Introduction
Hepatocellular carcinoma (HCC) is a cancer
originating from the liver. Its prognosis is poor despite the
advancements in treatment (1).
Treatments for HCC include local ablation, surgery, transcatheter
arterial chemoembolization, and chemotherapy (2,3).
Molecular therapy has also been established as a treatment option
(4). To develop a new molecular
therapy, research has focused on signaling pathways (5).
The Wnt pathway is involved in cell proliferation
and differentiation (6). Wnt
proteins bind to their receptor, frizzled, and its co-receptors,
low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6),
to form a complex (7,8). β-catenin is degraded by the glycogen
synthase kinase-3β complex (9).
When Wnt binds to its receptor complex, the degradation of
β-catenin is inhibited. β-catenin then accumulates in the cytoplasm
and the Wnt pathway is activated. β-catenin is a co-factor of the
T-cell factor (TCF)/lymphoid enhancer factor (LEF). When the Wnt
pathway is activated, the accumulated β-catenin translocates to the
nucleus, binds the promoter of target genes with TCF/LEF (10). In HCC, β-catenin is mutated and
overexpressed, which suggests that the Wnt pathway is
constitutively activated (11).
Therefore, β-catenin is a potential target in the exploration of
molecular therapy (12). The
inhibition of frizzled-9 suppresses the proliferation and motility
of HCC cells (13). Niclosamide is
a drug used for the treatment of tapeworm infections. It is an
inhibitor of the Wnt signaling pathway and it suppresses the
proliferation of HCC cells (14).
Previous reports indicate that the Wnt pathway is a promising
target in the treatment of HCC.
FH535 is a small molecule that inhibits the Wnt
signaling pathway and peroxisome proliferator-activator receptor
signaling (15). One of its unique
characteristics is that it inhibits the recruitment of β-catenin.
Therefore, FH535 is expected to be a potent inhibitor of the Wnt
signaling pathway.
In this study, we investigated the effects of FH535
on motility and proliferation of HCC cells.
Materials and methods
Cell culture
HLF cells and PLC/PRF/5 cells, human HCC cells, were
obtained from RIKEN Cell Bank (Tsukuba, Japan) and cultured in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis,
MO, USA) supplemented with 10% fetal bovine serum (FBS; Life
Technologies, Grand Island, NY, USA). They were cultured in 10-cm
dishes (Asahi Techno Glass, Funabashi, Japan) with 5% carbon
dioxide at 37°C in a humidified chamber.
Cell proliferation assay
Cells were trypsinized, harvested, and spread onto
96-well plates (Asahi Techno Glass) at a density of 1,000
cells/well. They were cultured in DMEM supplemented with 10% FBS.
The cells were cultured for 72 h with 0, 0.5, 1.5, 5, 15 or 50 μM
FH535 (Merck, Darmstadt, Germany) and subjected to
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) assay, according to the manufacturer's
instructions (Promega Corp., Madison, WI, USA). MTS is reduced by
cells to a colored formazan product that has an absorbance maximum
at 490 nm. Absorbance was measured using an iMark Microplate
Absorbance Reader (Bio-Rad, Hercules, CA, USA).
Real-time quantitative polymerase chain
reaction
Total RNA (5 μg), which was isolated using Isogen
(Nippon Gene, Tokyo, Japan), was used for the first-strand cDNA
synthesis with SuperScript III and oligo(dT) following the
manufacturer's instructions (Life Technologies). Real-time
quantitative PCR was performed using Fast SYBR Green Master Mix
(Life Technologies) with MiniOpticon (Bio-Rad). The results were
analyzed using the MiniOpticon system. Real-time quantitative PCR
was performed for 40 cycles, with 5 sec of denaturation and 5 sec
of annealing/extension. The primer sequences are listed in Table I. RPL19 was used as an internal
control as the target gene is a constitutively expressed
house-keeping gene (16).
 | Table IThe primer sequences. |
Table I
The primer sequences.
