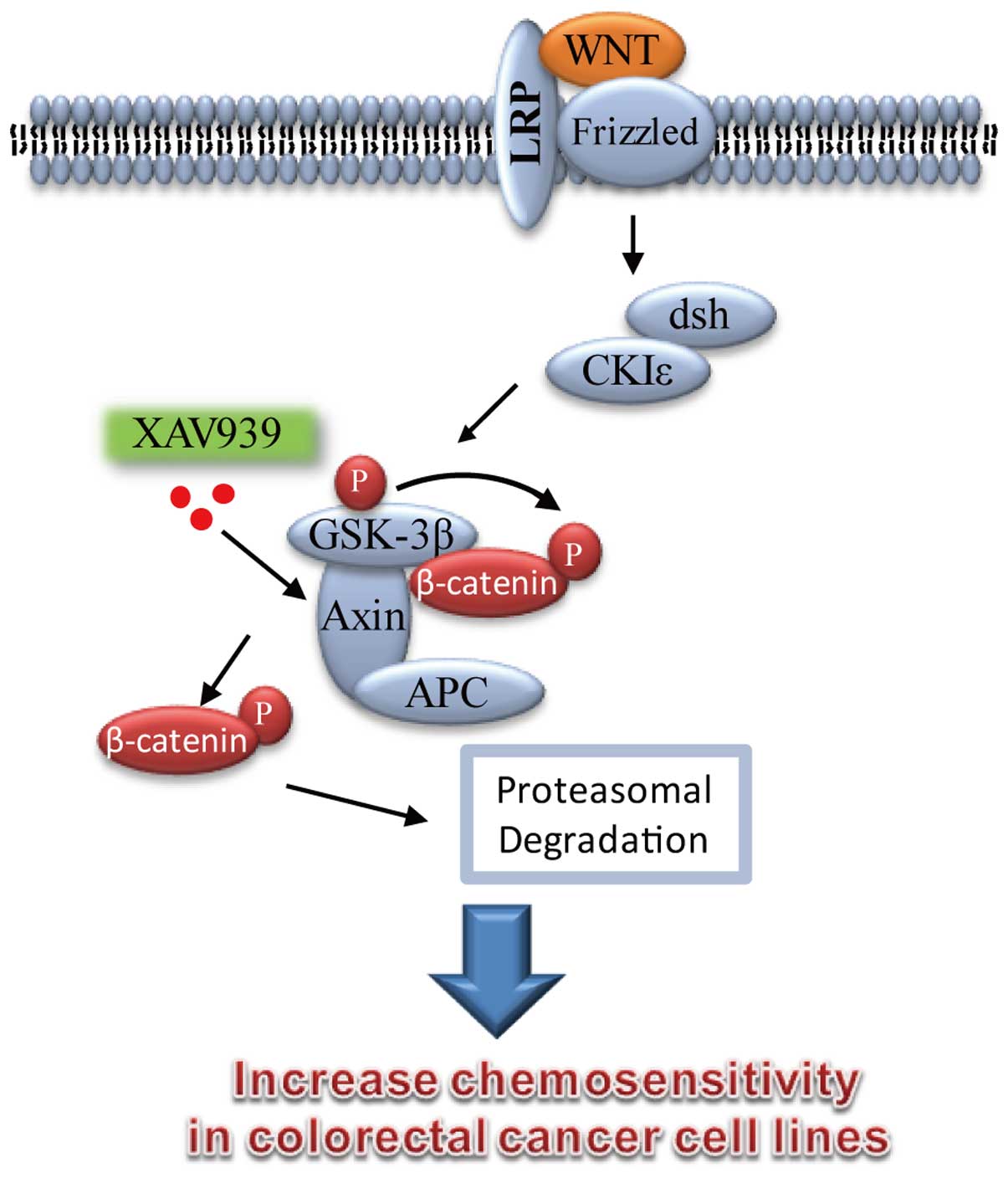
Introduction
Colorectal cancer (CRC) is the second most commonly
diagnosed cancer in females and the third in males (1). 5-Fluorouracil (5-FU) and cisplatin
(DDP) are commonly used in the chemotherapy of CRC, but typical
patient response rates for treatment with those anticancer agents
are between 10% and 30%, allowing cancer progression (2).
Current evidence suggests the drug resistance and
treatment failure are due to properties of tumor cells called
cancer stem cells (CSCs) (3). Only
small populations of tumor cells are CSCs within a tumor, and
responsible for the initiation, growth, and development of tumor,
pending on the animal model (4–6).
Similar to somatic tissue stem cells, CSCs proliferate slowly, they
are in dormant or slow-growing phase of the cell cycle. This
partially accounts for their therapeutic refractoriness to
chemo/radiation therapy and tumor relapse (7).
WNT proteins are a family of secreted, glycosylated,
and palmitoylated peptides that mediate a wide variety of processes
during embryogenesis by regulating stem cell division, migration,
and integrity of the stem cell niche (8,9).
Accumulating evidence indicates a critical role of Wnt, Notch, Shh
and Bmi-1 signaling pathway in the self-renewal and drug resistance
of CSCs (5). Aberrant Wnt
signaling pathway is associated with a wide array of tumor types
and plays an important role in the maintenance of stemness of CSCs
(5,10). Research over the last decade has
shown that cells harbor loss-of-function mutations involving
components of the Wnt signaling cascade, such as APC, β-catenin and
Axin, are considered universal in CRC (11–13).
The mutations cause aberrant transcriptional induction of
Wnt/β-catenin target genes (14,15).
Recent studies indicated that XAV939 is a small
molecule inhibitor of WNT signaling pathway, it broke Wnt signaling
pathway in cancer cell lines through binding to tankyrase (TNKS)
catalytic poly-ADP-ribose polymerase (PARP) domain, and then
resulted in marked stabilization of the Axin protein, finally
leading to increased β-catenin destruction (16–18).
Therefore, the aim of the present study was to
investigate the mechanisms and effects of WNT/β-catenin pathway on
drug resistance in CRC. In the present study, the colon cancer
cells SW480 and SW620 were treated with XAV939 for 24 h, the CD133
positive cell population, apoptosis and cell cycle of these cells
were detected by flow cytometry. The β-catenin, Axin and
CSC-related molecules were detected by western blotting. Our
results suggest that the WNT pathway small molecule modulator
XAV939 affected apoptosis induced by 5-FU/DDP, accompanied by the
protein expression level alteration of β-catenin, Axin and CSC
markers in colon cancer cells.
