Introduction
Recently, a number of studies identified cell
surface markers, such as CD44v3 and CD24, as CSC markers (1–7).
Oral squamous cell carcinoma (OSCC) is the eighth most prevalent
cancer worldwide and shows high morbidity and poor survival rates
(8). Despite advances in
therapeutic procedures and various combinations of chemotherapeutic
agents that have improved quality of life, mortality from this
disease remains high because of the development of distant
metastasis and the emergence of local and regional recurrences.
Such treatment failure may be due to a small population of cells
[cancer stem cells (CSCs)] that are responsible for tumorigenesis
and contribute to resistance to conventional therapy, such as
chemotherapy and radiotherapy. Therefore, identification of CSCs or
cancer stem cell-like cells (CSC-LCs) may lead to the development
of effective treatment. Many reports have verified the existence of
CSCs in various solid neoplasms (7,9–12).
With regard to OSCC, Prince et al (13) identified a population of CD44
positive tumor initiating cells in OSCC. Chen et al
(14) reported that OSCC harbored
potential CSC characterized by ALDH1.
CD44v3 is an alternative splicing form variant of
CD44, which is a multifunctional transmembrane glycoprotein
expressed in many types of cancer. With regard to OSCC, several
studies have reported that CD44v3 is associated with drug
resistance and unfavorable clinical outcomes (15,16).
CD24 is a 27-amino-acid single-chain protein that is
O- and N-glycosylated and is bound to the extracellular matrix
(17) and the extracellular
membrane by a glycosylphosphatidylinositol anchor (18). Although several studies have
reported that CD24 is associated with invasion, metastasis and
tumor differentiation (19,20),
whether CD24 expression is upregulated or downregulated with tumor
invasion remains unclear. CD24 has also been studied in combination
with CD44. Several studies have reported that
CD44+/CD24− cells showed CSC properties in
breast and prostate cancer (4–6). On
the other hand, several studies have reported that
CD44+/CD24+ was the CSC phenotype of
pancreatic and colorectal cancer (7,21).
With regard to OSCC, a few reports have shown that
CD44+/CD24− may be the CSC phenotype
(22,23).
In the present study, we focused on CD44v3 and CD24
and examined whether these markers have CSC properties by using two
human OSCC cell lines and 50 human OSCC tissues.
Materials and methods
Cell lines and media
Two OSCC cell lines, SAS and OSC20, both derived
from primary lesions of a patient with OSCC, were used in the
experiment. SAS was purchased from the Health Science Research
Resources Bank. OSC20 was donated by the Research Center for
Innovative Cancer Therapy, Molecular Targeting Therapeutics
Division, Kurume University, School of Medicine. SAS was grown in
Dulbecco's modified Eagle's medium (DMEM; Nissui Seiyaku Co., Ltd.,
Tokyo, Japan) and Ham's F12 medium supplemented with
heat-inactivated (56°C, 30 min) 5% fetal bovine serum (FBS;
Bioserum, Victoria, Australia), 100 U/ml, penicillin and 100 μg
streptomycin (Gibco-BRL/Life Technologies Inc., Gaitherburg, MD,
USA). OSC20 was grown in Eagle's minimum essential medium (EMEM;
Gibco, BRL/Life Technologies Inc.) with 5% FBS. Cells were cultured
in an atmosphere of 5% CO2 in air at 37°C.
Flow cytometric analysis and
separation
SAS and OSC20 cells with 80% confluence were washed
once with phosphate-buffered saline (PBS), detached with accutase
(Innovative Cell Technologies, Inc., San Diego, CA, USA), suspended
at 1×106 cells/ml in PBS supplemented with 2% FBS,
incubated with 10 μg/ml human IgG (R&D Systems, Inc.,
Minneapolis, MN, USA) for 15 min at room temperature, and then
incubated with allophycocyanin (APC)-conjugated mouse anti-human
CD44v3 (cat. no. FAB5088A; R&D Systems) combined with
phycoerythrin (PE)-conjugated mouse anti-human CD24 (cat. no.
555428; BD Biosciences, San Jose, CA, USA) at 4°C for 45 min.
Samples were washed, centrifuged at 500 × g for 3 min, resuspended
in 2 ml cold PBS supplemented with 2% FBS, then 1 μg/ml propidium
iodide (PI; BD Biosciences) was added and the cells were filtered
through a 40-μm cell strainer (BD Biosciences). Analysis and
separation were carried out with a FACSAria II (BD
Biosciences).
Cell growth assay
A total of 2,500 cells of each of the four cell
fractions, i.e., CD44v3+/CD24−,
CD44v3+/CD24+,
CD44v3−/CD24− and
CD44v3−/CD24+ cells isolated from two cell
lines were plated in 96-well plates and cultured in a
CO2 incubator. The cells were harvested at 24, 48, 72 or
96 h and the proliferation was examined in colorimetric assays
using 3-(4,5-dimethylthiazol-2yl-yl-)-2, 5-dimethyl tetrazolium
bromide (MTT) cell growth assay kits (Chemicon, Temecula, CA, USA)
as described elsewhere (24).
Sphere forming assay
Four cell fractions isolated from two cell lines
(5,000 cells/dish) were cultured in serum-free medium including 10
ng/ml epidermal growth factor (EGF; Sanko Junyaku Co., Ltd., Tokyo,
Japan) and 20 ng/ml basic fibroblast growth factor (bFGF)
(PeproTech, Rocky Hill, NJ, USA) using ultra-low attachment 6-well
plates (Corning Inc., Corning, NY, USA) for 1 week. Sphere
formation was assessed by counting the number of spheres (>3
cells) under a microscope (x200).
Drug treatment assay
The four isolated cell fractions sorted from two
cell lines were plated at 2,500 cells/well in 96-well plates, and
the effect of CDDP (1 or 5 μM) (Nihon Kayaku, Tokyo, Japan),
5-fluorouracil (5-FU) (10 or 100 μM) (Kyowa Hakko, Tokyo, Japan),
and cetuximab (100 or 1,000 nM) (Merck Serono Co., Ltd., Tokyo,
Japan) was examined. Drug resistance was determined after treatment
for 96 h by MTT assay.
