Introduction
Solid tumors are composed of not only tumor cells
but also a complex array of stromal cells (1). Accumulating evidence has demonstrated
that tumor-associated stromal cells play important roles in
providing support for tumor cells, thus contributing to tumor
initiation and progression (2,3). One
frequent component of the tumor stroma is tumor-associated
fibroblasts (TAFs) that express α-smooth muscle actin (α-SMA) and
has been reported to exert fundamental effects on tumor progression
(4). Tumors could recruit
mesenchymal stem cells (MSCs) into their microenvironment, where
they become TAFs and affect tumor cell survival, angiogenesis and
metastasis (5,6). MSCs are defined as multipotent stem
cells that have the capacity to give rise to adipocytes,
osteoblasts and chondrocytes (7).
MSCs can be isolated from a number of tissues including bone
marrow, adipose tissue and umbilical cord blood. Several studies
have pointed to MSCs as an important source of TAFs (1,8). The
precise role of MSCs in tumor initiation and progression is still
controversial because both pro- and anti-tumorigenic effects have
been reported.
Interactions between tumor cells and MSCs within the
local microenvironment could be mediated by cell-cell contact and
by paracrine mechanisms through release of a variety of bioactive
molecules such as growth factors, cytokines and inflammatory
mediators (9,10). Recently, a novel way emerged of
cell-cell communication mediated through exosomes, which are small
membrane vesicles secreted by a variety of cell types, including
tumor cells. Numerous reports showed that tumor exosomes are
associated with tumor development, chemoresistance and capacity to
escape from immune surveillance (11–13).
Although the biological functions are not well-defined, exosomes
are known to deliver diverse molecules to target cells ranging from
mRNAs, miRNAs, to proteins. Recent studies demonstrated that
tumor-derived exosomes can function in communication between tumor
cells and MSCs in the neoplastic tumor microenvironment (14–16).
However, the precise mechanisms underlying interactions between
MSCs and tumor exosomes remain largely unknown. Unraveling these
mechanisms is of great significance because they may lead to novel
preventive or therapeutic paradigms.
In this study, we explored the potential involvement
of long non-coding RNAs (lncRNAs) in crosstalk between lung tumor
cell derived exosomes and MSCs. We performed a comprehensive lncRNA
and mRNA profiling through microarray. lncRNAs (9.1%) (2775 out of
30586) and 9.3% of protein-coding mRNA (2439 out of 26109) were
differentially expressed (fold-change ≥2; P-value ≤0.05) in lung
tumor derived exosome stimulated MSCs. Furthermore, we
characterized the differentially expressed lncRNAs through their
classes and length distribution and correlated them with
differentially expressed mRNA. Of note, GO analysis of biological
process showed that upregulated mRNAs were enriched in mRNA
metabolic process, while downregulated ones were enriched in
detection of mechanical stimulus involved in sensory perception.
Pathway analysis indicated that 32 pathways were upregulated while
7 were down-regulated in A549 exosome treated MSCs. To the best of
our knowledge, this is the first study that gives a comprehensive
overview of the lncRNA transcriptome changes in MSCs after
stimulation with tumor derived exosomes, which will bring new
insights into the mechanisms underlying interactions between tumor
cells exosomes and its environmental component the MSCs.
Materials and methods
Cell culture
Adipose tissues were obtained from patients
undergoing liposuction according to procedures approved by the
Ethics Committee at the Chinese Academy of Medical Sciences and
Peking Union Medical College. MSCs were isolated and
culture-expanded as previously reported (17). Passage 3 cells were used for
following experiments. Lung cancer cell line A549 was purchased
from cell bank at the Chinese Academy of Medical Sciences. A549
cells were cultured in DF12 containing 10% fetal bovine serum
(FBS), penicillin (100 U/ml) and streptomycin (100 lg/ml) at 37°C
in humidified air with 5% CO2.
Exosome extraction
Exosome extraction was performed as previously
described (18). Briefly, A549
culture medium was collected and centrifuged at 800 × g for 5 min
and additional 2,000 × g for 10 min to remove lifted cells. The
supernatant was subjected to filtration on a 0.1-mm pore
polyethersulfone membrane filter (Corning) to remove cell debris
and large vesicles, followed by concentration by a 100,000 MW
cut-off membrane (CentriPlus-70, Millipore). The volume of
supernatant was reduced from approximately 250–500 ml to
approximately 30 ml. The supernatant was then ultracentrifuged at
100,000 × g for 1 h at 4°C using 70Ti rotor (Beckman Coulter). The
resulting pellets were resuspended in 6 ml PBS and ultracentrifuged
at 100,000 × g for 1 h at 4°C using 100Ti rotor (Beckman
Coulter).
