Introduction
Currently, a rapidly growing body of research
demonstrates that breast cancer arises from a small population of
cancer cells termed as ‘cancer stem cells’ (CSCs) or
‘tumor-initiating cells’ (TICs) (1). CSCs are endowed with self-renewing
and unlimited proliferation potential (2,3), and
it is conceivable that CSCs also share with normal stem cells
several properties such as the relative quiescence, resistance to
drugs or toxins through expression of drug transporters, a better
ability to repair DNA and resistance to apoptosis and hypoxia,
which is critical to enable them to survive for extended periods
(4–7). As a result, typical
chemo-radiotherapies could only eliminate the bulk of the tumor,
but CSCs would survive and develop into a new tumor over time.
Therefore, the discovery and development of specific therapies that
target CSCs has the potential to revolutionize the treatment of
malignant tumors (4,8).
Signaling pathways that support stem cell
self-renewal appear to be promising cancer treatment candidates for
personalized therapy. Several developmental pathways, such as
Notch, Sonic Hedgehog (Shh), WNT are involved in regulation of
self-renewal of normal stem cells. However, dysregulation of these
pathways also contributes to the maintenance of CSCs (9–13).
In fact, numerous ‘stemness’ related genes are also oncogenes, and
many genes that inhibit self-renewal are also tumor suppressor
genes. These observations suggest the CSCs originate from normal
stem cells with somatic mutants accumulation during aging, and
cancer is essentially a disease of ‘stemness’ gone awry (14). Compounds that converge on these
cell-intrinsic pathways may overcome the dynamic nature of CSCs and
thereby prevent the evolution of CSC clones that drive tumor
initiation, maintenance, and relapse (15,16).
Notably, some researchers have reported
pyrviniumpamoate (PP), a well-known anthelmintic drug, exhibited a
potent antitumor activity against several cancers including myeloma
(17), glioblastoma (15), colon cancer (18) and lung cancer (19) as a selective WNT pathway inhibitor.
Although the WNT signaling pathway is important for cell
proliferation and differentiation, cell movement and polarity, and
maintenance of self-renewal in CSCs (20), whether pharmacologic blocking of
the WNT signaling pathway with PP in breast cancer could provide
therapeutic possibility by inhibiting breast cancer stem cells
(BCSCs) remains to be elucidated.
Importantly, breast tumors are comprised of
phenotypically diverse populations of breast cancer cells (21). In the past decade, the genomic
studies have established at least five different breast cancer
subtypes with difference in incidence, drug response and survival:
the luminal A and B, Her-2 overexpressing (OE), basal-like, and
normal breast-like tumors (22).
Moreover, BCSCs are also heterogeneous and the existence of various
BCSC subpopulations which would lead to a rapid relapse after
primary treatments might pose a problem for cancer therapy
(23,24). Virtually, therapeutic failure is in
part due to the heterogeneity imparting phenotypic diversity within
the CSCs (25,26). Each cancer subtype contains
distinct CSC subpopulations expressing different CSC markers, and
the molecular difference of the CSCs also imply different outcome
in response to the current treatment (24,27).
In the present study, PP was tested for its ability
to suppress the self-renewal and mammosphere-formation ability of
BCSCs derived from distinct molecular subgroups (luminal: MCF7,
Her-2 OE: SK-BR3, claudin-low: MDA-MB-231, basal-like: MDA-MB-468).
Moreover, we also evaluated the efficacy of PP suppressing breast
cancer motility and EMT process in vitro. Additionally, the
ability of PP to suppress BCSC self-renewal in vivo and the
potential mechanisms involved were also examined.
Materials and methods
Antibodies and reagents
Rabbit monoclonal anti-NANOG, anti-SOX2, anti-GAPDH,
anti-E-cadherin, anti-Ki67 and anti-vimentin were from Cell
Signaling Technology. Rabbit polyclonal anti-OCT4, monoclonal
anti-N-cadherin were from Abcam. Goat anti-rabbit IgG-HRP was from
Santa Cruz Biotechnology. Antibodies to FITC-conjugated CD44, and
PE-conjugated CD24 were from Miltenyi Biotec. Pyrvinium pamoate was
purchased from Sigma-Aldrich, and it was dissolved in DMSO at a
concentration of 1 μmol/l and was stocked in aliquots at −20°C.
Cell culture
Human breast cancer cell lines MCF-7, MDA-MB-231,
MDA-MB-468 and SkBr-3 were obtained from American Type Culture
Collection (www.atcc.org). These cells above were routinely
cultured in their recommended media containing 10% fetal bovine
serum (FBS) (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin
(Invitrogen) at 37°C in a humidified chamber with 5%
CO2.
Mammosphere formation assay
For mammosphere assay, a single cell suspension was
prepared at a density of 104/ml in culture medium and
were plated in the ultra-low attachment 6-well plates (Corning).
The mammosphere culture medium was serum-free DMEM/F-12 (1:1)
(Hyclone) supplemented with 20 ng/ml human basic fibroblast growth
factor (Gibco), 20 ng/ml human epidermal growth factor (Gibco),
1xB27 (Invitrogen), and 5 μg/ml insulin (Sigma-Aldrich). Culture
medium was replenished every 3 days and images were taken at day
7.
Colony formation assay
Cells were resuspended in culture medium containing
10% FBS with or without PP and seeded at a density of
1×103/dish into a 6-cm dish. Cells were kept for two
weeks and monitored for colony formation. To evaluate the colony
formation, cells were fixed and then stained with crystal violet
(Beyotime) for 15 min after the culture period. The clones
consisting of a minimum of 50 cells were counted.
In vitro proliferation assay
Cells (1×104) were suspended in 200 μl
culture medium and then seeded into 96-well plates (Corning) in
quintuplicate overnight. Cells were treated with indicated
concentrations of PP (0–8,000 μM). After incubating for 3 days,
Cell Counting kit-8 (CCK8) assay was conducted according to the
manufacturer's protocol (28).
CCK8 (10 μl) (Dojindo) was added into each well and incubated at
37°C for 1 h. The absorbance was measured using a microplate reader
at 450 nm (Tecan). The measured optical density (OD) values were
directly proportional to the number of viable cells. Then,
dose-response curves were fitted to the data and the half-maximal
inhibitory concentrations (IC50s) were calculated using
SPSS software package (v19.0; IBM Corp., Armonk, NY, USA). All
experiments were repeated at least three times. The cell
proliferation rate was calculated as follows: Cell proliferation
rate (%) = Experimental group OD value/Control group OD value ×
100% (1).
CD44+/CD24−/low
cell population
Cells were resuspended as single cell in PBS with 5%
FBS and incubated with FITC mouse anti-human CD44 (#130-095-195,
1:100, Miltenyi Biotec) and PE mouse anti-human CD24 (#130-095-953,
1:100, Miltenyi Biotec) for 15 min at 4°C in the dark. Analysis was
performed using a FACS Aria II cell sorter (BD Biosciences).
ALDEFLUOR assay
The ALDEFLUOR kit (Stem Cell Technologies) was used
to detect the cell populations with high aldehyde dehydrogenase
(ALDH) enzyme activity according to the manufacturer's
instructions. After incubating with or without PP for 72 h, cells
were resuspended at a density of 106/ml in ALDH assay
buffer containing the ALDH substrate BAAA (1 mM) and incubated for
30 min at 37°C. As a negative control, a sample of cells was
incubated with 50 mM of diethylaminobenzaldehyde (DEAB), a specific
ALDH inhibitor. A FACS Aria II cell sorter was used to analyse the
ALDH-positive cell population.
Migration/invasion assay
Migration assays were performed in 24-well Falcon
tissue culture plate with non-coated membrane transwells (pore
size, 8.0 μm, Merck Millipore). PP-pretreated (1×105;
MDA-MB-231: 500 nM, 72 h; MDA-MB-468: 100 nM, 72 h) or untreated
breast cancer cells were seeded on the top chamber and starved
overnight, and then incubated for 24 h using 10% FBS DMEM as
chemoattractant. Then the cells on the top of the insert were
removed with a cotton swab. The invasion assay was performed as
described for migration assay by using 1.5×105 cells and
Matrigel-coated membrane. Migrated cells on the lower surface were
fixed with ice-cold 4% paraformaldehyde, stained with 0.1% crystal
violet and then photographed and counted.