Materials and methods
Chemicals
XAV939 was purchased from Sigma (St. Louis, MO,
USA), and 5-FU was from Shanghai Xudong Haipu Pharmaceutical Co.,
Ltd. (Shanghai, China). DDP was purchased from Yunnan Biovalley
Dengzhanhua Pharmaceutical Co., Ltd (China).
Cell culture
Human colon adenocarcinoma SW480 and SW620 cells
were obtained from American Type Culture Collection. The cells were
cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) with
10% fetal bovine serum (TBD, China) at 37°C under an atmosphere of
5% CO2.
Cell cycle and apoptosis analysis
Following treatment with different drugs for 24 h,
the cells were harvested and fixed in 70% ice-cold ethanol at 4°C
for 30 min. After washing with PBS, the cells were incubated with
propidium iodide staining buffer (BD Pharmingen, San Diego, CA,
USA) for 15 min and analyzed by FACSAria flow cytometer. The
experiment was performed in triplicate.
For apoptosis analysis, the cells were treated for
24 h with XAV939 (8 μmol/l), 5-FU (50 μmol/l), XAV939 (8 μmol/l) +
5-FU (50 μmol/l), DDP (8 μmol/l) and XAV939 (8 μmol/l) + DDP (8
μmol/l), separately. The cells were measured using FACSAria flow
cytometer (BD Biosciences, USA), and Annexin V (+) cells were
counted for apoptotic cells after Annexin V-fluorescein
isothiocyanate/propidium iodide (FITC/PI) (BD Pharmingen) double
staining. The experiment was performed in triplicate.
Flow cytometry analysis of CD133-positive
cell population
Human colon adenocarcinoma SW480 and SW620 cell
lines (1×106) were detached by treatment with 0.25%
trypsin/EDTA and washed twice with phosphate-buffered saline. The
cells were then resuspended in 100 μl of Staining Buffer containing
1% fetal bovine serum and place on ice for 20 min to block Fc
receptors. After incubating with primary phycoerythrin anti-human
CD133 antibody (Milteny Biotec, Bergisch Gladbach, Germany) for
another 10 min on ice in the dark, the cells were washed twice with
1 ml of ice-cold Staining Buffer and centrifuged (300 × g) for 10
min at 4°C. Cells resuspended in 0.3 ml of 2% formaldehyde fixation
buffer were analyzed using a FACSAria flow cytometer and Cell Quest
software (BD Biosciences). All flow cytometry results were obtained
from two independent experiments performed in triplicate.
Western blot analysis
Following treatment with different drugs for 24 h,
the cells were collected and lysed. Protein content was measured by
