Introduction
Epithelial ovarian cancer is the most lethal
gynecological cancer and has the highest mortality rate in the
world (1,2). Due to the asymptomatic nature of
early stages of this disease and lack of a reliable method for
early diagnosis, majority of patients with ovarian cancer are not
effectively treated in the early stage (3,4).
Although epithelial ovarian cancer is a chemosensitive tumor, with
initial overall response rate to systemic therapy exceeds 80%, the
development of acquired platinum-resistance has become a major
obstacle for the clinical management of ovarian cancer (5,6).
Platinum-resistance has resulted in a decrease in the overall
survival rate, with a significant decrease to 30% for patients in
advanced stages (5,7).
To improve its diagnosis and survival, it is
necessary to better understand the mechanism of ovarian cancer
development and to find novel ways for more effectively diagnose
and treatm ovarian cancer. Based on the fact that cap-dependent
translation of protein synthesis is a key regulatory step in the
flow of genetic information from cellular genome to proteome, it is
generally accepted that cap-dependent translation plays a central
role in tumorigenesis or other tumor phenomenon (8,9). On
the initiation of cap-dependent translation, the eukaryotic
translation initiation factor 4E (eIF4E) protein recognizes the
7-methyl-GTP (m7-GTP) cap structure at the 5′-end of
mRNA molecules and associates with the eukaryotic translation
initiation factor 4G (eIF4G) protein to form the translation
initiation complex. Thus, eIF4E protein plays a central role in the
process of protein synthesis.
The most frequently aberrant change in the
translational apparatus is the upregulated levels of eIF4E
expression, which selectively affects transport of specific
transcripts, increases cap-dependent translation, suppresses
apoptosis and induces malignant transformation (10). Although the mechanism whereby eIF4E
acts in tumorigenesis is still not understood, it has been
established to be rate-limiting for cell growth and transformation
in many types of human tumors, such as breast cancer, lung cancer,
colon carcinoma, bladder carcinoma, and cervical carcinoma
(11–13). Several research groups have
reported eIF4E overexpression was correlated with malignant
progression and poor prognosis in various cancers (14–16).
In tissue culture and xenograft mouse models, eIF4E overexpression
led to oncogenic transformation, and to increased tumor size,
invasion, and metastases (17,18).
Inhibition of eIF4E effectively suppresses tumor growth and
invasiveness in many types of cancers (19,20).
The elevated eIF4E greatly increases translation of some mRNA
encoding proteins contributing to angiogenesis and proliferation
such as vascular endothelial growth factor (VEGF), basic fibroblast
growth factor (FGF-2) and cyclin D1, which are key stimulators of
migration and angiogenesis in tumors (21,22).
These results suggest that eIF4E may play an important role
in malignant progression and even drug resistance of ovarian cancer
cells. However, the precise function and mechanisms of eIF4E
in tumorigenesis of ovarian cancer has not been characterized well
so far.
To directly examine the biological effects of eIF4E
in epithelial ovarian cancer cells, specific shRNA targeting
eIF4E gene was used in our study to knock down eIF4E
expression and disturb its binding to eIF4G, blocking the
cap-dependent translation initiation. To investigate the functional
relevance of eIF4E to resistance of ovarian cancer cells, our
comparison studies between platinum-sensitive ovarian cancer cells
A2780 and the platinum-resistant cell line C200 were explored with
shRNA/cisplatin combinative treatment in this study. Finally, we
wished to confirm that the deregulation of initiation of
cap-dependent translation was required to develop ovarian
tumorigenesis and maintain the resistance to platinum in ovarian
cancer cells, and to demonstrate that the inhibitors of
cap-dependent translation may be a therapeutic target against
epithelial ovarian cancer.
Materials and methods
Cell culture and reagents
Paired isogenic cisplatin-sensitive human ovarian
cancer cell line A2780 and its cisplatin-resistant clone C200 were
used in this study (23). As the
control cells an immortalized normal human ovarian epithelial cell
line (IOSE) was used. All cells were generously provided by Dr S.
Ramakrishnan (Minnesota University). The cancer cells were
maintained in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS, Gibco), insulin and 1% penicillin/streptomycin at 37°C in a
humidified 5% CO2 atmosphere. IOSE was maintained in 50%
M199, 50% MCDB105 medium supplemented with 5% FBS and 0.1%
NaHCO2. Primary antibodies against eIF4E (C46H6, rabbit
mAb, #9742), eIF4G (C65H5, rabbit mAb, #9742), 4E-BP1
(53H11, Rabbit mAb, #9644), PARP (46D11, rabbit mAb, #9532) and
goat peroxidase (HRP)-conjugated secondary antibodies (#7074) and
antibody against β-actin (D6A8, rabbit mAb, #8457) were all
purchased from Cell Signaling Technology.
eIF4E/eIF4G interaction inhibitor 4EGI-1 was
purchased from Selleck Chemicals (Selleck China, S7369). 4egi-1 was
dissolved in DMSO at concentration of 10 mM, and stored at −80°C.
Cisplatin was purchased from Sigma-Aldrich (P4394), and was
dissolved in 0.9% NaCl solution to make a 1 mM stock solution.
Chemiluminescence substrate was obtained from Pierce. The DC
Bio-Rad protein quantization reagents were from Bio-Rad.
m7-GTPcap binding assays
A total of 5×106 cells were lysed in 300
μl lysis buffer. The supernatants were incubated with 20 μl
Sepharose beads (Amersham Pharmacia Biotech) at 4°C for 1 h, and
washed twice in 1 ml PBS buffer. Also, 10 μl of
m7-GTP-Sepharose beads were washed with 500 μl of PBS
buffer three times. Then m7-GTP-Sepharose beads were
added into the cell extracts pre-cleared with Sepharose beads, and
rotated overnight at 4°C. The beads were washed three times with
PBS buffer. Then beads were denatured, and the supernatants were
resuspended in Laemmli sample buffer with 2% β-ME and were resolved
on SDS-PAGE for western blot analysis.
Plasmid and transfection
The pGIPZ shRNA plasmid and recombinant pGIPZ shRNA
plasmid targeting eIF4E and non-silencing negative control shRNA
plasmid were kindly provided by Dr S. Ramakrishnan (Minnesota
University). DNA template oligonucleotides corresponding to
eIF4E gene (GenBank NM_001968.3) was designed as follows:
eIF4E-shRNA1 (sense, 5′-AAGCAAACCUGCGGCUGAUCU-3′),
eIF4E-shRNA2 (sense, 5′-ACAGCAGAGACGAAGUGAC-3′), and a
non-specific shRNA (sense, 5′-GGACGUGGUCGCCA CCCUGCCC-3′). These
recombinant plasmids were named pGIP-4e1, pGIP-4e2 and pGIP-NS. For
temporal transfection, 2×105 skov-3 cells were seeded in
60-mm plates. The cells (60–70% confluent) were transfected with 2
μg of plasmid using Lipofectamine 2000 (Invitrogen) according to
the manufacturer's instructions.
Selecting a population of cells that
stably express siRNA
Cells (2×105) were plated into 6-well
plates. Total 2 μg of pGIP-4e and vector plasmid of eIF4E was,
respectively, transfected into C200 and A2780 cells using
Lipofectamine 2000 (Invitrogen) and cultured for 24 h without
antibiotic selection. Then the cells were cultured in medium
containing 5 μg/ml puromycin (Sigma) until all the non-transfected
cells were killed. The antibiotic-resistant cells were pooled and
passaged in medium containing 5 μg/ml puromycin. Stable
transfectants were termed as C200-4e1 or A2780-4e1 (transfected
with pGIP-4e1 plasmid), C200-NS or A2780-NS (transfected with
pGIP-NS plasmid) respectively.
