Introduction
The proper stimulation of the immune system and
targeting action require the application of various forms of
therapy including cytostatic drug therapy. Cyclophosphamide (CY) is
a well-characterized DNA-alkylating agent, widely used in the
treatment of malignancies (e.g., breast, ovarian, small cell lung
cancer and leukemias). Both the tumor-eradicating and
immunomodulating effects of CY are dose-dependent. At high doses,
CY induces mainly a cytotoxic effect, whereas at low doses it
stimulates the differentiation of effector CD4+ T cells
and inhibits the regulatory T (Treg) cell activity (1,2). The
influence of CY on the promotion of T helper (Th)1 cell activity
has been confirmed in rodent tumor models (3). This was demonstrated by an increase
in the production of cytokines specific for Th1 (mainly IL-2 and
IFN-γ) but a decrease in the secretion of IL-10 (4).
In order to improve the effectiveness of the
treatment with cytostatics, attempts are made to combine
chemotherapy with immunotherapy. The reasonable forms of the latter
are dendritic cell (DC)-based vaccines. In some animal models and
early clinical trials, the DC-based vaccines were used for
stimulation of immune response and for improving the antitumor
effect initiated by CY administration. Such therapy affected the
inhibition of Treg cells and the increase of cellular cytotoxicity
against tumor cells. Furthermore, it prolonged the survival of
colorectal cancer-bearing mice as well as stimulated the production
of IFN-γ by lymphocytes derived from these animals (5,6).
It is well known that DCs influence the
differentiation, migration and activation of CD4+ T
cells using cell-to-cell contact and cytokine production. For
antitumor immunotherapy based on DCs, various strategies of antigen
loading have been proposed (including whole tumor-cell lysates). It
is believed that the simultaneous cytokine use can support DC
maturation. Balkow and coworkers reported (7) that DC matured by anti-CD40 monoclonal
antibodies in the presence of IL-12 and IL-18 exhibited potent
activity of these cells against growing tumors. Brunner et
al (8) showed that DC
stimulated with tumor antigens and TNF-α, expressed the MHC class
II, CD80 and CD86 molecules at higher level than cells stimulated
only with tumor antigens. However, complete in vitro
maturation of DCs causes the high expression of MHC class II
antigens and costimulatory molecules, but the application in
vivo of fully matured DCs has led to decrease in DC-mediated
T-cell activation (9). Thus,
various levels of activation of antigen-specific T cells during the
formation of antitumor response can result from diverse maturity of
the DC contained in vaccines. The use of different viruses as
carriers of antigenic protein genes has also been reported
(10). Several lines of evidence
indicate that genetically modified DC involved in cellular vaccines
are capable of triggering a long-lasting tumor growth delay along
with an increase in the number of cytotoxic T cells as well as
cytokine-producing lymphocytes. Genetic modifications of DCs for
expressing cytokine genes (e.g., interleukin 2) (IL-2) may enhance
their activity (11). However, the
effectiveness of the clinical protocols employing various types of
DC-based vaccines is still unsatisfactory and needs further
investigation. DCs are believed to stimulate naive CD4+
T cells which are a key element of numerous immune mechanisms. Th1
cell subpopulation containing the IFN-γ-producing cells supports
cellular immunity; IL-4-producing cells representing the Th2 cell
subset is associated with humoral immunity. The Th17 cells,
secreting IL-17A and IL-17F, are responsible for pro-inflammatory
activity. The Treg cells play a critical role in active suppression
of immune response and are believed to be the main subpopulation of
cells able to secrete IL-10.
Many experimental and clinical results confirm that
the presence of CD4+ T cells is required during
development of antitumor response, and their infiltration into the
tumor tissue can connote a good prognosis in many types of cancers.
However, based on the type of tumor tissue and cytokine environment
the migration and activation of different subpopulation of
CD4+ T cells can be observed.
There is still only limited evidence demonstrating
the immune mechanisms responsible for the effect of the combined CY
and DC-vaccine therapy on differentiation of T cells involved in
the response against growing tumor. For this reason, the aim of the
present study is to elucidate whether the various types of DC-based
vaccines applied after CY administration caused diversity in
CD4+ T cell subpopulations directly related with the
inhibition of murine MC38 colon cancer growth. This was achieved by
analyzing the changes in CD4+ lymphocyte infiltration
into tumor tissue, ability of these cells to express T-bet, GATA3,
RORγt and FoxP3 transcription factors and to produce specific
cytokines. The alteration in systemic response was represented by
trends in splenic reactivity: cytokine secretion and diversity in
transcription factor expression. The applied treatment resulted in
the increase in the number of Th1 and Th2 cells followed by
time-dependent activation of CD8+ cells and a decrease
in the number of Th17 and Treg lymphocytes.
The observed alteration in the ration of
CD4+ T cell subpopulations may have a great value as a
prognostic factor and be the basis for future development of
anticancer therapy.
Materials and methods
Animals
Female C57BL/6 mice were obtained from the Center
for Experimental Medicine of the Medical University of Bialystok
(Bialystok, Poland). Mice were housed under specific pathogen-free
conditions, and were transferred to a conventional environment two
weeks before the experiment. All animal experiments were approved
by the Local Ethics Committee.
Preparation of dendritic cell-based
vaccines
Dendritic cells generated from bone marrow of
healthy mice were cultured for 8 days in RPMI-1640 medium (Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
Sigma-Aldrich, Seelze, Germany) and 40 ng/ml GM-CSF (Invitrogen,
Carlsbad, CA, USA) and 10 ng/ml IL-4 (ImmunoTools, Friesoythe,
Germany). On the seventh day, cells were activated with MC38 tumor
cell lysate (10% v/v) and stimulated with 50 ng/ml TNF-α
(Invitrogen) (BMDC/TAgTNF-α). Dendritic cells of JAWS II
cell line (CRL-11904) (obtained from ATCC, Manassas, VA, USA)
transduced with retroviral vector carrying mIL-2 gene (JAWS
II/IL-2 cells) or neomycin resistance gene (JAWS II/Neo, used as a
control of transduction) were maintained in RPMI-1640 and αMEM
(Gibco) (1:1) culture medium supplemented with 10% FBS and 5 ng/ml
GM-CSF (11).
BMDC/TAgTNF-α alone or with genetically modified JAWS II
cells were used as antitumor vaccines.
For cell characterization, cell surface molecule
expression and cytokine production were analyzed. DCs were stained
with anti-CD80 (conjugated with fluorochrome APC; clone 16-10A1,
hamster anti-mouse), anti-CD86 (PE-Cy7; clone GL1, rat anti-mouse),
and anti-I-Ab (FITC; clone 25-9-17, mouse anti-mouse)
monoclonal antibodies (all from BD Biosciences, San Jose, CA, USA).
The expression of molecules was measured by FACSCalibur flow
cytometer (BD Biosciences) and analyzed by Flowing software 2.5.1.
The concentrations of IL-2, IL-12, IL-6 and IL-10 cytokines in 48 h
cell culture supernatants were measured with commercially available
ELISA kits (BD Biosciences) according to the manufacturer's
instructions.
Mouse treatment schedule
Eight- to ten-week-old C57BL/6 mice were inoculated
subcutaneously (s.c.), into right flank, with MC38/0 cells
(1×106/0.2 ml/mouse). After 14 days, the MC38 colon
carcinoma-bearing mice were treated intraperitoneally (i.p.) with
CY (Baxter, Deerfield, IL, USA) (150 mg/kg body weight). On days
17, 24 and 31, mice were administered peritumorally (p.t.)
BMDC/TAgTNF-α, JAWS II/IL-2, JAWS II/Neo cells
(1.5×106 cells/mouse), or their combination
(1×106 BMDC/TAgTNF-α + 0.5×106
JAWS II cells/mouse). Twice a week tumor growth was assessed
morphometrically using electronic calipers, and tumor volumes were
calculated according to the formula: (α2 × b) ÷ 2, where
a is the shorter and b is a longer tumor diameter. Spleen and tumor
tissue were collected on day 31, 38 and 45 of the experiment. Mice
were monitored and the median of tumor volume were estimated.
