Introduction
Hepatocellular carcinoma (HCC) has a high incidence
rate worldwide (1). It is treated
with local ablation, surgery, transcatheter arterial
chemoembolization, and systemic chemotherapy (2). Despite the advances in therapy,
prognosis of HCC remains poor (3).
Hence, molecular therapy is an emerging trend in the treatment of
HCC (4).
The Wnt pathway is involved in the carcinogenesis in
HCC (5). Without stimulation,
Axin, Dishevelled (DVL), and glycogen synthase kinase-3 β form a
complex and degrade β-catenin (6).
Wnt proteins bind to their receptor, frizzled (Fz), and its
co-receptors, low-density lipoprotein receptor-related proteins 5
and 6 (LRP5/6), to form a complex (7,8).
This complex traps AXN and DVL. As a result, β-catenin is not
degraded and instead accumulates in the cytoplasm (9). Accumulated β-catenin translocates to
the nuclei and binds to the promoter of target genes with T-cell
factor/lymphoid enhancer factor (TCF/LEF) (10). In HCC, β-catenin is mutated and
overexpressed, which suggests that the Wnt pathway is
constitutively activated (11).
Not all HCC cases, however, harbor a mutation of β-catenin or Axin
(6). Hence, targets other than
β-catenin should be investigated in the Wnt pathway. Proliferation
of HCC cells is known to be suppressed by short-hairpin RNA of Fz2
(shRNA-Fz2) (12); however, it is
not clear whether shRNA-Fz2 suppresses the motility of HCC cells.
Another issue is the method of introduction of shRNA-Fz2. To this
end, methods should be developed to introduce shRNA-Fz2 into HCC
cells.
Ultrasound (US) generates cavitation bubbles in
liquids that eventually collapse (13). When the cavitation bubbles
collapse, the nearby cell membrane is rendered porous, and genetic
materials or small molecules can enter the cells through these
pores (14). This phenomenon is
called ‘sonoporation’. The strength of the US irradiation is
measured in terms of the mechanical index (MI). MI is calculated as
the negative peak pressure divided by the square root of the
frequency (15). US with a higher
MI would exhibit stronger biological effects. Plasmids and short
interference RNA have been introduced into cultured HCC cells via
US irradiation with a diagnostic US device (16,17).
One advantage of using diagnostic US is that its safety for the
human body has been established. Another advantage is that the
irradiation field can be monitored with a display of the US device,
which would enable the introduction of therapeutic genes
specifically into the target area. One major problem of irradiation
with a diagnostic US device is that the efficiency of the
introduction of plasmids into cultured cells is low (16).
Microbubbles have conventionally been used to
provide a strong contrast of the target area against the
surrounding background (18).
Microbubbles collapse when irradiated with US, thereby enhancing
sonoporation (19).
Perfluorobutane microbubbles (Sonazoid™; Daiichi-Sankyo,
Tokyo, Japan) are produced with albumin and are clinically applied
to diagnose HCC (20).
With the available background information, in this
study, we attempted to evaluate the possibility of suppressing HCC
cell motility with shRNA-Fz2. We, furthermore, investigated whether
shRNA-Fz2 suppressed the proliferation of HCC cells irradiated with
a diagnostic US device, which was enhanced with Sonazoid.
Materials and methods
Cell culture
Human HCC cell lines, HLF and PLC/PRF/5, were
purchased from RIKEN Cell Bank (Tsukuba, Japan). HLF cells and
PLC/PRF/5 cells were used for the study because Fz2 is expressed in
both these cells (21). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS) (Life Technologies, Grand Island, NY, USA). The
cells were cultured in 10-cm dishes (Asahi Techno Glass, Funabashi,
Japan) in an atmosphere containing 5% CO2 at 37°C in a
humidified chamber.
Ultrasound (US) irradiation
Cultured cells or the starchiodide mixture in black
96-well fluoroimmunoassay (FIA) plates (FIA plates) (Greiner
Bio-One, Frickenhausen, Germany) were irradiated with US, as
illustrated in Fig. 1. The bottom
surfaces of the FIA plates consist of a transparent polystyrene
film measuring 190±19 μm in thickness. The bottom surfaces of the
FIA plates allow the penetration of US to a greater extent than
that achieved with the bottom surfaces of other 96-well plates that
are relatively thicker. An 8.0-MHz linear-array probe (PLT-805AT;
Toshiba Medical Systems, Ohtawara, Japan) was attached to the
bottom of the FIA plates (Fig.
