Introduction
MicroRNAs (miRNAs) are short non-coding RNA
molecules of 19–24 nucleotides (nt) involved in
post-transcriptional regulation of genes expression (1). miRNAs participate in many significant
biological processes including cell proliferation, apoptosis,
migration, invasion, differentiation, initiation and progression of
various cancers (2–4). In recent years, miRNAs were
investigated in body fluids, such as plasma, serum, urine, and
saliva, and in tissues, and used as biomarkers in diverse diseases
including many cancers (5). It was
reported that miR-382 was downregulated in stage III/IV epithelial
ovarian carcinoma compared with the normal group (6,7).
Recently, miR-382 was reported downregulated in human ovarian
cancer tissues (8). Yet, limited
research has been carried out on the function and mechanism of
miR-382 in ovarian cancer.
ROR1 belongs to the RTKs, which are a large family
of cell surface glycoproteins (9).
It was reported that ovarian cancers patients with high expression
levels of ROR1 had a higher rate of relapse and a shorter median
survival than ovarian cancers patients who expressed low or
negligible levels of ROR1, which indicated that ovarian CSCs
express ROR1 that contributed to their abilities to form tumors,
thus, making ROR1 a potential target for the treatment of ovarian
cancer patients (10).
Furthermore, others reported the expression of ROR1 was related to
malignant characteristics of ovarian cancer, and ROR1 may act as a
novel prognostic biomarker in ovarian cancer (11). These studies suggested ROR1 may
function crucially in ovarian cancer. However, whether miR-382
targeted ROR1 or not in ovarian cancer cells remained unclear.
We studied the expression, functions and mechanism
of miR-382 in ovarian cancer, and the biological functions
including cellular proliferation, migration, invasion and
epithelial-mesenchymal transition (EMT) process in vitro, as
well as the involved molecular mechanisms including its target
relationship with ROR1. Our aim was to provide novel insights to
improve therapy and prevention of ovarian cancers.
Materials and methods
Cell culture and transfection
Ovarian cancer cell lines HO8910, A2780, SKOV3, and
COV434 were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA), human ovarian surface epithelium HOSE
cells were purchased from Pricells (Wuhan, China) and cultured in
DMEM medium containing 10% fetal bovine serum (FBS). HO8910, A2780,
SKOV3 cells were cultured in RPMI-1640 medium containing 10% FBS
and 1% antibiotic-antimycotic solution (100 U/ml penicillin and 100
μg/ml streptomycin). COV434 cell were cultured in McCoys 5A culture
media. Cells were maintained at 37°C in a humidified atmosphere
containing 5% CO2. Transfection was carried out by
Lipofectamine 2000 reagent (Invitrogen). The plasmids of miR-382
mimics (product no. HmiR-AN0480), miR-382 inhibitor (product no.
HmiR-AN0480-SN-10), miRNA NC mimics (product no. CmiR-AN0001) and
miRNA NC inhibitor (product no. CmiR-AN0001-SN) were purchased from
Fulen Gen (Guangzhou, China). The plasmid of pCDNA3.1-ROR1 was
constructed by AuraGene (Changsha, China).
miRNA target prediction
Prediction of miR-382 target sites was performed by
the online software MicroRNA.org - Targets and Expression
(http://www.microrna.org/microrna/home.do). The related
function of the target was also considered.
Quantitative real-time PCR
Total RNA was extracted by TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA) methods, and was reverse
transcribed as cDNA by using reverse transcription system
(Fermentans, Canada). The primers were designed using Primer 5.0,
and the forward and reverse primer sequences are showed in Table I. The quantitative real-time PCR
(qRT-PCR) was performed using the SYBR Green qPCR (Toyobo, Japan)
for studying the quantitative expression of various genes (ROR1,
E-cadherin, vimentin, N-cadherin and Snail) according to the
manufacturer's protocol. Three repetitions were tested for qRT-PCR.
The relative expressions of miR-382 and mRNAs were normalized to
those of internal reference U6 or β-actin, and were calculated by
the 2−ΔΔCt method.
 | Table IForward and reverse primer sequences
of genes. |
Table I
Forward and reverse primer sequences
of genes.
| Primer | Sequences (or product
number) |
|---|
| miR-382 | HmiRQP0480 (product
number) |
| U6 | HmiRQP9001 (product
number) |
| ROR1 | F:
AGATCACAGCTGCCTTCACTAT
R: GACATTCTCCAGGATTTCACAT |
| E-cadherin | F:
CTCGGCCTGAAGTGACTCGTAAC
R: CAGCAACGTGATTTCTGCATTTC |
| Vimentin | F:
GACGCCATCAACACCGAGTT
R: CTTTGTCGTTGGTTAGCTGGT |
| N-cadherin | F:
CAGTATCCGGTCCGATCTGC
R: GTCCTGCTCACCACCACTAC |
| Snail | F:
GCCTTCAACTGCAAATACTGC
R: CCTCATGTTTGTGCAGGAGA |
| β-actin | F:
AGGGGCCGGACTCGTCATACT
R: GGCGGCACCACCATGTACCCT |
MTT assay
Approximately 5×103 cells per well were
plated into 96-well plates (Costar, USA). After culturing for 24,
48, or 72 h, the cells were treated with fresh serum-free medium
and 10 μl/well MTT (Biosharp, Hefei, China) solution (10 mg/ml in
PBS) according to the manufacturer's protocol. DMSO 100 μl was
added to every well after the incubation for 4 h. Following
incubation at 37°C for 10 min, the absorbance was measured by
microplate reader (Thermo, USA) at 570 nm at room temperature.
Colony formation assay
Cells were trypsinized and suspended in medium
including 0.3% agar and 10% serum, then were plated onto a bottom
layer with 0.6% agar. The cells were plated at 300 cells/well into
6-well plates (Costar). The number of colonies were counted after
14 days by Giemsa staining (Solarbio, Beijing, China) via the
equation colony forming efficiency = (number of colonies/number of
inoculated cells) × 100%.