The values of two parameters were calculated: ΔTRV,
the difference in the median time required for the tumor to reach a
volume of 1 cm3 compared to the group of untreated mice
and TGI, tumor growth inhibition. The TGI was used to demonstrate
the percentage of the tumor volume inhibition calculated for groups
of treated mice compared to the untreated mice (TGI1) or
to the CY-treated control (TGI2).
Lymphocytes in spleen and tumor
tissue
Single cell suspension was prepared from tumor
nodules or spleens. Cells were incubated with anti-CD45 (PE-Cy7,
clone 30-F11) and anti-CD4 (APC, clone RM4-5) monoclonal antibodies
(mAbs) or anti-CD8 (FITC, clone 53–6.7) (all rat anti-mouse and all
from BD Biosciences), and analyzed by flow cytometer FACSCalibur
(BD Biosciences) and Flowing software 2.5.1.
Lymphocyte stimulation and intracellular
staining
Splenocytes or CD4+ cells isolated from
tumor tissue by anti-CD4 antibody-coated magnetic beads (BD IMag™),
were cultured in RPMI supplemented with 10% FBS and 0.5 μg/ml
concanavalin A (Con A; Sigma-Aldrich). Cell culture supernatants
were used for estimation of cytokine production by stimulated cells
(ELISA). For cytokine and transcription factor expression analysis,
brefeldin A (10 μg/ml) and monensin (2 μM) (both from eBiosciences,
San Diego, CA, USA) were added to the cell culture for the last 6
h. Cells were stained with anti-CD4 antibody (FITC, clone RM4-5),
fixed and incubated with anti-mouse CD16/CD32 mAb, and stained for
cytokine and/or transcription factor expression with the
intracellular staining kit from eBiosciences. The following
fluorochrome-conjugated mAbs were used: anti-T-bet (PE-Cy 7, clone
4B10), anti-GATA3 (PE, clone TWAJ), anti-RORγt (PE, clone AFKJS-9),
anti-FoxP3 (APC or PE, clone FJK-16s), or their appropriate isotype
control (all anti-human/mouse, all from eBiosciences). Flow
cytometry was performed using FACSCalibur cytometer (BD
Biosciences). All data were analyzed with Flowing software version
2.5.
Cytokine production
IFN-γ, IL-4, IL-10 (BD Biosciences) and IL-17A
(eBiosciences), and production by splenocytes or CD4+
lymphocytes isolated from tumor tissue was measured with
commercially available ELISA kits according to the manufacturer's
instructions.
Estimation of spleen cell
cytotoxicity
Spleen cells obtained from mice after
chemoimmunotherapy or from healthy mice, untreated MC38-bearing
mice and CY-treated MC38-bearing mice were isolated and co-cultured
(restimulated) for 4 days with mitomycin C-treated MC38 cells
(mitomycin C were obtainted from Sigma-Aldrich) (50 mg mitomycin
C/3×106 cells/ml/30 min at 37°C) in the presence of IL-2
as described (12). The source of
murine IL-2 was X63mIL-2 plasmacytoma cell supernatant used at 5%
concentration, corresponding to 100 U/ml. After 4 days of
restimulation the cytotoxicity of the spleen cells was tested by
flow cytometry. Target MC38/0 cells were stained with
DiOC18 lipophilic dye (Molecular Probes, Eugene, OR,
USA) for 30 min at 37°C and then washed with PBS. After
restimulation, spleen cells were incubated with labeled target
cells for 4 h at 37°C at a 10:1 ratio of effector to target cells.
The cells were then washed and dead cells were stained with
propidium iodide (PI; Molecular Probes). Samples were analyzed
using FACSCalibur flow cytometer and Flowing software 2.5.1.
Percentage of cytotoxicity was determined according to formula: the
percentage of dead target MC38/0 cells previously incubated with
splenocytes minus the percentage of dead MC38/0 cells cultured
alone.
Statistical analysis
Results are presented as the median (volume of tumor
nodules) or mean ± SD (all other results). Data were analyzed by
the non-parametric Mann-Whitney U test and Kruskal-Wallis ANOVA by
ranks (Statistica 10 software). The values of P<0.05 were
considered as statistically significant.
Results
Dendritic cells used for the preparation
of DC-based vaccines
The present study was carried out with the use of
two types of DCs: genetically modified cells of JAWS II line and
bone marrow-derived DCs. For the characterization of dendritic
cells, a surface antigen expression as well as cytokine production
were analyzed. JAWS II dendritic cells exhibited low expression of
co-stimulatory (CD80, CD86) and MHC class II molecules. JAWS II
cells modified to produce IL-2 (JAWS II/IL-2) revealed slight
increase in the expression of CD86 and MHC class II molecules
compared to parental JAWS II cells or JAWS II/Neo cells being a
control of transduction (Fig. 1),
making them able to present tumor antigens only marginally.
Interleukin 2 was only produced by JAWS II/IL-2 cells (for the
needs of the present study, 17.3 ng/ml). Whereas, IL-6 was produced
by JAWS II/Neo cells in higher amounts (18.9 ng/ml) than by
unmodified JAWS II (13.08 ng/ml) cells and JAWS II/IL-2 cells (4.08
ng/ml) (data not shown). JAWS II cells, in general, turned out to
be unable to produce IL-12 and IL-10. Thus, JAWS II/IL-2 cells
administered into mice, acted mainly as a source of IL-2 and only
to a small extent as the antigen-presenting DCs.
The bone marrow-derived DCs after ex vivo
stimulation with tumor antigens and TNF-α
(BM-DC/TAgTNF-α) were exploited for presentation of TAg.
These cells exhibited higher expression of costimulatory molecules
(CD80 and CD86) and MHC class II antigens compared to other groups
of BM-DC (Fig. 1). The
BM-DC/TAgTNF-α were able to produce IL-6 (19.1 ng/ml)
and small amounts of IL-12 (78 pg/ml), but did not secrete IL-10
and IL-2 (data not shown). Although expression of surface antigens
and secretion of proinflammatory cytokines increased
insignificantly, the BM-DC/TAgTNF-α were likely able to
activate T cells in host.
The use of DC-based vaccines improves the
antitumor CY effect
In order to analyze the effect of the CY treatment
followed by DC-based vaccines, the C57BL/6 mice bearing advanced
MC38 colon carcinoma were used. Fourteen days after MC38/0 cell
inoculation, when the tumors became palpable, the mice were treated
with single dose of CY (150 mg/kg body weight). Starting three days
later, cellular vaccines (1.5×106/mouse) were
administered three times, at weekly intervals, as is shown in
Fig. 2A. Vaccines consisted of
BM-DC/TAgTNF-α, JAWS II/IL-2, JAWS II/Neo cells, or
their combinations (BM-DC/TAgTNF-α + JAWS II/IL-2 cells,
BM-DC/TAgTNF-α + JAWS II/Neo cells with cell ratio 2:1).
Fig. 2 illustrates the therapeutic
effect on the tumor growth delay in one of the three independent
experiments (Fig. 2B). The data
were completed by statistical significance of the treatment
estimated on day 45 of the experiment (Fig. 2C). Using Kruskal-Wallis test as
well as Mann-Whitney U test, the statistically significant
differences were observed between group of mice obtaining CY and
groups treated with CY + DC/TAg (P=0.034) and/or JAWS II/IL-2 (CY
vs. CY + JAWS II/IL-2 at P=0.001; CY vs. CY + BM-DC/TAg + JAWS II/
IL-2 at P=0.0002). However, differences between CY + JAWS II/IL-2
cells and CY + BM-DC/TAg + JAWS II/IL-2 cell treatment proved to be
insignificant (P=0.78565).
