC patients, such as cisplatin, VP-16, gemcitabine,
paclitaxel and docetaxel (9).
However, there is lack of a mature and effective way for NSCLC
because most of the elderly patients refuse or are unable to
complete the treatment. So how to choose a drug with good effect
and little side reaction is a hot spot in tumor research (10,11).
Docetaxel is a cell cycle specific antitumor drug which is applied
to cells at the M phase. It is a relatively new type of
anti-microtubule taxane drug, and it is the only one approved for
first- and second-line chemotherapy for NSCLC drug treatment by the
U.S. Food and Drug Administration (FDA) and the EU. According to
the existing clinical data of current studies, DTX has been proved
very effective for NSCLC (12,13).
However, the low solubility of DTX severely limits
its clinical application. Polysorbate 80 (Tween-80) in saline
solution was used to enhance the solubility of DTX in commercial
products, such as Taxotere® and Aisu®, it can
induce hemolysis reaction and allergic reaction which is very
common in clinical use. DTX in these two commercial products
(Taxotere and Aisu) showed non-specific toxicities to normal
organs, which led to intolerable side-effects such as peripheral
neuropathy, hemolytic reaction and hypersensitivity reactions
(14). Furthermore, the short
elimination half-life of DTX in commercial products cause to more
frequent injection in clinic which increase inconvenience and pain
for the patients (15). Therefore,
removing the side-effects and improving the half-life of DTX is
getting ihncreased attention (16).
Biodegradable albumin nanoparticles have received
attention in cancer targeted therapeutics during the past few
decades (17). Previous reports
have proven that drug-loaded polymeric nanoparticles could
accumulate in certain tumors more efficiently than other carriers
by enhancing permeability and retention (EPR) effect (18,19).
Another advantage is the long circulating half-life and lower
systemic toxicity which is superior to conventional drug
formulations (20–23).
The molecular formula of polyethylene glycol (PEG)
is H (CH2CH2O) nOH, it is one of medicinal synthetic polymer
injections which can be used for humans approved by FDA. There are
many advantages of PEG drugs such as: i) extending biologic
half-life of drug, enhancing long-acting and sustained-release
effect; ii) improving the solubility and stability of the drug;
iii) reducing immunogenicity and antigenicity; iv) reducing enzyme
degradation; v) enhancing the targeting function of drugs; vi)
reducing the toxicity of some drugs. Based on the above advantages,
we chose PEG to formulate polymeric nanoparticles because of its
excellent biocompatibility and biodegradability, then we made a
comparison between DANPs and PEG-DANPs to see their effect against
NSCLC.
Aiming to develop a good nanoparticle carrier for
DTX, we synthesized PEG-DANPs via the emulsion-evaporation
cross-link method, and we carried out a series of experiments in
vitro, the results demonstrated that PEG-DANPs were a promising
modality for NSCLC.
Materials and methods
Materials
All reagents and solvents were used as received,
without further purification. Monomethoxy polyethylene glycol with
a molecular weight of 20,000 kDa (mPEG 20,000), D,L-lactide, and
stannous octoate were purchased from Sigma-Aldrich Chemical Corp.
(Shanghai, China); DTX was purchased from Beijing Norzer
Pharmaceutical Co., Ltd. (China, Beijing); free DTX
(Aisu®) was manufactured by Jiangsu Hengrui Medicine
Co., Ltd. (Jiangsu, China); and 3-(4,5)-dimethyl-thiazol(-z-y1)-3,5-di-phenyl-tetrazolium
bromide (MTT) was obtained from Amresco LLC (Solon, OH, USA);
Annexin V-FITC apoptosis detection kit was purchased from 4A
Biotech Co., Ltd (Beijing, China); VivoGlo® luciferin
was purchased from Promega Corp. (Madison, WI, USA). Trypsin, fetal
bovine serum (FBS) and RPMI-1640 medium were purchased from HyClone
Laboratories (Logan, UT, USA) and culture flasks and dishes were
from Corning Incorporated (Corning, NY, USA).
Cell line and animals
Human non-small lung cancer A549 cell line was
provided by the Department of Pathology in the Institute of
Medicinal Biotechnology in Peking Union Medical College. A549 cells
were cultured in RPMI-1640 medium supplemented with 10% heat
inactivated FBS and incubated in a humidified atmosphere of 5%
CO2 and 95% air at 37°C. Female BalB/c mice (6–8-week
old) were used for antitumor efficacy studies and were purchased
from Beijing Vital River Laboratories (Beijing, China).
Animals were acclimatized in the holding facility
prior to the beginning of the study. All animal procedures were
approved by the Institutional Animal Care and Use Committee of the
Chinese Academy of Medical Sciences. All surgeries were performed
under sodium pentobarbital anesthesia (5 mg/ml solution) and all
efforts were made to minimize suffering. Lung tumor and other
sections were routinely stained with hematoxylin and eosin
(H&E) and evaluated under a light microscope.
Preparation of DANPs and PEG-DANPs
DTX was dissolved in chloroform and ethanol to form
solution A, albumin was dissolved in sterile water to form solution
B. The solution A and B was mixed and stirred by homogenate machine
for 5 min to form raw milk, which was high pressure homogenized,
under 20,000 psi for 12 cycles. The chloroform of mixture was
eliminated by using rotary evaporator for 25 min and followed by
filtration through a 0.22-μm filter.
DANPs and mPEG (20,000 kDa) were added to the
solution of boric acid buffer (0.1 mol/l, pH 9.0) according to the
ratio of 3:1 for stirring and the reaction was terminated via
adding glycine (1 mg/ml) 3 h later. Unbound HAS and PEG were
removed by ultrafiltration (MWCO: 70 kDa) (24,25).
Determination via PAGE gel electrophoresis: DANPs
and PEG-DANPs were determined via PAGE gel electrophoresis with
iodine staining and mas blue staining.
