Introduction
Cancer is one of most lethal diseases, and surgical
resection or surgery coupled with systemic chemotherapy and
radiotherapy are the main approaches currently available for cancer
therapy. Despite advanced progress in technologies to diagnose and
treat cancers, the global burden of cancer continues to increase
greatly (1). Therefore, improved
therapeutic approaches are needed for more efficacious control of
the malignancy.
Ras genes, one of the earliest oncogenes
discovered in human tumors, play an important role in development
of many kinds of human tumors (2).
Mutations have been identified by which ras can mediate
tumorigenesis. Point mutation in the ras gene results in an
alteration of a single amino acid in the ras gene product,
p21Ras (3), and ras
mutations are present in ~30% of all human cancers (4). Activated Ras and the signaling
contributes significantly to several aspects of the malignant
phenotype, including the deregulation of tumor-cell growth,
programmed cell death and invasiveness, and the ability to induce
new blood-vessel formation (5).
These results clearly demonstrate that ras oncogenes offer an
excellent target for a therapeutic intervention (6). Since Ras signaling depends on
the p21Ras precise localization to the inner face of the plasma
membrane (4), disturbance and
blockade of intracellular Ras signaling may be one of most
impressive strategies for ras-driven tumors. Strategies currently
being developed mainly target the specific inhibition of Ras
farnesylation (7,8) or are based upon neutralizing of ras
proteins in cancer cells (9).
Among the latter approaches, intracellular expression of
single-chain antibody fragment (scFv) has made it possible to
neutralize a variety of intracellular harmful molecules (10,11).
In previous years, several anti-p21Ras scFv antibodies were
reported (6,12–14).
However, all of them were derived from mutant p21Ras, and attempts
to develop drugs that target mutant Ras proteins have, so far, been
unsuccessful (15–17).
Besides mutation, overexpression of wild-type p21Ras
is also one of the major cause of cancer development including
colon, pancreas, prostate, bladder, breast cancers, and gliomas
(18–26). We supposed that the scFv antibody
derived from wild-type p21Ras could have wider range of
applications and potent antitumoral effects in ras-driven tumors.
However, there was no scFv antibody against wild-type p21Ras
reported. In our previous studies, we prepared an anti-p21Ras scFv
using wild-type p21Ras proteins as immunogen. Immunohistochemistry
demonstrated that this scFv antibody could bind wild-type and
mutated H-p21Ras, K-p21Ras, N-p21Ras and react specifically with
human cell lines and primary tumor tissues. In the present study,
we investigated whether it could express within cells and inhibit
human ras-driven cancer growth in vitro and in
vivo.
Materials and methods
Cell lines
The following human cell lines were employed to
observe the antitumor effects of the intracellular scFv antibody
against p21Ras. Human colon cancer cell line SW480, human ovarian
cancer cell line OVCAR-3, human liver cancer cell line BEL-7402,
human ovarian cancer cell line SKOV3, human breast cancer cell line
MDA-MB-231 and human embryo lung fibroblast line HLFI were
purchased from Shanghai Cell Collection (Shanghai, China). All the
cells were cultured in RPMI-1640 medium containing 20% fetal bovine
serum (BSA) at 37°C in 5% CO2, except HLFI cells which
were cultured in MEM medium with 10% BSA.
Western blot analysis
Western blot analysis was used to detect expression
of p21Ras in the above-mentioned cell lines. Briefly, cultured
cells were harvested when the cell density was 5×105
cells/well, lysed in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1%
Triton X-100, 1 mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS) with a
protease inhibitor cocktail containing phenylmethylsulfonylfluoride
(PMSF) for 30 min to obtain tumor cell total proteins. Equal
amounts of cellular proteins were electrophoresed on SDA-PAGE gel
and then transferred to PVDF membranes at 4°C for 2 h, sealed with
5% fat-free milk. The membrane was incubated with anti-p21Ras
monoclonal antibodies prepared by our laboratory, followed by
incubation with goat anti-mouse IgG/HRP (ZSGB-BIO) at 1:5,000
dilution. Finally, the signals were detected by enhanced
chemiluminescence (ECL) according to the manufacturer's
recommendations and quantitated by densitometry, with β-actin
serving as an internal control.
Construction of anti-p21Ras scFv antibody
gene
pMD18-T-KGHR1-scFv plasmids contained anti-p21Ras
scFv gene was constructed by our laboratory (unpublished data).
Briefly, wild-type H-p21Ras, K-p21Ras and N-p21Ras were expressed
in E. coli. BALB/c mice were immunized with H-p21Ras as
antigen. Hybridoma producing anti-p21Ras monoclonal antibody KGH-R1
was isolated by screening with wild-type H-p21Ras, K-p21Ras,
N-p21Ras. Finally anti-p21Ras scFv gene was generated from the
hybridoma cDNA by phage scFv display techniques.
Preparation of recombinant adenovirus
carrying anti-p21Ras scFv gene
Anti-p21Ras scFv gene (750 bp) was amplified from
the plasmids pMD18-T-KGH-R1-scFv by sense primer with restriction
enzyme BglII: 5′-TTGCGGCCGCAACCGTTTGATT-3′ and antisense
primer with XhoI: 5′-CCCTCGAGGGCCGCCCGTTTGATT-3′. Then, the
PCR was performed by the following parameters: 95°C for 5 min; 94°C
for 30 sec; 55°C for 40 sec, 72°C for 40 sec × 35 cycles; 72°C for
10 min. After BglII and XhoI double digestion, scFv
gene was subcloned into the BglII and XhoI sites of
the pShuttle-IRES-hrGFP-1 vector, then identified by PCR
amplification using the universal primers on the shuttle vector,
pShuttle-F: 5′-CTCACGGGGATTTCCAAGTC-3′; pShuttle-R:
5′-ATGCAGTCGTCGAGGAATTG-3′. The PCR was performed by the following
parameters: 95°C for 5 min, 94°C for 30 sec, 56°C for 40 sec; 72°C
for 40 sec × 35 cycles; 72°C for 10 min. After PmeI
restriction and CIAP treatment, the pShuttle-IRES-hrGFP-ScFv
underwent linearization and phosphorylation. Then the shuttle
plasmid was electro-transformed into competent cells BJ5183-AD-1,
which contain adenovirus backbone vector pAdEasy-1 to generate
recombinant adenovirus vectors Ad-KGHR1-scFv.
Then recombinant adenovirus vectors were identified
by PacI restriction, and were transfected into HEK293 cells
by Lipofectamine 2000 (Invitrogen, USA) to become the packaged
recombinant adenovirus, called KGHV100. After cytopathic effects
appeared, the cells underwent freeze thawing four times to release
the recombinant adenovirus. The recombinant adenovirus KGHV100 was
identified by PCR amplification using the universal primers on the
shuttle vector to confirm that KGHV100 was successfully
constructed. The wild-type adenovirus was excluded through PCR
amplification using E1 region as the specific primers. The
replication-deficient adenovirus AdEasy-GFP (Ad-hrGFP) was used as
a control, which contained a green fluorescent protein (GFP) gene
under the control of a cytomegalovirus promoter. All of the viral
particles were prepared by enlargement cultivation and purified by
cesium chloride density gradient centrifugation and titered by
TCID50 method. Adenoviral infection was performed
according to the standard protocol (27). All infection experiments were
performed at a multiplicity of infection (MOI) of 100 (this work
was done in our early experiments) plaque forming units (pfu).