Scratch assay
Cells (closely 1×105 cells) were seeded
into 12-well plates (Costar), and were incubated at 37°C until
cells reached a confluence of ≥90%. A scratch was generated by use
of a sterile 10-μl pipette tip, and this time was considered as 0 h
for each experiment. Then, at 48 h, photographic images were
acquired with an inverted microscope (Motic, Xiamen, China). Thus,
migration distance = the gap of 0 h - the gap of 48 h the gaps of 0
h, so the migration distances are presented a reverse relationship
with the gap of 48 h (Figs. 3 and
7).
Invasion assay
Invasion assay were performed in 24-well plates with
8-μm pore size chamber inserts (BD, Frankin Lakes, NJ, USA).
Approximately 1×105 cells/well were resuspended in 200
μl of medium without fetal bovine serum (FBS; Gibco), and seeded on
the upper chamber with the Matrigel-coated membrane. In addition,
500 μl medium supplemented with 10% FBS was added into the lower
chamber. After 24-h incubation at 37°C and 5% CO2, the
membranes were stained with 0.1% crystal violet. The cell numbers
were counted via an inverted microscope (Motic). Each assay was
performed three independent times.
Western blotting
Protein extracts from SKOV3 and COV434 cells were
prepared using RIPA lysis buffer (AuraGene) according to the
manufacturer's protocol. The protein concentration was confirmed in
accordance with the Bradford protein assay reagent (Beyotime
Biotechnology, Suzhou, China), and bovine serum albumin was
utilized as a standard. Western blot analysis was subsequently
carried out to evaluate the levels of ROR1 (1:200, BM0049, ABZOOM),
E-cadherin (1:200, YT1453, Immunoway, Changsha, China), vimentin
(1:1,000, YT4881, Immunoway), N-cadherin (1:1,000, ab18203, Abcam,
China) and Snail (1:200, ab180714, Abcam). Equal amounts of lysate
were resolved via SDS-PAGE, and transferred to a PVDF membrane
(Millipore, Bedford, MA, USA) through a semidry transfer method.
The PVDF membrane was blocked with 5% non-fat milk in TBST buffer
for 2 h at room temperature, and then was incubated overnight with
primary antibodies, and then was incubated for 1 h using a suitable
secondary antibody (1:2,000, Jackson, USA).
Electrochemiluminescence was performed with a Gel Documentation and
Analysis System.
Luciferase reporter assay
Luciferase assays were performed in SKOV3 and COV434
cells. SKOV3 and COV434 cells were transfected with each of the
plasmids [empty vector (MOCK), ROR1 UTR wild-type (WT) and ROR1 UTR
mutant (MUT), as a miR-382 binding site] together with miR-382
mimics, miR-382 inhibitor, and negative control RNA in 24-well
plates. Two days after transfection, cells were harvested and
lysed. Dual luciferase reporter gene assay kit (BioVision,
Milpitas, CA, USA) was used to determine the luciferase activities
on a luminometer (Roche, Basel, Switzerland). Renilla luciferase
activity was normalized to firefly luciferase activity.
Statistical analysis
All statistical tests were conducted with SPSS 17.0
software. The variance between two groups and multiple groups were
compared by Student's t-test and ANOVA, respectively. The data are
expressed as mean ± standard deviation (SD). The p-values <0.05
were regarded as statistically significant.
Results
miR-382 is downregulated in human ovarian
cancer tissues and cell lines
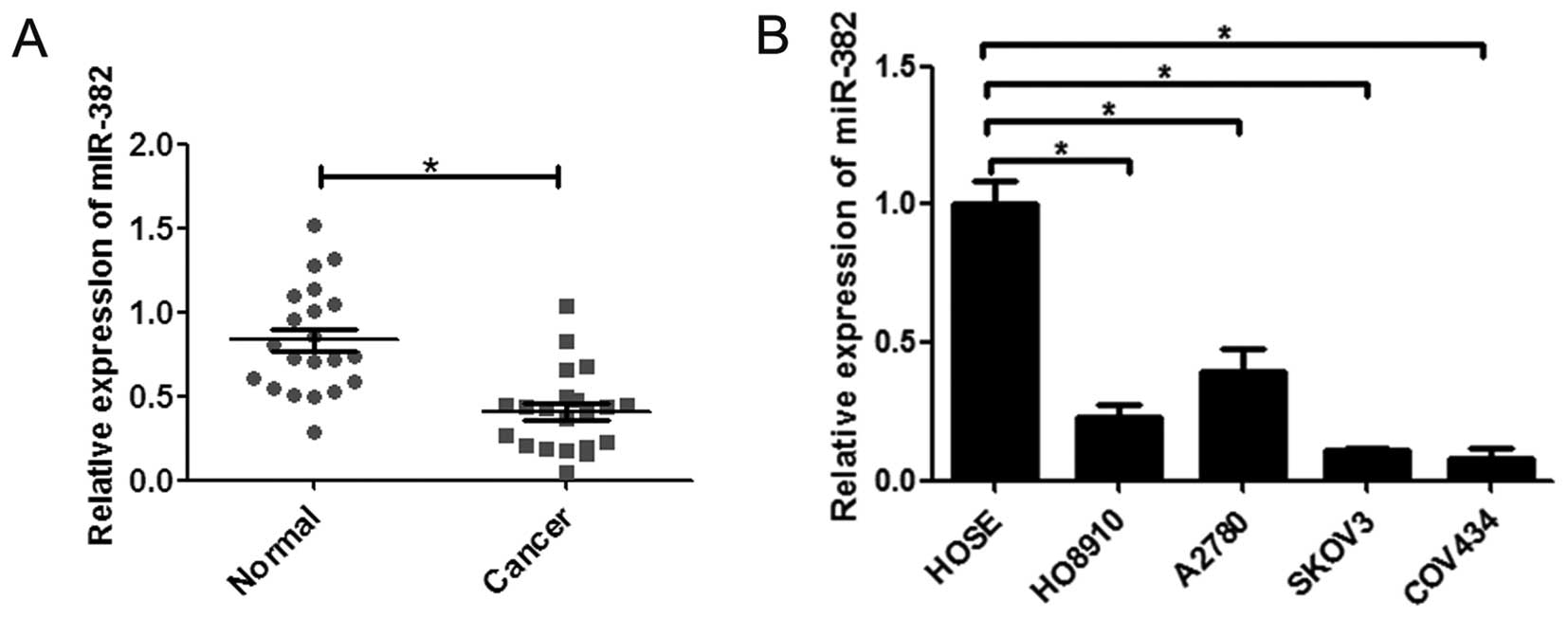
To investigate the role of miR-382 in ovarian
cancer, the expression of miR-382 was examined in tissues and cell
lines by qRT-PCR assays. We found the expression of miR-382 was
significantly downregulated in human ovarian cancer tissues
compared to adjacent non-cancerous tissues (n=20) (Fig. 1A). Moreover, the expression of
miR-382 in ovarian cancer cell lines (H08019, A2780, SKOV3 and
COV434) was markedly less than in ovarian epithelial cells HOSE
(Fig. 1B). These results indicate
that miR-382 is downregulated in both human ovarian cancer tissues
and cancer cell lines.