Encapsulation efficiency and size
distribution
Loading capacity was defined as the percentage of
DTX by weight in the freeze-dried nanoparticles, and encapsulation
efficiency was defined as the percentage of DTX by weight
incorporated in the nanoparticles compared with the initial weight
of DTX (26). Both were determined
by high performance liquid chromatography (HPLC) (27). The content of DTX was assayed on an
Agilent 1200LC HPLC system (Agilent Technologies, Santa Clara, CA,
USA). The mobile phase consisted of a mixture of acetonitrile and
water (55:45, v/v) delivered at a flow rate of 0.5 ml/min. The
injection volume was 20 μl and the wavelength was set at 230 nm.
The column temperature was 25°C. The concentration of DTX was
determined based on the peak area (standard curve as
y=22.122x+45.3424, R=0.9996). Drug loading content (DLC) = Content
of physically loaded DANPs (PEG-DANPs)/Content of DANPs (PEG-DANPs)
× 100%. Encapsulation efficiency (EE) = Content of physically
loaded DANPs (PEG-DANPs)/Content of DANPs (PEG-DANPs) initially
added × 100%.
The mean diameter, size distribution and zeta
potential of the DANPs and PEG-DANPs were measured by a Malven Nano
ZS laser particle analyzer. All the analysis were performed three
times (28,29). The morphology of the DANPs and
PEG-DANPs nanoparticles was analyzed by transmission electron
microscopy (TEM).
In vitro drug release
In vitro release of drugs were assayed using
dialysis diffusion method (30,31).
Typically, 0.5 ml of Aisu, DANPs and PEG-DANPs at DTX concentration
of 1 mg/ml was sealed into a dialysis bag (MWCO 8000 kDa; Pierce).
The bag was immersed in 40 ml PBS (140 mmol/l, pH 7.4) at 37°C with
a shaking rate of 100 rpm (27).
At predetermined time-points, 0.2 ml samples were taken from the
bags and the same volume of fresh medium was added. Each sample was
centrifuged at 10,000 rpm and the supernatant was assayed by
HPLC.
Cell viability
The in vitro cytotoxic activity of DANPs and
PEG-DANPs was evaluted by the MTT assay. Briefly, the A549 cells
(8×104 cells/ml) were seeded in 96-well plates and
incubated for 24 h to allow cell attachment. The cells were then
treated with a series of PBS (control), Aisu, DANPs and PEG-DANPs,
at 37°C (0.001, 0.01, 0.1, 1, 10 and 100 μg/ml). At the determined
incubation time-points of 48 h, 20 μl of MTT (5 mg/ml) was added
and incubated for 4 h, MTT was aspirated and 180 μl/well of DMSO
was added to dissolve the formazan crystals, and the plate was
gently shaken for 10 min. The optical density (OD) was measured at
490 nm by Synergy H1m monochromator-Based multi-mode microplate
reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). The cell
inhibition was calculated according to the formula: Cell inhibition
(%) = [1−(ODsample −
ODblank)/(ODsample − ODblank)] ×
100%. The results are expressed as means ± SD of 3 measurements. No
precipitation of DTX was found during the incubation procedure
(32).
Cell apoptosis assay
Apoptotic cells were determined by dual staining
with an Annexin V and propidium iodide (PI) kit (4A Biotech)
according to the manufacturer's instructions (33). After 48 h of incubation in the
exponential stage, A549 cells seeded in 12-well plates were treated
for a further 48 h with 10 nmol/ml Aisu, DANPs and PEG-DANPs,
respectively. After treatment, cells were washed twice with warm
PBS, detached by trypsin without EDTA, then the following steps of
collection, centrifuge, washing with warm PBS were performed, and
further stained with PI and Annexin V-FITC for 15 min at room
temperature in the dark. Apoptosis was then analyzed using a
FACScan cytometer. Quadrant analysis was performed and cells that
stained positive for both Annexin V-FITC and PI were designated as
apoptotic, while unstained cells were designated as viable.
Cellular uptake of coumarin 6(C6)-loaded
SPM (C6-SPM)
For in vitro fluorescence imaging, the
near-infrared fluorescent probe C6 was loaded into DANPs and
PEG-DANPs to yield C6-SPM. Briefly, the polymers and excess C6 were
co-dissolved in CHCl3 and a thin film was formed by the
evaporation of CHCl3. PBS (pH 7.4) was added, followed
by vortexing for 10 min. The nanoparticles were extruded through a
sterile membrane of pore size 220 nm (Millipore) to remove free C6.
A549 cells in exponential-stage growth were incubated with C6-SPM
at 37°C for 5, 10, 20 and 30 min, respectively, then the plate was
rinsed three times with cold PBS and fixed with 4% paraformaldehyde
for 10 min. Finally, cells were observed by confocal laser scanning
microscopy (CLSM, TCS SP2; Leica Microsystems GmbH, Wetzlar,
Germany). Images were examined using differential interference
contrast and C6-SPM was recorded with the green channel (C6) with
excitation at 488 nm.
Pharmacokinetics
Twelve male Bal B/c mice were divided into three
equal groups, and then injected with Aisu, DANPs and PEG-DANPs (20
mg/kg) through the tail vein. At 0.5, 1, 2, 4, 8, 10, 12 and 24 h,
following i.v. injection, 0.3 ml blood from the carotid artery was
collected into heparinized tubes at predetermined time-points.
Blood was immediately centrifugated at 4,000 rpm for 10 min to
isolate the plasma, then 100 μl of the plasma and 3 ml of
supernatant was mixed together and centrifuged at 3,000 rpm for 10
min. Then organic phase was processed in a similar manner to the
above, blow drying with nitrogen at 60°C water bath was carried
out, and HPLC was used for the determination (34).
Hemolysis test
Fresh whole blood was collected from male guinea
pigs. The blood was stirred quickly to take out fibrousprotein from
the blood, 0.9% saline was added and centrifuged at 2,000 rpm for
15 min. The blood was washed repeatedly until the supernatant did
not turn red. The prepared mixture was made into 2% (v/v) red cell
suspensions. Aisu, DANPs and PEG-DANPs were diluted to different
concentrations with 0.9% saline (0.1, 0.25, 0.5 and 0.75 mg/ml).