PKH67-labeled exosomes to AD-MSCs
Purified A549-exosomes were labeled with 1 μM Dil
(Invitrogen) as previously described (19). Pelleted exosomes were washed to
remove unbound PKH67, resuspended in PBS/5% BSA and then added to
AD-MSC medium for 4 h. AD-MSCs were then washed in PBS, fixed in 4%
paraformaldehyde, and imaged by microscopy.
Transmission electron microscopy
Purified exosomes were fixed with 1% glutaraldehyde
in PBS (pH 7.4). After rinsing, a 20 μl drop of the suspension was
loaded onto a formvar/carbon-coated grid, negatively stained with
3% (w/v) aqueous phosphotungstic acid for 1 min, and observed by
transmission electron microscopy.
Microarray and data analysis
Microarray and data analysis were performed by
KangChen Biotechnology, Shanghai, China. The company used Arraystar
Human LncRNA Microarray V3.0 which is designed for the global
profiling of human lncRNAs and protein-coding transcripts.
Approximately 30,586 lncRNAs and 26,109 coding transcripts can be
detected by this third-generation lncRNA microarray.
RNA labeling and array hybridization: sample
labeling and array hybridization were performed according to the
Agilent One-Color Microarray-Based Gene Expression Analysis
protocol (Agilent Technology) with minor modifications. Briefly,
mRNA was purified from total RNA after removal of rRNA (mRNA-ONLY™
Eukaryotic mRNA Isolation kit, Epicentre). Then, each sample was
amplified and transcribed into fluorescent cRNA along the entire
length of the transcripts without 3′ bias utilizing a random
priming method (Arraystar Flash RNA Labeling kit, Arraystar).
The labeled cRNAs were purified by RNeasy Mini kit
(Qiagen). The concentration and specific activity of the labeled
cRNAs (pmol Cy3/μg cRNA) were measured by NanoDrop ND-1000. One
microgram of each labeled cRNA was fragmented by adding 5 μl 10X
Blocking Agent and 1 μl of 25X Fragmentation Buffer, then heated
the mixture at 60°C for 30 min, finally 25 μl 2X GE Hybridization
buffer was added to dilute the labeled cRNA. Hybridization solution
(50 μl) was dispensed into the gasket slide and assembled to the
LncRNA expression microarray slide. The slides were incubated for
17 h at 65°C in an Agilent Hybridization Oven. The hybridized
arrays were washed, fixed and scanned with using the Agilent DNA
Microarray Scanner (part number G2505C).
Data analysis: slides were scanned at 5 lm/pixel
resolution using an Axon GenePix 4000B scanner (Molecular Devices
Corp.) piloted by GenePix Pro 6.0 software (Axon). Scanned images
(TIFF format) were then imported into NimbleScan software (version
2.5) for grid alignment and expression data analysis. Expression
data were normalized through quantile normalization and the Robust
Multichip Average (RMA) algorithm included in the NimbleScan
software. The probe level files and mRNA level files were generated
after normalization. All gene level files were imported into
Agilent GeneSpring GX software (version 11.5.1) and normalized by
the quantile method; then, Combat software was used to adjust the
normalized intensity to remove batch effects. Hierarchical
clustering was performed using Agilent GeneSpring GX software
(version 11.5.1). Agilent Feature Extraction software (version
11.0.1.1) was used to analyze acquired array images.
Results
Characterization of lung tumor cell
A549-derived exosomes
Exosomes were isolated from the culture supernatants
of lung tumor cell line A549 through a series of centrifugation and
filtration steps. Fig. 1A shows
the morphology of A549 cells under a light microscope. The exosome
concentration in A549 culture medium was 21.2±3.2 μg/ml. Under
transmission electron microscopy, the exosomes were observed to be
round vesicles of approximately 30–100 nm in size (Fig. 1B). CD63 and HSP70, typical protein
markers of exosomes, were detectable in the A549-derived exosomes
(Fig. 1C). To examine whether
A549-exosomes could be transferred into AD-MSCs, the exosomes were
fluorescently labeled with PKH67 and incubated with MSCs for 4 h.