Overexpression of miR-382 suppresses
proliferation of ovarian cancer cells in vitro
To better understand the role of miR-382 in ovarian
cancer, we used retroviral vectors to establish ovarian cancer cell
lines stably overexpressing or silencing miR-382. The expression
levels of miR-382 in the SKOV3 and COV434 cell lines were examined
by qRT-PCR (Fig. 2A and B). We
also measured the growth-promoting effect of miR-382 on ovarian
cancer cells by MTT and colony formation assays. MTT assays
revealed that overexpression of miR-382 significantly suppressed
proliferation of ovarian cancer cells and silencing miR-382 in
ovarian cancer cells dramatically promoted proliferation (Fig. 2C and D). In colony formation assay,
overexpression of miR-382 significantly inhibited the viability of
indicated cells which formed less and smaller clones in SKOV3
(Fig. 2E) and COV434 cells
(Fig. 2F). These findings suggest
that miR-382 suppresses proliferation of ovarian cancer cells in
vitro.
Overexpression of miR-382 suppresses
migration and invasion of ovarian cancer cells in vitro
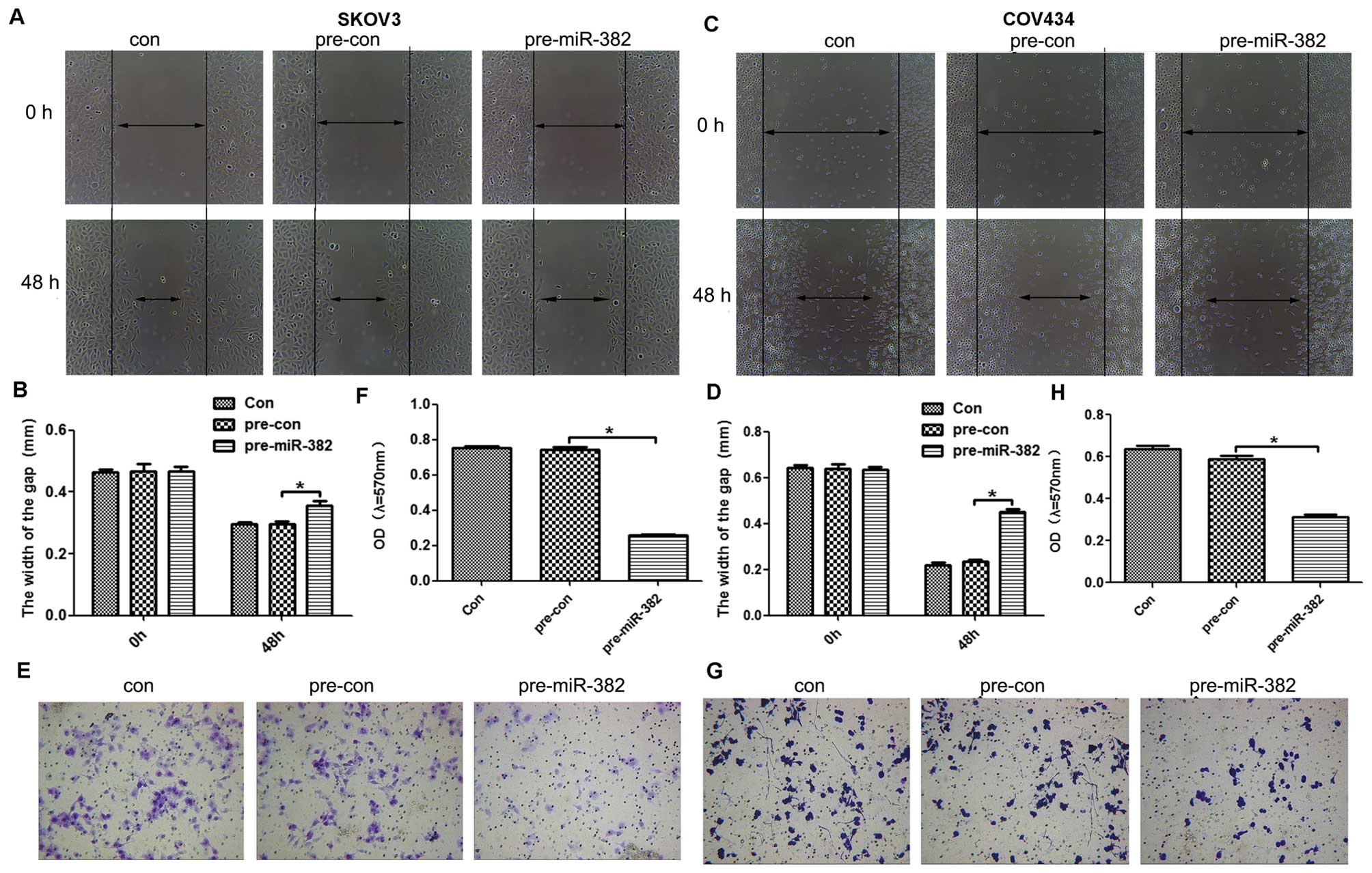
To evaluate the function of miR-382 in the migration
and invasion efficiency of ovarian cancer cells, the scratch test
and Transwell assays were performed. In scratch assay,
overexpression of miR-382 significantly promoted wound healing of
SKOV3 (Fig. 3A and B) and COV434
cells (Fig. 3C and D). Moreover,
Transwell assay was used to evaluate the invasive ability of
ovarian cancer cells. The results indicated that ectopic expression
of miR-382 significantly decreased the invasion rate of SKOV3
(Fig. 3E and F) and COV434 cells
(Fig. 3G and H).