1A). US was generated and monitored with SSA-700A (Toshiba
Medical Systems). The linear probe covered all the eight wells
present in a single row of the FIA plate (Fig. 1B). The FIA plates were irradiated
from below for 1 min. The irradiated field was monitored with the
display of the SSA-700A (Fig. 1C).
MI (0.1, 0.4 and 0.8) was not measured but were actually selected
with the US device.
Cell proliferation assay
The cells were trypsinized, harvested, and spread on
96-well plates (Asahi Techno Glass) or FIA plates at a density of
1,000 cells/well. The cells were cultured in DMEM supplemented with
10% FBS. The cells were transfected with shRNA of Fz2 (OriGene,
Rockville, MD, USA) (10 ng or 100 ng in 25 μl of Opti-MEM 1 Reduced
Serum Media (Life Technologies) per well) by using Lipofectamine
LTX (Life Technologies), following manufacturer's instructions. For
irradiation with US, 100 ng of shRNA-Fz2 was added in 25 μl of
Opti-MEM 1 Reduced Serum Media per well and irradiated with US, as
described above. Scrambled shRNA (100 ng/well) was used as a
negative control (OriGene). Mock transfection involved carrying out
the same transfection procedure with Lipofectamine LTX without
using any plasmids. After transfection/irradiation with US, 25 μl
of DMEM supplemented with 10% FBS was added to each well. The cells
were cultured for 72 h and subjected to
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) assay, according to the manufacturer's
instructions (Promega Corp., Madison, WI, USA). MTS is reduced by
the cells to a colored formazan product with an absorbance maximum
at 490 nm. The absorbance was measured using an iMark Microplate
Absorbance Reader (Bio-Rad, Hercules, CA, USA).
Real-time quantitative polymerase chain
reaction
Cells were cultured in DMEM supplemented with 10%
FBS in 6-well plates (Asahi Techno Glass) and transfected with
shRNA-Fz2 (0.25 or 2.5 μg) by using Lipofectamine LTX. Scrambled
shRNA (2.5 μg/well) was used as a negative control (OriGene). Mock
transfection involved carrying out the same transfection procedure
with Lipofectamine LTX without using any plasmids. Total RNA (5 μg)
was isolated with Isogen (Nippon Gene, Tokyo, Japan) and subjected
to the synthesis of the first-strand cDNA with SuperScript III and
oligo(dT), following the manufacturer's instructions (Life
Technologies). Total RNA isolated from an adult liver was purchased
from Promega Corp. Real-time quantitative PCR was performed using
Fast SYBR Green Master Mix (Life Technologies) with MiniOpticon
(Bio-Rad). The results were analyzed using the MiniOpticon system
(Bio-Rad). Real-time quantitative PCR was performed for 40 cycles,
with 5 sec of denaturation and 5 sec of annealing-extension.
Table I shows the primer
sequences. RPL19 was used as an internal control, since it is a
housekeeping gene that is constitutively expressed (22).
 | Table IPrimers for real-time quantitative
polymerase chain reaction. |
Table I
Primers for real-time quantitative
polymerase chain reaction.
| Primer name | Sequence | Description | Product size
(bp) | Annealing
temperature | Cycle | GenBank accession
no. |
|---|
| OMC355 |
5′-AGAGGCGGAGGAGAACAAACAG-3′ | Cyclin D1,
forward | 180 | 60 | 40 | NM_053056 |
| OMC356 |
5′-AGGCGGTAGTAGGACAGGAAGTTG-3′ | Cyclin D1,
reverse | | | | |
| OMC749 |
5′-CCTGGGCAGATTCCAAACCT-3′ | MMP9, forward | 89 | 60 | 40 | NM_004994 |
| OMC750 |
5′-GCAAGTCTTCCGAGTAGTTTTGGAT-3′ | MMP9, reverse | | | | |
| OMC321 |
5′-CGAATGCCAGAGAAGGTCAC-3′ | RPL19, forward | 157 | 60 | 40 | BC095445 |
| OMC322 |
5′-CCATGAGAATCCGCTTGTTT-3′ | RPL19, reverse | | | | |
Scratch assay
The cells were plated on 4-well chamber slides
(Becton Dickinson, Franklin Lakes, NJ, USA) and scratched with a
sterile razor on reaching confluence. Immediately after the scratch
was applied, the cells were transfected with shRNA-Fz2 (50 or 500
ng) by using Lipofectamine LTX. Scrambled shRNA (2.5 μg/well) was
used as a negative control (OriGene). Mock transfection involved
carrying out the same transfection procedure with Lipofectamine LTX
without using any plasmids. After 48 h of incubation, the cells
were subjected to hematoxylin and eosin staining. The slides were
observed under an AX80 microscope (Olympus, Tokyo, Japan). The
distance between the scratch line and the growing edge of the cell
layer was measured at five different points.
