Introduction
Breast cancer is one of the most frequently
diagnosed cancers in women (1),
and >1.6 million new cases of breast cancer were identified in
2010 over the world (2). The
incidence of breast cancer has continued to rise in the last
several decades (2). The prognosis
of patients with local breast cancer has improved by recent
advances in therapy, however, that of patients with distant
metastasis is still poor (3). Most
patients with metastatic lesions are not treated by surgery since
the presence of one metastatic lesion often indicates the presence
of widespread systemic disease (3). Chemotherapy, hormonal, and radiation
therapies are used for palliative purposes in the metastasis
patients, and these therapeutic effects are modest, but achieve
statistically significant prolongation of survival (3). If there were more effective therapies
for metastasis, the survival rate of patients with metastasis is
expected to be further improved. More understanding of the
mechanisms of the complicated process of metastasis should help
develop more effective therapies. The metastasis process is thought
to consist of a serial, interlinked, and selective steps, namely,
metastatic cancer cells invade the surrounding stroma, intravasate,
survive in the circulation, become arrested in a distant capillary,
extravasate, and proliferate in target organ(s) (4). One of the major concepts in
metastasis research, the ‘seed’ and ‘soil’ theory, was proposed by
Stephen Paget in 1889 (5). Paget
hypothesized that the interaction between tumor cells (seed) and
host environment (soil) determines metastatic outcome (5). This hypothesis predicted that the
microenvironment of disseminated tumor cells might promote tumor
metastasis and contribute to the organ selectivity (3). Experimental animal models would be
powerful tools for understanding of the molecular events of
multistep process of metastasis. Several previous studies reported
that various highly metastatic cells were established and used for
elucidating metastatic mechanisms (6–8). In
these studies, a luciferase-based bioluminescence imaging technique
was used to detect metastatic sites and growth of tumor cells.
Bioluminescence imaging has high sensitivity, specificity, and
capacity for quantitative analysis. Although this imaging technique
is suitable for whole-body imaging, it has poor spatial resolution
(1–2 mm) due to diffusely scattered photons and results in
difficulty in detecting precise localization of tumor cells
(9). In contrast, fluorescence
imaging has high-resolution and provides localization of tumor
cells and molecules associated with metastasis at cellular and
subcellular resolutions (10).
Thus, a tumor cell expressing both luciferase and fluorescent
protein has the potential to be a more powerful tool for
elucidation of tumor metastasis mechanisms. We previously
established luciferase-expressing MDA-MB-231-5a-D (5a-D-Luc), a
highly metastatic breast cancer cell line derived from MDA-MB-231
(6). In the present study, we
introduced a green fluorescent protein ZsGreen1 to 5a-D-Luc cells,
and evaluated the utility for in vivo imaging with several
optical imaging methods.
Materials and methods
Cell culture and transfection
5a-D-Luc cells, a highly bone metastatic activity
cell line derived from MDA-MB-231 (6), were maintained in DMEM medium
supplemented with 5% FBS (JRH Biosciences, Lenexa, KS, USA) in a
humidified incubator maintained at 37°C with 5% CO2. The
5a-D-Luc cells were transfected with pZsGreen1-C1 vectors
(Clontech, Mountain View, CA, USA) using Lipofectamine 2000
Transfection Reagent (Life Technologies, Carlsbad, CA, USA), and
maintained in DMEM with 5% FBS and 1 mg/ml G418 (Roche, Basel,
Switzerland) to obtain stable transfectants. We subsequently
isolated five subclones of the transfectants stably expressing
ZsGreen1 protein (5a-D-Luc-ZsGreen).
In vitro evaluation of 5a-D-Luc-ZsGreen
cells
Cell proliferation assay was performed using a
sulforhodamine B as described previously (11). To evaluate fluorescence and
bioluminescence intensities in vitro, 5a-D-Luc-ZsGreen cells
were serially diluted from 105 to 104 cells
in 96-well plates. The fluorescence intensity was measured with a
preclinical in vivo imaging system IVIS Lumina (Xenogen,
Alameda, CA, USA). Then, the bioluminescence intensity (luciferase
activity) was measured using Steady-Glo Luciferase Assay system
(Promega, Madison, WI, USA) with the IVIS Lumina.
