Introduction
More than 200,000 deaths from colorectal cancer
(CRC) are reported in Europe annually (1) with little variation over the last 20
years in the incidence of hepatic metastatic disease as a first
presentation (2). Despite the
introduction of newer chemotherapies and targeted therapeutics as
adjuvant treatments following radical colorectal resection, 60% of
patients will eventually develop liver metastases (CRC LM)
(3). Most of these patients have
disseminated disease and if untreated, they have a median survival
of <1 year. For the minority of patients with disease confined
to the liver, hepatic resection with negative (R0) resection
margins remains the only potentially curative therapy (4,5).
Despite a more aggressive surgical approach towards hepatic
metastases, most patients with CRC LM even without demonstrable
extrahepatic disease are not candidates for resection either
because of bilobar disease that is not amenable to complete
extirpation or where the extent of metastatic disease precludes the
ability to leave a sufficiently functioning liver remnant. In an
attempt to enhance resectability, several selective strategies have
been tried including the use of neoadjuvant chemotherapy, 2-stage
hepatectomy and preoperative portal vein embolization (6,7) with
only a limited number deemed suitable for either a formal
hepatectomy or a non-anatomical wedge resection.
Interstitial thermo-ablative techniques such as
radiofrequency ablation (RFA) permit parenchyma-sparing treatment
of hepatic tumours in these clinical scenarios (8) with several studies reporting
long-term survival following cryosurgical ablation (CSA) in
selected cases (9,10) although in this technique the
instrumentation has proven cumbersome and substantial complication
rates have been reported (9,11).
Radiofrequency ablation is currently the most commonly used
ablative method with a number of studies reporting its inherent
safety (12–14). Although RFA has traditionally been
used in a palliative setting for CRC LM, it has been also utilized
as an adjunct to resection designed to improve metastasis
resectability. In this context the current data of combined
resection plus thermo-ablation are limited and the oncologic
outcome of this approach is uncertain (14,15–23).
The aim of the present study is to review our experience of
patients undergoing hepatic resection for CRC LM combined with
intraoperative thermo-ablation and to analyze the safety, utility
and oncologic outcome of this operative strategy.
Materials and methods
Data were obtained from a prospectively collated
hepatobiliary surgery database in a dedicated oncology institute
identifying a total of 360 patients with resected CRC LM was
included in this analysis, treated during January 1994-December
2014. Of this total, 80 patients (22%) underwent hepatic resection
combined with additional thermo-ablative procedures as the primary
treatment modalities. All patients routinely underwent preoperative
abdominal and pelvic computed tomographic (CT) scans, chest
roentgenograms or chest CT and colonoscopy. Other imaging studies
such as ultrasonography, magnetic resonance imaging and positron
emission tomography (PET) were obtained at the discretion of the
treating surgeon. Preoperative chest CT and PET scanning was
performed in 320 (89%), and 307 (85%) patients, respectively, in
accordance with our previously published guidelines (24). Liver function was evaluated with
standard serum biochemical tests and by the Childs-Pugh
classification. No patients with biochemical evidence of cirrhosis
were included in this series analysis.
A standardized approach to hepatic resection was
used, which has been published (4,24).
Briefly, this method involves the use of low central venous blood
pressure, vascular control, and parenchymal transection using a
clamp-crushing technique with an intermittent Pringle maneuver.
Intraoperative ultrasound (IOUS) was carried out in all patients
and was also used to guide placement of the RFA needle electrode.
With the application of radiofrequency energy, the electrode
delivers a high-frequency alternating current to the immediate
surrounding tissues. Inflow arrest was not routinely performed for
ablations. The RFA was administered by means of 17-gauge
multi-tined expandable RF needle electrodes (Med Italia Biomedica,
Medolla, Italy) using an RF generator (RF2000; Boston Scientific,
Marlborough, MA, USA). The formation of the typical hyperechoic
peri-lesional cloud was monitored by IOUS.
Unit indications/contraindications for
combined resection plus intraoperative RFA
Intraoperative thermal ablation was combined with
liver resection (when deemed necessary), so as to avoid extensive
hepatectomies particularly for deep liver metastases <4 cm in
diameter. For each patient, the decision to perform RFA in
association with surgical ablation of the liver metastases was
taken by a multidisciplinary team involving the surgeon, an
interventional radiologist and an oncologist and was based upon
both technical feasibility of the combined procedure and its
potential cost-effectiveness.
If extrahepatic disease was present, liver resection
was indicated for: i) resectable/ablatable pulmonary metastases;
ii) resectable/ablatable isolated extrahepatic sites (e.g., ovary
and lung); iii) local direct extension of liver metastases to the
diaphragm and/or the adrenal gland that can be resected.
Contraindications to liver resection included
uncontrolled extrahepatic disease such as widespread pulmonary
disease; diffuse peritoneal disease; extensive nodal disease (such
as retroperitoneal, mediastinal or portal nodes) or central nervous
system metastases. All patients submitted to intraoperative RFA
were followed-up by CT scan one month after the procedure to verify
the completeness of necrosis of the lesion. For the purposes of the
present study we divided patients into 2 groups: hepatic resection
only (group 1) and hepatic resection plus RFA (group 2).
Definitions
Resections were defined in accordance with the
Couinaud classification (25).
Resection of segments 4 through 8 is referred to as an extended
right hepatectomy; resection of segments 2 through 5 and 8 is an
extended left hepatectomy. A right hepatectomy is a resection of
segments 5 through 8; a left hepatectomy is resection of segments 2
through 4. Major hepatectomy was defined as resection of 3 or more
segments. The largest resection was labelled as the primary
procedure and additional smaller resections and ablations were
labelled as secondary procedures. Bilobar tumour involvement was
defined as tumour(s) involving any segments of the left and right
hemiliver. Failure of ablative treatment was defined as incomplete
ablation (judged on IOUS). In situ recurrence was defined as
radiologic (CT or magnetic resonance imaging) and/or histologic
(needle biopsy) detection of recurrent tumour at the original
ablation site during follow-up. Radiologic proof was evaluated by
sequential imaging demonstrating progression of disease.
Synchronous disease was defined as the identification of liver
metastases within 1 year from the date of resection of the primary
colorectal carcinoma.
All complications and deaths within 30 days of
surgery were considered as postoperative morbidity and mortality.
Complications were graded on a scale of 1–5 according to a
previously published grading system (26). Grade 1 complications are those that
require only supportive care. Grade 2 complications require
moderate interventions such as intravenous medications or prolonged
tube feeding. Grade 3 complications require invasive surgical or
radiologic intervention. Grade 4 complications produce chronic
disability, and grade 5 complications result in death. Grades 1 and
2 were grouped as minor complications and grades 3–5 as major
complications.
Statistical analysis
SPSS statistical software, version 12 (SPSS Inc,
Chicago, IL, USA) was used for data analysis. Categorical variables
were compared using the χ2 test and continuous variables
were analyzed with the Wilcoxon rank-sum test. Survival comparisons
were performed by the Kaplan-Meier method with comparisons made
using the log-rank test where survival data were measured from the
time of resection of the hepatic metastases. Results are reported
as medians with ranges unless otherwise stated and P-values
<0.05 are considered significant.