The administration of CY (or CY + JAWS II/Neo cells)
induced tumor growth delay reaching ~10.4 days compared to
untreated group (Fig. 2D). Further
increase in the ΔTRV was observed after the use of CY with
BM-DC/TAgTNF-α or with BM-DC/TAgTNF-α + JAWS
II/Neo cells (12.7 days). However, the replacement of JAWS II/Neo
cells by JAWS II/IL-2 cells resulted in the increase of ΔTRV to
15.4 days, whereas, the application of BM-DC/TAgTNF-α +
JAWS II/IL-2 cells elicited an increase to 17 days.
The differences in TGI illustrate the influence of
DC-based vaccines on the final effect of the treatment. On the 31st
day of the experiments, the slight diversification among the groups
of mice was observed compared to untreated mice (TGI1
ranged from 84.1 to 89.9%), whereas TGI2 reached 37.9%,
but only in case of the mice receiving CY +
BM-DC/TAgTNF-α and transduced JAWS II cells. The
obtained result highlighted that ternary combinations were able to
hasten the antitumor response already in its early phase but the
significant effects of such therapy were found on the 45th day (14
days after the third vaccination). The differences between the
medians of tumor volumes calculated on this day proved to be
statistically significant, but the most intensive response against
the tumor was revealed after the use of CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cells (TGI2
amounted to 65.6%).
Changes in lymphocyte infiltration into
the tumor tissue generated by combined treatment
Taking into consideration the multidirectional
activity of the T cell subpopulations participating in tumor
antigen (TAg)-specific response, it was important to determine
whether the combined treatment affected the changes in
CD4+ or CD8+ T lymphocyte infiltration into
the MC38 tumor tissue. For this purpose, tumor nodules were
harvested from untreated mice on the 31st day of the experiments
and from mice of treated groups on the 31st, 38th and 45th day. We
determined a percentage of CD4+ cells among leukocytes
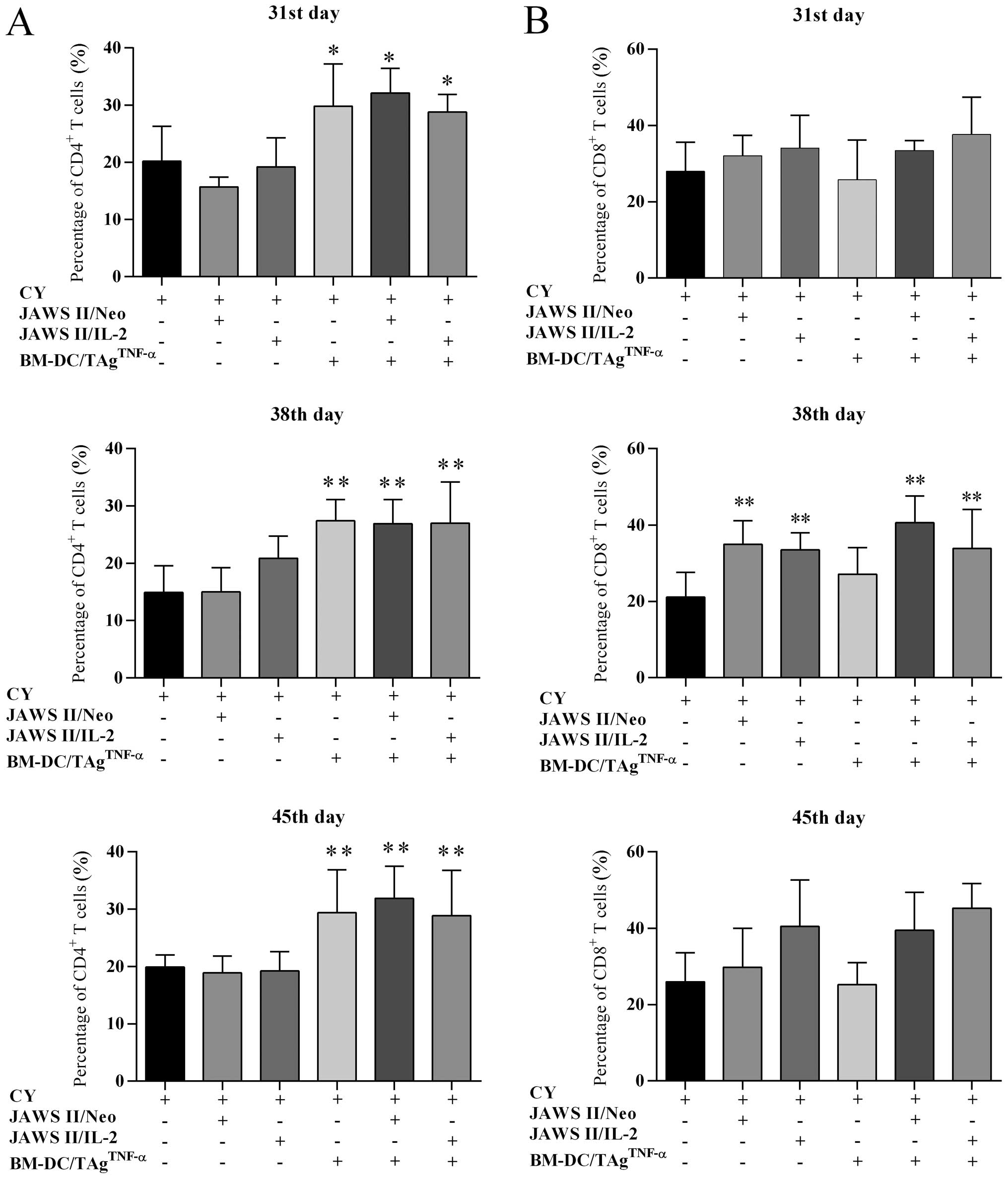
by flow cytometry (Fig. 3A). In
the case of CY-treated mice, the CD4+ cell percentage
among CD45+ leukocytes amounted to 20.2% (the 31st day),
14.9% (the 38th day) and 19.9% (the 45th day). The use of CY +
transduced JAWS II cells did not increase the percentage of
CD4+ T cells. Only after the replacement of JAWS II
cells by BM-DC/TAgTNF-α ± transduced JAWS II cells the
statistically significant growth in the percentage of infiltrating
CD4+ T cells compared to CY-treated control was noted
(Fig. 3A).
Changes in the percentage of CD8+ T tumor
infiltrating lymphocytes were dependent on the presence of
transduced JAWS II cells in DC-vaccines (Fig. 3B). Each day of the harvesting of
experiments, the lowest percentage of these lymphocytes in mice
treated with CY ± BM-DC/TAgTNF-α was found. Whereas, the
supplementation of cytostatic treatment by transduced JAWS II cells
resulted in the gradual increase of percentage of CD8+ T
cells. Although this process was dependent on duration of the
experiments, a statistically significant increase of the
CD8+ T cell percentage (at P<0.01, compared to
CY-treated control) was on the 38th day. However, the most
intensive growth of CD8+ T cell subpopulation was
observed on the 45th day of experiments after application of CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cells (45.2% of
CD45+ cells).
Overall, the application of CY followed by DC-based
vaccines resulted in a significant delay of MC38 tumor growth,
which was accompanied by considerable changes in CD4+
and CD8+ tumor infiltrating lymphocytes (TILs). The
intensity of the influx of TILs and their maintenance in tumor
tissue depended to a great extent on the nature of the cellular
vaccines; a CD4+ cell infiltration was associated with
the presence of BM-DC/TAgTNF-α in vaccines, whereas the
increase of CD8+ cell influx, with the presence of JAWS
II/IL-2 cells.
