Introduction
Hepatocellular carcinoma (HCC) accounts for 90–95%
of all kinds of liver cancers, with nearly 500,000 new patients
each year. It is a common malignant tumor in the digestive system,
and the incidence is ranked the sixth of all cancers in the world.
Patients with HCC are often diagnosed in the late stages due to the
undetectable onset, so the overall prognosis is poor, making it the
third ranked based on mortality in the world (1–4).
Partial hepatectomy is currently preferred to treat HCC. However,
only less than 30% of patients with HCC can finally be operated,
because 60–90% of the patients have viral hepatitis and liver
cirrhosis. Furthermore, the recurrence rate is 80% after operation
at five years, it seriously affects the surgical curative effect
(1,5,6).
Although liver transplantation can cure liver cancer without
considering the cirrhosis condition and improve the total 5-year
survival rate to 70–80%, it is restricted to be carried out widely
because of high technical requirements, the shortage of graft
source for transplantation, and the risk of health damage to donors
during living donor liver transplantation and other disadvantages
(5,7). In order to get good therapeutic
effects, all criteria of liver transplantation have limitations on
the number and size of the tumors, the vascular invasion and the
distant metastasis (8). Chemical
or thermal ablation, radiotherapy, transcatheter arterial
chemoembolization, drug treatment are palliative therapeutic
modalities for advanced liver cancer, but they still have many
disadvantages, such as impaired organic function, bone marrow
suppression and drug resistance (5).
As one of the main components of antitumor
traditional Chinese medicine from toad venom, bufalin has been
confirmed to have antitumor activity, which has an obviously
inhibiting role in various kinds of blood and solid malignancies
including liver cancer (9). In
recent research it is shown that bufalin is able to reverse
multi-drug resistance of HCC cells and strengthen the ability of
sorafenib to suppress proliferation of HCC cells (10,11).
Our preliminary study indicated that bufalin could restrain the
proliferation of human hepatoma BEL-7402 cells in a time- and
dose-dependent manner and the IC50 value of 72 h was
0.085 μg/ml. Yet, the mechanism of inhibiting the proliferation,
invasion and metastasis of HCC cells by bufalin still remains
unclear (12). The abnormal
activation of Wnt/β-catenin signaling pathway plays an important
role in the occurrence and development of many tumors (13). Multiple antitumor drugs resist HCC
cells through regulating the activity of Wnt/β-catenin signaling
pathway (14–16). The anti-HCC cell effect of bufalin
correlating with the abnormal activation of Wnt/β-catenin signaling
pathway is unclear.
The expression of associated factors in
Wnt/β-catenin signaling pathway of human hepatoma BEL-7402 cells,
α-fetoprotein (AFP) and albumin (ALB) protein expression are,
respectively, detected after bufalin is used in vitro. The
present study aims to reveal the mechanism of bufalin against the
proliferation, invasion and metastasis of HCC cells.
Materials and methods
Materials
Bufalin was purchased from Shanghai Bogoo
Biotechnology Co., Ltd. (Shanghai, China). Human hepatoma carcinoma
cell line BEL-7402 were provided by the Institute of Biochemistry
and Cell Biology (SIBS; Shanghai, China). RPMI-1640 medium (Gibco,
Grand Island, NY, USA), fetal bovine serum (FBS; Hangzhou Tianhang
Biological Technology, Co., Ltd., Hangzhou, China), trypsin (Sigma,
St. Louis, MO, USA), 0.1% crystal violet staining solution
(Beyotime Institute of Biotechnology, Shanghai, China) and Matrigel
basement membrane matrix (BD Biosciences, Bedford, MA, USA) were
purchased. Adenomatous polyposis coli (APC), E-cadherin, cyclin D1,
metalloproteinase-7 (MMP-7), cyclooxygenase-2 (COX-2), GSK-3β,
β-catenin, p-GSK-3β Ser9, AFP and ALB monoclonal antibodies were
purchased from Bioworld Technology Inc. (St. Louis Park, MN, USA);
β-actin antibody was purchased from Cell Signaling Technology
(Danvers, MA, USA). Other materials including the cell lysis
solution containing protease inhibitors (Beyotime Institute of
Biotechnology), BCA protein quantitative kit (Shanghai Weiao
Biotech, Ltd., Shanghai, China), 5X loading buffer (Shanghai Weiao
Biotech), Western blocking solution (Shanghai Weiao Biotech), IgG
(H+L) (HRP-labeled goat anti-rabbit IgG) (Beyotime Institute of
Biotechnology); Triton (Solabio, Beijin, China),
fluorescent-labeled goat anti-rabbit IgG (Jackson ImmunoResearch
Labs, West Grove, PA, USA), dihydrochloride (DAPI; Beyotime
Institute of Biotechnology) were purchased.
Cell cultures
BEL-7402 cells were cultured in RPMI-1640 medium
containing 10% fetal bovine serum in the incubator of 37°C,
saturated humidity and 5% CO2 in vitro.
Clony formation assay
BEL-7402 cells growing in log phase were seeded into
60-mm dishes at a density of 2,000 cells/dish. After cultured for
24 h, the cells were treated with bufalin (0.05 μg/ml) in
vitro. The medium was replaced with fresh medium containing
bufalin every 3 days. Nine days later, the culture medium was
removed and cell colonies were stained with 0.1% crystal violet.
Colonies including <15 cells and faintly stained cells were
ignored. The number of colonies in 10 random fields was counted
under an inverted microscope (Carl Zeiss, Oberkochen, Germany).
Cell migration assay
After cultured with bufalin (0.085 μg/ml) for 72 h
and with serum-free RPMI-1640 medium for 12 h in vitro,
BEL-7402 cells were harvested and prepared to 1×106/ml
cell suspension in serum-free RPMI-1640 medium. RPMI-1640 medium
(600 μl) with 10% fetal calf serum (FBS) was added into the lower
chamber of the Transwell (Corning Incorporated, Corning, NY, USA),
and 100 μl of cell suspension was added into the upper chamber. The
experiment was performed in triplicate. After incubated at 37°C
with 5% CO2 for 24 h, the cells that did not migrate
through the membrane were gently removed. Cells that migrated
through the membrane were stained with 0.1% crystal violet. Six
fields were randomly selected and observed under the inverted
microscope for stained cell number counting and image
collection.
Cell invasion assay
After the culture with bufalin (0.085 μg/ml) for 72
h and with serum-free RPMI-1640 for 12 h in vitro, BEL-7402
cells were harvested and prepared to 1×106/ml cell
suspension in serum-free RPMI-1640 medium. RPMI-1640 (600 μl) with
10% FBS was added into the lower chamber of the Transwell, and 100
μl of cell suspension was added into the upper chamber with a
filter coated with 100 μl Matrigel at 1:8 dilutions in serum-free
medium. The experiment was performed in triplicate. After incubated
at 37°C with 5% CO2 for 48 h, the cells that did not
invade the membrane were gently removed. Cells that invaded the
membrane were stained with 0.1% crystal violet. Six fields were
randomly selected and observed under an inverted microscope for
counting the cell number of stained cells and image collection.