Quantification of
H2O2 generation
Generation of H2O2 was
quantified using the starch-iodide method (23). Briefly, 100 μl of a mixture of
potassium iodide (0.05 M) and starch (5 mg/ml) was placed into each
well of the FIA plate. US irradiation generates
H2O2, and the generated
H2O2 oxidizes I- into I2, which then reacts
with starch to form a purple-colored complex. The absorbance of the
resulting complex was analyzed at 490 nm by using an iMark
Microplate Absorbance Reader.
Statistical analysis
One-way analysis of variance (ANOVA) was used for
statistical analysis with the JMP 10.0.2 software (SAS Institute,
Cary, NC). P-values <0.05 were determined to be statistically
significant.
Results
As reported in our previous study, shRNA-Fz2
suppresses proliferation of HLF cells (12); however, the experiments were not
repeated with HLF cells in the present study. To confirm that
PLC/PRF/5 cells expressed Fz2 at higher levels than those in an
adult liver, real-time quantitative PCR (qPCR) was performed
(Fig. 2A). PLC/PRF/5 cells
expressed higher levels of Fz2 than did the adult liver. Next,
suppression of the proliferation of PLC/ PRF/5 cells with shRNA-Fz2
was investigated (Fig. 2B). PLC/
PRF/5 cells were transfected with shRNA-Fz2 and subjected to MTS
assay. Cell proliferation was suppressed with shRNA-Fz2. To confirm
that the expression levels of Fz2 were those suppressed by the
transfection with shRNA-Fz2, RNA was isolated from the cells and
subjected to qPCR after transfection of shRNA-Fz2 into PLC/PRF/5
cells (Fig. 2C). Cyclin D1 is
involved in cell proliferation (24); hence, expression levels of cyclin
D1 were analyzed with qPCR after transfection of shRNA-Fz2 into
PLC/PRF/5 cells (Fig. 2D). The
expression levels of Fz2 decreased with the transfection of
shRNA-Fz2.
To clarify the suppression of cell motility with
shRNA-Fz2, the scratch assay was performed using HLF cells
(Fig. 3A–D) and PLC/PRF/5 cells
cultured in 4-well chamber slides (Fig. 3E–H). After the scratch was made
with a sterile razor, the cells were transfected with negative
control of shRNA at 500 ng/well (Fig.
3B and F), shRNA-Fz2 at 50 ng/well (Fig. 3C and G), or 500 ng/well (Fig. 3D and H). Cells subjected to mock
transfection were used as controls (Fig. 3A and E). Distance between the
growing edge of the cell layer and the scratch line was measured at
five different points for HLF cells (Fig. 3I) and PLC/PRF/5 cells (Fig. 3J). The distances were significantly
decreased with transfection of shRNA (P<0.05).
Matrix metalloproteinase 9 (MMP9) is involved in
cell motility (25), and its
expression levels increase in HCC tissues (26). The expression levels of MMP9 were
therefore, investigated. HLF cells (Fig. 4A) and PLC/PRF/5 cells (B) were
transfected with negative control of shRNA at 2.5 μg/well,
shRNA-Fz2 at 0.25 μg/well, or 2.5 μg/well. RNA was isolated from
the cells and subjected to qPCR to analyze MMP9 expression after 48
h culture. The expression levels of MMP9 significantly decreased by
transfection with shRNA-Fz2 (P<0.05).