the BCA protein assay kit (Beyotime Institute of Biotechnology,
Shanghai, China) and 20 μg protein per lane was separated by 8–12%
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto nitrocellulose membranes. Specific
protein bands were achieved with an ECL detection reagent (Pierce,
Rockford, IL, USA). Anti-axin (Cell Signaling Technology, Danvers,
MA, USA) dilution was 1:500. Anti-β-catenin (Cell Signaling
Technology) dilutions were 1:1,000. Anti-DCAMKL-1 and anti-TERT
(Abcam, Cambridge, MA, USA) dilutions were 1:300 and 1:800.
Anti-EpCAM and anti-α-tubulin (Cell Signaling Technology) dilutions
were 1:500 and 1:1,000. Horseradish peroxidase (HRP)-conjugated
goat anti-rabbit and goat anti-mouse IgqudG antibodies (ProteinTech
Group, Chicago, IL, USA) dilutions were 1:3,000. α-tubulin was used
as a protein loading control. The images were captured with
ChemiDocTM CRS+ Molecular Imager (Bio-Rad,
Hercules, CA, USA). The density of the protein band was quantitated
using Quantity One software (Bio-Rad). The experiment was performed
in triplicate.
Statistical analysis
Statistical analysis was performed with the SPSS13.0
software package (SPSS Inc., Chicago, IL, USA). Data are presented
as mean ± SD. One way analysis of variance (ANOVA) was used for
apoptosis, cell cycle and western blot data analyses. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of XAV939 on apoptosis induced by
5-FU/DDP in SW480 and SW620 cells
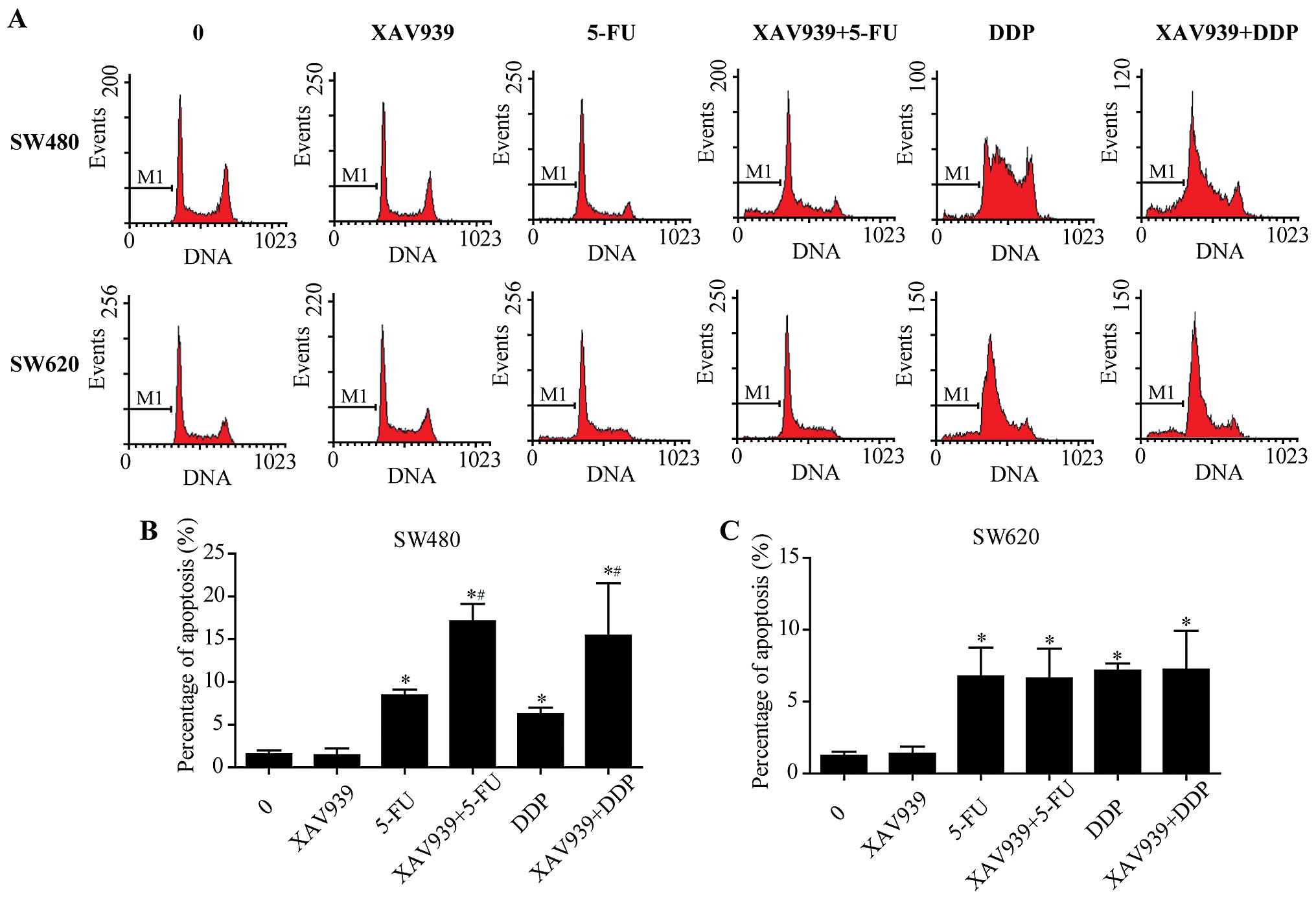
After incubation with either control/5-FU/DDP alone
or combined with XAV939, the effect of XAV939 on the apoptosis
induced by 5-FU/DDP of SW480 and SW620 cells was examined using
flow cytometry after Annexin V-FITC/PI staining (Fig. 1A). We found that XAV939
significantly increased apoptosis induced by 5-FU/DDP (Fig. 1B, P<0.05), whereas the effects
were slight in SW620 cells (Fig.
1C, P>0.05).
Effect of XAV939 on cell cycle
progression induced by 5-FU/DDP in SW480 and SW620 cells
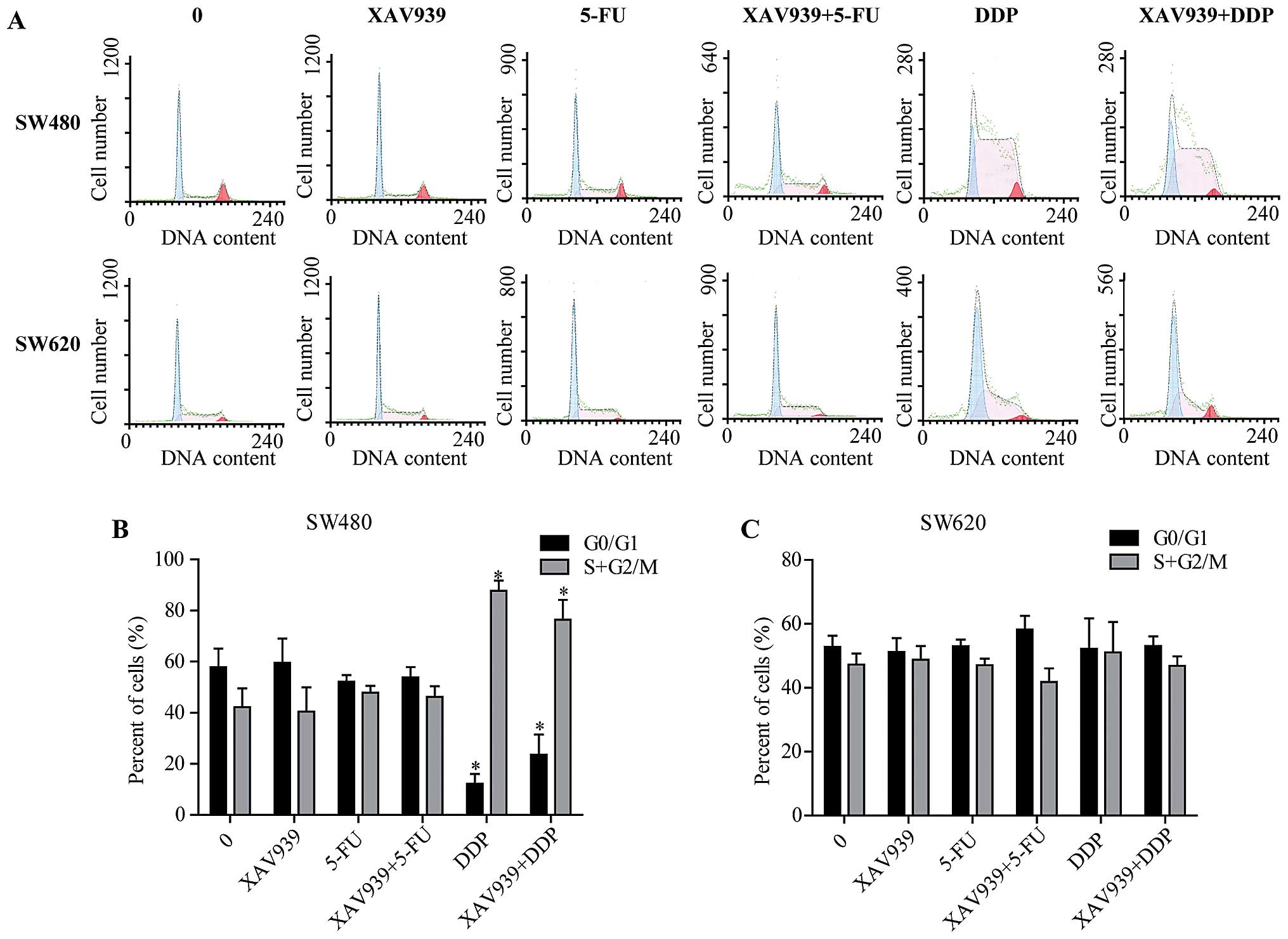
Consistently, after either incubated with
control/5-FU/DDP alone or combining with XAV939, SW480 and SW620
cells were stained and cell cycle distribution was determined by
flow cytometry (Fig. 2A). In SW480
cells, the percentage of G0/G1 phase cells were decreased, while
the percentage of cells at S+G2/M phase of the cell cycle was
increased after incubated with DDP (Fig. 2B, P<0.05). However, these
effects were not observed in SW620 cells (Fig. 2C, P<0.05).
