Introduction
Worldwide, lung cancer is the most frequently
diagnosed cancer and the leading cause of cancer deaths (1). Approximately 85–90% of lung cancer is
non-small cell lung cancer (NSCLC) which is characterized by
relatively low growth rate and poor responsiveness upon
chemotherapy, compared to small cell lung cancer (SCLC) (2). Although platinum or taxol-based
chemotherapy has been the standard treatment for NSCLC patients,
the intrinsic and acquired resistances to these drugs are the major
obstacles to achieve the successful long-term outcomes. To overcome
the resistance, the second line or combination chemotherapy
regimens have been used (3–5), but
the overall survival benefits of various chemotherapies in NSCLC is
not yet satisfactory.
The large receptor tyrosine kinase (RTK) family in
the human genome contains 58 RTKs and is divided into 20
subfamilies. One of the subfamilies is TAM family composed of three
RTK members which are Tyro3 (also referred to Brt, Dtk, Rse, Sky or
Tif ), Axl (also referred to Ark, Tyro7 or Ufo) and Mer (also
referred to Eyk, Nyk or Tyro12) (6–9).
Each of them has similar structural features, which are
extracellular domains, two immunoglobin-like and two fibronectin
type III domains and cytoplasmic kinase domain (10,11).
Among several ligands including growth arrest-specific 6 (Gas6),
protein S, tubby and tulip, Gas6 is the only one able to bind and
activate all three TAM RTKs, which evokes transduction of many
extracellular signals to cause cell growth, survival,
proliferation, migration and inhibition of apoptosis (11,12).
Since the first identification of Axl in 1988,
Axl cDNA was cloned in 1991 from chronic myelogenous
leukemia patients as a novel RTK (7). Axl overexpression and its activation
upon Gas6 stimulation have been reported in many types of cancer
such as acute leukemia (13),
breast (14), colon (15), esophageal, lung (16), ovarian (17), prostate (18) and thyroid cancer (19), which subsequently signals for cell
survival and proliferation (20–22).
For example, in more than half of non-small cell lung cancer
(NSCLC) cell lines, the levels of Axl, Mer and ligands, Gas6 and
protein S, were elevated (23–25).
In 48.3% of clinical samples of lung adenocarcinoma, Axl
overexpression was observed, which was also associated with disease
stages and lymph node metastasis (25). Therefore, Axl has been receiving
increased attention as a potent therapeutic target for cancer
treatment.
Curcumin, a polyphenolic compound extracted from
Curcuma longa, is a well-known natural product with
anti-oxidant, anti-inflammatory and anticancer activities (26,27).
Recent clinical investigations demonstrated that curcumin has not
only chemo-preventive, but also therapeutic potential in many types
of cancer. Moreover, oral administration of up to 12 g/day of
curcumin was reported to be safe enough, suggesting its low
toxicity at effective dose. Curcumin has also been demonstrated to
modulate many signal transduction pathways involved in survival,
carcinogenesis and apoptosis (28–30).
In the present study, we examined the effect of
curcumin on expression and activation of Axl RTK in NSCLC cells,
which subsequently inhibits cell proliferation and overcomes
chemo-resistance via both induction of p21 and reduction of
X-linked inhibitor of apoptosis (XIAP), suggesting that Axl RTK is
a novel target of curcumin to exert its anticancer activity.
Materials and methods
Reagents and antibodies
Curcumin was obtained from Sigma-Aldrich (St. Louis,
MO, USA). A549 and H460 cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). Primers for Axl were
synthesized by the domestic company, Bioneer Corp. (Daejeon,
Korea). TRI reagent was obtained from Solgent Co., Ltd. (Daejeon,
Korea). AmpliTaq DNA polymerase and Lipofectamine 2000 were
obtained from Roche Diagnostics Corp. (Indianapolis, IN, USA) and
Invitrogen (Carlsbad, CA, USA), respectively. G418 was from
Gibco-BRL (Gaithersburg, MD, USA). The plasmid, pGL3-basic vector
and the Dual-Glo luciferase assay kit were purchased from Promega
Corp. (Madison, WI, USA). For western blot analysis, specific
antibodies against Axl, cyclin D1, p21, XIAP and GAPDH, as well as
secondary antibodies were obtained from Santa Cruz Biotechnology
(Dallas, TX, USA).