The above results clearly showed that cell
proliferation and motility were suppressed by shRNA-Fz2. It was
confirmed that shRNA-Fz2 would be suitable as a therapeutic agent
of HCC. The study also aimed to evaluate methods for the
introduction of shRNA into HCC cells. Starch-iodide method was
applied to detect H2O2, which was generated
as a result of sonoporation (13).
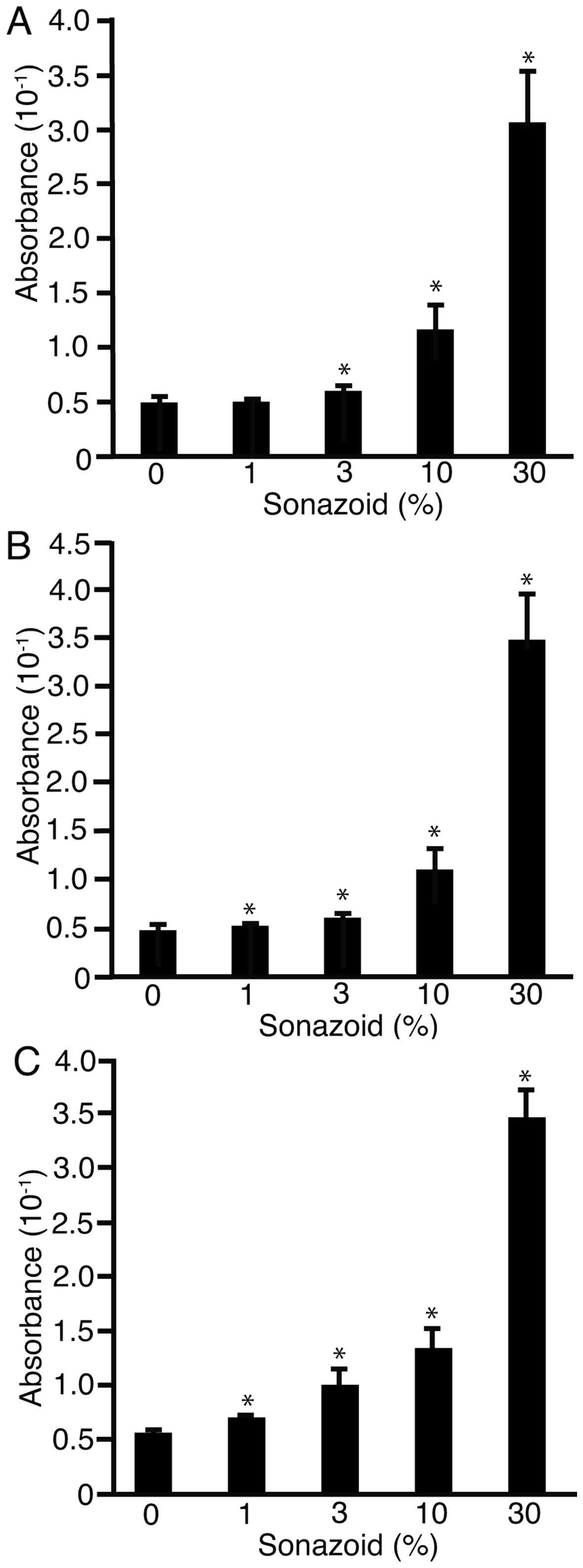
Sonazoid was added to the water at 0, 1, 3, 10 or 30% in FIA plates
after 1 min of US irradiation MI values of 0.1 (Fig. 5A), 0.4 (B), or 0.8 (C). MI was not
monitored but was set on the US device (SSA-700A). Absorbance of
the liquid in the well was analyzed at 490 nm. The absorbance
significantly decreased with the addition of Sonazoid (P<0.05).
These results suggested that US irradiation with Sonazoid generated
H2O2 and caused sonoporation.
