Introduction
Ulcerative colitis (UC) patients are well known to
carry a higher risk of developing colorectal dysplasia and cancer.
These lesions develop from chronic inflamed mucosa and progress
through dysplasia to adenocarcinoma, termed the
‘inflammation-dysplasia-carcinoma sequence’ (1,2). In
clinical settings, early detection of the neoplasia is the key to
improving the prognosis just as in most cancers. However,
UC-associated neoplasias often develop in flat or mildly elevated
lesions and are distributed multifocally within an area of
intestinal inflammation, making it hard to detect them in early
phase during colonoscopy (3–5).
The benefit of neoplasia surveillance colonoscopy in
UC has been established. At present, the recommended surveillance
strategy involves frequent random biopsies aimed at detecting
dysplasia/cancer (6–9). However, this method is limited by
sampling error, requires considerable time and cost, and has
resulted in only a modest reduction in cancer incidence and
mortality (10). Previously, the
use of a targeting biopsy on conventional white light imaging
(WLI), in which tissue specimens are obtained only when endoscopic
findings indicate the possibility of neoplasia, thereby yielding a
smaller number of samples, has been proposed (11,12).
However, it is still controversial whether a targeted biopsy should
replace a random biopsy.
Several advanced endoscopic imaging techniques, such
as chromoendoscopy (CE) with dye-spraying (13–15),
narrowband imaging (NBI) (16,17)
and autofluorescence imaging (AFI) (18,19),
which provide a more detailed visualization of the mucosa by
enhancing morphology and vascularization, have been developed to
improve upon the accuracy afforded by conventional WLI. In sporadic
colorectal tumors, it has been clearly shown that these imaging
techniques facilitate early detection allowing for the removal of
the lesions, thus avoiding the need for surgery (13). Advanced endoscopic imaging may also
provide better definition and delineation of early stage neoplastic
lesions even in UC, increased yield of detection and a decrease in
the number of biopsies taken. However, little is known about the
role of these techniques in UC-associated neoplastic lesions
(20–22).
This pilot study was conducted to analyze the
endoscopic characteristics of neoplastic lesions associated with UC
using advanced endoscopic imaging techniques. In particular, we
validated the role of AFI quantification for the first time.
Patients and methods
Patient selection and study design
A total of 11 patients (7 men and 4 women; median
age, 63 years) who underwent total colonoscopy at Kurume University
Hospital between April 2003 and March 2014, who had one or more
UC-associated neoplastic lesions that satisfied all of the
following inclusion criteria for the study, were enrolled: i)
Clinically and histologically diagnosed as having UC; ii) Mucosal
status was in remission stage (0 or 1 point in the Mayo endoscopic
score) because histopathological distinction between inflammation
and neoplasia can be extremely difficult in active inflammation;
iii) Lesions which were subsequently managed by surgical resection;
iv) Lesions in which histopathological evaluation was possible; and
v) Lesions which could be excluded as being advanced colorectal
cancer. Table I summarized the
characteristics of the UC patients studied. The ethics committee of
our hospital approved the study protocol and written informed
consent was obtained from each of the study participants.
 | Table ICharacteristics of the patients
studied. |
Table I
Characteristics of the patients
studied.
|
Characteristics | Data |
|---|
| Total number of
patients | 11 |
| Gender,
male/female | 7/4 |
| Age, years (median,
range) | 63 (33–74) |
| Disease duration,
years (median, range) | 14 (3–32) |
| Area involved |
| Extensive | 11 |
| Left-sided | 0 |
| Mayo score |
| Endoscopy | 1 (0–2) |
| Total | 2 (0–8) |
| Treatments |
| None | 0 |
| 5-aminosalicylic
acid | 8 |
| Prednisolone | 8 |
|
Immunomodulators | 2 |
| Anti-tumor
necrosis factor | 0 |
| Complication of
primary sclerosing cholangitis | 0 |
Endoscopic procedure
All patients underwent preparation for colonoscopy
by ingesting 2 liters of polyethylene glycolelectrolyte solution on
the morning of the procedure. In some cases, scopolamine
butylbromide (10 mg) was administered intravenously to avoid bowel
movements prior to the examination in those patients in whom this
agent was not contraindicated.