In addition, there was no significant difference in
the cell cycle distribution of SW480 cells between treated with
XAV939-DDP and DDP alone (Fig. 2B,
P>0.05), and no significant difference was found in the cell
cycle distribution of SW480 and SW620 cell treatments with or
without XAV939 (Fig. 2B and C,
P>0.05). Collectively, these results suggest that XAV939 has no
effect on cell cycle distribution.
Effect of XAV939 on proteins involved in
WNT/β-catenin signaling pathway
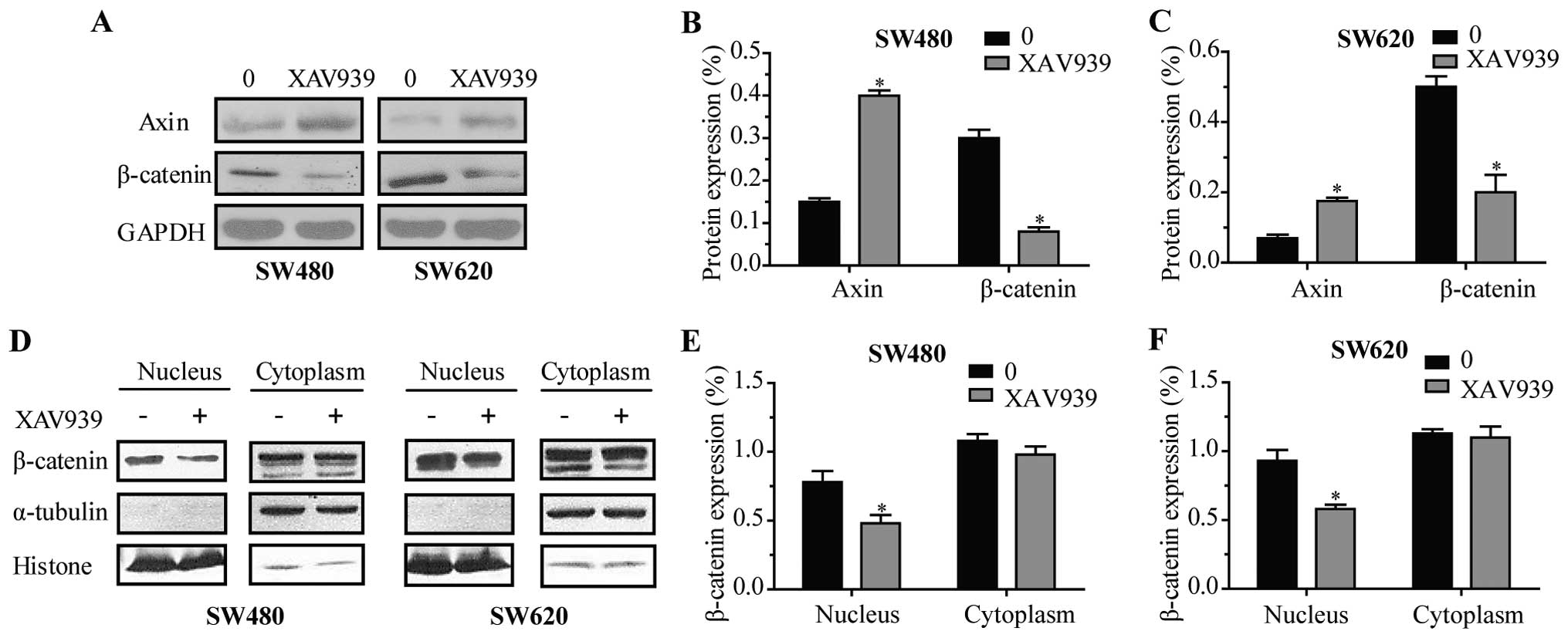
To further investigate the effect of XAV939 on
proteins involved in WNT/β-catenin signaling pathway expression,
total protein lysates were prepared and analyzed by western
blotting. As shown in Fig. 3A,
following 24 h of treatment of XAV939, the expression levels of
Axin were elevated, while the levels of total β-catenin decreased
in SW480 (Fig. 3B, P<0.05) and
SW620 cells (Fig. 3C, P<0.05),
respectively. Western blot analysis of β-catenin proteins both
cytoplasmic and nuclear (Fig. 3D)
displayed that β-catenin was downregulated in the nuclei of SW480
(Fig. 3E, P<0.05) and SW620
cells (Fig. 3F, P<0.05) after
treatment with XAV939 for 24 h, respectively.