In vivo xenograft assay
NOD/SCID mice were housed under aseptic conditions
in individually ventilated cages. For xenografting,
5×106 PP-pretreated or untreated breast cancer cells
(MDA-MB-231) were resuspended in a 1:1 mixture of culture medium
and Matrigel (BD Biosciences) and then transplanted into the fourth
pair of mammary fat pads of mice (4–6-week-old) as previously
described (29). After injection,
tumor size was measured by calipers each day and tumor growth was
plotted. Upon reaching the endpoint, mice were sacrificed and
tumors were harvested. All the tumors were formalin-fixed, and
paraffin-embedded for hematoxylin and eosin (H&E) and
immunohistochemical (IHC) staining. All staining was performed with
standard protocols and analyzed by a pathologist (Xiaochun Fei) who
specializes in breast cancer. The rabbit anti-Ki67 monoclonal
antibody (#9027S, 1:200) used for IHC was purchased from Cell
Signaling Technology. All experiments were performed in accordance
with guidelines of Shanghai Jiaotong University (SJTU) animal care
and use committee.
qPCR assay
Total RNA was extracted using TRIzol reagent
(Invitrogen) according the manufacturer's instructions. RNA
integrity was verified using the Experion automated electrophoresis
station (Bio-Rad), and the RNA concentration was measured at 260
nm. The qPCR assays were conducted with the aid of a FastStart
Universal SYBR Green Master kit (Roche) and an ABI PRISM 7900HT
sequence detection system (Applied Biosystems). The cycler protocol
was 5 min at 95°C, 40 cycles with 15 sec at 95°C, 60 sec at 60°C,
and 5 min at 72°C. Gene of interest expression was normalized to
the reference genes GAPDH, and fold expression was calculated with
the 2−ΔΔCt method (30). The primers used in the present
study are listed in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primers | Sapiens |
|---|
| GAPDH | Forward: 5′ GGA GCG
AGA TCC CTC CAA AAT 3′ | Homo |
| Reverse: 5′ GGC TGT
TGT CAT ACT TCT CAT GG 3′ | Homo |
| N-cadherin | Forward: 5′ AGC CAA
CCT TAA CTG AGG AGT 3′ | Homo |
| Reverse: 5′ GGC AAG
TTG ATT GGA GGG ATG 3′ | |
| E-cadherin | Forward: 5′ ATT TTT
CCC TCG ACA CCC GAT 3′ | Homo |
| Reverse: 5′ TCC CAG
GCG TAG ACC AAG A 3′ | |
| Slug | Forward: 5′ TGT GAC
AAG GAA TAT GTG AGC C 3′ | Homo |
| Reverse: 5′ TGA GCC
CTC AGA TTT GAC CTG 3′ | |
| Snail | Forward: 5′ ACT GCA
ACA AGG AAT ACC TCA G 3′ | Homo |
| Reverse: 5′ GCA CTG
GTA CTT CTT GAC ATC TG 3′ | |
| Twist1 | Forward: 5′ GTC CGC
AGT CTT ACG AGG AG 3′ | Homo |
| Reverse: 5′ GCT TGA
GGG TCT GAA TCT TGC T 3′ | |
| ZEB1 | Forward: 5′ TTA CAC
CTT TGC ATA CAG AAC CC 3′ | Homo |
| Reverse: 5′ TTT ACG
ATT ACA CCC AGA CTG C 3′ | |
| ZEB2 | Forward: 5′ GCG ATG
GTC ATG CAG TCA G 3′ | Homo |
| Reverse: 5′ CAG GTG
GCA GGT CAT TTT CTT 3′ | |
| NANOG | Forward: 5′ TTT GTG
GGC CTG AAG AAA ACT 3′ | Homo |
| Reverse: 5′ AGG GCT
GTC CTG AAT AAG CAG 3′ | |
| OCT4 | Forward: 5′ CTT GAA
TCC CGA ATG GAA AGG G 3′ | Homo |
| Reverse: 5′ GTG TAT
ATC CCA GGG TGA TCC TC 3′ | |
| SOX2 | Forward: 5′ TAC AGC
ATG TCC TAC TCG CAG 3′ | Homo |
| Reverse: 5′ GAG GAA
GAG GTA ACC ACA GGG 3′ | |
| KLF4 | Forward: 5′ CAG CTT
CAC CTA TCC GAT CCG 3′ | Homo |
| Reverse: 5′ GAC TCC
CTG CCA TAG AGG AGG 3′ | |
| ABCG2 | Forward: 5′ ACG AAC
GGA TTA ACA GGG TCA 3′ | Homo |
| Reverse: 5′ CTC CAG
ACA CAC CAC GGA T 3′ | |
| ALDH1 | Forward: 5′ GCA CGC
CAG ACT TAC CTG TC 3′ | Homo |
| Reverse: 5′ CCT CCT
CAG TTG CAG GAT TAA AG 3′ | |
| CD44 | Forward: 5′ CTG CCG
CTT TGC AGG TGT A 3′ | Homo |
| Reverse: 5′ CAT TGT
GGG CAA GGT GCT ATT 3′ | |
| WNT1 | Forward: 5′ CGA TGG
TGG GGT ATT GTG AAC 3′ | Homo |
| Reverse: 5′ CCG GAT
TTT GGC GTA TCA GAC 3′ | |
| WNT7B | Forward: 5′ GAA GCA
GGG CTA CTA CAA CCA 3′ | Homo |
| Reverse: 5′ CGG CCT
CAT TGT TAT GCA GGT 3′ | |
| MYC | Forward: 5′ CAC CTT
GTA GCA CGT CCT G 3′ | Homo |
| Reverse: 5′ GAC TCC
CCA AGA TGT GGT GG 3′ | |
| LRP5 | Forward: 5′ TGG CCC
GAA ACC TCT ACT G 3′ | Homo |
| Reverse: 5′ GCA CAC
TCG ATT TTA GGG TTC T 3′ | |
| FZD1 | Forward: 5′ AGC CAT
CCA GTT GCA CGA G 3′ | Homo |
| Reverse: 5′ GAG TCG
GGC CAC TTG AAG TT 3′ | |
| FZD10 | Forward: 5′ GGC GGT
GAA GAC CAT CCT G 3′ | Homo |
| Reverse: 5′ GGC GGT
GAA GAC CAT CCT G 3′ | |
| CTNNB1 | Forward: 5′ CAT CTA
CAC AGT TTG ATG CTG CT 3′ | Homo |
| Reverse: 5′ GCA GTT
TTG TCA GTT CAG GGA 3′ | |
| Gli1 | Forward: 5′ GTG CAA
GTC AAG CCA GAA CA 3′ | Homo |
| Reverse: 5′ ATA GGG
GCC TGA CTG GAG AT 3′ | |
| Gli2 | Forward: 5′ CAT GGA
GCA CTA CCT CCG TTC 3′ | Homo |
| Reverse: 5′ CGA GGG
TCA TCT GGT GGT AAT 3′ | |
| HES1 | Forward: 5′ TCA ACA
CGA CAC CGG ATA AAC 3′ | Homo |
| Reverse: 5′ GCC GCG
AGC TAT CTT TCT TCA 3′ | |
Western blotting
Cultured cells were washed with ice-cold PBS three
times, harvested, and lysed in RIPA buffer (Pierce) for immunoblot
analysis. In brief, the supernatants containing 10 μg total protein
were electrophoresed on 10–12% gradient sodium dodecyl
sulfate-polyacrylamide gels (SDS-PAGE) and then transferred to
polyvinylidene fluoride membranes (Bio-Rad). Membranes were blocked
in 5% (w/v) skim milk for 1 h at room temperature and then
incubated at 4°C overnight with the primary antibodies. Membranes
were then incubated with horseradish peroxidase-conjugated
secondary antibodies (Santa Cruz Biotechnology) for 1 h at room
temperature and detected using ECL Prime Western Blotting Detection
Reagent (GE Healthcare). Images were obtained using a LAS-3000
Imager (Fuji film). The primary antibodies used were NANOG (#4903S,
1:1,000, Cell Signaling Technology), SOX2 (#3579S, 1:1,000, Cell
Signaling Technology), GAPDH (#5174, 1:2,000, Cell Signaling
Technology), OCT4 (#ab109183, 1:1,000, Abcam), E-cadherin (#3195,
1:1,000, Cell Signaling Technology), N-cadherin (#ab18203, 1:1,000,
Abcam) and vimentin (#5741, 1:1,000, Cell Signaling
Technology).