Tumorigenicity assay
Various numbers of cells (1×103,
1×104, or 1×105) were injected subcutaneously
into 4-week-old female non-obese diabetic/severe combined
immunodeficiency (NOD/SCID) mice (n=5 in each group). Tumorigenic
capacity was judged 8 weeks after injection. The animal procedures
were approved by the Ethics Review Committee for Animal
Experimentation of Kurume University School of Medicine.
cDNA preparation and quantitative
real-time RT-PCR for gene expression assay
After the four fractions were isolated, total RNA
was extracted using an RNAqueous Micro kit (Life Technologies,
Carisbad, CA, USA), and complementary DNA (cDNA) was synthesized
using the Reverse Transcription System (Promega, Madison, WI, USA)
according to the manufacturer's instructions. Quantitative
real-time RT-PCR (RT-qPCR) was performed to examine the expression
of CSC-LC property-related genes [e.g., ABC transporter genes
(ABCB1 and ABCG2), anti-apoptosis genes (BCL2 and CFLAR),
self-replication genes (Oct4 and Nanog) and hypoxia-related genes
[hypoxia inducible factor 1α (HIF1α)] with ABI PRISM 7500 (Applied
Biosystems, Foster City, CA, USA). Gene expression assays and
primer and probe mixes were used for ABCB1, ABCG2, ALDH1A1, BCL2,
CFLAR, Oct4, Nanog, HIF1α, and β-actin [assay IDs (Hs 00184500_m1,
Hs01053790_m1, Hs00946916_m1, Hs00608023_m1, Hs00153439_m1,
Hs03666771, Hs04260366, Hs00153153_m1, and Hs99999903_m1,
respectively; Applied Biosystems)], and thermal conditions were as
follows: initial incubation at 95°C for 10 min, then 40 cycles
alternating in turn with 95°C for 10 sec, 60°C for 20 sec, and 72°C
for 15 sec, and then maintained at 72°C for 10 min. Comparative
gene expression analysis was performed using the
2(−ΔΔCq) method with normalization to the level of
internal control gene, β-actin.
Tissue samples
OSCC tissue samples for immunohistochemistry were
obtained from 50 patients who underwent surgical resection at
Kurume University Hospital between 2007 and 2008. These specimens
were fixed in 10% buffered formalin followed by paraffin embedment.
None of the patients had previously received any treatments,
including chemotherapy or radiotherapy. This cohort was composed of
33 men and 17 women aged from 48 to 87 years (median age, 70
years). The average observation time for overall survival was 52
months for patients still alive at the time of analysis, and ranged
from 1 to 95 months.
Immunohistochemical staining
All tissues were immunohistochemically examined for
expression of CD44v3 and CD24 using mouse anti-human CD44v3
monoclonal antibody (VFF-327v3, 1:50 dilution; Novocastra, New
Castle upon Tyne, UK) and mouse anti-human CD24 monoclonal antibody
(528807, 1:100 dilution; R&D Systems). BenchMark XT (Ventana
Medical Systems, Inc., Tucson, AZ, USA) was used for
immunostaining.
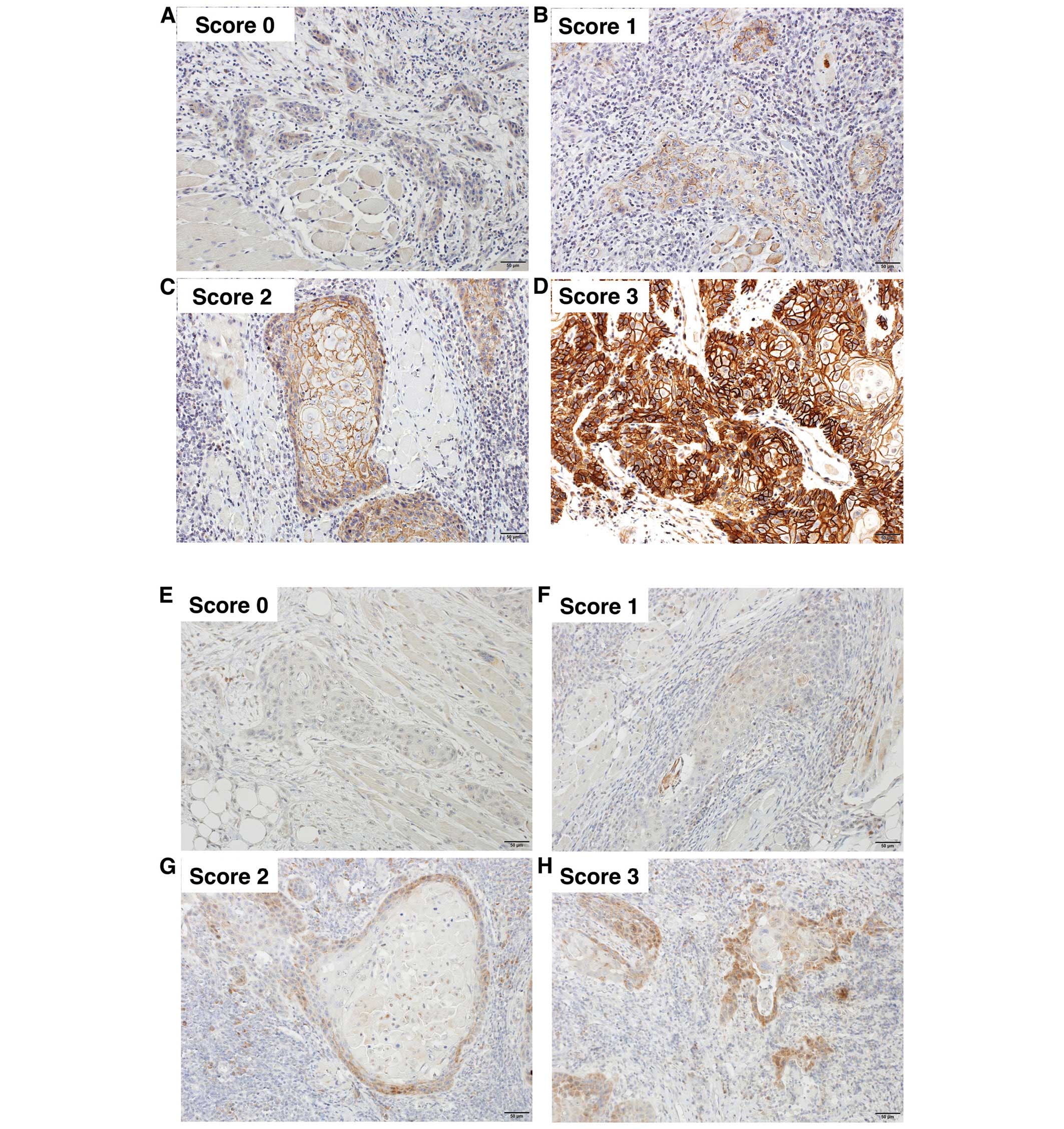
Evaluation of staining
All slides were evaluated by two of the authors
(K.T. and J.A.). CD44v3 and CD24 expressions were evaluated
according to staining intensity and staining area in the
non-invasive portion and invasive portion within the tumor. The
staining intensity was graded as 0, negative; 1, weakly positive;
2, moderately positive; or 3, strongly positive. The staining
intensity in the normal epithelium was used as an internal control.
The staining area was graded as 1, 0–20%; 2, 20–40%; 3, 40–60%; 4,
60–80%; and 5, 80–100%. The scores of staining intensity and area
were calculated. The expression scores of CD44v3 and CD24 were
compared between the non-invasive portion and invasive portion. The
expression scores of CD44v3 and CD24 were categorized as either
negative or positive. The median value of the expression score was
used to separate the negative and positive groups. The relationship
between isolated CD44v3 and isolated CD24 immunoexpression or
CD44v3/CD24 immunophenotype and each clinicopathological feature of
OSCC, such as T stage, nodal status, mode of invasion (25) and histological grade, was
analyzed.