Animal models
The animal experiments were approved by the
Institutional Animal Care and Use Committee of the National
Institute of Radiological Sciences, and all animal experiments were
conducted in accordance with the institutional guidelines regarding
animal care and handling. To evaluate fluorescence and
bioluminescence intensities in vivo, female BALB/c-nu/nu
mice (5-week-old; CLEA Japan, Tokyo, Japan) were subcutaneously
inoculated with a 1:1 mixture of 5a-D-Luc-ZsGreen cells
(1×103, 1×104, 5×104 and
1×105 cells) and Matrigel (Becton-Dickenson Bioscience,
Bedford, MA, USA) on the dorsal side. For intracardiac injection,
female nude mice (6-week-old) were anesthetized by inhalation of 3%
isoflurane and injected with 5a-D-Luc or 5a-D-Luc-ZsGreen cells
(1×105 cells) in 100 μl PBS into the left cardiac
ventricle as described previously (6).
In vivo bioluminescence imaging
Mice were injected with 2.5 mg/mouse D-luciferin
(Wako Pure Chemical Industries, Osaka, Japan) into the tail vain,
and the bioluminescence images were acquired with the IVIS Lumina
under isoflurane anesthesia (3%). Mean radiance
(photon/s/cm2/sr) in region of interest (ROI) drawn over
bioluminescence signals was measured using Living Image software
(Xenogen).
Ex vivo fluorescence imaging
Brain, bone, and other organs with bioluminescence
signals were resected from the mice that received intracardiac
injection of 5a-D-Luc-ZsGreen cells at 7, 14, 21 and 28 days after
injection. The resected tissues were observed with a fluorescence
stereoscopic microscope MZ16F (Leica Microsystems, Wetzlar,
Germany).
Two-photon laser-scanning microscopic
imaging
For the surgical procedure, a mixture of air,
oxygen, and isoflurane (1.5–2%) anesthesia was given via face-mask.
The head of mouse was fixed with a stereotactic frame, and cranial
windows were prepared according to the ‘Seylaz-Tomita method’
(12). The cranial window was
attached over the left side of the somatosensory cortex using
dental cement (Luxaflow, DMG, Hamburg, Germany) and centered at 1.8
mm caudal and 2.5 mm lateral from the bregma. A custom-made metal
plate was affixed to the front of the central skull (12,13).
For real-time tracking imaging of tumor cells just after
inoculation, mice were anesthetized with 1.5% isoflurane and was
placed supine on a custom-made apparatus. Needle (30 G: Terumo,
Tokyo, Japan) with thin plastic tube (catheter) was inserted into
left cardiac ventricle of mice and was fixed with dental cement
(Ionosit, DMG). The head of a mouse intraperitoneally administered
10 mM SR101 (8 μl/g body weight) was placed under an objective lens
of two-photon laser scanning microscopy (TCS-SP5 MP, Leica
Microsystems). Immediately after initiation of two-photon imaging,
1×105 of 5a-D-Luc-ZsGreen cells were injected into the
mouse through the catheter. The wavelength of excitation laser was
900 nm. An emission signal was separated by a beam splitter (560/10
nm) and simultaneously detected through a band-pass filter for
SR101 (610/75 nm) and tumor cells (525/50 nm). A single image plane
consisted of 1,024 by 1,024 pixels, and in-plane pixel-size was
0.25–0.45 μm depending on an instrumental zoom factor. The images
were acquired at depth of 0.2–0.4 mm from the cortical surface. To
observe the moving cancer cells and cerebral vasculature on the
surface over the barrel cortex, dynamic imaging was started just
after inoculation and conducted at a rate of 0.15 sec per frame for
60 sec. For temporal imaging to observe change of tumor cells and
blood vessels until day 21 after injection, the mice without a
catheter were imaged as described above.