Colonoscopy was performed using WLI and AFI of the
colon by experienced colonoscopists. The AFI system (Olympus
Medical Systems, Tokyo, Japan) used in this study consisted of a
light-source system (CLV-260SL), a processor (CV-260SL), a
liquid-crystal display monitor, and a specialized video endoscope
for AFI detection (CF-FH260AZI). When colorectal lesions were
detected, they were observed by switching to the NBI mode by the
press of a button in the control head of the endoscope.
Subsequently, the lesions were observed by CE using 0.4% indigo
carmine and the WLI mode. After washing the indigo carmine with
water insufflation, real-time color analysis on the AFI images was
conducted using a personal computer with software for color
analysis connected to the endoscopy system. Finally, magnifying
endoscopy with NBI and CE using crystal violet was performed to
estimate the detail of the encountered lesion.
Conventional endoscopic analysis
Conventional endoscopic features were classified
based on size, shape, location and color (18,23).
Chromoendoscopic analysis
Using magnifying CE, pit patterns were classified
into types I through V based on the classification of Kudo et
al (24,25) Type I represents regular round
crypts, type II represents stellar or papillary crypts, type III
represents small tubular or roundish crypts (IIIS) or
large tubular or roundish crypts (IIIL), type IV
consists of branch- or gyrus-like crypts, and type V consists of
irregular crypts (VI) or non-structural crypts
(VN). Type I and II lesions are mostly non-neoplastic,
whereas type III, IV and V lesions are mostly neoplastic.
Narrow-band imaging analysis
Using NBI, the lesion was classified using the Sano
capillary pattern classification (26,27),
with type I (faintly visible micro-vessels surrounding the pits)
representing a non-neoplasm, type II (elongated and increased
thicker vessels surrounding the pits) representing adenoma, and
type III (type IIIa, increased thick vessels unevenly sized with
branching and curtailed irregularity; type IIIb, nearly avascular
or loose vessels with fragmentation) indicating the detection of
cancer.
Autofluorescence imaging analysis
The lesion was assessed for color on AFI (18,19).
The color of mucosa was divided into green as non-neoplastic or
purple as neoplastic. Due to individual variations in the AFI
intensity, the AFI intensity of any identified lesion was also
compared with the intensity of the non-neoplastic area (both
UC-involved and -uninvolved area) in each individual, and the
corrected value, termed green/red (G/R) ratio, was estimated by
dividing the green color tone intensity values by the red color
tone intensity values, based on the method previously reported
(28). Color tone intensity
analysis was carried out with still images of the colorectal
lesions using software developed by Rasband, WS, Image J, U.S.
National Institutes of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/, 1997–2012.
Histopathology
Surgically resected specimens were immediately fixed
in 10% buffered formalin solution and subsequently stained with
hematoxylin and eosin (H&E). All specimens were evaluated
histopathologically by specialized pathologists who were blinded to
the endoscopic diagnosis. The pathological findings were
categorized as low-grade dysplasia, high-grade dysplasia (HGD) and
cancer.
Statistical analysis
The paired t-test and Spearman's correlation test
were used as appropriate. For all studies, p-values of <0.05
were set to determine the statistical significance. Statistical
analysis was performed using SPSS, version 11.5, software (SPSS
Inc., Chicago, IL, USA).
Results
As shown in Table
I, all 11 patients had extensive colitis with the median
disease duration of 14 years. The background mucosal status of all
patients was in the remission stage according to the Mayo
endoscopic score (0 or 1 point).