Statistical analysis
JMP software version 11.0 was used for all
statistical analysis. Comparisons of cell growth assay, sphere
forming assay, drug resistance assay, and quantitative real-time
RT-PCR assay were normally distributed and assessed by the
Shapiro-Wilk test, and then, a comparison of each in vitro
assay except for the sphere forming assay was performed using
one-way ANOVA with Dunnet post hoc comparisons. A comparison of the
sphere forming assay was performed using one-way ANOVA with the
Tukey post hoc comparisons. The expression score data of the
invasive portion were compared with that of the non-invasive
portion using a Mann-Whitney U test. Correlation of isolated CD44v3
and CD24 immunoexpression or the CD44v3/CD24 immunophenotypes with
the clinicopathological parameters was assessed by standard Chi
square tests or the Fisher's exact test. The overall survival rate
was defined as the interval between the diagnosis and the date of
death (uncensored data) or the date of the last available clinical
information (censored data). Comparison and estimation of
cumulative survival rates were performed using the Kaplan-Meier
curves and the log rank test. All tests were two-sided and a value
of P<0.05 was considered significant.
Results
Expression of CD44v3/CD24 in SAS and
OSC20
In SAS, the proportion of
CD44v3+/CD24−,
CD44v3+/CD24+,
CD44v3−/CD24− and
CD44v3−/CD24+ cells was 10.7, 48.2, 7.8 and
33.3%, respectively. Whereas, the proportion of
CD44v3+/CD24−,
CD44v3+/CD24+,
CD44v3−/CD24− and
CD44v3−/CD24+ cells was 24.1, 52.0, 11.2 and
12.7%, respectively (Fig. 1).
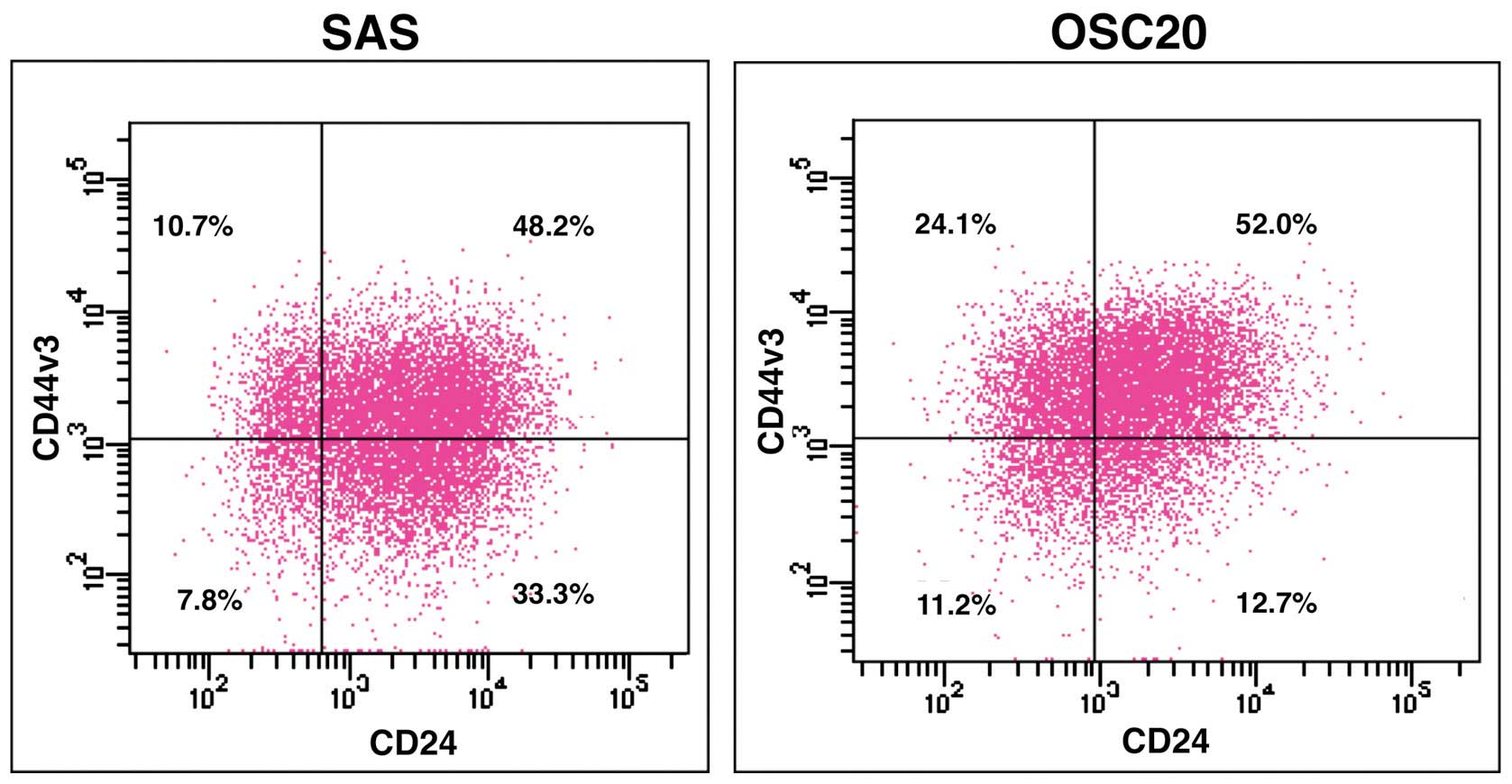 | Figure 1Expression of CD44v3/CD24 in SAS and
OSC20. SAS and OSC20 are labeled with CD44v3 and CD24, and then
analyzed by FCM. In SAS, the proportion of
CD44v3+/CD24−,
CD44v3+/CD24+,
CD44v3−/CD24− and
CD44v3−/CD24+ cells is 10.7, 48.2, 7.8 and