Histological analysis
After ex vivo imaging, the bone tissues were
fixed in 10% neutral-buffered formalin, decalcified with EDTA
treatment and embedded in paraffin. The tissue sections (1-μm
thick) were deparaffinized and stained with H&E or tartrate
resistant acid phosphatase (TRAP) staining kit (Wako Pure Chemical
Industries). After TRAP staining, the sections were counterstained
with hematoxylin. The brains were resected, embedded in frozen
section compound (Leica Microsystems) and frozen for cryostat
sectioning (8-μm thick). Immunofluorescence staining was conducted
using rat anti-CD31 antibody (BD Pharmingen, San Diego, CA, USA)
and Alexa Fluor 594-labeled anti-rat IgG (Molecular Probes, Eugene,
OR, USA) or rabbit anti-claudin-5 antibody (Abcam, Cambridge, UK)
and Alexa Fluor 594-labeled anti-rabbit IgG (Molecular Probes). The
slides were mounted in Vectashield mounting medium with DAPI
(Vector Laboratories, Burlingame, CA, USA). The stained tissues
were observed with a fluorescence microscope BX53 (Olympus, Tokyo,
Japan).
Statistical analysis
Statistical differences was analyzed by ANOVA with
the Dunnett's multiple comparison test, or by Student's t-test.
Correlation between the number of cells, and bioluminescence or
fluorescence intensity was calculated by Pearson's product
correlation coefficient.
Results
5a-D-Luc-ZsGreen cells
We transfected the ZsGreen1-expressing vector into
5a-D-Luc cells derived from MDA-MB-231 (6,7),
selected stably ZsGreen1-expressing cells, and then isolated five
subclones. One of the five subclones that showed the strongest