The same volume (200 μl) of drug solution and red cell suspension
was mixed at 37°C for 1 h. The mixture was then centrifuged for 5
min at 2,000 rpm s to separate the supernatant. Finally, the
detection of the supernatant was at 576 nm with the formula:
Hemolysis (%) = [(ABS − ABS 0)/(ABS 100 − ABS 0)] × 100%. Here, ABS
100 and ABS 0 are the A values of the solution at 100 and 0%
hemolysis, respectively. Results were reported as means ± SD.
Tumor study and in vivo antitumor
efficacy
All experimental procedures were performed in
conformity with institutional guidelines and protocols for the care
and use of laboratory animals. We chose 40 Bal B/c mice and
randomly divided into four groups, respectively, the negative
control group (glucose injection group), positive control group
(Aisu group), DANPs group and PEG-DANPs group with 10 animals of
each. The lung cancer A549 cells were suspended in BD Matrigel, and
the mice in each group were subcutaneously implanted with
3×106 cells to establish the transplantation tumor model
(35,36). When the tumor volume reached ~120
mm3, the mice were treated 4 times at 7-day intervals
with 5% glucose injection (negative control), Aisu, DANPs or
PEG-DANPs, respectively. All formulations were injected
intravenously via the tail vein at a DTX dose of 20 mg/kg. The body
weight and tumor volume were measured simultaneously. Tumor volume
was calculated using the equation of V = w2×l/2. Here w
and l are the width and length of the tumor. Forty-eight hours
after the last treatment, the mice were sacrificed. The tumors with
lung and the other major organs (including heart, liver, spleen and
kidney) were removed, fixed in 10% formalin solution, and subjected
to paraffin embedding for H&E staining.
Statistical analysis
Results are presented as means ± SD. Statistical
comparisons were made by t-test or ANOVA analysis. The level of
significance was set at P<0.05.
Results
Determination of DANPs and PEG-DANPs via
PAGE gel electrophoresis
The PAGE gel electrophoresis shows that PEG-DANPs
with two bands, PEG and protein, respectively; there is only one
band in DANPs representing the protein (Fig. 1).
Characterization of nanoparticles
The characterization of DANPs and PEG-DANPs is
presented in Table I. The DANPs
had an average particle size of 163.4±3.76 nm (Fig. 2A), a zeta potential of −19.4±0.18
mV, a polydispersity index of 0.14±0.03; compared with the average
particle size of PEG-DANPs of 169.19±2.36 nm (Fig. 2B), zeta potential −18.2±0.21 mV,
with a polydispersity index of 1.56±0.05. The results of the size
distribution indicated that the size of DANPs and PEG-DANPs was
similar (37). The morphology
images of TEM indicated that DANPs (Fig. 3A) and PEG-DANPs (Fig. 3B) were spherical with smooth
surfaces. The drug loading of DANPs is 8.71±0.98%, and an
encapsulation efficiency 93.58±0.86%, and PEG-DANPs is 8.72±1.05
and 95.4±5.5%, respectively.
 | Table ICharacterization of DANPs and
PEG-DANPs. |
Table I
Characterization of DANPs and
PEG-DANPs.
| Average size
(nm) | Zeta potential
(mV) | DLC (%) | EE (%) |
|---|
| DANPs | 163.4±3.76 | −19.4±0.18 | 8.71±0.98 | 93.58±0.86 |
| PEG-DANPs | 169.19±2.36 | −18.2±0.21 | 8.72±1.05 | 95.4±5.50 |
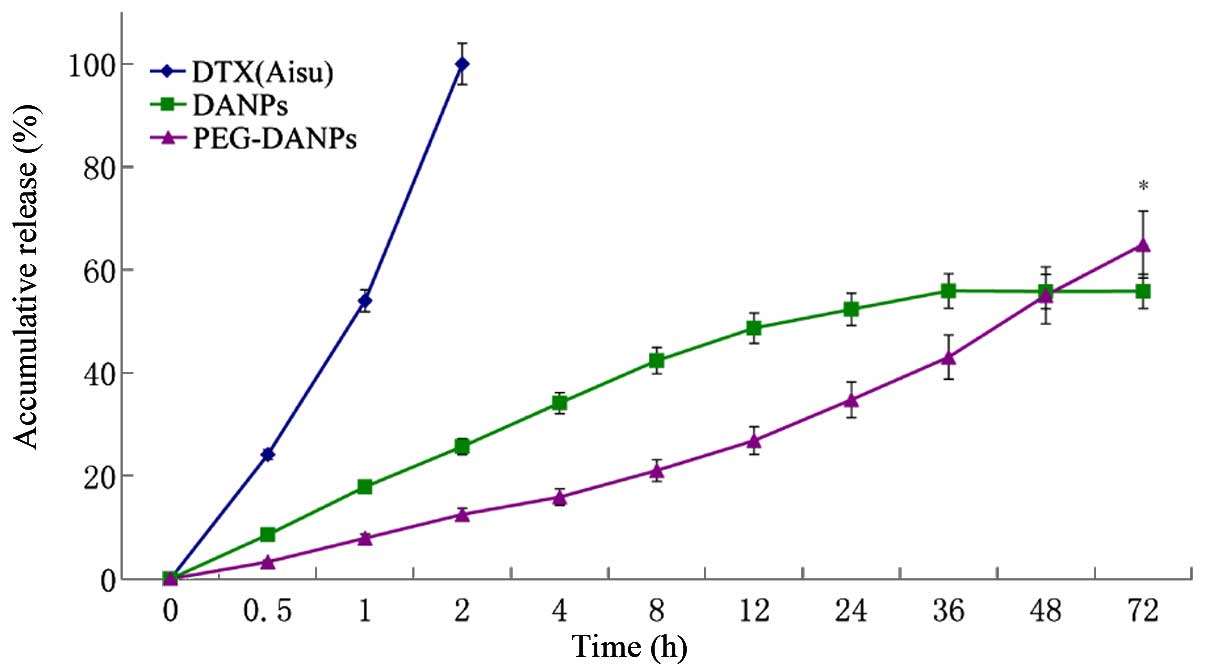
In vitro drug release
Fig. 4 shows the
release profile of Aisu, DANPs and PEG-DANPs. PEG-DANPs showed a
slower and continuous release of the whole process compared to Aisu
and DANPs. In this experiment, pH 7.4 PBS was selected to simulate
the environment of blood. The release medium contained 0.5% of
Tween-80 in which the DTX was easily soluble.