Intracellular expression of anti-p21Ras
scFv
MDA-MB-231 cell lines were seeded on slides and
cultured in dishes at 37°C with 5% CO2. When 80%
confluent cells formed, recombinant adenovirus KGHV100 was added
into cell lines at a multiplicity of infection (MOI) of 100 and
cultured continuously for 24 h. Then, the cells on slides were
fixed with 4% para-formaldehyde, and permeabilised with 0.5%
Tween-20. After blocking with BSA, the slides were incubated
overnight at 4°C with primary anti-Flag Tag rabbit polyclonal
antibodies, then incubated for 1 h at room temperature in the dark
with TRITC-conjugated goat anti-rabbit antibody (ZSGB-BIO), washed
for 5 min with PBS and mounted on coverslips. TRITC fluorescence
signals were analyzed with an Olympus IV fluorescence confocal
microscope (Olympus, Tokyo, Japan).
Colony formation analysis
The SW480, MDA-MB-231, BEL-7402, OVCAR-3 and SKOV3
cell lines were cultured in 24-well plates at 37°C with 5%
CO2 and were infected with recombinant adenovirus
KGHV100 at a MOI of 100 and were cultured for 24 h. Then, the tumor
cells were digested with 0.25% trypsin and suspended in 20% FBS.
Five hundred cells were cultured in 2.5 ml RPMI-1640 medium
containing 20% fetal bovine serum in the 6-wells plates for 2–3
weeks. The culture solutions were changed when the color changes of
the solutions were observed and cell lines growth were terminated
when culture clones were observed macroscopically. Cells were
washed with PBS and immobilized with methanol for 15 min. Following
1% Giemsa stain for 10–30 min, the cells were washed with water and
dried in air. Colony-forming efficiency was calculated using the
formula: Colony-forming efficiency = (numbers of clones/inoculated
cell counts) × 100%.
Transwell invasion assay
Cell invasion ability was assayed in a Transwell
cell culture chamber (Corning, USA) according to the manufacturer's
protocol (28). Briefly,
MDA-MB-231, OVCAR3, SW480, BEL-7402 and SKOV3 cell lines were
inoculated into 24-well plates, when 80% of confluent cells formed,
recombinant adenovirus KGHV100 was added into cell lines at a MOI
of 100 and cultured continuously for 24 h and then cell lines were
grow in RPMI-1640 without serum for 12 h. The lower chamber of the
Transwell was coated with 50 mg/l Matrigel (BD, USA), and was
filled with 500 μl RPMI-1640 supplemented with 20% fetal bovine
serum (FBS) as a chemoattractant. Cells (3×104) (~200 μl
cell suspension) were added into upper layer of the invasion
chamber, for static culture for 48 h at 37°C. The non-invasive
cells on the upper surface of the Matrigel were removed with a
cotton swab. The lower changer cells of the microwells were fixed
with 95% ethanol, stained with hematoxylin, then washed with pure
ethanol and distilled water, separately, and dried at room
conditions. The migrated cells were observed and counted under a
microscope by random selection of 10 different microscopical
visions.
Cell viability and apoptosis-related gene
assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay was performed as previously described (29). Briefly, SW480, MDA-MB-231, BEL-7402
and SKOV3 cells were seeded into 96-well plates at a density of
5×103 cells/well. Twenty-four hours later, the cells
were infected with the recombinant scFv-adenovirus KGHV100 at a MOI
of 100, the control blank group was also prepared. At 1, 2, 3, 4
and 5 days after infection, 20 μl of MTT (5 mg/ml) was added to
each well. After 4 h of incubation with MTT, DMSO (100 μl/well) was
added for 10 min. The optical density (OD) value of each well was
measured at 490 nm by enzyme-linked immunosorbent assay.
For apoptosis-related gene caspase-3 assay, SW480,
MDA-MB-231, BEL-7402 and SKOV3 cell were infected with the KGHV100
at a MOI of 100. After 48 h total cellular RNA was extracted from
cells, 1 μg of total RNA was used for reverse transcription with
the GoScript™ Reverse Transcription system (Promega, Beijing,
China). Expressions of caspase-3 was detected by quantitative
real-time PCR (qRT-PCR) using primer caspase-3-F and caspase-3-R
(Table I).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
internal control, with the primers GAPDH-F/GAPDH-R (Table I).
 | Table IPrimers used in qRT-PCR. |
Table I
Primers used in qRT-PCR.
| Primer no. | Primer
sequence |
|---|
| Caspase-3-F |
ATCTCCAAGCAATGTTCA |
| Caspase-3-R |
ACTACCATATAGCGTGTTC |
| PI3K-F |
GTGAAAGACGCCACGACAAT |
| PI3K-R |
TCTCCACTGCTGCCTGAAAC |
| PLCɛ-F |
CCAGGGGAGACAGCATCATT |
| PLCɛ-R |
GCATTTTCATTCAGCGACGA |
| RALGDS-F |
GCCTCACCTCTTGGTGTTCC |
| RALGDS-R |
GTGCTCCTTGCCCTTCTTGT |
| MAPK-F |
AAAGGGTCATCATCTCTG |
| MAPK-R |
GCTGTTGTCATACTTCTC |
| GAPDH-F |
GGACCTGACCTGCCGTCTAG |
| GAPDH-R |
GAGTGGGTGTCGCTGTTGAA |
Cell cycle analysis
The tumor cell lines SW480, MDA-MB-231, BEL-7402 and
SKOV3 were seeded into the 25-cm2 flasks at 37°C with 5%
CO2, until 80% confluent cells formed the cell were
infected with KGHV100 at a MOI of 100. After 48 h, digested with
0.25% trypsin, cells were harvested. In total, 106 cells
were washed twice with ice-cold PBS, immobilized overnight with 75%
ethanol, then washed twice again with ice-cold PBS, and stained
with 0.5 ml PI (Beckman, USA) for 30 min in the dark. Samples were
processed on a flow cytometer according to the manufacturer's
standard protocol. Data were analysed using MultiCycle DNA
software. The proliferation index of tumor cells infecting KGHV100
and the adenovirus of Ad-hrGFP were calculated using the following
formula: (proliferation index, PI) = (S+G2/M)/(G0/G1+S+G2/M) ×
100%. All experiments were completed in triplicate.
Quantitative real-time PCR (qRT-PCR)
analysis for downstream genes of ras
BEL-7402 cells were cultured in 6-well plates and
were infected with KGHV100 at a MOI of 100, with Ad-hrGFP infected
and uninfected cells as control. Forty-eight hours after infection,
total cellular RNA was extracted from cells using the Eastep™ Total
RNA Extraction kit (Promega). Total RNA (1 μg) was used for reverse
transcription with the GoScript™ Reverse Transcription system
(Promega). qRT-PCR was used to detect expression of downstresm
genes of ras, MAPK1, PI3K, PLCɛ and RALGDS, using
corresponding primers (Table I).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
internal control, with the primers GAPDH-F/GAPDH-R. A negative
control with no template was included for each reaction series. The
cycles were as follows: 95°C for 10 min; 95°C for 10 sec, 58°C for
20 sec, 72°C for 20 sec, for 45 cycles. The amplification products
were separated by 2% agarose gel electrophoresis and visualized by
SYBR Green staining (Bio-Rad, USA). Data were analyzed with Bio-Rad
CFX96 Manager software.
