Introduction
Melanoma, a cancer originating from melanocytes, is
one of the most aggressive forms of skin cancers with a high
frequency of metastasis. Estimates suggest that 76,690 new cases of
melanoma were diagnosed in year 2013 in USA alone, leading to ~9480
deaths. Solar ultraviolet (UV) radiation exposure is a recognized
risk factor for the development of cutaneous malignancies including
the risk of melanoma. The incidence of melanoma is increasing in US
and is increasing rapidly in children (1,2)
because of the increase in recreational UV light exposure. Although
melanoma is less common than non-melanoma, however, majority of
skin cancer-related deaths are due to melanoma. As, melanoma is a
highly malignant cancer with a potent capacity to metastasize to
distant organs, an approach that inhibits its growth and
progression may facilitate the development of an effective strategy
for its prevention or treatment.
Natural plant products offer promising tools for the
prevention of cancer growth and metastasis. Grape seed
proanthocyanidins (GSPs) are bioactive phytochemicals that have
shown strong anti-carcinogenic activity in various animal tumor
models and appear to exhibit minimal toxicity in laboratory animals
(3,4). GSPs are readily extracted from
grape-seeds, and are a mixture of dimers, trimers, tetramers, and
oligomers of monomeric catechins and/or (-)-epicatechins (3,4).
GSPs have been shown to have anti-inflammatory, anti-oxidant and
anti-metastatic properties in both in vitro and in
vivo models (3–7). They inhibit UV radiation- and
chemical carcinogen-induced skin carcinogenesis in mouse models
(3,8). Dietary administration of GSPs
resulted in a dose-dependent inhibition of the growth of tumor
xenografts of cancer cells of lungs (9), pancreas (10) and head and neck (11). Recently, we showed that GSPs
inhibit the invasive potential of melanoma cells (6). However, the anticarcinogenic
potential of GSPs against melanoma growth and progression is
largely unexplored.
β-catenin, a key component of Wnt signaling pathway,
is a complicated dual function protein. It participates in
formation of adherens junctions via formation of a stable complex
with the cell adhesion proteins of the cadherin family, while in
free non-phosphorylated state, β-catenin interacts with the T-cell
factor transcription factors to control expression of target genes
that are involved in cell proliferation, differentiation and
metastasis. Though various studies have implicated nuclear
accumulation of β-catenin occurring as a result of constitutively
active Wnt/β-catenin signaling in growth and progression of cancers
of various organs (12–14), the view that β-catenin is
‘uniformly oncogenic’ is far from acceptable in the scientific
community. Studies have shown that forced expression of a
melanocyte-specific, non-degradable, constitutively active
β-catenin mutant in either transgenic or Cre/lox systems is not
sufficient enough to induce melanoma in mice (15). Most importantly studies in human
melanoma patients suggest a positive correlation between increased
levels of nuclear β-catenin and an improved rather than poorer
prognosis of melanoma indicate that Wnt/β-catenin signaling may not
be oncogenic, but rather is required to prevent early melanoma
transformation (14,16–19).
Overall, in view of limited information concerning β-catenin, the
oncogenic/tumor suppressive role of β-catenin in case of melanoma
may best be regarded as contextual i.e. dependent on the model
system employed for the study.
In the present study, we determined growth
inhibitory effect of GSPs on melanoma using two different human
melanoma cell lines, namely A375 (BRAF-mutated) and Hs294t
(wild-type for BRAF gene, non-BRAF-mutated). For this purpose both
in vitro and in vivo tumor xenograft models were
used. Results of the present study indicate a pro-oncogenic role of
β-catenin in melanoma and also suggest that GSPs inhibit melanoma
growth by targeting β-catenin in our model system.
Materials and methods
Chemicals and antibodies
The purified fraction of proanthocyanidins from
grape seeds were obtained from the Kikkoman Corp. (Noda, Japan).
The β-cateninS33Y pcDNA plasmid bearing FLAG tag used
for the overexpression of non-degradable, constitutively active
mutant form of β-catenin was obtained from Addgene (Cambridge, MA,
USA), while β-catenin siRNA kit for knocking down the expression
level of β-catenin along with the siRNA transfection reagents, and
antibodies specific to PCNA, cyclin D1, cyclin D2, Cdks (2,4,6),
Cip1/p21, Kip1/p27, β-catenin, β-actin, histone H3, horseradish
peroxidase conjugated rabbit anti-goat and goat anti-rabbit
secondary antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). The antibodies specific for Bax, Bcl-2,
Bcl-xl, cleaved caspase-3, caspase-9, PARP, casein kinase 1α
(CK1α), glycogen synthase kinase-3β (GSK-3β), and phospho forms of
β-catenin were obtained from Cell Signaling Technology (Beverly,
MA, USA). Annexin V-conjugated Alexa Fluor 488 apoptosis detection
kit was purchased from Molecular Probes, Inc. (Eugene, OR,
USA).
Cell lines and cell culture
conditions
The human melanoma cells lines, Mel928, Mel1011 and
Mel1241, were a kind gift from Dr Paul Robbins (Center of Cancer
Research, National Cancer Institute, Bethesda, MD, USA), while A375
and Hs294t human melanoma cell lines were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). No
further authentication of cells was done by the authors. Cells were
grown in cell culture media as previously detailed (6). For treatment of cells, GSPs were
dissolved in a small amount (100 μl) of dimethylsulfoxide (DMSO),
which was then added to the complete cell culture medium. The
maximum concentration of DMSO in cell culture media was not
>0.1% (v/v). Cells treated with the same concentration of DMSO
only served as a vehicle control.
Cell viability assay or MTT assay
The effect of GSPs on the viability of human
melanoma cells was determined using MTT assay as previously
described (20). The effect of
GSPs on cell viability was calculated and presented in terms of
percent of control, which was arbitrarily assigned a value of 100%
viability. All treatment concentrations were replicated at least in
6-wells.
Apoptotic cell death analysis by flow
cytometry
GSPs induced apoptotic cell death in melanoma cells
was quantitatively determined by flow cytometry using the Annexin
V-conjugated Alexa Fluor 488 (Alexa 488) apoptosis detection kit
following the manufacturer's protocol, as was previously used and
described (20). Briefly, after
treatment of cells with GSPs (0, 20, 40 and 60 μg/ml) for 48 h,
cells were harvested, washed with PBS and stained with Alexa 488
and propidium iodide. The cells were then analyzed by fluorescence
activated cell sorting using the FACSCalibur instrument (BD
Biosciences, San Jose, CA, USA) and CellQuest 3.3 software, as
previously described (20).
siRNA knockdown of β-catenin in melanoma
cells
The β-catenin expression was knocked down in Mel1241
melanoma cells using β-catenin siRNA kit obtained from Santa Cruz
Biotechnology following the manufacturer's instruction. Briefly,
Mel1241 cells (3×105/well) were seeded in a 6-well plate
and allowed to grow to 70% confluency. The β-catenin siRNA mixed
with transfection reagents was overlaid on the cells for 6 h at
37°C and transferred into 2X growth medium for ~18–20 h. At 24 h
post-transfection, fresh medium was added to the cells and the
cells were incubated for an additional 48 h. The knockdown of
β-catenin expression in cells after transfection was confirmed by
western blot analysis using β-catenin-specific antibody.