Quantitative analysis of proteins in
Wnt/β-catenin signaling pathway and E-cadherin/β-catenin complex in
BEL-7402 cells
BEL-7402 cells were treated with bufalin (0.085
μg/ml) for 72 h, then rinsed with ice-cold 0.01 M PBS, cell lysis
solution containing protease inhibitors was added. After cell
lysis, lysis suspension was collected and centrifuged at 4°C with
speed of 16,000 × g for 15 min to draw off supernatant solution.
Afterwards, microplate reader (Bio-Rad Laboratories Inc., Hercules,
CA, USA) was used to measure the absorbance value of protein
samples and the reference standard samples in BCA protein
quantitative kit at ~562 nm, and then protein contents in various
groups were calculated. The protein content in unit volume of
extract was balanced, using 0.01 M PBS as supplement. Protein
solution was mixed with 5X loading buffer at 1:4 ratio, then the
protein mixture was denatured in water bath at 100°C for 5 min in
the various groups. Electrophoretic separation was conducted using
10% SDS polyacrylamide gels. The protein bands after
electrophoresis were transferred to polyvinylidene fluoride (PVDF)
membrane which was blocked with western blocking solution for 1 h.
Then this membrane was, respectively, incubated in monoclonal
antibodies APC (1:500), E-cadherin (1:500), cyclin D1 (1:500),
MMP-7 (1:500), COX-2 (1:500), GSK-3β (1:500), β-catenin (1:500),
p-GSK-3β Ser9 (1:500) and β-actin (1:1,000) at 4°C for 18 h. Then,
the membrane was rinsed with PBST three times and incubated in IgG
(H+L) at 37°C for 2 h. The PVDF membrane was again rinsed with PBST
three times prior to treatment with enhanced chemiluminescence
western blotting detection kit (Amersham Biosciencse, Piscataway,
NJ, USA). The images of protein bands were visualized on X-ray film
(Kodak, Rochester, NY, USA) and analyzed with the Bio-Rad Quantity
One software (Bio-Rad Laboratories) 3 times.
Localization of proteins in Wnt/β-catenin
signaling pathway and E-cadherin/β-catenin complex in BEL-7402
cells
BEL-7402 cells were treated with bufalin (0.085
μg/ml) for 72 h in vitro, and then cells were treated with
PBS-Triton solution (40 ml PBS + 0.1% Triton 50 μl), rinsed with
0.01 M PBS. The processed cells were, respectively, incubated with
rabbit anti-human monoclonal antibodies APC (1:100), E-cadherin
(1:100), cyclin D1 (1:100), MMP-7 (1:100), COX-2 (1:100), GSK-3β
(1:100), β-catenin (1:100) and p-GSK-3β Ser9 (1:100) at 4°C for 18
h. Afterwards, the cells were rinsed and incubated with
fluorescent-labeled goat anti-rabbit IgG at 37°C for 2 h. The cell
nucleus was stained with DAPI, then observed by a fluorescent
inverted microscope (Carl Zeiss) for image collection and
analysis.
Quantitative analysis of AFP and ALB
expression in BEL-7402 cells
BEL-7402 cells were treated with bufalin (0.085
μg/ml) for 1 week, then rinsed with ice-cold 0.01 M PBS, and cell
lysis solution containing protease inhibitors was added. After cell
lysis, lysis suspension was collected and centrifuged at 4°C with
speed of 16,000 × g for 15 min to draw off supernatant solution.
Then, microplate reader was used to measure the absorbance value of
protein samples and the reference standard samples in BCA protein
quantitative kit at ~562 nm, and then protein contents in various
groups were calculated. The protein content in unit volume of
extract was balanced, using 0.01 M PBS as supplement. Protein
solution was mixed with 5x loading buffer at 1:4 ratio, then the
protein mixture was denatured in water bath at 100°C for 5 min in
various group. Electrophoretic separation was conducted using 10%
SDS polyacrylamide gels. The protein bands after electrophoresis
were transferred to PVDF membrane which was blocked with western
blocking solution for 1 h. The membrane was then, respectively,
incubated in monoclonal antibodies AFP (1:500), ALB (1:500) and
β-actin (1:1,000) at 4°C for 18 h. The membrane was rinsed with
PBST three times and incubated in IgG (H+L) at 37°C for 2 h. The
PVDF membrane was again rinsed with PBST three times prior to
photographic developing and fixing. Digital images of protein bands
were obtained using ChemiDoc system and analyzed with the Bio-Rad
Quantity One software 3 times.
Statistical analysis
The SPSS 19.0 statistical software (IBM, Armonk, NY,
USA) was used for statistical processing. Quantitative variables
were expressed by mean ± SD and analyzed with t-test. P-value
<0.05 was considered statistically significant.
