Introduction
Malignant pleural mesothelioma (MPM) is an
aggressively growing neoplasm, which disseminates into the thoracic
cavity and frequency produces a malignant pleural effusion
(1,2). MPM is most often caused by asbestos
exposure with a long latency period, often exceeding 20 years
between the first exposure to asbestos and a diagnosis of the
disease (3,4). Despite the fact that this type of
tumor was once considered rare, the current incidence is predicted
to increase globally and peak in the coming decades particularly in
developing countries where the use of asbestos is still prevalent
(5,6).
Many therapeutic modalities have been applied for
the treatment of MPM including surgery, radiotherapy and
chemotherapy (7). Chemotherapy is
considered the main therapeutic option for patients with both
unresectable and resectable tumors. Chemotherapy alone or when used
in combination with other adjuvant therapeutic modalities such as
radiotherapy, might be the only therapeutic modality currently in
use for MPM (8,9). Nevertheless, chemo-therapeutic
regimens used against MPM have not proven effective to date,
because MPM is often resistant to chemotherapy (10,11).
Pemetrexed (PMX), an anti-folate agent, is one of
the currently approved chemotherapeutic agents for the first line
care of patients with MPM (12,13).
It acts mainly via inhibiting the multiple folate-dependent enzymes
that are involved in DNA synthesis and repair: thymidylate synthase
(TS), dihydrofolate reductase (DHFR), and glycinamide
ribonucleotide formyltransferase (GARFT) (14,15).
However, PMX has shown only modest activity in patients with MPM
either when used alone or in combination with other
chemotherapeutic agents such as cisplatin (16). Many studies have reported that a
high expression of TS, the rate-limiting enzyme of de novo
DNA synthesis, can significantly predict both poor sensitivity and
resistance to PMX-based chemotherapy (17,18).
Therefore, the development of strategies to downregulate the
expression of TS is anticipated to enhance the cytotoxic efficacy
of PMX against MPM, and, thus, improve the therapeutic outcome in
patients treated with PMX.
RNA interference (RNAi), a novel regulatory process
in which double-stranded RNA (dsRNA) induces a specific degradation
of its target mRNA, has rapidly become a highly specific and
powerful tool to silence target genes (19–21).
Many studies have revealed that RNAi can be a novel tool for
clarifying gene function and applicable to gene-specific
therapeutics (22–24). In the present study, therefore, we
assumed that downregulation of the TS gene by RNAi might be
efficient in enhancing the chemosensitivity of MPM cells to PMX. To
validate our assumption, we evaluated the efficacy of a TS gene
knockdown via a chemically synthesized short hairpin RNA (shRNA)
designed against TS to enhance the cytotoxicity of PMX against the
human mesotheliomal cell line MSTO-211H, in vitro. In
addition, we evaluated the in vivo antitumor efficacy of a
combination therapy with TS-shRNA lipoplex and PMX in an orthotopic
xenograft mouse model.
Materials and methods
Materials
Pemetrexed disodium (PM X; Alimta®) was
purchased from Eli Lilly (Indianapolis, IN, USA).
Dioleoylphosphatidylcholine (DOPC) and
dioleoyl-phosphatidylethanolamine (DOPE) were generously donated by
NOF Inc. (Tokyo, Japan). Cholesterol (CHOL) and D-luciferin
potassium salt were purchased from Wako Pure Chemical (Osaka,
Japan). 1,1′-Dioctadecyl-3,3,3′,3′-tetramethyl indotricarbo-cyanine
iodide (DiR) was purchased from Invitrogen (OR, USA). A cationic
lipid, O,O'-ditetradecanoyl-N-(α-trimethyl ammonioacetyl)
diethanolamine chloride (DC-6-14) was purchased from Sogo
Pharmaceutical (Tokyo, Japan).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was purchased from Nacalai Tesque (Kyoto, Japan). All other
reagents were of analytical grade.
Animals and tumor cell line
5-week-old male BALB/c nu/nu mice were
purchased from Japan SLC (Shizuoka, Japan). All animal experiments
were evaluated and approved by the Animal and Ethics Review
Committee of Tokushima University (permission No. 13086). A human
malignant pleural mesothelioma (MPM) cell line, MSTO-211H
expressing firefly luciferase (MSTO-211H-Luc) generated by stable
transfection with the firefly luciferase gene (pGL3 Basic plasmid;
Promega, Madison, WI, USA) was generously supplied by Dr Masashi
Kobayashi, Department of Thoracic Surgery, Faculty of Medicine,
Kyoto University (Kyoto, Japan) and was maintained in RPMI-1640
medium (Wako Pure Chemical).