fluorescence intensity was selected for the following experiments
and was named 5a-D-Luc-ZsGreen. In the 5a-D-Luc-ZsGreen cells, the
signal of green fluorescence was observed in the cytoplasm
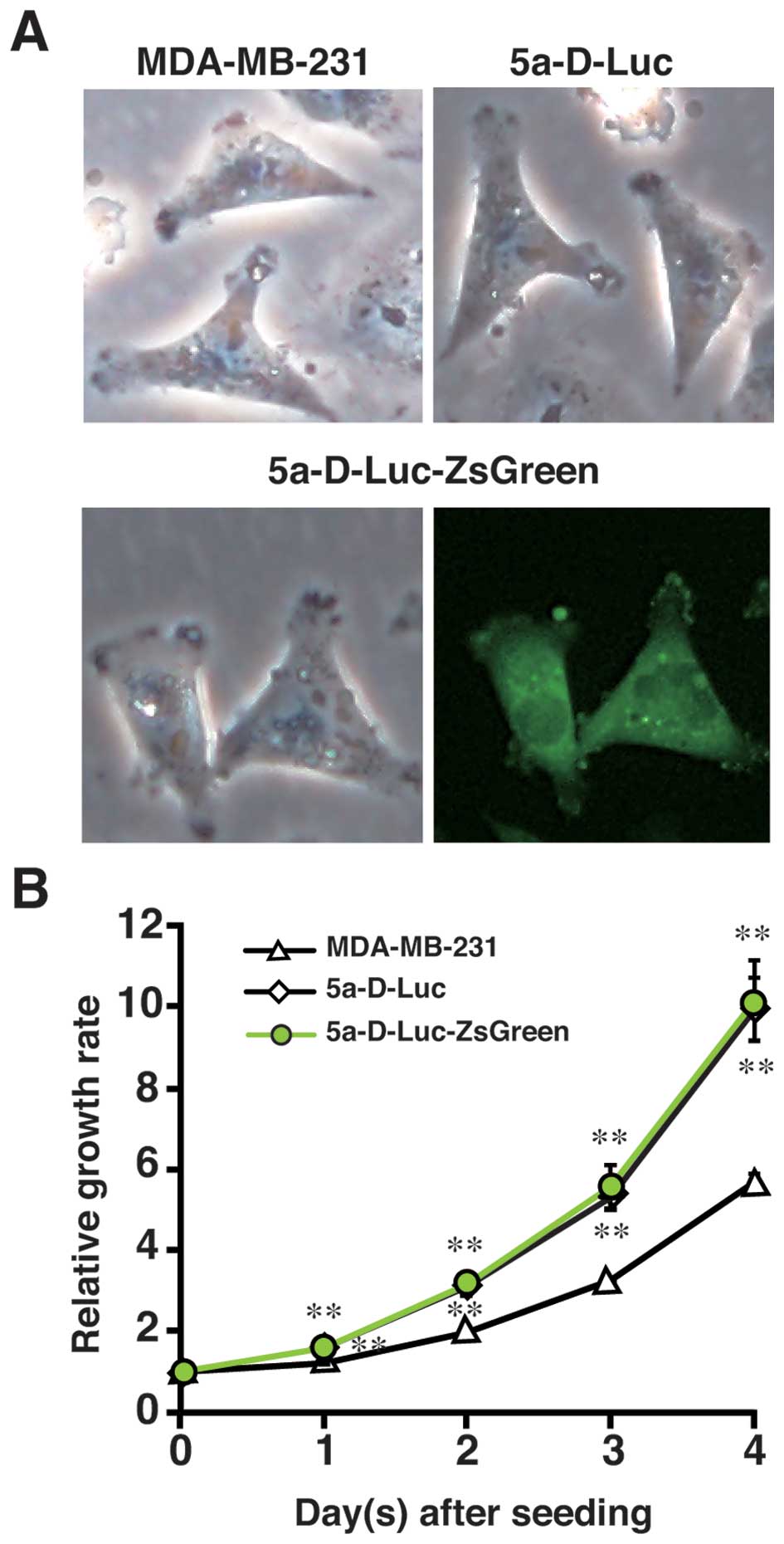
(Fig. 1A) and the fluorescence
intensity seemed to be stably maintained ≥12 passages in vitro
(data not shown). There were no morphological differences
between MDA-MB-231, 5a-D-Luc, and 5a-D-Luc-ZsGreen cells (Fig. 1A). The 5a-D-Luc and
5a-D-Luc-ZsGreen cells proliferated more rapidly than the parental
MDA-MB-231 cells (P<0.01) (Fig.
1B). No significant difference in growth rate was observed
between 5a-D-Luc and 5a-D-Luc-ZsGreen cells (Fig. 1B).
In vitro evaluation of
5a-D-Luc-ZsGreen
The bioluminescence and fluorescence intensities of
5a-D-Luc-ZsGreen were measured in vitro. Both
bioluminescence and fluorescence intensities were detected when
>10,000 cells were used. The number of cells was significantly
correlated with bioluminescence (R2 = 0.92, P<0.01)
and fluorescence intensity (R2 = 0.99, P<0.01)
(Fig. 2A and B).
In vivo evaluation of
5a-D-Luc-ZsGreen
To evaluate the correlation of the number of cells
with the bioluminescence and fluorescence intensities in
vivo, 3 h after subcutaneous inoculation of 5a-D-Luc-ZsGreen
cells into nude mice, the bioluminescence and fluorescence
intensities were measured. Both bioluminescence and fluorescence
intensities were detected when ≥10,000 cells were injected
(Fig. 2C and E). There were
significant correlations between the number of cells, and
bioluminescence (R2 = 0.89, P<0.01) or fluorescence
intensity (R2 = 0.84, P<0.01) (Fig. 2D and F).