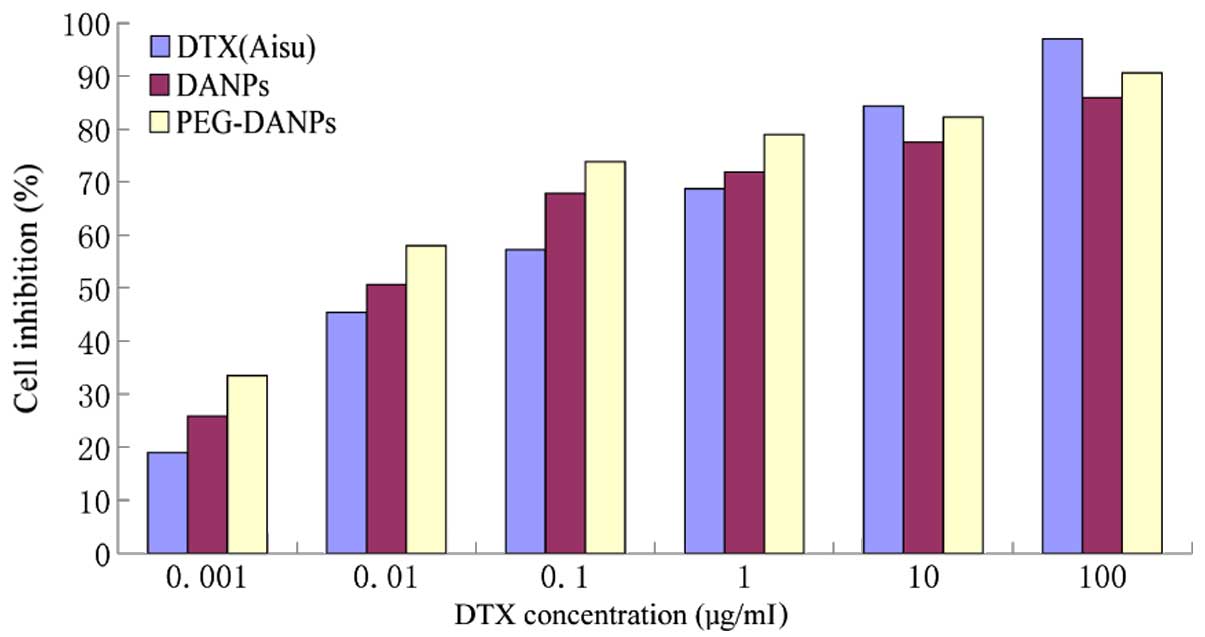
Cell viability
Fig. 5 shows the
result of the cytotoxicity of Aisu, DANPs and PEG-DANPs against
A549 lung cancer cells. A549 cells were exposed to a series of
equivalent concentrations of Aisu, DANPs and PEG-DANPs for 48 h,
and the inhibition rates were determined via the MTT method. The
cell survival rate had a dose-dependent inverse relationship with
the drug concentrations. PEG-DANPs accelerated cellular uptake of
the drug and induced higher cytotoxicity in cancer cells than Aisu
and DANPs, especially at lower DTX concentrations (0.001–0.1
μg/ml). However, A549 cells were more sensitive to Aisu than DANPs
and PEG-DANPs at higher concentrations (1–100 μg/ml). Nanoparticles
are internalized into cancer cells via endocytic mechanisms
(38), while the free drug
diffuses into cells according to the concentration gradient between
the intracellular and extra-cellular environments. This is the
reason why Aisu is more cytotoxic at higher concentrations.
PEG-DANPs increase DTX-induced apoptosis
in A549 cells
DTX was described as an antimitotic agent which
could bind to β-tubulin, resulting in block of the cell cycle at
the G2/M phase and apoptosis of cells (39,40).
According to a previous study (41), encapsulation of DTX in
nanoparticles further increased apoptosis of prostate cancer cells.
Given that PEG-DTX-HANPs demonstrated stronger in vitro
cytotoxicity than DANPs and Aisu, we performed apoptosis assays
using Annexin V-FITC and PI staining to compare induction of
apoptosis. As predicted, PEG-DANPs (55.65%) increased late
apoptosis in A549 cells compared with DANPs and Aisu (25.85 and
43.00%) (Fig. 6).
The comparison of in vitro cellular
uptake
Cellular uptake is very important in the nanodrug
delivery system. Poor cellular uptake may result in low levels of
intracellular DTX, ultimately leading to unsatisfactory therapeutic
effects. Cellular uptake of C6-SPM was qualitatively visualized by
CLSM and the internalization speed was roughly estimated. The CLSM
images of A549 cells after incubation with C6-SPM for 5, 10, 20 and
30 min are shown in Fig. 7A. CLSM
images at 5 and 10 min showed that C6-SPM fluorescence (green) was
closely located around the membrane, indicating that C6-SPM was not
internalized into A549 cells. However, when the incubation time was
extended to 20 min, C6-SPM was successfully internalized into A549
cells. Nanoparticles have previously been reported to be
internalized into the cytoplasm together with the entrapped drug
via an endocytic mechanism (42).
The process demonstrated in Fig.
7B indicated that PEG-DANPs could be absorbed sooner and easier
into the nucleus than DANPs.