Forced-overexpression of mutant β-catenin
in melanoma cells
For the forced overexpression of mutant β-catenin,
expression plasmid (β-cateninS33Y pcDNA) was transfected
in Mel1011 cells (inactivated β-catenin cell line) using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the
manufacturer's protocol and stable clones were obtained through
1000 μg/ml G418 (Invitrogen) selection. Mutant forced-β-catenin
overexpression was checked by western blotting using monoclonal
anti-FLAG antibody (Sigma, St. Louis, MO, USA).
Western blot analysis
Following treatment of melanoma cells with or
without GSPs the cells were harvested, cell lysates were prepared
using ice-cold lysis buffer supplemented with protease inhibitors,
as previously detailed (20). In
case of tumor xenografts, tumor lysates were also prepared
following the same procedure after homogenizing the tumor samples
in lysis buffer. Western blot analysis was conducted as previously
detailed (11,20). The membrane was stripped and
re-probed with anti-β-actin antibody to verify equal protein
loading on the gel.
Athymic nude mice and tumor xenograft
model
Female athymic nude mice of 4–5 weeks of age were
purchased from the National Cancer Institute (NCI; Bethesda, MD,
USA) and housed in the Animal Resource Facility at the University
of Alabama at Birmingham in accordance with the Institutional
Animal Care and Use Committee (IACUC) guidelines. The mice were
given control AIN76A diet with or without supplementation with GSPs
(0.2 and 0.5%, w/w) and drinking water ad libitum throughout
the experiment. The animal protocol used in the present study was
approved by the IACUC. To determine the in vivo
chemotherapeutic efficacy of GSPs against tumor xenograft growth,
exponentially growing A375 cells (3×106 in 100 μl PBS)
or other cell lines were injected subcutaneously in the right flank
of each mouse. One day after tumor cell inoculation, mice were
divided randomly into different treatment groups with five mice per
group. One group of mice received the AIN76A control diet, while
other groups of mice received a 0.2 or 0.5% (w/w) GSPs-supplemented
AIN76A control diet in pellet throughout the experiment protocol.
The tumor growth/size and body weight of each mouse was recorded on
weekly basis. Tumor size was measured using Vernier calipers and
volumes were calculated using the hemiellipsoid model formula:
tumor volume = ½ (4π/3) (l/2) (w/2) h, where l is the length, w is
the width and h is the height. At the termination of the
experiment, mice were sacrificed, tumor from each mouse was excised
and the wet weight of each tumor was recorded on digital balance. A
part of the tumor was used to prepare tumor lysates for western
blot analysis and the other part of the tumor tissues was
paraffin-embedded and used for immunohistochemical analysis.
Immunohistochemical detection and
analysis of PCNA+ and activated caspase-3+
cells
Paraffin-embedded tumor sections (5 μm thick) were
deparaffinized, rehydrated and then antigen retrieval was carried
out, as previously described (21). The non-specific binding sites were
blocked with 3% bovine serum albumin in PBS buffer before
incubation with anti-PCNA or anti-cleaved caspase-3 specific
antibodies. After washing, the sections were incubated with
biotinylated secondary antibody followed by horseradish
peroxidase-conjugated streptavidin. The sections were further
incubated with 2,4-diaminobenzidine substrate and counterstained
with hematoxylin. The PCNA-positive and activated
caspase-3-positive positive cells in sections were counted in at
least 4–5 different fields and photographed using an Olympus
microscope (Model BX40F4; Olympus Tokyo, Japan) fitted with a
Q-Color 5 Olympus camera.
Statistical analysis
The statistical significance of the difference
between the values of control and treatment groups was determined
by either Student's t-test or simple one-way ANOVA using GraphPad
Prism version 4.00 for Windows, (GraphPad software, San Diego, CA,
USA). In each case, P<0.05 was considered statistically
significant.
Results
GSPs exert cytotoxic effects on melanoma
cells and inhibit their viability
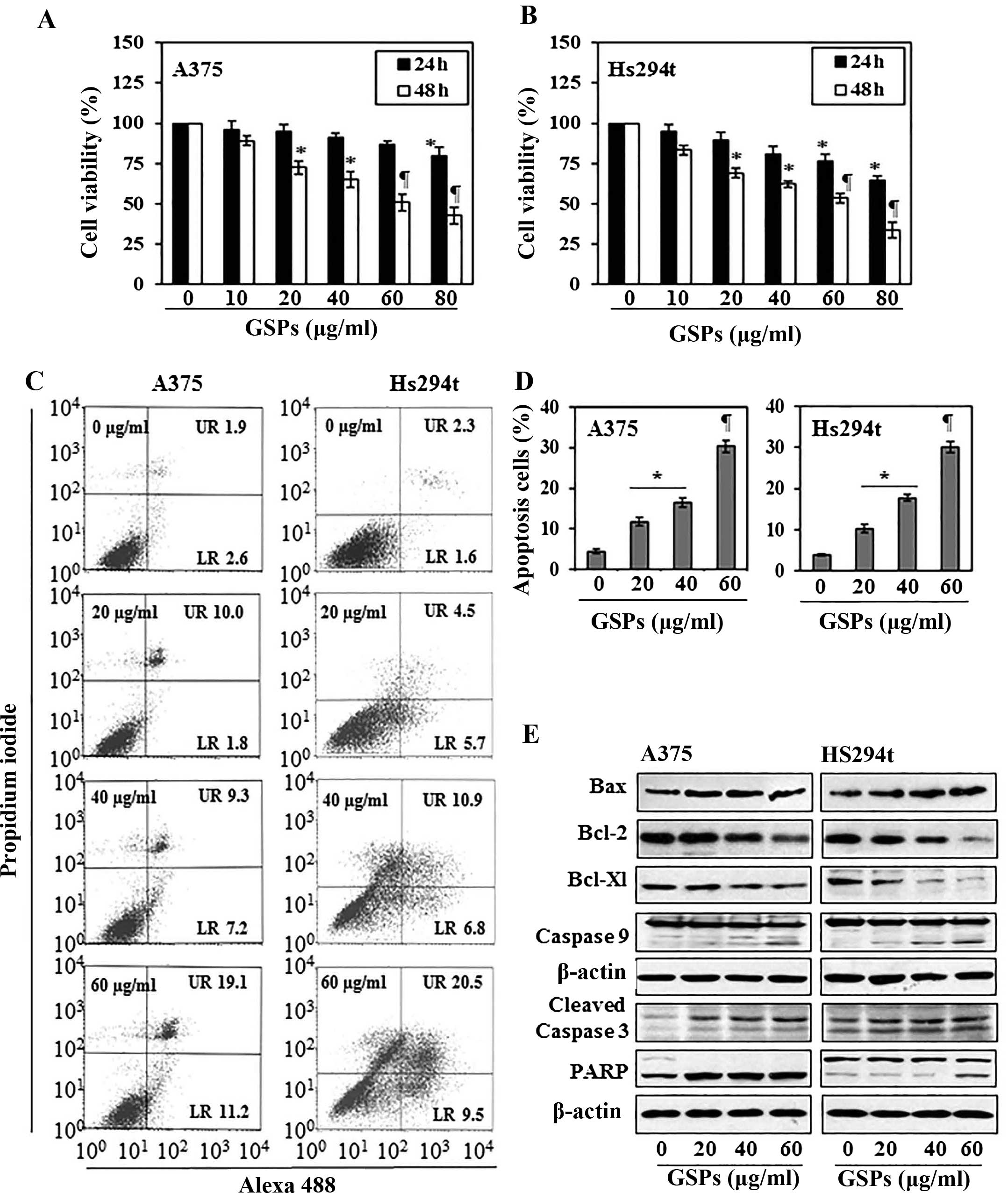
The cytotoxic effects of GSPs on melanoma cells,
A375 and Hs294t, were determined using MTT assay. As shown in
Fig. 1A, compared with the
non-GSP-treated control cells, treatment of A375 cells with GSPs
for 24 and 48 h resulted in a significant dose-dependent decrease