Western blot analysis
Approximately 5×105 cells were harvested
and boiled in 200 μl lysis buffer containing protease inhibitors.
Samples were subjected to SDS-PAGE and electrophoretically
transferred to a PVDF (polyvinylidene difluoride) membrane.
Membranes were incubated with the primary antibodies (1:1,000) at
4°C overnight, washed with TBST buffer, and incubated again with an
appropriate HRP-conjugated secondary antibody (1:5,000) at room
temperature for 1 h. The membranes were washed and examined by
chemiluminescence detection.
Cell proliferation assay
The cell proliferation was analyzed with
5-bromo-2′-deoxy-uridine (BrdU) incorporated into the newly
synthesized DNA of replicating cells. In brief, cells were cultured
in a 96-well plate for 24 h and pulse-labeled with BrdU for 4 h
according to the manufacturer's instructions. The BrdU label in the
DNA was detected using a peroxidase and FITC-conjugated anti-BrdU
second antibody subjected to immunodetection and immunofluorescence
assays (24). For
peroxidase-conjugated anti-BrdU immunodetection, the values of
relative proliferation was quantified with a peroxidase substrate
and measured at 370 nm with a wavelength of 540 nm. For BrdU-FITC
immunofluorescence assay, cells were cultured on cover slips until
reaching 60% confluence, and then BrdU incorporated into cellular
DNA was detected after shRNA plasmid transfection for 24 h with
immunofluorescence assay kit (Roche Applied Science, USA). The
cells were analyzed using a Nikon confocal microscope, at the
wavelength of excitation and emission of 488 and 525 nm,
respectively. The positive BrdU cells were counted from five
randomly selected fields by direct counting cells in each sample in
a blinded manner. The percentage of positive cells was calculated
as the number of positive cells divided by the number of total
cells. Each assay was repeated at least 3 times.
Adherent colony formation assay
For cell colony formation assay on plastic surface,
cells were trypsinized and plated in triplicate in 6-well culture
plates at 500 cells per dish. Cells were allowed to grow at 37°C,
5% CO2 with media changes every 3–4 days until colonies
were visible by eye. After 14-day incubation, cells were fixed with
4% paraformaldehyde at 4°C, then stained with 0.1% crystal violet
for 20 min. Cells were washed and plates were scanned, and colonies
consisting of ≥30 cells were counted using ImageJ software.
Flow cytometric analysis of cell cycle
and apoptosis
Cells were collected and fixed with 70% ice-cold
ethanol overnight at −20°C. Cells were centrifuged, resuspended in
1 ml of PBS mixed with propidium iodide (PI) and RNaseA (10 μg of
propidium iodide, 10 μg of RNase A, and 0.5% Tween-20), and
incubated at 37°C for 40 min. Cell cycle distribution was
determined by analyzing 10,000–20,000 cells using a FACScan flow
cytometer and CellQuest software (Becton-Dickinson, San Jose, CA,
USA). The percentage of apoptotic cells was determined by the
subG1 peak in the DNA histogram.
Growth inhibition assay
For the cisplatin-siRNA combination experiments, a
3-(4,5-dimethythazolz-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay was used to analyzed the effects of eIF4E-shRNA on
cell viability in vitro. All cells were pre-treated with
eIF4E-shRNA plasmid for 24 h before cisplatin treatment.
Drug-treated cells were incubated for 48 h and then MTT was added
to the cultures for an additional 2 h. The results were assessed in
a 96-well microreader (Bio-Rad Co.) by measuring the absobance at a
wavelength of 570 nm. The inhibitory rate of cell growth was
calculated as: inhibitory rate = (1−Atreated
group/Auntreated group) × 100%. The IC50 was
determined based on the dose response curves by Graphpad Prism
software. Combination index (CI) for drug interaction was
calculated using CompuSyn software (CompuSyn, Inc.). The
experiments were performed three times independently.
Cell migration and invasion assays
In vitro cell migration and invasion assays
were performed using Transwell chambers. For the migration assays,
2×104 cells were added to the upper chamber of 8-μm pore
size Transwells (BD Biosciences, Franklin Lakes, NJ, USA). For the
invasion assays, 1×105 cells were added to the upper
chamber of 8-μm pore size Tranwells precoated with Matrigel (BD
Biosciences). In these assays, cells were suspended in RPMI-1640
medium containing 10%, FBS was added to the upper chambers, and
RPMI-1640 medium containing 10% FBS was placed in the lower well,
and then incubated at 37°C in 5% CO2. After 24 h of
incubation, non-migrated or non-invaded cells were removed
carefully. The filters were then fixed in 4% paraformaldehyde and
stained with crystal violet. Five random fields were counted per
chamber using an inverted microscope (Olympus Corp., Tokyo, Japan)
at ×200 magnification for each membrane. All of the analysis was
performed in triplicate.
Statistical analysis
All statistical analyses were performed using SPSS
(version 16, SPSS Inc., Chicago, IL, USA). The values were
expressed as the means ± SE and statistical significance was
analyzed using two-tailed Student's t-test. A p-value of <0.05
was considered as significant and indicated by asterisks in the
figures.
Results
Cisplatin-resistant ovarian cancer cells
display a hyper-activated eIF4E and elevated cap-dependent
translation
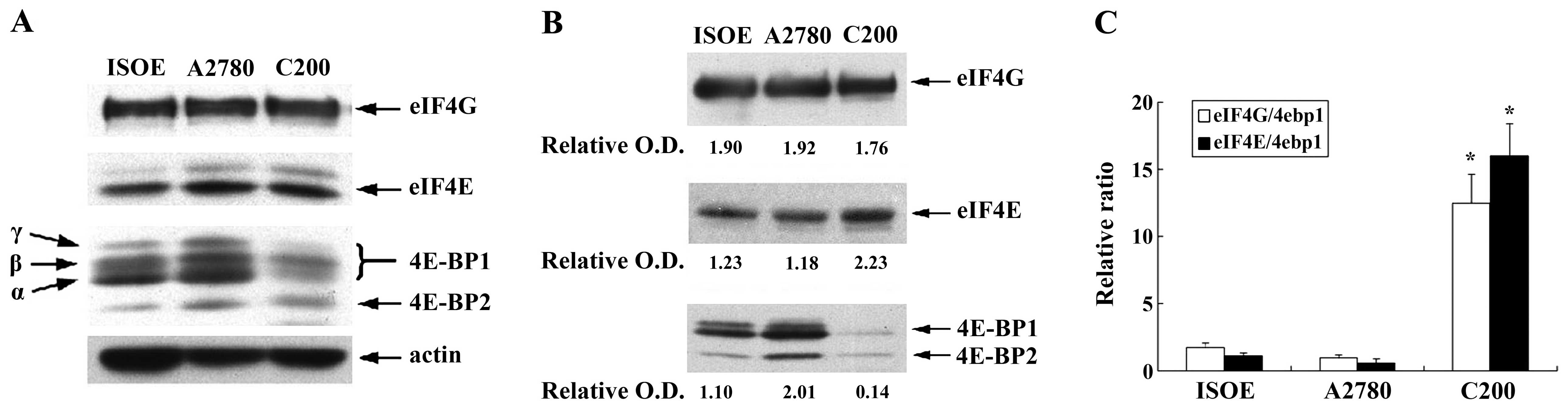
We first detected eIF4E and 4E-BP1/2
protein expression in A2780 and C200 cells in comparison with an
immortalized normal human ovarian epithelial cell line (IOSE) with
western blot analysis. As presented in Fig. 1A, we found there were significant
differences between IOSE cells and carcinoma cell lines, the levels
of eIF4E were substantially increased in A2780 and C200 cell lines.