In vivo tumor-killing assay
This study was approved by the Ethics Board of
Kunming General Hospital, and is in accordance with the Helsinki
Declaration of 1975. Animals involved in the study were cared for
in accordance with institutional guidelines. BEL-7402, SW480 and
SKOV3 tumor xenograft models were established by subcutaneous
(s.c.) inoculation of 5×106 cells into the left
subaxillary of 4-week-old female SPF BALB/c nude mice (Vital River
Laboratory Animal Co. Ltd., Beijing, China), respectively. When
tumors reached an average diameter of 0.5 cm, mice were assigned
randomly to treatment group (KGHV100) and control group (treated
with Ad-hrGFP virus). Pre-established tumors were then injected
with 3×108 plaque-forming units (pfu) of KGHV100 (with
Ad-hrGFP virus as control) in 100 μl solution. The injections were
repeated seven times every three days. The conditions of each mouse
was carefully observed every two days. Tumor growth was monitored
by periodic measurements with calipers and tumor volume was
calculated using the following formula: (maximal length) ×
(perpendicular width)2/2. Tumor growth curve was draw
using the data.
Mice were sacrificed at day 56 after injection, and
the tumors were removed immediately and fixed in formalin, embedded
in paraffin, sectioned at 4 μm and stained with hematoxylin and
eosin (H&E). Immunohistochemically (IHC), sections were
deparaffinized in xylene, hydrated in serial dilutions of ethanol
and subsequently immersed in 1.5% H2O2 to
quench endogenous peroxidase activity. The sections were heated in
citrate buffer (10 mmol/l citric acid, pH 6.0) for antigen
retrieval, then incubated with Ultra V Block (Thermo, USA) at room
temperature for 30 min. The sections were incubated with 1:400
diluted primary anti-Flag Tag rabbit polyclonal antibodies
overnight at 4°C. After three successive rinsing, the tissue
sections were incubated with biotinylated goat anti-polyvalent plus
for 30 min at room temperature. The slides were then washed and
streptavidin peroxidase plus was added for 10 min. After washing
three times, the chromogen was developed for 5 min with liquid
3,3′-diaminobenzidine (Thermo). Finally, the slides were
counterstained with hematoxylin, dehydrated and mounted with balsam
for examination. Images were captured using the charge-coupled
camera (Olympus, DP11) equipped with a microscope (Olympus, BX50),
and processed using Adobe Photoshop software, version 5.0.
Measurement of tissue apoptosis
Tumor apoptosis was determined by terminal
deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
(TUNEL) method according to the instructions of the manufacturer
(In Situ Cell Death Detection kit; Roche Diagnostics). The
apoptotic indices were determined by counting the percentages of
positive cells from five randomly selected high power fields.
Data analysis
Each result is expressed as the mean value ±
standard deviation of the mean. All statistical analyses were
performed using SPSS Version 11.5. Student's t-test was applied for
paired analyses to reveal statistical significance. Differences
between multiple groups were analyzed by one-way analysis of
variance (ANOVA). Survival rates were analyzed by the Kaplan-Meier
method. Statistical significance is indicated by a P-value
<0.05.
Results
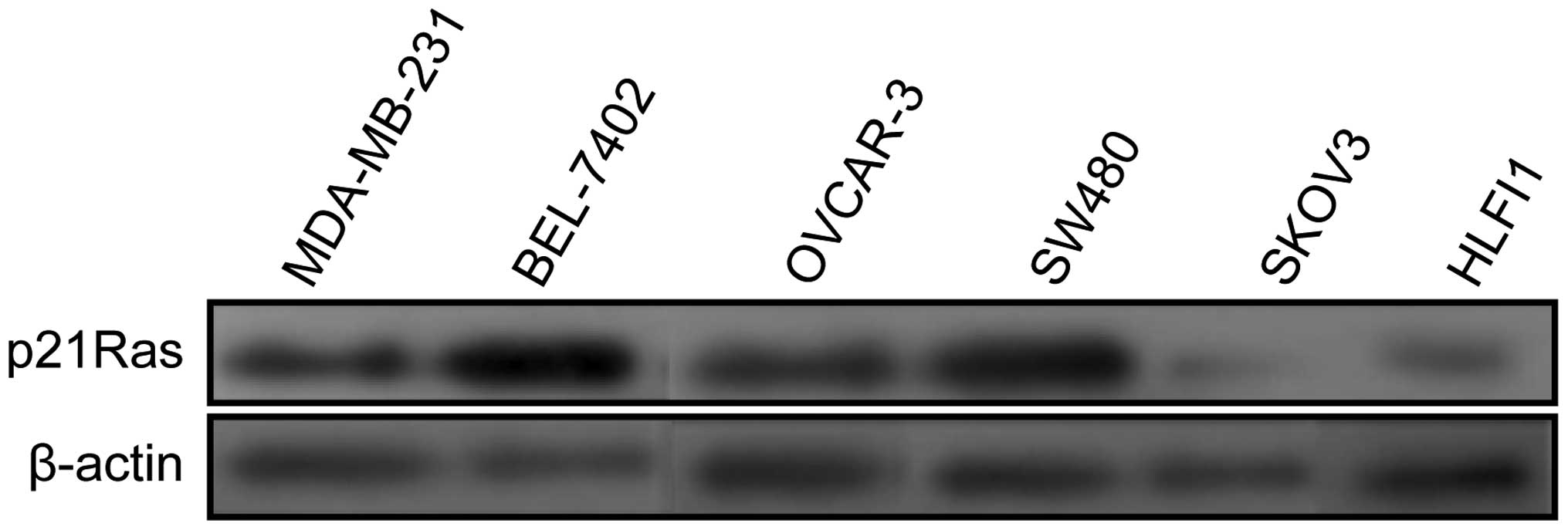
Expression of p21Ras in tumor cell
lines
Western blot analysis showed that p21Ras protein
displayed high expression level in SW480, MDA-MB-231, OVCAR-3,
BEL-7402 cell lines, but the low level of p21Ras expression was
observed in SKOV3 cell line. In human lung fibroblast HFL1 cell
line, p21Ras protein displayed a relatively basic expression level
(Fig. 1).