Influence of DC-based vaccines on
diversity of CD4+ cell subpopulations infiltrating the
tumor tissue
In the following part of the investigation, we
focused especially on the ability of CD4+ T cell
populations to trigger the antitumor response. For this purpose,
after magnetic isolation of cells from tumor tissue they were
stimulated with concanavalin A (Con A). Analysis of their activity
was based on cytokine secretion and changes in the percentage of
cells expressing transcription factors specific for main
CD4+ subpopulations (T-bet, GATA3, RORγt and FoxP3).
Between the first and last harvesting day of the
experiments, CD4+ T cells isolated from mice treated
with CY ± transduced JAWS II cells produced <0.13 ng/ml IFN-γ
(Fig. 4). On day 31, the
application of CY + BM-DC/TAgTNF-α caused a significant
increase of the production which amounted to 0.37 ng/ml, but the
highest amount of IFN-γ was released by CD4+ T cells
derived from mice treated with CY + BM-DC/TAgTNF-α +
JAWS II/IL-2 cells (0.45 ng/ml). In both cases, the differences in
cytokine production level were statistically significant compared
to CY-treated group. However, the largest alterations in the level
of IFN-γ production were found on the 45th day. Administration of
CY + BM-DC/TAgTNF-α + JAWS II/ Neo cells, caused
production of IFN-γ at a level of 1.0 ng/ml (P=0.015). Whereas, the
use of the CY + BM-DC/TAgTNF-α + JAWS II/IL-2 cells
induced the highest IFN-γ secretion amounting to 1.7 ng/ml (P=0.004
compared to CY-treated control).
The level of IL-4 production by CD4+ T
cells, likewise, was diversified in the vaccine- and time-dependent
manner. CD4+ T cells isolated from tumor tissue of mice
treated with CY or mice receiving CY + JAWS II/Neo cells, generally
produced less IL-4 than 0.03 ng/ml (Fig. 4). On the 38th day, the
administration of CY + JAWS II/IL-2 cells caused a significant
increase in the cytokine production. However, the treatment with CY
+ BM-DC/TAgTNF-α or with ternary combinations was more
potent for CD4+ T cell activation. Lymphocytes isolated
from mice treated with CY + BM-DC/TAgTNF-α + transduced
JAWS II cells on the 45th day produced a very similar amount of
cytokine. The diversity in the level of IL-4 production was
statistically significant (P<0.01 vs. CY-treated control). Of
note, regardless of the level of cytokine production, the treatment
with ternary combinations resulted in the long-lasting activity of
both IFN-γ- and IL-4-producing CD4+ T cells which in
further perspective could affect the polarization of immune
response towards Th1 or Th2.
According to the potential role of IL-10-producing T
regulatory cells in modulation of immune response against growing
colon cancer, we decided to determine the level of IL-10 production
by CD4+ T cells separated from tumor tissues. The cells
originated from control mice or mice treated with CY + JAWS II/Neo
cells produced <0.08 ng/ ml of IL-10 (Fig. 4). On day 31, only the application
of CY + BM-DC/TAgTNF-α + JAWS II/IL-2 cells caused a
statistically significant increase in the IL-10 production compared
to the CY-treated control (0.58 ng/ml, P=0.004). On the 38th day,
the amount of IL-10 significantly increased after application of
both ternary combinations and ranged from 0.9 to 1.85 ng/ ml (at
P<0.05 compared to CY-treated mice). On the 45th day, the
application of CY + BM-DC/TAgTNF-α + JAWS II/ IL-2 cells
caused the highest cytokine production by CD4+ TILs
(2.13 ng/ml, P=0.004). Thus, the secretion of IL-10 was
time-dependent, and related to the nature of cellular vaccines in a
similar manner to IFN-γ and IL-4 production. It was also associated
with the largest therapeutic effect.
The activity of the CD4+ T lymphocytes
after stimulation with Con A was confronted also with the
percentage of cells expressing transcription factors specific for
analyzed Th cell subpopulations (Fig.
5).
Tumor tissue from treated mice was infiltrated with
growing number of TILs. The percentage of
CD4+T-bet+ cells on the 31st day ranged from
8.0% (CY-treated control) and 17.7% (CY + BM-DC/TAgTNF-α
+ JAWS II/IL-2 cells), while on the 38th day reached 24.7% (CY +
BM-DC/TAgTNF-α) and 32.0% (CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cells) (data not shown).
However, the number of CD4+T-bet+ cells in
these groups decreased on the 45th day (Fig. 5A) and ranged between 12.3% (CY +
JAWS II/Neo cells) and 23.9% (CY + JAWS II/IL-2 cells), compared to
the CY-treated mice (29.7%). Only the treatment with CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cells resulted in
considerable increase in percentage of
CD4+T-bet+ lymphocytes (37.2%). As stated
previously, the differences in the size of
CD4+T-bet+ cell subpopulation were dependent
on time and the nature of vaccines.
The obtained results are essential in the light of
our assumption that the BM-DC/TAgTNF-α + JAWS II/IL-2
cells were involved in stimulation of Th1-type response which was
accompanied by the increase in IFN-γ production to mediate the
response polarization.
The second TIL-subpopulation was
CD4+GATA3+ cells. On the 38th day, the
percentage of CD4+GATA3+ cells was higher
than on 31st day of experiments and increased to 20.1% (CY +
BM-DC/TAgTNF-α) or 21.7% (BM-DC/TAgTNF-α +
JAWS II/IL-2 cells) (data not shown). On the 45th day (Fig. 5A), the size of
CD4+GATA3+ cell population increased to 30.2%
only in the case of mice treated with CY +
BM-DC/TAgTNF-α whereas the application of other types of
vaccines diminished or remained at constant the percentage of
CD4+GATA3+ cells. The strongest effect of
treatment with BM-DC/TAgTNF-α highlighted the
relationship between the nature of the vaccine and
CD4+GATA3+ cell population
differentiation.
On the 31st day of the experiments the percentage of
CD4+RORγt+ cells was relatively low and
ranged from 9.4% (for CY + JAWS II/IL-2 group) to 15.2% (for
CY-treated group). One week later (38th day) we observed ~2-fold
increase in all groups (from 18.3% for CY +
BM-DC/TAgTNF-α, 20.8% for CY + JAWS II/IL-2 group, to
30.8% for CY-treated group) (data not shown). The statistically
significant diversity in the number of
CD4+RORγt+ T cells was found only on the 45th
day (Fig. 5B). When mice were
treated with CY, the percentage of lymphocytes amounted to 29.7%.
The application of CY with transduced JAWS II cells or with
BM-DC/TAgTNF-α diminished the percentage to 24.7 or
27.7%, respectively. However, a significant decrease in percentage
of CD4+RORγt+ T cells was noted after the
application of CY + BM-DC/TAgTNF-α + JAWS II/IL-2 cells
(10.1%, P=0.004) or CY + BM-DC/TAgTNF-α + JAWS II/Neo
cells (9.1%, P=0.008), compared to CY-treated control. This
decrease of CD4+RORγt+ percentage was perhaps
associated with differentiation of cells during the Th1 or Th2
polarization of immune response at least in the case of CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cell application.
CD4+ T lymphocytes expressing FoxP3
transcription factor were the fourth analyzed subpopulation. On the
31st day as well as on the 38th day of the experiments in tumor
tissue derived from treated mice a gradual decrease in percentage
of CD4+FoxP3+ cells was noted (data not
shown). Especially, when mice were administered CY +
BM-DC/TAgTNF-α or CY + BM-DC/TAgTNF-α + JAWS
II/IL-2 cells, a reduction in the number of TILs (3.4 and 4.1%,
respectively) was observed compared to 9.5% for CY-treated control
(data not shown). On the 45th day (Fig. 5B), the application of CY +
BM-DC/TAgTNF-α ± transduced JAWS II cells caused
statistically significant decrease in the percentage of
CD4+FoxP3+ lymphocytes amounting to 2.0%
(P=0.004) or 2.9% (P=0.015) compared to CY-treated control. The
differences found in the percentage of
CD4+FoxP3+ cells turned out to be similar to
those observed for CD4+RORγt+ T cell
subpopulation. However, considering the decrease of
CD4+FoxP3+ cell percentage and the increase
of IL-10 production by CD4+ TILs, we postulate that
other subpopulations of CD4+ cells than
CD4+FoxP3+ cells are also able to produce
this cytokine during the long-lasting fight against tumor.