Comparison of metastatic ability of
5a-D-Luc-ZsGreen and 5a-D-Luc
To compare the metastatic ability between 5a-D-Luc
and 5a-D-Luc-ZsGreen, these cells were injected into the left
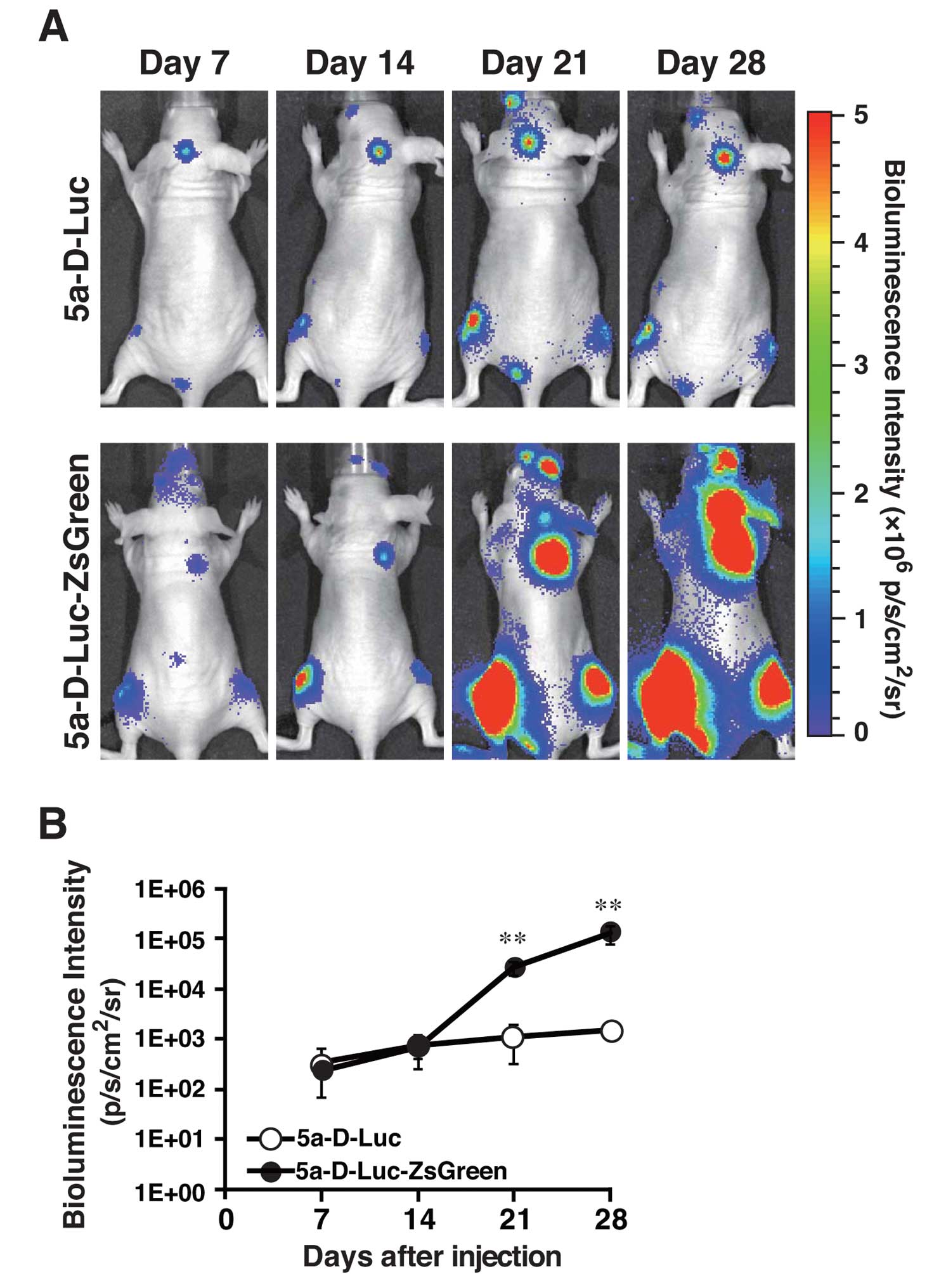
cardiac ventricle. As shown in Fig.
3A, bioluminescence signals were detected at 7 days after
inoculation with 1×105 cells, and the signal intensity
and the number of metastatic sites increased over time. The signal
intensity in metastatic regions in 5a-D-Luc-ZsGreen-inoculated mice
became stronger than that in 5a-D-Luc-inoculated mice 21 and 28
days after inoculation (P<0.01) (Fig. 3B). According to ex vivo
imaging and histological analysis, the 5a-D-Luc-ZsGreen cells
metastasized to bone (all of five mice), brain (all of five mice),
adrenal gland (two of five mice), and lymph node (one of five mice)
by day 28 after intracardiac inoculation. The 5a-D-Luc cells
metastasized to bone (all of five mice) and brain (two of five
mice), other tissue metastasis was not observed by day 28 after
intracardiac inoculation.