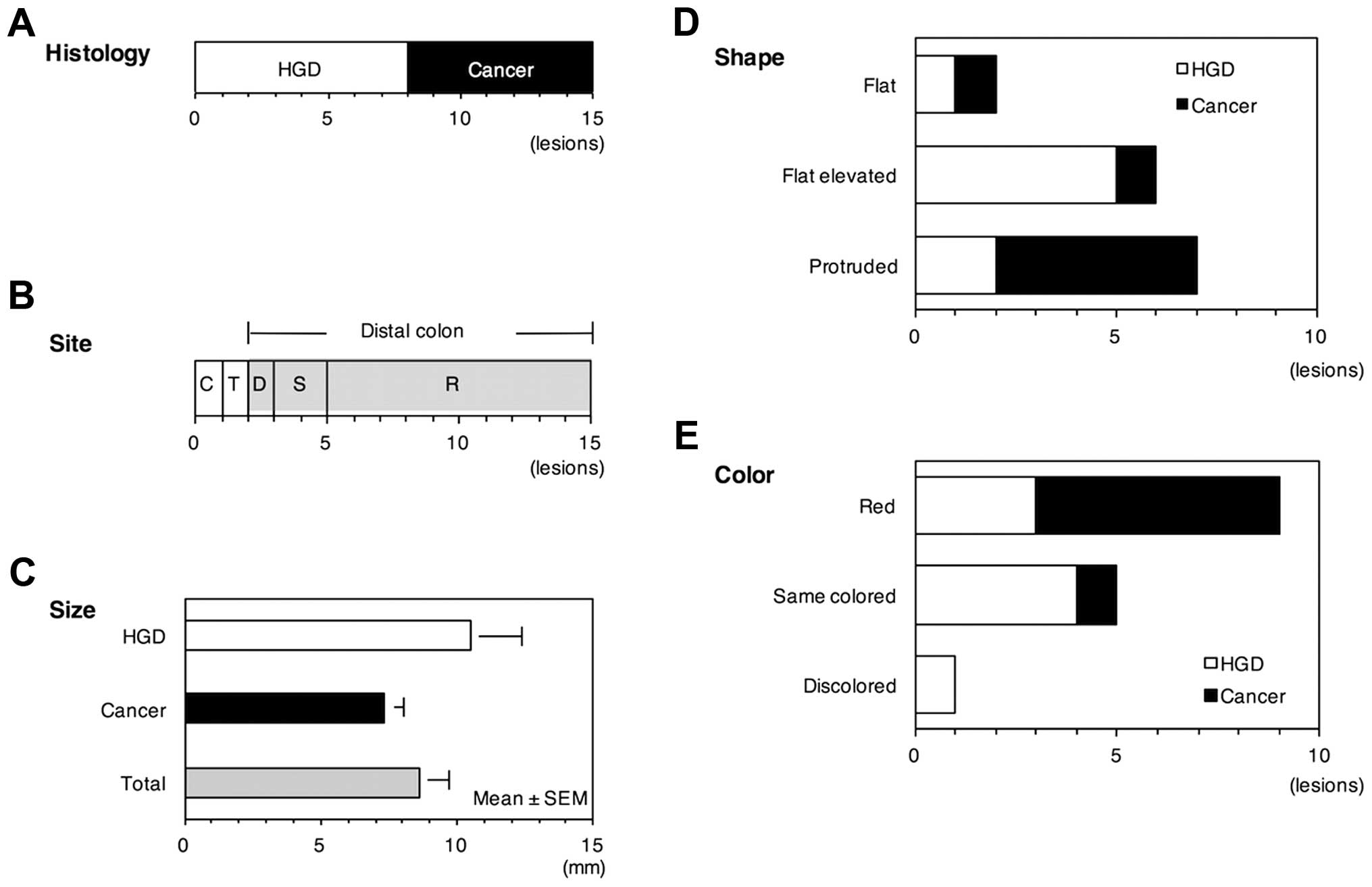
Clinicopathological features of the neoplastic
lesion are presented in Fig. 1.
All of the 15 neoplastic lesions were macroscopically identified,
including 8 HGD and 7 cancer. The lesion was mostly located in the
distal colon (13/15, 86.7%) with the mean size of 8.6 mm. The shape
was protruding in 46.7% (7/15), flat elevated in 40.0% (6/15) and
flat in 13.3% (2/15). The color was red in 60.0% (9/15), same
colored in 33.3% (5/15) and discolored in 6.7% (1/15).
We next analyzed the macroscopic features of the
neoplastic lesions using advanced endoscopic imaging techniques. On
CE, the lesion was classified into pit patterns type I to V with
type I and II representing non-neoplastic pattern and type III, IV
and V representing neoplastic pattern. Of 15 lesions examined in
this study, 13 lesions (86.7%) showed neoplastic pit patterns in
the order of type IV (branch- or gyrus-like crypts) > type
IIIL (large tubular or roundish crypts) > type
VI (irregular crypts) (Fig.
2). Two of the lesions could not be classified into pit
patterns and the pattern was thus regarded as unclassified.