Results
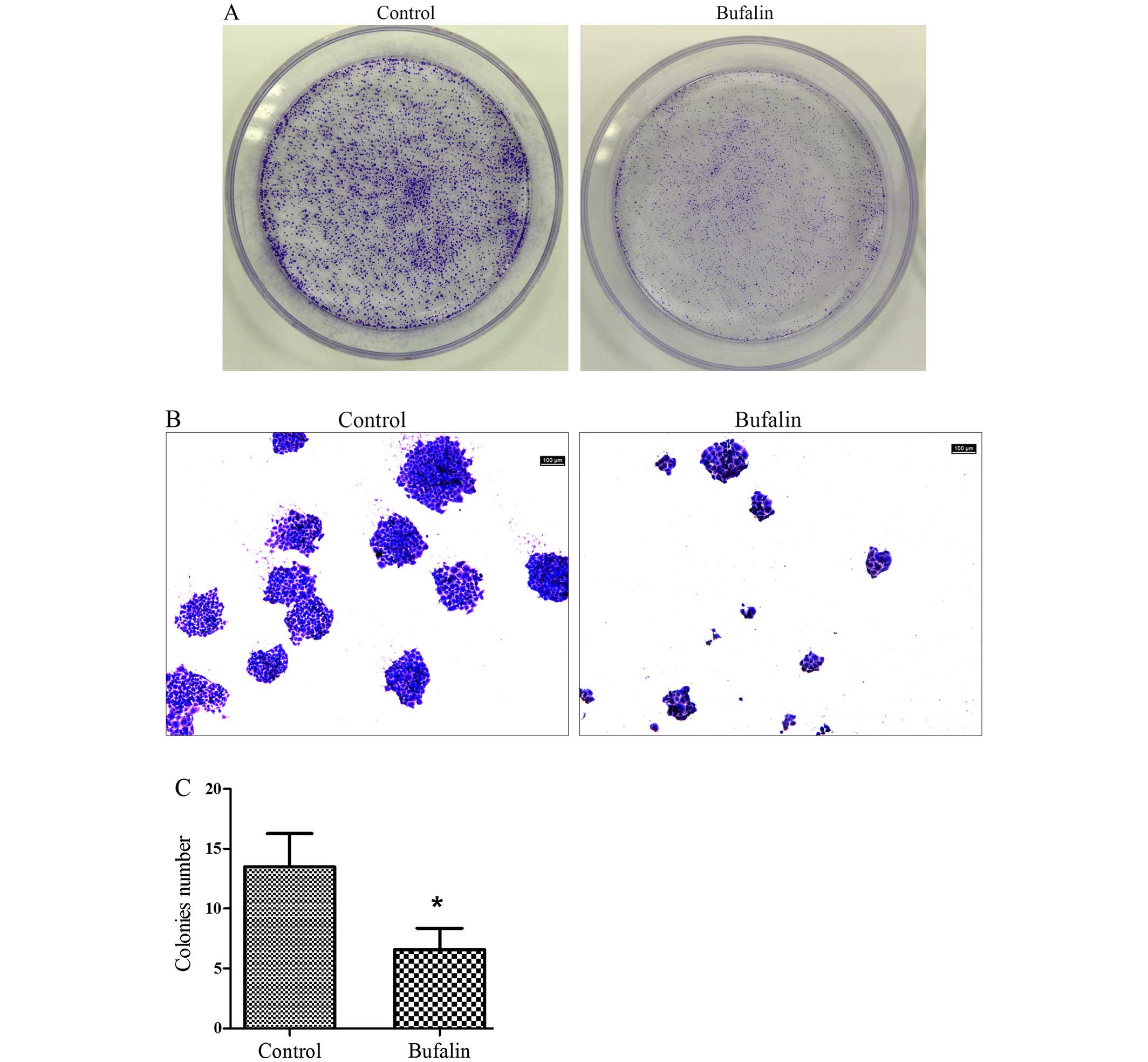
Effect of bufalin on colony formation of
HCC cells
The number of the colonies in the bufalin group
(0.05 μg/ml) was lower than that of control group (Fig. 1A). The colonies formed in bufalin
group (0.05 μg/ml) and the cells in colonies of bufalin group were
clearly less than those of control group under microscope (Fig. 1B). Quantitative analysis of the
colonies showed that compared with the control group, the colony
formation ability of BEL-7402 cells was significantly inhibited by
bufalin (P<0.05; Fig. 1C).
Influence of bufalin on migration and
invasion of HCC cells
Compared with the control group, the migration and
invasion ability of the BEL-7402 cells was significantly inhibited
by bufalin (Fig. 2A and C).
Quantitative analysis of the migrating and invasive cells showed
that compared with the control group, the migration and invasion
number of BEL-7402 cells having been treated with bufalin (0.085
μg/ml) for 72 h was reduced significantly (both P<0.05; Fig. 2B and D).
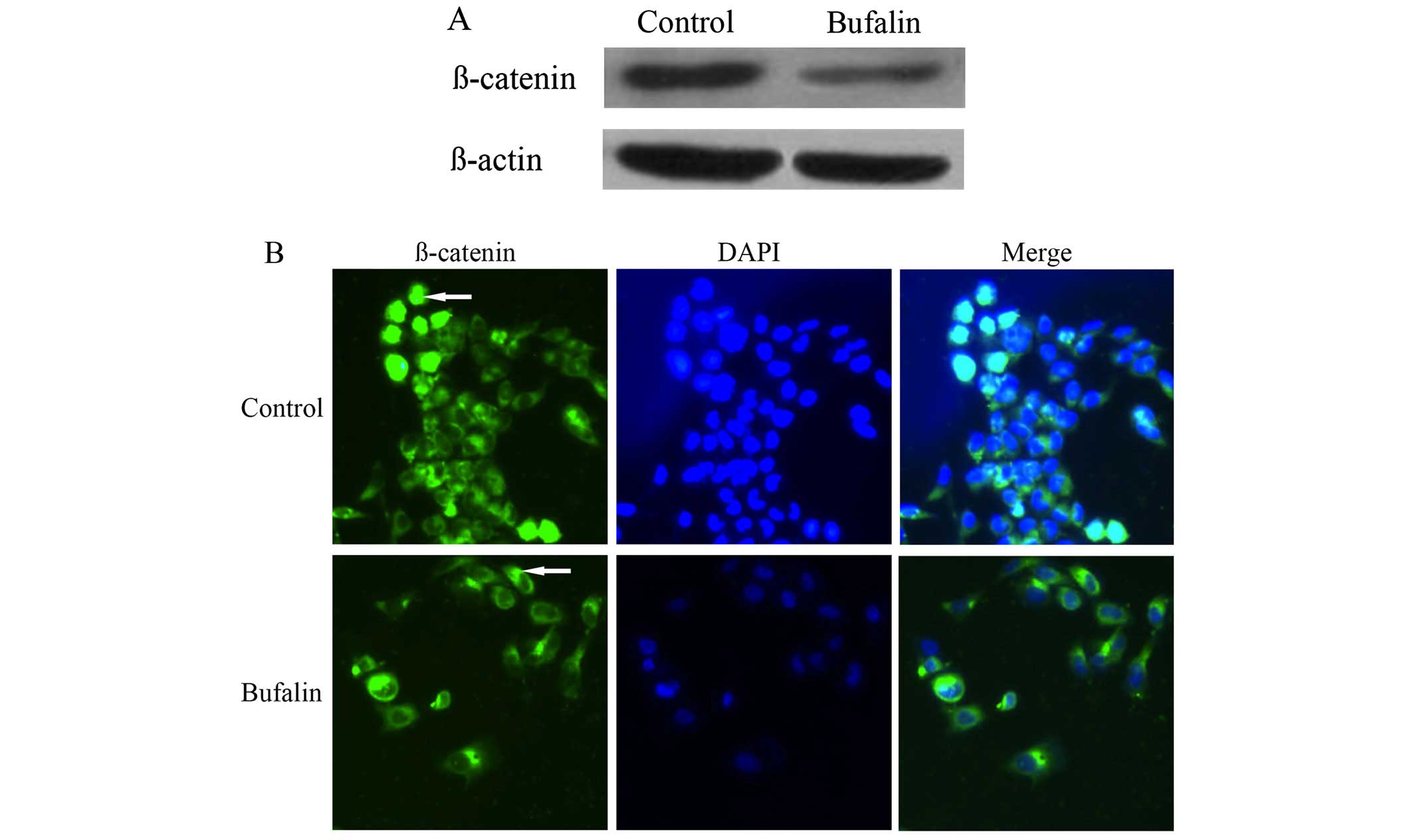
Effect of bufalin on the protein
expression and localization of β-catenin in HCC cells
Compared with the control group, the protein
expression of β-catenin in BEL-7402 cells of bufalin group after
exposure to bufalin (0.085 μg/ml) for 72 h decreased significantly
(P<0.05) (Table I and Fig. 3A).