Results and Discussion
Over the 20-year period there were 168 males and 162
females included with separation of cases into two main groups;
group 1 with 280 patients undergoing resection only (mean age, 60
years; range 25–86 years) and group 2 with 80 patients undergoing
combined resection and RFA treatment (mean age, 58 years; range,
50–83 years). Table I shows the
patient characteristics and distribution of metastases amongst the
two groups. In 61 patients (76.5%), intraoperative RFA was used for
centrally located tumours on the contralateral side of the primary
resection that could not be safely removed by resection. Ten
patients (12.5%) had extensively diseased parenchyma (steatosis)
precluding further resection and 2 patients (2.5%) had tumour
proximate to the inferior vena cava precluding a margin-negative
resection. In 11 patients (13.75%), the primary tumour was
associated with regional lymph node metastases.
 | Table IClinical features of patients sorted
by treatment group. |
Table I
Clinical features of patients sorted
by treatment group.
| Features | Resection-only
(n=280) | Resection + RFA
(n=80) | P-value |
|---|
| Age in years, mean
(range) | 60 (25–86) | 58 (50–83) | 0.538 |
| Gender |
| Male, n (%) | 131 (47.1) | 37 (48) | 1.000 |
| Female, n (%) | 149 (52.9) | 43 (52) | |
| ASA score |
| 1–2, n (%) | 33 (11.8) | 0 | 0.132 |
| 3–4, n (%) | 247 (88.2) | 80 (100) | |
| T stage |
| T1 or T2, n
(%) | 48 (16.6) | 5 (5.9) | 0.479 |
| T3 or T4, n
(%) | 132 (83.4) | 75 (94.1) | |
| N stage |
| 0, n (%) | 86 (30.6) | 14 (17.6) | 0.038 |
| 1, n (%) | 133 (47.5) | 23 (29.4) | |
| 2, n (%) | 61 (21.9) | 43 (52.9) | |
| Timing of mts |
| Synchronous, n
(%) | 110 (39.1) | 56 (70.6) | 0.018 |
| Metachronous, n
(%) | 170 (60.9) | 24 (29.4) | |
| CEA before
resection, mean, ng/ml | 6 (0–786) | 2.5 (0.7–274) | 0.315 |
| Biggest metastatic
volume in cm, mean | 3 (0.3–16) | 2.3 (0.4–8) | 0.01 |
| Single/multiple
metastases |
| Single, n (%) | 201 (71.8) | 0 (0) | <0.001 |
| Multiple, n
(%) | 79 (28.2) | 80 (100%) | |
| Number of treated
lesions mean (range) | 1 (1–6) | 4 (2–10) | <0.001 |
| Metastatic
distribution |
| Bilobar, n
(%) | 36 (12.9) | 61 (76.5) | <0.001 |
| Uni-lobar, n
(%) | 244 (87.1) | 19 (23.5) | |
| Total treated
lesions | 280 | 198 | |
| Resection, n
(%) | 280 (100) | 100 (50.5) | |
| RFA, n (%) | 0 (0) | 98 (49.5) | |
| Neoadjuvant
chemotherapy, n (%) | 148 (52.9) | 47 (58.8) | 0.800 |
| Neoadjuvant
chemotherapeutic response |
| Regression, n
(%) | 59 (40.2) | 33 (70) | 0.152 |
| Stable, n (%) | 54 (36.8) | 4 (8) | |
| Progression, n
(%) | 34 (23) | 10 (22) | |
| Simultaneous
primary resection, n (%) | | 21 (26) | |
| Preop. Portal vein
embolization, n (%) | | 7 (8.75) | |
| Extrahepatic
resection, n (%) | | 5 (6) | |
| Local invasion, n
(%) | | 3 (4) | |
| Histologic
infiltration, n (%) | | 2 (2) | |
The median preoperative serum carcinoembryogenic
antigen (CEA) level at the time of partial hepatectomy was 12 ng/ml
(range, 3–21 ng/ml), with 29 patients (36%) recording a level
>15 ng/ml. Sixty-nine patients (86%) presented with synchronous
hepatic metastases. Neoadjuvant chemotherapy was administered in 18
patients (22.5%), for a median period of 3 months (range, 2–6
months). Adjuvant chemotherapy following hepatic resection was
administered to 69 patients (86%). Overall, a total of 478 tumours
were ablated; 98 (30.6%) by RFA with 380 resections. Group 2
patients had a higher incidence of multiple metastases (100% in
group 2 vs. 28.2% in group 1, respectively; P<0.001) and bilobar
involvement (76.5% in group 2 vs. 12.9% in group 1, respectively;
P<0.001) with a correspondingly higher mean number of lesions
treated per patient (mean = 4, range 2–10 in group 2 vs. mean = 1,
range 1–6 in group 1; P<0.001). The timing of liver metastasis
presentation influences the treatment type where synchronous
metastases are more likely to be treated by combined therapy (70.6
vs. 39.1%, respectively; P=0.018).
Table II shows the
type and extent of ablative procedure amongst the two groups. A
total of 219 wedge resections were performed. This type of
resection was the commonest type of procedure performed in group 2
cases (86/100). Overall, amongst the major hepatectomies, multiple
segmentectomy was the most commonly performed procedure (39 cases
or 24% of the total hepatic resections) with 25 such procedures
being performed in group 1 cases (8% of total hepatic resections).
Extended hepatectomies and hemihepatectomies were performed in 13
and 8 patients, respectively (all within group 1) representing 8
and 10%, respectively, of all resections performed. Histologically
involved surgical margins were found in 67 (24.1%) of the resection
only group 1 cases and in 19 (23.5%) of the combined treatment
group 2 cases.
 | Table IIClinical results sorted by group. |
Table II
Clinical results sorted by group.
| Clinical data | Resection-only
(n=280) | Resection + RFA
(n=80) | P-value |
|---|
| In-hospital stay,
days, mean (range) | 8 (5–28) | 9 (5–32) | 0.01 |
| Surgical procedure
duration, min, mean (range) | 120 (90–150) | 220 (180–300) | |
| Pringle maneuver
duration min, mean (range) | 15 (12–30) | 15 (12–30) | |
| Major
hepatectomies, n (% of total resections) | 147 (37.5) | 14 (8) | |
| Extended
hepatectomies, n (%) | 13 (8) | 0 (0) | |
| Hemi-hepatectomies,
n (%) | 16 (10) | 0 (0) | |
| Segmentectomies
(>2 seg), n (%) | 25 (15.5) | 14 (8) | |
| Minor resections, n
(% of total resections) | 133 (34.5) | 86 (23) | |
| Lesser
complications, n (%) | 8 (2.8) | 0 (0) | 0.564 |
| Severe
complications, n (%) | 10 (3.5) | 0 (0) | |
| Blood loss in ml,
mean (range) | 442 (100–4000) | 288 (100–800) | 0.269 |
| Patients requiring
transfusion, n (%) | 39 (14.7) | 0 (0) | 0.089 |
| Histologically +
resection margins (R1), n (%) | 67 (24.1) | 19 (23.5) | |
| Hepatic in
situ recurrence, n (%; % R1) | 4 (1.5; 6) | 2 (2.5; 10) | |
Concerning perioperative data for the resectional
group, the median operative time was 120 min (range, 90–150 min)
for group 1 patients whereas combined resection and RFA treatment
took a median of 220 min (range, 180–300 min). The median duration
of the Pringle maneuver (when used) for both groups was 15 min
(range, 10–32 min). The median intraoperative estimated blood loss
was 250 ml (range, 50–1200 ml), with a median postoperative
hospital stay of 8 days (range, 5–28) for resection only cases and
9 days (5–32) for those who had RFA in addition to
resection. There was no perioperative mortality with a
per-operative complication rate of 6% (18/280 cases) which included
5 bile leaks, 3 patients with atrial fibrillation, 1 patient with a
postoperative myocardial infarction, 2 patients with transient
hepatic dysfunction and a further 2 cases with postoperative ileus.