in cell viability. Treatment for 24 h resulted in a 4–20% decrease
in cell viability, while treatment for 48 h resulted in 11–57%
(P<0.01–0.001) decrease in cell viability. Under identical
conditions, GSPs exerted similar inhibitory effects on the
viability of Hs294t cells, as shown in Fig. 1B. However, the growth of normal
human epidermal melanocytes was not significantly affected by
GSPs-treatment under identical experimental conditions.
GSPs induce apoptosis in human melanoma
cells
To examine whether inhibition of cell viability in
A375 and Hs294t melanoma cells by GSPs is due to the induction of
apoptosis in these cells, the A375 and Hs294t cells were treated
with varying concentrations of GSPs (0, 20, 40 and 60 μg/ml) for 48
h and apoptotic cells were analyzed using the Alexa 488/PI
apoptotic cell detection kit. Apoptotic cell death was determined
in terms of early-stage and late-stage apoptotic cells, which are
shown respectively in the lower right (LR) and upper right (UR)
quadrants of the FACS histograms (Fig.
1C). Treatment of the A375 and Hs294t cells with GSPs for 48 h
resulted in significant induction of apoptosis in both cell lines.
The percentage of total apoptotic cells (in UR+LR quadrants) in
A375 cells after treatment with GSPs was as follows: 4.5%
(vehicle-treated control), 11.8% (20 μg/ml, P<0.05), 16.5% (40
μg/ml, P<0.01), 30.3% (60 μg/ml, P<0.001) as summarized in
Fig. 1D. Similarly, GSP-induced
apoptosis was also observed when Hs294t cells were treated with
GSPs for 48 h, as shown in Fig. 1C and
D.
GSPs affect the protein expression of
Bcl-2 family and activate caspase-3 and poly (ADP-ribose)
polymerase (PARP) in human melanoma cells
The proteins of the Bcl-2 family play critical roles
in regulation of apoptosis by acting as promoters or inhibitors of
cell death process (22,23). Therefore, the effect of GSPs on the
proteins of Bcl-2 family in both A375 and Hs294t cells was
determined. For this purpose, A375 and Hs294t cells were treated
with GSPs (0, 20, 40 and 60 μg/ml) for 48 h, and cell lysates were
prepared and subjected to western blot analysis. Western blot
analysis revealed that treatment of cells with GSPs resulted in a
dose-dependent reduction in the levels of the Bcl-2 and Bcl-xl
proteins with a concomitant increase in the levels of Bax compared
with the cells that were not treated with GSPs (Fig. 1E). These data indicate that GSPs
treatment can alter the protein levels of key members of the Bcl-2
family in a manner that contribute to the susceptibility of
melanoma cells to GSP-induced apoptosis (Fig. 1E). Western blot analysis also
revealed that treatment of A375 and Hs294t cells with GSPs enhanced
the activation or cleavage of caspase-3 and PARP when compared with
the cells which were not treated with GSPs (Fig. 1E). These events contribute to the
induction of cancer cell apoptosis.
GSPs reduce cellular accumulation of
β-catenin in melanoma cells
Studies have indicated that aberrant activation of
Wnt signaling leading to β-catenin overexpression or accumulation
is involved in cancers of various organs including melanoma.
Therefore, we determined the effect of GSPs on the levels of
β-catenin protein in both A375 and Hs294t cells using western blot
analysis. For this purpose melanoma cells were treated with GSPs
for 48 h and cytosolic and nuclear fractions were prepared. Western
blot analysis revealed that treatment of A375 and Hs294t cells with
GSPs resulted in reduced expression levels of β-catenin in both
nucleus as well as in cytoplasm of the cells (Fig. 2A). Since, cellular accumulation of
β-catenin is inversely correlated with phosphorylation at certain
key residues of β-catenin (Ser45, Ser33,
Ser37 and Thr41), we checked the effect of
GSPs on the levels of β-catenin phosphorylation at these sites.
Western blot analysis of the cytoplasmic fraction of melanoma cells
revealed that treatment of A375 and Hs294t cells with GSPs
increased the phosphorylation of β-catenin at Ser45, and
Ser33/Ser37/Thr41 in both melanoma
cell lines (Fig. 2A). Furthermore,
GSP treatment of melanoma cells resulted in a dose-dependent
increase of CK1α and GSK-3β. Both CK1α and GSK-3β are known to
target β-catenin for proteasomal degradation via combined
phosphorylation at key residues of β-catenin (24). These results suggest that GSPs
enhance melanoma cell apoptosis by targeting β-catenin in both A375
and Hs294t cells.