 | Table IEffect of bufalin on the protein
expression in Wnt/β-catenin signal pathway and E-cadherin/β-catenin
complex in BEL-7402 cells (gray value, mean ± SD, n=3). |
Table I
Effect of bufalin on the protein
expression in Wnt/β-catenin signal pathway and E-cadherin/β-catenin
complex in BEL-7402 cells (gray value, mean ± SD, n=3).
|
Protein/parameter | Bufalin group | Control group | t-value | P-value |
|---|
| β-catenin | 1328±23a | 2302±35 | 40.281 | <0.001 |
| p-GSK-3β Ser9 | 1254±38a | 2017±34 | 25.918 | <0.001 |
| E-cadherin | 3968±28a | 2077±46 | −60.821 | <0.001 |
| APC | 2109±45 | 2034±64 | −1.660 | 0.172 |
| GSK-3β | 2798±37 | 2775±26 | −0.881 | 0.428 |
| MMP-7 | 1224±41a | 2187±55 | 24.314 | <0.001 |
| COX-2 | 1021±61a | 1908±43 | 20.585 | <0.001 |
| Cyclin D1 | 1814±44a | 2987±45 | 32.282 | <0.001 |
The immunofluorescence imaging showed that β-catenin
mainly localized in the cytoplasm and nuclei of BEL-7402 cells in
the control group. After exposure to bufalin (0.085 μg/ml) for 72
h, the fluorescence intensity of β-catenin increased significantly
on cell membrane, whereas the fluorescence intensity of β-catenin
decreased in the cytoplasm and nuclei of BEL-7402 cells (Fig. 3B).
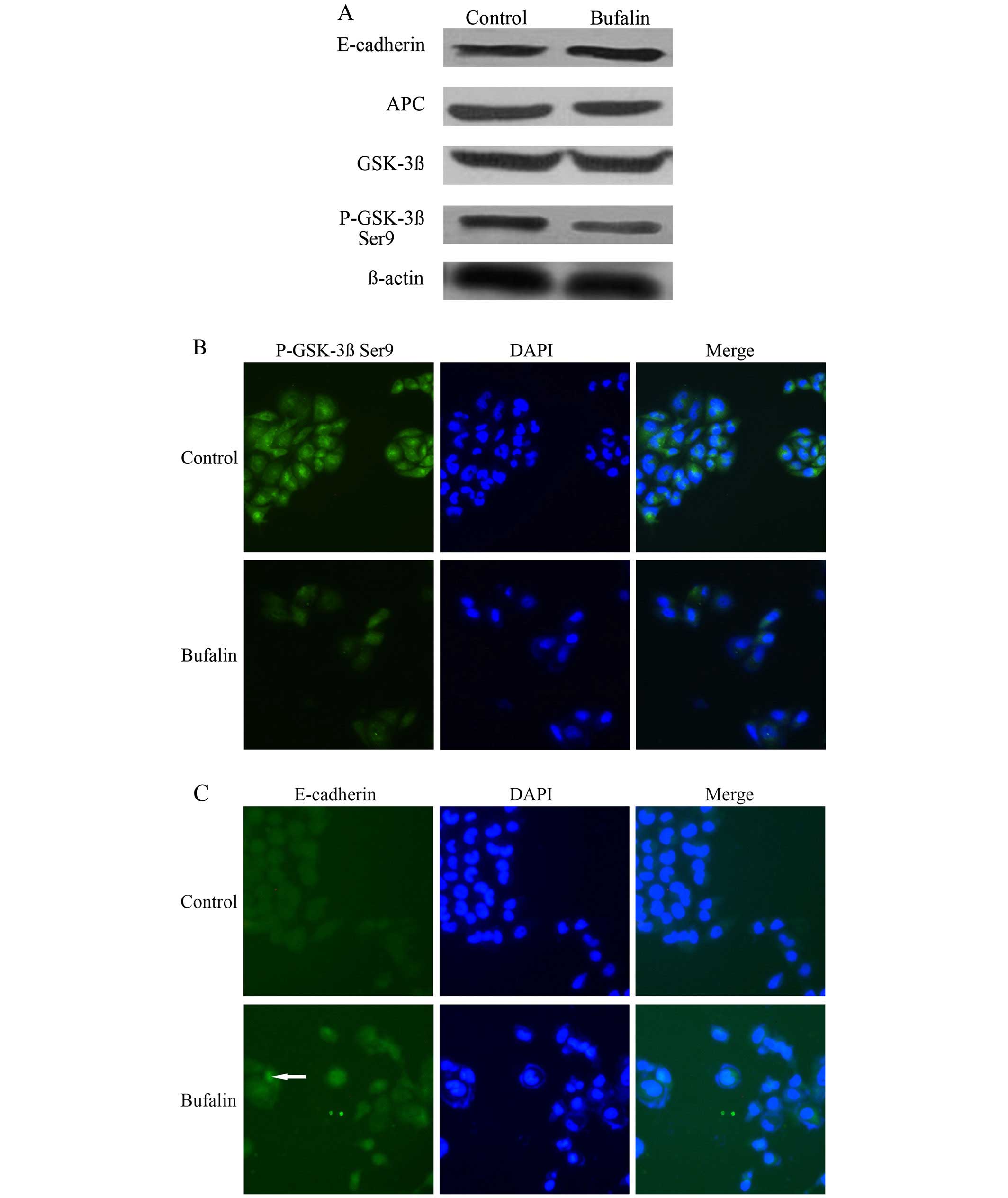
Effect of bufalin on the protein
expression of p-GSK-3β Ser9, E-cadherin, APC and GSK-3β in
wnt/β-catenin signal pathway of HCC cells
Compared with the control group, the protein
expression of p-GSK-3β Ser9 in BEL-7402 cells of the bufalin group
after exposure to bufalin (0.085 μg/ml) for 72 h decreased
significantly. The protein expression of E-cadherin in BEL-7402
cells increased significantly after bufalin treatment (P<0.05).
The protein expression of APC and GSK-3β in BEL-7402 cells was not
significantly different between the control group and the bufalin
group (P>0.05) (Table I and
Fig. 4A).