Bone metastasis of 5a-D-Luc-ZsGreen
cells
In all of the 5a-D-Luc-ZsGreen-injected mice, bone
metastases in femora, tibiae, and spine were observed. Other bone
metastases were also observed in mandibles (two of five mice),
scapula (three of five mice), wrist joint (three of five mice), and
incisor (two of five mice). The ZsGreen1 fluorescence was clearly
detected in the metastasized regions by ex vivo imaging by
fluorescence stereomicroscopy (Fig.
4A). The ZsGreen1 fluorescence was detected in the sections of
metastasized bone after formalin fixation and decalcification
(Fig. 4B and C). By the
histological analysis of bone metastasis sites, bone marrow cavity
was almost completely replaced by metastatic tumor cells at day 28
after intracardiac injection (Fig.
4B). Fluorescence imaging and TRAP staining showed clearly many
osteoclasts were localized between the tumor cells and bone
(Fig. 4C).
Multifocal metastasis of 5a-D-Luc-ZsGreen
cells in the brain
As shown in Fig.
5A, the green fluorescence of the tumor cells was widely
distributed in the whole brain just after intracardiac injection.
At 7 days after injection, only small and weak fluorescence foci
were detected, and subsequently the intensity and size of
metastatic foci increased over time (Fig. 5A). These findings were consistent
with the results in bioluminescence imaging as shown in Fig. 3. The 5a-D-Luc-ZsGreen cells
developed multifocal metastasis in the cerebrum, the cerebellum,
and leptomeninges. This feature is common to brain metastasis in
breast cancer patients (14).
Immunofluorescence staining of CD31 showed a close
association of vessels with tumor cells. At 5 min after injection,
tumor cells were detected within brain microvessels, especially at
vascular branching points (Fig.
5B, upper left panel). At day 14, tumor cells were extravasated
into surrounding tissues, and no cell was observed within the blood
vessels (Fig. 5B, upper right
panel). At day 21, tumor cells proliferated along microvessels
(Fig. 5B, lower left panel), and
alterations of vessel morphology and density were also observed at
day 28 (Fig. 5B, lower right
panel). By immunostaining for claudin-5, a tight junction marker
used for evaluating the integrity of blood-brain barrier (BBB), the
lack of claudin-5 expression was noted in a part of microvessels
near tumor metastatic sites at day 28 (Fig. 5C).
Real-time tracking and temporal imaging
of 5a-D-Luc-ZsGreen cells in the brain
Finally, in vivo two-photon laser-scanning
microscopy allowed real-time tracking of circulating tumor cells in
bloodstream of the brain. The tumor cells traveled through the
bloodstream in brain vessels just after intracardiac injection
(Fig. 5D). At 20 sec after
injection, some tumor cells were plugged in a blood vessel
branching point (Fig. 5D), and
most of these cells disappeared by day 5 after injection (data not
shown). Temporal imaging using two-photon microscopy revealed that:
i) tumor cells proliferated in the brain parenchyma and
microvessels formed in a single direction, and ii) microvessel
density increased as tumor cells proliferated until day 21 after
intracardiac injection (Fig.
5E).
Discussion
In the present study, we transfected an expressing
vector of ZsGreen1 into a metastatic cell line 5a-D-Luc (6,7)
which is a subclone of the MDA-MB-231 breast cancer cell line
expressing luciferase, and successfully established a new tumor
cell line 5a-D-Luc-ZsGreen expressing both luciferase and ZsGreen1.
Our in vivo experiments showed that the metastatic sites of
5a-D-Luc-ZsGreen and 5a-D-Luc cells were detected by
bioluminescence imaging from at least day 7 after intracardiac
injection. The 5a-D-Luc-ZsGreen cells developed multiple metastases
at high frequencies in bones and the brain like the parental cell
line 5a-D-Luc. Unexpectedly, the signal intensity of
bioluminescence in 5a-D-Luc-ZsGreen tumors was significantly
stronger than that of 5a-D-Luc tumors at 21 and 28 days after
injection, even though in vitro cell growth of the two lines
was not different. The bioluminescence intensity of
5a-D-Luc-ZsGreen and 5a-D-Luc cells was linearly correlated with
the number of cells in vitro and in vivo. These
results suggest that the 5a-D-Luc-ZsGreen cells have a higher
growth rate of tumor in vivo compared with 5a-D-Luc cells.