On NBI, the lesion was classified into type I to III
capillary patterns with type I representing a non-neoplasm, type II
representing adenoma and type III (subdivided into IIIa and IIIb)
indicating cancer. Of the 11 lesions examined, one lesion (0.9%)
was identified as type I (faintly visible microvessels surrounding
the pits), 3 lesions (27.3%) as type II (elongated and increased
thicker vessels surrounding the pits) and 7 lesions (63.6%) as type
IIIa (increased thick vessels unevenly sized with branching and
curtailed irregularity) (Fig.
3).
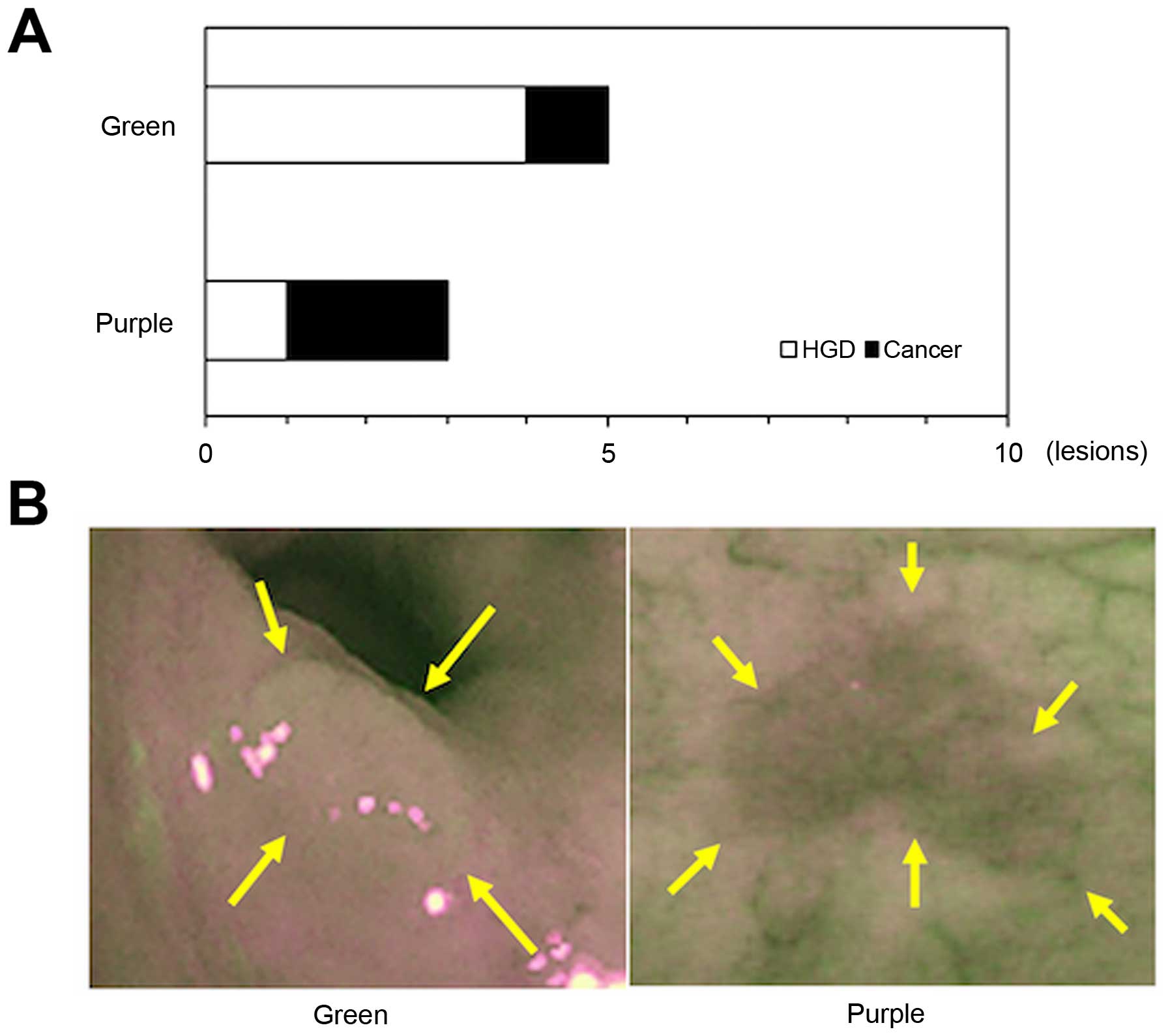
On AFI, in general the non-neoplastic lesions appear
green and neoplastic lesions appear purple. In the present study,
information was available for 8 lesions from a total of 15 lesions
because 7 lesions were excluded due to insufficient bowel
preparation which may disrupt autofluorescence. Of these, 5 lesions
(62.5%) were colored green and 3 lesions (37.5%) colored purple
(Fig. 4).