The immunofluorescence imaging showed that compared
with the control group, the fluorescence intensity of p-GSK-3β Ser9
in the cytoplasm decreased (Fig.
4B), and the fluorescence intensity of E-cadherin on cell
membrane increased after exposure to bufalin (0.085 μg/ml) for 72 h
(Fig. 4C). Whereas the
fluorescence intensity of APC and GSK-3β in cytoplasm was,
respectively, not significantly different between the bufalin group
and the control group (Fig. 4D and
E).
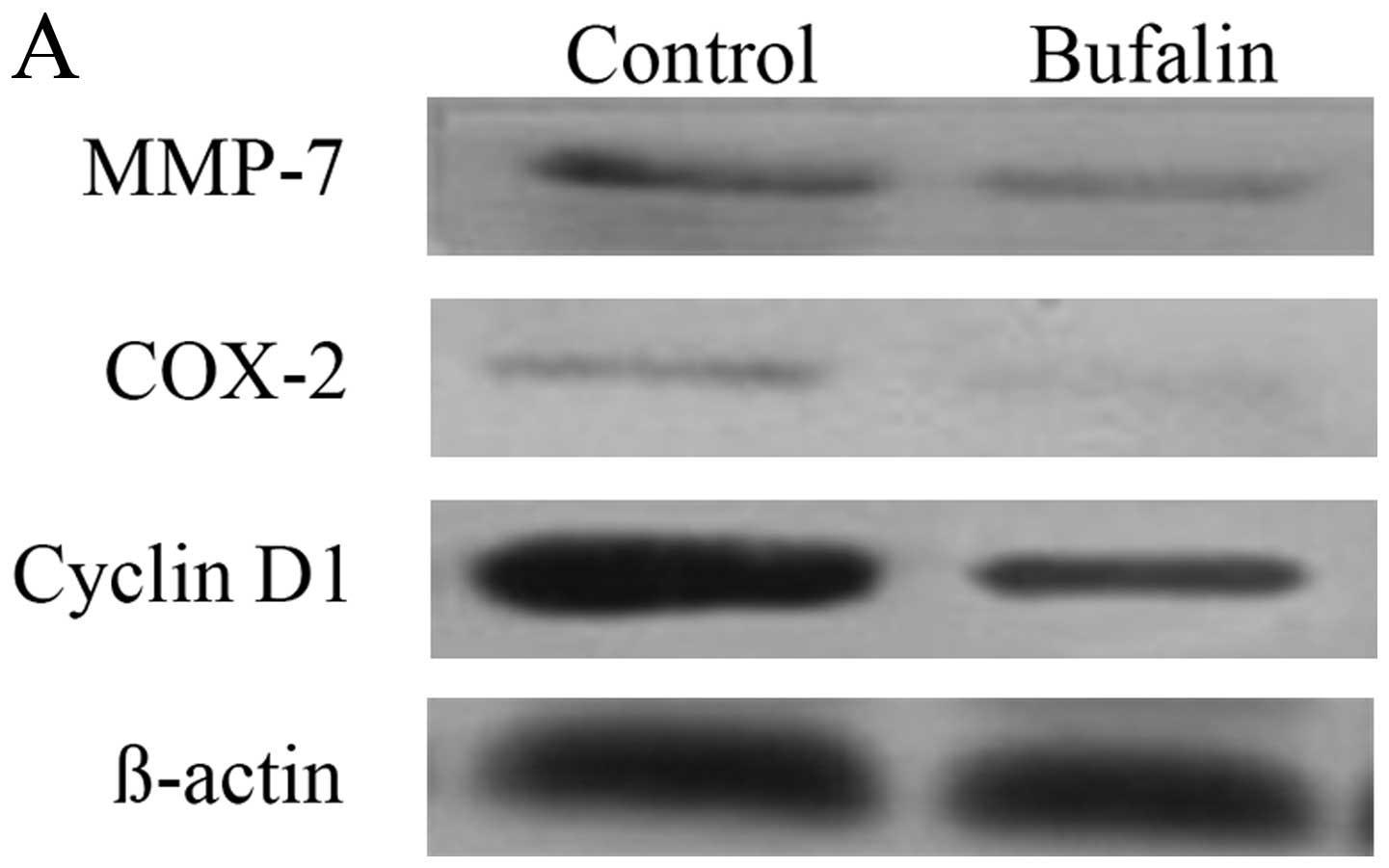
Effect of bufalin on the protein
expression of MMP-7, COX-2, and cyclin D1 in the downstream
molecules of wnt/β-catenin signal pathway in HCC cells
Compared with the control group, the protein
expression of MMP-7, COX-2 and cyclin D1 in BEL-7402 cells of
bufalin group after exposure to bufalin (0.085 μg/ml) for 72 h
decreased significantly (P<0.05) (Table I and Fig. 5A).
The immunofluorescence imaging showed that when
compared with the control group, the fluorescence intensity of
MMP-7, COX-2 and cyclin D1 in cytoplasm of BEL-7402 cells decreased
after exposure to bufalin (0.085 μg/ml) for 72 h (Fig. 5B–D).
Effect of bufalin on the protein
expression of AFP and ALB in HCC cells
Compared with the control group, the protein
expression of AFP in BEL-7402 cells of bufalin group after exposure
to bufalin (0.085 μg/ml) for 1 week obviously decreased, and the
expression levels of ALB increased significantly (both P<0.05)
(Table II and Fig. 6).
 | Table IIEffect of bufalin on the protein
expression of AFP and ALB in BEL-7402 cells (gray value, mean ± SD,
n=3). |
Table II
Effect of bufalin on the protein
expression of AFP and ALB in BEL-7402 cells (gray value, mean ± SD,
n=3).
|
Protein/parameter | Bufalin group | Control group | t-value | P-value |
|---|
| AFP | 7341±643a | 11043±947 | 5.605 | 0.005 |
| ALB | 10031±376a | 4752±470 | 15.19 | <0.001 |
Discussion
Intracellular signal transduction pathway is a
complex network system that transduces extracellular signals into
intracelluar downstream molecules. However, these accurately
regulating signal networks occur some abnormal change in malignant
tumor cells as a result of gene mutation, which eventually results
in a series of changes in cellular metabolism and the occurrence of
new features. The change of cell signaling pathway can give rise to
various malignant behavior, such as uncontrollable proliferation,
anti-apoptosis, invasion and metastasis of tumor cells (17,18).