Changes in systemic antitumor response
generated by CY and DC-based vaccines
Firstly, we decided to analyze the effect of the
therapy on an alteration in size of two main spleen cell
populations. On the 31st day the percentage of CD4+
splenocytes obtained from treated mice ranged between 13.3% (CY +
BM-DC/TAgTNF-α + JAWS II/Neo cells) and 17.7% (CY +
BM-DC/TAgTNF-α) vs. 16.8% of CY-treated mice (Fig. 6A). A slight increase in the size of
CD4+ T cell population ranged from 17.9% (CY + JAWS
II/IL-2) to 21.8% (CY + BM-DC/TAgTNF-α) was observed on
the 38th day. On the 45th day of experiments, in most of the
treated groups the size diminished to 14.6% (CY + JAWS II/Neo
cells) or 16.9% (CY + BM-DC/TAgTNF-α), except from mice
treated with CY + JAWS II/IL-2 cells, in which the percentage of
CD4+ T splenocytes reached 20.6%. Thus, on consecutive
harvesting days, only slight differences, independent on the nature
of vaccines, could be observed. In contrast to CD4+ T
splenocytes, changes in CD8+ cell percentage depended to
a great extent on the vaccines (Fig.
6B). On the 31st day, percentage of CD8+ T cell
subpopulation obtained from treated mice ranged between 17.9% (CY +
BM-DC/TAgTNF-α or CY + JAWS II/IL-2 cells) and 19.2% (CY
+ JAWS II/Neo cells). However, on the next harvesting day, the
percentage of these splenocytes diminished after treatment with CY
± single cell vaccines and increased when mice were treated with
ternary combinations (23.9 and 24.4%). On the 45th day of
experiments, in all groups the percentage of CD8+ cells
was similar to that previously recorded. No statistically
significant changes were found but the most visible changes were
exhibited on the 38th day, this is 7 days after the third
vaccination.
Taking into consideration the inhibition of tumor
growth and significant differences in number and activity of
CD4+ TILs we decided to analyze the level of spleen cell
reactivity, within the cytotoxic activity of splenocytes harvested
from treated mice. On the 31st day of experiments, when CY +
BM-DC/TAgTNF-α + JAWS II/Neo cells were applied, the
cytotoxicity amounted to 23.8% (P=0.03; compared to CY-treated
group) (Table I). On the 38th day
of experiments, the splenocytes cytotoxicity increased considerably
in all groups, and after treatment with CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cells were on average 39%
and remained similar to those observed on the 45th day (37.6%). On
the contrary, the use of CY + JAWS II/IL-2 cell vaccines elicited
the weakest but permanently growing cytotoxicity of spleen cells.
Overall, the cytotoxic activity of spleen cells gradually increased
in the consecutive harvesting days, excluding therapy with CY +
BM-DC/TAgTNF-α + JAWS II/IL-2, which induced the highest
and stable cytotoxicity on the 38th day. The data shed light on the
strong relationship between kinetics of systemic antitumor reaction
and the nature of the vaccines.
 | Table ITumor-specific cytotoxic activity of
splenocytes. |
Table I
Tumor-specific cytotoxic activity of
splenocytes.
| Percentage of
cytotoxicity ± SD (%) |
|---|
|
|
|---|
| Therapy | 31st day | 38th day | 45th day |
|---|
| CY (Control) | 13.84±6.3 | 24.88±11.4 | 28.43±8.4 |
| CY + JAWS
II/Neo | 19.62±9.2 | 28.03±5.6 | 34.02±13 |
| CY + JAWS
II/IL-2 | 14.82±9.9 | 20.93±10.9 | 25.50±10.1 |
| CY +
BM-DC/TAgTNF-α | 18.34±9.4 | 32.94±9.7 | 32.86±7.2 |
| CY +
BM-DC/TAgTNF-α + JAWS II/Neo |
23.77±5.8a | 31.21±5.6 | 36.41±12.5 |
| CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 | 19.27±7.1 | 39.00±9.9 | 37.56±13.0 |
In order to determine the effect of the combined
treatments on the spleen cell ability for cytokine production, the
cells were stimulated with Con A, and concentrations of IFN-γ,
IL-4, IL-17A and IL-10 in supernatants from the above cell cultures
were measured. Splenocytes from healthy mice were also stimulated
and only IFN-γ production was found (3.7 ng/ml).
Splenocytes from treated tumor-bearing mice elicited
strong differences in IFN-γ production. On the 31st day (Fig. 7), the concentration of IFN-γ ranged
from 4.6 ng/ml (CY-treated mice) to 13.6 ng/ml (CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cells). On the 38th day,
this production ranged from 3.1 ng/ml (CY-treated mice) to 11.2
ng/ml (CY + BM-DC/TAgTNF-α + JAWS II/IL-2 cells).
Whereas, on the 45th day, it was 6.4 ng/ml (CY + JAWS II/IL-2 cell)
to 13.9 ng/ml (CY + BM-DC/TAgTNF-α + JAWS II/IL-2
cells). This increase was statistically significant (P<0.01)
compared to CY-treated control (Fig.
7).
The ability of spleen cells to produce IL-4 was
changed. On 31st or 38th days of the experiments we observed that
the combined treatments affected the splenocytes to secrete a
significantly higher IL-4 concentration than CY-treated group
(Fig. 7). The statistically
significant increase in the cytokine production was observed when
mice received CY + BM-DC/TAgTNF-α ± transduced JAWS II
cells (P<0.01). However, the highest IL-4 concentration was
noted after treatment with CY + BM-DC/TAgTNF-α + JAWS
II/IL-2 cells. Spleen cells harvested on the 45th day secreted
<0.3 ng/ml of IL-4.
The level of IL-10 production by splenocytes was
strongly associated with the nature of the treatment of mice. Cells
from CY-treated mice secreted IL-10 in a gradually growing manner
(from 0.1 to 0.2 ng/ml to 1.5 ng/ml respectively, on the
consecutive harvesting days). On the 38th day, the application of
CY + transduced JAWS II cells caused a significant increase (vs.
CY-treated group) in the IL-10 production amounting to 1.7 or 3.1
ng/ml. After treatment with ternary combinations, IL-10 production
was 10.7 or 9.2 ng/ml. However, only after treatment with CY +
BM-DC/TAgTNF-α they exhibited the highest secretion of
cytokine (15.2 ng/ml). On the 45th day, the decrease in the
production was observed. Of note, the use of CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cells, regardless of
harvesting day, resulted in extended IL-10 production on a moderate
level.
It has to be highlighted that the concentration of
IL-17A in culture supernatants of splenocytes was very low (<0.2
ng/ml) and did not differ significantly among groups and in time
(data not shown).
The changes in the level of cytokine production
revealed that long-lasting spleen cell activity depended on the
nature of the vaccines. The highest IL-4 and IL-10 production was
elicited on the 38th day, whereas the IFN-γ secretion increased up
to the 45th day of the experiments.
Influence of DC-based vaccines on
diversity of CD4+ cell subpopulations in spleen
The observed changes in the cytokine secretion
seemed to be associated with the stage of CD4+ T
lymphocyte differentiation. Therefore, the estimation of the
percentage of T-bet+, GATA3+,
RORγt+ and FoxP3+ cells among harvested
splenocytes was reasonable.