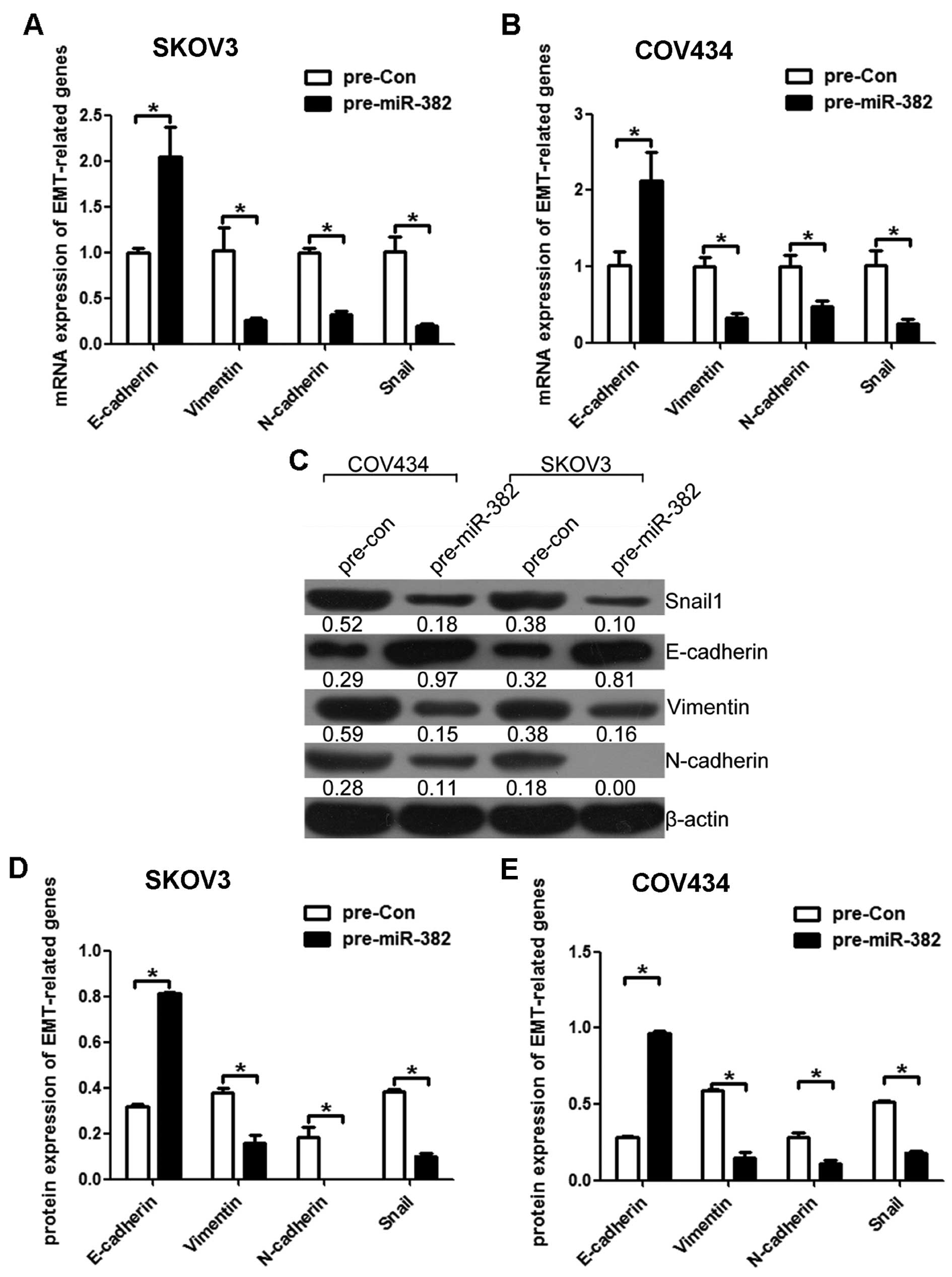
EMT is taken for a key mechanism by which cancer
cells acquire their migratory and invasive capabilities. To further
investigate whether the inhibitory effect of miR-382 on migration
and invasion was mediated by epithelial to mesenchymal transition
(EMT), we examined the expression of several EMT markers by qRT-PCR
and western blot assays. Both in mRNA and protein level as
expected, miR-382 overexpression increased the expression level of
epithelial marker (E-cadherin) and decreased the levels of
mesenchymal markers in SKOV3 (Fig. 4A,
C and D) and COV434 cells (Fig.