Six patients in the hepatic resection only group developed
postoperative infectious complications (1 bile leak, 3 pleural
effusions, 1 bronchopneumonia and 1 wound infection). There were no
RFA-associated complications or complications in the combined
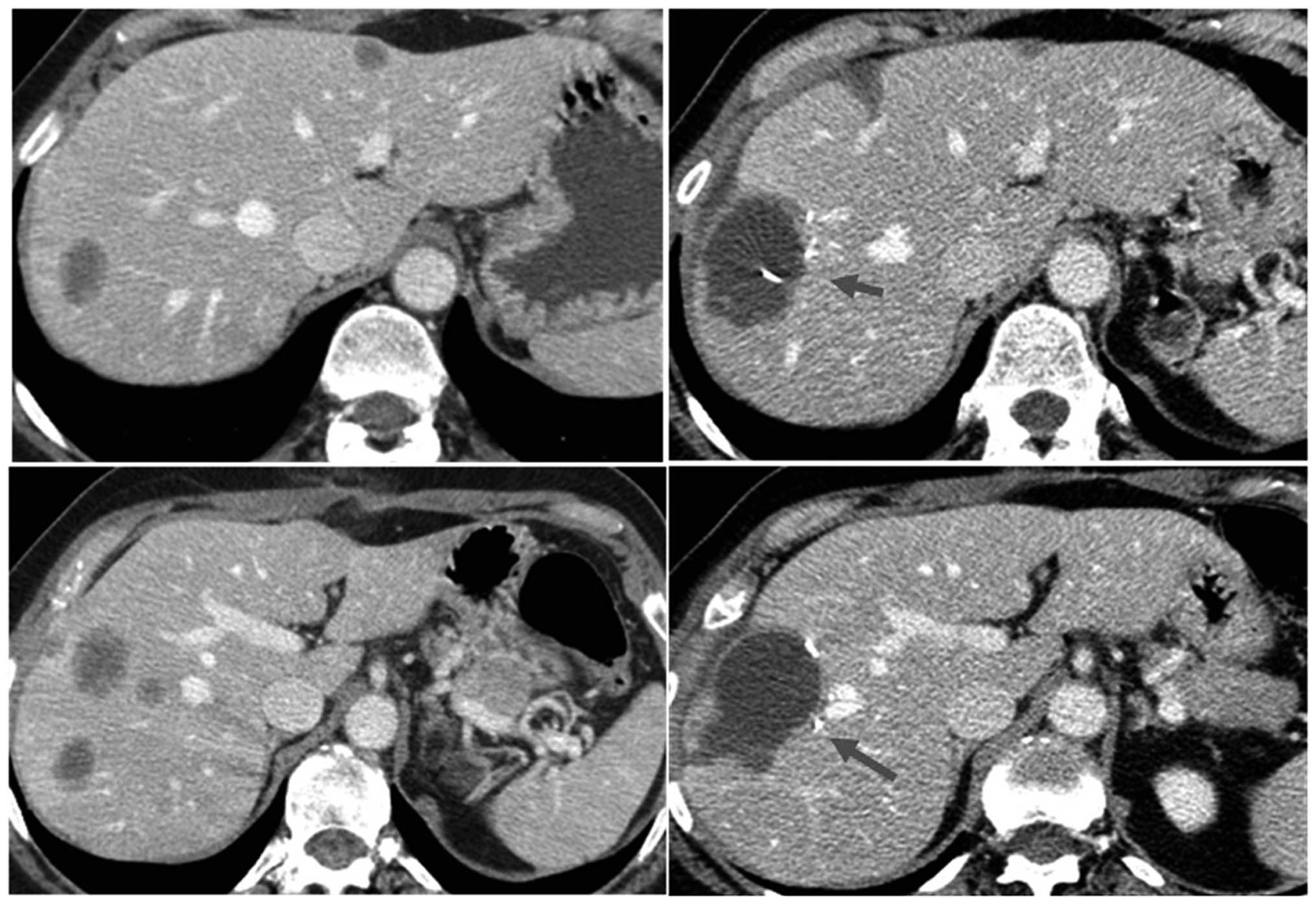
treatment group. Fig. 1 shows an
example of the effect of RFA thermo-ablation on different hepatic
lesions in a single patient treated with combined resection and RFA
(segments V, VII and VII), as they appear on CT scan ten days after
surgery.
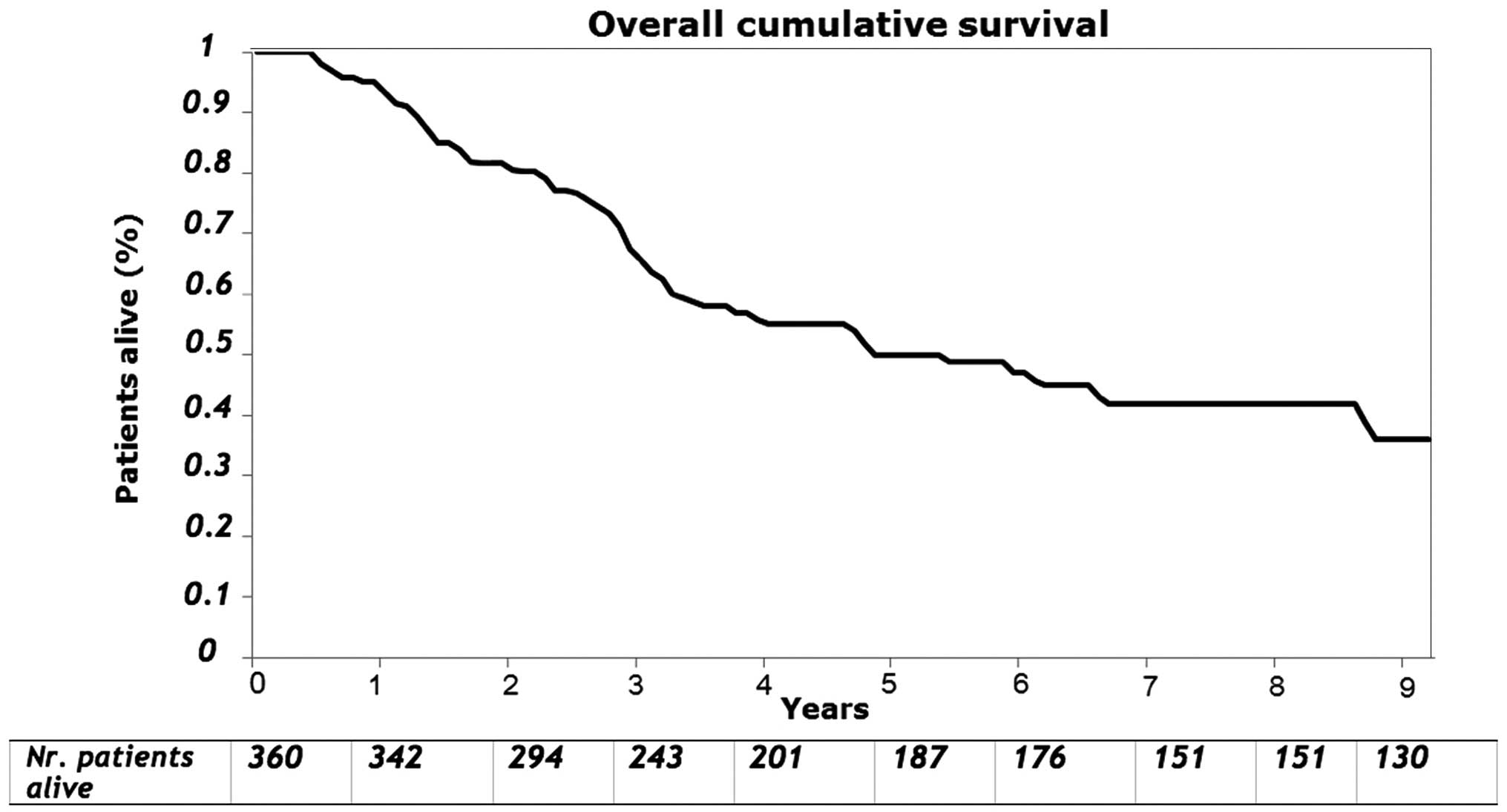
The median follow-up for survivors was 90 months
(range, 1–180 months). For the entire group as measured from the
time of hepatic resection, the overall 3- and 5-year survival was
70 and 50%, respectively (Fig. 2).