On the 31st and the 38th days of the experiments,
the percentage of CD4+T-bet+ lymphocytes
increased gradually accordingly to the type of treatment (data not
shown). On the 45th day (Fig. 8),
the percentage grew significantly and ranged from 17.4% (CY-treated
mice) to 28.0% (CY + BM-DC/TAgTNF-α + JAWS II/Neo cells)
and 31.0% (CY + BM-DC/TAgTNF-α + JAWS II/IL-2 cells).
However, the highest percentage of CD4+T-bet+
lymphocytes (32.6%) was induced by application of CY +
BM-DC/TAgTNF-α (statistically significant vs. CY-treated
control at P<0.05). The obtained results indicate that one-third
of the splenocytes were able to express the T-bet transcription
factor and probably to provide the high IFN-γ production.
On the 31st day of the experiments the percentage
of CD4+GATA3+ cells was not varied among
treated groups (from 5.1% for CY-treated group, 6.5% for CY +
BM-DC/TAgTNF-α + JAWS II/Neo, to 8.0% for CY +
BM-DC/TAgTNF-α + JAWS II/ IL-2 group), but on the next
harvesting day, it grew approximately two times after application
of CY + BM-DC/TAgTNF-α ± transduced JAWS II cells (14.8%
for CY + BM-DC/TAgTNF-α + JAWS II/Neo group and 13.1%
for CY + BM-DC/TAgTNF-α + JAWS II/IL-2 group) and was
statistically significant (at P<0.01) vs. CY-treated control
(data not shown). On the 45th day (Fig. 8), the use of CY alone or followed
by transduced JAWS II cells resulted in a slight increase in the
percentage of splenocytes; treatment with CY +
BM-DC/TAgTNF-α, CY + BM-DC/TAgTNF-α + JAWS
II/Neo cells caused additional increase of the percentage to 22.9
or 19.9%, respectively. The increase of percentage of
CD4+GATA3+ cells to 28.2% (P=0.019 vs.
CY-treated control) was a result of application of CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cells and highlighted the
role of this vaccine in differentiation of CD4+ cells
towards GATA3+ Th2 cells. Of note, the use of any
combination of treatment did not affect the changes in the number
of CD4+RORγt+ spleen cells (data not shown)
that was consistent with the lack of IL-17A production.
The relatively high level of IL-10 production by
splenocytes, suggested the presence of the Treg cell population in
spleen. To confirm this assumption, the size of
CD4+FoxP3+ splenocytes subpopulation was
analyzed. On the 31st and 38th day of experiments, we noted
considerable decrease in these percentages compared to CY-treated
groups (data not shown). A similar process was observed on the 45th
day (Fig. 8). The use of CY +
BM-DC/TAgTNF-α resulted in a decrease in the lymphocyte
percentage to 4.5%, compared to 8.7% for CY-treated mice. The
application of CY + BM-DC/ TAgTNF-α + JAWS II/Neo cells
induced a further reduction of the lymphocyte population (3.9%) but
the lowest percentage of CD4+FoxP3+ cells was
demonstrated after the application of CY +
BM-DC/TAgTNF-α + JAWS II/IL-2 cells (2.8%). These
changes were statistically significant vs. CY-treated control
(P<0.05).
The inverse relationship between the number of
CD4+GATA3+ cells and
CD4+FoxP3+ cells associated with application
of CY + BM-DC/TAgTNF-α + JAWS II/IL-2 cells suggested
that other subpopulations of CD4+ cells were able to
produce IL-10 during the long-lasting fight against tumor. The
treatment with ternary combinations turned out to be necessary for
effective stimulation of both Th1-type and Th2-type response in
spleen and creation of the opportunity to reduce the percentage of
Treg cells but not other CD4+ T cell subpopulations
participating in the prolonged IL-10 production.
Discussion
Cyclophosphamide (CY) is DNA-alkylating agent
widely used in the treatment of malignant diseases. Acting in
dose-dependent manner, it induces cytotoxic effect, promotes Th1
cells, and inhibits the T regulatory cell activity (1,3). In
the present study, CY was administered at a single dose of 150
mg/kg body weight to reduce the volume of the MC38 tumor nodules,
and to eliminate suppressor cells.
The potency of DCs for tumor growth inhibition has
been confirmed both in experiments with animal tumor models and in
clinical trials (13). Despite
extensive research on the harnessing of DCs for immunotherapy, its
effects are still unsatisfactory (14–16).
DCs, exploited as therapeutic tools, are often subjected to ex
vivo antigenic stimulation (17,18).
In the present study, bone marrow-derived DC stimulation was
induced by the MC38/0 cell lysate (TAg) and TNF-α
(BM-DC/TAgTNF-α). After the BM-DC maturation, an
increase in expression of CD80 and slight CD86 was observed. In
light of the results obtained by Kuchroo and Ranger (19,20)
the DCs with high expression of CD80, especially due to their
capacity to produce the inflammatory cytokines, should be able to
induce differentiation of Th1 more than Th2 lymphocytes. The JAWS
II cells transduced with IL-2 gene (JAWS II/IL-2) were used as
element of vaccines responsible for tumor growth inhibition
(11,21). As in our previous studies these
cells were exploited mainly as a source of IL-2 supporting
antitumor response.
Chemo-immunotherapy using CY and DC-based vaccines
is considered as an effective tool in the fight against cancer,
including the generation of immune memory to prevent cancer
recurrence (3,5,6). In
the MC38-model, we observed that such treatment affected
statistically significant tumor growth inhibition (Fig. 2) which was the most spectacular on
the 45th day of the experiment, 14 days after the third application
of CY + BM-DC/TAgTNF-α + JAWS II/L-2 cells (65.6% of TGI
and TRV amounted to 17 days).
Based on our earlier study and achievements
presented by other authors (11,21–25)
we recognized an infiltration of lymphoid cells into tumor tissue
as an important prognostic factor. High lymphocyte influx was
observed already on the 31st day of the experiments, especially
after the application of CY + BM-DC/TAgTNF-α ±
transduced JAWS II cells (Fig. 3).
Such treatment resulted in increased number of CD4+ and
CD8+ T infiltrating tumor lymphocytes (TILs). These
results were consistent with our previous observation in which
immunotherapy with various combined cellular vaccines elicited more
intense influx than vaccines containing only one type of DCs
(11,21,26).
For further analysis of the relationship between
nature of cellular vaccines and stimulation of lymphoid immune
cells, we attempted to determine the effect of IL-2-producing
and/or stimulated DCs, included in the vaccines supporting the
CY-therapy, on differentiation of the main CD4+ T cell
subpopulations and their prolonged presence in the tumor tissue.
For this purpose, we estimated the production of Th-specific
cytokines and the expression of corresponding transcription
factors. We found changes in the number and reactivity of the Th1
(T-bet+), Th2 (GATA3+), Th17
(RORγt+), and Treg (FoxP3+) subpopulations
corresponding with a creation of antitumor response, both in tumor
tissue and in the spleens. The present study showed that the level
of cytokine production was time-dependent. Regardless of the level
of cytokine production, the treatment with ternary combinations
resulted in the long-lasting activity of both IFN-γ- and
IL-4-producing CD4+ TILs (Fig. 4) and in consequence would be
responsible for Th1 or Th2 polarization of the immune response.
These results were relevant in light of our assumption that the
BM-DC/TAgTNF-α + JAWS II/IL-2 cells contribute to
stimulation of dominant Th1 cell activity. It was consistent with
the observation that the treatment with ternary combinations
elicited the rise of splenocyte ability to produce IFN-γ as well as
with the data obtained in other investigations both on material
harvested from patients with cancers (27–29)
and in experimental models (3,6,30).
Production of IL-4 and GATA3 expression are both
believed to be features of Th2-type cells. The production of this
cytokine by CD4+ TILs remained constant after the 38th
day in contrast to splenocytes which were not able to produce IL-4
on the 45th day. On the last harvesting day, diversity of
CD4+GATA3+ cells, similarly to
CD4+T-bet+ cells, depended on the nature of
cellular vaccines. However, the strongest
CD4+GATA3+ cell response in tumor tissue was
elicited by the application of CY + BM-DC/TAgTNF-α,
whereas, the highest percentage of spleen-derived
CD4+GATA3+ cells was found after treatment
with CY + BM-DC/TAgTNF-α + JAWS II/IL-2 cells.