Statistical analysis
All graphs and statistical analyses were made using
Prism 5 statistical software (GraphPad Software, Inc.), unless
otherwise stated. Student's t-test was employed for two-group
comparisons. The results are expressed as mean ± standard deviation
(SD). All experimental data were obtained from at least three
experimental repeats and P-values <0.05 were considered
statistically significant. Bar graphs show mean values with 95%
confidence intervals.
Results
PP inhibits proliferation of different
breast cancer cells
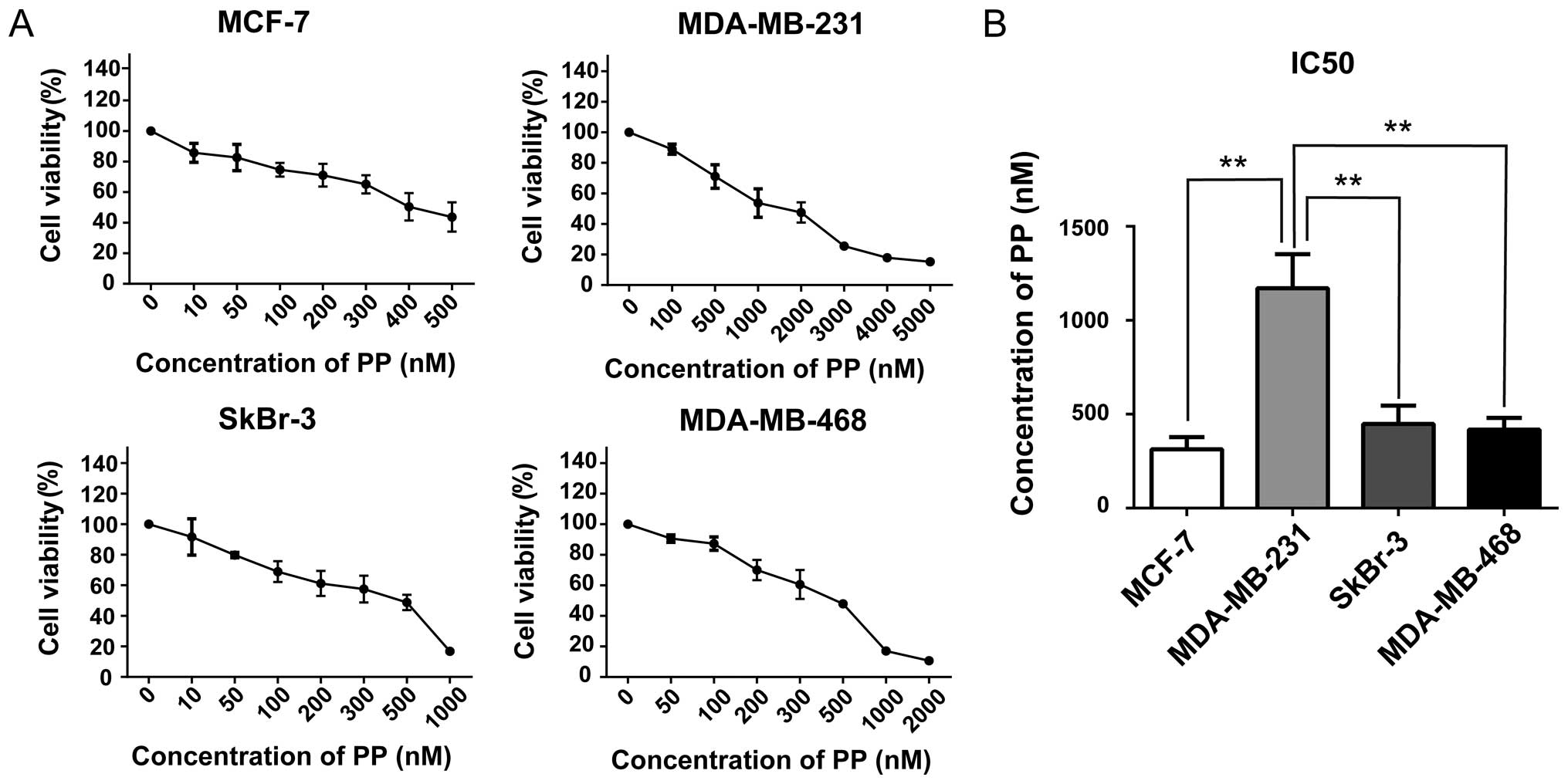
In order to evaluate the effects of PP on
proliferation in breast cancer cells, cell viabilities were
examined after exposing four breast cancer cell lines to varying
concentrations of PP for 3 days. We found PP efficiently decreased
the viabilities on MCF-7 (luminal), MDA-MB-231 (claudin-low),
MDA-MB-468 (basal-like) and SkBr3 (HER2-OE) cells in a
dose-dependent manner (Fig. 1A).
The half-maximal inhibitory concentrations (IC50s) of PP
were used at a nanomole concentration and they vary considerably
among diverse subtypes. Of interest, MDA-MB-231, a claudin-low
breast cancer cell line, was relatively insensitive to the PP
treatment with a IC50 value of 1170±105.0 nM (Fig. 1B).
PP inhibits self-renewal capacity of
BCSCs in vitro
As the cardinal property of stemness, self-renewal
is defined by the ability of a cell, at each cell division, to
generate an identical copy of itself and a cell of the same or
different phenotype (31).
Moreover, because the mammosphere culture mirrors in vitro
tumorigenic capacity and it can also retrospectively identify CSCs
that develop from single stem cell-like clones (32,33),
mammosphere formation assay was utilized in our study. As shown in
Fig. 2A, mammospheres were
successfully generated from MCF-7, MDA-MB-468, SkBr3, and
MDA-MB-231 cells. Furthermore, we evaluated whether PP could exert
influence on self-renewal capacity of BCSCs. In the mammosphere
formation assay, PP was shown to significantly reduce both the
number and size of mammospheres in vitro (Fig. 2B). As a consequence of these
findings, we next tested its effect on colony formation. As
expected, our findings also directly illustrated that PP was
effective against colony formation across all four cell lines
tested (Fig. 2C and D). Taken
together, our results demonstrated that PP significantly inhibits
self-renewal and proliferation of BCSCs.
 | Figure 2PP effectively inhibits self-renewal
of BCSCs. (A) Morphology of mammospheres derived from different
breast cancer cell lines. Cells were cultured in non-adherent
culture conditions for 7 days, and images were captured by a
microscope. (B) Representative images of mammosphere formation
assay of four breast cancer cell lines in the absence or presence
of PP. The dose of PP used for MCF-7, SkBr-3, MDA-MB-231, and
MDA-MB-468 was 100, 200, 500 and 200 nM, respectively. (C) Cell
counting results of mammosphere formation assay. (D) Colony
formation assay of different breast cancer cell lines in the
absence or presence of PP. The dose of PP used for MCF-7,
MDA-MB-231, SkBr-3 and MDA-MB-468 was 100, 200, 100 and 100 nM,
respectively. (E) Cell counting results of colony formation assay.
Data are reported as means ± SD of three independent experiments.