To quantify AFI intensity, the G/R ratio was
determined for each lesion after adjustments for the surrounding
mucosal values. Of note, the G/R ratio was significantly lower in
the neoplastic area than the adjacent non-neoplastic area involved
in UC (p=0.00014) and the non-neoplastic area uninvolved in UC
(p=0.00651) (Fig. 5). The G/R
ratio was not associated with the size or histological type of the
lesion. Fig. 6 exemplifies the
endoscopic and histological images of colorectal cancer in one
patient with long-standing extensive colitis.
Discussion
Currently, several advanced endoscopic imaging
techniques have been attempted to improve the limitation of
conventional endoscopy (13–19).
In this pilot study, we analyzed the endoscopic characteristics of
neoplastic lesions associated with UC using these advanced
techniques, including CE, NBI and AFI.
A better understanding of cancer risk factors may
allow endoscopic resources to be more focused on patients at higher
risk. It is well established that the risk for colorectal cancer
increases with the duration (more than 7 years) and anatomic extent
of UC (1). In line with this
established observation, our study showed that neoplastic lesion
tended to develop in patients with a long history of extensive
colitis. Another risk, a history of primary sclerosing cholangitis,
was not observed in this study probably due to the small number of
the patients in this study.
The neoplastic lesions in our study were
predominantly located in the distal colon (86.7%), appeared
protruded (46.7%) or flat elevated (40.0%), and were colored red
(60.0%). These findings are consistent with previous reports
describing how dysplasia and early cancer were characterized by low
protruding or flat mucosa, often associated with redness (29–33).
CE is used to better define the superficial mucosa.
CE with dye agents enhances the mucosal detail and permits a more
precise characterization. Kudo et al classified the pit
patterns into types I to V (24,25).
As reported by Sada et al (29), some neoplastic lesions associated
with UC have a neoplastic type IIIS to IIIL
or type IV-type pit patterns. In contrast, Hata et al
(30,31) reported that type III or IV pit
patterns were not observed in some dysplastic lesions. Our study
showed that 13 of the 15 dysplasia/cancer lesions (86.7%) were
representative of the neoplastic pit pattern, suggesting that CE is
helpful to detect and discriminate neoplastic lesions in UC
although coexisting inflammatory changes may modify the mucosal
detail.
NBI is an endoscopy system, which enables a clear
visualization of the microvasculature of colorectal lesions
(26,27). Previous investigations have shown
that NBI is effective in distinguishing neoplastic colorectal
lesions in sporadic settings (16,17).
Our present study showed that 7 of the 11 dysplasia/cancer lesions
(63.6%) were representative of the neoplastic capillary pattern.
Together with the results from recent randomized trials
demonstrating that NBI was a reasonable alternative to CE (34–37),
NBI is one of the available modalities to distinguish neoplastic
lesion associated with UC.
AFI is a novel technique that is based on the fact
that tissues exhibit fluorescence when exposed to ultraviolet
(<400 nm) or shorter waveband visible light (mostly blue)
(19). Recent investigations have
demonstrated the usefulness of AFI in discriminating colorectal
neoplasia in sporadic setting (18). However, the available data on AFI
for UC surveillance is sparse.
On AFI, neoplastic tissue is visible as a purple
lesion on a green background fluorescence of normal colonic tissue
(18,19). Our study showed that a purple
lesion was observed only in 37.5% of the neoplastic lesions,
indicating that the qualitative analysis of the AFI color, green or
purple, is not so helpful for detecting neoplasia associated with
UC. One possible explanation is that the AFI color is altered
according to the grade of inflammation (38,39).