Cell culture and establishment of
cisplatin and paclitaxel-resistant cells
The A549 and H460 cells were grown in RPMI-1640
medium (Gibco-BRL) containing 10% fetal bovine serum (FBS), 2 mM
L-glutamine, 10 U/ml penicillin and 10 g/ml streptomycin at 37ºC in
5% CO2 in a water-saturated atmosphere. The variants of
A549 and H460 cells which are resistant to cisplatin (A549/CisR and
H460/CisR) or paclitaxel (A549/TR and H460/TR cells) were
established by stepwise exposure of the parental cells to
escalating concentrations of cisplatin (ranging from 0.5 to 2 mM)
and paclitaxel (ranging from 3 to 24 nM), respectively.
Reverse transcription PCR (RT-PCR)
Cells (2×105) were seeded in a 60-mm
culture dish and grown overnight. They were then treated with the
indicated concentrations (0, 5, 10 and 20 μM) of curcumin for 24 h.
Total RNA was extracted using TRI reagent and subjected to cDNA
synthesis and PCR. The specific primers were as follows: Axl sense,
5′-AACCTT CAACTCCTGCCTTCTCG-3′ and antisense, 5′-CAGCTTCT
CCTTCAGCTCTTCAC-3′; GAPDH sense, 5′-GGAGCCAA AAGGGTCATCAT-3′ and
antisense, 5′-GTGATGGCATG GACTGTGGT-3′. The mRNA level of Axl was
normalized to that of GAPDH.
Promoter activity test
The promoter reporter plasmid, pGL3-Axl, which
contains the Axl promoter region ranging from −887 to +7 bp
of the transcriptional start site was amplified by PCR and
subcloned into the pGL3-basic vector, the luciferase reporter
plasmid. The constructed promoter reporter plasmid was
co-transfected into cells (3×105 cells in a 60-mm dish)
with Renilla luciferase vectors, pRL-SV40, as an internal
control. Luciferase activity was measured using a Dual-Glo
luciferase assay system.
Western blot analysis
Total cell lysates were prepared from the parental
or chemoresistant cells treated with the indicated concentrations
(0, 5, 10 and 20 μM) of curcumin using lysis buffer [1% Triton
X-100, 50 mM Tris (pH 8.0), 150 mM NaCl, 1 mM PMSF, 1 mM
Na3VO4 and protease inhibitor cocktail].
Untreated cells were used as controls. Protein concentrations were
determined using Bio-Rad protein assays. Proteins from the cell
lysates (20–40 μg) were separated by 12% SDS-PAGE, and
electrotransferred onto nitrocellulose membranes. The membranes
were blocked for 30 min at room temperature in Tris-buffered saline
with 0.05% Tween-20 (TTBS) containing 5% non-fat dry milk, and then
incubated with TTBS containing a primary antibody for 4 h at room
temperature. After 3×10 min washes in TTBS, the membranes were
incubated with peroxidase-conjugated secondary antibody for 1 h.
Following 3 additional 10-min washes with TTBS, the protein bands
of interest were visualized using an enhanced chemiluminescence
detection system (Amersham™ ECL™ Prime Western Blotting Detection
reagent; GE Healthcare, Piscataway, NJ, USA).
Cell viability measurement
To assess cell viability, the number of viable cells
was counted using Trypan blue. Briefly, 3×103 cells were
seeded into 60-mm culture dish, grown overnight and then treated
with the indicated concentrations (0, 5, 10 and 20 μM) of curcumin
for 24 h. After curcumin treatment, cells were harvested and
stained with 0.4% Trypan blue solution. Dye-excluding viable cells
were counted under the microscope. Cell viability was also
expressed as a percentage of the viable cells with respect to
untreated control cells.
Clonogenic assay
Cells were seeded into 24-well plates
(1×102 cells/well) and treated with the indicated
concentrations (0, 5, 10 and 20 μM) of curcumin for 24 h.
Curcumin-treated cells were then cultured for the next 7–10 days to
form colonies. Colonies of >50 cells were stained with Crystal
violet (in 60% methanol; Junsei Chemical Co., Ltd., Tokyo, Japan)
and images were acquired using the RAS-3000 Image Analysis System
(FujiFilm, Tokyo, Japan).
Ectopic expression of Axl
To ectopically express Axl, the recombinant plasmid,
pcDNA3-Axl, was constructed by cloning the Axl cDNA into the
EcoRI and BamHI sites of the pcDNA3 vector and 2 μg
of purified plasmids were transfected into the A549 cells
(3×105 cells in a 60 mm dish) using Lipofectamine 2000
(Invitrogen). To establish stable cell lines, which constitutively
express Axl, the transfected cells were cultured in the presence of
400 μg/ml of G418. The RPMI-1640 medium containing G418 was
refreshed every 3 days. After 3–4 weeks, the Axl-expressing cells
were enriched and the Axl expression in these cells was analyzed by
western blot analysis.