Interestingly, 5a-D-Luc-ZsGreen cells metastasized not only to
femur and the brain but also to incisor, adrenal gland, and lymph
node, unlike 5a-D-Luc cells. These findings suggest that
5a-D-Luc-ZsGreen cells acquired a more invasive and highly
metastatic phenotype compared with 5a-D-Luc cells. Further
comprehensive characterization of the two lines may provide new
insight into the molecular mechanisms of metastasis.
The approximate sites of metastatic 5a-D-Luc-ZsGreen
tumors were easily detected in live animal by bioluminescence
imaging, while the precise sites in excised organs and tissues were
easily detected by fluorescence stereoscopic microscopy. It allows
us to do efficient sampling of appropriate tissues for further
pathologic analysis. In tissue sections, the strong fluorescence of
5a-D-Luc-ZsGreen cells enables us to easily evaluate tumor spread
and localization at the cellular level. Wang et al
established MDA-MB-231 cells expressing both eGFP and luciferase
for an orthotopic mammary tumor model, and assessed the expression
of eGFP and luciferase in vitro and in vivo (15). Those cells were used only for
frozen sections (15), while the
5a-D-Luc-ZsGreen cells could be used not only for frozen sections
but also decalcified and paraffin-embedded sections in the present
study because the fluorescence intensity of ZsGreen1 remains after
paraffin fixation and decalcification (16). In addition, the ZsGreen1 protein
provides higher fluorescence intensities compared with eGFP
(17), suggesting that
5a-D-Luc-ZsGreen cells would be detected more easily in
vitro and in vivo. The above suggests that
5a-D-Luc-ZsGreen cells could be applied to all kinds of
histological techniques and useful for research on the interaction
of tumor cells with osteoclasts and the influence of tumor cells on
BBB integrity at cellular level in vivo.
Our results showed that two-photon laser-scanning
microscopy enabled real-time tracking and temporal imaging of
5a-D-Luc-ZsGreen cells at single cell level in vivo. In our
previous study, we showed that two-photon laser-scanning microscopy
allowed for three-dimensional volume imaging of rhodamine-labeled
cerebral microvessels up to a depth of 800 μm in the cortex of
anesthetized mice (18). In the
present study, 5a-D-Luc-ZsGreen cells were successfully detected up
to a depth of 600 μm over 21 days after intracardiac injection.
Moreover, we succeeded in temporal imaging of increased
angiogenesis accompanied by tumor growth at metastatic sites. The
results confirmed the stable and bright fluorescence of
5a-D-Luc-ZsGreen cells in vivo. The bright fluorescence was
also supported by our results of real-time tracking of circulating
5a-D-Luc-ZsGreen cells in the brain tissue microcirculation. Taken
together, two-photon laser-scanning microscopy in 5a-D-Luc-ZsGreen
cells enabled us to determine the detailed behavior of tumor cells
during the process of metastasis. Future studies should focus on
each process of tumor cell adhesion to microvessels, extravasation,
colonization, and influences on brain parenchymal cells, and these
analyses may provide new insight into the relationship of
circulating tumor cells with blood flow and vascular walls.
In conclusion, 5a-D-Luc-ZsGreen cells allowed for
easy detection of metastatic sites in the whole body and detailed
histological evaluation of the relationship between tumor cells and
molecules involved in metastasis at single cell level. Moreover,
the strong fluorescence of the cells enabled real-time tracking
imaging of circulating tumor cells immediately after intracardiac
injection and temporal imaging of tumor proliferation and
angiogenesis in vivo. These data demonstrated that our cells
would be a useful and powerful tool to further understanding the
mechanisms of tumor invasion, such as extravasation and
infiltration to tissue, at single cell level. Interestingly,
5a-D-Luc-ZsGreen cells are more metastatic compared with the
parental 5a-D-Luc cells, suggesting that further comprehensive
characterization of the two lines may provide a new insight into
mechanisms of metastasis.