33.3%, respectively. Whereas, the proportion of
CD44v3+/CD24−,
CD44v3+/CD24+,
CD44v3−/CD24− and
CD44v3−/CD24+ cells is 24.1, 52.0, 11.2 and
12.7%, respectively. |
Biological features of sorted cell
fractions in SAS and OSC20 in vitro
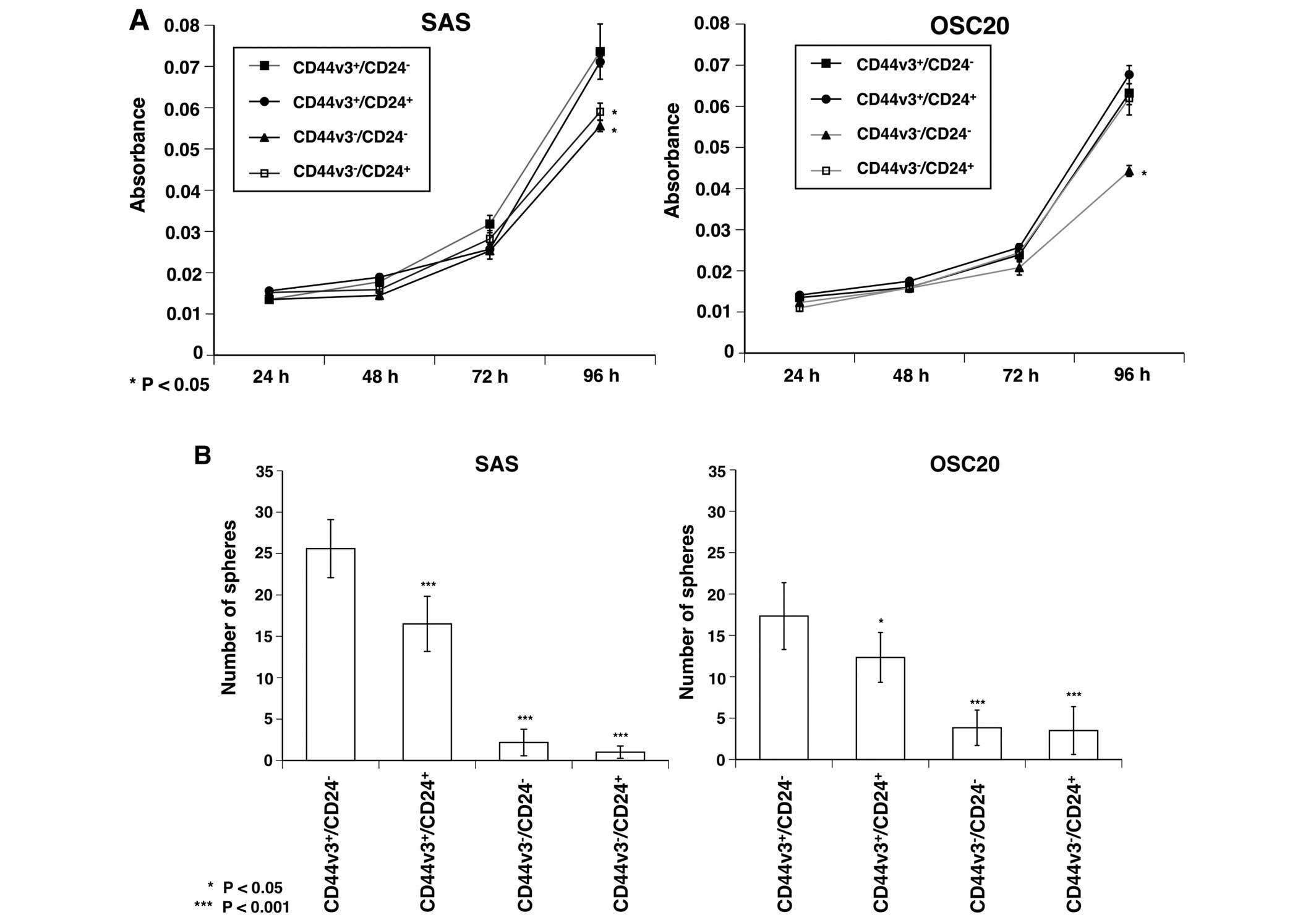
CD44v3+/CD24− cells in SAS
showed a significantly higher proliferative ability than that of
CD44v3−/CD24− and
CD44v3−/CD24+ cells after culturing for 96 h
(P<0.05), and, CD44v3+/CD24− cells in
OSC20 showed a significantly higher proliferative ability than that
of CD44v3−/CD24− cells (P<0.05) (Fig. 2A).
The sphere forming ability of
CD44v3+/CD24− cells in both SAS and OSC20 was
significantly higher than the other fractions (P<0.05 or
P<0.001). There was also a significant difference in sphere
forming ability between the two fractions, for example, between
CD44v3+/CD24+ and
CD44v3−/CD24−, and between
CD44v3+/CD24+ and
CD44v3−/CD24+ cells (P<0.001) (Fig. 2B).
After 96-h treatment with CDDP, 5-FU, or cetuximab,
the sensitivity to each drug was assessed with the MTT assay in SAS
and OSC20. Cell growth was significantly suppressed in cells
treated with CDDP, 5-FU and cetuximab, as compared with control
cells in both SAS and OSC20 (Fig.
2C).
In SAS, CD44v3+/CD24− cells
had a significantly higher resistance in CDDP (1 μM) or 5-FU (10 or
100 μM) treatment than the other fractions (P<0.05 or
P<0.001), and had a significantly higher resistance in CDDP (5
μM) or cetuximab (100 or 1,000 nM) treatment than
CD44v3+/CD24+ and
CD44v3−/CD24+ cells (P<0.001) (Fig. 2C).
In OSC20, CD44v3+/CD24− cells
had a significantly higher resistance in CDDP (1 or 5 μM) or 5-FU
(100 μM) or cetuximab (100 nM) treatment than
CD44v3+/CD24+ cells and/or
CD44v3−/CD24+ cells (P<0.05, P<0.01 or
P<0.001). There was no significant difference in sensitivity in
5-FU (10 μM) or cetuximab (1,000 nM) treatment among the cell
fractions (Fig. 2C).