Effect of XAV939 on CD133+
SW480 and SW620 cells
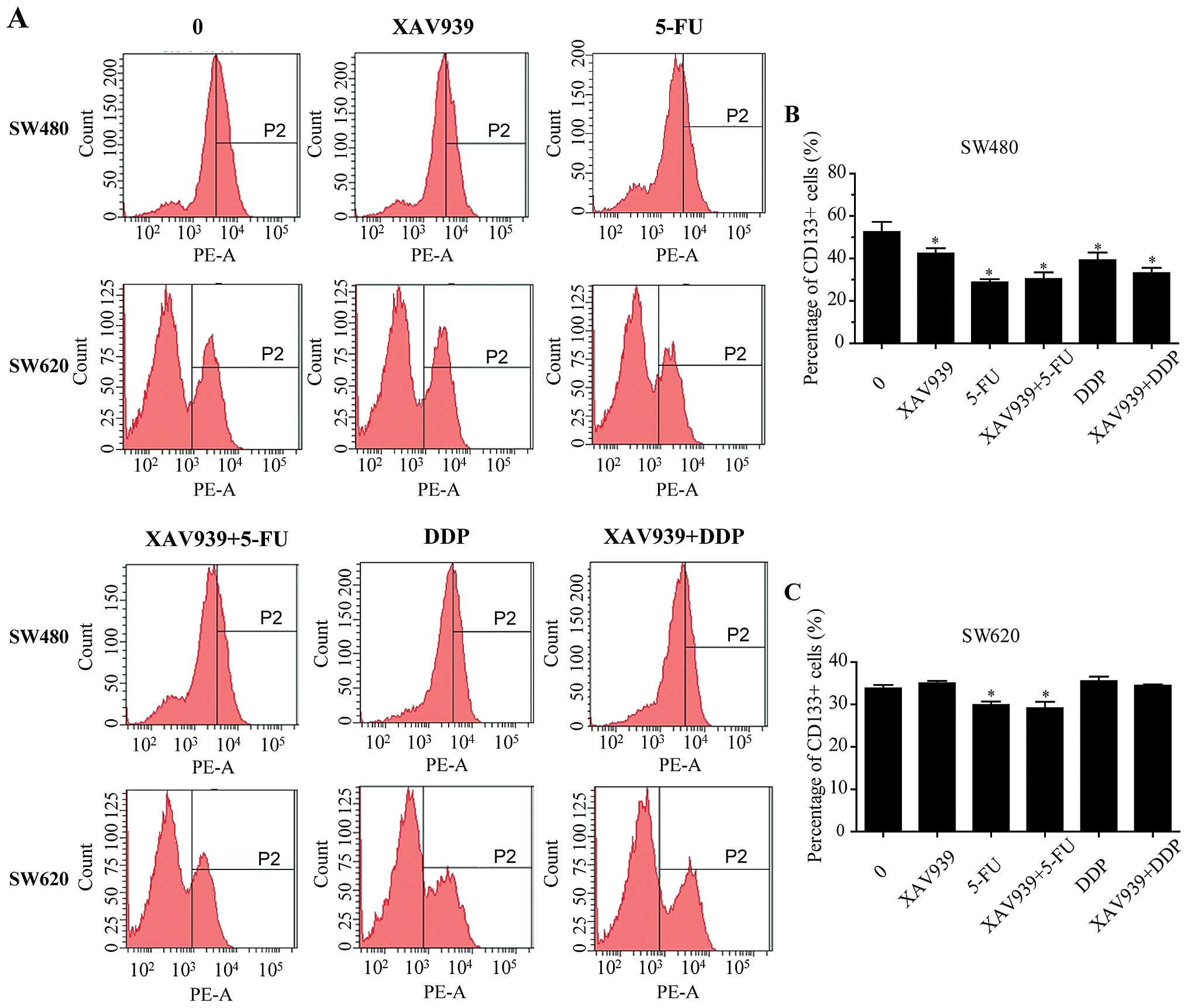
After either treated with 5-FU/DDP alone or combined
with XAV939 for 24 h, the effect of XAV939 on the proportion of
CD133+ SW480 and SW620 cells was examined using flow
cytometry (Fig. 4A). As shown in
Fig. 4B and C, the proportions of
CD133+ SW480 cells were decreased when either XAV939,
5-FU or DDP was added (P<0.05). There was no significant
difference in the proportions of CD133+ SW480 cells
between treated with 5-FU/DDP alone and combining with XAV939
(Fig. 4B, P>0.05). In SW620
cells, either treated with 5-FU alone or combined with XAV939 for
24 h, the proportions of CD133+ cells were decreased
compared with control (Fig. 4C,
P<0.05), but there was no significant difference between the two
groups. In addition, either treated with DDP alone or combining
with XAV939 had no effect on the proportions of CD133+
SW620 cells (Fig. 4C,
P>0.05).
Effect of XAV939 on proteins involved in
CSC markers in SW480 and SW620 cells
To further investigate the effect of XAV939 on
proteins involved in CSC marker expression, total protein lysates
were prepared and analyzed by western blotting (Fig. 5A and B). As shown in Fig. 5C–F, following 24-h treatment of
XAV939, the expression levels of Axin were elevated, and the levels
of total β-catenin were decreased in SW480 and SW620 cells
(P<0.05), respectively, whereas the effects were slight in
5-FU/DDP treated cells (P>0.05). The treatment of SW480 and
SW620 cells with XAV939 or 5-FU for 24 h resulted in downregulation
in the levels of EpCAM protein (P<0.05), whereas these effects
were slight in DDP treated cells (P>0.05). Following 24 h of
treatment combining 5-FU/DDP with XAV939 resulted in significantly
lower expression levels of EpCAM protein compared with treatment
either with 5-FU, XAV939 or DDP alone (P<0.05).
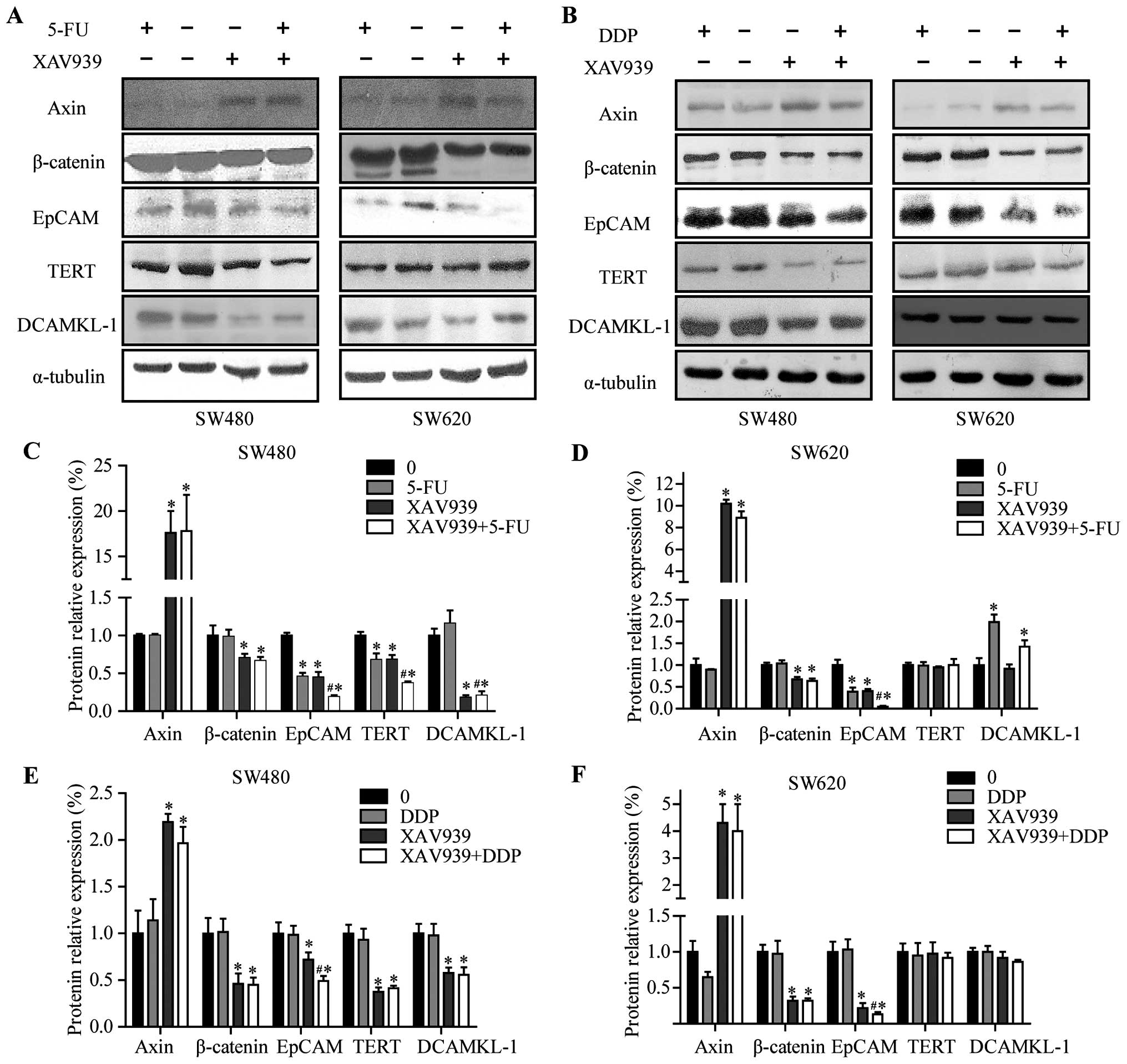 | Figure 5Expression levels of Axin, β-catenin,
EpCAM, TERT and DCAMKL-1 in SW480 and SW620 cells before and after
treatment with XAV939, 5-FU, DDP for 24 h. (A and B) Western blot
analyses of β-catenin, EpCAM, TERT, DCAMKL-1 and Axin in the
indicated SW480/SW620 cell groups. (C and D) Quantitated analysis
of β-catenin, EpCAM, TERT, DCAMKL-1 and Axin proteins in SW480 and
SW620 cells after treated with 5-FU alone or combining with XAV939
for 24 h. (E and F) Quantitated analysis of β-catenin, EpCAM, TERT,
DCAMKL-1 and Axin proteins in SW480 and SW620 cells after treated
with DDP alone or combined with XAV939 for 24 h. α-tubulin was used
as an internal loading control. Values are expressed as
protein/α-tubulin. The density of the protein band was assessed
using Quantity One software (Bio-Rad, Hercules, CA, USA). The data
are expressed as means ± SD of three experiments.
*P<0.05 compared with the control.
#P<0.05 compared with 5-FU-treated or DDP-treated
cells. |
Following 24-h treatment of SW480 cells with XAV939
or 5-FU resulted in downregulation in the levels of TERT protein
(Fig. 5C and E, P<0.05). The
treatment combining 5-FU with XAV939 resulted in significantly
lower expression levels of TERT protein compared with treated
either 5-FU or XAV939 alone (Fig.
5C, P<0.05) in SW480 cells. However, these effects were
slight in both SW620 and DDP treated cells (Fig. 5D and 5F, P>0.05). Finally,
following 24-h treatment with XAV939, the expression levels of
DCAMKL-1 were decreased in SW480 cells (Fig. 5C and E, P<0.05). The treatment