Wnt signaling pathway plays a crucial role in
embryonic development and tissue stability (19). To date, Wnt signaling pathway is
classified into two categories: canonical Wnt pathway and
non-canonical Wnt pathway. The activation of non-canonical Wnt
pathway does not rely on the participation of β-catenin, including
Wnt/Ca2+ pathway and Wnt/PCP pathway (20). Wnt/β-catenin pathway, known as a
canonical Wnt signaling pathway, consists of Wnt signaling ligands,
Fz receptor family, and co-receptor lipoprotein receptor-related
proteins 5 and 6 (LRP5/6), disheveled (Dsh), axin, β-catenin, APC,
casein kinase 1a and GSK-3β. Among them, β-catenin acts as the key
protein in function implementation of Wnt/β-catenin signaling
pathway, β-catenin accumulation in cytoplasm predicts that
Wnt/β-catenin pathway is in the activated state. In normal mature
cells, due to lack of Wnt factor stimulation, most of β-catenin
combines with E-cadherin on cell membrane, while a small amount of
free β-catenin will be quickly phosphorylated in degradation
complex (consisting of APC, axin and GSK-3β), modified by ubiquitin
and then hydrolyzed by protease. In this case, the quantity of free
casein kinase 1a-catenin in cytoplasm usually remains at a very low
level. As required in embryonic development or tissue regeneration,
receptor complexes on cell membrane (including Fz receptor family
and co-receptor LRP5/6) will combine with extracellular Wnt
factors, and degrade the degradation complex under the mediation of
Dsh protein. Thus, β-catenin will accumulate in cytoplasm and pass
through the nuclear membrane to conjugate with transcription
factor/lymphoid enhancer-binding factor (TCF/LEF) in the nucleus,
then activate the transcription of downstream target protein genes
that are related to cell proliferation, apoptosis, matrix
dissolution and angiogenesis, such as cyclin D1, MMP-7, COX-2,
c-myc, survivin and vascular endothelial growth factor (VEGF)
(21–23). Among these downstream target
proteins, the cyclin D1 can regulate and control the cell
transformation from G1 phase to S phase to interfere with the
process of cell proliferation, by combining with cyclin-dependent
kinase and activating it in G1 phase (24). MMP-7, a member of the family of
matrix metalloproteinases, is not only involved in various
extracellular matrix lysis, but also can damage diversified
tumor-suppressor compositions on the cell surface (25). COX-2 can suppress tumor cell
apoptosis, promote tumor cell proliferation, stimulate matrix
metalloproteinases production and enhance tumor cell invasion and
metastasis abilities (26). The
overexpression of cyclin D1, MMP-7 and COX-2 plays an important
role in the proliferation, invasion and metastasis of liver cancer
(27–29).
The abnormal activation of Wnt/β-catenin signal
pathway has important biological effects on the occurrence and
development of primary liver cancer (30). E-cadherin, mainly expressed in
epithelial cells, can combine with intracellular β-catenin, and
form a connected composite structure to participate in maintaining
cell-cell and cell-extracellular matrix adhesion stability.
Therefore, the low expression of E-cadherin will result in a
prominent increase of free β-catenin in the cytoplasm (31). GSK-3β, the main function domain of
complex degradation, possesses two phosphorylation sites with
opposite regulating effects (including Tyr216 and Ser9).
Phosphorylated Tyr216 can strengthen GSK-3β activity, while
phosphorylated Ser9 site will lead to GSK-3β deactivation (32). The higher phosphorylation at GSK-3β
ser9 site and lower expression of E-cadherin is regarded as major
causes of activation of Wnt/β-catenin pathway in HCC cells
(33). In addition,
epithelial-mesenchymal transition (EMT) also plays a key role in
cancer metastasis process. As a phenotypic conversion, EMT promotes
embryonic development and organ formation. However, EMT makes polar
epithelial cells to convert into mesenchymal cells, increasing the
loss of tight junctions between cells, promotes metastasis,
invasion and enhances the anti-apoptotic ability of cells (34,35).
In the present study, through clone formation assay,
we verified our previous experimental results that bufalin could
inhibit HCC cell proliferation. In order to further explore the
anti-HCC effect of bufalin, the tumor invasion and metastasis
environment in vitro were established through Transwell
migration and invasion assay (36). The results showed that bufalin was
able to inhibit the migration and invasion ability of BEl-7402
cells. Further experimental results showed that bufalin increased
the local expression of β-catenin significantly on cell membrane,
whereas, decreased the local expression of β-catenin in the
cytoplasm and the nuclei of BEL-7402 cells, decreased the
expression of p-GSK-3β Ser9 in the cytoplasm, increase the
expression of E-cadherin and β-catenin on the cell membrane of
BEL-7402 cells, decreased the expression of cyclin D1, MMP-7 and
COX-2 in cytoplasm of BEL-7402 cells. It indicated that bufalin
could inhibit the phosphorylation of GSK-3β Ser9 site and
translocation of β-catenin in HCC cells, then influenced the
extranuclear signal transduction and endonuclear target molecules,
ultimately resulting in lower expressions of cyclin D1, MMP-7 and
COX-2 in HCC cells. It might be an important biological mechanism
of bufalin by which it inhibits the HCC cell proliferation,
invasion and metastasis. Since bufalin increased the expression of
E-cadherin and β-catenin on the cell membrane, the quantity of
E-cadherin/β-catenin complex increased as well. The complex between
cells is an important factor to maintain the stability of the
epithelial cells, having an inhibitory effect on EMT during the
metastasis of tumor cells (37).
Based on considerable research, when the EMT of tumor cells are
restricted, the invasion and metastasis ability of tumor cells
markedly decrease (38–40). Thus, enhancing the stability
between the membrane of epithelial cells and inhibiting the EMT are
also important mechanisms by which bufalin inhibits the HCC cell
invasion and migration.
AFP develops during fetal development, the AFP
concentration decreases rapidly after birth. In normal adult, the
AFP concentration is very low, and AFP gene is in a hermit state,
but will be activated when high quantity of HCC cells appear. Thus,
it is used widely in clinical diagnosis of HCC, and it is an
important malignant phenotype of HCC cells. The overexpression of
AFP is often associated with HCC metastasis, vascular invasion, and
other related malignant behavior (41,42).
In contrast with AFP, ALB produced by the liver cells has an
important role in maintaining body's normal physiological
activities (43). Determination of
ALB synthesis reflects liver cell function and it is an indicator
of liver cancer cell differentiation in vitro (44,45).
Recent studies have found that AFP can promote cell growth of HCC
while ALB inhibits HCC cell proliferation (46,47).
After bufalin was added into HCC cells in vitro, the result
showed that bufalin could significantly decrease expression of AFP,
while bufalin increased expression of ALB in BEL-7402 cells. It
indicates that bufalin reverses the cell malignant phenotype,
promoting cell differentiation and maturation, and regulating the
expression of AFP and ALB in BEL-7402 cells, thus, suggesting that
bufalin could inhibit the malignant biological behavior of HCC
cells.