Transfection of siRNA
To reduce Axl expression, RNA interference-mediated
gene silencing was performed. Cells (3×105) were seeded
in 60-mm culture dishes, grown overnight and then transfected with
50 nM siRNA targeting Axl (sense, 5′-AAGAUUUGGAGAdACACACUGA-3′ and
antisense, 5′-UCAGUGUGUUCUCCAAAUCUU-3′), as previously described
(30) or control siRNA. The cells
were harvested for 24 and 48 h after transfection and used to
evaluate protein expression and cell proliferation,
respectively.
Statistical analysis
Data were expressed as the means ± SD of triplicate
samples or at least three independent experiments. To determine
statistical significance, the Student's t-test was used with a
P-value threshold of <0.05.
Results
Curcumin suppresses expression of Axl
receptor tyrosine kinase at transcriptional level
We first examined if curcumin alters expression of
Axl receptor tyrosine kinase (RTK) in the lung cancer A549 and H460
cells. After treatment of cells with 5, 10 and 20 μM curcumin for
24 h, Axl protein level was determined by western blot analysis.
The results of western blot analysis showed that Axl protein level
in curcumin-treated cells was reduced in the dose-dependent manner
(Fig. 1A). Additionally, this
inhibitory effect of curcumin on Axl expression was also
time-dependent, since Axl protein level was found to gradually
decrease, when these cells were treated with 20 μM curcumin for 6,
12 and 24 h (Fig. 1B).
Downregulation of Axl expression by curcumin was
further demonstrated by RT-PCR. Consistent with western blot
results, Axl mRNA levels of A549 and H460 cells were also markedly
and dose-dependently diminished by the indicated concentrations of
curcumin (Fig. 1C). Moreover, the
effect of curcumin on transcription of the Axl gene was
examined using the Axl promoter-luciferase reporter plasmid,
pGL3-Axl. As shown in Fig. 1D,
luciferase activities of A549 cells transfected with pGL3-Axl and
treated with 5, 10 and 20 μM curcumin for 6 h were significantly
declined, indicating that curcumin inhibits Axl expression at the
transcriptional level.
Curcumin inhibits activation of Axl upon
the growth arrest-specific gene 6 stimulation
Binding of growth arrest-specific gene 6 (Gas6), a
validated ligand, to Axl results in its activation, that is the
phosphorylation of tyrosine residues at intracellular kinase domain
(31,32). We next examined the effect of
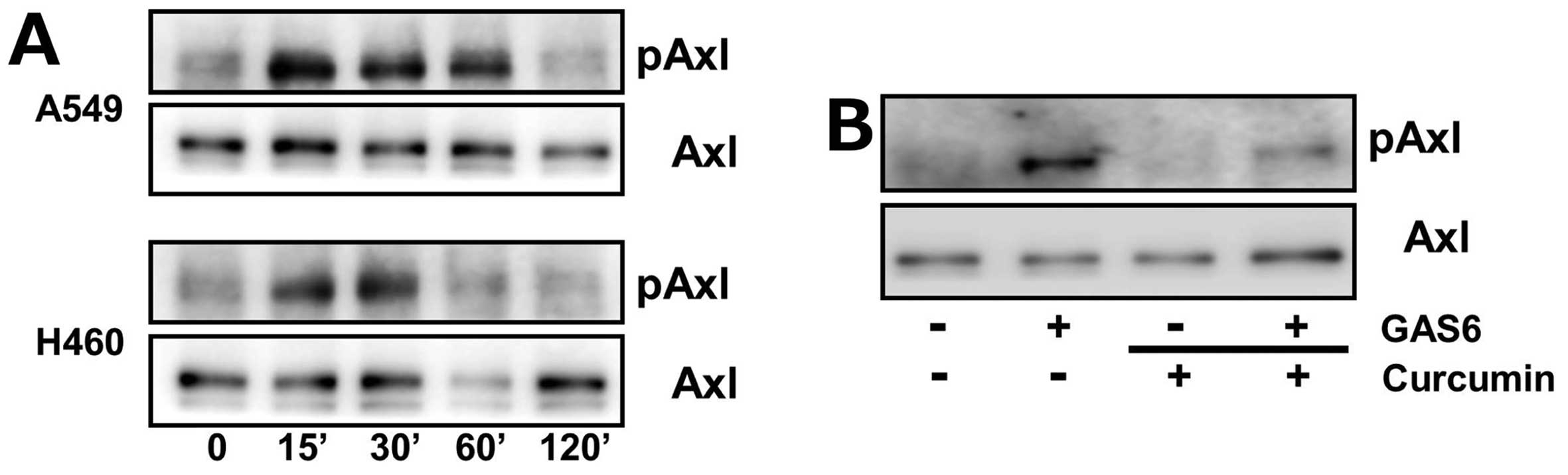
curcumin on Axl phosphorylation after Gas6 treatment.