Chemically synthesized shRNAs
All shRNAs, chemically synthesized and purified by
high performance liquid chromatography, were purchased from
Hokkaido System Science (Sapporo, Japan). The sequence of shRNA
against thymidylate synthase (TS shRNA) was
5′-GUAACACCAUCGAUCAUGAUAGUGCUCCUGGUUGUCAUGAUCGAUGGUGUUACUU-3′ and
for a nonspecific shRNA nonspecific (NS shRNA, not to target any
gene in either the human or mouse genome) was
5′-CUUAAUCGCGUAUAAGGCUAGUGCUCCUGGUUGGCCUUAUACGCGAUUAAGAUU-3′.
shRNAs were dissolved in RNase-free TE buffer at a final
concentration of 100 nmol/ml.
shRNA transfection
MSTO-211H cells were transfected with shRNA using
Lipofectamine® RNAiMAX (LfRNAiMAX, Invitrogen, Carlsbad,
CA, USA) according to the manufacturer's instructions. Briefly, 300
pmol shRNA and 15 μl LfRNAiMAX were diluted in Opti-MEM I
(Invitrogen) to a total volume of 500 μl. The diluted shRNA and
LfRNAiMAX were mixed and incubated at room temperature for 20 min
to form the shRNA/LfRNAiMAX complex. The shRNA/LfRNAiMAX complex
was then applied to the cells followed by incubation for the
indicated time interval.
Real-time quantitative reverse
transcriptase (qRT)-PCR analysis
RNA isolation and cDNA synthesis were performed
according to the manufacturer's instructions. Briefly, in 6-well
plates, 5×104 cells were seeded for 24 h before shRNA
transfection. The cells were transfected with 5 or 10 nM of either
TS shRNA or NS shRNA, as described above. At 72-h
post-transfection, the total RNA of the MSTO-211H cells was
isolated using an RNaqueous-micro kit (Ambion, Austin, TX, USA). To
conduct the reverse transcription reaction, 2 μl of RNA was
converted to cDNA with a total volume of 20 μl, including 500 nM of
Oligo(dT)20, 500 μM dNTP, 1 μl of RNase inhibitor, and 1 μl of
ReverTra Ace (Toyobo, Osaka, Japan). Real-time PCR was performed on
a StepOnePlus real-time PCR system (Applied Biosystems, CA, USA)
with a FastStart TaqMan Probe Master and Universal ProbeLibrary
(Roche Diagnostics GmbH, Manheim, Germany) according to the
manufacturer's instructions. The TS primers and a probe were from
the Assay-on-Demand gene expression assay mix (TS assay ID
Hs00426591-ml, PCR product size 87 bp; Applied Biosystems). The
GAPDH primers and a probe were designed using ProbeFinder software
(Roche Diagnostics GmbH). The GAPDH primers and the probes were as
follows: GAPDH primers and probe (forward
5′-GCTCTCTGCTCCTCCTGTTC-3′ and reverse 5′-ACGACCAAATCCGTTGACTC-3′,
probe #60). The amplification conditions were as follows: 10 min at
95°C, followed by 40 cycles at 95°C for 15 sec and at 60°C for 1
min. The results were expressed as the threshold cycle
(CT), and the relative expression levels for each primer
set were normalized to the internal control expression of GAPDH
using the 2−ΔΔCT method. The TS mRNA expression level of
non-transfected cells was set at 100%. Three independent
experiments were performed with the same results.
In vitro cytotoxicity assay
MSTO-211H cells were seeded onto 96-well plates at a
density of 2,000 cells per well 24 h before shRNA transfection. The
cells were transfected with 5 nM of either TS shRNA or NS shRNA for
24 h. After transfection, the culture medium was replaced with
fresh medium containing various concentrations of PMX, range:
0.001–1,000 ng/ml. Following 72 h incubation at 37°C, media were
discarded and MTT assay was conducted as described previously
(25). Cell viability (%) was
calculated from the ratio between the percentage of viable treated
cells and viable untreated cells.
Preparation of cationic liposomes
Cationic liposome composed of DOPE:DOPC:DC-6-14
(3:2:5 molar ratio) was prepared as previously described (23). To follow the in vivo
distribution of shRNA lipoplexes, 0.1 mol% of the fluorescent dye
DiR was incorporated in the lipid mixture. The mean diameter and
zeta potential for cationic liposomes was 102.8±32.7 nm and
49.1±3.7 mV (n=3), respectively, as determined with a NICOMP 370
HPL submicron particle analyzer (Particle Sizing System, Santa
Barbara, CA, USA). The concentration of phospholipids was
determined by colorimetric assay (26).