In conclusion, metastasis of malignant tumors is a
continuous complicated process, including the tumor growth in the
original site, cells invading into the body circulation after EMT,
and replantation into the new organ tissue from the circulatory
system followed by proliferation (48,49).
Our research results show that by inhibiting GSK-3β Ser9 site
phosphorylation, bufalin weakened the activity of the Wnt/β-catenin
signaling pathway. By increasing inter-membrane
E-cadherin/β-catenin complex, bufalin inhibited the EMT of HCC
cells. It indicates that they are the key mechanisms of bufalin
against cell proliferation, invasion and metastasis of HCC cells.
However, due to poor water solubility, high toxicity, and short
half-life the clinical application of bufalin is restricted
(50). It is necessary to further
develop and prepare the new drug dosage form of bufalin such as
nano-drugs via combining bufalin with polymer nano-carrier, the new
dosage form can not only reduce the bufalin toxicity, but also have
slow-release and active targeting properties. The present study
provide very important theoretical basis for further developing the
new molecular targeted drugs aimed at specific site of the
Wnt/β-catenin signaling pathway. The new dosage form of bufalin and
molecular targeted drugs will improve the anti-HCC effects of
bufalin.
Acknowledgements
The present study was supported by a grant from the
Programs of Key Hepatic-Biliary-Pancreatic Surgery Foundation of
Putuo District, Shanghai, China (no. 2012-B-162).
References
|
1
|
Song MJ and Bae SH: Newer treatments for
advanced hepatocellular carcinoma. Korean J Intern Med. 29:149–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Shen Z, Zhu Z, Han R and Huai M
and Huai M: Clinical values of AFP, GPC3 mRNA in peripheral blood
for prediction of hepatocellular carcinoma recurrence following
OLT: AFP, GPC3 mRNA for prediction of HCC. Hepat Mon. 11:195–199.
2011.PubMed/NCBI
|
|
3
|
Zhao YJ, Ju Q and Li GC: Tumor markers for
hepatocellular carcinoma. Mol Clin Oncol. 1:593–598. 2013.
|
|
4
|
Jo S and Shim HK: A patient who has
survived for a long period with repeated radiotherapies for
multifocal extrahepatic metastases from hepatocellular carcinoma.
Radiat Oncol J. 31:267–272. 2013. View Article : Google Scholar
|
|
5
|
Lin S, Hoffmann K and Schemmer P:
Treatment of hepatocellular carcinoma: A systematic review. Liver
Cancer. 1:144–158. 2012. View Article : Google Scholar
|
|
6
|
Belghiti J and Fuks D: Liver resection and
transplantation in hepatocellular carcinoma. Liver Cancer. 1:71–82.
2012. View Article : Google Scholar
|
|
7
|
Vitale A, Volk M and Cillo U: Transplant
benefit for patients with hepatocellular carcinoma. World J
Gastroenterol. 19:9183–9188. 2013. View Article : Google Scholar
|
|
8
|
Chan SC: Liver transplantation for
hepatocellular carcinoma. Liver Cancer. 2:338–344. 2013. View Article : Google Scholar
|
|
9
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar
|
|
10
|
Gu W, Liu L, Fang FF, Huang F, Cheng BB
and Li B: Reversal effect of bufalin on multidrug resistance in
human hepatocellular carcinoma BEL-7402/5-FU cells. Oncol Rep.
31:216–222. 2014.
|
|
11
|
Gao Y, Li HX, Xu LT, Wang P, Xu LY, Cohen
L, Yang PY, Gu K and Meng ZQ: Bufalin enhances the
anti-proliferative effect of sorafenib on human hepatocellular
carcinoma cells through downregulation of ERK. Mol Biol Rep.
39:1683–1689. 2012. View Article : Google Scholar
|
|
12
|
Gai JQ, Qin JM and Fan YZ: The effect of
bufalin on proliferation and invasion of human liver cancer cells.
World Chin J Digestology. 22:1921–1927. 2014.
|
|
13
|
Pandurangan AK: Potential targets for
prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt
pathways. Asian Pac J Cancer Prev. 14:2201–2205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu MX, Zhao L, Deng C, Yang L, Wang Y, Guo
T, Li L, Lin J and Zhang L: Curcumin suppresses proliferation and
induces apoptosis of human hepatocellular carcinoma cells via the
wnt signaling pathway. Int J Oncol. 43:1951–1959. 2013.PubMed/NCBI
|
|
15
|
Wang F, He L, Dai WQ, Xu YP, Wu D, Lin CL,
Wu SM, Cheng P, Zhang Y, Shen M, et al: Salinomycin inhibits
proliferation and induces apoptosis of human hepatocellular
carcinoma cells in vitro and in vivo. PLoS One. 7:e506382012.
View Article : Google Scholar
|
|
16
|
Thompson MD, Dar MJ and Monga SP:
Pegylated interferon alpha targets Wnt signaling by inducing
nuclear export of β-catenin. J Hepatol. 54:506–512. 2011.
View Article : Google Scholar :
|
|
17
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bachmann J, Raue A, Schilling M, Becker V,
Timmer J and Klingmüller U: Predictive mathematical models of
cancer signalling pathways. J Intern Med. 271:155–165. 2012.
View Article : Google Scholar
|
|
19
|
Di Maio A, Setar L, Tiozzo S and De Tomaso
AW: Wnt affects symmetry and morphogenesis during post-embryonic
development in colonial chordates. Evodevo. 6:172015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sastre-Perona A and Santisteban P: Role of
the wnt pathway in thyroid cancer. Front Endocrinol (Lausanne).
3:312012.
|
|
21
|
Saito-Diaz K, Chen TW, Wang X, Thorne CA,
Wallace HA, Page-McCaw A and Lee E: The way Wnt works: Components
and mechanism. Growth Factors. 31:1–31. 2013. View Article : Google Scholar :
|
|
22
|
Ren S, Johnson BG, Kida Y, Ip C, Davidson
KC, Lin SL, Kobayashi A, Lang RA, Hadjantonakis AK, Moon RT, et al:
LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in
pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl
Acad Sci USA. 110:1440–1445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fuchs SY, Ougolkov AV, Spiegelman VS and
Minamoto T: Oncogenic beta-catenin signaling networks in colorectal
cancer. Cell Cycle. 4:1522–1539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagasundaram N, Hailong Z, Jiming L,
Karthick V, George Priya Doss C, Chirangib C and Luonan C:
Analysing the effect of mutation on protein function and
discovering potential inhibitors of CDK4: Molecular modelling and
dynamics studies. PLoS One. 10:e01339692015. View Article : Google Scholar
|
|
25
|
Wu J, Guan X, Zhang K, Li YT, Bai P and Wu
J: A/G polymorphism of matrix metalloproteinase 7 gene promoter
region and cancer risk: A meta-analysis. Biomed Rep. 1:792–796.