After treatment, over 80% of the MSCs cells exhibited green
fluorescence (Fig. 1D).
A549-derived exosomes inhibited
osteogenic and adipogenic differentiation of AD-MSCs
To investigate the biological function of A549
exosomes on adipogenic and osteogenic differentiation of AD-MSCs,
200 μg/ml A549-derived exosomes were added into AD-MSC culture
medium. AD-MSCs treated with A549 exosomes were then cultured in
osteogenic induction medium or adipogenic induction medium. A549
exosome treatment inhibited osteogenic differentiation, which was
indicated by the decrease of ALP activity (Fig. 2A), as well as decreased mineral
deposition detected by Alizarin red staining (Fig. 2B). Moreover, lower mRNA expression
levels of osteo-specific markers were detected in exosome treated
AD-MSCs (Fig. 2D). According to
the results of Oil red O staining by day 10 of differentiation, the
accumulation of lipid droplets decreased significantly after
treatment with A549 exosomes (Fig.
2C). In addition, mRNA expression levels of adipogenic
transcription factors and adipocyte-specific markers PPARγ and LPL
decreased remarkably in cells treated with A549 exosomes compared
with control group (Fig. 2E). The
results above suggested that A549 acted as a negative regulator in
osteogenic and adipogenic differentiation of AD-MSCs.
A549-derived exosomes did not induce
fibroblastic differentiation of AD-MSCs
To determine whether or not lung tumor-derived
exosomes could contribute to generation of TAFs from MSCs, we
treated AD-MSCs with A549-derived exosomes. MSCs were cultured in
medium containing 200 μg/ml A549-derived exosomes for 6 days. No
morphological changes such as elongated cellular processes were
observed (Fig. 3A). Additionally,
we tested the expression of myofibroblastic/fibroblastic markers
such as α-SMA and FAP. FAP was increased in MSCs incubated with
A549-derived exosomes compared to controls incubated in medium
without exosomes after 6 days (Fig.
3B), but expression of α-SMA was not changed. These results
suggested that A549-derived exosomes did not induce fibroblastic
differentiation of AD-MSCs, at least within 6 days.
Overview of lncRNA profiles in A549
exosome-treated ADMSCs and control ADMSCs
To examine the lncRNA expression profiles in AD-MSCs
treated with or without A549 exosomes, we used Arraystar Human
LncRNA Microarray V3.0 which contains 30,586 lncRNA probes
collected from Ref Seq, UCSC known genes and Gencode and 26,109
mRNA probes. Total RNA was extracted and examined for quality
control before array. The OD260/OD280 ratios were approximately
2.0, and the OD260/OD230 ratios were >1.8. The overview of
lncRNA expression profiles is summarized in Table I and Fig. 4A and B. Overall, we found that 9.1%
of lncRNAs (2775 out of 30586) and 9.3% of protein-coding mRNA
(2439 out of 26109) were differentially expressed (fold-change ≥2;
P-value ≤0.05) between A549 exosome treated AD-MSCs and control
AD-MSCs.
 | Table ISummary of microarray analysis
results. |
Table I
Summary of microarray analysis
results.