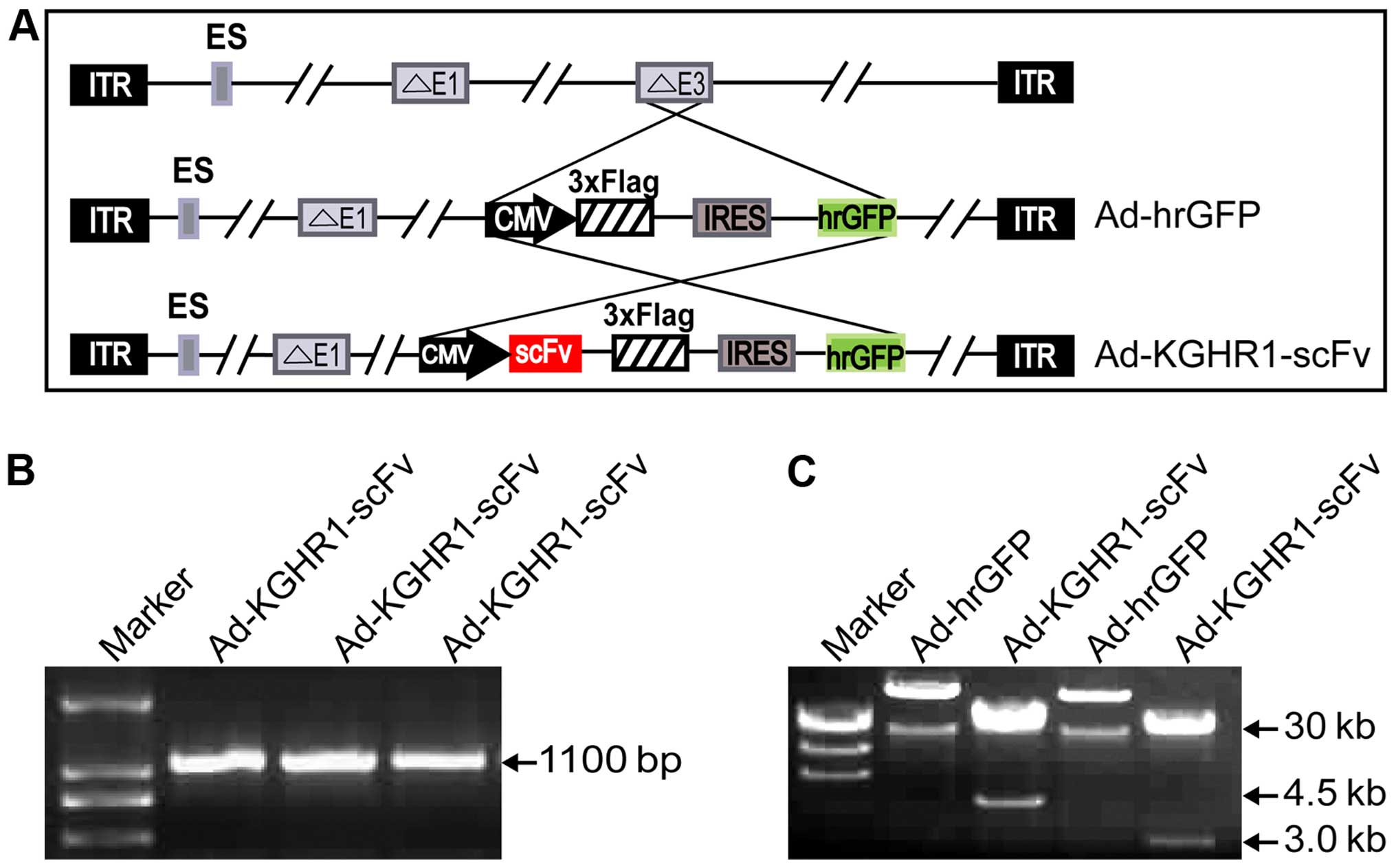
Preparation of recombinant adenovirus
KGHV100
We successfully constructed the recombinant
adenovirus vector Ad-KGHR1-scFv (Fig.
2A) by subcloning anti-p21Ras scFv gene, KGHR1-scFv, into
shuttle vector of replication-defective adenovirus, in which the
adenovirus E1 regions were deleted, and E3 gene was replaced by CMV
promoter, Flag, IRES and hrGFP gene, then recombined with backbone
vector pAdEasy-1in BJ5183-AD-1 cells. PCR amplification
demonstrated the vector contained scFv gene (Fig. 2B). PacI restriction showed
different sized plasmid segments of 30 and 4.5 kb (homologous
recombination occurred at the left and right arm) or 30 and 3 kb
(homologous recombination occurred at replication starting section
and the right arm) (Fig. 2C). By
transfecting Ad-KGHR1-scFv and packaging in HEK293 cells we
obtained the recombinant adenovirus KGHV100.
Intracellular expression of anti-p21Ras
scFv fragments in tumor cells
Immunofluorescence studies revealed very distinct
intracellular expression of anti-p21Ras scFv antibody in tumor
cells infected with recombinant adenovirus KGHV100. Fig. 3 shows that KGHV100 was able to
infect MDA-MB-231 cells and express reporter gene protein, green
fluorescent proteins (GFP) and anti-p21Ras scFv antibody. The
combination of the two fluorescence patterns is shown in Fig. 3C. The signal GFP (green) and the
Flag signal (red) were combined and the two chromophores were
superimposed in the same image with a green/red color scale,
leading to yellow color in case of colocalization. The
intracellular antibody displayed a cytoplasmic/perinuclear
pattern.
KGHV100 inhibits the studied tumor cell
lines in vitro
To validate the function of anti-p21Ras
intracellular scFv antibody on tumor cell growth regulation, the
tumor cell lines mentioned above were used in analysis in
vitro. Colony formation assay indicated that colony-forming
efficiency in BEL-7402 cells infected KGHV100 were decreased to
44.9%, but in the control groups (the cells were infected Ad-hrGFP)
was 66.2%, and the numbers of clones from tumor cells were reduced
greatly after the infection with the KGHV100. Obviously, the
difference of the two groups was statistically significant
(P<0.05). Similar results were found in other tumor cell lines
with high p21Ras expression. The colony-forming efficiency in
MDA-MB-231, SW480 and OVCAR3 cells infected by KGHV100,
respectively, was 21.7, 25.0 and 26.0%, but in the control groups
the colony-forming efficiency was 29.9, 35.5 and 35.9%,
respectively. The clone formation capacity of tumor cell lines with
high p21Ras expression was reduced by infection with KGHV100, the
activities of cell replication were inhibited. However, the
differences between the control and the experimental groups were
not statistically significant in p21Ras lowly expressing SKOV3
cells (Fig. 4A).
Consistently, Transwell invasion assay showed that
KGHV100 infection could significantly inhibit the invasive ability
of the tumor cells of high p21Ras expression compared with Ad-hrGFP
transfected groups. In contract, there was no apparent inhibition
of invasive ability in SKOV3 cells of low p21Ras expression
infected by either KGHV100 or Ad-hrGFP (Fig. 4B).
KGHV100 inhibits the tumor cell line
viability and induces apoptosis in vitro
MTT assay was performed to detect cell viability
after infection of KGHV100. As showed in Fig. 5A, there was no significant
difference in proliferation between Ad-hrGFP and uninfected groups
of the four lines studied. However, at 1, 2, 3, 4 and 5 days after
KGHV100 infection, the cell proliferation of MDA-MB-231, BEL-7402
and SW480 was significantly suppressed. Interestingly, a similar
inhibitory effect was not observed in SKOV3 cells with low p21Ras
expression. These results indicated that the growth of tumor cells
with high p21Ras expression was inhibited by KGHV100 infection.
The expression of apoptosis-related gene caspase-3
increased significantly in MDA-MB-231, BEL-7402 and SW480 in
KGHV100 infected cells compared with the control groups. However,
there was no significant difference in expressions of caspase-3 in
the SKOV3 cell lines between KGHV100 and Ad-hrGFP groups (Fig 5B). This result showed that the
KGHV100 was able to induce apoptosis in the three cell lines with
high p21Ras expression.