Specially, we detected much lower amounts of 4E-BP1/2
protein in C200 cells. It indicates that C200 cells possessed much
higher levels of eIF4E than A2780 cells, and an increasing activity
of eIF4F in C200 cells. Interestingly, we found that all cells
expressed similar levels of eIF4G in addition to eIF4E (Fig. 1A).
To further compare the pattern and integrity of
eIF4F in IOSE and the other two carcinoma cell lines, we assessed
formation of the m7GTP-eIF4F complex in an
m7-GTP cap binding assay. The levels of cap-bound eIF4E,
eIF4G and 4E-BP1/2 were analyzed by immunoblotting. The
relative amounts of cap captured eIF4E and eIF4G in cells could be
regarded as indicator of the integrity and functional potency of
eIF4F. The amounts of cap-associated 4E-BP1 showed
negative impact on eIF4E assembly and function. The cap-bound
fraction from IOSE and A2780 cells contained significant amounts of
4E-BP1 (Fig. 1B),
indicating that in these cells, cap-dependent translation was under
strong 4E-BP1-mediated negative control. In contrast,
the cap-bound complexes from C200 cells were at a significantly
lower level of 4E-BP1/2 indicating that
cisplatin-resistant cells existed in a translationally activated
state. These results clearly indicated that eIF4E expression was
upregulated in platinum-resistant ovarian cancer cells. Moreover,
eIF4E and 4E-BPs displayed a deregulated activity in
platinum-resistant ovarian cancer cells.
Knockdown of eIF4E inhibits the
proliferation of A2780 and C200 cells in vitro
If elevated eIF4E was critical for the protein
synthesis of ovarian cancer cells, we hypothesized that
downregulation of eIF4E would result in inhibition of the growth of
ovarian cancer cells. To verify this, we employed two different
sequences of eIF4E shRNA plasmid pGIP-4e1 and pGIP-4e2 to
knock down eIF4E expression and determined its impact on the growth
in our study. The efficiency of silencing and the expression level
of eIF4E protein was measured with expression of green fluorescent
proteins. In order to analyze the downregulation of eIF4E
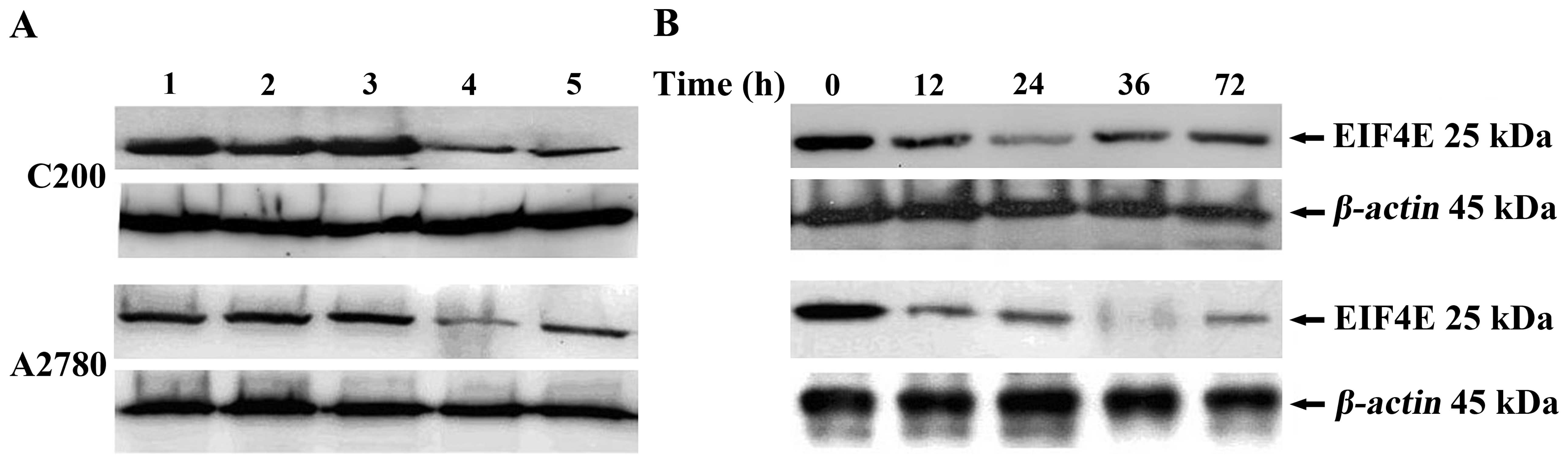
expression in cells, western blot assay was performed. As shown in
Fig. 2A, the levels of eIF4E
protein expression in A2780 and C200 cells were both significantly
reduced by pGIP-4e1 and pGIP-4e2 in comparison with its negative
control cells, and the inhibitory rates were ~65% and 55%
(p<0.05) in C200 cells, and 75 and 55% (p<0.05) in A2780
cells, respectively. Moreover, pGIP-4e1 showed stronger inhibition
effect in both cell lines. Thus, we focused our further analyses on
the pGIP-4e1 plasmid at the next biological assay. By the
time-course analysis, we observed that downregulation of eIF4E
protein expression level occurred at 12 h after transfection. The
eIF4E reduction peak was at 24 h in C200 cells and 36 h in A2780
cells, respectively. The eIF4E reduction in both A2780 and C200
cells was sustained up to 72 h (Fig.
2B). The results indicated successful knockdown of eIF4E by
pGIP-4e1 in ovarian cancer cells. In addition, no effects of shRNA
plasmid were observed on the expression of β-action used as an
internal control gene in this study.