combining 5-FU with XAV939 resulted in significantly lower
expression levels of DCAMKL-1 protein compared with treatment with
5-FU alone (Fig. 5C, P<0.05) in
SW480 cells.
Discussion
Intensive study of the Wnt signaling pathway shows
accumulating evidence suggesting that activation of WNT signaling
pathway in CSCs contribute to their chemoresistance. Therefore,
targeting WNT signaling pathway to reverse CSC multidrug resistance
in CRC cells is a promising way for improving chemotherapeutic
effects (19–22).
The β-catenin destruction complex, which consists of
Apc, Axin, Ck1, and GSK-3β, promotes proteasome-mediated
proteolysis of phosphorylated β-catenin (23). Axin is a negative regulator of the
Wnt signaling pathway, which promotes the phosphorylation and
degradation of β-catenin. XAV939, a small molecule inhibitor of WNT
signaling pathway was able to block Wnt signaling through
stabilizing Axin protein and increasing β-catenin destruction in
colon cancer cell lines (16–18).
Our results showed that the expression levels of Axin protein were
elevated while the levels of total and nuclei β-catenin protein
were decreased in SW480 and SW620 cells after treatment with
XAV939, and suggested that XAV939 stabilized the Axin protein,
finally leading to decreasing nuclear accumulation of β-catenin and
improving β-catenin destruction, which is consistent with the
results of Renna et al (24) and Bao et al (25).
Consistent with our results, Li et al
revealed that HOTAIR induced cisplatin resistance by activating the
WNT/β-catenin pathway, which could be reversed by pre-treatment
with the inhibitor XAV939 in human ovarian cancer (26). Although no significant difference
was found in the apoptosis ratio of CRC cells after treatment with
XAV939, combined XAV939 with 5-FU or DDP could significantly
enhance the apoptosis ratio of CRC cells, suggested that XAV939
increases the sensitivity of tumors to chemotherapy. Renna et
al (24) also reported that
statistically significant difference in mortality rate was not
detected between XAV939 treated cells and DMSO control cells, but
the co-administration of XAV939 and ionizing radiation (IR)
inhibited MB cells proliferation and clonogenic capacity, decreased
their efficacy in repairing DNA damage, and increased IR-induced
cell mortality. These results showed XAV939-induced TNKS PARP
activity inhibition leading to the WNT pathway inhibition, the
DNA-PKcs instability and caused radiosensitivity, and suggested
Wnt/β-catenin signaling pathway plays an important role in tumor
anti-apoptosis. Consistent with the experimental results of Botting
et al (27), our results
also showed that no significant difference was found in the cell
cycle distribution of CRC cells after treatment with XAV939,
suggesting that XAV939 alone has slight effect on CRC cell
proliferation. However, Ma and colleagues showed that XAV939
inhibited cell proliferation and colony formation in hepatocellular
carcinoma cells (28), suggesting
that XAV939 may have different effects on different tumor cell
proliferation. It is widely accepted that genetic heterogeneity is
present among different tumors as well as between primary lesions
and metastases colorectal cancer (29), accordingly, we found that XAV939
could significantly increase the apoptosis induced by 5-FU/DDP in
primary CRC cell line SW480, however, the effects were slight in
metastatic CRC cell line SW620, indicated that WNT/β-catenin
signaling pathway has different downstream effects on different
kinds of CRC cells.