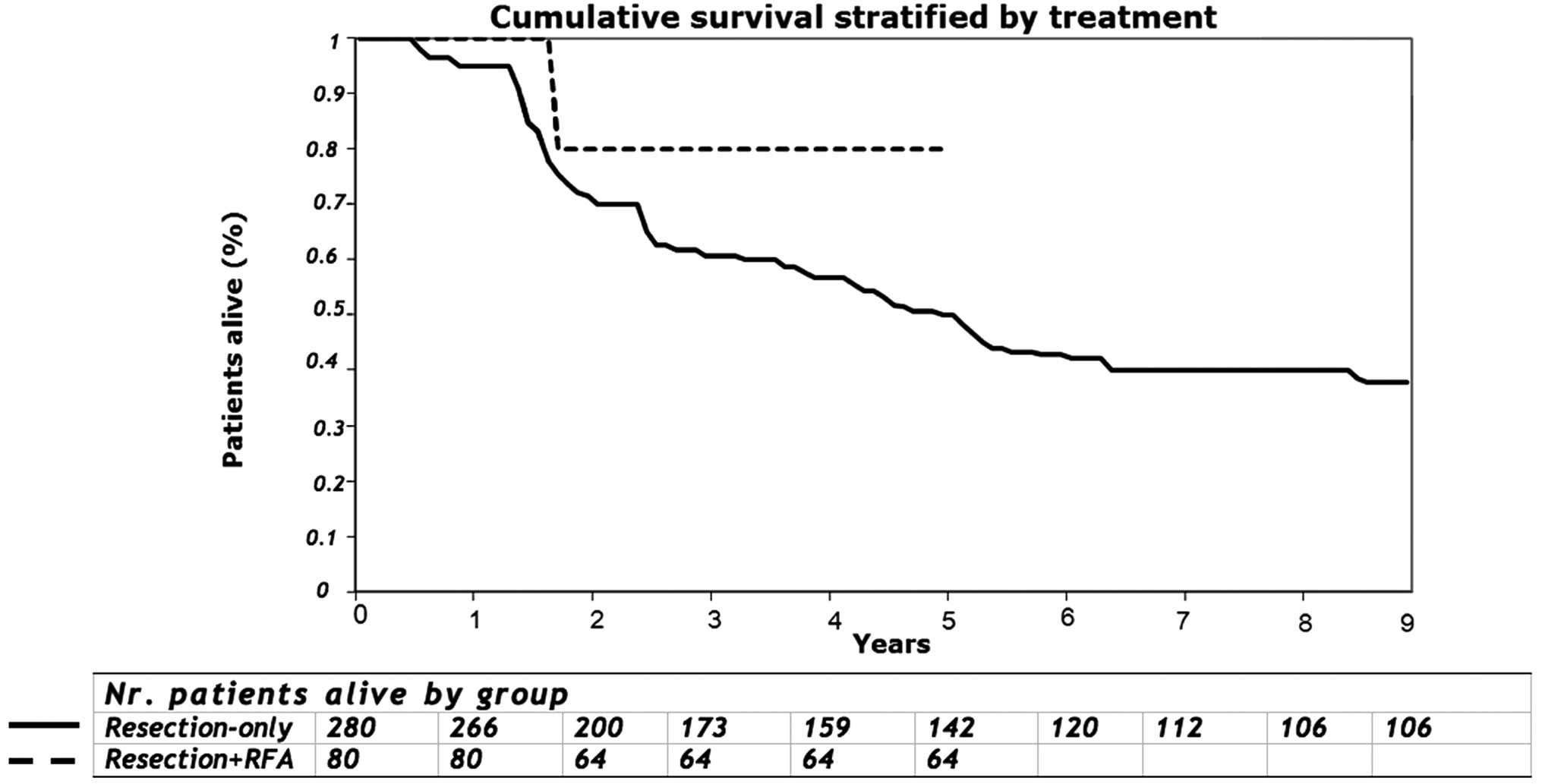
The 5-year survival for group 1 and group 2 was 49 and 80%,
respectively (P=0.193; Fig. 3). On
univariate analysis, tumour size exceeding 5 cm, a positive
resection margin, positive nodal status of the primary, tumour
number and the preoperative CEA level were not associated with
survival (Table III). For those
undergoing RFA treatment, the number of lesions, a size of lesion
exceeding 2 cm in maximal diameter or proximity to major vessels
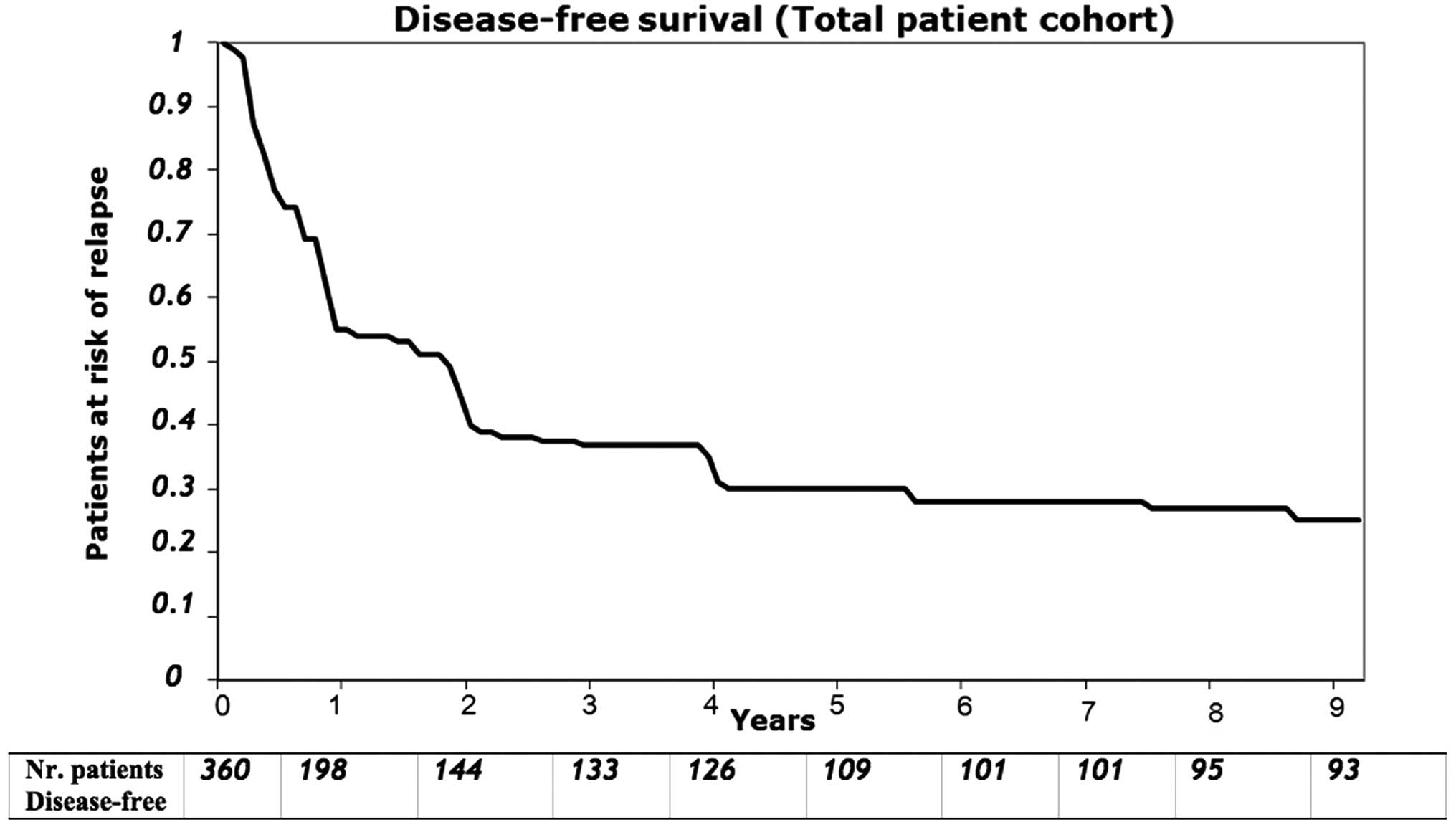
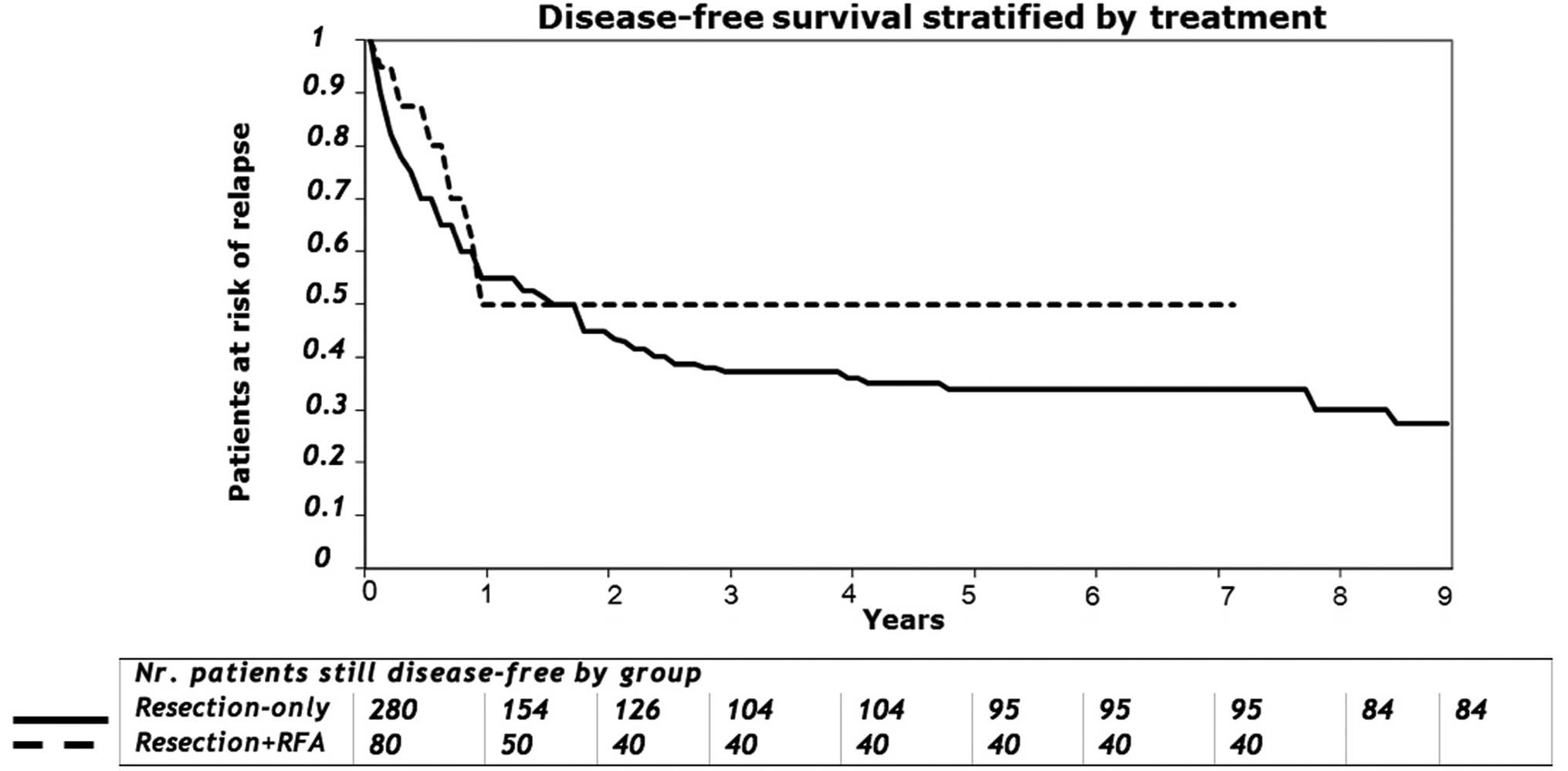
were not associated with overall survival. The median disease-free
survival measured from the time of hepatic resection for the
patients with complete gross resection was 30 months (range, 1–180
months), with an actuarial disease-free survival of 50% at 1 year,
43% at 2 years and 34% at 3 years (Fig. 4). The median hepatic disease-free
survival was 20 months (range, 0–160 months) where 91 patients
(66%) had hepatic recurrence at the time of last follow-up
(Fig. 5). The disease-free
survival of patients treated with combined resection and RFA was
higher than that of the resection-alone group at 3 years (50 vs.
37.14%, respectively; P=0.48) and at 5 years (50 vs. 33.9%,
respectively; P=0.069). Overall, disease recurred in 169 of the 280
patients undergoing complete gross resection (70%) where 111
patients (30%) had no signs of recurrence at their last follow-up.