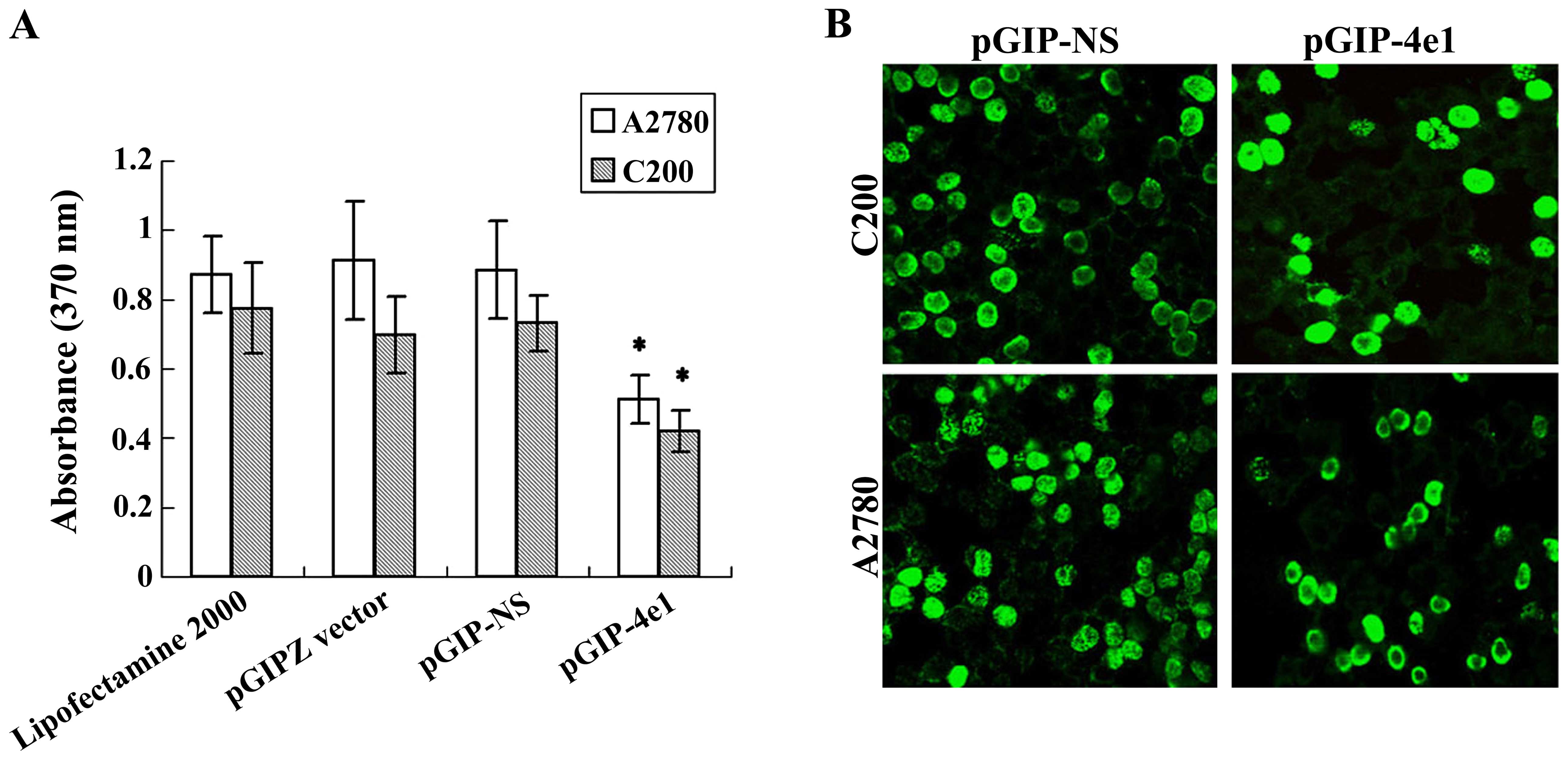
We examined the anti-proliferate response of
pGIP-4e1 in ovarian cancer cell lines C200 and A2780 with BrdU
proliferation assay. The results showed that the growth of both
A2780 and C200 cells were inhibited by pGIP-4e1 plasmid after
transfection for 24 h (Fig. 3A).
BrdU-FITC immunofluorescence assay indicated BrdU incorporation was
significantly decreased in cells that were transfected with
pGIP-4e1 and displayed less density than that of control cells
(Fig. 3B).
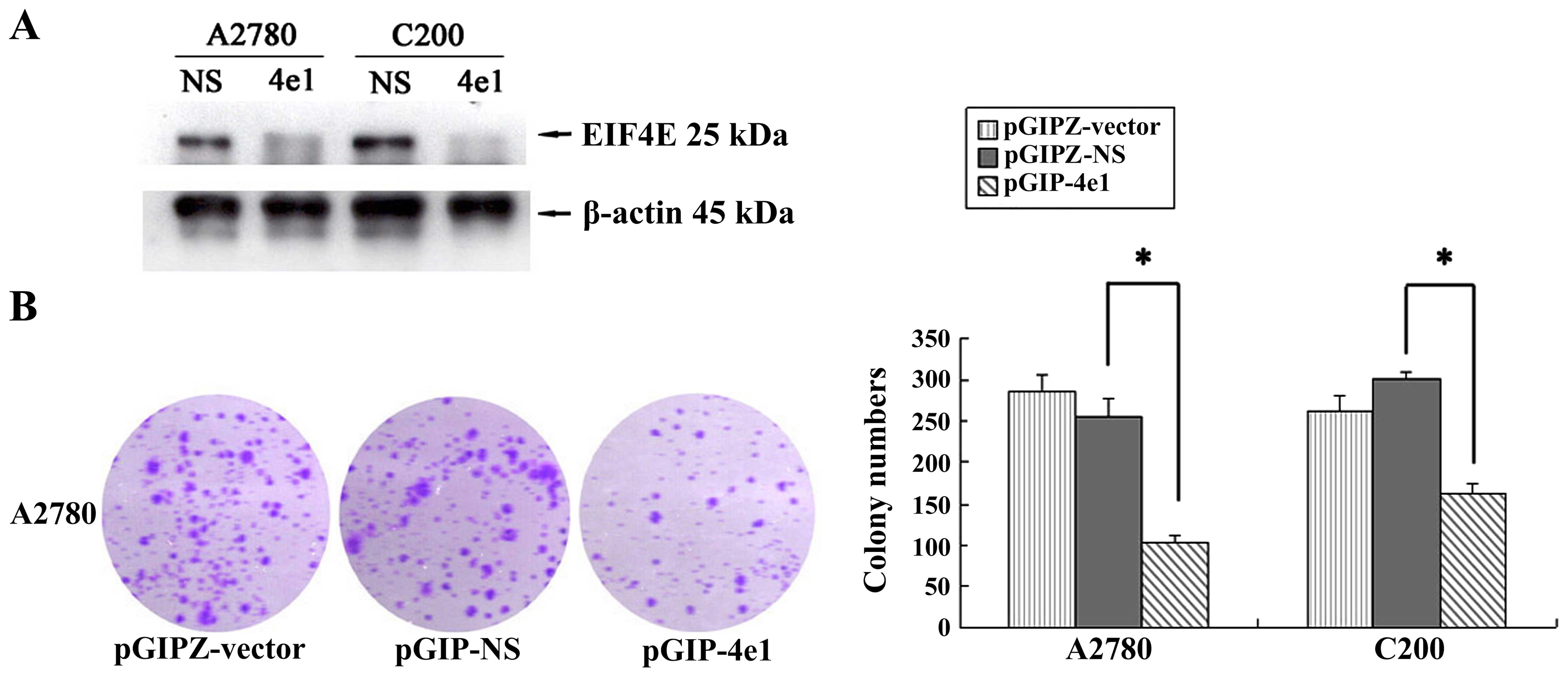
Knockdown of eIF4E inhibits the colony
formation of C200 and A2780 cells
To investigate the potential of knockdown of
eIF4E in reducing colony formation of tumor cells in
vitro, we examined the stably transfected C200 and A2780 cells.
First, we generated stable cell lines expressing shRNA specific to
eIF4E, C200-4e1 and A2780-4e1, C200-NS and A2780-NS
(negative plasmid control). The expression of eIF4E protein was
analyzed by western blot analysis to test the inhibitive effect of
pGIP-4e1 in these stable cell lines. Results of western blot
analysis showed that eIF4E protein level was significantly
decreased by pGIP-4e1shRNA in ovarian cancer cells (Fig. 4A). Colony formation assay was
analyzed two weeks after plating. All cells were fixed in
paraformaldehyde and stained with crystal violet (Fig. 4B). The mean values from plates are
shown in Fig. 4B. In our results,
pGIP-4e1 reduced the colony formation, the number of colonies of
both cancer cells was much lower than those of control cell lines,
respectively (p<0.05). It further indicated that inhibition of
eIF4E expression suppresses the growth of cancer cells.