To further evaluate if β-catenin is a molecular
target of GSPs, we used melanoma cell lines that differ in the
activation status of β-catenin and compared the cytotoxic effects
of GSPs. For this purpose Mel928 and Mel1011 cell lines were
treated with GSPs (0, 20, 40, 60, 80 and 100 μg/ml) for 24 and 48
h. Cells were harvested and cytotoxicity of GSPs was evaluated
using trypan blue staining assay. Mel928 cells exhibit
constitutively activated β-catenin signaling, while Mel1011 cells
exhibit inactivated β-catenin. As shown in Fig. 2B, treatment of Mel928 cells with
GSPs significantly enhanced cell death (P<0.01–0.001) in a dose-
and time-dependent manner. Resultant data on cell death is
summarized in terms of percentage of dead cells ± SD for different
treatment groups. However, GSPs failed to exert significant
cytotoxic effects in Mel1011 cells (Fig. 2B, right panel).
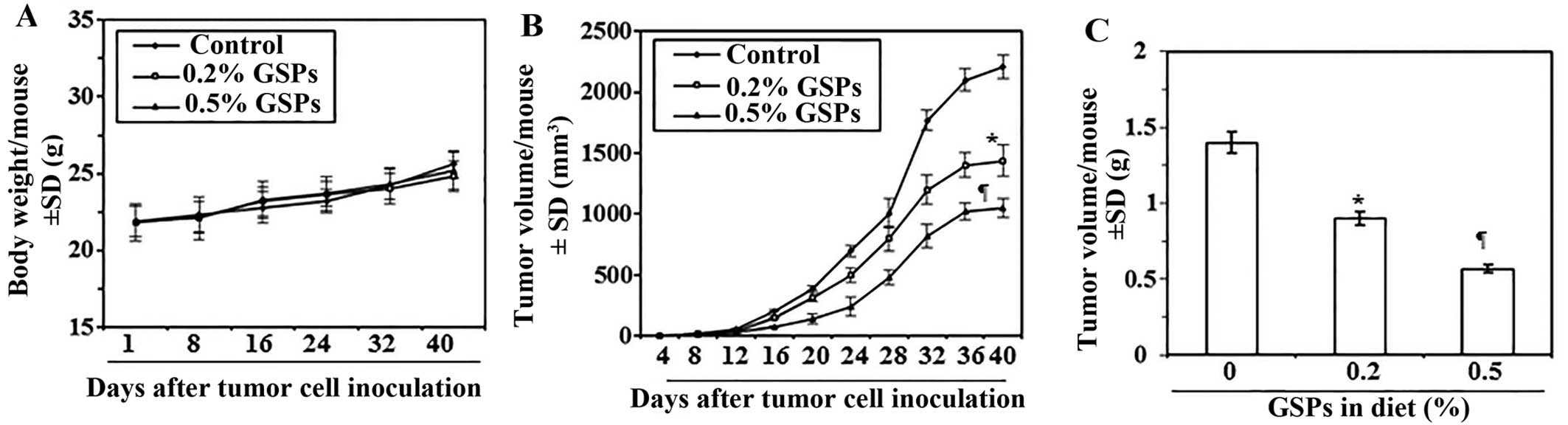
Dietary GSPs inhibit the growth of tumor
xenograft of melanoma cells in nude mice
Next, we sought to determine whether dietary
administration of GSPs inhibit in vivo tumor xenograft
growth in a nude mouse model. As the growth inhibitory effect of
GSPs on both A375 and Hs294t cell lines were identical, we selected
the A375 cell line for further in vivo studies. The mice
were given the AIN76A control diet alone or the same diet
supplemented with GSPs (0.2 and 0.5%, w/w). The dietary doses of
GSPs used in the present study are based on our previous reports of
tumor growth inhibitory effects of GSPs in non-melanoma and
non-small cell lung cancer models (3,9). The
average body weights of mice which received control AIN76A or GSPs
supplemented diet were comparable throughout the experimental
protocol (Fig. 3A). The mice that
were given GSPs in diet did not exhibit any physical sign of
toxicity or abnormal behavior (data not shown). Weekly measurement
of the tumor volume indicated that the average tumor growth in
terms of tumor volume/mouse was lower in the GSPs-fed mice than the
control diet group (Fig. 3B). As
shown in Fig. 3B, on termination
of the study at day 40 the average tumor volume in mice that
received 0.2% GSPs and 0.5% GSPs in diet was, respectively, 35 and
52% less (P<0.01–0.001) than average volume of tumors from mice
which were given control diet. The experiment was terminated at 40
days after tumor cell implantation. At this time the mice were
sacrificed, the tumors harvested, and the wet weight of the
tumor/mouse in each treatment group was recorded. As shown in
Fig. 3C, the wet weight of the
tumors was 36–60% lower (P<0.01–0.001) in mice which were given
GSPs (0.2 and 0.5%, w/w) supplemented diet as compared to the
tumors from mice given control AIN76A diet. As the chemotherapeutic
effect of 0.5% GSP-supplemented diet on tumor growth was >0.2%
GSPs in diet, the tumor xenografts tissues from 0.5% GSP-treated
group were subsequently used for comparison of biochemical
parameters with the tumor xenografts from control AIN76A diet-fed
group.
Dietary GSPs enhance apoptotic cell death
of melanoma cells in tumor xenograft tissues
To determine whether GSP-induced inhibition of tumor
xenograft growth is due to induction of apoptosis in cancer cells,
we determined the effect of GSPs on the markers of apoptosis in
xenograft samples. Western blot analysis revealed that tumor
xenografts of mice which were given GSPs showed enhanced expression
of proapoptotic protein Bax while the expression of anti-apoptotic
proteins Bcl-2 and Bcl-xl were decreased compared with tumor
xenografts of mice which were given control AIN76A diet (Fig. 4A). Compared to tumor xenografts of
the control diet group, the enhanced expression of caspase-3,
caspase-9 and cleavage of PARP proteins was also observed in the
melanoma tumor xenograft of mice given GSPs in diet (Fig. 4A). The proapoptotic effects of GSPs
were further confirmed by immunohistochemical detection of
activated caspase-3-positive cells in tumor xenograft samples
(Fig. 4B). The percentage of
activated caspase-3-positive cells in tumor samples from mice that
were given GSPs was significantly higher (P<0.001) than the
percentage of caspase-3-positive cells in the tumors of the
untreated control mice (Fig.
4C).