The relationship of IFN-γ and IL-10 secretion by
CD4+ TILs and this observed in the case of spleen cells
was more complicated, due to their inverse nature (Figs. 4 and 7): CD4+ TILs were able to
produce more IL-10 than IFN-γ while spleen cells produced much more
IFN-γ than IL-10. This finding suggests that duration of
CD4+ T lymphocyte activity could be associated with the
induction of their polarization by vaccines containing
BM-DC/TAgTNF-α and enhanced by JAWS II/IL-2 cells.
Admittedly, administration of CY is believed to
reduce percentage of Treg cells, but in the present study, its
effect was not observed. This could be due to the fact that
cytostatic effect was short-term, especially that the time interval
between single CY administration and the last day of material
harvesting amounted to 31 days. Thus, gradual decrease in
percentage of the CD4+FoxP3+ and also
CD4+RORγt+ T cells was strongly determined by
multiplicity application and nature of vaccines not only in the
tumor tissue but also in the spleen (Figs. 5 and 8). The inverse relationship between the
percentage of CD4+FoxP3+ cells and
CD4+T-bet+ or
CD4+GATA3+ T cells might be associated with
an inhibition of differentiation of Treg cells or their ability to
convert to the other subpopulations, as was suggested by Zhu and
Paul (31). Additionally, in a
report by Yamamoto et al (29) the elimination of Treg cells in the
spleen effectively induced an increase in the number of Th1 cells
(producing IFN-γ) and reduced the number of Th2 cells (producing
IL-10). In another case, the application of autologous DC-based
vaccine together with low dose of IL-2 resulted in generation of
response against kidney cancer associated with a decrease in the
number of CD4+CD25+ Treg cells and a
promotion of a Th1-type response (32). Furthermore, polarization of
anti-tumor response induced by administration of DC vaccines was
associated with the elimination of Treg cells, and increased the
cytotoxic activity of splenocytes in vitro and the number of
IFN-γ-secreting CD4+ and CD8+ cells (26,33).
The cytotoxic activity of spleen cells was also revealed by us. It
gradually increased in the consecutive harvesting days (Table I) but after ternary combinations
the highest cytotoxicity was observed on the 38th day.
Interesting relationships were found between the
expression of FoxP3 and the secretion of IL-10, both believed to be
features of Treg cells. The greatest difference in IL-10 production
by CD4+ TILs was found on the 45th day. The growing body
of evidence points out that among CD4+ lymphocytes, Treg
cells are not the only ones that are able to produce IL-10. For
example, IL-10 can be secreted by IFN-γ-producing cells
representing T-bet+ cell subpopulation (34) or by GATA3+ cells
(35). Shoemaker et al
(36) found a positive correlation
between the level of IL-10 production and GATA3 expression by
splenocytes. They demonstrated that splenic Th2 lymphocytes from
healthy mice can produce IL-10. Even after treatment with
DC-vaccines producing IL-12 the splenocytes are able to produce
IL-10 (26). Taking into
consideration that the percentage of CD4+ T cells
expressing GATA3 was higher than cells expressing FoxP3, we assume
here that in the MC38-tumor model CD4+GATA3+
T cell are mainly responsible for IL-10 production and
CD4+FoxP3+ lymphocytes only slightly
contribute to this process. The inverse relationship between the
number of CD4+GATA3+ cells and
CD4+FoxP3+ cells associated with application
of CY + BM-DC/TAgTNF-α + JAWS II/IL-2 cells suggested
that other subpopulations of CD4+ cells were able to
produce IL-10 during the long-lasting fight against tumor. Under a
proper stimulation Th2 cells secrete IL-4 responsible for
stabilization the Th2 status of CD4+ T cells, lack of
cytotoxic of the CD8+ T cells and protection lymphocytes
from apoptosis. On the contrary, IL-4 can promote tumor growth due
to presence of IL-4R on surface of many types of tumor cells
(37). Thus, the temporary
potential of CD4+ lymphocytes to produce IL-4 in our
model can be associated with an increase of CD8+ cell
activation direct towards tumor cells. Some authors show that IL-10
exploited the generation of immunosuppression, while others
demonstrate its immunostimulatory properties. This functional
duality of IL-10 is substantial in the context of the regulation of
tumor growth (35). Additionally,
it is observed that relatively high systemic doses of IL-10, were
not immunosuppressive and, when given in combination with IL-4,
countered its suppressive effect.
In conclusion, the administration of CY followed by
multiple application of various DC-based vaccines into MC38 colon
carcinoma-bearing mice elicited MC38 tumor growth delay. This was
accompanied by an increase in the number and activity of Th1 and
Th2 cells and a decrease in the number of Th17 and Treg cells. The
gradual changes in Th1 and Th2 cell populations of both
tumor-derived CD4+ lymphocytes and spleen cells were
followed by the increase in the production of IFN-γ, IL-10 and
temporarily IL-4. This was also manifested by an increase in the
percentage of T-bet+ and GATA3+ T cells, a
reduction in the number of FoxP3+ cells (but in late
stage of immune response). Vaccines consisting of
BM-DC/TAgTNF-α appeared to be the most effective in the
stimulation of Th2-type response, while BM-DC/TAgTNF-α
together with JAWS II/IL-2 cells were an appropriate support for
the stimulation of Th1-type responses. Generally, the DC-based
vaccines were able to elicit the alteration in the tumor
environment and, in this way, they intensified the effect of
CY.
Acknowledgements
The present study was supported by the National
Science Center of Poland (decision number DEC-2011/01/N/NZ4/01725)
and Wroclaw Centre of Biotechnology, Programme The Leading National
Research Centre (KNOW) for years 2014–2018.
Abbreviations:
|
BM-DCs
|
bone marrow-dendritic cells
|
|
MC38
|
murine colon carcinoma
|
|
TAg
|
tumor antigens
|
|
TNF-α
|
tumor necrosis factor α
|
References
|
1
|
Ding ZC, Blazar BR, Mellor AL, Munn DH and
Zhou G: Chemotherapy rescues tumor-driven aberrant CD4+
T-cell differentiation and restores an activated polyfunctional
helper phenotype. Blood. 115:2397–2406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao J, Cao Y, Lei Z, Yang Z, Zhang B and
Huang B: Selective depletion of
CD4+CD25+Foxp3+ regulatory T cells
by low-dose cyclophosphamide is explained by reduced intracellular
ATP levels. Cancer Res. 70:4850–4858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sistigu A, Viaud S, Chaput N, Bracci L,
Proietti E and Zitvogel L: Immunomodulatory effects of
cyclophosphamide and implementations for vaccine design. Semin
Immunopathol. 33:369–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matar P, Rozados VR, Gervasoni SI and
Scharovsky GO: Th2/ Th1 switch induced by a single low dose of
cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol
Immunother. 50:588–596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu JY, Wu Y, Zhang XS, Yang JL, Li HL,
Mao YQ, Wang Y, Cheng X, Li YQ, Xia JC, et al: Single
administration of low dose cyclophosphamide augments the antitumor
effect of dendritic cell vaccine. Cancer Immunol Immunother.
56:1597–1604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Veltman JD, Lambers MEH, van Nimwegen M,
de Jong S, Hendriks RW, Hoogsteden HC, Aerts JG and Hegmans JP:
Low-dose cyclophosphamide synergizes with dendritic cell-based
immunotherapy in antitumor activity. J Biomed Biotechnol.