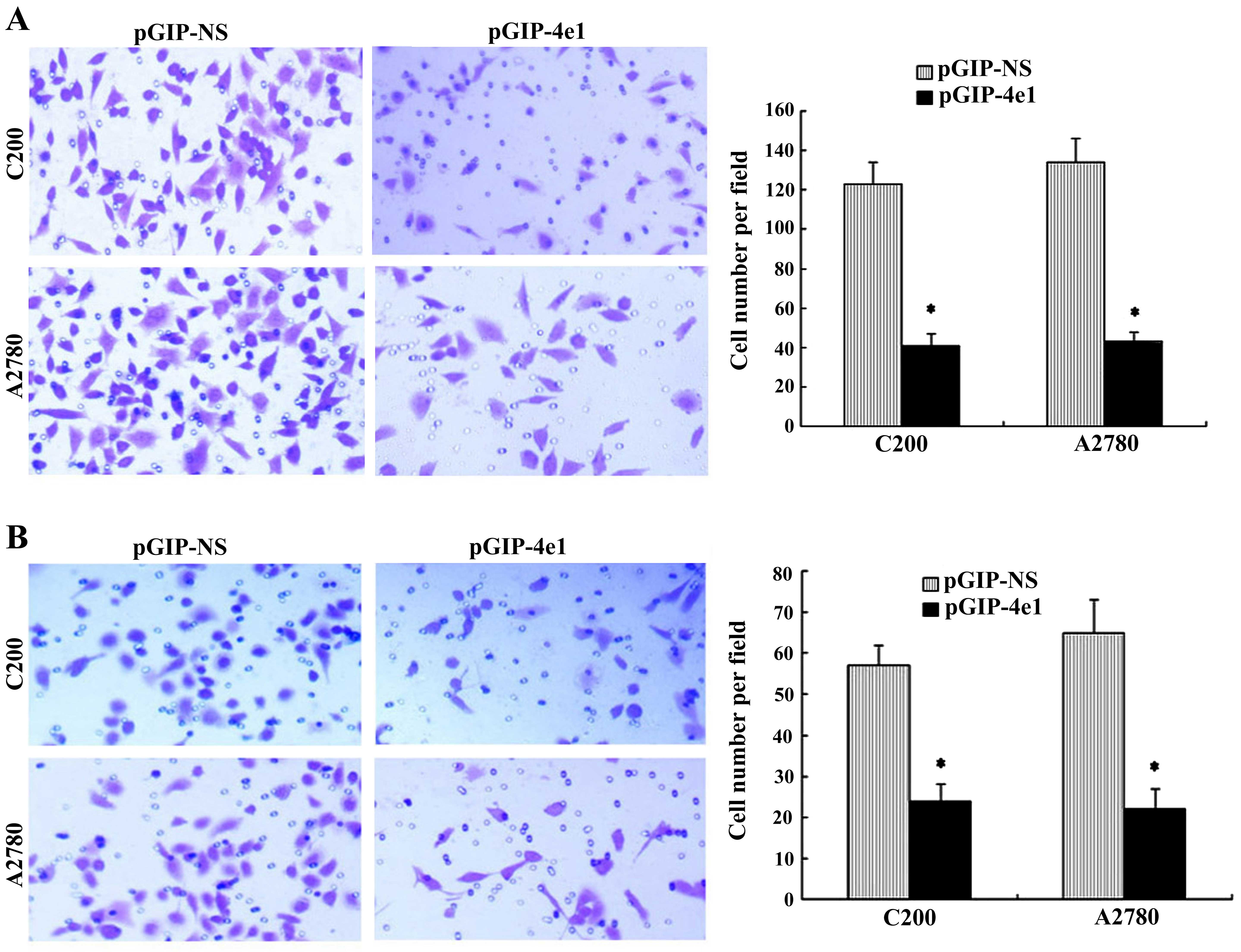
Knockdown of eIF4E inhibits the migration
and invasion of C200 and A2780 cells
Some research has shown that eIF4E might be involved
in regulation of cancer metastasis. Therefore, we evaluated whether
the role of eIF4E knockdown affected the migration and invasion of
ovarian cancer cells. The C200 and A2780 cells stably transfected
pGIP-4e1 shRNA were analyzed by Transwell assays. Western blot
analysis showed that eIF4E protein level was significantly
decreased by pGIP-4e1 shRNA in C200 and A2780 cells (Fig. 4A). The results of transwell assays
showed that knockdown of eIF4E significantly reduced the number of
migration and invasion in both cell lines compared with the control
cells (Fig. 5). It suggested that
elevated eIF4E expression was associated with positive regulation
of ovarian cancer cell migration and invasion.
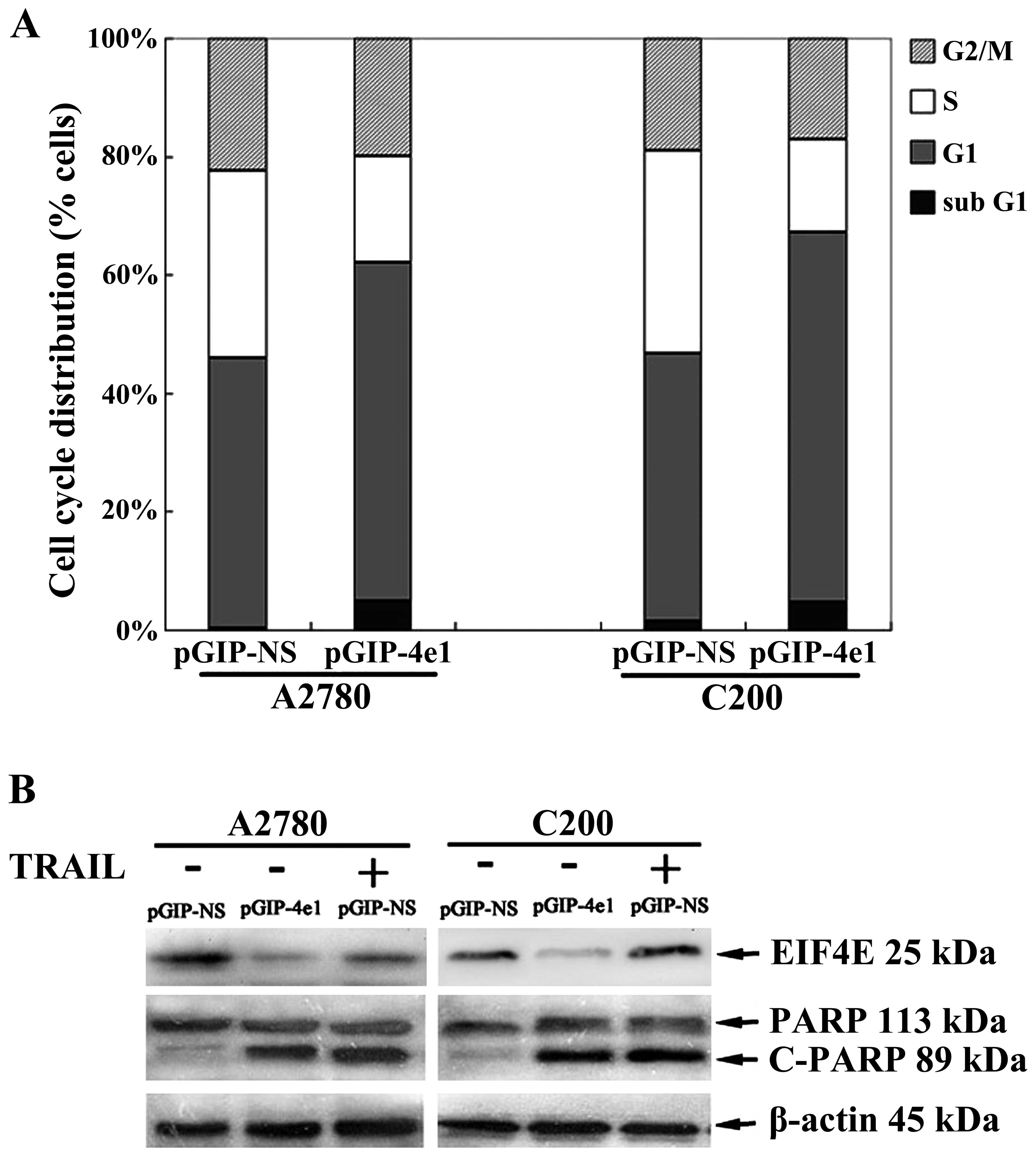
Knockdown of eIF4E arrests ovarian cancer
cells in G1 phase and induces apoptosis
To elucidate the mechanisms underlying the siRNA
mediated growth inhibition, further analysis was performed to test
the effects of eIF4E-shRNA on the cell cycle progression of C200
and A2780 cells and each assay was performed three times. The mean
values of triplicate experiments are shown in Fig. 6. Flow cytometric cell cycle
analysis of logarithmically growing C200 cells revealed a
distribution of continuously proliferating cells in G1
phase, S phase and the mitotic G2/M phase as evaluated
in Fig. 6A. Compared with that of
transfected C200-NS cells, the percentage of C200-4e cells in the
G1 phase increased by 17.2±2.1%, while the percentage of
C200-4e cells in S phase significantly reduced by 18.5±1.2%
(p<0.05). As shown in Fig. 6A,
cell cycle analysis showed a significantly greater distribution in
subG1 phase indicating more apoptotic cells in C200-4e
cells than in C200-NS cells (p<0.05). However, a significant
difference was not found in G2/M phase between C200-4e
and C200-NS cells, suggesting that knockdown of eIF4E induces
G1 cell arrest and apoptosis but does not increase
G2/M phase in which mitotic arrest occurs. Using cleaved
PARP as readout of apoptosis, we further determined whether
knockdown of eIF4E induces apoptosis in the tested cell lines. As
presented in Fig. 6B, we detected
cleaved form of PARP in C200-4e or A2780-4e cells. Interestingly,
we found the stronger expression of cleaved-PARP in C200-4e cells.
As a positive control, tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) induced strong cleavage of PARP
in the both cell lines.
Knockdown of eIF4E enhances cisplatin
cytotoxicity of ovarian cancer cells
The lower level of 4E-BP1/2 and elevated
eIF4E proteins indicated that cisplatin-resistant C200 cells showed
a translational activated state, and it was involved in development
of acquired resistance to cisplatin. We speculated that inhibition
of eIF4E would overcome cisplatin resistance and enhance the
sensitivity of ovarian cancer cells to cisplatin. To test this
hypothesis, an MTT assay was used to examine the impact on cell
response to cisplatin by knockdown of eIF4E in C200 and A2780
cells. Generally, the approximate cisplatin IC50 value
of A2780 cell line was 10–20 μM, and the equivalent IC50
value for C200 was ~200 μM. The C200 cell lines were ~20-fold
resistant to cisplatin compared with the A2780 (23). As presented in Fig. 7A, we tested the cisplatin
IC50 of stable transfected C200-4e1 cells compared with