Serum-starved A549 and H460 cells were treated with
Gas6 for 15, 30, 60 and 120 min and phosphorylated Axl levels were
determined by western blot analysis. As illustrated in Fig. 2A, Axl phosphorylation by Gas6
occurred within 15 min in both cells and returned back to each of
their basal levels by 120 min. However, we found that
pre-incubation of H460 cells with curcumin inhibited Gas6-induced
Axl phosphorylation (Fig. 2B),
suggesting the inhibitory effect of curcumin on Axl activation upon
Gas6 stimulation.
Curcumin targets Axl to inhibit cell
proliferation
Since overexpression and activation of Axl had been
reported to be involved in oncogenesis, cell survival,
proliferation and anti-apoptosis (13,14,20,22),
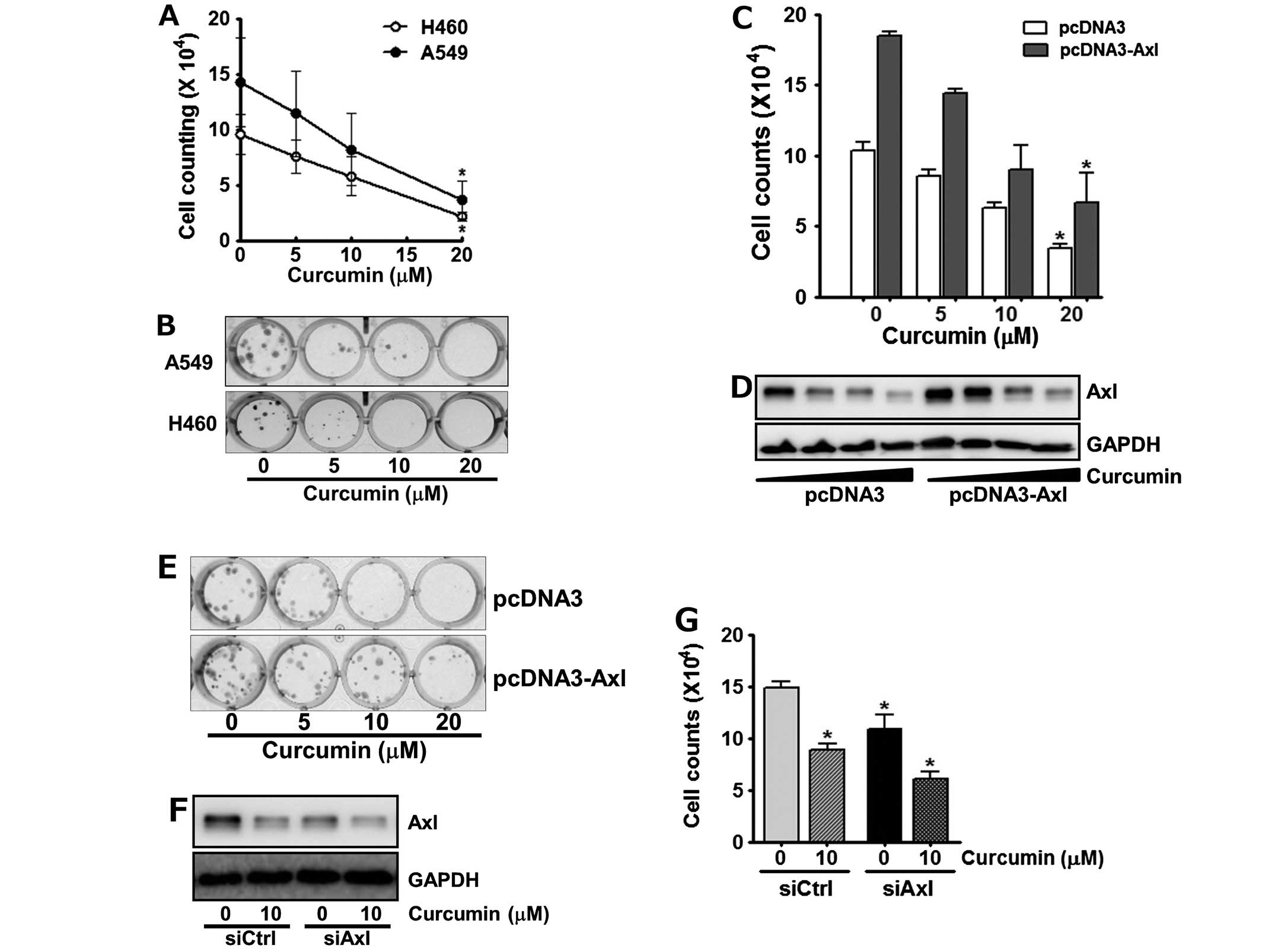
we next assessed if the down-regulation of Axl by curcumin affects
cell viability. The cells were incubated with 0, 5, 10 and 20 μM of
curcumin for 24 h, and the number of viable cells was then counted.