2013.
|
|
26
|
Wu KK, Cheng HH and Chang TC:
5-methoxyindole metabolites of L-tryptophan: Control of COX-2
expression, inflammation and tumorigenesis. J Biomed Sci.
21:172014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee MJ, Xu DY, Li H, Yu GR, Leem SH, Chu
IS, Kim IH and Kim DG: Pro-oncogenic potential of NM23-H2 in
hepatocellular carcinoma. Exp Mol Med. 44:214–224. 2012. View Article : Google Scholar :
|
|
28
|
Chen L, Li M, Li Q, Wang CJ and Xie SQ:
DKK1 promotes hepatocellular carcinoma cell migration and invasion
through β-catenin/MMP7 signaling pathway. Mol Cancer. 12:1572013.
View Article : Google Scholar
|
|
29
|
Sui W, Zhang Y, Wang Z, Wang Z, Jia Q, Wu
L and Zhang W: Antitumor effect of a selective COX-2 inhibitor,
celecoxib, may be attributed to angiogenesis inhibition through
modulating the PTEN/PI3K/Akt/HIF-1 pathway in an H22
murine hepatocarcinoma model. Oncol Rep. 31:2252–2260.
2014.PubMed/NCBI
|
|
30
|
Wands JR and Kim M: WNT/β-catenin
signaling and hepatocellular carcinoma. Hepatology. 60:452–454.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Somorjai IM and Martinez-Arias A: Wingless
signalling alters the levels, subcellular distribution and dynamics
of Armadillo and E-cadherin in third instar larval wing imaginal
discs. PLoS One. 3:e28932008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu W, Wang H, Wang Y, Li H and Ji L:
Metabolic factors-triggered inflammatory response drives
antidepressant effects of exercise in CUMS rats. Psychiatry Res.
228:257–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wolfe A, Thomas A, Edwards G, Jaseja R,
Guo GL and Apte U: Increased activation of the Wnt/β-catenin
pathway in spontaneous hepatocellular carcinoma observed in
farnesoid X receptor knockout mice. J Pharmacol Exp Ther.
338:12–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hou KZ, Fu ZQ and Gong H: Chemokine ligand
20 enhances progression of hepatocellular carcinoma via
epithelial-mesenchymal transition. World J Gastroenterol.
21:475–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HJ, Choi WJ and Lee CH:
Phosphorylation and reorganization of keratin networks:
Implications for carcinogenesis and epithelial mesenchymal
transition. Biomol Ther (Seoul). 23:301–312. 2015. View Article : Google Scholar
|
|
36
|
Wei X, Wang J, He J, Ma B and Chen J:
Biological characteristics of CD133+ cancer stem cells
derived from human laryngeal carcinoma cell line. Int J Clin Exp
Med. 7:2453–2462. 2014.
|
|
37
|
David JM and Rajasekaran AK: Dishonorable
discharge: The oncogenic roles of cleaved E-cadherin fragments.
Cancer Res. 72:2917–2923. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xia W, Ma X, Li X, Dong H, Yi J, Zeng W
and Yang Z: miR-153 inhibits epithelial-to-mesenchymal transition
in hepatocellular carcinoma by targeting Snail. Oncol Rep.
34:655–662. 2015.PubMed/NCBI
|
|
39
|
Liao ZJ, Guo YH, Zhao Z, Yao JT, Xu R and
Nan KJ: Gemcitabine inhibits the micrometastasis of non-small cell
lung cancer by targeting the EpCAM-positive circulating tumor cells
via the HGF/cMET pathway. Int J Oncol. 45:651–658. 2014.PubMed/NCBI
|
|
40
|
Quan MF, Xiao LH, Liu ZH, Guo H, Ren KQ,
Liu F, Cao JG and Deng XY: 8-bromo-7-methoxychrysin inhibits
properties of liver cancer stem cells via downregulation of
β-catenin. World J Gastroenterol. 19:7680–7695. 2013. View Article : Google Scholar
|
|
41
|
Hu Z and Zhao W: Novel insights into the
molecular mechanisms of α-fetoprotein expression and malignant
phenotypes of hepatocellular carcinoma. Cell Mol Immunol. 9:7–8.
2012. View Article : Google Scholar
|
|
42
|
Kim HA, Nam K, Lee M and Kim SW:
Hypoxia/hepatoma dual specific suicide gene expression plasmid
delivery using bio-reducible polymer for hepatocellular carcinoma
therapy. J Control Release. 171:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garcia-Martinez R, Caraceni P, Bernardi M,
Gines P, Arroyo V and Jalan R: Albumin: Pathophysiologic basis of
its role in the treatment of cirrhosis and its complications.
Hepatology. 58:1836–1846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
You J, Raghunathan VK, Son KJ, Patel D,
Haque A, Murphy CJ and Revzin A: Impact of nanotopography, heparin
hydrogel microstructures, and encapsulated fibroblasts on phenotype
of primary hepatocytes. ACS Appl Mater Interfaces. 7:12299–12308.
2015. View Article : Google Scholar :
|
|
45
|
Ohguchi S, Nakatsukasa H, Higashi T,
Ashida K, Nouso K, Ishizaki M, Hino N, Kobayashi Y, Uematsu S and
Tsuji T: Expression of alpha-fetoprotein and albumin genes in human
hepatocellular carcinomas: Limitations in the application of the
genes for targeting human hepatocellular carcinoma in gene therapy.
Hepatology. 27:599–607. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Toro A, Ardiri A, Mannino M, Arcerito MC,
Mannino G, Palermo F, Bertino G and Di Carlo I: Effect of pre- and
post-treatment α-fetoprotein levels and tumor size on survival of
patients with hepatocellular carcinoma treated by resection,
transarterial chemoembolization or radiofrequency ablation: A
retrospective study. BMC Surg. 14:402014. View Article : Google Scholar
|
|
47
|
Nojiri S and Joh T: Albumin suppresses
human hepatocellular carcinoma proliferation and the cell cycle.
Int J Mol Sci. 15:5163–5174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vernon AE and LaBonne C: Tumor metastasis:
A new twist on epithelial-mesenchymal transitions. Curr Biol.
14:R719–R721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yin P, Wang Y, Qiu Y, Hou L, Liu X, Qin J,
Duan Y, Liu P, Qiu M and Li Q: Bufalin-loaded mPEG-PLGA-PLL-cRGD
nanoparticles: Preparation, cellular uptake, tissue distribution,
and anticancer activity. Int J Nanomed. 7:3961–3969. 2012.
|