C200-NS cells, the cisplatin IC50 value of C200-4e1
cells was significantly decreased by 28.2% (p<0.05).
Accordingly, the cisplatin IC50 also significantly
decreased by 18.2% in A2780 cells (Fig. 7B). Thus, it was clear that
knockdown of eIF4E expression was able to enhance the effect of
cisplatin in resistant ovarian cancer cells.
As a small molecule inhibitor of eIF4E/eIF4G
interaction, 4egi-1 behaves as 4ebp1 mimetic to inhibit
cap-dependent translation initiation. Thus, we further analyzed
whether 4egi-1 could enhance the cytotoxic effects of cisplatin on
C200 cells. Our results showed that the combination of 4egi-1 and
cisplatin was more potent than either agent alone in inhibiting the
growth of C200 cells. The combination indexes were <1 for
combination treatments (Fig. 7C),
indicating synergy between 4egi-1 and cisplatin in cytotoxicity.
This result further suggested that inhibition of cap-dependent
translation indeed overcame cisplatin resistance and enhanced the
sensitivity of ovarian cancer cells to cisplatin.
Discussion
Activation of translation initiation is critical for
cancer cell growth, survival, and tumor progression. There is
increasing evidence in the literature linking altered activation of
the cap-dependent translation machinery to cell transformation and
human cancer (25,26). Here, our results showed that the
activation of cap-dependent translational initiation was
significantly different in platinum-sensitive and resistant ovarian
cancer cell lines. The exciting finding in this study was the
significantly higher activated eIF4E and lower level of
4E-BP1/2 in cisplatin-resistant C200 cells (Fig. 1A). It is well known that the
expression inhibition or hyper-phosphorylation of 4E-BP1
can cause a decreasing affinity to eIF4E sequence to release the
eIF4E to promote assembly of the eIF4F, and initiate translation
(27). It suggested that
platinum-resistant cells possessed a hyper-activated eIF4E and
eIF4F complex. To the best of our knowledge, this is the first
study that links eIF4E and cap-dependent translation to the
acquired cisplatin-resistant ovarian cancer cells.
As a rate-limiting factor in cap-dependent
translation and a focal downstream point of multiple signal
pathways in cells, eIF4E might be a rational target for novel
cancer therapeutics (28). It is
well known that the altered expression of eIF4E contributes to
cancer progression by enabling the translation of a limited pool of
mRNAs encoding key proteins involved in cellular malignancy.
Overexpression of eIF4E resulted in enhanced translation of mRNAs
containing extensive secondary structure in their 5′-UTR. These
mRNAs encode growth-promoting gene products such as cyclin D1,
EGF-R/erb-b, c-Myc, c-myc, and VEGF (21,29–31).
Thus, we hypothesized that eIF4E could plausibly serve as an
integrator and amplifier of a broad range of diverse neoplastic
phenomena in naturally occurring ovarian cancer. In this study, our
results showed that knockdown of eIF4E significantly suppressed
cell proliferation, colony formation, migration and invasion of
both A2780 and C200 cells in vitro. To explore the mechanism
of growth inhibition, we detected the changes of cell cycle in
A2780 and C200 cells stably expressing eIF4E shRNA with FCM assay.
Results indicated that the knockdown of eIF4E significantly induced
accumulation of the cells in the G1 phase, and induced
increased number of apoptotic cells in the subG1 phase.