Pharmacokinetics of DANPs and
PEG-DANPs
The compartment model was simulated using the
WinNonlin 4.0 program and the parameters of pharmacokinetics were
obtained (29,43). In the present study, after the
injection of DANPs and PEG-DANPs, the DTX concentration was still
measurable after 24 h, the concentration of PEG-DANPs is higher
than DANPs. On the contrary, the Aisu was not detectable even after
12 h. The difference in the DTX concentration between the three
drugs was that the nanoparticles were generally rather present in
blood in PEG-DANPs group than in DANPs and Aisu group. This is
probably due to the slower distribution of DTX from nanoparticles
into tissues because of its stability and sustained release. The
pharmacokinetic parameters of the Aisu, DANPs and PEG-DANPs group
are shown in Table II. The
PEG-DANPs significantly enhanced the half-life of DTX.
 | Table IIPharmacokinetic parameters of three
drugs (n=4, means ± SD). |
Table II
Pharmacokinetic parameters of three
drugs (n=4, means ± SD).
| Parameters |
Aisu® | DANPs | PEG-DANPs |
|---|
| T 1/2/h | 2.13±0.16 | 3.13±0.13 | 6.58±0.18a,b |
| AUC/mg.h.L-1 | 6.50±0.66 | 7.15±0.52 | 11.30±0.85a,b |
| Cl/mL.h-1 | 2.61±0.15 | 2.38±0.6 | 1.38±0.09a,b |
Hemolysis test
All the injections and other potential
immunohemolysis-causing or non-immunohemolysis-causing pharmaceutic
preparation need to be tested prior to use. Thus, we made the
exiperiments to test whether DANPs and PEG-DANPs are able to cause
the breakdown or destruction of red blood cells and red cell
aggregation. In addition, it can reflect the extent of the
breakdown of RBC wall caused by chemical agents during the
injection. In Fig. 8, the results
of the hemolysis test as the hemolysis percent vs. DTX
concentration are presented. Aisu displayed much more toxicity
toward red blood cells (RBC) than DANPs and PEG-DANPs at the same
concentration (P<0.05). The results indicated that PEG-DANPs
caused the lowest hemolysis between the three drugs.
Anticancer effects of PEG-DANPs in
vivo
Tumor-bearing nude mice were injected with Aisu,
DANPs and PEG-DANPs, and their therapeutic effects were examined by
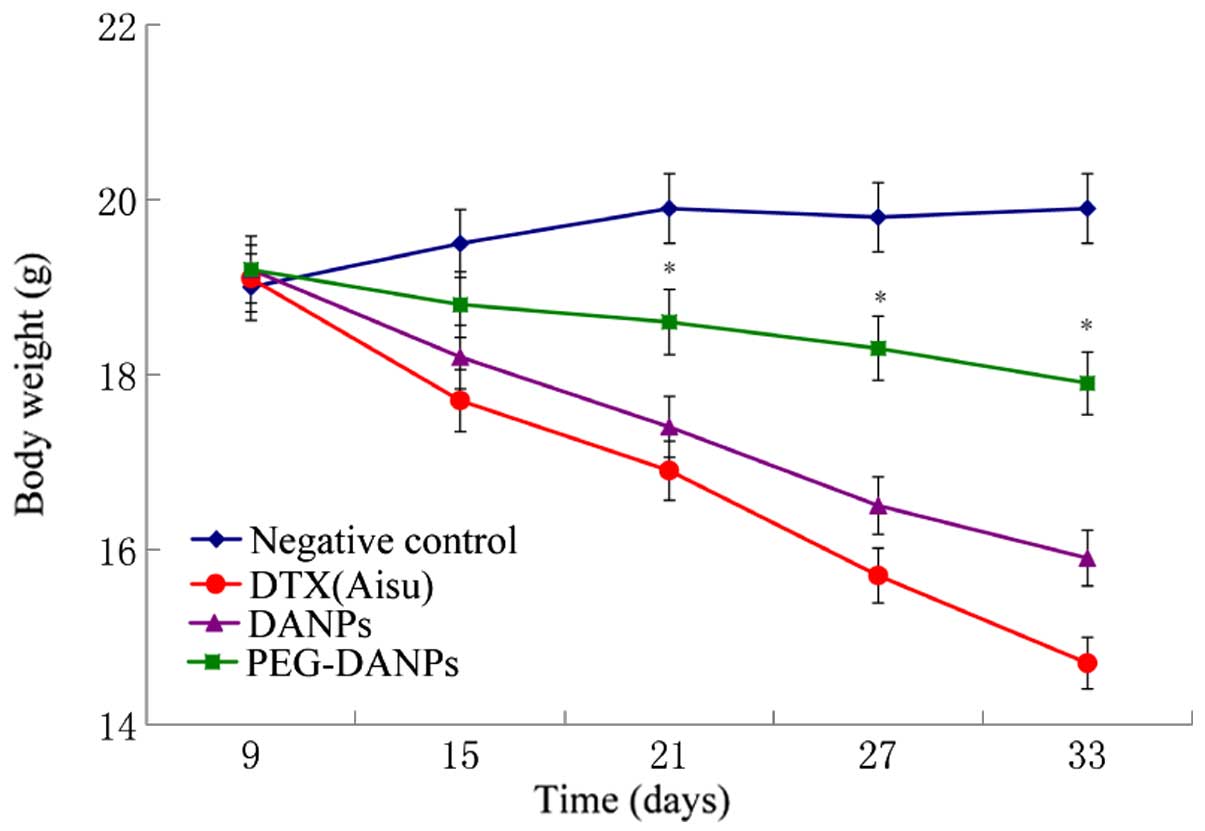
measuring the suppression of body weight and tumor growth. The body
weight of the mice in the negative control groups was basically
unchanged or slightly increased, while the mice in the drug groups
all lost weight in the process of the treatment. However, the
reduction range in the Aisu groups was more significant than the
other two groups (P<0.01) (Fig.