As shown in Fig. 3A, treatment of
cells with curcumin reduced cell viability in a dose-dependent
manner. Of note, following the incubation of A549 and H460 cells
with 20 μM curcumin, only 30% and 22% of the cells survived,
respectively.
The anti-proliferative effects of curcumin on the
A549 and H460 cells were also observed by clonogenic assay. The
cells were exposed to the indicated concentrations of curcumin for
24 h and then allowed to grow for the next 10 days. Curcumin
treatment was found to result in the dose-dependent decline of
colony formation (Fig. 3B).
Specifically, H460 cells as well as A549 cells failed to form
visible colonies at 10 and 20 μM curcumin, respectively, indicating
that curcumin seems to be more cytotoxic toward H460 cells than
A549 cells.
To further confirm the involvement of Axl in the
antiproliferative effects of curcumin, we examined the cytotoxic
effect of curcumin on cells manipulated to enhance or reduce Axl
expression. As shown in Fig. 3C,
A549 cells transfected with pcDNA3-Axl, a recombinant plasmid
containing Axl cDNA for its overexpression, were less sensitive to
curcumin treatment compared to the control cells transfected with
pcDNA3 vector, indicating that Axl overexpression reduced the
antiproliferative effects of curcumin. Western blot analysis
consistently showed that the Axl level of the pcDNA3-Axl
transfected A549 cells was higher than that of their control cells
even after curcumin treatment (Fig.
3D). Colony formation assay also manifested that in contrast to
the control cells, Axl-overexpressing A549 cells formed more
colonies and were relatively less affected by curcumin treatment
(Fig. 3E). Subsequently, H460
cells were transfected with Axl specific siRNA, siAxl, or control
siRNA, siCtrl and then treated with curcumin for 24 h. We found
that Axl targeting by siAxl significantly decreased Axl expression
(Fig. 3F), which resulted in the
augmentation of anti-proliferative effect of curcumin (Fig. 3G). Taken together, these results
demonstrated that Axl protein level tightly correlates with cell
proliferation and verified that curcumin inhibits cell
proliferation via down-regulation of Axl expression.
Curcumin suppresses proliferation of both
cisplatin- and paclitaxel-resistant lung cancer cells and results
in the elevation of p21 as well as reduction of XIAP
expression
Next, we asked if curcumin could be cytotoxic in the
cisplatin- and paclitaxel-resistant NSCLC cells which are the
variants of A549 and H460 cells. Each of the variants was
established by stepwise exposure of parental cells to increasing
concentrations of cisplatin (A549/CisR and H460/CisR cells) or
paclitaxel (A549/TR and H460/TR cells), respectively. As shown in
Fig. 4A, curcumin reduced the Axl
protein levels of A549/TR, A549/CisR, H460/TR and H460/CisR cells
in a dose-dependent manner. Notably, in H460/TR cells, the Axl
protein level was found to be fairly low and still decreased by
curcumin treatment.
In accordance with the western blot results, the
viability of both paclitaxel- and cisplatin-resistant cells were
dose-dependently declined by curcumin treatment. Especially,
exposure of cells with 20 μM curcumin for 24 h was found to result
in only 41.3% (A549/TR), 62.8% (A549/CisR), 15.6% (H460/TR), 27%
(H460/CisR) survival of these cells, respectively (Fig. 4B and C). Colonogenic activity of
curcumin-treated the variants of A549 and H460 cells further showed
cytotoxicity of curcumin on these chemoresistant cells. As shown in
Fig. 4D, treatment of these cells
with curcumin reduced the number of colonies as well as the size of
each colony. In contrast to chemoresistant A549 cells, H460/TR and
H460/CisR cells were more profoundly affected by curcumin
treatment, which are consistent with the result from cell viability
measurement.