Preparation of shRNA lipoplexes
For the preparation of shRNA/cationic liposome
complex (shRNA lipoplex), shRNA and cationic liposome were mixed at
a molar ratio of 2000/1 (lipid/shRNA, molar ratio), and the mixture
was vigorously vortexed for 10 min at room temperature to form
shRNA lipoplex. The mean diameter and zeta potential of the shRNA
lipoplex was 395.3±32.2 nm and 30.6±1.9 mV (n=3), respectively. To
detect the absence of free-shRNA in the prepared shRNA lipoplex,
electrophoresis was performed on 2% agarose gel.
Intrapleural orthotopic implantation
model
For the development of the orthotopic implantation
model, five-week-old male BALB/c nu/nu mice were
anaesthetized with 2,2,2-tribromo-ethanol (Avertin; Sigma-Aldrich)
and injected directly into the left pleural cavity with
1×106 MESTO-211H-Luc cells in 100 μl of PBS. IVIS was
used to monitor the development of thoracic tumors. When
establishment of thoracic tumors was ensured, the mice were
randomized into control and treatment groups (n=6/group).
In vivo distribution study of DiR-labeled
lipoplex
To follow the in vivo distribution of
lipoplex in orthotopic tumor-bearing mice (n=5), mice were
intrapleurally injected with 50 μl of DiR-labeled shRNA lipoplex
(20 μg shRNA/mouse). At selected post-injection time points (5 and
30 min; 1, 2, 6, 12, 24, 48, 72, 96 and 120 h), mice were
anesthetized by isoflurane and the in vivo distribution of
DiR-labeled shRNA lipoplex was visualized using an in vivo
imaging system (IVIS, Xenogen, CA, USA). The fluorescence images
were acquired using an exposure time of 1/8 sec. For the ex
vivo fluorescence imaging analyses, five major organs (liver,
spleen, lung, kidney and heart) were harvested after the final
measurement and immediately subjected to fluorescence imaging using
the IVIS imaging system.
Therapeutic efficacy in an orthotopic
tumor mouse model
For the therapeutical experiments, at day 7
post-tumor cells implantation, when the establishment of pleural
metastasis was ensured, the mice were divided into 6 groups (n=6):
a control group treated with sucrose and 5 groups treated with
either NS shRNA lipoplex, TS shRNA lipoplex, free PMX, NS shRNA
lipoplex + free PMX, or TS shRNA lipoplex + free PMX. In groups
treated with shRNA lipoplex, mice were intrathoracically injected
with shRNA lipoplex (20 μg/mouse) on days 7, 9, 11, 13, 15 and 17.
In the groups treated with free PMX, PMX (25 mg/kg) was
intraperitoneally (i.p.) administered on days 7, 8, 9, 10, 11, 14,
15, 16, 17 and 18 after tumor cell implantation. The antitumor
efficacy was evaluated in terms of both the mean survival time
(MST) and the percentage of increased life span [ILS (%)]. The MST
(day) was identified by recording the mortality on a daily basis
for 45 days, and the ILS (%) was calculated using the following
equations: MST (day) = day of the first death + day of the last
death/2 (27); ILS (%) = [(mean
survival time of treated group/mean survival time of control group)
−1] ×100 (28).
Bioluminescence imaging with IVIS
Seventy-two hours after the last treatment (on day
21), the mice were anesthetized with isoflurane inhalation, and
were subsequently i.p.-injected with 100 μl of 7.5 mg/ml
D-luciferin potassium salt. Bioluminescence in vivo imaging
was initiated 5 min after injection and bioluminescence from the
region of interest (ROI) was defined manually. Background
photon-flux was defined using a ROI from a mouse that was not given
an i.p. injection of D-luciferin potassium salt. All bioluminescent
data were collected and analyzed using IVIS. Following in
vivo imaging procedures, the mice were euthanized, the thoracic
tumors were carefully removed, and the in vivo gene
knockdown effect of treatments was determined by qRT-PCR as
described above.
Statistical analysis
All values are expressed as the mean ± SD.
Statistical analysis was performed with a two-tailed unpaired
Student's t-test and one way ANOVA using GraphPad InStat View
software (GraphPad Software, San Diego, CA, USA). The level of
significance was set at p<0.05.