9), suggesting severe systemic toxicity in addition to tumor
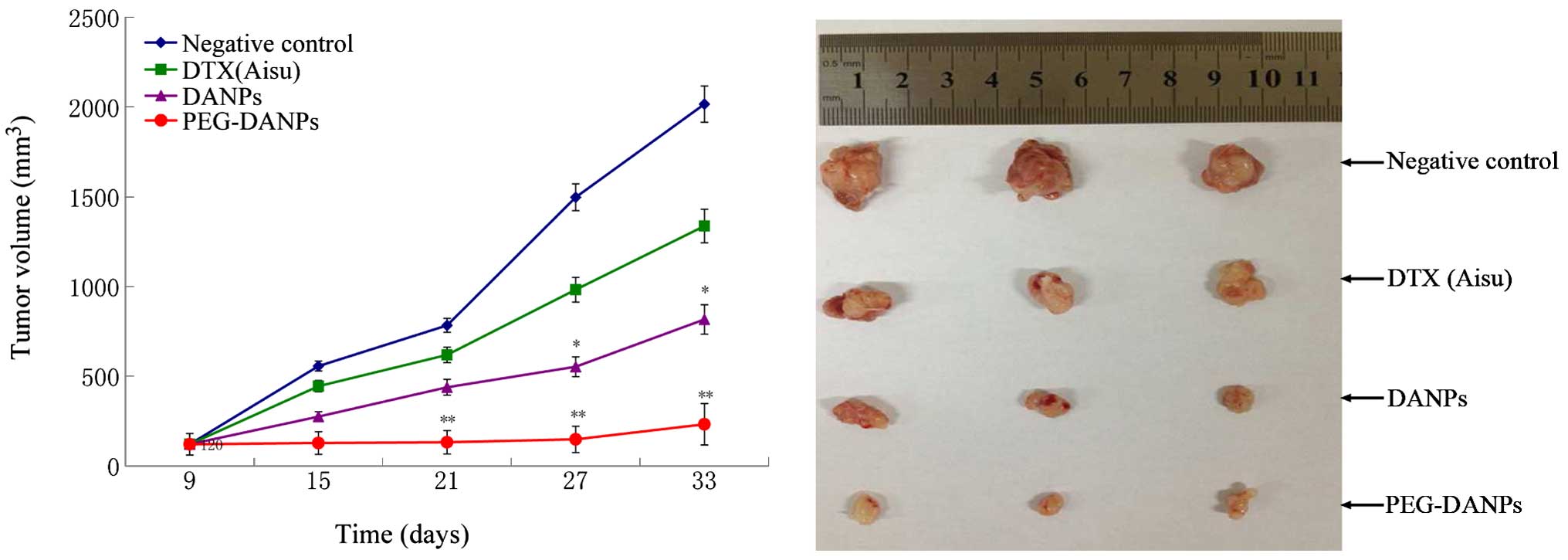
toxicity. It was found that all the tumor volumes treated with
Aisu, DANPs and PEG-DANPs were much smaller than those of negative
control groups treated with the same dose (P<0.05), and the
tumors of PEG-DANPs groups were obviously smaller than those of
Aisu groups (Fig. 10), indicating
that PEG-DANPs are the most effective in inhibiting tumor growth
among the three.
Discussion
In the present study, zeta potential of DANPs and
PEG-DANPs was −19.4±0.18 and −18.2±0.21 mV, respectively, which
indicated that they had a negative surface charge. Our experiments
indicated that the DANPs presented high drug loading and
encapsulation efficiency of 8.71±0.98 and 93.58±0.86%; for
PEG-DANPs, the values are 8.72±1.05 and 95.4±5.5%. In vitro
release, compared with Aisu, DANPs and PEG-DANPs presented a more
sustained manner of release, this is because DTX is encapsulated in
the core portion, it has to go through the process of diffusion
before release which lead to the delayed effect. In the present
study, the gradual degradation of the copolymer and diffusion of
drug are presented for the release of the loaded drug, this is the
same as previous research results (44). Furthermore, Fig. 3 indicates that PEG-DANPs are
superior to DANPs in vitro drug release, this is due to the
PEG which in the surface of the nanoparticles. PEG-DANPs could be
also used as a platform for the incorporation of active targeting
moiety (39). PEG-DANPs could not
only minimize the exposure of normal tissues but also increased the
accumulation of the therapeutic drug in the tumor site compared to
Aisu (43), all the
characteristics show its potential applicability as a drug delivery
system. The results of the pharmacokinetics indicated that
PEG-DANPs had the longest circulation time, which was the same as
in in vitro sustained release of DTX from the nanoparticles.
As a result, the enhanced half-life of the PEG-DANPs was due to its
longer circulation effect and residence time in vivo. In
addition, the incorporation of DTX in nanoparticles produced better
drug solubilization than that in Tween-80 in vitro release
test. The PEG chain of PEG-DANPs is hydrophilic, it is currently
thought to act as a protector to achieve long circulation time of
drugs in the blood. It was able to accumulate in liver, spleen and
lung, and was finally released from these organs to blood
circulation according to the drug concentration gradient (40), which resulted in a sustained blood
level compared to Aisu and DANPs. From that discussed above, we can
make a conclusion that PEG-DANPs can not only increase the
concentration and uptake of anti-tumor drugs in the tumors but also
prolong the time that drugs are sustained in the blood (45,46),
all of which are the main approaches to increase antitumor activity
and inhibit tumor growth in chemotherapy.
PEG-DANPs with an appropriate particle size can
significantly accumulate in the tumor via the EPR effect, this is
called size-dependent passive targeting. The size of PEG-DANPs is
~169 nm, it can preferential accumulate and stay in tumor tissues
compared to normal tissues. Moreover, from the pharmacokinetic
test, we concluded that the DTX concentration in blood of the
PEG-DANPs group was higher than that of DANPs and Aisu group. These
points may well explain why PEG-DANPs delayed tumor development
significantly better than the two other drugs. Fig. 8, shows the body weight changes in
the tested mouse groups. The body weight loss of the mice in Aisu
groups was significantly more than the DANPs and PEG-DANPs groups,
especially the PEG-DANPs group. These results show that PEG-DANPs
has less toxicity to normal organs and less systemic toxicity.
In summary, in our experiments the PEG-DANPs were
fabricated by emulsion-evaporation cross-link method against NSCLC.