The only intraoperative thermo-ablation failure was for a large
lesion 5 cm in maximal diameter. Amongst the group 1 patients where
there was histological margin involvement (R1 resections) there
were 4 in-liver recurrences representing 1.5% of the total
resection group or 6% of R1 resections. Amongst the group 2 cases
there were 19 with histological involvement of their resection
margins with 2 cases of in liver recurrence (both at the site of
the previous resection), representing recurrence in only 2.5% of
group 2 patients or 10% of all R1 resections.
 | Table IIIGlobal 5-year survival predictors -
univariate analysis. |
Table III
Global 5-year survival predictors -
univariate analysis.
| Survival
predictors | Global 5-year
survival (%) | P-value |
|---|
| Procedure |
|
Resection-only | 48.6 | 0.193 |
| Resection +
RFA | 80 | |
| Gender |
| Male | 39 | 0.271 |
| Female | 55.4 | |
| N-positive
primitive tumour |
| No | 57.8 | 0.331 |
| Yes | 47.2 | |
| Metastatic
timing |
| Syncronous | 43.4 | 0.539 |
| Metacronous | 51 | |
| Bilateral
distribution |
| Yes | 66 | 0.162 |
| No | 47 | |
| Number of
lesions |
| >3 | 48.9 | 0.41 |
| <3 | 50.9 | |
| Volume of lesions
(cm) |
| <5 | 52.7 | 0.161 |
| >5 | 34.7 | |
| Histologically
involved margins |
| No | 51 | |
| Yes | 46 | 0.73 |
| Neoadjuvant
chemotherapy |
| No | 51.8 | 0.794 |
| Yes | 45.3 | |
| Adjuvant
chemotherapy |
| No | 65.4 | 0.015 |
| Yes | 43.1 | |
This single unit retrospective analysis showed
similar overall and disease-free survival between patients with
hepatic colorectal metastases undergoing hepatic resection and
those receiving combined hepatic resection with RFA treatment,
despite the finding that patients having the combined treatment
overall had more hepatic lesions. Colorectal cancer (CRC) is the
3rd leading cause of cancer death, with one-quarter of CRC patients
presenting with metastases where over recent years there has been a
generally more aggressive attitude towards surgical metastasis
excision (5,27). In this context, R0 margin-free
surgical resection remains the only potentially curative treatment
available although only between 10 and 20% of patients will
actually be suitable for this approach. A variety of ablative
therapies including chemical ablation, cryosurgery, thermo-ablation
(radiofrequency ablation-RFA and microwave therapy) and
electroporation have been advocated for hepatic metastatic CRC with
each modality having particular indications and contraindications
(28). The commonest treatments
with available outcome data are RFA and cryoablation. Such ablative
therapies have traditionally been used in the treatment of hepatic
metastatic CRC for the local control of unresectable disease,
however, they have also been employed selectively as an adjunct to
hepatic resection in an attempt to enhance the potential
resectability of some metastatic tumors (29).
Several clinical questions remain concerning the
wider use of RFA in hepatic metastatic CRC. The first question is
whether RFA is equivalent to resection in clearly resectable cases.
In this respect, there are considerable data showing higher local
recurrence and reduced survival for RFA-only treated cases although
it is accepted that patients undergoing potentially curative
hepatic resections and those treated with ablative therapies are
not strictly comparable (30).
Concerning this point, however, a recent Cochrane Library review
concluded that the therapeutic use of RFA as a definitive
alternative to resection remains unproven (31). The second question is whether RFA
can extend the pool of resectable cases. Attempts to assess this
question through randomized controlled trials have failed with the
French FFCD trial which compared RFA with resection closing in 2004
(32) and the EORTC-CLOCC trial
which compared RFA with chemotherapy in cases deemed unresectable
being downscaled from a phase III to a phase II study and closing
early in 2006 because of poor recruitment (33). The extended use of RFA as an
adjunct to resection appears to be gaining worldwide acceptance
with a respectable safety profile (8,11)
although its efficacy is controversial because of relatively high
reported rates of intrahepatic recurrence either at or distant from
the ablation sites (34).
Data specifically assessing the role of combined
resection plus RFA in metastatic CRC are currently limited and
non-randomized (Table IV) and the
reported oncologic outcomes are mixed. Early studies by Elias et
al (14,15) and Pawlik et al (16) reported the safety of single-arm
studies which combined resection with RFA; an effect confirmed in a
later non-randomized single-arm analysis by Evrard et al
(21) which assessed RFA use in a
single surgical unit for unresectable hepatic disease. Although
patients can achieve prolonged survival with this approach, the low
early event-free survival rate reported in this study suggests that
they are rarely cured from their hepatic disease if it is initially
deemed unresectable.
 | Table IVAvailable literature comparing
hepatic resection with combined resection and radiofrequency
ablation for patients with metastatic colorectal cancer. |
Table IV
Available literature comparing
hepatic resection with combined resection and radiofrequency
ablation for patients with metastatic colorectal cancer.
| Author, year
(Ref.) | Trial design | N | Follow-up
(months) | Intrahepatic
recurrence rate (%) | Overall 3-year
survival (%) |
|---|
| Elias et al,
2000 (15) | R + RFA | 21 | 17 | 42 | 94.7 |
| Pawlik et
al, 2003 (16) | R + RFA | 172 | 21.3 | 47 | 65.2 |
| Abdalla et
al, 2004 (17) | R | 190 | 21 | 11 | 73 |
| R + RFA | 101 | | 28 | 43 |
| Kornprat et
al, 2007 (18) | R +
Ablationa | 39 | 21.1 | 14 | 47 |
| Gleisner et
al, 2008 (19) | R | | | | |
| R + RFA | 192 | N.R. | 14.8 | 74.1 |
| | 55 | | 50.9 | 44.9 |
| Leung et al,
2010 (20) | R | | | | |
| R + RFA | 84 | 37 | 55.9 | 54 |
| | 16 | | 62.5 | 38 |
| Evrard et
al, 2012 (21) | R + RFA | 42 | 35 | 57 | 43 |
| Karanicolas et
al, 2013 (22) | R | 141 | 44 | N.R. | 67 |
| R + RFA | 95 | 23 | | 77 |
| Eltawil et
al, 2014 (23) | R | 150 | 35 | 25.3 | 65.5 |
| R + RFA | 24 | 36 | 50 | 61.4 |
| Current series | R | 280 | 90 | 60 | 70 |
| R + RFA | 80 | 84 | 50 | 50 |
Abdalla et al (17) and Kornprat and colleagues (18) both independently showed higher
intrahepatic recurrence rates and survival disadvantage if
combination therapies were used whereas similar to the present
study, others have been unable to demonstrate any deleterious
survival effect if RFA is added to resection specifically if it is
applied in patients presenting with bilobar disease (20,22).
The present study showed an in liver recurrence rate that did not
differ between resection only and combined management cases and
that was similar to other studies (19–21,23).
Variations in the patterns of recurrence in the differently managed
groups will most likely reflect a worse tumour biology where
recurrences are relatively uncommon at the RFA-treated sites but
where RFA-managed patients tend to have higher rates of
extrahepatic failure (19,30).
These studies should be interpreted with caution as
they have considerable heterogeneity, with variations in the
technical radiofrequency approach (percutaneous, laparoscopic or
open) and with the type of RFA probe used (35–37).
Also, these variant RFA approaches are not strictly comparable and
each has particular advantages and disadvantages. Percutaneous RFA
for example, is unable to directly assess the zone of thermal
injury and is less reliable when a lesion is subcapsular or
adjacent to other viscera. By contrast, open RFA can be used
concomitantly with intra-abdominal staging, at the time of
temporary hepatic inflow occlusion and during selective synchronous
resections of the colorectal primary.