This is probably due to several factors that modify the AFI color
both in inflammation and neoplasia, such as tissue architecture,
light absorption and scattering properties, the biochemical
content, or metabolic status of the tissue (40,41).
Inomata et al demonstrated that quantitative
analysis of the AFI color using the G/R ratio was effective in
distinguishing sporadic neoplastic lesions (28). The most important observation in
the present study is that we found for the first time that the G/R
ratio is helpful in discriminating dysplasia/cancer in UC as well.
The G/R ratio was not associated with the lesion size or
histological type, supporting the idea that this index is available
even for smaller lesions. Quantitative AFI, rather than
qualitative, has therefore the potential for detection of early
neoplastic changes in UC. The field of AFI is still young and
multiple questions remain unanswered. However, our results in AFF
quantification are very promising but as we gain more experience,
these will be better defined, hence the need for further
investigations.
There are several limitations to our study. First,
this was a single-center analysis involving only a limited number
of patients with a retrospective design. Second, because of a
retrospective design, the diagnosis in the study subjects had been
established. Although advanced endoscopic imaging such as CE, NBI
and AFI look very promising, large-scale, prospective study will be
needed to assess their place in the surveillance of UC
patients.
In conclusion, this pilot study suggested that
endoscopic analysis based on advanced imaging, in particular AFI
quantitation, is helpful to detect early stage neoplastic lesions
in long standing UC.
Acknowledgements
The present study was supported in part by a
Grant-in-Aid from the Japanese Ministry of Education, Culture and
Science (no. 25460964) and Health and Labour Sciences Research
Grants for research on intractable diseases from the Ministry of
Health, Labour and Welfare of Japan.
References
|
1
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: A meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ullman TA and Itzkowitz SH: Intestinal
inflammation and cancer. Gastroenterology. 140:1807–1816. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morson BC and Pang LS: Rectal biopsy as an
aid to cancer control in ulcerative colitis. Gut. 8:423–434. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernstein CN, Shanahan F and Weinstein WM:
Are we telling patients the truth about surveillance colonoscopy in
ulcerative colitis? Lancet. 343:71–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blackstone MO, Riddell RH, Rogers BH and
Levin B: Dysplasia-associated lesion or mass (DALM) detected by
colonoscopy in long-standing ulcerative colitis: An indication for
colectomy. Gastroenterology. 80:366–374. 1981.PubMed/NCBI
|
|
6
|
Farrell RJ and Peppercorn MA: Ulcerative
colitis. Lancet. 359:331–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eaden JA and Mayberry JF; British Society
for Gastroenterology; Association of Coloproctology for Great
Britain and Ireland. Guidelines for screening and surveillance of
asymptomatic colorectal cancer in patients with inflammatory bowel
disease. Gut. 51(Suppl 5): V10–V12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winawer S, Fletcher R, Rex D, Bond J, Burt
R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, et al;
Gastrointestinal Consortium Panel. Colorectal cancer screening and
surveillance: Clinical guidelines and rationale - Update based on
new evidence. Gastroenterology. 124:544–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kornbluth A and Sachar DB: Ulcerative
colitis practice guidelines in adults. American College of
Gastroenterology, Practice Parameters Committee. Am J
Gastroenterol. 92:204–211. 1997.PubMed/NCBI
|
|
10
|
Rutter MD: Surveillance programmes for
neoplasia in colitis. J Gastroenterol. 46(Suppl 1): 1–5. 2011.
View Article : Google Scholar
|
|
11
|
Watanabe T, Ajioka Y, Matsumoto T,
Tomotsugu N, Takebayashi T, Inoue E, Iizuka B, Igarashi M, Iwao Y,
Ohtsuka K, et al: Target biopsy or step biopsy? Optimal
surveillance for ulcerative colitis: A Japanese nationwide
randomized controlled trial. J Gastroenterol. 46(Suppl 1): 11–16.
2011. View Article : Google Scholar
|
|
12
|
Matsumoto T, Iwao Y, Igarashi M, Watanabe
K, Otsuka K, Watanabe T, Iizuka B, Hida N, Sada M, Chiba T, et al:
Endoscopic and chromoendoscopic atlas featuring dysplastic lesions
in surveillance colonoscopy for patients with long-standing
ulcerative colitis. Inflamm Bowel Dis. 14:259–264. 2008. View Article : Google Scholar
|
|
13
|
Basu S, Torigian D and Alavi A: The role
of modern molecular imaging techniques in gastroenterology.