To demonstrate the intracellular effectors which are
involved in the curcumin-mediated downregulation of Axl expression
and result in the inhibition of cell proliferation, we assessed the
levels of cell cycle regulator, p21 and apoptosis related protein,
the X-linked inhibitor of apoptosis protein (XIAP). The H460,
H460/TR and H460/CisR cells were treated with 10 μM curcumin for 24
h and western blot analysis showed that curcumin induced the
expression of the cyclin dependent kinase inhibitor p21, which
causes cell cycle arrest, but reduced that of XIAP, which inhibits
apoptosis (Fig. 4E). Collectively,
these results indicate that curcumin downregulates Axl expression,
subsequently increases p21 protein level and decreases XIAP protein
level, which result in the inhibition of cell proliferation.
Discussion
Standard chemotherapy for NSCLC has been the
combination of platinum-based agents (cisplatin or carboplatin) and
a second drug (pemetrexed, gemstabine, paclitaxel or vinorelbine),
but low response rate (20–35%) and eventual development of
chemoresistance among initial responders have been the main causes
of poor prognosis (33,34). Linger et al (24) have demonstrated that Mer or Axl
inhibition enhanced sensitivity of NSCLC cells to various cytotoxic
agents such as cisplatin, carboplatin, doxorubicin or etoposide by
promoting apoptosis. Since TAM family members of RTKs have been
reported to play important roles in cell survival, proliferation
and apoptosis (35), targeting of
these RTKs seems to be a potent strategy to improve standard
chemotherapy regimens.
In the present study, we observed that curcumin had
inhibitory effects on Axl expression, Gas6-dependent Axl
phosphorylation, and Axl promoter activity in NSCLC cells
(Figs. 1 and 2). Silencing of Axl expression by RNA
interference or specific monoclonal antibodies against Axl have
been demonstrated to inhibit cell proliferation, metastasis and
xenograft tumor growth in NSCLC (36,37).
Consistent with previous reports, we also observed that curcumin
decreased the viability of NSCLC cells (A549 and H460). Moreover,
anti-proliferative effect of curcumin was reduced by ectopic
expression of Axl and augmented by Axl knockdown using siRNA,
respectively, suggesting that curcumin abrogates these A549 and
H460 cell proliferation via downregulation of Axl expression and
further confirming that Axl is a new target of curcumin that
contributes to its anticancer effects which have been known to be
due to negative regulation of diverse intracellular molecules
including transcription factors (38), growth factors, protein kinases
(39) and oncogenic proteins
(40), resulting in cell cycle
arrest and/or apoptosis.
Several reports have shown that curcumin could
affect each stage of cancer such as initiation, promotion and
progression, ingestion of curcumin was even found to significantly
inhibit the activity of lymphocytic glutathione S-transferase, a
phase II detoxification enzyme, involved in the development of
chemoresistance (41,42). The growing body of evidence also
indicates that overexpression and/or activation of Axl is a novel
mechanism to induce the acquired resistance to various anticancer
drugs including cytotoxic agents and various tyrosine kinase
inhibitors, especially EGF receptor inhibitors (gefitinib or
erlotinib) (43,44). Consistently, our data also showed
that the viabilities of cisplatin/taxol-resistant cells (A549/CisR,
H460/CisR, A549/TR and H460/TR) were decreased by curcumin
treatment (Figs. 3A and B and
4B–D) and Axl protein levels of
each cell type were also declined by curcumin, implying again that
Axl plays a critical role in proliferation of both parental and
chemoresistant NSCLC cells and the acquisition of resistance
against chemotherapeutic drugs.
In summary, our data indicate that curcumin has
inhibitory effects on Axl expression and the activation in response
to Gas6 binding, which are associated with its anti-proliferative
activity in parental as well as each type of
cisplatin/paclitaxel-resistant NSCLC cells. Thus, Axl seems to be a
potent therapeutic target of curcumin to inhibit cell proliferation
and to overcome chemoresistance of NSCLC cells.
Acknowledgements
The present study was supported by the 2014 Yeungnam
University Research Grant (no. 214A380116).
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
Gas6
|
growth arrest-specific 6
|
|
NSCLC
|
non-small cell lung cancer
|
|
RTK
|
receptor tyrosine kinase
|
|
XIAP
|
X-linked inhibitor of apoptosis
|
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type:
male:female differences diminishing and adenocarcinoma rates
rising. Int J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group. Comparison of four chemotherapy
regimens for advanced non-small cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lai C and Lemke G: An extended family of
protein-tyrosine kinase genes differentially expressed in the
vertebrate nervous system. Neuron. 6:691–704. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Bryan JP, Frye RA, Cogswell PC, Neubauer
A, Kitch B, Prokop C, Espinosa R III, Le Beau MM, Earp HS and Liu
ET: axl, a transforming gene isolated from primary human myeloid
leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell
Biol. 11:5016–5031. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai C, Gore M and Lemke G: Structure,
expression, and activity of Tyro 3, a neural adhesion-related
receptor tyrosine kinase. Oncogene. 9:2567–2578. 1994.PubMed/NCBI
|
|
9
|
Graham DK, Dawson TL, Mullaney DL,
Snodgrass HR and Earp HS: Cloning and mRNA expression analysis of a
novel human protooncogene, c-mer. Cell Growth Differ. 5:647–657.