The in vitro release and in vivo pharmacokinetics
studies showed a sustained and continuous release effect; the in
vitro cytotoxicity study proved the dose- and time-dependent
manner against A549 lung cancer cells; the cellular uptake test
in vitro demonstrated that PEG-DANPs could be absorbed
faster and easier into the nucleus; the hemolysis test indicated
PEG-DANPs had the lowest side-effects compared to Aisu and DANPs;
the in vivo evaluation pointed to PEG-DANPs having superior
antitumor effect and relatively lower side-effects compared with
Aisu and DANPs. PEG-DANPs exerted promising therapeutic effects on
NSCLC, and it is a good drug-delivery platform for the treatment of
NSCLC.
References
|
1
|
Gridelli C, Maione P, Comunale D and Rossi
A: Adjuvant chemotherapy in elderly patients with non-small cell
lung cancer. Cancer Contr. 14:57–62. 2007.
|
|
2
|
Wu SH: Effect of docetaxel with cisplatin
in treating advanced non-small cell lung cancer. J Hainan Medical
College. 16:1615–1617. 2010.(In Chinese).
|
|
3
|
Li XT: Docetaxel and cisplatin treatment
of non-small cell lung cancer analysis. Chin J Modern Drug Appl.
4:34–35. 2010.
|
|
4
|
Lu XM and Mao GX: A study of docetaxel
plus cisplatin versus gemcitabine plus cisplatin in treating
advanced non-small cell lung cancer. J Basic Clin Oncol.
22:308–310. 2009.
|
|
5
|
Xu Y: Docetaxel combined with cisplatin in
the treatment of advanced non-small cell lung cancer. Chin J Modern
Drug Applic. 4:26–28. 2010.
|
|
6
|
Shao JH: Efficacy of docetaxel combined
with nedaplatin or cisplatin in patients with advanced non-small
cell lung cancer. Chin J New Drugs. 19:599–601. 2010.
|
|
7
|
Zhang CH, Ren ZH, Li M, et al: Cisplatin
plus docetaxel combination in the first-line treatment of advanced
non-small cell lung cancer. Chin J Clin Oncol Rehab. 17:54–56.
2010.
|
|
8
|
Stineheombe TE and Socinski MA:
Considerations for second-line therapy of non-small cell lung
cancer. Oncologist. 13(Suppl 1): 28–36. 2008. View Article : Google Scholar
|
|
9
|
Xu YZ and Xu LW: The exploration of 70
years of age or older chemotherapy against non-small cell lung
cancer cells. Zhejiang Clin Med. 9:1226–1227. 2007.(In
Chinese).
|
|
10
|
Li JJ: A study of cisplatin plus docetaxel
or gemcitabine in treating advanced non small cell lung cancer. Mod
Med. 18:54–55. 582011.
|
|
11
|
Xiong Y, Zhou TC, Liu Y, Wang ZW, Lin XL,
Song XL, Shi XY and Liao ZW: Clinical analysis of efficacy in
docetaxel plus cisplatin chemotherapy with 3-DCRT treating the
patients with locally advanced NSCLC. Chin J Cancer Prev Treat.
17:699–702. 2010.
|
|
12
|
Greco FA, Spigel DR, Burris HA III,
Shipley DL, Farley C, Gandhi J, Houston GA and Hainsworth JD:
Weekly docetaxel versus docetaxel/gemcitabine in elderly/poor
performance status (PS) patients (pts) with stage III B/IV
non-small cell lung cancer (NSCLC): Randomized phase III trial of
the Minnie Pearl Cancer Research Network. J Clin Oncol. 25(Suppl):
75342007.
|
|
13
|
Kudoh S, Takeda K, Nakagawa K, Takada M,
Katakami N, Matsui K, Shinkai T, Sawa T, Goto I, Semba H, et al:
Randomized phase study of docetaxel versus vinorelbine for elderly
patients with advanced non-small cell lung cancer (NSCLC): Results
of a west Japan thoracic oncology group trial(WJTOG 9904). J Clin
Oncol. 24:3657–3663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singer EA and Srinivasan R: Intravenous
therapies for castration-resistant prostate cancer: Toxicities and
adverse events. Urol Oncol. 30(Suppl 4): S15–S19. 2012. View Article : Google Scholar :
|
|
15
|
Jin MJ, Piao SJ, Jin TX, Jin ZH, Yin XZ
and Gao ZG: Improved anti-tumor efficiency against prostate cancer
by docetaxel-loaded PEG-PCL micelles. J Huazhong Univ Sci Technolog
Med Sci. 34:66–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yared JA and Tkaczuk KH: Update on taxane
development: New analogs and new formulations. Drug Des Devel Ther.
6:371–384. 2012.PubMed/NCBI
|
|
17
|
Liu Q, Li R, Zhu Z, Qian X, Guan W, Yu L,
Yang M, Jiang X and Liu B: Enhanced antitumor efficacy,
biodistribution and penetration of docetaxel-loaded biodegradable
nanoparticles. Int J Pharm. 430:350–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghosh SC, Neslihan Alpay S and
Klostergaard J: CD44: A validated target for improved delivery of
cancer therapeutics. Expert Opin Ther Targets. 16:635–650. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao W, Xiang B, Meng TT, Liu F and Qi XR:
Chemotherapeutic drug delivery to cancer cells using a combination
of folate targeting and tumor microenvironment-sensitive
polypeptides. Biomaterials. 34:4137–4149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang F, Zhang D, Zhang Q, Chen Y, Zheng D,
Hao L, Duan C, Jia L, Liu G and Liu Y: Synergistic effect of
folate-mediated targeting and verapamil-mediated P-gp inhibition
with paclitaxel-polymer micelles to overcome multi-drug resistance.
Biomaterials. 32:9444–9456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ganesh S, Iyer AK, Gattacceca F, Morrissey
DV and Amiji MM: In vivo biodistribution of siRNA and cisplatin
administered using CD44-targeted hyaluronic acid nanoparticles. J
Control Release. 172:699–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iyer AK, Greish K, Seki T, Okazaki S, Fang
J, Takeshita K and Maeda H: Polymeric micelles of zinc
protoporphyrin for tumor targeted delivery based on EPR effect and
singlet oxygen generation. J Drug Target. 15:496–506. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang J, Nakamura H and Maeda H: The EPR
effect: Unique features of tumor blood vessels for drug delivery,
factors involved, and limitations and augmentation of the effect.