CSCs are a small subset of cancer cells within the
tumor that showed stem cell characteristics such as self-renewal,
the potential to proliferate extensively and the capability to
develop into multiple lineages. Many researches have indicated CSCs
are the key elements in drug resistance and tumor recurrence
(3,4). Colon CSCs are characterized by a
typical profile of different markers such as CD133 (30–32),
ALDH1 (33), EpCAM (34,35),
TERT and DCAMKL-1 (36). Geng
et al (37) revealed that
overexpression of hTERT mRNA may contribute to primary
drug-resistance of tumors. In the present study, the treatment with
XAV939 alone for 24 h resulted in down-regulation in the levels of
EpCAM, TERT and DCAMKL-1 protein in SW480 cells, as well as EpCAM
in SW620 cells, suggested that EpCAM, TERT and DCAMKL-1 protein is
the downstream effector of WNT signaling pathway. Furthermore, we
found that the treatment combining 5-FU with XAV939 resulted in
significantly lower expression levels of DCAMKL-1, EpCAM and TERT
protein compared with treatment with 5-FU alone in SW480 cells, as
well as EpCAM in SW620 cells. Compared with treatment with DDP
alone, combining DDP with XAV939 caused significantly lower
expression levels of EpCAM in SW480 and SW620 cells. This may be
the one of the underlying molecular mechanism of XAV939 impacting
5-FU/DDP curative effects. Yamashita and colleagues (38) reported that GSK-3β inhibitor BIO
upregulated EpCAM and TERT proteins in HuH1 and HuH7 cells, and the
sensitivity to 5-FU chemotherapy were distinct between
EpCAM+ and EpCAM− cells. Wang et al
found that silencing β-catenin by RNA interference resulted in
downregulation of TERT (39).
Femia and colleagues reported that DCAMKL-1 was significant
correlated with the nuclei β-catenin. Bisson and Prowse found that
treatment with WNT inhibitors reduced both pros-tasphere size and
self-renewal in prostate cancer cells (40,41).
In 2011, Wang et al showed that XAV939 can robustly induce
cardiomyogenesis in mouse ES cells (42). Collectively, these results suggest
that the Wnt/β-catenin signaling pathway can affect the
characteristics of tumor stem cells to a certain extent.
In conclusion, we showed for the first time that the
WNT signaling pathway inhibitor XAV939 could significantly increase
apoptosis induced by 5-FU/DDP, accompanied by the protein
expression level alteration of β-catenin, Axin and CSC markers in
colon cancer cells (Fig. 6).
WNT/β-catenin signaling pathway is involved in the drug resistance
of colon CSC. Axin protein, an important component of Wnt/β-catenin
signaling pathway, could be a potential molecular target for
reversing multidrug resistance in colon cancer.
Acknowledgements
This study was financially supported by Guangdong
Natural Science Foundation (no. 2014A030307007) and Sci-Tech
Project Foundation of Qingyuan City (no. 2013A009).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peters GJ, Backus HH, Freemantle S, van
Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J,
Calvert AH, Marsh S, et al: Induction of thymidylate synthase as a
5-fluorouracil resistance mechanism. Biochim Biophys Acta.
1587:194–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crea F, Danesi R and Farrar WL: Cancer
stem cell epigenetics and chemoresistance. Epigenomics. 1:63–79.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bohl SR, Pircher A and Hilbe W: Cancer
stem cells: Characteristics and their potential role for new
therapeutic strategies. Onkologie. 34:269–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanabe Y, Yoshimura K, Yoshikawa K,
Tsunedomi R, Shindo Y, Matsukuma S, Maeda N, Kanekiyo S, Suzuki N,
Kuramasu A, et al: A stem cell medium containing neural stimulating
factor induces a pancreatic cancer stem-like cell-enriched
population. Int J Oncol. 45:1857–1866. 2014.PubMed/NCBI
|
|
7
|
McCubrey JA, Steelman LS, Abrams SL,
Misaghian N, Chappell WH, Basecke J, Nicoletti F, Libra M, Ligresti
G, Stivala F, et al: Targeting the cancer initiating cell: The
ultimate target for cancer therapy. Curr Pharm Des. 18:1784–1795.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi H, Sun B, Zhao X, Du J, Gu Q, Liu Y,
Cheng R and Dong X: Wnt5a promotes vasculogenic mimicry and
epithelial-mesenchymal transition via protein kinase Cα in
epithelial ovarian cancer. Oncol Rep. 32:771–779. 2014.PubMed/NCBI
|
|
10
|
Wang WJ, Wu MY, Shen M, Zhi Q, Liu ZY,
Gong FR, Tao M and Li W: Cantharidin and norcantharidin impair
stemness of pancreatic cancer cells by repressing the β-catenin
pathway and strengthen the cytotoxicity of gemcitabine and
erlotinib. Int J Oncol. 47:1912–1922. 2015.PubMed/NCBI
|
|
11
|
Liu W, Dong X, Mai M, Seelan RS, Taniguchi
K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C,
et al: Mutations in AXIN2 cause colorectal cancer with defective
mismatch repair by activating beta-catenin/TCF signalling. Nat
Genet. 26:146–147. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyoshi Y, Nagase H, Ando H, Horii A,
Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T and Nakamura Y:
Somatic mutations of the APC gene in colorectal tumors: Mutation
cluster region in the APC gene. Hum Mol Genet. 1:229–233. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Segditsas S and Tomlinson I: Colorectal
cancer and genetic alterations in the Wnt pathway. Oncogene.
25:7531–7537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian XH, Hou WJ, Fang Y, Fan J, Tong H,
Bai SL, Chen Q, Xu H and Li Y: XAV939, a tankyrase 1 inhibitior,
promotes cell apoptosis in neuroblastoma cell lines by inhibiting
Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 32:1002013.