*P<0.05, **P<0.01 and
***P<0.001. Scale bar, 100 μm. PP, pyrvinium pamoate;
BCSC, breast cancer stem cell. |
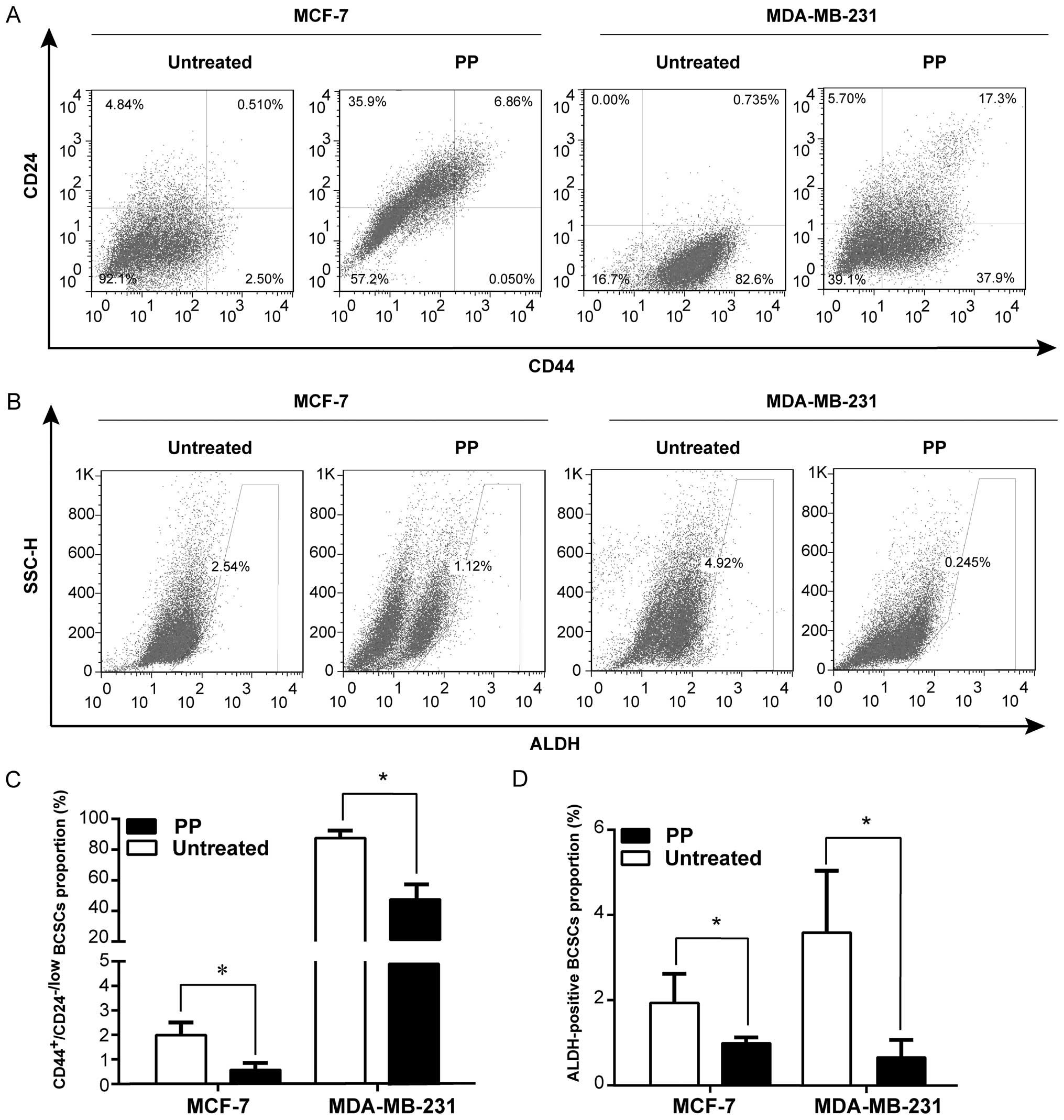
PP decreases different BCSC
subpopulations
Both CD44+/CD24−/low and
ALDH-positive have been widely used as specific markers to identify
the BCSCs from breast cancer tissues, and the putative BCSCs are
capable of self-renewal and generating tumors resembling breast
cancer (34). To this end, we
further evaluated whether PP was able to eliminate the BCSCs with
CD44+CD24−/low or ALDH-positive phenotype
directly. Results of the flow cytometric assay depicted that after
3-day treatment, PP markedly reduced the
CD44+CD24−/low population in different breast
cancer cell lines (MCF-7, MDA-MB-231: Fig. 3A and B; MDA-MB-468: data not
shown), compared with the control (P<0.05). Similarly, a decline
of ALDH-positive cell population was also observed in PP-treated
cells (MCF-7, MDA-MB-231: Fig. 3C and
D; SkBr-3, MDA-MB-468: data not shown). Actually, recent
studies have identified CD44+CD24−/low and
ALDH-positive phenotypes probably refer to different BCSC
populations (2,35). Our results therefore indicated PP
can suppress BCSC population with a distinct phenotype.
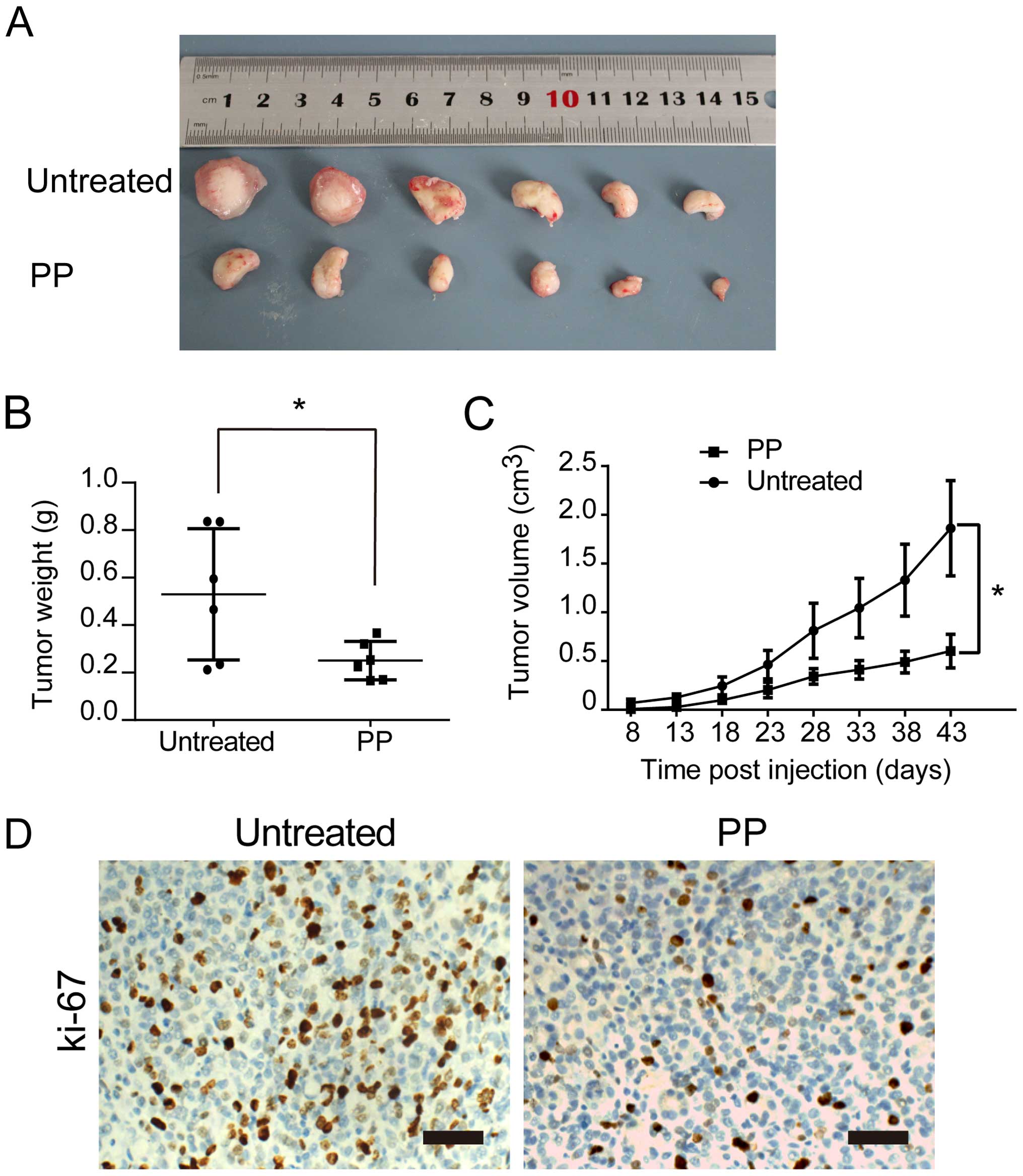
PP reduces tumorigenicity of BCSCs in
vivo
In our xenograft model, 5×106
PP-pretreated or untreated breast cancer cells (MDA-MB-231) were
injected into the cleared mammary fat pads of NOD/SCID mice. All
the tumor tissues were confirmed with hematoxylin and eosin
(H&E) staining. We observed that PP-pretreatment strongly
delayed tumor size and tumor weight (Fig. 4A and B). Furthermore, the tumor
growth curves demonstrated that the tumor volume of PP-pretreated
group was markedly decreased, compared with control group
(P<0.05) (Fig. 4C).