| Primer name | Sequence | Description | Product size
(bp) | Annealing
temperature | Cycle | GenBank |
|---|
| OMC355 |
5′-AGAGGCGGAGGAGAACAAACAG-3′ | Cyclin D1,
forward | 180 | 60 | 40 | NM_053056 |
| OMC356 |
5′-AGGCGGTAGTAGGACAGGAAGTTG-3′ | Cyclin D1,
reverse | | | | |
| OMC749 |
5′-CCTGGGCAGATTCCAAACCT-3′ | MMP9, forward | 89 | 60 | 40 | NM_004994 |
| OMC750 |
5′-GCAAGTCTTCCGAGTAGTTTTGGAT-3′ | MMP9, reverse | | | | |
| OMC321 |
5′-CGAATGCCAGAGAAGGTCAC-3′ | RPL19, forward | 157 | 60 | 40 | BC095445 |
| OMC322 |
5′-CCATGAGAATCCGCTTGTTT-3′ | RPL19, reverse | | | | |
Scratch assay and hematoxylin and eosin
staining
Cells were plated on 4-well chamber slides
(Becton-Dickinson, Franklin Lakes, NJ, USA). When the cells reached
confluence, they were scratched with a sterile razor. The cells
were incubated with 0 or 50 μM FH535 for 48 h and stained with
hematoxylin and eosin (H&E). For the analysis of apoptosis, the
cells were plated in 4-well chamber slides (Becton-Dickinson). The
cells were incubated with 0 or 50 μM FH535 for 48 h and then
stained with H&E. The stained slides were observed under an
AX80 microscope (Olympus, Tokyo, Japan) for the apoptosis analysis
and scratch assay. In the scratch assay, the distance between the
scratched line and the growing edges of the cells was measured at
five points.
Statistical analysis
Data were analyzed by one-way analysis of variance
(ANOVA) using JMP 10.0.2 software (SAS Institute, Cary, NC, USA).
P-values <0.05 were considered statistically significant.
Results
To analyze the suppression of cell proliferation,
HLF cells (Fig. 1A) and PLC/PRF/5
cells (Fig. 1B) were cultured with
FH535. After 72 h, the cells were subjected to MTS assay.
Proliferation was found to be significantly suppressed in both cell