2010:7984672010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balkow S, Loser K, Krummen M, Higuchi T,
Rothoeft T, Apelt J, Tuettenberg A, Weishaupt C, Beissert S and
Grabbe S: Dendritic cell activation by combined exposure to
anti-CD40 plus interleukin (IL)-12 and IL-18 efficiently stimulates
anti-tumor immunity. Exp Dermatol. 18:78–87. 2009. View Article : Google Scholar
|
|
8
|
Brunner C, Seiderer J, Schlamp A,
Bidlingmaier M, Eigler A, Haimerl W, Lehr HA, Krieg AM, Hartmann G
and Endres S: Enhanced dendritic cell maturation by TNF-alpha or
cytidine-phosphate-guanosine DNA drives T cell activation in vitro
and therapeutic anti-tumor immune responses in vivo. J Immunol.
165:6278–6286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossowska J, Pajtasz-Piasecka E, Szyda A,
Krawczenko A, Zietara N and Dus D: Tumour antigen-loaded mouse
dendritic cells maturing in the presence of inflammatory cytokines
are potent activators of immune response in vitro but not in vivo.
Oncol Rep. 21:1539–1549. 2009.PubMed/NCBI
|
|
10
|
Pajtasz-Piasecka E and Indrová M:
Dendritic cell-based vaccines for the therapy of experimental
tumors. Immunotherapy. 2:257–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rossowska J, Pajtasz-Piasecka E, Ryśnik O,
Wojas J, Krawczenko A, Szyda A and Duś D: Generation of antitumor
response by IL-2-transduced JAWS II dendritic cells. Immunobiology.
216:1074–1084. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rossowska J, Pajtasz-Piasecka E, Anger N,
Wojas-Turek J, Kicielińska J, Piasecki E and Duś D:
Cyclophosphamide and IL-12-transduced DCs enhance the antitumor
activity of tumor antigen-stimulated DCs and reduce Tregs and MDSCs
number. J Immunother. 37:427–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steinman RM: Decisions about dendritic
cells: Past, present, and future. Annu Rev Immunol. 30:1–22. 2012.
View Article : Google Scholar
|
|
14
|
Coquerelle C and Moser M: DC subsets in
positive and negative regulation of immunity. Immunol Rev.
234:317–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalinski P, Urban J, Narang R, Berk E,
Wieckowski E and Muthuswamy R: Dendritic cell-based therapeutic
cancer vaccines: What we have and what we need. Future Oncol.
5:379–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palucka K, Ueno H, Roberts L, Fay J and
Banchereau J: Dendritic cell subsets as vectors and targets for
improved cancer therapy. Curr Top Microbiol Immunol. 344:173–192.
2011.
|
|
17
|
Fong L, Brockstedt D, Benike C, Breen JK,
Strang G, Ruegg CL and Engleman EG: Dendritic cell-based
xenoantigen vaccination for prostate cancer immunotherapy. J
Immunol. 167:7150–7156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nencioni A, Grünebach F, Schmidt SM,
Müller MR, Boy D, Patrone F, Ballestrero A and Brossart P: The use
of dendritic cells in cancer immunotherapy. Crit Rev Oncol Hematol.
65:191–199. 2008. View Article : Google Scholar
|
|
19
|
Kuchroo VK, Das MP, Brown JA, Ranger AM,
Zamvil SS, Sobel RA, Weiner HL, Nabavi N and Glimcher LH: B7-1 and
B7-2 costimulatory molecules activate differentially the Th1/Th2
developmental pathways: Application to autoimmune disease therapy.
Cell. 80:707–718. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ranger AM, Das MP, Kuchroo VK, Glimcher LH
and Ploegh H: B7-2 (CD86) is essential for the development of
IL-4-producing T cells. Int Immunol. 8:1549–1560. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wojas-Turek J, Pajtasz-Piasecka E,
Rossowska J, Piasecki E and Duś D: Antitumor effect of murine
dendritic and tumor cells transduced with IL-2 gene. Folia
Histochem Cytobiol. 50:414–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cannon MJ, Goyne H, Stone PJB and
Chiriva-Internati M: Dendritic cell vaccination against ovarian
cancer - tipping the Treg/TH17 balance to therapeutic advantage?
Expert Opin Biol Ther. 11:441–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pajtasz-Piasecka E, Rossowska J, Szyda A,
Krawczenko A and Dus D: Generation of anti-tumor response by JAWS
II mouse dendritic cells transduced with murine interleukin 12
genes. Oncol Rep. 17:1249–1257. 2007.PubMed/NCBI
|
|
25
|
Lança T and Silva-Santos B: The split
nature of tumor-infiltrating leukocytes: Implications for cancer
surveillance and immunotherapy. Oncoimmunology. 1:717–725. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rossowska J, Anger N, Kicielińska J,
Pajtasz-Piasecka E, Bielawska-Pohl A, Wojas-Turek J and Duś D:
Temporary elimination of IL-10 enhanced the effectiveness of
cyclophosphamide and BMDC-based therapy by decrease of the
suppressor activity of MDSCs and activation of antitumour immune
response. Immunobiology. 220:389–398. 2015. View Article : Google Scholar
|
|
27
|
Escobar A, López M, Serrano A, Ramirez M,
Pérez C, Aguirre A, González R, Alfaro J, Larrondo M, Fodor M, et
al: Dendritic cell immunizations alone or combined with low doses
of interleukin-2 induce specific immune responses in melanoma
patients. Clin Exp Immunol. 142:555–568. 2005.PubMed/NCBI
|
|
28
|
Matias BF, de Oliveira TM, Rodrigues CM,
Abdalla DR, Montes L, Murta EFC and Michelin MA: Influence of
immunotherapy with autologous dendritic cells on innate and
adaptive immune response in cancer. Clin Med Insights Oncol.
7:165–172. 2013.PubMed/NCBI
|
|
29
|
Yamamoto M, Kamigaki T, Yamashita K, Hori
Y, Hasegawa H, Kuroda D, Moriyama H, Nagata M, Ku Y and Kuroda Y:
Enhancement of anti-tumor immunity by high levels of Th1 and Th17
with a combination of dendritic cell fusion hybrids and regulatory
T cell depletion in pancreatic cancer. Oncol Rep. 22:337–343.
2009.PubMed/NCBI
|
|
30
|
Guo C, Manjili MH, Subjeck JR, Sarkar D,
Fisher PB and Wang XY: Therapeutic cancer vaccines: Past, present,
and future. Adv Cancer Res. 119:421–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu J and Paul WE: Heterogeneity and
plasticity of T helper cells. Cell Res. 20:4–12. 2010. View Article : Google Scholar
|
|
32
|
Baek S, Kim CS, Kim SB, Kim YM, Kwon SW,
Kim Y, Kim H and Lee H: Combination therapy of renal cell carcinoma
or breast cancer patients with dendritic cell vaccine and IL-2:
Results from a phase I/II trial. J Transl Med. 9:1782011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saha A and Chatterjee SK: Combination of
CTL-associated antigen-4 blockade and depletion of CD25 regulatory
T cells enhance tumour immunity of dendritic cell-based vaccine in
a mouse model of colon cancer. Scand J Immunol. 71:70–82. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cope A, Le Friec G, Cardone J and Kemper
C: The Th1 life cycle: Molecular control of IFN-γ to IL-10
switching. Trends Immunol. 32:278–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kicielińska J and Pajtasz-Piasecka E: The
role of IL-10 in the modulation of the immune response in normal
conditions and the tumor environment. Postepy Hig Med Dosw Online.
68:879–892. 2014.(In Polish). View Article : Google Scholar
|
|
36
|
Shoemaker J, Saraiva M and O'Garra A:
GATA-3 directly remodels the IL-10 locus independently of IL-4 in
CD4+ T cells. J Immunol. 176:3470–3479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koller FL, Hwang DG, Dozier EA and
Fingleton B: Epithelial interleukin-4 receptor expression promotes
colon tumor growth. Carcinogenesis. 31:1010–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|