| Probe class | Total | Differentially
expressed (fold change ≥2) |
|---|
| LncRNA | 30586 | 2775 (9.1%) |
| mRNA | 26109 | 2439 (9.3%) |
| Combined | 56695 | 5214 (9.2%) |
Fig. 4C shows the
hierarchical cluster of lncRNAs expression between A549 exosome
treated AD-MSCs and control AD-MSCs. Among the 2775 differentially
expressed lncRNAs, 1263 lncRNAs were upregulated in experimental
group compared to the control group, while 1512 lncRNAs were
downregulated. The top 10 up- and down-regulated lncRNAs in A549
exosome treated AD-MSCs compared to control AD-MSCs are shown in
Fig. 4D and Table II. We classified these
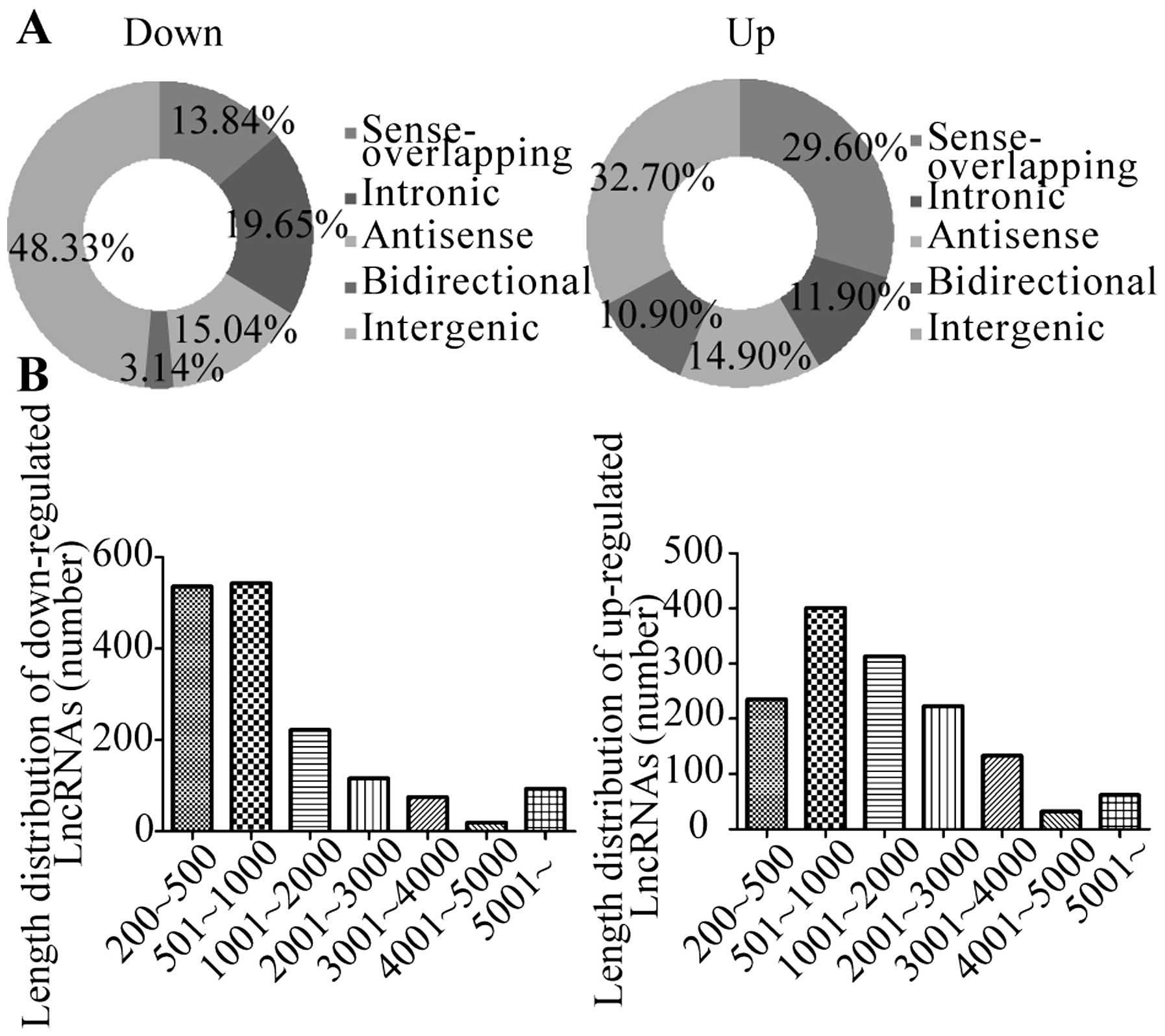
differentially expressed lncRNAs into 5 groups:
‘sense-overlapping’, the lncRNA exon is overlapping a coding
transcript exon on the same genomic strand; ‘intronic’, the lncRNA
is overlapping the intron of a coding transcript on the same
genomic strand; ‘antisense’, the lncRNA is transcribed from the
antisense strand; ‘bidirectional’, the lncRNA is oriented head to
head to a coding transcript within 1000 bp; ‘intergenic’: there are
no overlapping or bidirectional coding transcripts near the
lncRNA.
 | Table IIThe top 10 up- and down-regulated
lncRNAs in A549 exosome treated AD-MSCs versus control AD-MSCs. |
Table II
The top 10 up- and down-regulated
lncRNAs in A549 exosome treated AD-MSCs versus control AD-MSCs.