Immunohistochemical staining also found significantly lower Ki-67
expression in the PP-pretreated group (Fig. 4D), supporting our hypothesis of PP
effectively targeting self-renewal and proliferation of BCSCs in
vitro and in vivo.
PP inhibits breast cancer cell
invasiveness and EMT process
To extend our analysis of the role of PP in cell
motility, we applied Transwell assays to evaluate the breast cancer
cell migratory and invasive potential of two of the most aggressive
breast cancer cell lines (MDA-MB-231, MDA-MB-468) in the absence or
presence of PP. As shown in Fig.
5A–D, PP significantly inhibited cell motility and reduced the
number of cells that migrated through the membrane. Because EMT has
a major role in cancer metastasis and maintenance of BCSCs, the
expression levels of epithelial and mesenchymal markers were also
examined. We found PP greatly increased the expression of the
epithelial marker E-cadherin. For mesenchymal markers N-cadherin
and vimentin, however, PP treatment effectively decreased their
expression both at translational and transcriptional levels
(Fig. 5E and F). Moreover, a high
mRNA expression of well-known transcriptional repressors of
E-cadherin (such as Snail, ZEB1 and Twist1) were also observed
(Fig. 5E). Collectively, these
results indicated PP attenuates the migratory and invasive
properties of cancer cells and EMT process.
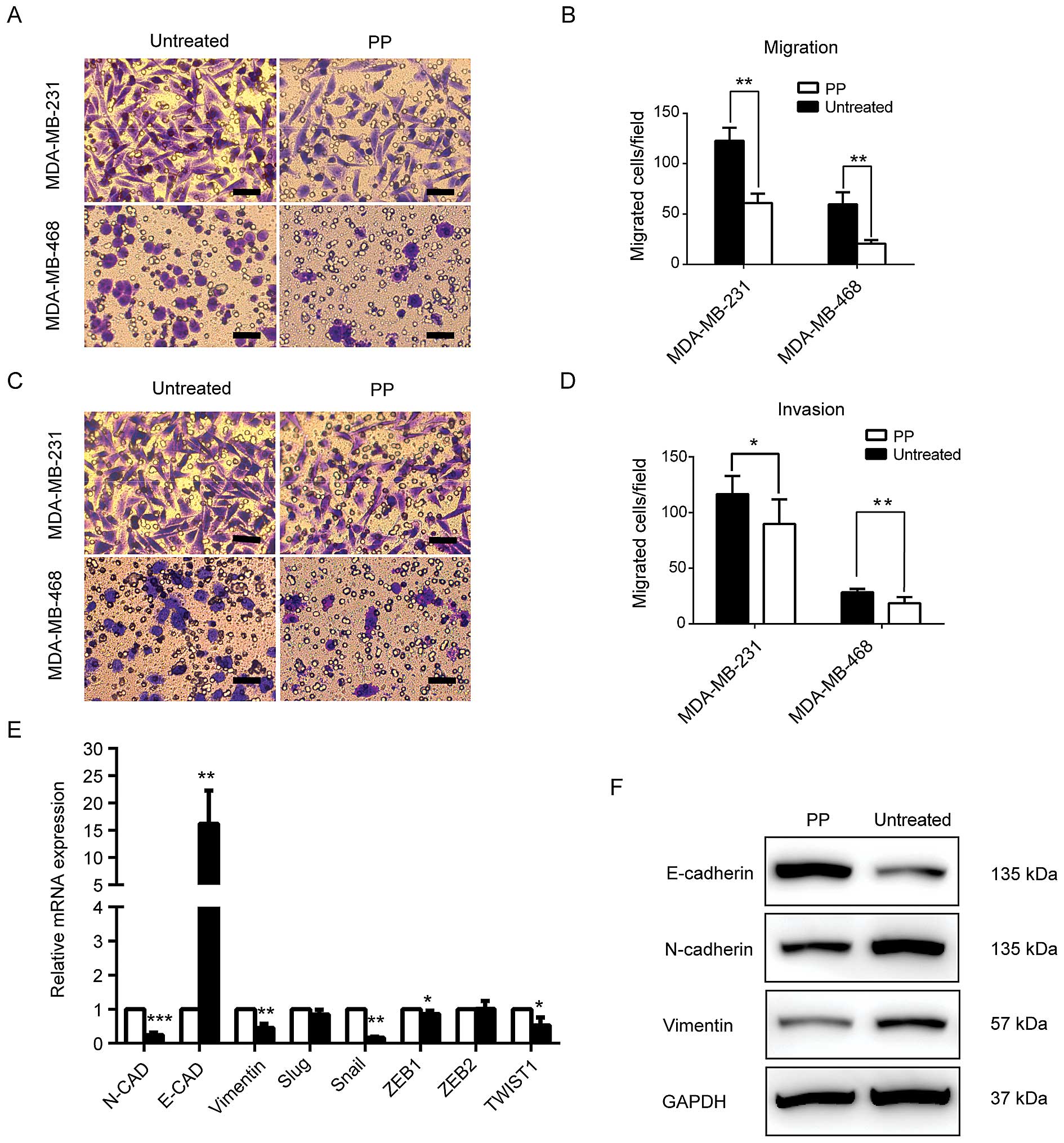 | Figure 5PP suppresses metastatic potential
and EMT process in breast cancer. Transwell assay was used to
evaluate the inhibitory effect of PP on migratory (A and C) and
invasive potential (B and D) of MDA-MB-231 and MDA-MB-468 breast
cancer cells. (E) Real-time PCR was used to analyse the gene
expression of E-cadherin, N-cadherin, vimentin, Snail, Slug, ZEB1,
ZEB2 and Twist1. (F) Western blot analysis of EMT-related markers.