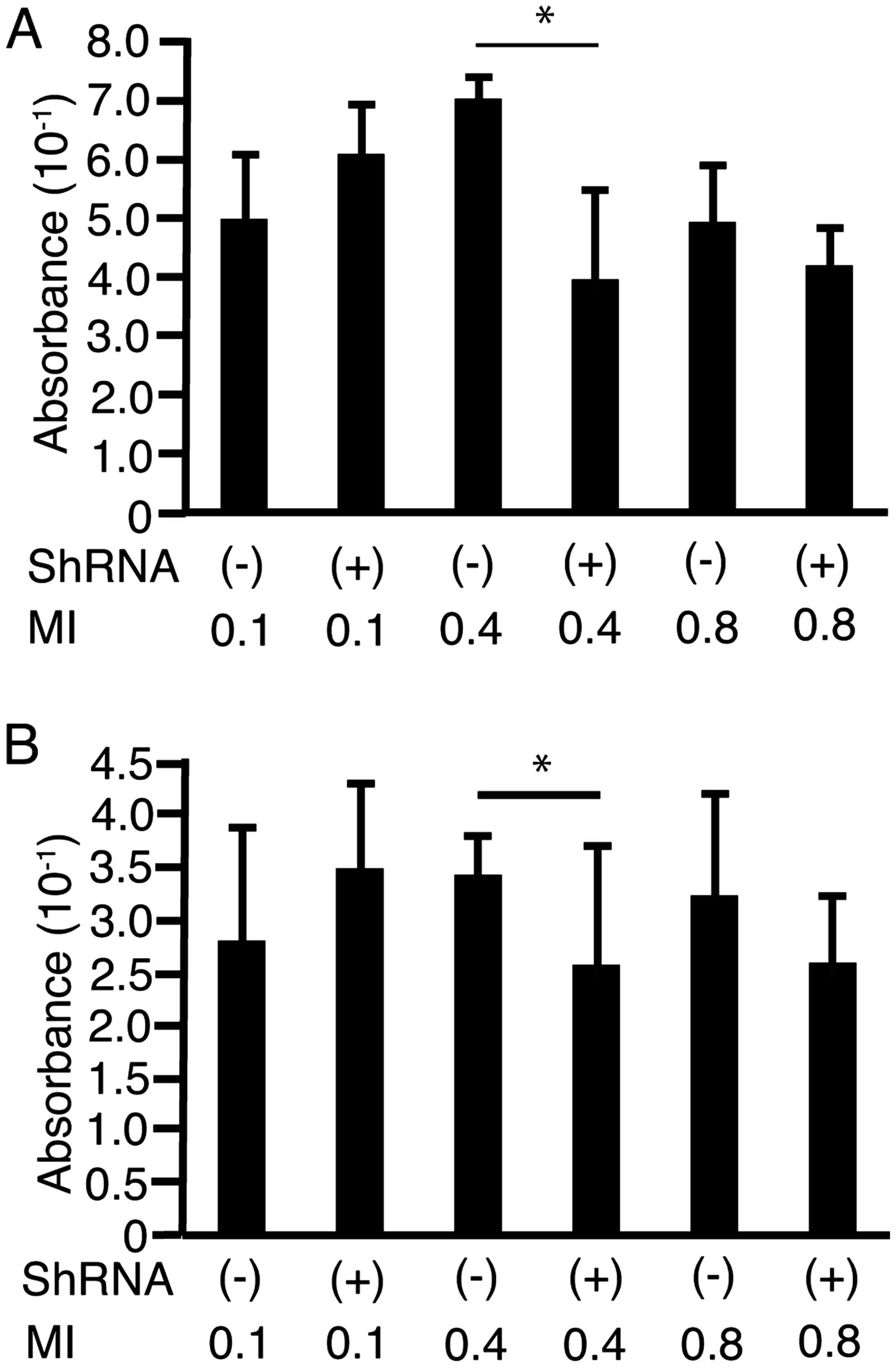
To address the possibility that shRNA-Fz2 suppressed
cell proliferation on irradiation with US, HLF cells (Fig. 6A) and PLC/PRF/5 cells (Fig. 6B) were cultured in FIA plates. The
cells were irradiated with US at MI values of 0.1, 0.4, or 0.8
after the addition of shRNA-Fz2 (100 ng/well) with or without 30%
Sonazoid. MI was not monitored but was set on the US device. At a
MI of 0.4, shRNA-Fz2 suppressed cell proliferation in both cell
lines.