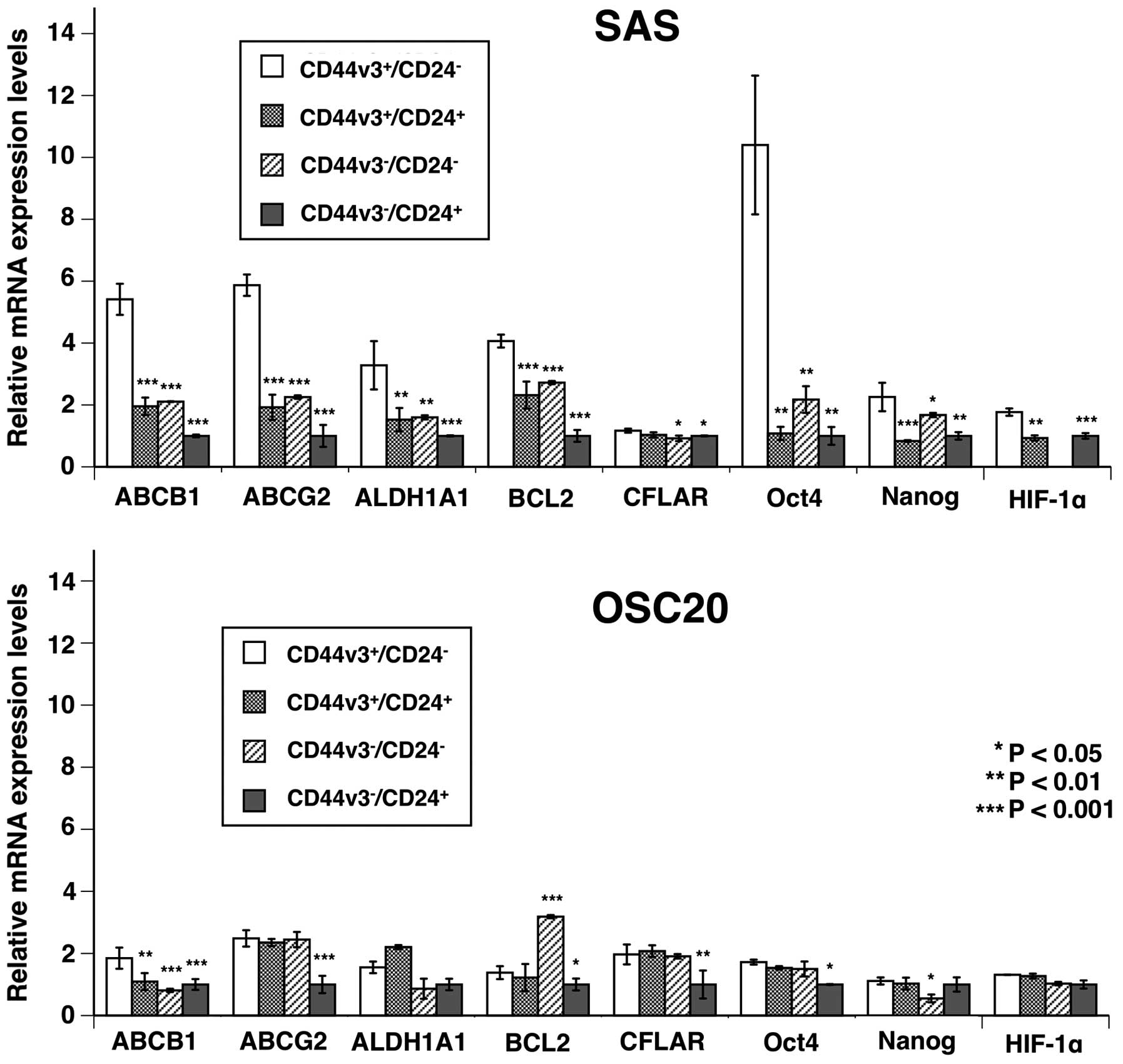
Analysis of CSC-LC property-related gene
expression in sorted cell fractions in SAS and OSC20 by
RT-qPCR
We performed RT-qPCR analysis to compare CSC-LC
property related gene expression in
CD44v3+/CD24− cells and the other cell
fractions in SAS and OSC20 cells. In SAS,
CD44v3+/CD24− cells showed significantly
higher mRNA expression of transporter-related genes (ABCB1 and
ABCG2), ALDH1A1, anti-apoptotic gene (BCL2), and self-replication
genes (Oct-4, Nanog), than the other fractions (Fig. 3).
Tumorigenicity assays in vivo in sorted
cell fractions in SAS
Injection of 1×103 cells from all of the
cell fractions produced no tumors in NOD/SCID mice. In contrast,
two mice that received 1×104
CD44v3+/CD24− cells or
CD44v3+/CD24+ cells developed tumors 8 weeks
after the inoculation. In addition, injection of 1×105
cells from all of the cell fractions produced tumors in NOD/SCID
mice. The ratio of tumorigenicity among
CD44v3+/CD24− cell fractions and the other
fractions in SAS was not significantly different (Table I).
 | Table ITumorigenicity of sorted cell
fractions in SAS. |
Table I
Tumorigenicity of sorted cell
fractions in SAS.
| Injected cell
number |
|---|
|
|
|---|
|
1×103 |
1×104 |
1×105 |
|---|
|
CD44v3+/CD24− | 0/5 (0%) | 2/5 (40%) | 2/5 (40%) |
|
CD44v3+/CD24+ | 0/5 (0%) | 2/5 (40%) | 3/5 (60%) |
|
CD44v3−/CD24− | 0/5 (0%) | 0/5 (0%) | 1/5 (20%) |
|
CD44v3−/CD24+ | 0/5 (0%) | 0/5 (0%) | 2/5 (40%) |
Immunohistochemical findings and their
relationship to clinicopathological features
CD44v3 and CD24 were predominantly expressed on the
cell membrane and in the cytoplasm, respectively. A representative
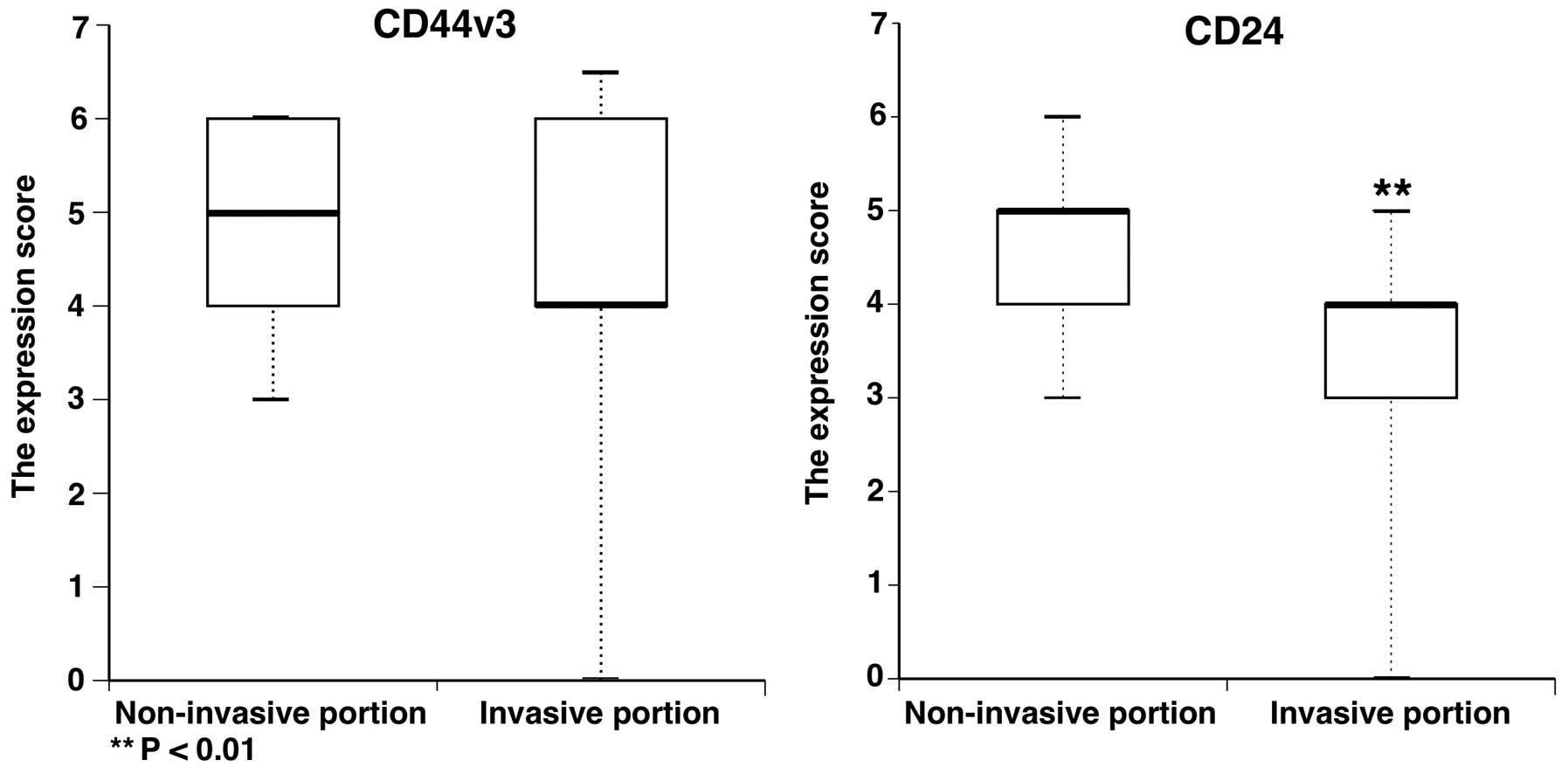
immunostaining photomicrograph of each grade is shown in Fig. 4. CD44v3 and CD24 immunoscoring of
the non-invasive portion and invasive portion is shown in Fig. 5. A significant difference was
observed between the expression scores of CD24 in the non-invasive
portion and those in the invasive portion (P<0.01) (Fig. 5).