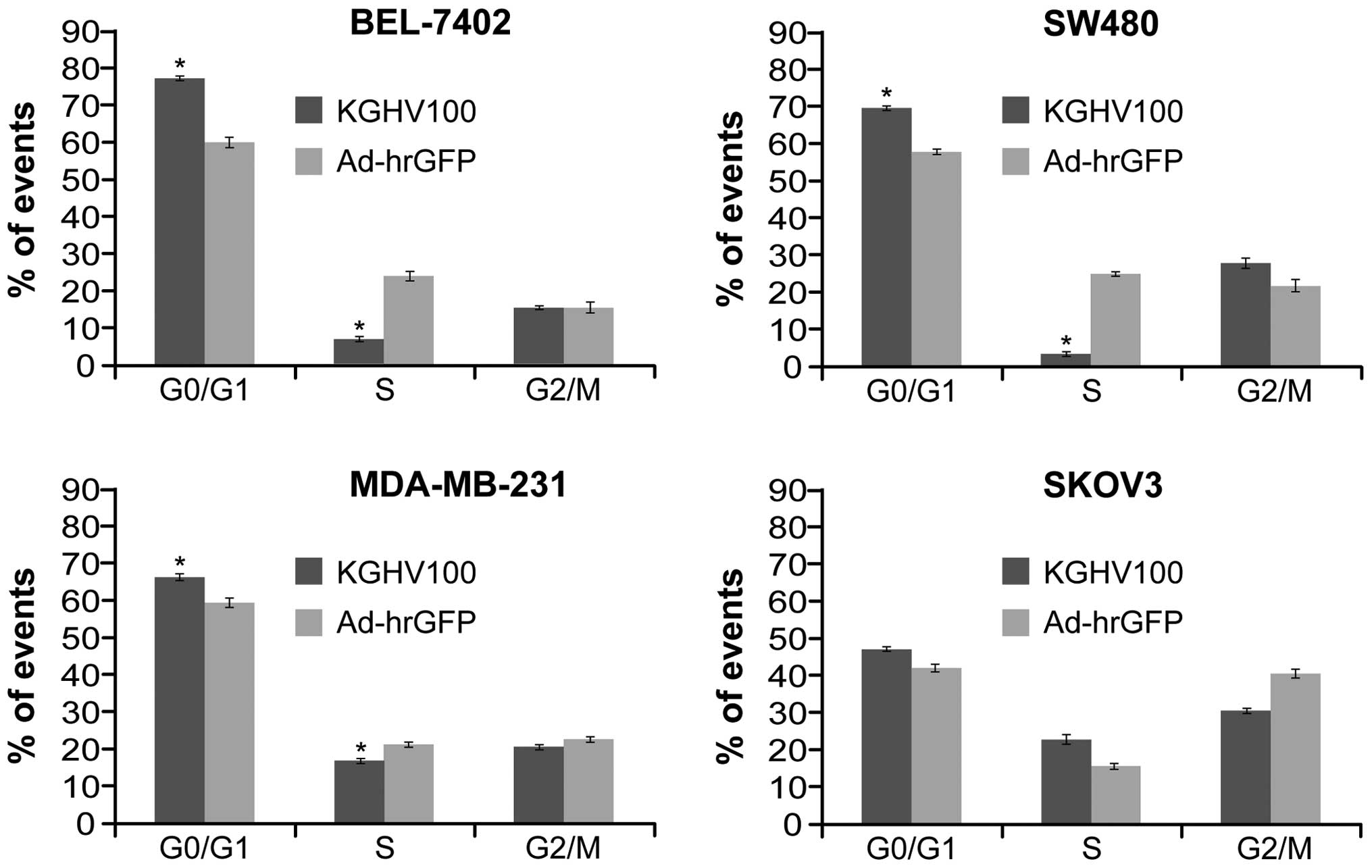
The KGHV100 induces G0/G1 cell cycle
arrest in the tumor cells
To examine the effect of KGHV100 on the cell cycle,
flow cytometry was performed on the four tumor cell lines at 48 h
after infection. The phase distribution of three lines infected
with KGHV100 was markedly different from that of Ad-hrGFP groups.
There was a significant increase in the percentage of G0/G1 phase
cells and an obvious decrease in S phases cells in KGHV100 groups
compared with Ad-hrGFP groups in the three cell lines with high
p21Ras expression (P<0.05). Whereas, there was no significant
difference in SKOV3 cells with low p21Ras expression compared with
control group (P>0.05) (Fig.
6). Thus, the KGHV100 could block cell cycle progression of the
three tumor cell lines at G0/G1 phase.
KGHV100 inhibits the downstream gene
expression of Ras
The expression of the ras downstream genes MAPK1,
PI3K, PLCɛ and RALGDS in BEL-7402 cell line infected by
KGHV100 was detected by qRT-PCR. As shown in Fig. 7, there were no significant
difference in expression of MAPK1, PI3K, PLCɛ and
RALGDS in the Ad-hrGFP and the non-infected groups. However,
the expression of MAPK1, PI3K and PLCɛ decreased
significantly in tumor cells after infection with KGHV100. The
expression of RALGDS gene increased in KGHV100 group
compared to the control. Obviously, KGHV100 could inhibit the
expression of the downstream genes of ras (Fig. 7).
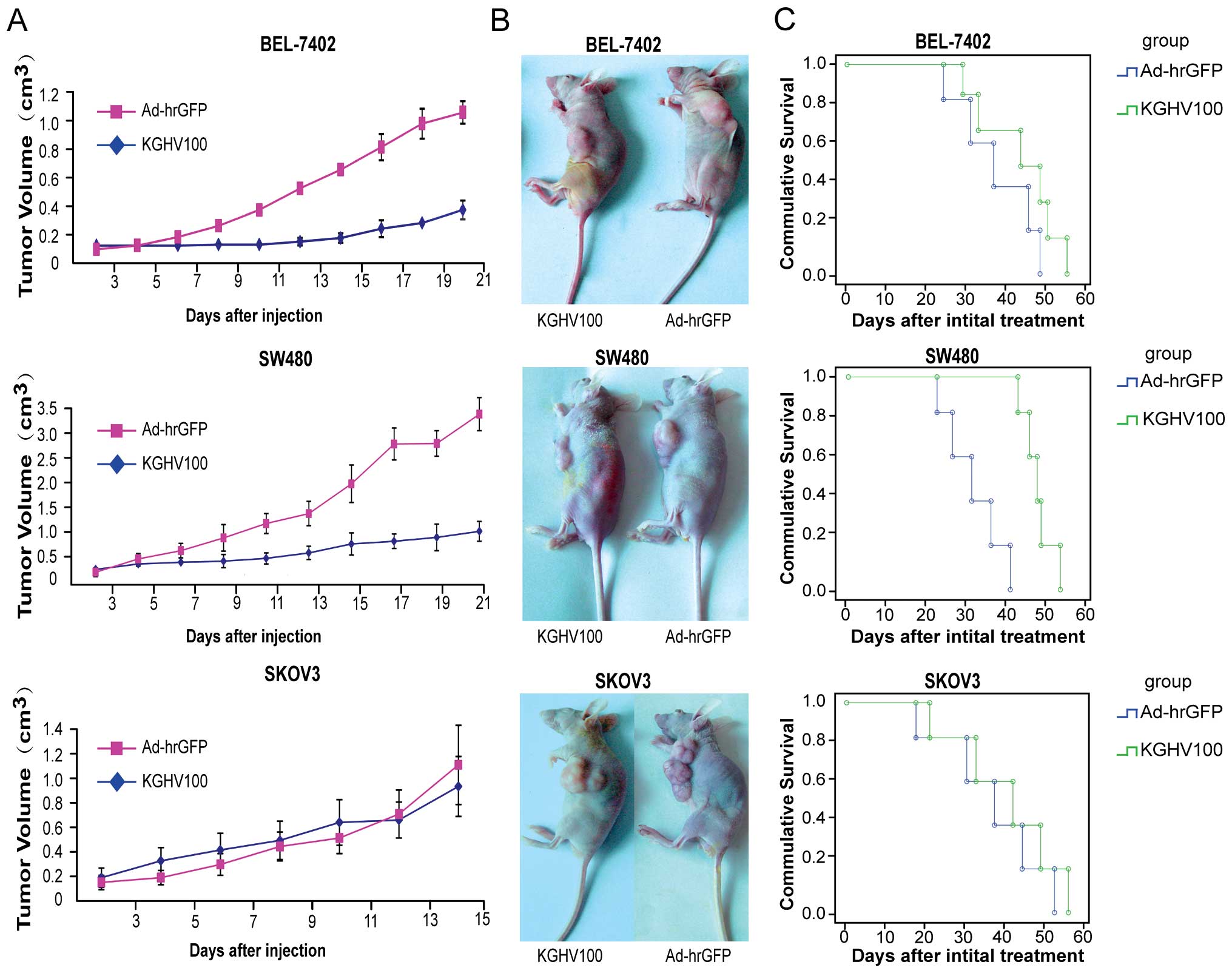
KGHV100 slows down the growth of tumor
xenografts in nude mice and induces apoptosis
To further investigate the therapeutic efficacy of
KGHV100 in vivo, three kinds of tumor xenograft nude mouse