Discussion
Prognosis of HCC becomes significantly poorer with
metastasis (27). Pathologically,
HCC cells invade the surrounding normal tissues, suggesting that
the motility of HCC cells is the major factor underlying metastasis
(28). Hence, suppression of HCC
cell motility is expected to improve the prognosis of HCC. Our
results clearly indicated that shRNA-Fz2 suppressed the motility of
HLF cells and PLC/PRF/5 cells. Given that our previous report
showed that shRNA-Fz2 suppresses HCC cell proliferation (12), the present findings and those of
our previous study together show that shRNA-Fz2 is a potential
candidate for the treatment of HCC.
Plasmids can be experimentally introduced into
cultured cells or tissues (29);
however, the efficiency of this introduction is low, which is a
major problem (16). To improve
the efficiency, three commercially available microbubbles,
SonoVue™ (Bracco Imaging, Courcouronnes, France),
Optison™ (GE Healthcare, Little Chalfont, UK), and
Sonazoid, have been compared for their efficiency in introducing
genes into the skeletal muscle (30). The authors report that introduction
efficiency does not depend on the size or composition of the
microbubbles or the stability of the microbubbles but on their
concentration. In the present study, introduction efficiency
increased as the concentration of Sonazoid was raised. Sonazoid has
been used to introduce plasmids into cultured cells (31); in that study, the authors used a US
generator for experimental purposes. In our study, a diagnostic US
device was used. Our data showed that introduction of plasmids into
cultured cells could be possible with irradiation from a diagnostic
US device and by using Sonazoid, which is commercially
available.
In the present study, the scratch assay was not
performed after US irradiation with the addition of shRNA-Fz2 and
Sonazoid, since US did not permeate or weaken the bottom surface of
the glass 4-chamber slides. Wells larger than those on FIA plates
were not commercially available.
In the future, the strength of US irradiation would
be monitored with a hydrophone, and HCC cells would be cultured in
larger plates to analyze the expression levels of cyclin D1 and
MMP9 after sonoporation with shRNA-Fz2, which was enhanced with
Sonazoid.
In conclusion, motility of HLF cells and PLC/PRF/5
cells was suppressed with shRNA-FZ2. Sonazoid enhanced sonoporation
mediated by the diagnostic US device and suppression of the
proliferation of both cell types with shRNA-Fz2.
References
|
1
|
McGlynn KA, Petrick JL and London WT:
Global epidemiology of hepatocellular carcinoma: An emphasis on
demographic and regional variability. Clin Liver Dis. 19:223–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knox JJ, Cleary SP and Dawson LA:
Localized and systemic approaches to treating hepatocellular
carcinoma. J Clin Oncol. 33:1835–1844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tejeda-Maldonado J, García-Juárez I,
Aguirre-Valadez J, González-Aguirre A, Vilatobá-Chapa M,
Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-Ávila JF and
Carrillo-Pérez DL: Diagnosis and treatment of hepatocellular
carcinoma: An update. World J Hepatol. 7:362–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen C and Wang G: Mechanisms of
hepatocellular carcinoma and challenges and opportunities for
molecular targeted therapy. World J Hepatol. 7:1964–1970. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bogaerts E, Heindryckx F, Vandewynckel YP,
Van Grunsven LA and Van Vlierberghe H: The roles of transforming
growth factor-β, Wnt, Notch and hypoxia on liver progenitor cells
in primary liver tumours (Review). Int J Oncol. 44:1015–1022.
2014.PubMed/NCBI
|
|
6
|
Pez F, Lopez A, Kim M, Wands JR, Caron de
Fromentel C and Merle P: Wnt signaling and hepatocarcinogenesis:
Molecular targets for the development of innovative anticancer
drugs. J Hepatol. 59:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka SS, Kojima Y, Yamaguchi YL,
Nishinakamura R and Tam PP: Impact of WNT signaling on tissue
lineage differentiation in the early mouse embryo. Dev Growth
Differ. 53:843–856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi-Yanaga F: Activator or
inhibitor? GSK-3 as a new drug target. Biochem Pharmacol.