| Top 10 lncRNAs | Chromosomal
localization | RNA length | Start locus | Start locus | Associated gene
name | Relationship |
|---|
| Upregulated |
| HMlincRNA1636+ | chrx | 8519 | 68199500 | 68208019 | | Intergenic |
| NR_046464 | chr14 | 1687 | 101292444 | 101327360 | | Intergenic |
| NR_052024 | chr20 | 803 | 33866708 | 33872520 | EIF6 |
Sense-overlapping |
| ENST00000420309 | chr2 | 573 | 70223961 | 70313407 | | Intergenic |
| NR_046466 | chr14 | 1506 | 101292444 | 101327360 | | Intergenic |
| NR_045370 | chr20 | 4760 | 62507483 | 62512243 | TPD52L2 |
Sense-overlapping |
| NR_024596 | chr11 | 1129 | 86014397 | 86056985 | C11orf73 |
Sense-overlapping |
| ENST00000520714 | chr14 | 1351 | 101292454 | 101311828 | | Intergenic |
| uc010hbj.3 | chr22 | 1172 | 51222224 | 51238065 | RABL2B | Bidirectional |
| ENST00000440436 | chr10 | 480 | 6067940 | 6078390 | IL2RA | Antisense |
| Downregulated |
|
ENST00000428453 | chr15 | 4383 | 20588367 | 20711414 | | Intergenic |
|
ENST00000426501 | chr15 | 2874 | 20587868 | 20659133 | | Intergenic |
|
TCONS_00006633 | chr3 | 2229 | 125984828 | 125994041 | | Intergenic |
|
ENST00000440714 | chr21 | 292 | 40400460 | 40401053 | | Intergenic |
| uc010ciy.1 | chr16 | 2079 | 89978911 | 89981576 | RP11-566K11.2 |
Sense-overlapping |
| NR_048550 | chr1 | 1604 | 218066241 | 218094146 | DSCR3 | Intergenic |
| uc011aef.2 | chr21 | 458 | 38580954 | 38592893 | MSH2 | Antisense |
| uc002rwa.2 | chr2 | 2000 | 47713159 | 47715691 | CPA3 | Antisense |
|
ENST00000488190 | chr3 | 1414 | 148568719 | 148677899 | | Antisense |
| CB112975 | chr13 | 379 | 30229248 | 30229615 | | Intergenic |
Fig. 5A shows the
distribution of the five classes of lncRNAs with changed expression
in A549 exosome treated AD-MSCs. The lncRNAs are mainly between 200
and 3000 bp in length. Fig. 5B
shows the length distribution of differentially expressed lncRNAs.
The majority of the differentially expressed lncRNAs have a length
between 500 and 1000 bp.
GO and pathway analysis of differentially
expressed genes in AD-MSCs treated with A549 exosomes
The microarray also detected 2439 differentially
expressed mRNA (absolute fold-change ≥2; P-value ≤0.05). Among
them, 1940 mRNAs were upregulated in AD-MSCs treated with A549
exosomes compared to the control group, while 499 mRNAs were
downregulated. Fig. 6A showed the
hierarchical cluster of mRNA expression between A549 exosome
treated AD-MSCs and control AD-MSCs. Specifically, we picked out
the top 10 up- and down-regulated mRNAs. (Fig. 6B). Moreover, we performed GO
analysis to determine the gene and gene product enrichment in
biological processes, cellular components and molecular functions.
We found that the highest enriched GOs targeted by upregulated
mRNAs in A549 exosome treated AD-MSCs were mRNA metabolic process
(ontology: biological process) (Fig.
7A), intracellular part (ontology: cellular component)
(Fig. 7B) and structural
constituent of ribosome (ontology: molecular function) (Fig. 7C). The highest enriched GOs
targeted by the downregulated transcripts in A549 exosome treated
AD-MSCs were detection of mechanical stimulus involved in sensory
perception (ontology: biological process) (Fig. 7D), primary cilium (ontology:
cellular component) (Fig. 7E) and
acetylgalactosaminyltransferase activity (ontology: molecular
function) (Fig. 7F). Pathway
analysis indicated that 32 pathways were upregulated and 7 were
downregulated in A549 exosome treated MSCs. Fig. 7G and H show the top 10 of the
upregulated and 7 of the down-regulated pathways, respectively.