lines (P<0.05).
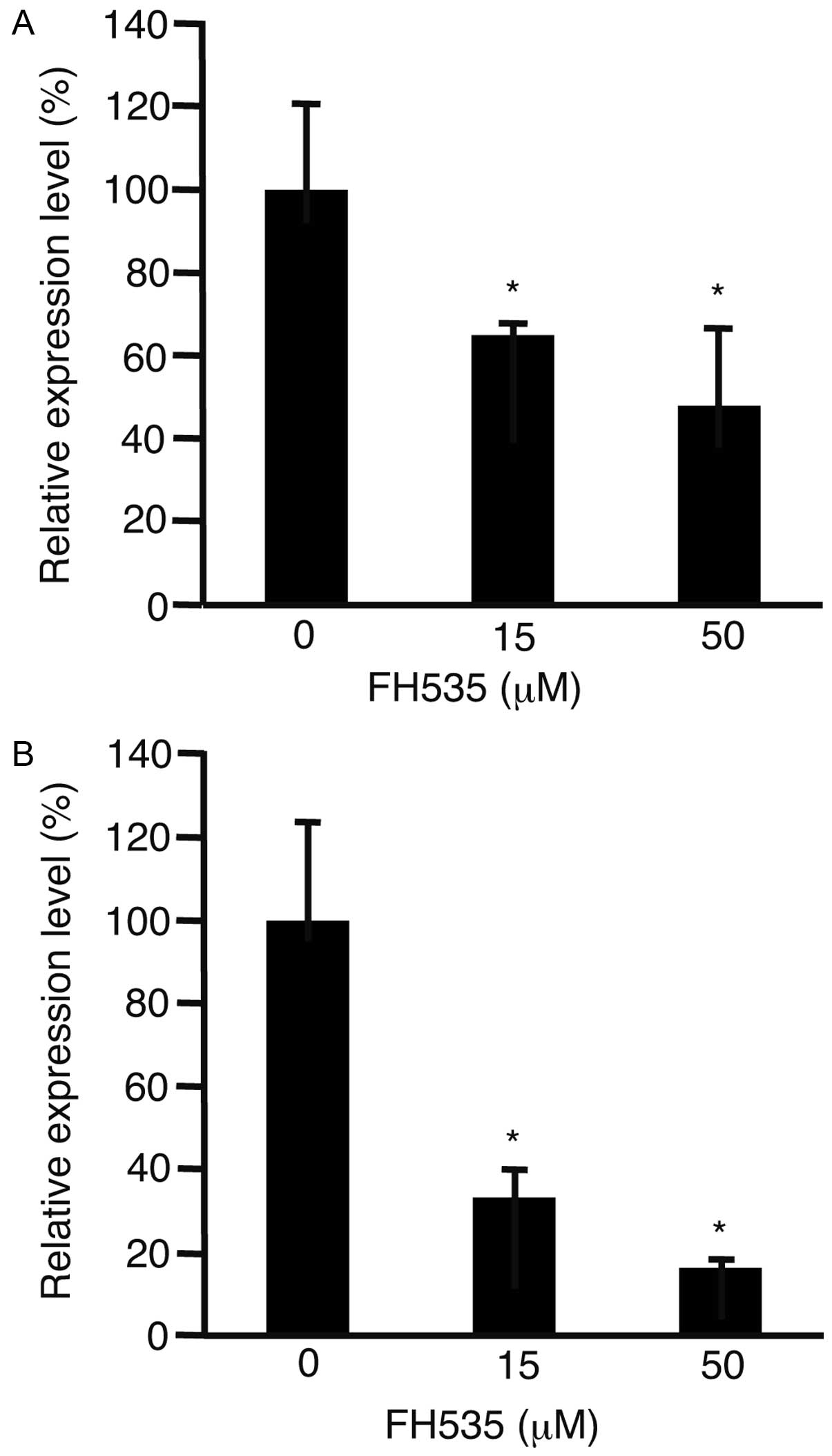
Cylin D1 is involved in the regulation of cell cycle
progression (17). To evaluate the
expression levels of cyclin D1, HLF cells (Fig. 2A) and PLC/PRF/5 cells (Fig. 2B) were incubated with FH535. After
48 h, RNA was isolated from the cells and subjected to real-time
quantitative PCR. The expression levels of cyclin D1 were
significantly suppressed in both cell lines (P<0.05).
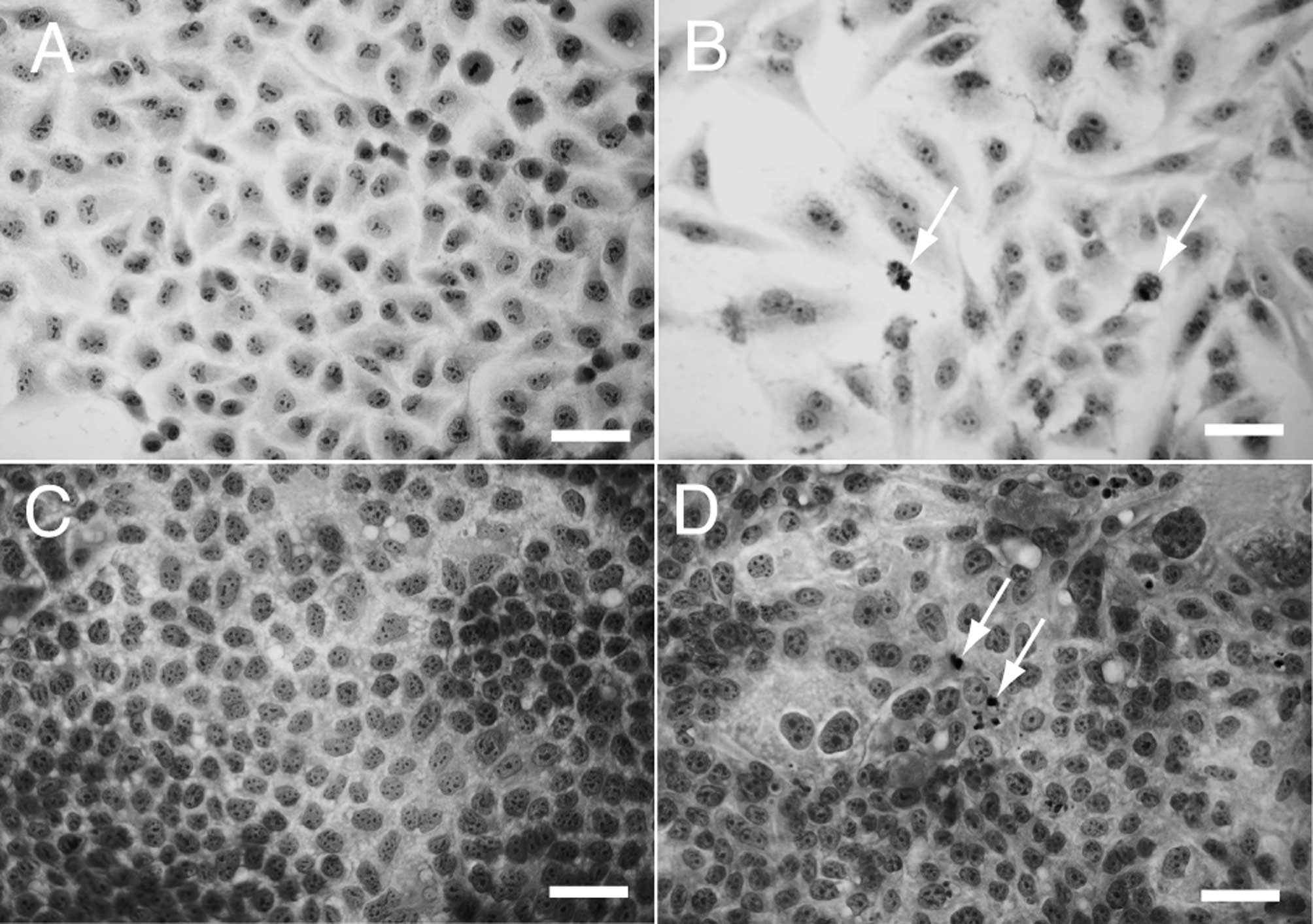
To clarify the involvement of apoptosis in the
suppression of cell proliferation, HLF (Fig. 3A and B) and PLC/PRF/5 cells
(Fig. 3C and D) were incubated
with FH535 at 0 μM (Fig. 3A and C)
or 50 μM (Fig. 3B and D) and
subjected to H&E staining. Pyknotic nuclei (arrows) were
observed in the cells cultured with 50 μM FH535. These results
indicate that the cells underwent apoptosis with FH535 at 50
μM.
To address the possibility that FH535 suppressed
cell motility, HLF cells (Fig. 4A and
C) and PLC/PRF/5 cells (Fig. 4B
and D) were cultured with FH535 at 0 μM (Fig. 4A and C) or 50 μM (Fig. 4B and D). The distance between the
growing edges of the cells and the scratched line significantly
decreased with the addition of 50 μM FH535 (Fig. 4E) (P<0.05).
The expression levels of matrix metalloproteinase 9
were analyzed because this gene is involved in cancer metastasis
(18). The expression levels of
matrix metalloproteinase 9 were significantly suppressed in the HLF
cells (Fig. 5A) and PLC/PRF/5
cells (Fig. 5B) (P<0.05).
Discussion
FH535 suppresses the proliferation of cancer cells.
Specifically, in this study, it was observed to suppress the
proliferation of HLF cells and PLC/PRF/5 cells. The expression
levels of cyclin D1 decreased in both cell types after incubation