Gastroenterology. 135:1055–1061. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Axelrad AM, Fleischer DE, Geller AJ,
Nguyen CC, Lewis JH, Al-Kawas FH, Avigan MI, Montgomery EA and
Benjamin SB: High-resolution chromoendoscopy for the diagnosis of
diminutive colon polyps: Implications for colon cancer screening.
Gastroenterology. 110:1253–1258. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu KI, Sano Y, Kato S, Fujii T, Nagashima
F, Yoshino T, Okuno T, Yoshida S and Fujimori T: Chromoendoscopy
using indigo carmine dye spraying with magnifying observation is
the most reliable method for differential diagnosis between
non-neoplastic and neoplastic colorectal lesions: A prospective
study. Endoscopy. 36:1089–1093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sano Y, Muto M, Tajiri H, Ohtsu A and
Yoshida S: Optical/digital chromoendoscopy during colonoscopy using
narrow-band image system. Dig Endosc. 17(Suppl 1): pp. S43–S48.
2005, http://onlinelibrary.wiley.com/doi/10.1111/j.1443-1661.2005.00511.x/abstract.
View Article : Google Scholar
|
|
17
|
Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS,
Lin JT, Shun CT and Wang HP: A prospective comparative study of
narrow-band imaging, chromoendoscopy, and conventional colonoscopy
in the diagnosis of colorectal neoplasia. Gut. 56:373–379. 2007.
View Article : Google Scholar
|
|
18
|
Arita K, Mitsuyama K, Kawano H, Hasegawa
S, Maeyama Y, Masuda J, Akagi Y, Watanabe Y, Okabe Y, Tsuruta O, et
al: Quantitative analysis of colorectal mucosal lesions by
autofluorescence endoscopy: Discrimination of carcinomas from other
lesions. Oncol Rep. 26:43–48. 2011.PubMed/NCBI
|
|
19
|
Panjehpour M, Overholt BF, Vo-Dinh T,
Haggitt RC, Edwards DH and Buckley FP III: Endoscopic fluorescence
detection of high-grade dysplasia in Barrett's esophagus.
Gastroenterology. 111:93–101. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gabbani T, Manetti N, Bonanomi AG, Annese
AL and Annese V: New endoscopic imaging techniques in surveillance
of inflammatory bowel disease. World J Gastrointest Endosc.
7:230–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Subramanian V and Bisschops R:
Image-enhanced endoscopy is critical in the surveillance of
patients with colonic IBD. Gastrointest Endosc Clin N Am.
24:393–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Efthymiou M, Taylor AC and Kamm MA: Cancer
surveillance strategies in ulcerative colitis: The need for
modernization. Inflamm Bowel Dis. 17:1800–1813. 2011. View Article : Google Scholar
|
|
23
|
Hasegawa S, Mitsuyama K, Kawano H, Arita
K, Maeyama Y, Akagi Y, Watanabe Y, Okabe Y, Tsuruta O and Sata M:
Endoscopic discrimination of sessile serrated adenomas from other
serrated lesions. Oncol Lett. 2:785–789. 2011.
|
|
24
|
Kudo S, Hirota S, Nakajima T, Hosobe S,
Kusaka H, Kobayashi T, Himori M and Yagyuu A: Colorectal tumours
and pit pattern. J Clin Pathol. 47:880–885. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kudo S, Tamura S, Nakajima T, Yamano H,
Kusaka H and Watanabe H: Diagnosis of colorectal tumorous lesions
by magnifying endoscopy. Gastrointest Endosc. 44:8–14. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uraoka T, Saito Y, Ikematsu H, Yamamoto K
and Sano Y: Sano's capillary pattern classification for narrow-band
imaging of early colorectal lesions. Dig Endosc. 23(Suppl 1): pp.
112–115. 2011, http://dx.doi.org/10.1111/j.1443-1661.2011.01118.x.
View Article : Google Scholar
|
|
27
|
Sakamoto T, Saito Y, Nakajima T and
Matsuda T: Comparison of magnifying chromoendoscopy and narrow-band
imaging in estimation of early colorectal cancer invasion depth: a
pilot study. Dig Endosc. 23:pp. 118–123. 2011, http://dx.doi.org/10.1111/j.1443-1661.2010.01049.x.