Discussion
Interactions between tumor cells and other cell
components in the tumor microenvironment play profound roles in
driving tumor initiation and progression. Recently, accumulating
evidence provides clues for the involvement of exosomes in
mediating such interactions (20–22).
Tumor derived exosomes usually carry tumor antigens which make them
useful as powerful anticancer vaccines (23). They also play active roles in
various biological behaviors of tumor cells such as immune
modulation and drug resistance. For instance, breast cancer-derived
exosomes are capable of inducing an inflammatory response in
macrophages, which may ultimately result in an enhanced rate of
metastatic tumor development (24). Xiao et al found A549 cells
exosomes are involved in the decrease of the sensitivity of A549
cells to DDP (25).
Tumor exosomes cause a myriad of biological changes
in target cells with the transfer of mRNAs or proteins. Our study
demonstrated that lung tumor cell A549 derived exosomes could
inhibit osteogenic and adipogenic differentiation of AD-MSCs. MSCs
may support tumor propagation or dissemination by preventing
recognition of the tumor cells by the immune system or by promoting
tumor cell invasiveness (26,27).
Cross-talk between MSCs within tumor stroma and cancer cells has
been identified to contribute to tumor progression and metastasis
through stromal formation or modulation of cell proliferation.
Recent studies demonstrated that tumor-derived exosomes can
function in mediating such cross-talk between MSCs and cancer
cells. However, the precise mechanisms by which tumor exosomes
affect MSCs remain largely unknown.
Specifically, whether epigenetic regulators such as
long non-coding RNA are involved in this process remains unclear.
Long non-coding RNAs have been established to participate in
various biological processes that are crucial for development and
differentiation. Although the vast majority of their functions
remain unexplored, there is evidence that some lncRNAs are involved
in regulating stem cell properties. Herein, a major focus of our
study was to define the repertoire of lncRNAs in tumor exosome
treated AD-MSCs.
To our knowledge, this is the first study to give a
comprehensive overview of the lncRNA transcriptome changes in MSCs
after stimulation with tumor derived exosomes. We found 9.1% of
lncRNAs (2775 out of 30586) and 9.3% of protein-coding mRNA (2439
out of 26109) were differentially expressed (fold-change ≥2;
P-value ≤0.05) in lung tumor cell exosome treated MSCs.
Furthermore, we characterized the differentially expressed lncRNAs
through their classes and length distribution and correlated them
with differentially expressed mRNA. Noteworthy, GO analysis of
biological process showed that upregulated mRNAs were enriched in
mRNA metabolic process, while downregulated ones were enriched in
detection of mechanical stimulus involved in sensory perception.
Pathway analysis indicated that 32 pathways were upregulated while
7 were downregulated in A549 exosome treated MSCs. These results
suggest that tumor exosomes could stimulate MSCs into an active
state.
In summary, our study is the first to demonstrate
that a set of lncRNAs is significantly regulated in AD-MSCs upon
treatment with tumor derived exosomes, suggesting a role of lncRNAs
in the regulation of AD-MSCs. This will provide some new insights
into the involvement of lncRNAs in exosome-mediated crosstalk
between MSCs and tumor cells, which is a novel mechanism whereby
tumor cells educate MSCs to modulate the tumor
microenvironment.
Acknowledgements
This study was supported by grants from National
Natural Science Foundation of China (no. 30830052, 30700321,
30800429) and Program for Cheung Kong Scholars and Innovative
Research Team in University-PCSIRT (no. IRT0909).
References
|
1
|
Quante M, Tu SP, Tomita H, Gonda T, Wang
SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhowmick NA and Moses HL: Tumor-stroma
interactions. Curr Opin Genet Dev. 15:97–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pietras K and Ostman A: Hallmarks of
cancer: Interactions with the tumor stroma. Exp Cell Res.
316:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu
J, Cao W, Han C and Chen Y: Mesenchymal stem cells derived from
bone marrow favor tumor cell growth in vivo. Exp Mol Pathol.
80:267–274. 2006. View Article : Google Scholar
|
|
6
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roorda BD, ter Elst A, Kamps WA and de
Bont ES: Bone marrow-derived cells and tumor growth: Contribution
of bone marrow-derived cells to tumor micro-environments with
special focus on mesenchymal stem cells. Crit Rev Oncol Hematol.