Dietary GSPs inhibit the proliferative
potential and the expression of cell cycle regulatory proteins of
G0/G1 phase in melanoma tumor xenograft tissues
Uncontrolled proliferation of tumor cells is a
characteristic feature of most cancers. Therefore, to explain the
growth inhibitory effect of dietary GSPs on tumor xenografts, we
analyzed the melanoma tumor xenografts for the potential
antiproliferative effects of GSPs using immunohistochemical
detection of PCNA-positive cells. The results of the
immunohistochemical detection of PCNA-positive cells in tumor
xenograft tissues indicated that the percentage of proliferating
cells was significantly reduced (78%, P<0.001) in tumor
xenografts from mice given GSP-supplemented diet than tumor
xenografts of GSP-untreated control mice (Fig. 4D and E). Anti-proliferative effects
of GSPs were further verified by western blot analysis for PCNA and
markers of cell cycle regulatory proteins, such as cyclins (D1 and
D2) and Cdks. Western blot analysis revealed that the levels of
PCNA, cyclins and Cdks were lower in tumor xenografts of
GSP-treated mice compared with tumor xenografts from control mice
(Fig. 4F). Additionally, the
expression levels of tumor suppressor proteins, Cip1/p21 and
Kip1/p27, were restored or reactivated in tumors of GSP-treated
mice than the tumors from mice which were given control diet
(Fig. 4F, right panel).
GSPs reduce β-catenin levels in melanoma
tumor xenografts
As β-catenin has been involved in tumor progression,
we determined whether dietary GSPs have any effect on the
expression level of β-catenin in melanoma tumor xenografts. As
shown in Fig. 5, western blot
analysis revealed that treatment of GSPs reduced the nuclear as
well as cytosolic levels of β-catenin in tumor xenograft samples.
Similarly, the phosphorylation of β-catenin at Ser45,
and other target residues
(Ser33/Ser37/Thr41), and the
levels of CK1α and GSK-3β were higher in tumor xenografts of mice
which received GSPs in diet than the tumor xenografts of control
mice.
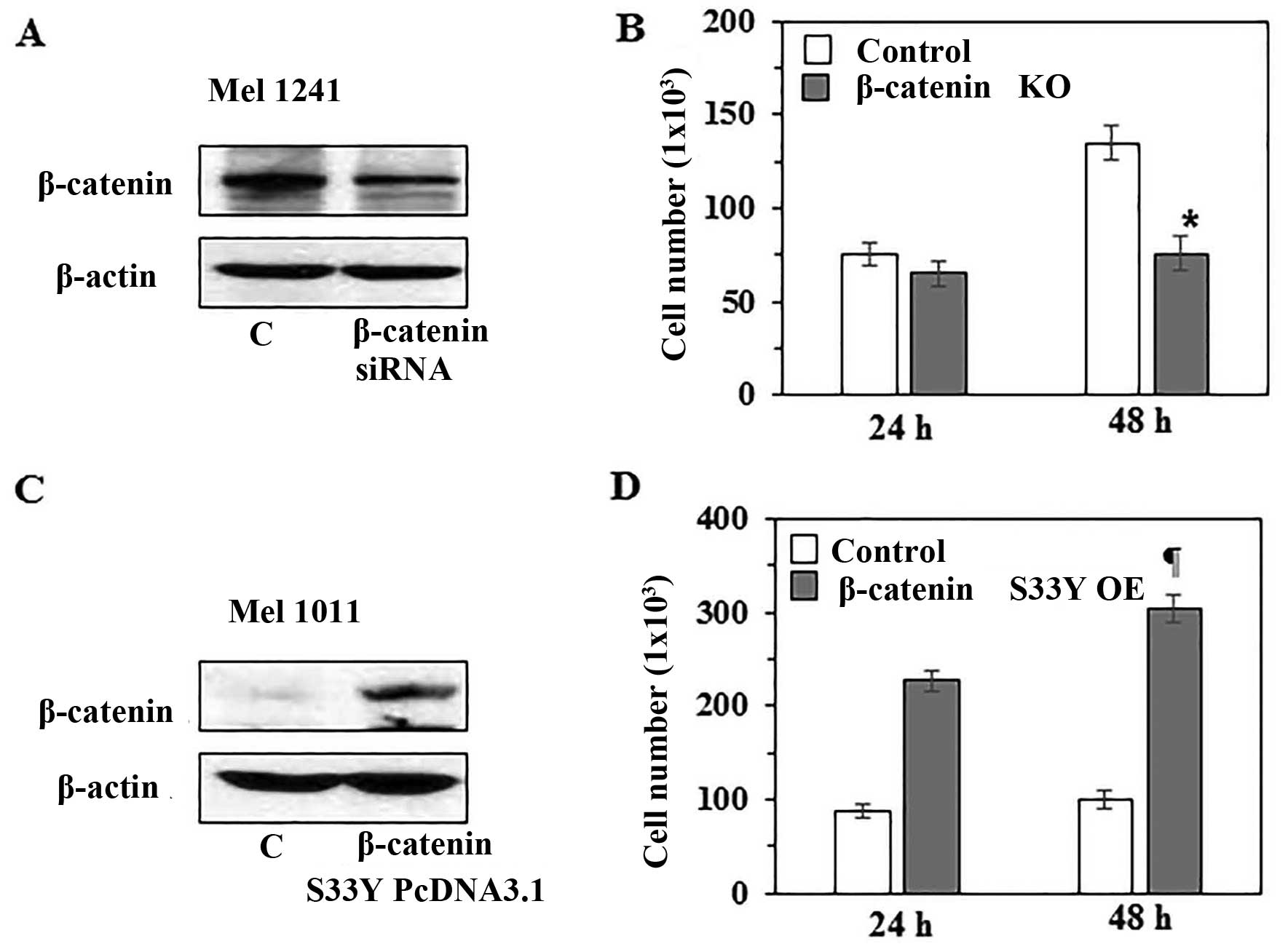
β-catenin level in human melanoma cells
determines the rate of melanoma cell growth
To further verify the role of β-catenin in melanoma
cell growth, in vitro experiments were conducted. For this
purpose, we knocked-down β-catenin in Mel1241 cells using β-catenin
specific siRNA kit (Santa Cruz Biotechnology), and this led to
reduced expression of β-catenin compared with scrambled RNA
transfected control Mel1241 cells, as analyzed by western blot
analysis (Fig. 6A). To determine
whether the knockdown of β-catenin affects cell proliferation,
control Mel1241 cells and β-catenin knockdown Mel1241 cells were
cultured for 24 and 48 h. Then, cells were harvested and counted
under microscope using hemocytometer. As shown in Fig. 6B, knockdown of β-catenin was
associated with reduced proliferation. The number of β-catenin
knockdown Mel1241 cells was significantly (44%, P<0.01) less
than the control Mel1241 cells after 48 h of culture. In another
experiment, we examined the effect of β-catenin
forced-overexpression in Mel1011 cells (β-catenin inactivated cell
line) by transfecting the cells with pcDNA3-β-catenin
S33Y plasmid. Forced overexpression of degradation
resistant mutant form of β-catenin (β-cateninS33Y) was
confirmed by western blotting using anti-FLAG antibody (Fig. 6C). Cells were cultured for 24 and
48 h, thereafter cells were harvested and counted under a
microscope. It was observed that forced overexpression of mutant
β-catenin in Mel1011 cells resulted in increased cell proliferation
rate compared with proliferation rate of control non-transfected
Mel1011 cells. As shown in Fig.