models were, respectively, established by subcutaneously injecting
BEL-7402, SW480 or SKOV3 cells. In the two models of BEL-7402 and
SW480 treated with KGHV100, the tumors grew remarkably slower and
displayed a significant growth retardation compared with those in
Ad-hrGFP-treated mice (P<0.05). However, SKOV3-derived tumors
displayed a significant growth regardless of injection by KGHV100
or Ad-hrGFP (Fig. 8A and B). This
means that KGHV100 was capable of inhibiting tumor growth in p21Ras
high expression tumor model. Kaplan-Meier analysis showed that high
p21Ras expression tumor mice (BEL-7402, SW480) had higher survival
rates in KGHV100 injected groups than in the control groups.
However, in low p21Ras expression tumor mice (SKOV3) the survival
rate showed no difference between the experimental and control
groups (Fig. 8C).
Model mice were sacrificed at day 56 after
injection, xenograft tumors were subjected to histopathological
examination. Extensive central necrosis, which indicated that the
tumors grew more quickly were observed in the tumor tissues from
the control group of xenograft mice, whereas the feature was not
displayed in the KGHV100 treated group (Fig. 9). IHC analysis demonstrated the
cytoplasmic/perinuclear expression of the scFv in the xenograft
tumors treated with KGHV100, but no scFv immune stain was found in
the control group (Fig. 9).
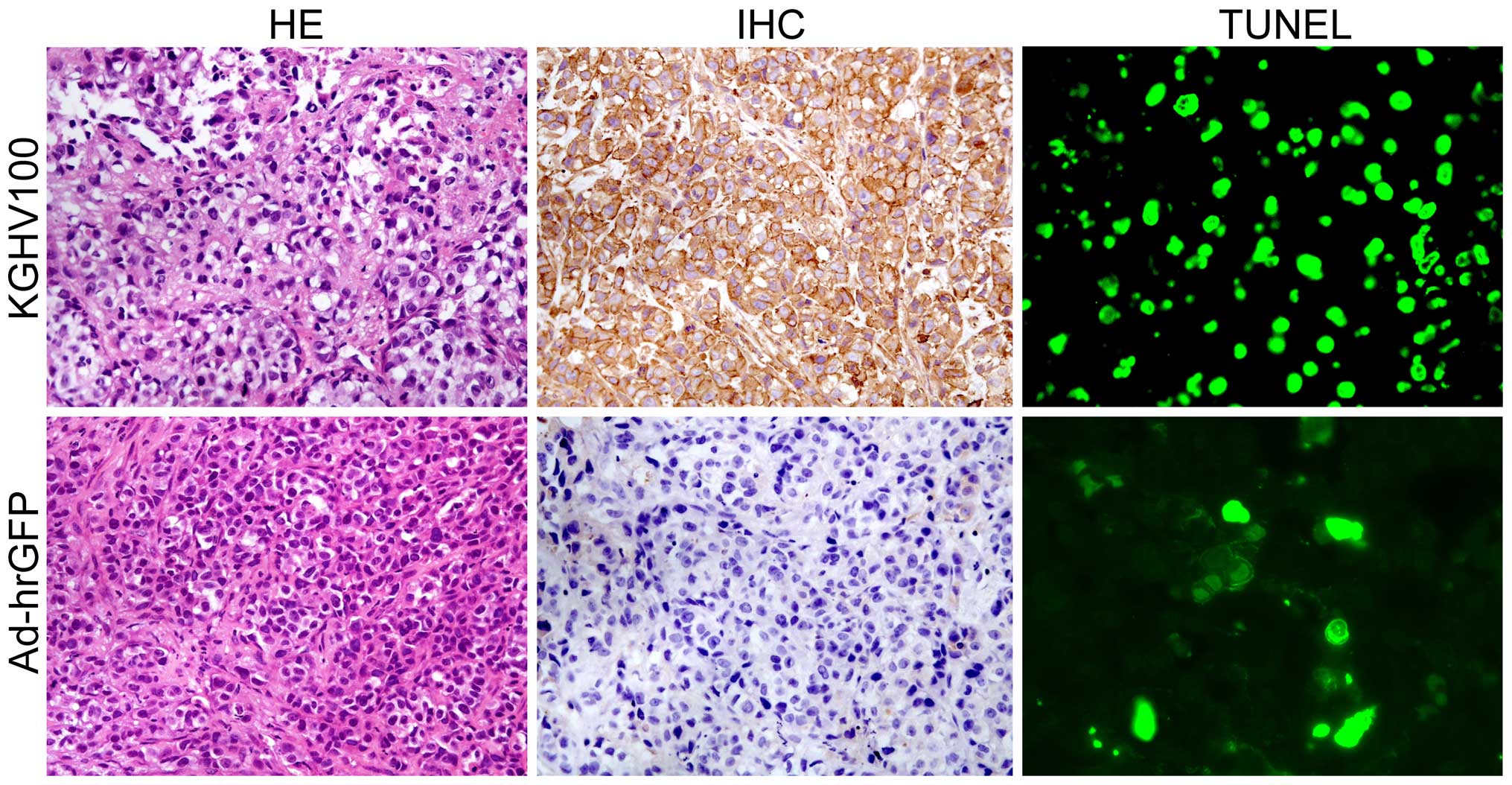 | Figure 9Histological and TUNEL staining for
xenograft tumor tissues of nude mice. Hematoxylin and eosin
(H&E) showed that there were nuclear condensation, shrinkage of
nuclear membrane and less mitotic tumors treated with KGHV100.