Coincidentally, we found that the expression of cleaved PARP was
significantly upregulated by eIF4E shRNA inducing downregulation of
eIF4E expression and apoptosis in ovarian cancer cells. Our study
suggested that eIF4E played a critical role in inhibiting the
growth of cancer cells through growth arrest or/and apoptosis in
both tested ovarian cancer cells lines. The exact mechanism of the
eIF4E shRNA-induced proliferation and apoptosis needs to be further
clarified.
Platinum-based chemotherapy plays a pivotal role as
first line chemotherapy option and is usually combined with taxanes
to treat human ovarian cancer (5,32,33).
However, the clinical therapeutic results of ovarian cancer are not
satisfactory due to development of resistance to chemotherapeutic
treatments, resulting in a dramatic decrease in the overall
survival rate (34,35). It has been reported that the
chemoresistance maybe a manifestation of inherent properties, but
also the ability of responding to an apoptotic stimulus that
rendered chemotherapy ineffective. Generally, the anticancer effect
of cisplatin was mediated by the formation of functionally lethal
intrastrand DNA cross-links, which resulted in many cellular
biological effects including DNA synthesis inhibition, RNA
transcription and proteins translation suppression, cell cycle
arrest, and apoptosis (5,36,37).
Despite the substantial data accumulated over the past years, the
mechanisms responsible for platinum-resistance remains unclear. As
a focal downstream point of multiple signal pathways, we
hypothesized eIF4E was an ideal breakthrough point to offer
valuable clues of platinum resistance or targets for
chemotherapeutics of epithelial ovarian cancer. Here, we
investigated the efficacy of eIF4E knockdown to cisplatin
cytotoxicity in different ovarian cancer cell subtypes including
cisplatin-sensitive and cisplatin-resistant ovarian cancer cells.
An important finding in our study was the anticancer activity of
eIF4E knockdown in cisplatin-resistant ovarian cancer cells. Our
results showed that the in stable transfected C200-4e1 cells
compared with C200-NS cells, the cisplatin IC50 value of
C200-4e1 cells was decreased by 28.2%. Others have found that the
overexpression of eIF4E induces the upregulated levels of cyclin D1
expression which confers many tumors cisplatin resistance (38,39).
It is likely that the aberration of eIF4E caused the alteration of
cap-dependent translation, and then resulted in the different
sensitivity to platinum in ovarian cancer cells due to the
alteration of platinum accumulation, detoxification and metabolism,
even DNA damage repair.
On the other hand, 4EGI-1, a small molecule
inhibitor that specially prevents eIF4E from binding to eIF4G, was
used in our study to block the cap-dependent translation initiation
and eIF4F assembly (40,41). Thus, we further determined whether
addition of 4EGI-1 would enhance the growth inhibitory effects of
cisplatin on C200 cells. The combination of cisplatin and 4EGI-1
was more potent than either agent alone in inhibiting the growth of
C200 cells. The combination indexes were <1 for all combination
treatments (Fig. 7C), indicating
synergy between cisplatin and 4EGI-1 in inhibiting the growth of
C200 cells. These data showed that cisplatin chemotherapy could be
more effective in combination with inhibition of translation
initiation or eIF4F assembly. As reported on other human
malignancies by other researchers, the data reported here provide
direct evidence that inhibition of translation initiation and eIF4F
assembly with eIF4E shRNA or 4EGI-1 could abrogate ovarian cancer
cell growth in vitro and synergistically led to enhancement
of chemosensitivity to cisplatin in ovarian cancer cells.
Taken together, our study demonstrated that eIF4E
expression was elevated in human ovarian cancer cells. An
increasing activity of eIF4F was detected in cisplatin-resistant
C200 cells. The elevated eIF4E expression was associated with
positive regulation of cell proliferation, migration and invasion
of ovarian cancer cells. Our results suggested that the sustained
activation of eIF4E in cancer cells might be essential for
expression of a transformed phenotype. Our study further suggested
that knockdown eIF4E could effectively lead to growth suppression,
apoptosis and enhancement of chemosensitivity to cisplatin of
ovarian cancer cells. Eukaryotic initiation factor eIF4E should be
an ideal breakthrough point to offer valuable clues of ovarian
tumorigenesis, and downregulated eIF4E expression might a useful
approach to improve the therapeutic responsiveness of ovarian
cancer.
Acknowledgements
We thank Dr Sundaram Ramakrishnan (Minnesota
University) for kindly providing ovarian cancer and control cells.
This study was supported by grants provided by the National Natural
Science Foundation of China (no. 81200220).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lloyd KL, Cree IA and Savage RS:
Prediction of resistance to chemotherapy in ovarian cancer: A
systematic review. BMC Cancer. 15:1172015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eckstein N, Servan K, Hildebrandt B,
Pölitz A, von Jonquières G, Wolf-Kümmeth S, Napierski I, Hamacher
A, Kassack MU, Budczies J, et al: Hyperactivation of the
insulin-like growth factor receptor I signaling pathway is an
essential event for cisplatin resistance of ovarian cancer cells.
Cancer Res. 69:2996–3003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eckstein N: Platinum resistance in breast
and ovarian cancer cell lines. J Exp Clin Cancer Res. 30:912011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller DS, Blessing JA, Krasner CN, Mannel
RS, Hanjani P, Pearl ML, Waggoner SE and Boardman CH: Phase II
evaluation of pemetrexed in the treatment of recurrent or
persistent platinum-resistant ovarian or primary peritoneal
carcinoma: A study of the Gynecologic Oncology Group. J Clin Oncol.
27:2686–2691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aletti GD, Dowdy SC, Podratz KC and Cliby
WA: Relationship among surgical complexity, short-term morbidity,
and overall survival in primary surgery for advanced ovarian
cancer. Am J Obstet Gynecol. 197:676.e1–676.e7. 2007. View Article : Google Scholar
|
|
8
|
De Benedetti A and Graff JR: eIF-4E
expression and its role in malignancies and metastases. Oncogene.
23:3189–3199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polunovsky VA and Bitterman PB: The
cap-dependent translation apparatus integrates and amplifies cancer
pathways. RNA Biol. 3:10–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clemens MJ: Targets and mechanisms for the
regulation of translation in malignant transformation. Oncogene.
23:3180–3188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Larsson O, Li S, Issaenko OA, Avdulov S,
Peterson M, Smith K, Bitterman PB and Polunovsky VA: Eukaryotic
translation initiation factor 4E induced progression of primary
human mammary epithelial cells along the cancer pathway is
associated with targeted translational deregulation of oncogenic
drivers and inhibitors. Cancer Res. 67:6814–6824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong K, Wang R, Wang X, Lin F, Shen JJ,
Gao P and Zhang HZ: Tumor-specific RNAi targeting eIF4E suppresses
tumor growth, induces apoptosis and enhances cisplatin cytotoxicity
in human breast carcinoma cells. Breast Cancer Res Treat.
113:443–456. 2009. View Article : Google Scholar
|
|
13
|
Oridate N, Kim HJ, Xu X and Lotan R:
Growth inhibition of head and neck squamous carcinoma cells by
small interfering RNAs targeting eIF4E or cyclin D1 alone or
combined with cisplatin. Cancer Biol Ther. 4:318–323. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Benedetti A and Graff JR: eIF-4E
expression and its role in malignancies and metastases. Oncogene.