Adv Drug Deliv Rev. 63:136–151. 2011. View Article : Google Scholar
|
|
24
|
Tang N, Du G, Wang N, Liu C, Hang H and
Liang W: Improving penetration in tumors with nano assemblies of
phospholipids and doxorubicin. J Natl Cancer Inst. 99:1004–1015.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li RY: Docetaxel-loaded PEG-albumin
nanoparticles for anti-metastasis in murine 4T1 breast cancer.
Yanbian University PhD Thesis. 2012
|
|
26
|
Li YF, Li PR, Jin MJ, Jiang C and Gao Z:
Docetaxel-encapsulating small-sized polymeric micelles with higher
permeability and its efficacy on the orthotopic transplantation
model of pancreatic ductal adenocarcinoma. Int J Mol Sci.
15:23571–23588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Zhao JP, Wang YN, Fu ZP, Guo N, Dong
JR and Xia Y: Experimental study of serum containing juglone
anti-tumor in vitro. Trans Beijing Inst Technol. 33:545–550.
2013.
|
|
28
|
Zheng D, Li X, Xu H, Lu X, Hu Y and Fan W:
Study on docetaxel-loaded nanoparticles with high antitumor
efficacy against malignant melanoma. Acta Biochim Biophys Sin
(Shanghai). 41:578–587. 2009. View Article : Google Scholar
|
|
29
|
Yuan Z, Chen D, Zhang S and Zheng Z:
Preparation, characterization and evaluation of docetaxel-loaded,
folate-conjugated PEG-liposomes. Yakugaku Zasshi. 130:1353–1359.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao Y, Yu HR and Wang HX: Preparation of
paclitaxel-loaded nanoparticles by albumin fragments. J Chongqing
Med Univ. 37:795–798. 2012.
|
|
31
|
Shi NQ, Gao W, Xiang B and Qi XR:
Enhancing cellular uptake of activable cell-penetrating
peptide-doxorubicin conjugate by enzymatic cleavage. Int J Nanomed.
7:1613–1621. 2012.
|
|
32
|
Li JQ, Yang ZZ, Meng TT and Qi XR: The use
of cationic liposomes to co-deliver docetaxel and siRNA for
targeted therapy of hepatocelluar carcinoma. J Chin Pharm Sci.
23:667–673. 2014. View Article : Google Scholar
|
|
33
|
Li YF, Jin MJ, Shao S, Huang W, Yang F,
Chen W, Zhang S, Xia G and Gao Z: Small-sized polymeric micelles
incorporating docetaxel suppress distant metastases in the
clinically-relevant 4T1 mouse breast cancer model. BMC Cancer.
14:329–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen W, Gu B, Wang H, Pan J, Lu W and Hou
H: Development and evaluation of novel itraconazole-loaded
intravenous nanoparticles. Int J Pharm. 362:133–140. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim D, Gao ZG, Lee ES and Bae YH: In vivo
evaluation of doxorubicin-loaded polymeric micelles targeting
folate receptors and early endosomal pH in drug-resistant ovarian
cancer. Mol Pharm. 6:1353–1362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao ZG, Fain HD and Rapoport N: Controlled
and targeted tumor chemotherapy by micellar-encapsulated drug and
ultrasound. J Control Release. 102:203–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L, Yang M, Wang Q, Li Y, Guo R,
Jiang X, Yang C and Liu B: 10-Hydroxy camptothecin loaded
nanoparticles: Preparation and antitumor activity in mice. J
Control Release. 119:153–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiao L, Xiong X, Sun X, Zhu Y, Yang H,
Chen H, Gan L, Xu H and Yang X: Role of cellular uptake in the
reversal of multidrug resistance by PEG-b-PLA polymeric micelles.
Biomaterials. 32:5148–5157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen W, Zhan C, Gu B, Meng Q, Wang H, Lu W
and Hou H: Targeted brain delivery of itraconazole via RVG29
anchored nanoparticles. J Drug Target. 19:228–234. 2011. View Article : Google Scholar
|
|
40
|
Mu L, Teo MM, Ning HZ, Tan CS and Feng S:
Novel powder formulations for controlled delivery of poorly soluble
anticancer drug: Application and investigation of TPGS and PEG in
spray-dried particulate system. J Control Release. 103:565–575.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Li R, Qian X, Ding Y, Tu Y, Guo R,
Hu Y, Jiang X, Guo W and Liu B: Superior antitumor efficiency of
cisplatin-loaded nanoparticles by intratumoral delivery with
decreased tumor metabolism rate. Eur J Pharm Biopharm. 70:726–734.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Danhier F, Lecouturier N, Vroman B, Jérôme
C, Marchand-Brynaert J, Feron O and Préat V: Paclitaxel-loaded
PEGylated PLGA-based nanoparticles: In vitro and in vivo
evaluation. J Control Release. 133:11–17. 2009. View Article : Google Scholar
|
|
43
|
Gong C, Xie Y, Wu Q, Wang Y, Deng S, Xiong
D, Liu L, Xiang M, Qian Z and Wei Y: Improving anti-tumor activity
with polymeric micelles entrapping paclitaxel in pulmonary
carcinoma. Nanoscale. 4:6004–6017. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Van Vlerken LE, Vyas TK and Amiji MM:
Poly(ethyleneglycol)-modified nanocarriers for tumor-targeted and
intracellular delivery. Pharm Res. 24:1405–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang F, Zhang D, Zhang Q, Guo S, Zheng D,
Hao L, Guo H and Li C: Tissue distribution and pharmacokinetics
evaluation of DOMC-FA micelles for intravenous delivery of PTX. J
Drug Target. 21:137–145. 2013. View Article : Google Scholar
|
|
46
|
Wang J, Huo MR and Zhang XY: Progress in
hyaluronic acid-based targeted nano-drug delivery systems. Chin J
Pharmaceut. 44:828–835. 2013.
|