There was no significant correlation between
isolated CD44v3 and isolated CD24 immunoexpression or CD44v3/CD24
immunophenotypes and each clinicopathological parameter in the
non-invasive portion (data not shown). The association between
CD44v3 and CD24 expression in the invasive portion and
clinicopathological parameters is summarized in Table II. CD44v3 expression was
significantly correlated with lymph node metastasis (P=0.039), but
not with age, gender, T stage, mode of invasion and tumor
differentiation. CD24 expression was significantly correlated with
gender (P=0.01), but not with the other clinicopathological
parameter. The association between CD44v3/CD24 immunophenotypes and
clinicopathological parameters in 50 OSCC patients is summarized in
Table III. When the 50 cases
were subdivided into four groups i.e.,
CD44v3+/CD24−,
CD44v3+/CD24+,
CD44v3−/CD24− and
CD44v3−/CD24+ cases, the four
immunophenotypes were not significantly correlated with each
clinicopathological parameter.
 | Table IIAssociation of CD44v3 and CD24
immunoexpression with clinicopathological parameters in 50 OSCC
patients. |
Table II
Association of CD44v3 and CD24
immunoexpression with clinicopathological parameters in 50 OSCC
patients.
| | Invasive
portion | | Invasive
portion | |
|---|
| |
| |
| |
|---|
| | Immunoreactive
score of CD44v3 | | Immunoreactive
score of CD24 | |
|---|
| |
| |
| |
|---|
| Variables | Total no. | + | − | P-value | + | − | P-value |
|---|
| No. of
patients | 50 | 23 | 27 | | 11 | 39 | |
| Age (years) | | | | 0.969 | | | 0.705 |
| ≤65 | 14 | 6 | 8 | | 2 | 12 | |
| >65 | 36 | 17 | 19 | | 9 | 27 | |
| Gender | | | | 0.897 | | | 0.01 |
| Male | 32 | 15 | 17 | | 3 | 29 | |
| Female | 18 | 8 | 10 | | 8 | 10 | |
| T
classification | | | | 0.763 | | | 0.688 |
| T1/T2 | 39 | 17 | 22 | | 8 | 31 | |
| T3/T4 | 11 | 6 | 5 | | 3 | 8 | |
| N
classification | | | | 0.039 | | | 0.64 |
| N0 | 43 | 17 | 26 | | 9 | 34 | |
| N1+N2 | 7 | 6 | 1 | | 2 | 5 | |
| Mode of
invasion | | | | 0.575 | | | 0.938 |
| 1+2 | 13 | 6 | 7 | | 3 | 10 | |
| 3 | 24 | 13 | 11 | | 6 | 18 | |
| 4 | 13 | 4 | 9 | | 2 | 11 | |
|
Differentiation | | | | 0.601 | | | 0.147 |
| Well | 34 | 17 | 17 | | 5 | 29 | |
| Moderate +
poor | 16 | 6 | 10 | | 6 | 10 | |
 | Table IIIAssociation of CD44v3/CD24
immunophenotype with clinicopathological parameters in 50 OSCC
patients. |
Table III
Association of CD44v3/CD24
immunophenotype with clinicopathological parameters in 50 OSCC
patients.
| | Invasive
portion | |
|---|
| |
| |
|---|
| | CD44v3/CD24
profile | |
|---|
| |
| |
|---|
| Variables | Total no | +/− | +/+ | −/− | −/+ | P-value |
|---|
| No. of
patients | 50 | 18 | 5 | 21 | 6 | |
| Age (years) | | | | | | 0.82 |
| ≤65 | 14 | 6 | 0 | 6 | 2 | |
| >65 | 36 | 12 | 5 | 15 | 4 | |
| Gender | | | | | | 0.139 |
| Male | 32 | 13 | 2 | 16 | 1 | |
| Female | 18 | 5 | 3 | 5 | 5 | |
| T
classification | | | | | | 0.882 |
| T1/T2 | 39 | 13 | 4 | 18 | 4 | |
| T3/T4 | 11 | 5 | 1 | 3 | 2 | |
| Nodal status | | | | | | 0.862 |
| N0 | 43 | 14 | 4 | 19 | 6 | |
| N1 + N2 | 7 | 4 | 1 | 2 | 0 | |
| Mode of
invasion | | | | | | 0.647 |
| 1 + 2 | 13 | 3 | 3 | 7 | 0 | |
| 3 | 24 | 11 | 2 | 7 | 4 | |
| 4 | 13 | 4 | 0 | 7 | 2 | |
|
Differentiation | | | | | | 0.505 |
| Well | 34 | 14 | 3 | 15 | 2 | |
| Moderate +
poor | 16 | 4 | 2 | 6 | 4 | |
Overall survival (OS) rates and
CD44v3/CD24 immunophenotypes in the invasive portion
Kaplan Meier analysis established the relationship
between CD44v3 expression in the invasive portion and OS (Fig. 6A). CD44v3+ cases tended
to show poor OS compared with CD44v3− cases (P=0.055).
The relationship between CD24 expression in the invasive portion
and OS is shown in Fig. 6B. There
was no significant difference in OS between CD24+ cases
and CD24− cases. The Kaplan Meier curves for OS in OSCC
patients that were subdivided into four groups according to
CD44v3/CD24 immunophenotypes are shown in Fig. 6C.
CD44v3+/CD24− cases showed significantly
worse OS than CD44v3+/CD24− cases
(P=0.029).
Discussion
In the present study,
CD44v3+/CD24− cell fractions in SAS and OSC20
were 10.7 and 24.1%, respectively. Although the
CD44v3+/CD24− cell fraction in SAS or OSC20
did not show a higher proliferative ability than each of the other
fractions (CD44v3−/CD24+,
CD44v3−/CD24− and
CD44v3−/CD24+ cells), the
CD44v3+/CD24− cell fraction showed
significantly higher sphere forming ability, a higher CDDP or 5-FU
resistance, suggesting that the CD44v3+/CD24−
cell fraction had CSC-LC properties in SAS or OSC20.