6D, after 48 h of culture the number of β-catenin
overexpressing Mel1011 cells was significantly higher (67%,
P<0.001) than control Mel1011 cells.
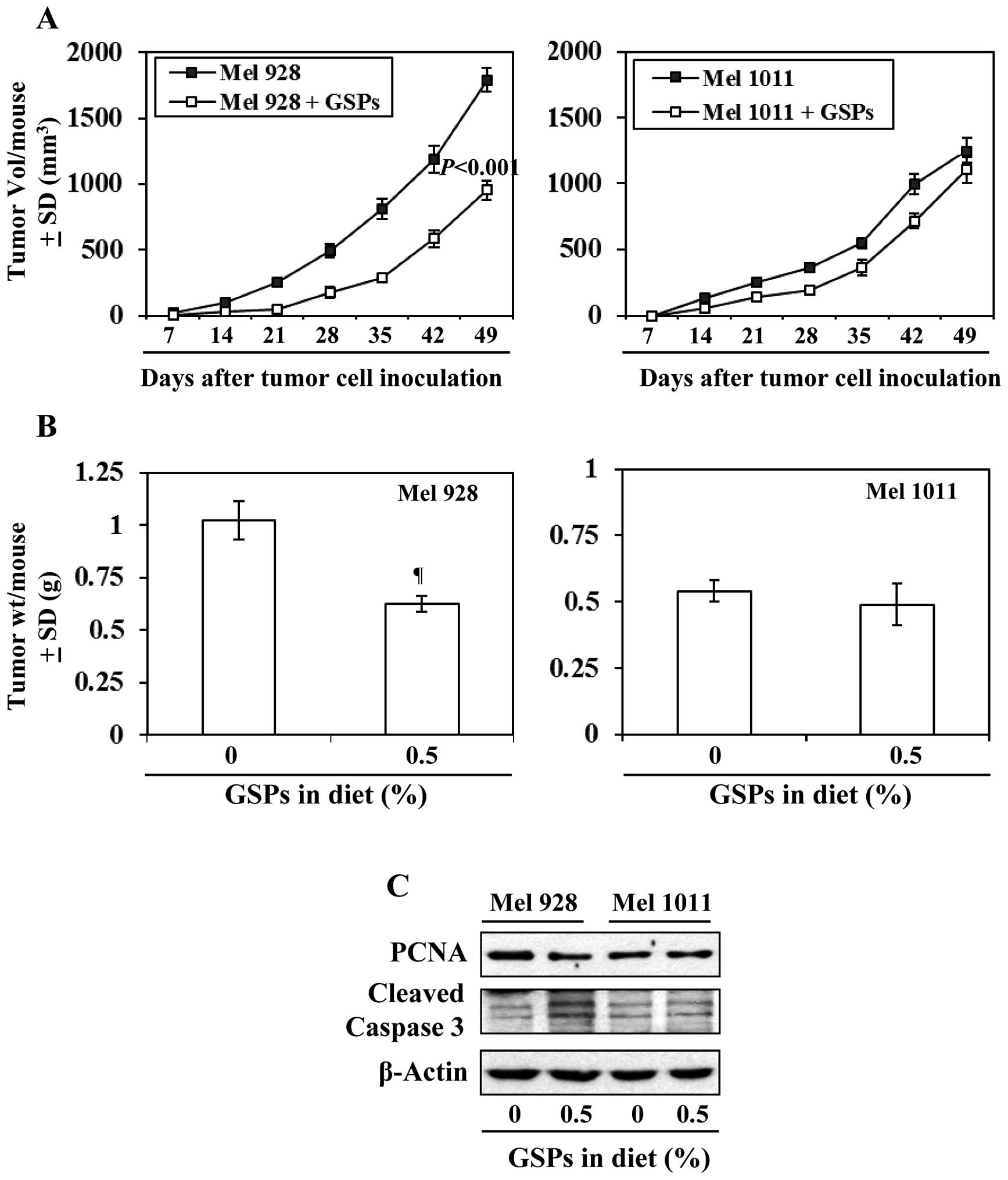
Effect of dietary GSPs on tumor xenograft
growth of β-catenin-proficient (active) and β-catenin-deficient
(inactive) melanoma cells
As inhibition of melanoma xenograft growth by GSPs
is associated with reduced levels of β-catenin, we further
determined and compared the efficacy of dietary GSPs (0.5%) on the
tumor xenograft growth of melanoma cells with a difference in
Wnt/β-catenin activity (Mel928 vs. Mel1011) and those cells were
subcutaneously implanted (2×106 cells/mouse) in nude
mice. Experiment was continued for 49 days based on IACUC
guidelines. As shown in Fig. 7A
(left panel), significant inhibition (P<0.001) in tumor
xenograft growth was observed in mice which were implanted with
Mel928 cells (β-catenin proficient) and fed with GSPs-supplemented
diet. At the termination of the experiment at day 49 the average
tumor volume in the mice which received 0.5% GSP supplemented diet
was 47% less (P<0.001) compared with the tumor volume of Mel928
tumor xenografts of mice which received control AIN76A diet
(Fig. 7A). However, GSPs failed to
inhibit the xenograft growth of Mel1011 cells (β-catenin-deficient)
in an identical experimental protocol (Fig. 7A). Additionally, the tumor
xenograft volume of Mel928 cells was greater than tumor xenograft
volume of Mel1011 at the termination of the experiment. The wet
weights of the tumors from each treatment group at the termination
of the experiment were recorded (Fig.
7B). It was observed that: i) dietary GSPs resulted in
significant reduction in weight (39%, P<0.01) of Mel928 tumor
xenografts. In contrast, GSPs did not have significant inhibitory
effect on the weight of tumor xenografts of Mel1011 cells, which
have inactivated β-catenin. ii) The wet weight of Mel928 tumor
xenografts recorded after 49 days of protocol was higher than the
wet weight of Mel1011 tumor xenografts. Additionally, western blot
analysis revealed that dietary GSPs reduced the expression of PCNA
and increased expression of activated caspase-3 in Mel928 tumor
xenografts, whereas this effect of GSPs was not observed in tumor
xenografts of Mel1011 cells (Fig.