However, the nuclear membrane was smooth, the nucleolis were
prominent and there were more mitosis in tumors treated with
Ad-hrGFP adenovirus (magnification, ×400). Immunohistochemical
staining (IHC) analysis showed high level of anti-p21Ras scFv
expression in the xenograft tumors treated with KGHV100, but no
anti-p21Ras scFv immunostain was found in the control group
(magnification, ×400). Apoptotic tumor cells were detected by TUNEL
staining in the KGHV100 group (magnification, ×1,000), whereas only
a few apoptotic cells were found in the control group
(magnification, ×1,000). |
More apoptosis of tumor cells were displayed in
KGHV100 treated groups than in the control group. In the
virus-treated groups, there were more obvious amounts of cancer
cells positive for TUNEL stainning. The apoptosis index was
54.2±8.2 in KGHV100 group, and 16.4±4.3 in the control group
(Fig. 9).
Discussion
The scFv antibody is an antigen-binding protein that
consists of the VH and VL regions of the variable antigen-binding
sites of immunoglobulin, connected by a short linker sequence
(30). The scFv are generally of
low molecular weights that improve their penetrability into cell
membrane (31), but do not remain
in cytoplasm for a long time. Intracellular antibody technology
provides a key method to solve this problem (32,33).
The intracellular antibody can neutralize intracellular antigens by
the ectopic expression of recombinant antibodies targeted to
different intracellular compartments (33). Intracellular antibodies are
synthesized inside of the cells by expressing vector such as
adenovirus, adeno-associated virus, herpes simplex virus,
retrovirus vector which carried the antibody gene (34). After the initial formal proof of
intracellular antibody expression and targeting within the cell in
1990 (32), the function of many
antigens has been successfully inhibited by intracellular
expressing antibodies in the cytoplasm or the nucleus (10,11,35,36),
and it has been prospected as tools for gene therapy (37) and functional genomics study
(38). Most present studies on
cancer therapy with intracellular antibodies are focused on
neutralizing certain proteins by intracellular scFv antibodies
(39,40). For example, recombinant adenovirus
vector carrying anti erbB2-scFv gene was used for ovarian cancer
therapy, which significantly prolonged the survival of
tumor-bearing animals (12).
Ras is a well-known important oncogene involved in
the development and progression of many human tumors, but no
antibody drugs targeting ras have been approved for clinical
application up to now. In a previous study (data not shown) we
constructed anti-p21Ras scFv gene and prepared anti-p21Ras scFv
antibody, KGH-R1, which was able to react with wild-type and
mutation H-p21Ras, N-p21Ras and K-p21Ras proteins. To our
knowledge, this is the first time using wild-type p21Ras as
immunogens to prepare scFv antibody. In the present study we
described a novel recombinant adenovirus KGHV100 by subcloning
KGH-R1-scFv gene in a replication-defective adenovirus vector and
packaging in HEK293 cells.
Fluorescence microscopy demonstrated that KGHV100
expressed anti-p21Ras intracellular scFv antibody in both the
cultured tumor cells and in transplantation tumors. MTT, Transwell
and colony formation analysis showed that KGHV100 caused
significant growth arrest in p21Ras high expressing tumor cells
MDA-MB-231, BEL-7402, and SW480 in vitro. Furthermore, flow
cytometry demonstrated KGHV100 can induce G0/G1 cell cycle arrest
in the studied tumor cell lines. However, the growth,
proliferation, invasion ability of the tumor cell line SKOV3 with
low p21Ras expression were not suppressed by KGHV100 compared with
the high p21Ras expression cell lines. qRT-PCR showed that KGHV100
inhibits the expression of ras downstream genes, MAPK1, PI3K
and PLCɛ. Another downstream gene, the RALGDS, was
slightly upregulated for, as yet, unclear reasons.
In vivo assays, we evaluated the antitumor
effect of KGHV100 and observed notable growth inhibitory effects.
Tumor cell lines with high p21Ras expression BEL-7402 and SW480
were selected to test the inhibition effects of the intrcellular
scFv antibody on tumor growth by injecting the recombinant
adenovirus KGHV100 into xenograft tumor established in Balb/c nude
mice through tumor cell line transferation. It showed that the
growth of tumors were significantly slower and the survival rates
were longer in experimental group than in the control group. The
intracellular antibody decreases tumor cell viability and invasion
ability and inhibits tumor growth significantly. In SKOV3 (low
p21Ras expression) mouse model, the tumor growth and survival rate
of nude mice were not be affected by KGHV100. These results
indicate that the adenovirus-mediated intracellular expression of
anti-p21Ras scFv exerted strong antitumor activity, implied that it
may be potentially used in gene therapy for cancers of p21Ras
overexpression.
Conditionally replicating adenovirus (oncolytic
adeno-virus) is currently the best vector to carry therapy gene.
However, it can proliferate and kill tumor cells, which make it
difficult to differentiate antitumor effects between therapy gene
and the adenovirus. The purpose of this study was to investigate
whether our anti-p21Ras scFv could express within tumor cells and
inhibit human ras-driven cancer cell growth. So we employed
replication-defective adenovirus to carry the scFv gene instead of
conditionally replicating adenovirus. In future, we will use
conditionally replicating adenoviruses, the E1a and E1b genes
controlled by the human telomerase reverse transcriptase (hTERT)
promoter and the hypoxia response element (HRE), respectively, to
increase the antitumor efficacy of this novel anti-p21Ras scFv.
Acknowledgements
This study was supported by the grants of National
Natural Science Foundation of China (no. 30872994), the Scientific
and Technological Key Project of Yunnan Province (no. 2006SG11),
and the Applied Foundation Key Project of Yunnan Province
(2013FA059).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernández-Medarde A and Santos E: Ras in
cancer and developmental diseases. Genes Cancer. 2:344–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reddy EP, Reynolds RK, Santos E and
Barbacid M: A point mutation is responsible for the acquisition of
transforming properties by the T24 human bladder carcinoma
oncogene. Nature. 300:149–152. 1982. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adjei AA: Blocking oncogenic Ras signaling
for cancer therapy. J Natl Cancer Inst. 93:1062–1074. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cochet O, Kenigsberg M, Delumeau I,
Virone-Oddos A, Multon MC, Fridman WH, Schweighoffer F, Teillaud JL
and Tocqué B: Intracellular expression of an antibody
fragment-neutralizing p21 ras promotes tumor regression. Cancer
Res. 58:1170–1176. 1998.PubMed/NCBI
|
|
7
|
Prendergast GC, Davide JP, deSolms SJ,
Giuliani EA, Graham SL, Gibbs JB, Oliff A and Kohl NE:
Farnesyltransferase inhibition causes morphological reversion of
ras-transformed cells by a complex mechanism that involves
regulation of the actin cytoskeleton. Mol Cell Biol. 14:4193–4202.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kohl NE, Omer CA, Conner MW, Anthony NJ,
Davide JP, deSolms SJ, Giuliani EA, Gomez RP, Graham SL, Hamilton
K, et al: Inhibition of farnesyltransferase induces regression of
mammary and salivary carcinomas in ras transgenic mice. Nat Med.