with FH535. In the HCC cells, FH535 decreased the expression levels
of cyclin D1 and suppressed the cell cycle (19). These results were consistent with
those of the previous reports. The data clearly showed that the
cells underwent apoptosis. The results indicated that FH535
suppressed cell proliferation by suppressing the cell cycle and
inducing apoptosis.
FH535 suppresses cell motility as evidenced in the
present study. In addition, FH535 is known to suppress the
metastasis of HCC and pancreatic cancer cells (19,20).
In this study, the expression levels of matrix metalloproteinase 9
decreased in HLF cells and PLC/PRF/5 cells. The previous reports
and our data indicated that FH535 suppresses the motility of cancer
cells by decreasing the expression levels of matrix
metalloproteinase 9.
One possible limitation of this study is that the
concentration of FH535 was relatively high. FH535 suppressed the
proliferation and migration of HCC cells at 50 μM with statistical
significance. In breast cancer cells, FH535 suppresses
proliferation and migration at 1 μM (21). A higher concentration might be
hazardous to cells, leading to adverse effects. To reduce this
risk, using a combination of FH535 and other reagents would be
desirable.
FH535 and sorafenib synergistically inhibit the
proliferation of Huh-7 cells, another HCC cell line, and cancer
stem cells (22). Another
application of FH535 is in irradiation therapy (23). In the future, FH535 should be
combined with other chemotherapeutic agents or small molecules.
In conclusion, FH535 suppressed cell proliferation
by decreasing the expression of cyclin D1 and by inducing
apoptosis. In addition, it suppressed cell motility by decreasing
the expression of matrix metalloproteinase.