Results
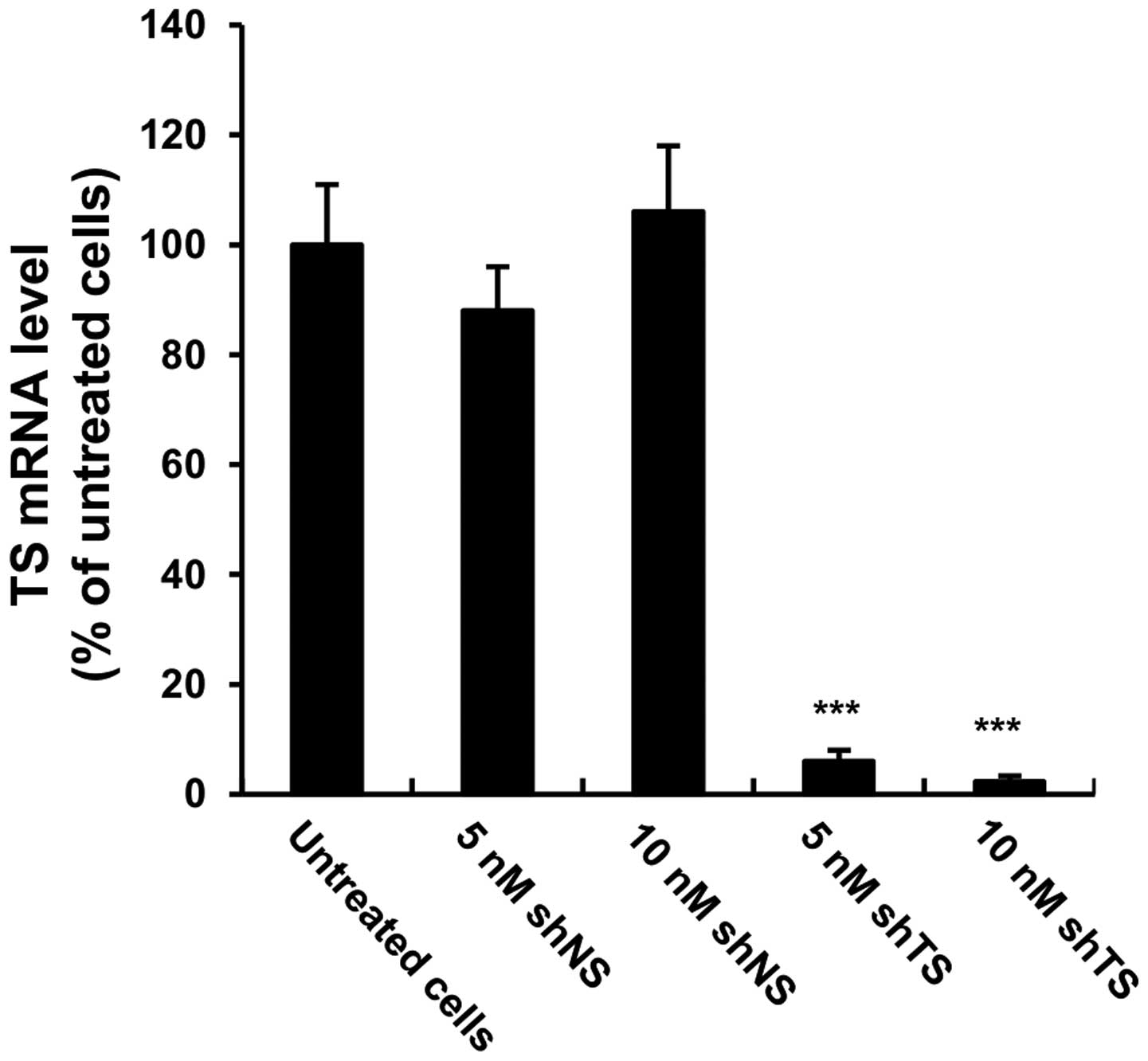
In vitro downregulation of TS mRNA
expression by chemically synthesized shRNA
To quantify the gene knockdown efficiency, MSTO-211H
cells were transfected with either 5 or 10 nM TS shRNA or NS shRNA
for 72 h, then the mRNA expression levels of TS were quantified
using qRT-PCR. Compared with untransfected cells, the mRNA
expression levels of TS were downregulated >90% (p<0.001) in
the TS shRNA transfected cells (Fig.
1). The NS shRNA-transfected cells exhibited no significant
change in TS mRNA expression compared with untreated cells.