69:187–198. 2009. View Article : Google Scholar
|
|
9
|
Ye J, Wu D, Wu P, Chen Z and Huang J: The
cancer stem cell niche: Cross talk between cancer stem cells and
their microenvironment. Tumour Biol. 35:3945–3951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egeblad M, Nakasone ES and Werb Z: Tumors
as organs: Complex tissues that interface with the entire organism.
Dev Cell. 18:884–901. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grange C, Tapparo M, Collino F, Vitillo L,
Damasco C, Deregibus MC, Tetta C, Bussolati B and Camussi G:
Microvesicles released from human renal cancer stem cells stimulate
angiogenesis and formation of lung premetastatic niche. Cancer Res.
71:5346–5356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen WX, Cai YQ, Lv MM, Chen L, Zhong SL,
Ma TF, Zhao JH and Tang JH: Exosomes from docetaxel-resistant
breast cancer cells alter chemosensitivity by delivering microRNAs.
Tumour Biol. 35:9649–9659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iero M, Valenti R, Huber V, Filipazzi P,
Parmiani G, Fais S and Rivoltini L: Tumour-released exosomes and
their implications in cancer immunity. Cell Death Differ. 15:80–88.
2008. View Article : Google Scholar
|
|
14
|
Chowdhury R, Webber JP, Gurney M, Mason
MD, Tabi Z and Clayton A: Cancer exosomes trigger mesenchymal stem
cell differentiation into pro-angiogenic and pro-invasive
myofibroblasts. Oncotarget. 6:715–731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haga H, Yan IK, Takahashi K, Wood J,
Zubair A and Patel T: Tumour cell-derived extracellular vesicles
interact with mesenchymal stem cells to modulate the
microenvironment and enhance cholangiocarcinoma growth. J Extracell
Vesicles. 4:249002015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Bucan V, Baehre H, von der Ohe J,
Otte A and Hass R: Acquisition of new tumor cell properties by
MSC-derived exosomes. Int J Oncol. 47:244–252. 2015.PubMed/NCBI
|
|
17
|
Cao Y, Sun Z, Liao L, Meng Y, Han Q and
Zhao RC: Human adipose tissue-derived stem cells differentiate into
endothelial cells in vitro and improve postnatal neovascularization
in vivo. Biochem Biophys Res Commun. 332:370–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohshima K, Inoue K, Fujiwara A, Hatakeyama
K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi
K, et al: Let-7 microRNA family is selectively secreted into the
extracellular environment via exosomes in a metastatic gastric
cancer cell line. PLoS One. 5:e132472010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hood JL, Pan H, Lanza GM and Wickline SA:
Consortium for Translational Research in Advanced Imaging and
Nanomedicine (C-TRAIN): Paracrine induction of endothelium by tumor
exosomes. Lab Invest. 89:1317–1328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y,
Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et
al: Exosome transfer from stromal to breast cancer cells regulates
therapy resistance pathways. Cell. 159:499–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Yuan X, Shi H, Wu L, Qian H and
Xu W: Exosomes in cancer: Small particle, big player. J Hematol
Oncol. 8:832015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang MK and Wong AS: Exosomes: Emerging
biomarkers and targets for ovarian cancer. Cancer Lett. 367:26–33.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS,
Ko JK, Koh JS, Kim YN and Kim CW: Exosomes: A new delivery system
for tumor antigens in cancer immunotherapy. Int J Cancer.
114:613–622. 2005. View Article : Google Scholar
|
|
24
|
Chow A, Zhou W, Liu L, Fong MY, Champer J,
Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, et al: Macrophage
immunomodulation by breast cancer-derived exosomes requires
Toll-like receptor 2-mediated activation of NF-κB. Sci Rep.
4:57502014. View Article : Google Scholar
|
|
25
|
Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu
Y and Feng J: Exosomes: Decreased sensitivity of lung cancer A549
cells to cisplatin. PLoS One. 9:e895342014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohno S, Tachibana M, Fujii T, Ueda S,
Kubota H and Nagasue N: Role of stromal collagen in
immunomodulation and prognosis of advanced gastric carcinoma. Int J
Cancer. 97:770–774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keleg S, Büchler P, Ludwig R, Büchler MW
and Friess H: Invasion and metastasis in pancreatic cancer. Mol
Cancer. 2:142003. View Article : Google Scholar : PubMed/NCBI
|