7C).
Discussion
The antitumor activity of GSPs has been shown in
some preclinical models (3–11),
however, the antitumor activity of GSPs has not been explored in
melanoma. We therefore determined the therapeutic effects of GSPs
on melanoma cell lines using both in vitro and in
vivo models. Here, we report that GSPs significantly decrease
the viability and induce apoptotic cell death of human melanoma
cell lines, which are BRAF-mutated (A375) and
non-BRAF-mutated but highly specific to metastasis (Hs294t).
Notably, GSPs did not exhibit significant cytotoxicity to normal
human epidermal melanocytes under identical experimental
conditions. A major apoptotic signal transduction cascade
associated with induction of apoptosis includes the proteins of
Bcl-2 family, which either promote cell survival or promote
apoptosis (25,26). We found that treatment of A375 and
Hs294t cells with GSPs resulted in a dose-dependent decrease in the
levels of anti-apoptotic proteins (Bcl-2, Bcl-xl) and a
simultaneous increase in the pro-apoptotic protein (Bax). It is
well known that the cleavage of caspases and PARP contributes in
cancer cell apoptosis. Our data suggest that GSPs-induced apoptosis
in melanoma cells is mediated through activation of caspase-3,
caspase-9 and PARP, and this may be a possible mechanism of
GSP-induced apoptosis in melanoma cells.
The molecular mechanisms underlying the progression
of melanoma remain unresolved. Various studies have implicated
constitutively active Wnt/β-catenin signaling in melanoma
progression and metastasis (12,13).
Nuclear β-catenin accumulation has been correlated with late stages
of tumor progression. The presence of mutated β-catenin is
associated with aggressive tumor growth and regulates expression of
various target genes that mediate cellular processes including
proliferation and migration (27,28).
In the canonical model of Wnt signaling, β-catenin is
phosphorylated at certain key residues by GSK-3β and casein kinase
1 α (CK1α) leading to its ubiquitination and subsequent degradation
(24). Like cancers of other
organs, the regulation of β-catenin is lost in melanoma (29–31).
This then leads to nuclear accumulation of β-catenin and subsequent
stimulation of downstream target genes, which includes the genes of
cell proliferation (e.g., PCNA, cyclins and cyclin-dependent
kinases) and tumor progression (e.g., matrix metalloproteinases)
(32–34). Our results show that inhibition of
melanoma cell growth by GSPs is associated with the reduction in
the accumulation of nuclear and cytosolic β-catenin in melanoma
cells. It has been shown that phosphorylation of β-catenin at
critical target residues (such as at Ser45,
Ser33/37 and Thr41) by GSK-3β and CK1α within
the cytosolic destruction complex leads to degradation of β-catenin
and thus reduces its nuclear accumulation (24). In the present study, we found that
treatment of melanoma cells with GSPs enhances the expression of
GSK-3β and CK1α, and β-catenin is phosphorylated at critical target
residues. This effect then leads to degradation of β-catenin within
the degradation complex resulting in its reduced nuclear and
cytosolic accumulation. It thus explains inhibitory effects of GSPs
against melanoma cell growth.
To verify therapeutic effects of GSPs obtained in
in vitro cell culture system, in vivo studies were
conducted using in vivo tumor xenograft model. The GSPs were
administered in the diet of the mice as this approach was proven
effective in other cancers (4,8–11).
The present study provides evidence that dietary administration of
GSPs inhibits the growth of A375 tumor xenografts in the athymic
nude mice without any apparent sign of toxicity. The identification
of molecular targets is an important consideration in terms of
monitoring the clinical efficacy of GSPs in suggesting potential
combinations with other agents or drugs. In this context, the
inhibitory effect of dietary GSPs on the growth of tumor xenograft
in athymic nude mice was studied and found to be associated with
the: i) induction of apoptotic cell death of tumor cells, as
indicated by the analysis of the proteins of Bcl-2 family and
activated caspase-3 and PARP proteins; ii) inhibition of PCNA; iii)
control of cell cycle regulatory proteins; and iv) reduction in the
levels of cytosolic and nuclear β-catenin in tumor xenograft
samples at the termination of the in vivo animal
experiments. In an attempt to further verify the role of GSPs in
melanoma growth by targeting β-catenin, the siRNA knockdown of
β-catenin in Mel1241 cells (β-catenin-activated cell line) resulted
in suppression of cell proliferation, while forced expression of
β-catenin in Mel1011 cells (β-catenin-inactivated) resulted in
enhanced proliferation rate of these melanoma cells. These
observations were further supported by another in vivo tumor
xenograft experiment in which Mel928 (β-catenin activated) and
Mel1011 (β-catenin-inactivated) cells were subcutaneously implanted
and mice given AIN76A control diet with and without supplementation
of GSPs (0.5%, w/w). Administration of dietary GSPs significantly
inhibited the growth of Mel928 melanoma tumor xenografts, but
failed to inhibit the xenograft growth of Mel1011, thus, further
supporting our hypothesis that inhibition of melanoma growth by
dietary GSPs is mediated through inhibition of accumulation of
β-catenin in melanoma cells.
In summary, the outcome of this study suggests that
GSPs have the ability to block or inhibit the growth potential of
melanoma cells, and this effect of GSPs is mediated through
targeting β-catenin and its signaling molecules in melanoma. Thus
intervention strategies targeting key molecules of the
Wnt/β-catenin pathway may represent promising strategies to inhibit
the growth and progression of melanoma. This new insight into the
anti-melanoma activity of GSPs could serve as the basis for
alternative therapy of malignant melanoma as a single agent or as a
combination with already known drugs of melanoma in high risk
individuals.
Acknowledgements
The present study was supported by grants from the
National Institutes of Health (NIH, CA166883) and the Veterans
Administration Merit Review Award (1I01BX001410) to S.K.K.
References
|
1
|
Hall HI, Miller DR, Rogers JD and Bewerse
B: Update on the incidence and mortality from melanoma in the
United States. J Am Acad Dermatol. 40:35–42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strouse JJ, Fears TR, Tucker MA and Wayne
AS: Pediatric melanoma: Risk factor and survival analysis of the
surveillance, epidemiology and end results database. J Clin Oncol.