In Transwell assay, the concentration of PP used for MDA-MB-231 and
MDA-MB-468 was 500 and 100 nM, respectively. The cell lysates for
the immunoblot assay were obtained from the PP-treated MDA-MB-231
(1,000 nM, 72 h) and control cells. Data are reported as mean ± SD,
*P<0.05, **P<0.01,
***P<0.001. Scale bar, 50 μm. PP, pyrvinium pamoate;
EMT, epithelial-mesenchymal transition. |
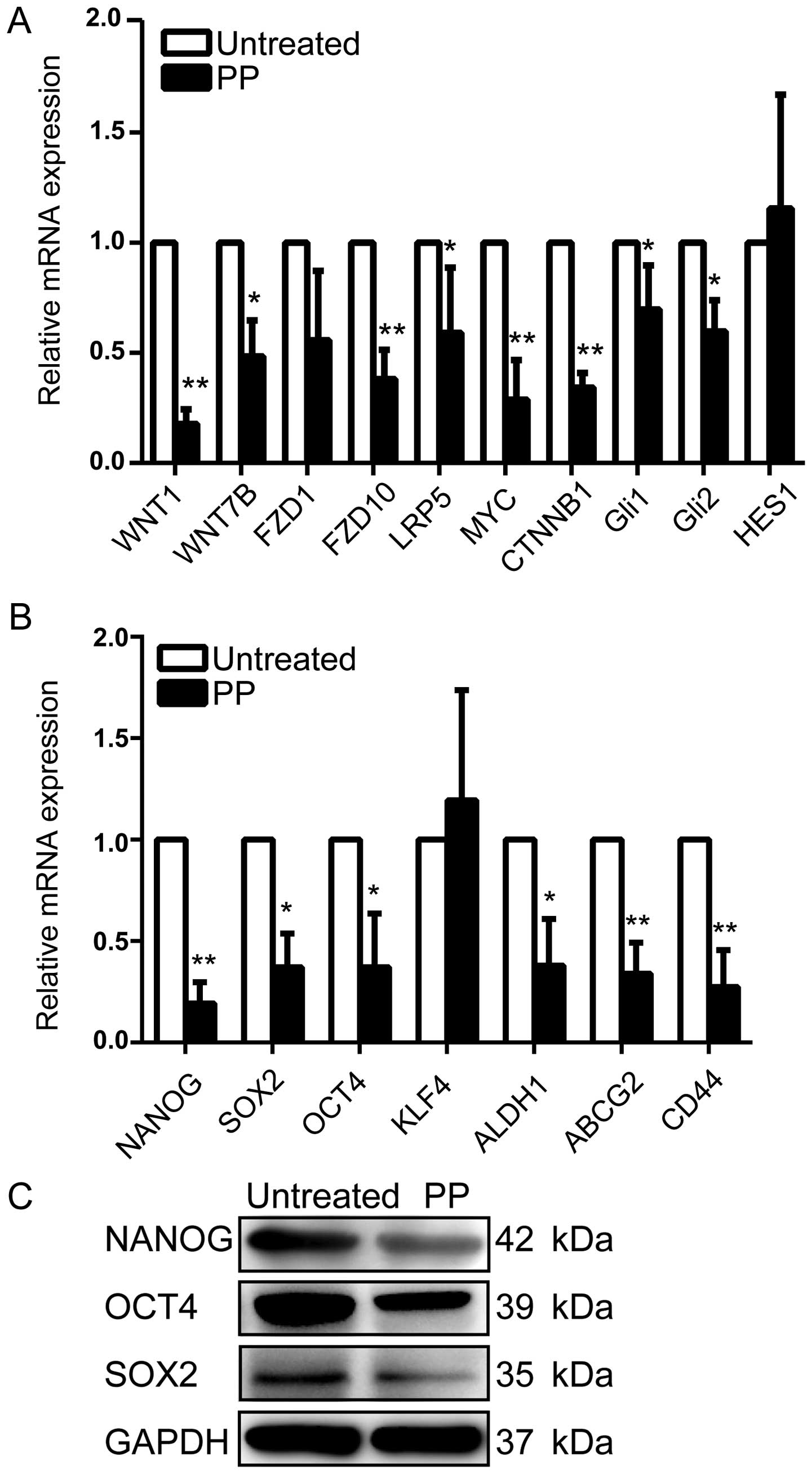
PP effectively attenuates WNT signaling
and downregulates stemness regulators
We next investigated the mechanisms underlying the
inhibitory effects of PP on BCSCs. Recently, WNT signaling pathway
was reported to play a pivotal role in sustaining self-renewal
potential and chemoresistance in CSCs (20). Aberrant activation of CTNNB1, MYC,
and LRP5 is known as key process of the WNT signaling pathway. As
shown in Fig. 6A, we observed that
PP significantly decreased average expression levels of FZD1,
FZD10, WNT1, WNT7B, CTNNB1, MYC, and LRP5 at transcriptional level
compared with control. Additionally, a prior study has reported
that pyrvinium is a potent inhibitor of Sonic Hedgehog (Shh)
signaling, which acts by reducing the stability of the Gli family
of transcription factors (36).
Interestingly, a decrease of Gli1 and Gli2 was also confirmed with
Q-PCR assay in the present study, whereas the mRNA level of HES1,
the target gene of Notch pathway, remained unaltered (Fig. 6A).
Because several self-renewal genes including NANOG,
OCT4 and SOX2 are key regulators of stemness in CSCs (37), we further explored whether PP
downregulates these stemness genes in vitro. Our data
revealed significantly lower expression of these stem cell markers
in the PP-treated breast cancer cells in comparison with untreated
cells both at mRNA and protein levels (Fig. 6B and C). Moreover, PP also
efficiently down-regulated the expression of other stemness genes
including ALDH1, CD44 and ABCG2 (Fig.
6B). To sum up, all these results pointed towards the
possibility that PP inhibits BCSC activity through attenuating WNT
pathway activity and down-regulating stemness regulators.
Discussion
In the past decade, evidence has mounted for the
role of the WNT signaling pathway during embryogenesis and
physiological organogenesis (38).
More recently, the WNT pathway also has been identified as an
important regulator of self-renewal capacity in CSCs and a
potential high-yield therapeutic target (20). Although no FDA-approved drugs that
regulate WNT signaling are available to date (18), improved drug-screening platforms
and new technologies have discovered agents that can alter WNT
signaling in preclinical models (39,40).
However, because the WNT pathway is shared with normal stem cells,
most of the compounds may be proved difficult not to damage normal
stem cells and thereby limit their use (8). Hence, pyrvinium pamoate (PP), a
classical anthelmintic drug, is attracting particular attention as
a novel WNT pathway inhibitor. Recent studies have demonstrated
that PP can exert a potent anticancer activity in several cancer
types via inhibiting WNT pathway activity and autophagy process
(15,17,19,41),
but reports describing the effect of PP on breast cancer cells,
especially on BCSCs, are scarce.
The CSC hypothesis has been proposed for years.
Theoretically, if BCSCs were totally eliminated, the remaining
non-stem tumor cells would be unable to re-grow or to promote new
tumors. Given that conventional agents fail to eliminate the BCSCs
that evade therapy to drive patient relapse, new potential targets
and drugs that kill both BCSCs and non-tumorigenic cancer cells are
now clinically warranted (42).
The present investigation examined the ability of PP
to inhibit breast cancer cells proliferation. Our cell viability
assays demonstrated that PP substantially suppressed proliferation
of four genetically different breast cancer cell lines (Fig. 1A). Interestingly, the claudin-low
breast cancer cell line MDA-MB-231 showed a relatively higher
IC50, compared with all other cell lines (Fig. 1B). These results may be partly
explained by two phenomena: the claudin-low subtype most closely
resembles the epithelial stem cells (43,44);
and the CD44+/CD24−/low/claudin-low profile
is increased in post-treatment samples after neoadjuvant
chemotherapy or hormonal therapy (45). These findings together suggest
different biological features (such as drug-resistance) associated
with BCSCs converging in the claudin-low phenotype (43). PP has been described to function as
a potent inhibitor of self-renewal via multiple mechanisms
(15,19,46).
Hence, we further explored the effect of PP on BCSC self-renewal
capacity. Similarly, we also validated that PP has the capacity to
inhibit mammosphere formation and colony formation of BCSCs
(Fig. 2B–E). Moreover, our
xenograft model also confirmed that the effect of PP on
tumorigenicity decreased although the result was limited to a
single breast cancer cell line (MDA-MB-231) (Fig. 4A and B). Indeed, MDA-MB-231 is one
of the most aggressive breast cancer cell lines and has the highest
CD44+CD24−/low BCSC frequency (~90%). Thus,
our in vivo study result may be more broadly applicable
including for less aggressive subtypes such as luminal breast
cancer. Taken together, these findings clearly demonstrated the
effectiveness of PP to overcome proliferation and self-renewal of
both anchorage-dependent cells and BCSCs. At this juncture, it is
quite logical to postulate that PP might emerge as a promising drug
for successful eradiation of breast cancer.