4B, C and E). Taken together, these findings suggest that
miR-382 was able to impede invasion mediated by EMT in
vitro.
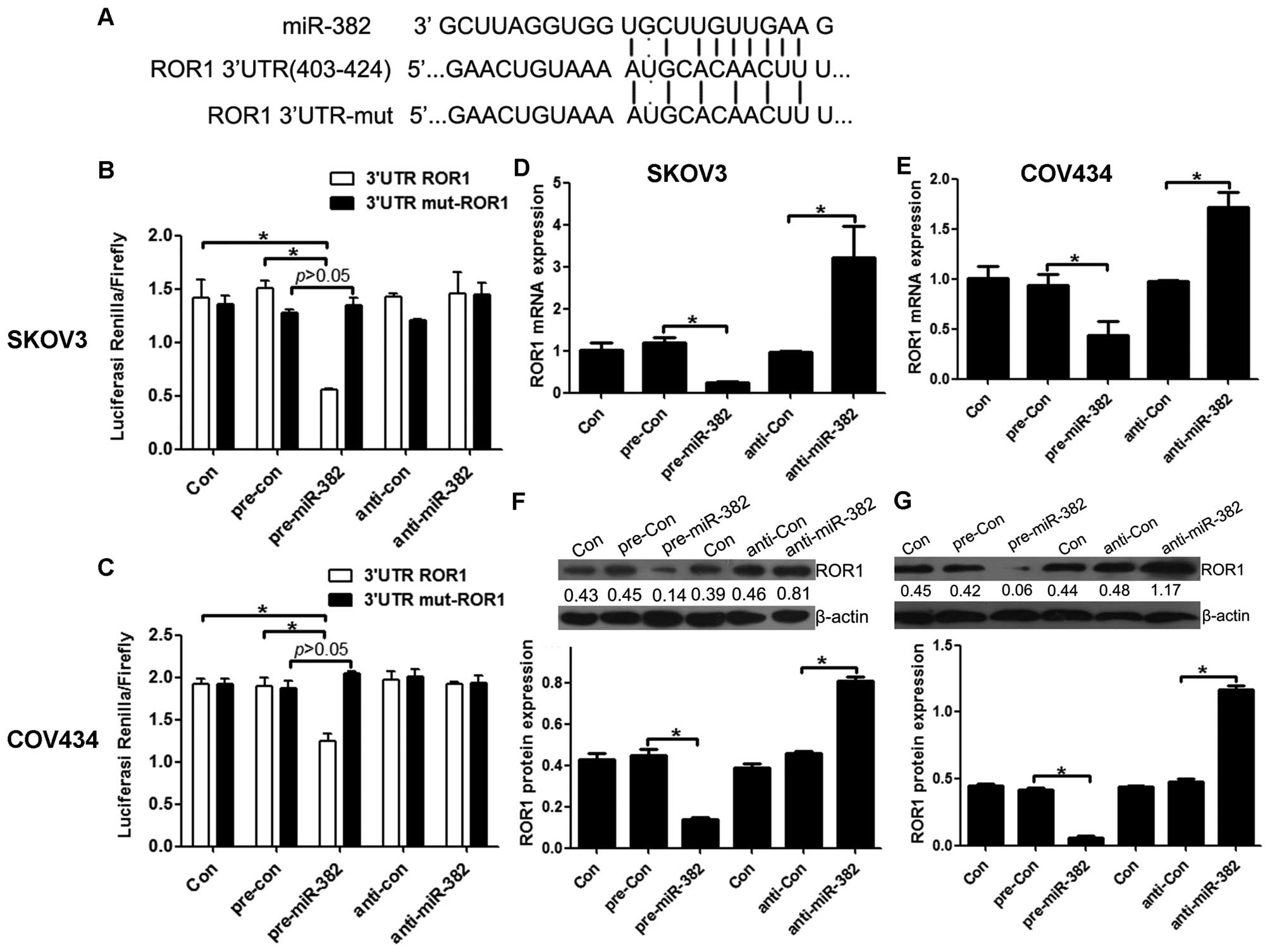
ROR1 is a direct target of miR-382 in
ovarian cancer cells
Many miRNAs have been reported to function by
binding a specific target. To examine whether miR-382 has a similar
mechanism, prediction of miRNA target sites was performed by the
online software MicroRNA.org - Targets and Expression (http://www.microrna.org/microrna/home.do), showing the
ROR1-3′-UTR regions contain the miR-382 complementary sequence
(Fig. 5A). In addition, it has
been reported that ROR1 function is crucial in ovarian cancer.
Thus, we focused on ROR1 as the primary candidate target of
miR-382, and examined firstly the direct binding between miR-382
and ROR1, we constructed the vectors ROR1-3′-UTR and
mut-ROR1-3′-UTR to observe their binding activity with miR-382.
miR-382 overexpression consistently and significantly reduced the
luciferase reporter activity by the ROR1-3′-UTR. However,
ROR1-3′-UTR luciferase reporter activity was unaffected by point
mutations in the miR-382-binding seed region (Fig. 5B and C). Collectively, these data
suggest that miR-382 may inhibit ROR1 expression by targeting its
3′-UTR. As predicted, qRT-PCR and western blot assays showed that,
at 48 h after transfection, the enhanced miR-382 in SKOV3 and
COV434 cells significantly repressed ROR1 RNA and protein
expression compared to cells transfected with a scrambled control.