View Article : Google Scholar
|
|
17
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan
CW, Wei S, Hao W, Kilgore J, Williams NS, et al: Small
molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi-Yanaga F and Kahn M: Targeting
Wnt signaling: Can we safely eradicate cancer stem cells? Clin
Cancer Res. 16:3153–3162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCubrey JA, Steelman LS, Abrams SL,
Misaghian N, Chappell WH, Basecke J, Nicoletti F, Libra M, Ligresti
G, Stivala F, et al: Targeting the cancer initiating cell: The
ultimate target for cancer therapy. Curr Pharm Des. 18:1784–1795.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ying J, Tsujii M, Kondo J, Hayashi Y, Kato
M, Akasaka T, Inoue T, Shiraishi E, Inoue T, Hiyama S, et al: The
effectiveness of an anti-human IL-6 receptor monoclonal antibody
combined with chemotherapy to target colon cancer stem-like cells.
Int J Oncol. 46:1551–1559. 2015.PubMed/NCBI
|
|
22
|
Wang B, Zou Q, Sun M, Chen J, Wang T, Bai
Y, Chen Z, Chen B and Zhou M: Reversion of trichostatin A
resistance via inhibition of the Wnt signaling pathway in human
pancreatic cancer cells. Oncol Rep. 32:2015–2022. 2014.PubMed/NCBI
|
|
23
|
Moon RT, Bowerman B, Boutros M and
Perrimon N: The promise and perils of Wnt signaling through
beta-catenin. Science. 296:1644–1646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Renna C, Salaroli R, Cocchi C and Cenacchi
G: XAV939-mediated ARTD activity inhibition in human MB cell lines.
PLoS One. 10:e01241492015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bao R, Christova T, Song S, Angers S, Yan
X and Attisano L: Inhibition of tankyrases induces Axin
stabilization and blocks Wnt signalling in breast cancer cells.
PLoS One. 7:e486702012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Yang S, Su N, Wang Y, Yu J, Qiu H
and He X: Overexpression of long non-coding RNA HOTAIR leads to
chemoresistance by activating the Wnt/β-catenin pathway in human
ovarian cancer. Tumour Biol. Sep 4–2015.(Epub ahead of print).
|
|
27
|
Botting GM, Rastogi I, Chhabra G, Nlend M
and Puri N: Mechanism of resistance and novel targets mediating
resistance to EGFR and c-Met tyrosine kinase inhibitors in
non-small cell lung cancer. PLoS One. 10:e01361552015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma L, Wang X, Jia T, Wei W, Chua MS and So
S: Tankyrase inhibitors attenuate WNT/β-catenin signaling and
inhibit growth of hepatocellular carcinoma cells. Oncotarget.
6:25390–25401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vakiani E, Janakiraman M, Shen R, Sinha R,
Zeng Z, Shia J, Cercek A, Kemeny N, D'Angelica M, Viale A, et al:
Comparative genomic analysis of primary versus metastatic
colorectal carcinomas. J Clin Oncol. 30:2956–2962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schneider M, Huber J, Hadaschik B, Siegers
GM, Fiebig HH and Schüler J: Characterization of colon cancer
cells: A functional approach characterizing CD133 as a potential
stem cell marker. BMC Cancer. 12:962012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang ZL, Zheng Q, Yan J, Pan Y and Wang
ZG: Upregulated CD133 expression in tumorigenesis of colon cancer
cells. World J Gastroenterol. 17:932–937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang BB, Li ZJ, Zhang FF, Hou HT, Yu JK
and Li F: Clinical significance of stem cell marker CD133
expression in colorectal cancer. Histol Histopathol. Oct
7–2015.(Epub ahead of print).
|
|
33
|
Vogler T, Kriegl L, Horst D, Engel J,
Sagebiel S, Schäffauer AJ, Kirchner T and Jung A: The expression
pattern of aldehyde dehydrogenase 1 (ALDH1) is an independent
prognostic marker for low survival in colorectal tumors. Exp Mol
Pathol. 92:111–117. 2012. View Article : Google Scholar
|
|
34
|
Kanwar SS, Yu Y, Nautiyal J, Patel BB and
Majumdar AP: The Wnt/beta-catenin pathway regulates growth and
maintenance of colonospheres. Mol Cancer. 9:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Imrich S, Hachmeister M and Gires O: EpCAM
and its potential role in tumor-initiating cells. Cell Adhes Migr.
6:30–38. 2012. View Article : Google Scholar
|
|
36
|
Nakanishi Y, Seno H, Fukuoka A, Ueo T,
Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et
al: Dclk1 distinguishes between tumor and normal stem cells in the
intestine. Nat Genet. 45:98–103. 2013. View
Article : Google Scholar
|
|
37
|
Geng M, Yin YC, Cao YC, Fu ZJ, Wang XY and
Tai YH: Anti-tumor effects of chemotherapeutic drugs on human
gastric cancer cells in vitro and the relationship with expression
of hTERT mRNA. Zhonghua Zhong Liu Za Zhi. 29:838–841. 2007.(In
Chinese).
|
|
38
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al:
EpCAM-positive hepatocellular carcinoma cells are tumor-initiating
cells with stem/progenitor cell features. Gastroenterology.
136:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang XH, Sun X, Meng XW, Lü ZW, Liu MN and
Pei FH: The role and significance of Wnt/beta-catenin signaling
pathway regulating the signaling molecules in hepatocellular
carcinoma. Zhonghua Gan Zang Bing Za Zhi. 18:672–675. 2010.(In
Chinese). PubMed/NCBI
|
|
40
|
Femia AP, Dolara P, Salvadori M and
Caderni G: Expression of LGR-5, MSI-1 and DCAMKL-1, putative stem
cell markers, in the early phases of 1,2-dimethylhydrazine-induced
rat colon carcinogenesis: Correlation with nuclear β-catenin. BMC
Cancer. 13:482013. View Article : Google Scholar
|
|
41
|
Bisson I and Prowse DM: WNT signaling
regulates self-renewal and differentiation of prostate cancer cells
with stem cell characteristics. Cell Res. 19:683–697. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang H, Hao J and Hong CC: Cardiac
induction of embryonic stem cells by a small molecule inhibitor of
Wnt/β-catenin signaling. ACS Chem Biol. 6:192–197. 2011. View Article : Google Scholar :
|