With continual refinement of massive parallel
sequencing (MPS) technologies markedly shorten the path to fully
personalized medicine, however, tumor heterogeneity will be one of
the greatest challenges to manage in this endeavor (47). Notably, Venugopal et al
revealed PP can selectively target TICs that drive tumor
heterogeneity in human glioblastoma (15). In breast cancer, a previous study
revealed that the overlap between
CD44+CD24−/low and ALDH-positive CSC
phenotypes seems to be very small (<1%) (48). Additionally, it has been proven
that their distributions among intrinsic breast cancer subtypes
were different (2,49). Basal-like tumors contained a higher
percentage of CSCs with CD44+CD24−/low
phenotype, whereas ALDH enzyme activity was mainly found in HER-OE
and basal/epithelial breast cancer cells. The
CD44+CD24−/low and ALDH-positive subsets seem
to identify CSCs with distinct levels of differentiation (2). Most importantly, previous studies
reported variable therapeutic responses on different CSC
populations (24,27). Due to these discrepancies, we next
analysed whether PP was able to reduce
CD44+/CD24−/low and ALDH-positive
subpopulation which are the most two consistently used methods to
identify and isolate BCSCs. Interestingly, we noted that both
CD44+CD24−/low and ALDH-positive
BCSCs population decreased after 3-day PP treatment (Fig. 3C and D). Thus, we provide direct
evidence that PP is an inhibitor of BCSCs and a novel agent to
overcome heterogeneity in both BCSCs and non-stem tumor bulk.
Unlike differentiated epithelial cells, when
detaching from the extracellular matrix during the migratory
process, CSCs can avoid apoptosis and survive in the blood stream.
Then, as it travels all over the body, CSCs are able to select a
suitable site distant from the primary tumor and thrive in its new
environment (50). Thus, it is
believed that CSCs are able to metastasize to distant organs where
they serve as seeds of metastatic lesions (35). Of note, our results showed that PP
significantly reduced metastatic capacity of two of the most
aggressive breast cancer cell lines (MDA-MB-231, MDA-MB-468)
(Fig. 5A–D). Consistent with the
above findings, a prior study also observed that intraperitoneal
injection of PP caused a trend toward decreased lung metastasis in
mice (46). Moreover, EMT-related
genes (N-cadherin, vimentin, Snail, Twist1) were also confirmed to
be downregulated in the presence of PP (Fig. 5E and F). Indeed, one key link
between CSCs and metastasis may be the EMT, the process by which
epithelial cells shed their epithelial characteristics in order to
become mesenchymal cells with enhanced motility and spindle cell
shape (7). Therefore, although
more studies are needed to provide more direct evidence for the
conclusions, our results point towards the possibility that PP
circumvents BCSC migratory and metastatic potential by suppressing
the EMT process.
As a WNT pathway inhibitor, PP has two targets in
the WNT signaling cascade (51).
On the one hand, PP was able to inhibit WNT pathway by enhancing
casein kinase 1α (CK-1α) activity (52). One the other hand, PP was also
reported to inhibit Pygopus (PYG), a co-transcription factor
of β-catenin, and thereby interfere with target gene transcription
(51). However, other researchers
also revealed PP is not a ‘bona fide’ activator of CK-1α but
promotes downregulation of Akt/PKB and GSK3 activation, thus,
regulates WNT signaling (53). To
delineate the mechanisms underlying the inhibitory effect of PP on
BCSCs, we focused on the critical pathways that regulate the
self-renewal of CSCs (WNT, Shh, and Notch signaling pathways).
Similarly, we also found PP successfully attenuated WNT pathway
activity and stemness regulator expression in BCSCs (Fig. 6A and B). Interestingly, the
inhibitory function of PP was also identified in the Shh pathway,
but not the Notch pathway (Fig.
6A). In line with our data, Li et al also showed that PP
was a potent Shh pathway inhibitor, which acts by reducing the
stability of the Gli family of transcription factors (36). Actually, crosstalk between the WNT
and Shh pathways has been evidenced in cancer (54,55).
On the basis of these findings, we speculate that PP targets the
overlapping components of WNT pathway and Shh pathway, and it
warrants further studies (such as microarray assay and rescue
experiment) to identify the target genes associated with the
inhibition of BCSCs by PP.
Our current findings have direct implications with
regard to evaluation of PP as a potent inhibitor of both BCSCs and
non-tumorigenic cancer cells. However, it will take time for this
information to be translated into clinic. Major concerns mainly
focus on the absorption, distribution and systemic toxicity of PP.
However, it is noteworthy that PP has been used as a classical
anthelmintic drug for more than fifty years and the doses (0–1,000
nM) used in our study were relatively low and safe. Another
challenge before us is that when taken orally, the absorption of PP
from the gut is minimal (56).
Thus, the development of pharmaceutical technology to improve the
drug delivery is urgently needed. Because BCSCs are also highly
associated with chemo-resistance behavior (57), PP combination therapy with current
chemotherapeutic agents (such as anthracyclines and taxanes) should
be evaluated in future studies both in vitro and in
vivo.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China grant (no. 81172522).
References
|
1
|
Chiba T, Kita K, Zheng YW, Yokosuka O,
Saisho H, Iwama A, Nakauchi H and Taniguchi H: Side population
purified from hepatocellular carcinoma cells harbors cancer stem
cell-like properties. Hepatology. 44:240–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ricardo S, Vieira AF, Gerhard R, Leitão D,
Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes
J: Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression
distribution within intrinsic molecular subtype. J Clin Pathol.
64:937–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Brien CA, Kreso A and Jamieson CH:
Cancer stem cells and self-renewal. Clin Cancer Res. 16:3113–3120.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lou H and Dean M: Targeted therapy for
cancer stem cells: The patched pathway and ABC transporters.
Oncogene. 26:1357–1360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishii H, Iwatsuki M, Ieta K, Ohta D,
Haraguchi N, Mimori K and Mori M: Cancer stem cells and
chemoradiation resistance. Cancer Sci. 99:1871–1877. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chuthapisith S, Eremin J, El-Sheemey M and
Eremin O: Breast cancer chemoresistance: Emerging importance of
cancer stem cells. Surg Oncol. 19:27–32. 2010. View Article : Google Scholar
|
|
7
|
Saadin K and White IM: Breast cancer stem
cell enrichment and isolation by mammosphere culture and its
potential diagnostic applications. Expert Rev Mol Diagn. 13:49–60.
2013. View Article : Google Scholar
|
|
8
|
Sehl ME, Sinsheimer JS, Zhou H and Lange
KL: Differential destruction of stem cells: Implications for
targeted cancer stem cell therapy. Cancer Res. 69:9481–9489. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malhotra GK, Zhao X, Band H and Band V:
Shared signaling pathways in normal and breast cancer stem cells. J
Carcinog. 10:382011. View Article : Google Scholar
|
|
10
|
Harrison H, Farnie G, Howell SJ, Rock RE,
Stylianou S, Brennan KR, Bundred NJ and Clarke RB: Regulation of
breast cancer stem cell activity by signaling through the Notch4
receptor. Cancer Res. 70:709–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farnie G and Clarke RB: Mammary stem cells
and breast cancer - role of Notch signalling. Stem Cell Rev.
3:169–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Czerwinska P and Kaminska B: Regulation of
breast cancer stem cell features. Contemp Oncol (Pozn). 19A:A7–A15.