By comparison, downregulation of miR-382 by inhibitors in SKOV3
(Fig. 5D and F) and COV434
(Fig. 5E and G) cells led to a
moderate increase in the ROR1 RNA and protein level. Together,
these data provide strong evidence that ROR1 is a specific target
of miR-382 in ovarian cancer cells.
miR-382 rescues the promotion effect of
ROR1 on migration and invasion of ovarian cancer cells
To clarify the roles of ROR1 in ovarian cancer, the
expression of ROR1 was investigated in tissues and cell lines. It
was found that the mRNA level of ROR1 was upregulated in human
ovarian cancer tissues (Fig. 6A),
and was negatively correlated with the downregulation of miR-382
(Fig. 6B). The mRNA and protein
levels of ROR1 in the human ovarian cancer cell lines HO8910, SKOV3
and COV434 were markedly higher than their expression in human
ovarian epithelial HOSE cells (Fig.
6C). To evaluate the function of ROR1 that acted as a target of
miR-382 in the migration and invasion efficiency of ovarian cancer
cells, the scratch test and the Transwel assays were performed. The
overexpression of ROR1 significantly increased cell migration,
which was rescued by overexpression of miR-382 in both SKOV3
(Fig. 7A and B) and COV434 cells
(Fig. 7C and D). Overexpression of
ROR1 promoted the cell invasion, while this effect was reversed by
overexpression of miR-382 in SKOV3 (Fig. 7E and F) and COV434 cells (Fig. 7G and H). Taken together, these
findings suggested that miR-382 inhibited migration and invasion by
directly targeting ROR1 in SKOV3 and COV434 cells.
miR-382 is able to rescue the effect of
ROR1 on EMT process of ovarian cancer cells
The above observations suggested that miR-382 could
inhibit ovarian cancer cell migration and invasion and exerted its
function by directly targeting ROR1; we further investigated the
mechanism by which miR-382 and ROR1 regulate the cell malignant
phenotype. To determine whether the typical molecular alternations
of EMT occurred, the RNA and protein levels of epithelial
(E-cadherin), mesenchymal (vimentin and N-cadherin) markers and
EMT-related transcription factor Snail were measured by qRT-PCR and
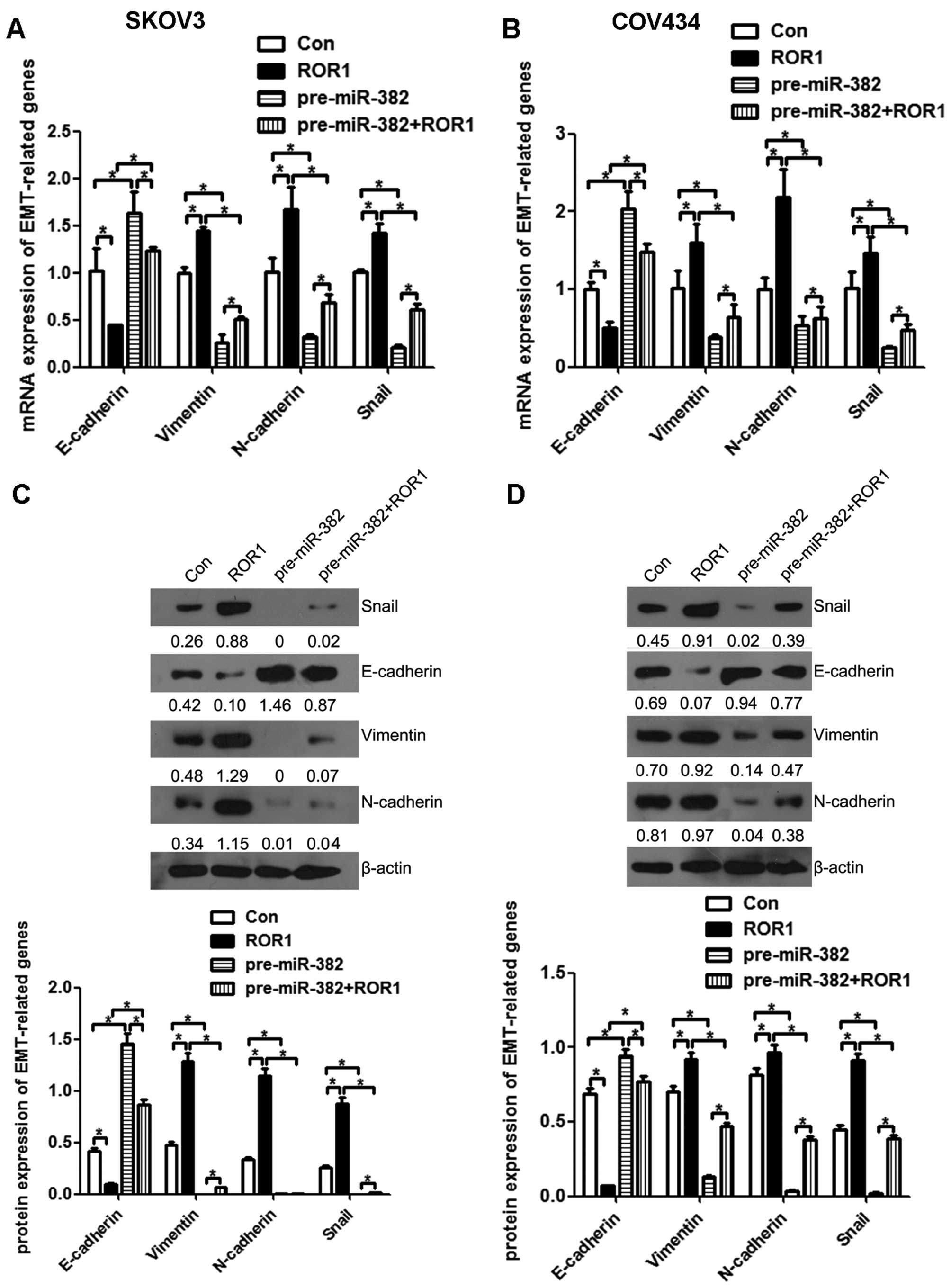
western blot assays. ROR1 overexpression reduced the mRNA
expression of the epithelial maker E-cadherin and increased the
mesenchymal makers N-cadherin, vimentin and EMT-related
transcription factor Snail, while the effect was reversed by
overexpression of miR-382 in SKOV3 (Fig. 8A) and COV434 cells (Fig. 8B). In line with mRNA expression,
ROR1 overexpression lowered the protein amounts of the epithelial
maker E-cadherin and increased the expression of the mesenchymal
makers N-cadherin, vimentin and EMT-related transcription factor
Snail, which was reversed markedly by overexpression of miR-382 in
SKOV3 (Fig. 8C) and COV434 cells
(Fig. 8D). The above findings
suggested that miR-382 and its target gene ROR1, could affect
ovarian cancer cell migration and invasion by regulating of the EMT
process.