Previous studies have reported that CSC-LCs have
drug transporter genes such as ABCB1 and ABCG2 (26,27).
Our real-time PCR assay found that drug transporter genes such as
ABCB1 and ABCG2 were significantly more highly expressed in the
CD44v3+/CD24− cell fraction than the other
cell fractions in SAS. However, ABCB1 and ABCG2 are not major
transporters of CDDP or 5-FU, and therefore, high expression of
ABCB1 and ABCG2 is unlikely to be the explanation for the increased
resistance to CDDP or 5-FU in SAS. Other studies have reported that
CSC-LCs have anti-apoptotic and drug-resistant properties due to
the expression of anti-apoptosis genes such as BCL2 and CFLAR
(28), which contribute to the
emergence of CDDP or 5-FU resistance (29–32).
Our real-time PCR assays found that anti-apoptotic genes such as
BCL2 and CFLAR were more highly expressed in the
CD44v3+/CD24− cell fraction than the other
cell fractions in SAS. These findings suggest that
CD44v3+/CD24− cells have anti-apoptotic
effects; therefore, they probably showed higher CDDP or 5-FU
resistance. Oct-4 and Nanog are associated with self-renewal
capacity (14,33). Our real-time PCR assays also found
that self-replication markers such as Oct4 or Nanog were
significantly more highly expressed in the
CD44v3+/CD24− cell fraction than the other
cell fractions in SAS.
Indeed, CD44v3+/CD24− showed
the highest sphere forming abilities compared with the other
fractions in both cell lines. Sphere forming assay is a
representative assay used to confirm self-renewal capacity in
vitro. Collectively, these findings suggest that the
CD44v3+/CD24− cell fraction had a higher
self-renewal capacity.
Before the presence of CSC was widely accepted in
solid tumors, immunohistochemical studies on CD44, including its
variant isoform such as CD44v3 and CD24 were conducted in various
tumors, including OSCCs. However, the relationship between these
markers and various clinicopathological findings was inconsistent
in several previous studies (34–40).
The present study demonstrated that CD44v3 expression in the
invasive portion was slightly downregulated compared with the
non-invasive portion. These findings are partially consistent with
several studies on CD44 isoforms in OSCCs (36,41–43).
Molecular cross-talk between tumor and host at the invasion front
is probably associated with these alterations of adhesion
molecules, such as CD44 isoforms (36,44,45).
Our present study demonstrated CD24 expression in the invasive
portion was significantly downregulated compared with expression in
the non-invasive portion. In some reports, the upregulation of CD24
expression was associated with an early event of carcinogenesis in
various carcinomas (35,39,46).
In the present study, CD24 expression in the invasive portion was
found in only 22% of cases and was not associated with prognosis.
However, out of the cases without CD24 expression, the cases with
unfavorable prognosis were enhanced when combined with
CD44v3+ expression. As we described before,
immunohistochemical analyses on CD44 and/or CD24 were inconsistent.
These inconsistent results may be due to extreme variations in
tissue material, varying specificity of the numerous different
antibodies used, and the diverse scoring and evaluation system
applied (36).
CD44v3+ cells showed a higher
tumorigenicity in vivo assay compared with
CD44v3− cells. These effects were almost independent of
CD24 status. Also, immunohistochemical findings demonstrated that
CD44v3 was more prognostic as a single marker in OSCCs.
Collectively, these findings suggest that CD44v3 may be a more
reliable marker for CSC-LC properties than CD24 as a single marker.
The interaction of CD44 isoforms and CD24 is probably associated
with each other directly or indirectly. Further studies on the
association between CD44 isoforms and CD24 should be conducted to
clarify the exact mechanisms.
In conclusion, the results suggest that the
CD44v3+/CD24− cell population shows CSC-LC
properties in a human OSCC cell line. Additionally, we presented
some evidence that isolated CD44v3 immunoexpression or
CD44v3+/CD24− immunophenotypes could give
prognostic information associated with unfavorable clinical
outcomes.
Acknowledgements
We thank Ms. Akemi Fujiyoshi for her assistance in
our experiments.
References
|
1
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalish ED, Iida N, Moffat FL and
Bourguignon LY: A new CD44V3-containing isoform is involved in
tumor cell growth and migration during human breast carcinoma
progression. Front Biosci. 4:A1–A8. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma F, Li H, Wang H, Shi X, Fan Y, Ding X,
Lin C, Zhan Q, Qian H and Xu B: Enriched
CD44+/CD24− population drives the aggressive
phenotypes presented in triple-negative breast cancer (TNBC).
Cancer Lett. 353:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hurt EM, Kawasaki BT, Klarmann GJ, Thomas
SB and Farrar WL: CD44+CD24− prostate cells
are early cancer progenitor/stem cells that provide a model for
patients with poor prognosis. Br J Cancer. 98:756–765. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petersen PE: Oral cancer prevention and
control - the approach of the World Health Organization. Oral
Oncol. 45:454–460. 2009. View Article : Google Scholar
|
|
9
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar
|
|
10
|
Kim CF, Jackson EL, Woolfenden AE,
Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT and Jacks T:
Identification of bronchioalveolar stem cells in normal lung and
lung cancer. Cell. 121:823–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen C, Wei Y, Hummel M, Hoffmann TK,
Gross M, Kaufmann AM and Albers AE: Evidence for
epithelial-mesenchymal transition in cancer stem cells of head and
neck squamous cell carcinoma. PLoS One. 6:e164662011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang SJ, Wong G, de Heer AM, Xia W and
Bourguignon LY: CD44 variant isoforms in head and neck squamous
cell carcinoma progression. Laryngoscope. 119:1518–1530. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bourguignon LY, Wong G, Earle C and Chen
L: Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes
miR-302 expression leading to self-renewal, clonal formation, and
cisplatin resistance in cancer stem cells from head and neck
squamous cell carcinoma. J Biol Chem. 287:32800–32824. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pirruccello SJ and LeBien TW: The human B
cell-associated antigen CD24 is a single chain sialoglycoprotein. J
Immunol. 136:3779–3784. 1986.PubMed/NCBI
|
|
18
|
Fischer GF, Majdic O, Gadd S and Knapp W:
Signal transduction in lymphocytic and myeloid cells via CD24, a
new member of phosphoinositol-anchored membrane molecules. J
Immunol. 144:638–641. 1990.PubMed/NCBI
|
|
19
|
Sano A, Kato H, Sakurai S, Sakai M, Tanaka
N, Inose T, Saito K, Sohda M, Nakajima M, Nakajima T, et al: CD24
expression is a novel prognostic factor in esophageal squamous cell
carcinoma. Ann Surg Oncol. 16:506–514. 2009. View Article : Google Scholar
|
|
20
|
Yang XR, Xu Y, Yu B, Zhou J, Li JC, Qiu
SJ, Shi YH, Wang XY, Dai Z, Shi GM, et al: CD24 is a novel
predictor for poor prognosis of hepatocellular carcinoma after
surgery. Clin Cancer Res. 15:5518–5527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeung TM, Gandhi SC, Wilding JL, Muschel R
and Bodmer WF: Cancer stem cells from colorectal cancer-derived
cell lines. Proc Natl Acad Sci USA. 107:3722–3727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YC, Chen YW, Hsu HS, Tseng LM, Huang
PI, Lu KH, Chen DT, Tai LK, Yung MC, Chang SC, et al: Aldehyde
dehydrogenase 1 is a putative marker for cancer stem cells in head
and neck squamous cancer. Biochem Biophys Res Commun. 385:307–313.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiu CC, Lee LY, Li YC, Chen YJ, Lu YC, Li
YL, Wang HM, Chang JT and Cheng AJ: Grp78 as a therapeutic target
for refractory head-neck cancer with
CD24−CD44+ stemness phenotype. Cancer Gene
Ther. 20:606–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hisaka T, Yano H, Ogasawara S, Momosaki S,
Nishida N, Takemoto Y, Kojiro S, Katafuchi Y and Kojiro M:
Interferon-alphaCon1 suppresses proliferation of liver cancer cell
lines in vitro and in vivo. J Hepatol. 41:782–789. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto E, Kohama G, Sunakawa H, Iwai M
and Hiratsuka H: Mode of invasion, bleomycin sensitivity, and
clinical course in squamous cell carcinoma of the oral cavity.