2015.
|
|
13
|
Zhao C, Chen A, Jamieson CH, Fereshteh M,
Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al:
Hedgehog signalling is essential for maintenance of cancer stem
cells in myeloid leukaemia. Nature. 458:776–779. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomasetti C and Vogelstein B: Cancer
etiology. Variation in cancer risk among tissues can be explained
by the number of stem cell divisions. Science. 347:78–81. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Venugopal C, Hallett R, Vora P, Manoranjan
B, Mahendram S, Qazi MA, McFarlane N, Subapanditha M, Nolte SM,
Singh M, et al: Pyrvinium targets CD133 in human glioblastoma brain
tumor-initiating cells. Clin Cancer Res. 21:5324–5337. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abetov D, Mustapova Z, Saliev T, Bulanin
D, Batyrbekov K and Gilman CP: Novel small molecule inhibitors of
cancer stem cell signaling pathways. Stem Cell Rev. 11:909–918.
2015.PubMed/NCBI
|
|
17
|
Harada Y, Ishii I, Hatake K and Kasahara
T: Pyrvinium pamoate inhibits proliferation of
myeloma/erythroleukemia cells by suppressing mitochondrial
respiratory complex I and STAT3. Cancer Lett. 319:83–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiegering A, Uthe FW, Hüttenrauch M,
Mühling B, Linnebacher M, Krummenast F, Germer CT, Thalheimer A and
Otto C: The impact of pyrvinium pamoate on colon cancer cell
viability. Int J Colorectal Dis. 29:1189–1198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Lou Y, Zheng X, Wang H, Sun J,
Dong Q and Han B: Wnt blockers inhibit the proliferation of lung
cancer stem cells. Drug Des Devel Ther. 9:2399–2407.
2015.PubMed/NCBI
|
|
20
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polyak K: Heterogeneity in breast cancer.
J Clin Invest. 121:3786–3788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lorico A and Rappa G: Phenotypic
heterogeneity of breast cancer stem cells. J Oncol.
2011:1350392011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang A, Chen L, Li C and Zhu Y:
Heterogeneity in cancer stem cells. Cancer Lett. 357:63–68. 2015.
View Article : Google Scholar
|
|
25
|
Chen J, Li Y, Yu TS, McKay RM, Burns DK,
Kernie SG and Parada LF: A restricted cell population propagates
glioblastoma growth after chemotherapy. Nature. 488:522–526. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Nenutil R, Appleyard MV, Murray K,
Boylan M, Thompson AM and Coates PJ: Lack of correlation of stem
cell markers in breast cancer stem cells. Br J Cancer.
110:2063–2071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bozkulak EC and Weinmaster G: Selective
use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol
Cell Biol. 29:5679–5695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Appleyard MV, Murray KE, Coates PJ,
Wullschleger S, Bray SE, Kernohan NM, Fleming S, Alessi DR and
Thompson AM: Phenformin as prophylaxis and therapy in breast cancer
xenografts. Br J Cancer. 106:1117–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu S and Labhasetwar V: Drug resistant
breast cancer cell line displays cancer stem cell phenotype and
responds sensitively to epigenetic drug SAHA. Drug Deliv Transl
Res. 3:183–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lombardo Y, de Giorgio A, Coombes CR,
Stebbing J and Castellano L: Mammosphere formation assay from human
breast cancer tissues and cell lines. J Vis Exp. Mar 22–2015.(Epub
ahead of print). View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hwang-Verslues WW, Lee WH and Lee EY:
Biomarkers to target heterogeneous breast cancer stem cells. J Mol
Biomark Diagn. (Suppl 8): 62012.PubMed/NCBI
|
|
35
|
Luo M, Brooks M and Wicha MS:
Epithelial-mesenchymal plasticity of breast cancer stem cells:
Implications for metastasis and therapeutic resistance. Curr Pharm
Des. 21:1301–1310. 2015. View Article : Google Scholar :
|
|
36
|
Li B, Fei DL, Flaveny CA, Dahmane N,
Baubet V, Wang Z, Bai F, Pei XH, Rodriguez-Blanco J, Hang B, et al:
Pyrvinium attenuates Hedgehog signaling downstream of smoothened.
Cancer Res. 74:4811–4821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bourguignon LY, Wong G, Earle C and Chen
L: Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes
miR-302 expression leading to self-renewal, clonal formation, and
cisplatin resistance in cancer stem cells from head and neck
squamous cell carcinoma. J Biol Chem. 287:32800–32824. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peifer M and Polakis P: Wnt signaling in
oncogenesis and embryogenesis - a look outside the nucleus.
Science. 287:1606–1609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar
|
|
40
|
Dihlmann S and von Knebel Doeberitz M:
Wnt/beta-catenin-pathway as a molecular target for future
anti-cancer therapeutics. Int J Cancer. 113:515–524. 2005.
View Article : Google Scholar
|
|
41
|
Deng L, Lei Y, Liu R, Li J, Yuan K, Li Y,
Chen Y, Liu Y, Lu Y, Edwards CK III, et al: Pyrvinium targets
autophagy addiction to promote cancer cell death. Cell Death Dis.
4:e6142013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gangopadhyay S, Nandy A, Hor P and
Mukhopadhyay A: Breast cancer stem cells: A novel therapeutic
target. Clin Breast Cancer. 13:7–15. 2013. View Article : Google Scholar
|
|
43
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12:R682010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Asiedu MK, Ingle JN, Behrens MD, Radisky
DC and Knutson KL: TGFbeta/TNF(alpha)-mediated
epithelial-mesenchymal transition generates breast cancer stem
cells with a claudin-low phenotype. Cancer Res. 71:4707–4719. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Creighton CJ, Li X, Landis M, Dixon JM,
Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A,
Herschkowitz JI, et al: Residual breast cancers after conventional
therapy display mesenchymal as well as tumor-initiating features.
Proc Natl Acad Sci USA. 106:13820–13825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu W, Lacerda L, Debeb BG, Atkinson RL,
Solley TN, Li L, Orton D, McMurray JS, Hang BI, Lee E, et al: The
antihelmintic drug pyrvinium pamoate targets aggressive breast
cancer. PLoS One. 8:e715082013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Swanton C, Burrell RA and Futreal PA:
Breast cancer genome heterogeneity: A challenge to personalised
medicine? Breast Cancer Res. 13:1042011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
49
|
de Beça FF, Caetano P, Gerhard R,
Alvarenga CA, Gomes M, Paredes J and Schmitt F: Cancer stem cells
markers CD44, CD24 and ALDH1 in breast cancer special histological
types. J Clin Pathol. 66:187–191. 2013. View Article : Google Scholar
|
|
50
|
Bill R and Christofori G: The relevance of
EMT in breast cancer metastasis: Correlation or causality? FEBS
Lett. 589:1577–1587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Thorne CA, Hanson AJ, Schneider J, Tahinci
E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson
AG, et al: Small-molecule inhibition of Wnt signaling through
activation of casein kinase 1α. Nat Chem Biol. 6:829–836. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Saraswati S, Alfaro MP, Thorne CA,
Atkinson J, Lee E and Young PP: Pyrvinium, a potent small molecule
Wnt inhibitor, promotes wound repair and post-MI cardiac
remodeling. PLoS One. 5:e155212010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Venerando A, Girardi C, Ruzzene M and
Pinna LA: Pyrvinium pamoate does not activate protein kinase CK1,
but promotes Akt/PKB down-regulation and GSK3 activation. Biochem
J. 452:131–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Song L, Li ZY, Liu WP and Zhao MR:
Crosstalk between Wnt/β-catenin and Hedgehog/Gli signaling pathways
in colon cancer and implications for therapy. Cancer Biol Ther.
16:1–7. 2015. View Article : Google Scholar
|
|
55
|
Yanai K, Nakamura M, Akiyoshi T, Nagai S,
Wada J, Koga K, Noshiro H, Nagai E, Tsuneyoshi M, Tanaka M, et al:
Crosstalk of hedgehog and Wnt pathways in gastric cancer. Cancer
Lett. 263:145–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Smith TC, Kinkel AW, Gryczko CM and Goulet
JR: Absorption of pyrvinium pamoate. Clin Pharmacol Ther.
19:802–806. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bartucci M, Dattilo R, Moriconi C,
Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi
G, Sperati F, et al: TAZ is required for metastatic activity and
chemoresistance of breast cancer stem cells. Oncogene. 34:681–690.
2015. View Article : Google Scholar
|