Discussion
Ovarian cancer is the most deadly gynecological
malignant tumor, and also the fifth most common cancer death of
female in the world (12,13). It is commonly diagnosed at an
advanced stage, and the overall survival rate of 5 years is ~20%
(14). The incidence of ovarian
cancer rises along with age, and is most universal at seventy to
eighty years of life (15,16). The exact cause of ovarian cancer is
still unknown. At present, the conventional therapy for ovarian
cancer contains surgery, combining chemotherapy with a
platinum-based (such as carboplatin) and a taxane-based (such as
paclitaxel) treatment (17,18).
Despite the advances in surgery and chemotherapy, the ovarian
cancer patients suffer from serious side-effects caused by
chemotherapy, and almost 70–80% of ovarian cancer may recur after
first-line chemotherapy (19).
Therefore, it is necessary and urgent to find new therapeutic
targets that could contribute to the clinical treatment of ovarian
cancer.
Increasing evidence exists that miRNAs play an
important role in early diagnosis, prognosis, evaluation of therapy
results and prevention of cancers (20–23).
Each cancer has certain specific miRNA alterations that can be used
as a cancer-specific ‘signature’ for potential clinical application
to improve the precision of diagnosis, prognosis and treatment
targets (24,25). Depending on the miRNA alterations
in cancer of an individual patient, targeted therapies for
personalized cancer treatment can be considered (20). Recently, it has been reported that
miR-382 was downregulated in human ovarian cancer tissues (8). However, the functions of miR-382 have
not previously been explored. Therefore, we studied miR-382 in
ovarian cancer. We found that miR-382 was downregulated in human
ovarian cancer cells and tissues, which was consistent with a
previous study (8). Also, the
expression of miR-382 and ROR1 was moderate in ovarian cancer cell
lines SKOV3 and COV434, so we selected these cells for our study.
Furthermore, we found that miR-382 could inhibit the proliferation,
migration, invasion and the EMT process of ovarian cancer cells.
These results demonstrated that miR-382 may act as a tumor
suppressor ovarian cancer.
Many miRNAs have been reported to function by
binding a specific target. We predicted that the miR-382 has
potential to bind ROR1 by bioinformatics methods. Given this, we
selected ROR1 as a candidate target of miR-382, and found that
miR-382 could target ROR1 by luciferase reporter assay.
Overexpression of miR-382 treatment declined the expression of ROR1
and silencing of miR-382 raised the expression of ROR1 in SKOV3 and
COV434 cells. These results suggested that ROR1 could act as a
target of miR-382. Furthermore, we showed miR-382 can rescue the
acceleration effect of ROR1 on the proliferation, migration and
invasion in SKOV3 and COV434 cells. These results indicated that
miR-382 suppressed cancer progression by targeting ROR1 in ovarian
cancer.
EMT is a vital step in the invasion and metastasis
of cancers, in which epithelial cells lose their apical-basal
polarity and cell-cell adhesion as well as gain migratory and
invasive properties (26,27). A recent study has shown that the
overexpression of miR-382 suppressed the EMT process, while
inhibition of miR-382 stimulated EMT in osteosarcoma (28). Cui et al (29) reported breast adenocarcinomas
expressing high levels of ROR1 were more likely to have gene
expression signatures associated with the EMT and higher rates of
metastasis than breast adenocarcinomas expressing low levels of
ROR1, suggesting ROR1 may regulate EMT and metastasis. We
speculated that both miR-382 and ROR1 were involved in the EMT
process. Thus, we determined whether ROR1 that acted as a target of
miR-382 was involved in the EMT process in ovarian cancer cells,
and found that miR-382 rescued the promotion effect of ROR1 on EMT
in SKOV3 and COV434 cells.
In conclusion, miR-382 inhibited the migration and
invasion of ovarian cancer cells through targeting ROR1 via
regulating EMT process in ovarian cancer cells. miR-382 functioned
as a tumor suppressor, and might be useful in therapeutics of
ovarian cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81172469), Science and
Technology Program of Hunan Provincial Science Technology
Department (no. 2014FJ3090) and Science and Technology Program of
Changsha City Science Technology Bureau (no. K1403050-31).
References
|
1
|
Macha MA, Seshacharyulu P, Krishn SR, Pai
P, Rachagani S, Jain M and Batra SK: MicroRNAs (miRNAs) as
biomarker(s) for prognosis and diagnosis of gastrointestinal (GI)
cancers. Curr Pharm Des. 20:5287–5297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruepp A, Kowarsch A and Theis F: PhenomiR:
microRNAs in human diseases and biological processes. Methods Mol
Biol. 822:249–260. 2012. View Article : Google Scholar
|
|
4
|
Tüfekci KU, Meuwissen RL and Genç S: The
role of microRNAs in biological processes. Methods Mol Biol.
1107:15–31. 2014. View Article : Google Scholar
|
|
5
|
Tricoli JV and Jacobson JW: MicroRNA:
Potential for cancer detection, diagnosis, and prognosis. Cancer
Res. 67:4553–4555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dahiya N and Morin PJ: MicroRNAs in
ovarian carcinomas. Endocr Relat Cancer. 17:F77–F89. 2010.