View Article : Google Scholar
|
|
28
|
Inomata H, Tamai N, Aihara H, Sumiyama K,
Saito S, Kato T and Tajiri H: Efficacy of a novel auto-fluorescence
imaging system with computer-assisted color analysis for assessment
of colorectal lesions. World J Gastroenterol. 19:7146–7153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sada M, Igarashi M, Yoshizawa S, Kobayashi
K, Katsumata T, Saigenji K, Otani Y, Okayasu I and Mitomi H: Dye
spraying and magnifying endoscopy for dysplasia and cancer
surveillance in ulcerative colitis. Dis Colon Rectum. 47:1816–1823.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hata K, Watanabe T, Motoi T and Nagawa H:
Pitfalls of pit pattern diagnosis in ulcerative colitis-associated
dysplasia. Gastroenterology. 126:374–376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hata K, Watanabe T, Kazama S, Suzuki K,
Shinozaki M, Yokoyama T, Matsuda K, Muto T and Nagawa H: Earlier
surveillance colonoscopy programme improves survival in patients
with ulcerative colitis associated colorectal cancer: Results of a
23-year surveillance programme in the Japanese population. Br J
Cancer. 89:1232–1236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hata K, Watanabe T, Shinozaki M, Kojima T
and Nagawa H: To dye or not to dye? That is beyond question!
Optimising surveillance colonoscopy is indispensable for detecting
dysplasia in ulcerative colitis. Gut. 53:17222004.PubMed/NCBI
|
|
33
|
Matsumoto T, Nakamura S, Jo Y, Yao T and
Iida M: Chromoscopy might improve diagnostic accuracy in cancer
surveillance for ulcerative colitis. Am J Gastroenterol.
98:1827–1833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pellisé M, López-Cerón M, Rodríguez de
Miguel C, Jimeno M, Zabalza M, Ricart E, Aceituno M,
Fernández-Esparrach G, Ginès A, Sendino O, et al: Narrow-band
imaging as an alternative to chromoendoscopy for the detection of
dysplasia in long-standing inflammatory bowel disease: A
prospective, randomized, crossover study. Gastrointest Endosc.
74:840–848. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Günther U, Kusch D, Heller F, Bürgel N,
Leonhardt S, Daum S, Siegmund B, Loddenkemper C, Grünbaum M, Buhr
HJ, et al: Surveillance colonoscopy in patients with inflammatory
bowel disease: Comparison of random biopsy vs. targeted biopsy
protocols. Int J Colorectal Dis. 26:667–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kiesslich R, Goetz M, Lammersdorf K,
Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR and
Neurath MF: Chromoscopy-guided endomicroscopy increases the
diagnostic yield of intraepithelial neoplasia in ulcerative
colitis. Gastroenterology. 132:874–882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kiesslich R, Fritsch J, Holtmann M,
Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR and
Neurath MF: Methylene blue-aided chromoendoscopy for the detection
of intraepithelial neoplasia and colon cancer in ulcerative
colitis. Gastroenterology. 124:880–888. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rubin DT and Turner JR: Surveillance of
dysplasia in inflammatory bowel disease: The
gastroenterologist-pathologist partnership. Clin Gastroenterol
Hepatol. 4:pp. 1309–1313. 2006, http://europepmc.org/abstract/MED/17110299.
View Article : Google Scholar
|
|
39
|
Matsumoto T, Moriyama T, Yao T, Mibu R and
Iida M: Autofluorescence imaging colonoscopy for the diagnosis of
dysplasia in ulcerative colitis. Inflamm Bowel Dis. 13:640–641.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zonios GI, Cothren RM, Arendt JT, Wu J,
Van Dam J, Crawford JM, Manoharan R and Feld MS: Morphological
model of human colon tissue fluorescence. IEEE Trans Biomed Eng.
43:113–122. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marchesini R, Pignoli E, Tomatis S,
Fumagalli S, Sichirollo AE, Di Palma S, Dal Fante M, Spinelli P,
Croce AC and Bottiroli G: Ex vivo optical properties of human colon
tissue. Lasers Surg Med. 15:351–357. 1994. View Article : Google Scholar : PubMed/NCBI
|