Acknowledgements
The authors thank Yuriko Ogawa for technical
assistance and the staff in the Laboratory Animal Sciences section
for animal management.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Talmadge JE and Fidler IJ: AACR centennial
series: the biology of cancer metastasis: historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
6
|
Ehata S, Hanyu A, Fujime M, Katsuno Y,
Fukunaga E, Goto K, Ishikawa Y, Nomura K, Yokoo H, Shimizu T, et
al: Ki26894, a novel transforming growth factor-beta type I
receptor kinase inhibitor, inhibits in vitro invasion and in vivo
bone metastasis of a human breast cancer cell line. Cancer Sci.
98:127–133. 2007. View Article : Google Scholar
|
|
7
|
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y,
Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K and Imamura T:
Bone morphogenetic protein signaling enhances invasion and bone
metastasis of breast cancer cells through Smad pathway. Oncogene.
27:6322–6333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bos PD, Zhang XH, Nadal C, Shu W, Gomis
RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, et
al: Genes that mediate breast cancer metastasis to the brain.
Nature. 459:1005–1009. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Servais EL, Colovos C, Bograd AJ, White J,
Sadelain M and Adusumilli PS: Animal models and molecular imaging
tools to investigate lymph node metastases. J Mol Med Berl.
89:753–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kain SR, Adams M, Kondepudi A, Yang TT,
Ward WW and Kitts P: Green fluorescent protein as a reporter of
gene expression and protein localization. Biotechniques.
19:650–655. 1995.PubMed/NCBI
|
|
11
|
Sudo H, Tsuji AB, Sugyo A, Ogawa Y, Sagara
M and Saga T: ZDHHC8 knockdown enhances radiosensitivity and
suppresses tumor growth in a mesothelioma mouse model. Cancer Sci.
103:203–209. 2012. View Article : Google Scholar
|
|
12
|
Tomita Y, Kubis N, Calando Y, Tran Dinh A,
Méric P, Seylaz J and Pinard E: Long-term in vivo investigation of
mouse cerebral microcirculation by fluorescence confocal microscopy
in the area of focal ischemia. J Cereb Blood Flow Metab.
25:858–867. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takuwa H, Masamoto K, Yamazaki K,
Kawaguchi H, Ikoma Y, Tajima Y, Obata T, Tomita Y, Suzuki N, Kanno
I, et al: Long-term adaptation of cerebral hemodynamic response to
somatosensory stimulation during chronic hypoxia in awake mice. J
Cereb Blood Flow Metab. 33:774–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weil RJ, Palmieri DC, Bronder JL, Stark AM
and Steeg PS: Breast cancer metastasis to the central nervous
system. Am J Pathol. 167:913–920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang K, Xie S, Ren Y, Xia H, Zhang X and
He J: Establishment of a bioluminescent MDA-MB-231 cell line for
human triple-negative breast cancer research. Oncol Rep.
27:1981–1989. 2012.PubMed/NCBI
|
|
16
|
Harrell JC, Dye WW, Allred DC, Jedlicka P,
Spoelstra NS, Sartorius CA and Horwitz KB: Estrogen receptor
positive breast cancer metastasis: Altered hormonal sensitivity and
tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer
Res. 66:9308–9315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bell P, Vandenberghe LH, Wu D, Johnston J,
Limberis M and Wilson JM: A comparative analysis of novel
fluorescent proteins as reporters for gene transfer studies. J
Histochem Cytochem. 55:931–939. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masamoto K, Tomita Y, Toriumi H, Aoki I,
Unekawa M, Takuwa H, Itoh Y, Suzuki N and Kanno I: Repeated
longitudinal in vivo imaging of neuro-glio-vascular unit at the
peripheral boundary of ischemia in mouse cerebral cortex.
Neuroscience. 212:190–200. 2012. View Article : Google Scholar : PubMed/NCBI
|