Overall, RFA appears to be a safe ablative modality
where decision making concerning the different forms of ablation
available reflects both inherent instrumental safety profiles and
specific clinical scenarios. By contrast to RFA, serious adverse
events have been reported with cryosurgical ablation (CSA),
including a cryoshock syndrome, significant haemorrhage following
ice ball cracking and the development of hepatic abscesses
(10,11). Limited retrospective data comparing
RFA with CSA have reported higher complication rates, more
extensive blood loss and a more prolonged length of hospital stay
in those patients treated with CSA (38). Non-thermal ablative techniques in
particular will have a role in those cases presenting with large
central tumours located near great vessels where RFA efficacy would
be diminished by a well-described ‘heat-sink’ effect of cooling
local blood flow (8,28,39,40).
The oncologic efficacy of thermo-ablative techniques
is influenced by various tumour-related factors, including the
number of lesions, their size and their proximity to larger vessels
(11,14,17,41).
In this regard, treatment by RFA of hepatic tumours exceeding 3 cm
in maximal diameter remains a significant challenge. The only RFA
in-liver recurrence in our series occurred at the ablation site of
a lesion which was 5 cm in size. Larger lesions are more amenable
to CSA because multiple probes can be placed simultaneously where
in this setting the hypoechoic changes induced by the ice-ball can
be readily visualized with ultrasound. The use of CSA is also
preferable to define margin enhancement when a lesion has been
excised with suboptimal margins making CSA currently the only
described method of achieving long-term survival in patients where
margins are histologically involved (42). Alternatively in those cases managed
with RFA, in order to minimize treatment failure in the smaller
tumors, future analysis will be required of the efficacy of the
newer electrodes which are capable of inducing larger coagulation
zones. For centrally located larger tumours on the contralateral
side of a primary resection that cannot be surgically removed
whilst preserving a sufficient functional liver remnant, RFA
combined with temporary hepatic inflow occlusion designed to
diminish ablative heat loss may potentially prove the best
management option (43).
In conclusion, the present study has the major
limitations of being a retrospective review of non-randomized
cases. Given that the number of tumour deposits and their size
correlate with a worse survival and that RFA-treated cases tend
more often to have multiple tumours, bilateral disease and larger
lesions, strict comparison of treated groups will be flawed because
of poor patient matching. This has resulted in an inability to
conduct randomized trials incorporating combined therapy in one
treatment arm (44) where closer
matching although resulting in enhanced internal comparability
actually reduces the generalizability of the data (30). In order to overcome this matching
bias, Gleisner et al (19)
used the more rigorous technique of propensity score analysis
designed to determine the likelihood that a patient will receive a
specific treatment by creating a single predictor which reflected
all of the confounding clinicopathological variables (45). Even this approach will have
limitations if the studied populations (resection vs. combined
resection plus RFA) have relatively poor overlap of their
characteristics such that the baseline features will be so
different between the groups that no causal conclusions concerning
the differential effects of the treatment can be effectively
drawn.
In summary, our data support the use of RFA when
complete resection of an hepatic metastasis from CRC cannot be
achieved. The combination of resection with ablation shows
equivalent recurrence rates when compared with resection alone and
does not appear to compromise cancer-specific survival. Despite an
inability to adequately randomize patients to different treatment
arms, a more extended use of RFA in cases initially deemed
unresectable will at least define standards during ablation both
for RFA technique and for real-time ultrasonographic monitoring
during such procedures.
Abbreviations:
|
RFA
|
radiofrequency ablation
|
|
CRC
|
colorectal cancer
|
|
LM
|
liver metastasis
|
|
IOUS
|
intraoperative ultrasound
|
|
CT
|
computed tomography
|
|
PET
|
positron emission tomography
|
|
CSAc
|
cryosurgical ablation
|
References
|
1
|
Boyle P and Ferlay J: Cancer incidence and
mortality in Europe, 2004. Ann Oncol. 16:481–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kemeny N: Management of liver metastases
from colorectal cancer. Oncology (Williston Park). 20:1161–1176.
1179discussion 1179–1180, 1185–1186. 2006.
|
|
3
|
Yoo PS, Lopez-Soler RI, Longo WE and Cha
CH: Liver resection for metastatic colorectal cancer in the age of
neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer.
6:202–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiappa A, Makuuchi M, Lygidakis NJ, Zbar
AP, Chong G, Bertani E, Sitzler PJ, Biffi R, Pace U, Bianchi PP, et
al: The management of colorectal liver metastases: Expanding the
role of hepatic resection in the age of multimodal therapy. Crit
Rev Oncol Hematol. 72:65–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akgül Ö, Çetinkaya E, Ersöz Ş and Tez M:
Role of surgery in colorectal cancer liver metastases. World J
Gastroenterol. 20:6113–6122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adam R, Miller R, Pitombo M, Wicherts DA,
de Haas RJ, Bitsakou G and Aloia T: Two-stage hepatectomy approach
for initially unresectable colorectal hepatic metastases. Surg
Oncol Clin N Am. 16:525–536. viii2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simoneau E, Hassanain M, Shaheen M,
Aljiffry M, Molla N, Chaudhury P, Anil S, Khashper A, Valenti D and
Metrakos P: Portal vein embolization and its effect on tumour
progression for colorectal cancer liver metastases. Br J Surg.
102:1240–1249. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lahat E, Eshkenazy R, Zendel A, Zakai BB,
Maor M, Dreznik Y and Ariche A: Complications after percutaneous
ablation of liver tumors: A systematic review. Hepatobiliary Surg
Nutr. 3:317–323. 2014.PubMed/NCBI
|
|
9
|
Kerkar S, Carlin AM, Sohn RL, Steffes C,
Tyburski J, Littrup P and Weaver D: Long-term follow up and
prognostic factors for cryotherapy of malignant liver tumors.
Surgery. 136:770–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seifert JK, Springer A, Baier P and
Junginger T: Liver resection or cryotherapy for colorectal liver
metastases: A prospective case control study. Int J Colorectal Dis.
20:507–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bilchik AJ, Wood TF, Allegra D, Tsioulias
GJ, Chung M, Rose DM, Ramming KP and Morton DL: Cryosurgical
ablation and radiofrequency ablation for unresectable hepatic
malignant neoplasms: A proposed algorithm. Arch Surg. 135:657–662;
discussion 662–664. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mulier S, Mulier P, Ni Y, Miao Y, Dupas B,
Marchal G, De Wever I and Michel L: Complications of radiofrequency
coagulation of liver tumours. Br J Surg. 89:1206–1222. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Curley SA, Marra P, Beaty K, Ellis LM,
Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H, et al:
Early and late complications after radiofrequency ablation of
malignant liver tumors in 608 patients. Ann Surg. 239:450–458.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elias D, Baton O, Sideris L, Boige V,
Malka D, Liberale G, Pocard M and Lasser P: Hepatectomy plus
intraoperative radiofrequency ablation and chemotherapy to treat
technically unresectable multiple colorectal liver metastases. J
Surg Oncol. 90:36–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elias D, Goharin A, El Otmany A, Taieb J,
Duvillard P, Lasser P and de Baere T: Usefulness of intraoperative
radiofrequency thermoablation of liver tumours associated or not
with hepatectomy. Eur J Surg Oncol. 26:763–769. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pawlik TM, Izzo F, Cohen DS, Morris JS and
Curley SA: Combined resection and radiofrequency ablation for
advanced hepatic malignancies: Results in 172 patients. Ann Surg
Oncol. 10:1059–1069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdalla EK, Vauthey JN, Ellis LM, Ellis V,
Pollock R, Broglio KR, Hess K and Curley SA: Recurrence and
outcomes following hepatic resection, radiofrequency ablation, and
combined resection/ablation for colorectal liver metastases. Ann
Surg. 239:818–825; discussion 825–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kornprat P, Jarnagin WR, DeMatteo RP, Fong
Y, Blumgart LH and D'Angelica M: Role of intraoperative
thermoablation combined with resection in the treatment of hepatic
metastasis from colorectal cancer. Arch Surg. 142:1087–1092. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gleisner AL, Choti MA, Assumpcao L, Nathan
H, Schulick RD and Pawlik TM: Colorectal liver metastases:
Recurrence and survival following hepatic resection, radiofrequency
ablation, and combined resection-radiofrequency ablation. Arch
Surg. 143:1204–1212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leung EY, Roxburgh CS, Leen E and Horgan
PG: Combined resection and radiofrequency ablation for bilobar
colorectal cancer liver metastases. Hepatogastroenterology.