23:3189–3199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Culjkovic B, Topisirovic I and Borden KL:
Controlling gene expression through RNA regulons: The role of the
eukaryotic translation initiation factor eIF4E. Cell Cycle.
6:65–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Topisirovic I, Siddiqui N, Orolicki S,
Skrabanek LA, Tremblay M, Hoang T and Borden KL: Stability of
eukaryotic translation initiation factor 4E mRNA is regulated by
HuR, and this activity is dysregulated in cancer. Mol Cell Biol.
29:1152–1162. 2009. View Article : Google Scholar :
|
|
17
|
Ko SY, Guo H, Barengo N and Naora H:
Inhibition of ovarian cancer growth by a tumor-targeting peptide
that binds eukaryotic translation initiation factor 4E. Clin Cancer
Res. 15:4336–4347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Graff JR and Zimmer SG: Translational
control and metastatic progression: Enhanced activity of the mRNA
cap-binding protein eIF-4E selectively enhances translation of
metastasis-related mRNAs. Clin Exp Metastasis. 20:265–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Fan S, Koo J, Yue P, Chen ZG,
Owonikoko TK, Ramalingam SS, Khuri FR and Sun SY: Elevated
expression of eukaryotic translation initiation factor 4E is
associated with proliferation, invasion and acquired resistance to
erlotinib in lung cancer. Cancer Biol Ther. 13:272–280. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Graff JR, Konicek BW, Carter JH and
Marcusson EG: Targeting the eukaryotic translation initiation
factor 4E for cancer therapy. Cancer Res. 68:631–634. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang SX, Hewitt SM, Steinberg SM, Liewehr
DJ and Swain SM: Expression levels of eIF4E, VEGF, and cyclin D1,
and correlation of eIF4E with VEGF and cyclin D1 in multi-tumor
tissue microarray. Oncol Rep. 17:281–287. 2007.PubMed/NCBI
|
|
22
|
Liang B, He Q, Zhong L, Wang S, Pan Z,
Wang T and Zhao Y: Circulating VEGF as a biomarker for diagnosis of
ovarian cancer: A systematic review and a meta-analysis. Onco
Targets Ther. 8:1075–1082. 2015.PubMed/NCBI
|
|
23
|
Solomon LA, Ali S, Banerjee S, Munkarah
AR, Morris RT and Sarkar FH: Sensitization of ovarian cancer cells
to cisplatin by genistein: The role of NF-kappaB. J Ovarian Res.
1:92008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan J, Lata C, Santilli A, Green D, Roy S
and Santilli S: Supplemental oxygen reverses hypoxia-induced smooth
muscle cell proliferation by modulating HIF-alpha and VEGF levels
in a rabbit arteriovenous fistula model. Ann Vasc Surg. 28:725–736.
2014. View Article : Google Scholar :
|
|
25
|
Soni A, Akcakanat A, Singh G, Luyimbazi D,
Zheng Y, Kim D, Gonzalez-Angulo A and Meric-Bernstam F: eIF4E
knockdown decreases breast cancer cell growth without activating
Akt signaling. Mol Cancer Ther. 7:1782–1788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Avdulov S, Li S, Michalek V, Burrichter D,
Peterson M, Perlman DM, Manivel JC, Sonenberg N, Yee D, Bitterman
PB, et al: Activation of translation complex eIF4F is essential for
the genesis and maintenance of the malignant phenotype in human
mammary epithelial cells. Cancer Cell. 5:553–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Castellvi J, Garcia A, Rojo F,
Ruiz-Marcellan C, Gil A, Baselga J and Ramon y Cajal S:
Phosphorylated 4E binding protein 1: A hallmark of cell signaling
that correlates with survival in ovarian cancer. Cancer.
107:1801–1811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meric F and Hunt KK: Translation
initiation in cancer: A novel target for therapy. Mol Cancer Ther.
1:971–979. 2002.PubMed/NCBI
|
|
29
|
Pinzaglia M, Montaldo C, Polinari D,
Simone M, La Teana A, Tripodi M, Mancone C, Londei P and Benelli D:
EIF6 over-expression increases the motility and invasiveness of
cancer cells by modulating the expression of a critical subset of
membrane-bound proteins. BMC Cancer. 15:1312015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rhoads RE: Signal transduction pathways
that regulate eukaryotic protein synthesis. J Biol Chem.
274:30337–30340. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brown EJ and Schreiber SL: A signaling
pathway to translational control. Cell. 86:517–520. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Metzger-Filho O, Moulin C and D'Hondt V:
First-line systemic treatment of ovarian cancer: A critical review
of available evidence and expectations for future directions. Curr
Opin Oncol. 22:513–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Han A, Chen E, Singh RK,
Chichester CO, Moore RG, Singh AP and Vorsa N: The cranberry
flavonoids PAC DP-9 and quercetin aglycone induce cytotoxicity and
cell cycle arrest and increase cisplatin sensitivity in ovarian
cancer cells. Int J Oncol. 46:1924–1934. 2015.PubMed/NCBI
|
|
35
|
Meng Q, Xia C, Fang J, Rojanasakul Y and
Jiang BH: Role of PI3K and AKT specific isoforms in ovarian cancer
cell migration, invasion and proliferation through the p70S6K1
pathway. Cell Signal. 18:2262–2271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arafa el-SA, Zhu Q, Barakat BM, Wani G,
Zhao Q, El-Mahdy MA and Wani AA: Tangeretin sensitizes
cisplatin-resistant human ovarian cancer cells through
downregulation of phosphoinositide 3-kinase/Akt signaling pathway.
Cancer Res. 69:8910–8917. 2009. View Article : Google Scholar
|
|
37
|
Johnson SW, Swiggard PA, Handel LM,
Brennan JM, Godwin AK, Ozols RF and Hamilton TC: Relationship
between platinum-DNA adduct formation and removal and cisplatin
cytotoxicity in cisplatin-sensitive and -resistant human ovarian
cancer cells. Cancer Res. 54:5911–5916. 1994.PubMed/NCBI
|
|
38
|
Zhou FF, Yan M, Guo GF, Wang F, Qiu HJ,
Zheng FM, Zhang Y, Liu Q, Zhu XF and Xia LP: Knockdown of eIF4E
suppresses cell growth and migration, enhances chemosensitivity and
correlates with increase in Bax/Bcl-2 ratio in triple-negative
breast cancer cells. Med Oncol. 28:1302–1307. 2011. View Article : Google Scholar
|
|
39
|
Bitterman PB and Polunovsky VA:
eIF4E-mediated translational control of cancer incidence. Biochim
Biophys Acta. 1849:774–780. 2015. View Article : Google Scholar
|
|
40
|
Chen L, Aktas BH, Wang Y, He X, Sahoo R,
Zhang N, Denoyelle S, Kabha E, Yang H, Freedman RY, et al: Tumor
suppression by small molecule inhibitors of translation initiation.
Oncotarget. 3:869–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hoeffer CA, Cowansage KK, Arnold EC, Banko
JL, Moerke NJ, Rodriguez R, Schmidt EK, Klosi E, Chorev M, Lloyd
RE, et al: Inhibition of the interactions between eukaryotic
initiation factors 4E and 4G impairs long-term associative memory
consolidation but not reconsolidation. Proc Natl Acad Sci USA.
108:3383–3388. 2011. View Article : Google Scholar : PubMed/NCBI
|