1:792–797. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Canevari S, Biocca S and Figini M: Re:
Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer
Inst. 94:1031–1032; author reply 1032. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mhashilkar AM, Bagley J, Chen SY, Szilvay
AM, Helland DG and Marasco WA: Inhibition of HIV-1 Tat-mediated LTR
transactivation and HIV-1 infection by anti-Tat single chain
intrabodies. EMBO J. 14:1542–1551. 1995.PubMed/NCBI
|
|
11
|
Duan L, Bagasra O, Laughlin MA, Oakes JW
and Pomerantz RJ: Potent inhibition of human immunodeficiency virus
type 1 replication by an intracellular anti-Rev single-chain
antibody. Proc Natl Acad Sci USA. 91:5075–5079. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deshane J, Siegal GP, Alvarez RD, Wang MH,
Feng M, Cabrera G, Liu T, Kay M and Curiel DT: Targeted tumor
killing via an intracellular antibody against erbB-2. J Clin
Invest. 96:2980–2989. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cochet O, Kenigsberg M, Delumeau I,
Duchesne M, Schweighoffer F, Tocqué B and Teillaud JL:
Intracellular expression and functional properties of an
anti-p21Ras scFv derived from a rat hybridoma containing specific
lambda and irrelevant kappa light chains. Mol Immunol.
35:1097–1110. 1998. View Article : Google Scholar
|
|
14
|
Lener M, Horn IR, Cardinale A, Messina S,
Nielsen UB, Rybak SM, Hoogenboom HR, Cattaneo A and Biocca S:
Diverting a protein from its cellular location by intracellular
antibodies. Eur J Biochem. 267:1196–1205. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gysin S, Salt M, Young A and McCormick F:
Therapeutic strategies for targeting ras proteins. Genes Cancer.
2:359–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Russell JS, Lang FF, Huet T, Janicot M,
Chada S, Wilson DR and Tofilon PJ: Radiosensitization of human
tumor cell lines induced by the adenovirus-mediated expression of
an anti-Ras single-chain antibody fragment. Cancer Res.
59:5239–5244. 1999.PubMed/NCBI
|
|
17
|
Van Etten B, Van Tiel ST, Ambagtsheer G,
Eggermont AM and Ten Hagen TL: Isolated limb perfusion based
anti-p21ras gene therapy in a rat rhabdomyosarcoma. Anticancer Res.
24:2295–2301. 2004.PubMed/NCBI
|
|
18
|
Saki M, Toulany M and Rodemann HP:
Acquired resistance to cetuximab is associated with the
overexpression of Ras family members and the loss of
radiosensitization in head and neck cancer cells. Radiother Oncol.
108:473–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thor A, Horan Hand P, Wunderlich D, Caruso
A, Muraro R and Schlom J: Monoclonal antibodies define differential
ras gene expression in malignant and benign colonic diseases.
Nature. 311:562–565. 1984. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wakita K, Ohyanagi H, Yamamoto K, Tokuhisa
T and Saitoh Y: Overexpression of c-Ki-ras and c-fos in human
pancreatic carcinomas. Int J Pancreatol. 11:43–47. 1992.PubMed/NCBI
|
|
21
|
Hamdy S, Aprikian A, Begin L, Fair W and
Bazinet M: Ras p21 overexpression is a late event in
prostate-cancer. Int J Oncol. 4:627–631. 1994.PubMed/NCBI
|
|
22
|
Murugan AK, Munirajan AK and Tsuchida N:
Ras oncogenes in oral cancer: The past 20 years. Oral Oncol.
48:383–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng ZY, Tian L, Bu W, Fan C, Gao X, Wang
H, Liao YH, Li Y, Lewis MT, Edwards D, et al: Wild-Type N-Ras,
Overexpressed in basal-like breast cancer, promotes tumor formation
by inducing IL-8 Secretion via JAK2 activation. Cell Reports.
12:511–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hand PH, Thor A, Wunderlich D, Muraro R,
Caruso A and Schlom J: Monoclonal antibodies of predefined
specificity detect activated ras gene expression in human mammary
and colon carcinomas. Proc Natl Acad Sci USA. 81:5227–5231. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gerosa MA, Talarico D, Fognani C, Raimondi
E, Colombatti M, Tridente G, De Carli L and Della Valle G:
Overexpression of N-ras oncogene and epidermal growth factor
receptor gene in human glioblastomas. J Natl Cancer Inst. 81:63–67.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Novara R, Coda R, Martone T and Vineis P:
Exposure to aromatic amines and ras and c-erbB-2 overexpression in
bladder cancer. J Occup Environ Med. 38:390–393. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pelletier AJ, Kunicki T and Quaranta V:
Activation of the integrin alpha v beta 3 involves a discrete
cation-binding site that regulates conformation. J Biol Chem.
271:1364–1370. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He XP, Su CQ, Wang XH, Pan X, Tu ZX, Gong
YF, Gao J, Liao Z, Jin J, Wu HY, et al: E1B-55kD-deleted oncolytic
adenovirus armed with canstatin gene yields an enhanced anti-tumor
efficacy on pancreatic cancer. Cancer Lett. 285:89–98. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaku Y, Noguchi A, Okutani A, Inoue S,
Tanabayashi K, Yamamoto Y, Hotta A, Suzuki M, Sugiura N and Yamada
A: Altered specificity of single-chain antibody fragments bound to
pandemic H1N1-2009 influenza virus after conversion of the
phage-bound to the soluble form. BMC Res Notes. 5:4832012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu D, Wang C, Li C, Zhang X, Zhang B, Mi
Z, An X and Tong Y: Production and characterization of a humanized
single-chain antibody against human integrin alphav beta3 protein.
J Biol Chem. 286:24500–24507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Biocca S, Neuberger MS and Cattaneo A:
Expression and targeting of intracellular antibodies in mammalian
cells. EMBO J. 9:101–108. 1990.PubMed/NCBI
|
|
33
|
Cattaneo A and Biocca S: Intracellular
Antibodies: Development and Applications. Springer-Verlag; Berlin:
pp. 1–196. 1997, View Article : Google Scholar
|
|
34
|
Thomas SM and Grandis JR: The current
state of head and neck cancer gene therapy. Hum Gene Ther.
20:1565–1575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biocca S, Pierandrei-Amaldi P and Cattaneo
A: Intracellular expression of anti-p21ras single chain Fv
fragments inhibits meiotic maturation of xenopus oocytes. Biochem
Biophys Res Commun. 197:422–427. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marasco WA, Haseltine WA and Chen SY:
Design, intracellular expression, and activity of a human
anti-human immunodeficiency virus type 1 gp120 single-chain
antibody. Proc Natl Acad Sci USA. 90:7889–7893. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rondon IJ and Marasco WA: Intracellular
antibodies (intrabodies) for gene therapy of infectious diseases.
Annu Rev Microbiol. 51:257–283. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Visintin M, Tse E, Axelson H, Rabbitts TH
and Cattaneo A: Selection of antibodies for intracellular function
using a two-hybrid in vivo system. Proc Natl Acad Sci USA.
96:11723–11728. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang W, Sima N, Kong D, Luo A, Gao Q, Liao
S, Li W, Han L, Wang J, Wang S, et al: Selective targeting of
HPV-16 E6/E7 in cervical cancer cells with a potent oncolytic
adenovirus and its enhanced effect with radiotherapy in vitro and
vivo. Cancer Lett. 291:67–75. 2010. View Article : Google Scholar
|
|
40
|
Chang Y, Li Y, Hu J, Guo J, Xu D, Xie H,
Lv X, Shi T and Chen Y: Adenovirus vector-mediated expression of
TMEM166 inhibits human cancer cell growth by autophagy and
apoptosis in vitro and in vivo. Cancer Lett. 328:126–134. 2013.
View Article : Google Scholar
|