23:4735–4741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mittal A, Elmets CA and Katiyar SK:
Dietary feeding of proanthocyanidins from grape seeds prevents
photocarcinogenesis in SKH-1 hairless mice: Relationship to
decreased fat and lipid peroxidation. Carcinogenesis. 24:1379–1388.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma SD, Meeran SM and Katiyar SK:
Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative
stress and activation of mitogen-activated protein kinases and
nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol
Cancer Ther. 6:995–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nandakumar V, Singh T and Katiyar SK:
Multi-targeted prevention and therapy of cancer by
proanthocyanidins. Cancer Lett. 269:378–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaid M, Singh T and Katiyar SK: Grape seed
proanthocyanidins inhibit melanoma cell invasiveness by reduction
of PGE2 synthesis and reversal of epithelial-to-mesenchymal
transition. PLoS One. 6:e215392011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Q, Prasad R, Rosenthal E and Katiyar
SK: Grape seed proanthocyanidins inhibit the invasive potential of
head and neck cutaneous squamous cell carcinoma cells by targeting
EGFR expression and epithelial-to-mesenchymal transition. BMC
Complement Altern Med. 11:134–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meeran SM, Vaid M, Punathil T and Katiyar
SK: Dietary grape seed proanthocyanidins inhibit 12-O-tetradecanoyl
phorbol-13-acetate-caused skin tumor promotion in
7,12-dimethylbenz[a] anthracene-initiated mouse skin, which is
associated with the inhibition of inflammatory responses.
Carcinogenesis. 30:520–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akhtar S, Meeran SM, Katiyar N and Katiyar
SK: Grape seed proanthocyanidins inhibit the growth of human
non-small cell lung cancer xenografts by targeting insulin-like
growth factor binding protein-3, tumor cell proliferation, and
angiogenic factors. Clin Cancer Res. 15:821–831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prasad R, Vaid M and Katiyar SK: Grape
proanthocyanidin inhibit pancreatic cancer cell growth in vitro and
in vivo through induction of apoptosis and by targeting the
PI3K/Akt pathway. PLoS One. 7:e430642012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prasad R and Katiyar SK: Bioactive
phytochemical proanthocyanidins inhibit growth of head and neck
squamous cell carcinoma cells by targeting multiple signaling
molecules. PLoS One. 7:e464042012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sinnberg T, Menzel M, Kaesler S,
Biedermann T, Sauer B, Nahnsen S, Schwarz M, Garbe C and Schittek
B: Suppression of casein kinase 1alpha in melanoma cells induces a
switch in beta-catenin signaling to promote metastasis. Cancer Res.
70:6999–7009. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Syed DN, Afaq F, Maddodi N, Johnson JJ,
Sarfaraz S, Ahmad A, Setaluri V and Mukhtar H: Inhibition of human
melanoma cell growth by the dietary flavonoid fisetin is associated
with disruption of Wnt/β-catenin signaling and decreased Mitf
levels. J Invest Dermatol. 131:1291–1299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lucero OM, Dawson DW, Moon RT and Chien
AJ: A re-evaluation of the ‘oncogenic’ nature of Wnt/beta-catenin
signaling in melanoma and other cancers. Curr Oncol Rep.
12:314–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Delmas V, Beermann F, Martinozzi S,
Carreira S, Ackermann J, Kumasaka M, Denat L, Goodall J, Luciani F,
Viros A, et al: Beta-catenin induces immortalization of melanocytes
by suppressing p16INK4a expression and cooperates with N-Ras in
melanoma development. Genes Dev. 21:2923–2935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chien AJ, Moore EC, Lonsdorf AS,
Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL
and Moon RT: Activated Wnt/beta-catenin signaling in melanoma is
associated with decreased proliferation in patient tumors and a
murine melanoma model. Proc Natl Acad Sci USA. 106:1193–1198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maelandsmo GM, Holm R, Nesland JM, Fodstad
Ø and Flørenes VA: Reduced beta-catenin expression in the cytoplasm
of advanced-stage superficial spreading malignant melanoma. Clin
Cancer Res. 9:3383–3388. 2003.PubMed/NCBI
|
|
18
|
Kageshita T, Hamby CV, Ishihara T,
Matsumoto K, Saida T and Ono T: Loss of beta-catenin expression
associated with disease progression in malignant melanoma. Br J
Dermatol. 145:210–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bachmann IM, Straume O, Puntervoll HE,
Kalvenes MB and Akslen LA: Importance of P-cadherin, beta-catenin,
and Wnt5a/frizzled for progression of melanocytic tumors and
prognosis in cutaneous melanoma. Clin Cancer Res. 11:8606–8614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mantena SK, Sharma SD and Katiyar SK:
Berberine inhibits growth, induces G1 arrest and apoptosis in human
epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin
cascade, disruption of mitochondrial membrane potential and
cleavage of caspase 3 and PARP. Carcinogenesis. 27:2018–2027. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaid M, Sharma SD and Katiyar SK:
Honokiol, a phytochemical from the Magnolia plant, inhibits
photocarcinogenesis by targeting UVB-induced inflammatory mediators
and cell cycle regulators: Development of topical formulation.
Carcinogenesis. 31:2004–2011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adams JM and Cory S: Life-or-death
decisions by the Bcl-2 protein family. Trends Biochem Sci.
26:61–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chao DT and Korsmeyer SJ: BCL-2 family:
Regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hockenbery D, Nuñez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gavert N and Ben-Ze'ev A: β-Catenin
signaling in biological control and cancer. J Cell Biochem.
102:820–828. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rimm DL, Caca K, Hu G, Harrison FB and
Fearon ER: Frequent nuclear/cytoplasmic localization of
beta-catenin without exon 3 mutations in malignant melanoma. Am J
Pathol. 154:325–329. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rubinfeld B, Robbins P, El-Gamil M, Albert
I, Porfiri E and Polakis P: Stabilization of beta-catenin by
genetic defects in melanoma cell lines. Science. 275:1790–1792.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Demunter A, Libbrecht L, Degreef H, De
Wolf-Peeters C and van den Oord JJ: Loss of membranous expression
of beta-catenin is associated with tumor progression in cutaneous
melanoma and rarely caused by exon 3 mutations. Mod Pathol.
15:454–461. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lowy AM, Clements WM, Bishop J, Kong L,
Bonney T, Sisco K, Aronow B, Fenoglio-Preiser C and Groden J:
β-Catenin/Wnt signaling regulates expression of the membrane type 3
matrix metalloproteinase in gastric cancer. Cancer Res.
66:4734–4741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li YJ, Wei ZM, Meng YX and Ji XR:
Beta-catenin up-regulates the expression of cyclinD1, c-myc and
MMP-7 in human pancreatic cancer: Relationships with carcinogenesis
and metastasis. World J Gastroenterol. 11:2117–2123. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qi J, Chen N, Wang J and Siu CH:
Transendothelial migration of melanoma cells involves
N-cadherin-mediated adhesion and activation of the beta-catenin
signaling pathway. Mol Biol Cell. 16:4386–4397. 2005. View Article : Google Scholar : PubMed/NCBI
|