86:191–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jamieson C, Sharma M and Henderson BR:
Targeting the β-catenin nuclear transport pathway in cancer. Semin
Cancer Biol. 27:20–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rizvi S and Gores GJ: Molecular profiling
and research of therapeutic targets. Dig Dis. 33:586–589. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Sueishi M: Short hairpin RNA of
frizzled-2 suppresses the proliferation of hepatocellular carcinoma
cells. Oncol Lett. 8:1519–1522. 2014.PubMed/NCBI
|
|
13
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Sueishi M: Sonoporation: Gene transfer
using ultrasound. World J Methodol. 3:39–44. 2013. View Article : Google Scholar
|
|
14
|
Tomizawa M, Ebara M, Saisho H, Sakiyama S
and Tagawa M: Irradiation with ultrasound of low output intensity
increased chemosensitivity of subcutaneous solid tumors to an
anti-cancer agent. Cancer Lett. 173:31–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Jong N: Mechanical index. Eur J
Echocardiogr. 3:73–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Plasmid DNA introduced into
cultured cells with diagnostic ultrasound. Oncol Rep. 27:1360–1364.
2012.PubMed/NCBI
|
|
17
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Short interference RNA
introduced into cultured cells with diagnostic ultrasound. Oncol
Rep. 27:65–68. 2012.
|
|
18
|
Salvatore V, Borghi A and Piscaglia F:
Contrast-enhanced ultrasound for liver imaging: Recent advances.
Curr Pharm Des. 18:2236–2252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Cock I, Zagato E, Braeckmans K, Luan Y,
de Jong N, De Smedt SC and Lentacker I: Ultrasound and microbubble
mediated drug delivery: Acoustic pressure as determinant for uptake
via membrane pores or endocytosis. J Control Release. 197:20–28.
2015. View Article : Google Scholar
|
|
20
|
Claudon M, Dietrich CF, Choi BI, Cosgrove
DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC,
et al: Guidelines and good clinical practice recommendations for
contrast enhanced ultrasound (CEUS) in the liver - update 2012: A
WFUMB-EFSUMB initiative in cooperation with representatives of
AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 34:11–29.
2013.
|
|
21
|
Fujimoto T, Tomizawa M and Yokosuka O:
SiRNA of frizzled-9 suppresses proliferation and motility of
hepatoma cells. Int J Oncol. 35:861–866. 2009.PubMed/NCBI
|
|
22
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping, and
novel promoter region. Genomics. 25:372–380. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kondo T and Yoshii G: Effect of intensity
of 1.2 MHz ultrasound on change in DNA synthesis of irradiated
mouse L cells. Ultrasound Med Biol. 11:113–119. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JK and Diehl JA: Nuclear cyclin D1: An
oncogenic driver in human cancer. J Cell Physiol. 220:292–296.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vandooren J, Van den Steen PE and
Opdenakker G: Biochemistry and molecular biology of gelatinase B or
matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev
Biochem Mol Biol. 48:222–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Shen Y, Cao B, Yan A and Ji H:
Elevated expression levels of androgen receptors and matrix
metalloproteinase-2 and -9 in 30 cases of hepatocellular carcinoma
compared with adjacent tissues as predictors of cancer invasion and
staging. Exp Ther Med. 9:905–908. 2015.PubMed/NCBI
|
|
27
|
Duseja A: Staging of hepatocellular
carcinoma. J Clin Exp Hepatol. 4(Suppl 3): S74–S79. 2014.
View Article : Google Scholar
|
|
28
|
Tomizawa M, Kondo F and Kondo Y: Growth
patterns and interstitial invasion of small hepatocellular
carcinoma. Pathol Int. 45:352–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horie S, Watanabe Y, Ono M, Mori S and
Kodama T: Evaluation of antitumor effects following tumor necrosis
factor-α gene delivery using nanobubbles and ultrasound. Cancer
Sci. 102:2082–2089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alter J, Sennoga CA, Lopes DM, Eckersley
RJ and Wells DJ: Microbubble stability is a major determinant of
the efficiency of ultrasound and microbubble mediated in vivo gene
transfer. Ultrasound Med Biol. 35:976–984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Tachibana R, Okamoto A, Azuma T,
Sasaki A, Yoshinaka K, Tei Y, Takagi S and Matsumoto Y:
Ultrasound-mediated gene transfection in vitro: Effect of
ultrasonic parameters on efficiency and cell viability. Int J
Hyperther. 28:290–299. 2012. View Article : Google Scholar
|