1994.PubMed/NCBI
|
|
10
|
Heiring C, Dahlbäck B and Muller YA:
Ligand recognition and homophilic interactions in Tyro3: Structural
insights into the Axl/Tyro3 receptor tyrosine kinase family. J Biol
Chem. 279:6952–6958. 2004. View Article : Google Scholar
|
|
11
|
Sasaki T, Knyazev PG, Clout NJ, Cheburkin
Y, Göhring W, Ullrich A, Timpl R and Hohenester E: Structural basis
for Gas6-Axl signalling. EMBO J. 25:80–87. 2006. View Article : Google Scholar
|
|
12
|
Lemke G and Rothlin CV: Immunobiology of
the TAM receptors. Nat Rev Immunol. 8:327–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rochlitz C, Lohri A, Bacchi M, Schmidt M,
Nagel S, Fopp M, Fey MF, Herrmann R and Neubauer A: Axl expression
is associated with adverse prognosis and with expression of Bcl-2
and CD34 in de novo acute myeloid leukemia (AML): Results from a
multicenter trial of the Swiss Group for Clinical Cancer Research
(SAKK). Leukemia. 13:1352–1358. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berclaz G, Altermatt HJ, Rohrbach V,
Kieffer I, Dreher E and Andres AC: Estrogen dependent expression of
the receptor tyrosine kinase axl in normal and malignant human
breast. Ann Oncol. 12:819–824. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Craven RJ, Xu LH, Weiner TM, Fridell YW,
Dent GA, Srivastava S, Varnum B, Liu ET and Cance WG: Receptor
tyrosine kinases expressed in metastatic colon cancer. Int J
Cancer. 60:791–797. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nemoto T, Ohashi K, Akashi T, Johnson JD
and Hirokawa K: Overexpression of protein tyrosine kinases in human
esophageal cancer. Pathobiology. 65:195–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rankin EB, Fuh KC, Taylor TE, Krieg AJ,
Musser M, Yuan J, Wei K, Kuo CJ, Longacre TA and Giaccia AJ: AXL is
an essential factor and therapeutic target for metastatic ovarian
cancer. Cancer Res. 70:195–203. 2010. View Article : Google Scholar
|
|
18
|
Sainaghi PP, Castello L, Bergamasco L,
Galletti M, Bellosta P and Avanzi GC: Gas6 induces proliferation in
prostate carcinoma cell lines expressing the Axl receptor. J Cell
Physiol. 204:36–44. 2005. View Article : Google Scholar
|
|
19
|
Ito T, Ito M, Naito S, Ohtsuru A, Nagayama
Y, Kanematsu T, Yamashita S and Sekine I: Expression of the Axl
receptor tyrosine kinase in human thyroid carcinoma. Thyroid.
9:563–567. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hutterer M, Knyazev P, Abate A, Reschke M,
Maier H, Stefanova N, Knyazeva T, Barbieri V, Reindl M, Muigg A, et
al: Axl and growth arrest-specific gene 6 are frequently
overexpressed in human gliomas and predict poor prognosis in
patients with glioblastoma multiforme. Clin Cancer Res. 14:130–138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun WS, Fujimoto J and Tamaya T:
Coexpression of growth arrest-specific gene 6 and receptor tyrosine
kinases Axl and Sky in human uterine endometrial cancers. Ann
Oncol. 14:898–906. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gustafsson A, Martuszewska D, Johansson M,
Ekman C, Hafizi S, Ljungberg B and Dahlbäck B: Differential
expression of Axl and Gas6 in renal cell carcinoma reflecting tumor
advancement and survival. Clin Cancer Res. 15:4742–4749. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wimmel A, Glitz D, Kraus A, Roeder J and
Schuermann M: Axl receptor tyrosine kinase expression in human lung
cancer cell lines correlates with cellular adhesion. Eur J Cancer.
37:2264–2274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linger RM, Keating AK, Earp HS and Graham
DK: Taking aim at Mer and Axl receptor tyrosine kinases as novel
therapeutic targets in solid tumors. Expert Opin Ther Targets.