57:41–46. 2010.PubMed/NCBI
|
|
21
|
Evrard S, Rivoire M, Arnaud JP, Lermite E,
Bellera C, Fonck M, Becouarn Y, Lalet C, Puildo M and
Mathoulin-Pelissier S: Unresectable colorectal cancer liver
metastases treated by intraoperative radiofrequency ablation with
or without resection. Br J Surg. 99:558–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karanicolas PJ, Jarnagin WR, Gonen M,
Tuorto S, Allen PJ, DeMatteo RP, D'Angelica MI and Fong Y:
Long-term outcomes following tumor ablation for treatment of
bilateral colorectal liver metastases. JAMA Surg. 148:597–601.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eltawil KM, Boame N, Mimeault R, Shabana
W, Balaa FK, Jonker DJ, Asmis TR and Martel G: Patterns of
recurrence following selective intraoperative radiofrequency
ablation as an adjunct to hepatic resection for colorectal liver
metastases. J Surg Oncol. 110:734–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiappa A, Bertani E, Makuuchi M, Zbar AP,
Contino G, Viale G, Pruneri G, Bellomi M, Della Vigna P, Zampino
MG, et al: Neoadjuvant chemotherapy followed by hepatectomy for
primarily resectable colorectal cancer liver metastases.
Hepatogastroenterology. 56:829–834. 2009.PubMed/NCBI
|
|
25
|
Couinaud C: Anatomic principles of left
and right regulated hepatectomy: Technics. J Chir (Paris).
70:933–966. 1954.in French.
|
|
26
|
Martin RC II, Brennan MF and Jaques DP:
Quality of complication reporting in the surgical literature. Ann
Surg. 235:803–813. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ito K, Govindarajan A, Ito H and Fong Y:
Surgical treatment of hepatic colorectal metastasis: Evolving role
in the setting of improving systemic therapies and ablative
treatments in the 21st century. Cancer J. 16:103–110. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singla S, Hochwald SN and Kuvshinoff B:
Evolving ablative therapies for hepatic malignancy. BioMed Res Int.
2014:2301742014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee KH, Kim HO, Yoo CH, Son BH, Park YL,
Cho YK, Kim H and Han WK: Comparison of radiofrequency ablation and
resection for hepatic metastasis from colorectal cancer. Korean J
Gastroenterol. 59:218–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsai S and Pawlik TM: Outcomes of ablation
versus resection for colorectal liver metastases: Are we comparing
apples with oranges? Ann Surg Oncol. 16:2422–2428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cirocchi R, Trastulli S, Boselli C,
Montedori A, Cavaliere D, Parisi A, Noya G and Abraha I:
Radiofrequency ablation in the treatment of liver metastases from
colorectal cancer. Cochrane Database Syst Rev. 2012.Article no.
CD006317. View Article : Google Scholar
|
|
32
|
Benoist S and Nordlinger B: Radiofrequency
ablation in liver tumours. Ann Oncol. 15(Suppl 4): iv313–iv317.
2004.PubMed/NCBI
|
|
33
|
Ruers T, Punt CJ, van Coeverden F, Borel
Rinkes I, Lederman JA, Poston GJ, Bechstein W, Lentz M, Mauer M,
Nordlinge B, et al: Final results of the EORTC intergroup
randomized study 40004 (CLOCC) evaluating the benefit of
radiofrequency ablation (RFA) combined with chemotherapy for
unresectable colorectal liver metastases (CRC LM). J Clin Oncol.
28(Suppl): 3526A2010.
|
|
34
|
Nielsen K, van Tilborg AA, Meijerink MR,
Macintosh MO, Zonderhuis BM, de Lange ES, Comans EF, Meijer S and
van den Tol MP: Incidence and treatment of local site recurrences
following RFA of colorectal liver metastases. World J Surg.
37:1340–1347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Machi J, Uchida S, Sumida K, Limm WM,
Hundahl SA, Oishi AJ, Furumoto NL and Oishi RH: Ultrasound-guided
radiofrequency thermal ablation of liver tumors: Percutaneous,
laparoscopic, and open surgical approaches. J Gastrointest Surg.
5:477–489. 2001. View Article : Google Scholar
|
|
36
|
Ahmad A, Chen SL, Kavanagh MA, Allegra DP
and Bilchik AJ: Radiofrequency ablation of hepatic metastases from
colorectal cancer: Are newer generation probes better? Am Surg.
72:875–879. 2006.PubMed/NCBI
|
|
37
|
Eisele RM, Neumann U, Neuhaus P and
Schumacher G: Open surgical is superior to percutaneous access for
radiofrequency ablation of hepatic metastases. World J Surg.
33:804–811. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pearson AS, Izzo F, Fleming RYD, Ellis LM,
Delrio P, Roh MS, Granchi J and Curley SA: Intraoperative
radiofrequency ablation or cryoablation for hepatic malignancies.
Am J Surg. 178:592–599. 1999. View Article : Google Scholar
|
|
39
|
Stoltz A, Gagnière J, Dupré A and Rivoire
M: Radiofrequency ablation for colorectal liver metastases. J Visc
Surg. 151(Suppl 1): S33–S44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pillai K, Akhter J, Chua TC, Shehata M,
Alzahrani N, Al-Alem I and Morris DL: Heat sink effect on tumor
ablation characteristics as observed in monopolar radiofrequency,
bipolar radiofrequency, and microwave, using ex vivo calf liver
model. Medicine (Baltimore). 94:e5802015. View Article : Google Scholar
|
|
41
|
Mulier S, Ni Y, Jamart J, Ruers T, Marchal
G and Michel L: Local recurrence after hepatic radiofrequency
coagulation: Multivariate meta-analysis and review of contributing
factors. Ann Surg. 242:158–171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dwerryhouse SJ, Seifert JK, McCall JL,
Iqbal J, Ross WB and Morris DL: Hepatic resection with cryotherapy
to involved or inadequate resection margin (edge freeze) for
metastases from colorectal cancer. Br J Surg. 85:185–187. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nikfarjam M, Muralidharan V,
Malcontenti-Wilson C, McLaren W and Christophi C: Impact of blood
flow occlusion on liver necrosis following thermal ablation. ANZ J
Surg. 76:84–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Curley SA: Radiofrequency ablation versus
resection for resectable colorectal liver metastases: Time for a
randomized trial? Ann Surg Oncol. 15:11–13. 2008. View Article : Google Scholar :
|
|
45
|
Rubin DB: Estimating causal effects from
large data sets using propensity scores. Ann Intern Med.
127:757–763. 1997. View Article : Google Scholar
|