Cancer. 51:2175–2180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rizzo S, Hersey JM, Mellor P, Dai W,
Santos-Silva A, Liber D, Luk L, Titley I, Carden CP, Box G, et al:
Ovarian cancer stem cell-like side populations are enriched
following chemotherapy and overexpress EZH2. Mol Cancer Ther.
10:325–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Nuchtern JG, Jax TW, Gobel U, Goodell MA and Brenner MK: A distinct
‘side population’ of cells with high drug efflux capacity in human
tumor cells. Proc Natl Acad Sci USA. 101:14228–14233. 2004.
View Article : Google Scholar
|
|
28
|
Yajima T, Ochiai H, Uchiyama T, Takano N,
Shibahara T and Azuma T: Resistance to cytotoxic
chemotherapy-induced apoptosis in side population cells of human
oral squamous cell carcinoma cell line Ho-1-N-1. Int J Oncol.
35:273–280. 2009.PubMed/NCBI
|
|
29
|
Yang Z, Liu Y, Liao J, Gong C, Sun C, Zhou
X, Wei X, Zhang T, Gao Q, Ma D, et al: Quercetin induces
endoplasmic reticulum stress to enhance cDDP cytotoxicity in
ovarian cancer: Involvement of STAT3 signaling. FEBS J.
282:1111–1125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karaayvaz M, Zhai H and Ju J: miR-129
promotes apoptosis and enhances chemosensitivity to 5-fluorouracil
in colorectal cancer. Cell Death Dis. 4:e6592013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Longley DB, Wilson TR, McEwan M, Allen WL,
McDermott U, Galligan L and Johnston PG: c-FLIP inhibits
chemotherapy-induced colorectal cancer cell death. Oncogene.
25:838–848. 2006. View Article : Google Scholar
|
|
32
|
Micheau O, Solary E, Hammann A and
Dimanche-Boitrel MT: Fas ligand-independent, FADD-mediated
activation of the Fas death pathway by anticancer drugs. J Biol
Chem. 274:7987–7992. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murakami S, Ninomiya W, Sakamoto E,
Shibata T, Akiyama H and Tashiro F: SRY and OCT4 are required for
the acquisition of cancer stem cell-like properties and are
potential differentiation therapy targets. Stem Cells.
33:2652–2663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oliveira LR, Oliveira-Costa JP, Araujo IM,
Soave DF, Zanetti JS, Soares FA, Zucoloto S and Ribeiro-Silva A:
Cancer stem cell immunophenotypes in oral squamous cell carcinoma.
J Oral Pathol Med. 40:135–142. 2011. View Article : Google Scholar
|
|
35
|
Abdulmajeed AA, Dalley AJ and Farah CS:
Putative cancer stem cell marker expression in oral epithelial
dysplasia and squamous cell carcinoma. J Oral Pathol Med.
42:755–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bánkfalvi A, Krassort M, Buchwalow IB,
Végh A, Felszeghy E and Piffkó J: Gains and losses of adhesion
molecules (CD44, E-cadherin, and beta-catenin) during oral
carcinogenesis and tumour progression. J Pathol. 198:343–351. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi D, Lee HW, Hur KY, Kim JJ, Park GS,
Jang SH, Song YS, Jang KS and Paik SS: Cancer stem cell markers
CD133 and CD24 correlate with invasiveness and differentiation in
colorectal adenocarcinoma. World J Gastroenterol. 15:2258–2264.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kristiansen G, Denkert C, Schlüns K, Dahl
E, Pilarsky C and Hauptmann S: CD24 is expressed in ovarian cancer
and is a new independent prognostic marker of patient survival. Am
J Pathol. 161:1215–1221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bircan S, Kapucuoglu N, Baspinar S, Inan G
and Candir O: CD24 expression in ductal carcinoma in situ and
invasive ductal carcinoma of breast: An immunohistochemistry-based
pilot study. Pathol Res Pract. 202:569–576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Darwish NS, Kim MA, Chang MS, Lee HS, Lee
BL, Kim YI and Kim WH: Prognostic significance of CD24 expression
in gastric carcinoma. Cancer Res Treat. 36:298–302. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Herold-Mende C, Seiter S, Born AI, Patzelt
E, Schupp M, Zöller J, Bosch FX and Zöller M: Expression of CD44
splice variants in squamous epithelia and squamous cell carcinomas
of the head and neck. J Pathol. 179:66–73. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fonseca I, Pereira T, Rosa-Santos J and
Soares J: Expression of CD44 isoforms in squamous cell carcinoma of
the border of the tongue: A correlation with histological grade,
pattern of stromal invasion, and cell differentiation. J Surg
Oncol. 76:115–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Masuda M, Kuratomi Y, Shiratsuchi H,
Nakashima T, Naonobu K and Komiyama S: Decreased CD44H expression
in early-stage tongue carcinoma associates with late nodal
metastases following interstitial brachytherapy. Head Neck.
22:662–665. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Williams HK, Sanders DS, Jankowski JA,
Landini G and Brown AM: Expression of cadherins and catenins in
oral epithelial dysplasia and squamous cell carcinoma. J Oral
Pathol Med. 27:308–317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bànkfalvi A and Piffkò J: Prognostic and
predictive factors in oral cancer: The role of the invasive tumour
front. J Oral Pathol Med. 29:291–298. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sagiv E, Memeo L, Karin A, Kazanov D,
Jacob-Hirsch J, Mansukhani M, Rechavi G, Hibshoosh H and Arber N:
CD24 is a new oncogene, early at the multistep process of
colorectal cancer carcinogenesis. Gastroenterology. 131:630–639.
2006. View Article : Google Scholar : PubMed/NCBI
|