View Article : Google Scholar :
|
|
7
|
Wyman SK, Parkin RK, Mitchell PS, Fritz
BR, O'Briant K, Godwin AK, Urban N, Drescher CW, Knudsen BS and
Tewari M: Repertoire of microRNAs in epithelial ovarian cancer as
determined by next generation sequencing of small RNA cDNA
libraries. PLoS One. 4:e53112009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thériault BL, Basavarajappa HD, Lim H,
Pajovic S, Gallie BL and Corson TW: Transcriptional and epigenetic
regulation of KIF14 overexpression in ovarian cancer. PLoS One.
9:e915402014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gschwind A, Fischer OM and Ullrich A: The
discovery of receptor tyrosine kinases: Targets for cancer therapy.
Nat Rev Cancer. 4:361–370. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Cui B, Lai H, Liu G, Ghia EM,
Widhopf GF II, Zhang Z, Wu CC, Chen L, Wu R, et al: Ovarian cancer
stem cells express ROR1, which can be targeted for
anti-cancer-stem-cell therapy. Proc Natl Acad Sci USA.
111:17266–17271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Qiu J, Ye C, Yang D, Gao L, Su Y,
Tang X, Xu N, Zhang D, Xiong L, et al: ROR1 expression correlated
with poor clinical outcome in human ovarian cancer. Sci Rep.
4:58112014.PubMed/NCBI
|
|
12
|
Böcker W: WHO classification of breast
tumors and tumors of the female genital organs: Pathology and
genetics. Verh Dtsch Ges Pathol. 86:116–119. 2002.In German.
|
|
13
|
Kan CW, Howell VM, Hahn MA and Marsh DJ:
Genomic alterations as mediators of miRNA dysregulation in ovarian
cancer. Genes Chromosomes Cancer. 54:1–19. 2015. View Article : Google Scholar
|
|
14
|
Vargas-Hernández VM, Moreno-Eutimio MA,
Acosta-Altamirano G and Vargas-Aguilar VM: Management of recurrent
epithelial ovarian cancer. Gland Surg. 3:198–202. 2014.PubMed/NCBI
|
|
15
|
Goff BA, Mandel L, Muntz HG and Melancon
CH: Ovarian carcinoma diagnosis. Cancer. 89:2068–2075. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou XM, Zhang H and Han X: Role of
epithelial to mesenchymal transition proteins in gynecological
cancers: Pathological and therapeutic perspectives. Tumour Biol.
35:9523–9530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang DH, Guo L and Wu XH: Checkpoint
inhibitors in immunotherapy of ovarian cancer. Tumour Biol.
36:33–39. 2015. View Article : Google Scholar
|
|
18
|
Muccioli M and Benencia F: Toll-like
Receptors in ovarian cancer as targets for immunotherapies. Front
Immunol. 5:3412014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garces AH, Dias MS, Paulino E, Ferreira CG
and de Melo AC: Treatment of ovarian cancer beyond chemotherapy:
Are we hitting the target? Cancer Chemother Pharmacol. 75:221–234.
2015. View Article : Google Scholar
|
|
20
|
Sethi S, Ali S, Sethi S and Sarkar FH:
MicroRNAs in personalized cancer therapy. Clin Genet. 86:68–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Casanova-Salas I, Rubio-Briones J,
Calatrava A, Mancarella C, Masiá E, Casanova J, Fernández-Serra A,
Rubio L, Ramírez-Backhaus M, Armiñán A, et al: Identification of
miR-187 and miR-182 as biomarkers of early diagnosis and prognosis
in patients with prostate cancer treated with radical
prostatectomy. J Urol. 192:252–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang C, Chen X, Alattar M, Wei J and Liu
H: MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis
of gastric cancer. Cancer Gene Ther. 22:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li SQ, Chen FJ and Cao XF: Distinctive
microRNAs in esophageal tumor: Early diagnosis, prognosis judgment,
and tumor treatment. Dis Esophagus. 26:288–298. 2013. View Article : Google Scholar
|
|
24
|
Gounaris-Shannon S and Chevassut T: The
role of miRNA in haematological malignancy. Bone Marrow Res.
2013:2691072013. View Article : Google Scholar
|
|
25
|
Sethi S, Kong D, Land S, Dyson G, Sakr WA
and Sarkar FH: Comprehensive molecular oncogenomic profiling and
miRNA analysis of prostate cancer. Am J Transl Res. 5:200–211.
2013.PubMed/NCBI
|
|
26
|
Koutsaki M, Spandidos DA and Zaravinos A:
Epithelial-mesenchymal transition-associated miRNAs in ovarian
carcinoma, with highlight on the miR-200 family: Prognostic value
and prospective role in ovarian cancer therapeutics. Cancer Lett.
351:173–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takai M, Terai Y, Kawaguchi H, Ashihara K,
Fujiwara S, Tanaka T, Tsunetoh S, Tanaka Y, Sasaki H, Kanemura M,
et al: The EMT (epithelial-mesenchymal-transition)-related protein
expression indicates the metastatic status and prognosis in
patients with ovarian cancer. J Ovarian Res. 7:762014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu M, Jin H, Xu CX, Sun B, Song ZG, Bi WZ
and Wang Y: miR-382 inhibits osteosarcoma metastasis and relapse by
targeting Y box-binding protein 1. Mol Ther. 23:89–98. 2015.
View Article : Google Scholar
|
|
29
|
Cui B, Zhang S, Chen L, Yu J, Widhopf GF
II, Fecteau JF, Rassenti LZ and Kipps TJ: Targeting ROR1 inhibits
epithelial-mesenchymal transition and metastasis. Cancer Res.
73:3649–3660. 2013. View Article : Google Scholar : PubMed/NCBI
|