14:1073–1090. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shieh YS, Lai CY, Kao YR, Shiah SG, Chu
YW, Lee HS and Wu CW: Expression of axl in lung adenocarcinoma and
correlation with tumor progression. Neoplasia. 7:1058–1064. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abe Y, Hashimoto S and Horie T: Curcumin
inhibition of inflammatory cytokine production by human peripheral
blood monocytes and alveolar macrophages. Pharmacol Res. 39:41–47.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oyama Y, Masuda T, Nakata M, Chikahisa L,
Yamazaki Y, Miura K and Okagawa M: Protective actions of
5′-n-alkylated curcumins on living cells suffering from oxidative
stress. Eur J Pharmacol. 360:65–71. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park W, Amin AR, Chen ZG and Shin DM: New
perspectives of curcumin in cancer prevention. Cancer Prev Res
(Phila). 6:387–400. 2013. View Article : Google Scholar
|
|
29
|
Sharma RA, Euden SA, Platton SL, Cooke DN,
Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer
SM, et al: Phase I clinical trial of oral curcumin: Biomarkers of
systemic activity and compliance. Clin Cancer Res. 10:6847–6854.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stitt TN, Conn G, Gore M, Lai C, Bruno J,
Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al: The
anticoagulation factor protein S and its relative, Gas6, are
ligands for the Tyro 3/Axl family of receptor tyrosine kinases.
Cell. 80:661–670. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Varnum BC, Young C, Elliott G, Garcia A,
Bartley TD, Fridell YW, Hunt RW, Trail G, Clogston C, Toso RJ, et
al: Axl receptor tyrosine kinase stimulated by the vitamin
K-dependent protein encoded by growth-arrest-specific gene 6.
Nature. 373:623–626. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stordal B, Pavlakis N and Davey R: A
systematic review of platinum and taxane resistance from bench to
clinic: An inverse relationship. Cancer Treat Rev. 33:688–703.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Linger RM, Keating AK, Earp HS and Graham
DK: TAM receptor tyrosine kinases: Biologic functions, signaling,
and potential therapeutic targeting in human cancer. Adv Cancer
Res. 100:35–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ye X, Li Y, Stawicki S, Couto S,
Eastham-Anderson J, Kallop D, Weimer R, Wu Y and Pei L: An anti-Axl
monoclonal antibody attenuates xenograft tumor growth and enhances
the effect of multiple anticancer therapies. Oncogene.
29:5254–5264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J,
Kallop D, Ludlam MJ and Pei L: Axl as a potential therapeutic
target in cancer: Role of Axl in tumor growth, metastasis and
angiogenesis. Oncogene. 28:3442–3455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sethi G and Tergaonkar V: Potential
pharmacological control of the NF-κB pathway. Trends Pharmacol Sci.
30:313–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dorai T, Gehani N and Katz A: Therapeutic
potential of curcumin in human prostate cancer. II Curcumin
inhibits tyrosine kinase activity of epidermal growth factor
receptor and depletes the protein. Mol Urol. 4:1–6. 2000.PubMed/NCBI
|
|
40
|
Seol DW, Chen Q and Zarnegar R:
Transcriptional activation of the hepatocyte growth factor receptor
(c-met) gene by its ligand (hepatocyte growth factor) is mediated
through AP-1. Oncogene. 19:1132–1137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sharma RA, Ireson CR, Verschoyle RD, Hill
KA, Williams ML, Leuratti C, Manson MM, Marnett LJ, Steward WP and
Gescher A: Effects of dietary curcumin on glutathione S-transferase
and malondialdehyde-DNA adducts in rat liver and colon mucosa:
Relationship with drug levels. Clin Cancer Res. 7:1452–1458.
2001.PubMed/NCBI
|
|
42
|
Townsend D and Tew K: Cancer drugs,
genetic variation and the glutathione-S-transferase gene family. Am
J Pharmacogenomics. 3:157–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Z, Lee JC, Lin L, Olivas V, Au V,
LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, et al:
Activation of the AXL kinase causes resistance to EGFR-targeted
therapy in lung cancer. Nat Genet. 44:852–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rho JK, Choi YJ, Kim SY, Kim TW, Choi EK,
Yoon SJ, Park BM, Park E, Bae JH, Choi CM, et al: MET and AXL
inhibitor NPS-1034 exerts efficacy against lung cancer cells
resistant to EGFR kinase inhibitors because of MET or AXL
activation. Cancer Res. 74:253–262. 2014. View Article : Google Scholar
|