References
|
1
|
Tejeda-Maldonado J, García-Juárez I,
Aguirre-Valadez J, González-Aguirre A, Vilatobá-Chapa M,
Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-Ávila JF and
Carrillo-Pérez DL: Diagnosis and treatment of hepatocellular
carcinoma: An update. World J Hepatol. 7:362–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lencioni R, Petruzzi P and Crocetti L:
Chemoembolization of hepatocellular carcinoma. Semin Intervent
Radiol. 30:3–11. 2013. View Article : Google Scholar :
|
|
3
|
Kim HY and Park JW: Clinical trials of
combined molecular targeted therapy and locoregional therapy in
hepatocellular carcinoma: Past, present, and future. Liver Cancer.
3:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furuse J, Ishii H, Nakachi K, Suzuki E,
Shimizu S and Nakajima K: Phase I study of sorafenib in Japanese
patients with hepatocellular carcinoma. Cancer Sci. 99:159–165.
2008.
|
|
5
|
Chen C and Wang G: Mechanisms of
hepatocellular carcinoma and challenges and opportunities for
molecular targeted therapy. World J Hepatol. 7:1964–1970. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bogaerts E, Heindryckx F, Vandewynckel YP,
Van Grunsven LA and Van Vlierberghe H: The roles of transforming
growth factor-β, Wnt, Notch and hypoxia on liver progenitor cells
in primary liver tumours (Review). Int J Oncol. 44:1015–1022.
2014.PubMed/NCBI
|
|
7
|
Tanaka SS, Kojima Y, Yamaguchi YL,
Nishinakamura R and Tam PP: Impact of WNT signaling on tissue
lineage differentiation in the early mouse embryo. Dev Growth
Differ. 53:843–856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi-Yanaga F: Activator or
inhibitor? GSK-3 as a new drug target. Biochem Pharmacol.
86:191–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jamieson C, Sharma M and Henderson BR:
Targeting the β-catenin nuclear transport pathway in cancer. Semin
Cancer Biol. 27:20–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rizvi S and Gores GJ: Molecular profiling
and research of therapeutic targets. Dig Dis. 33:586–589. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monga SP: β-catenin signaling and roles in
liver homeostasis, Injury, and tumorigenesis. Gastroenterology.
148:1294–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujimoto T, Tomizawa M and Yokosuka O:
SiRNA of frizzled-9 suppresses proliferation and motility of
hepatoma cells. Int J Oncol. 35:861–866. 2009.PubMed/NCBI
|
|
14
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S, Sueishi M and Yoshida T: Niclosamide
suppresses hepatoma cell proliferation via the Wnt pathway. Onco
Targets Ther. 6:1685–1693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Handeli S and Simon JA: A small-molecule
inhibitor of Tcf/beta-catenin signaling down-regulates PPARgamma
and PPARdelta activities. Mol Cancer Ther. 7:521–529. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping, and
novel promoter region. Genomics. 25:372–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gedaly R, Galuppo R, Daily MF, Shah M,
Maynard E, Chen C, Zhang X, Esser KA, Cohen DA, Evers BM, et al:
Targeting the Wnt/β-catenin signaling pathway in liver cancer stem
cells and hepatocellular carcinoma cell lines with FH535. PLoS One.
9:e992722014. View Article : Google Scholar
|
|
20
|
Wu MY, Liang RR, Chen K, Shen M, Tian YL,
Li DM, Duan WM, Gui Q, Gong FR, Lian L, et al: FH535 inhibited
metastasis and growth of pancreatic cancer cells. Onco Targets
Ther. 8:1651–1670. 2015.PubMed/NCBI
|
|
21
|
Iida J, Dorchak J, Lehman JR, Clancy R,
Luo C, Chen Y, Somiari S, Ellsworth RE, Hu H, Mural RJ, et al:
FH535 inhibited migration and growth of breast cancer cells. PLoS
One. 7:e444182012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galuppo R, Maynard E, Shah M, Daily MF,
Chen C, Spear BT and Gedaly R: Synergistic inhibition of HCC and
liver cancer stem cell proliferation by targeting RAS/RAF/MAPK and
WNT/β-catenin pathways. Anticancer Res. 34:1709–1713.
2014.PubMed/NCBI
|
|
23
|
Su H, Jin X, Zhang X, Zhao L, Lin B, Li L,
Fei Z, Shen L, Fang Y, Pan H, et al: FH535 increases the
radiosensitivity and reverses epithelial-to-mesenchymal transition
of radioresistant esophageal cancer cell line KYSE-150R. J Transl
Med. 13:1042015. View Article : Google Scholar : PubMed/NCBI
|