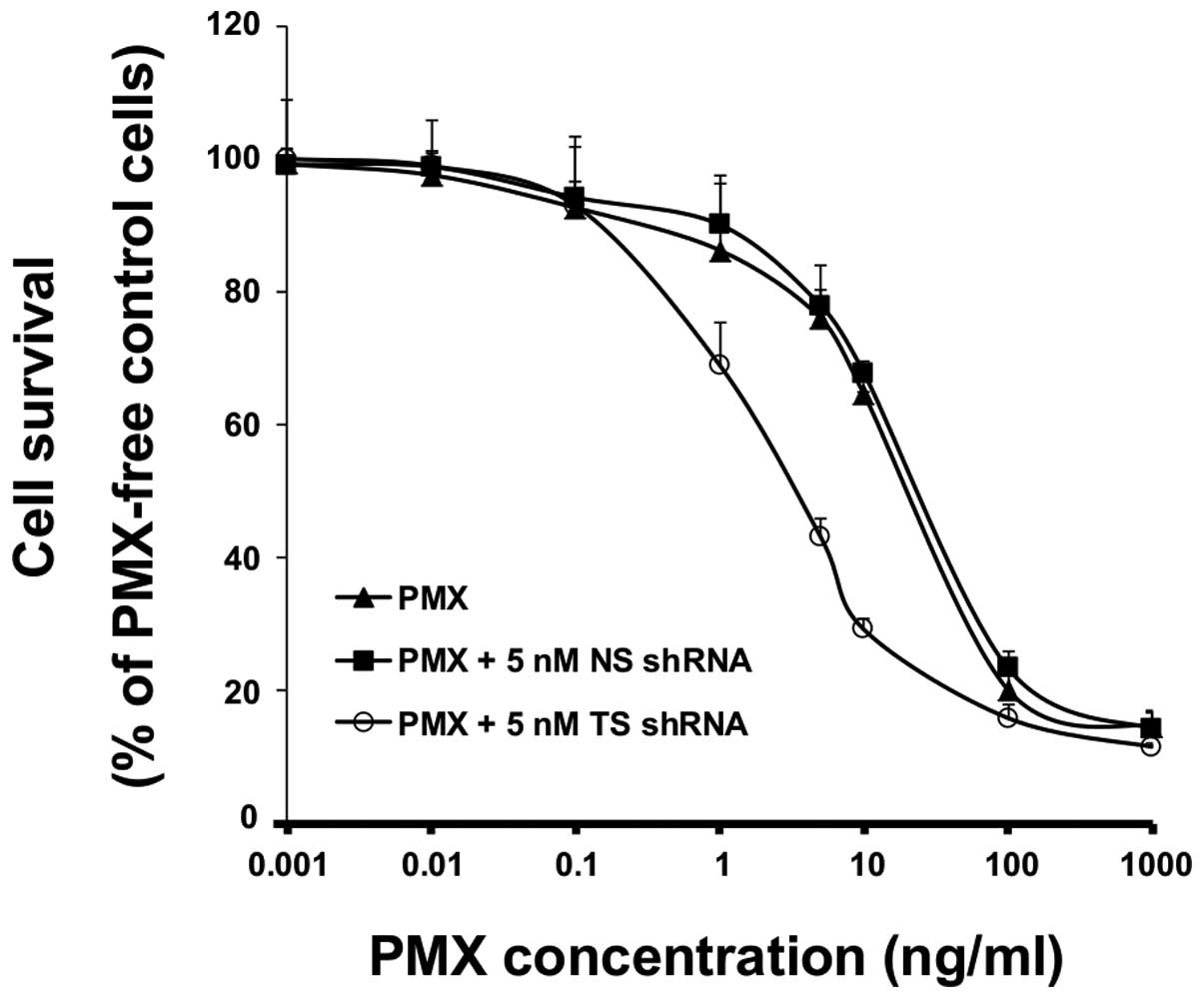
In vitro cell growth inhibition by a
combination of TS shRNA and PMX treatment
To investigate whether TS gene knockdown affects the
in vitro cytotoxicity of PMX against MSTO-211H cells, cell
viability after exposure to PMX alone, or to a combination of TS
shRNA and PMX, was evaluated via MTT assay. As shown in Fig. 2, PMX treatment decreased cell
viability in a dose-dependent manner. In addition, the combined
treatment with NS shRNA and PMX was as toxic as PMX alone, whereas
the combination of TS shRNA with PMX resulted in a significantly
higher cytotoxicity than that caused by PMX alone (IC50
2.74±0.20 ng/ml versus 38.82±1.70 ng/ml, respectively) (p<0.05),
suggesting the occurrence of a synergistic effect between TS shRNA
and PMX.
In vivo antitumor efficacy of the
combined therapy of TS-shRNA and PMX in an orthotopic implantation
model
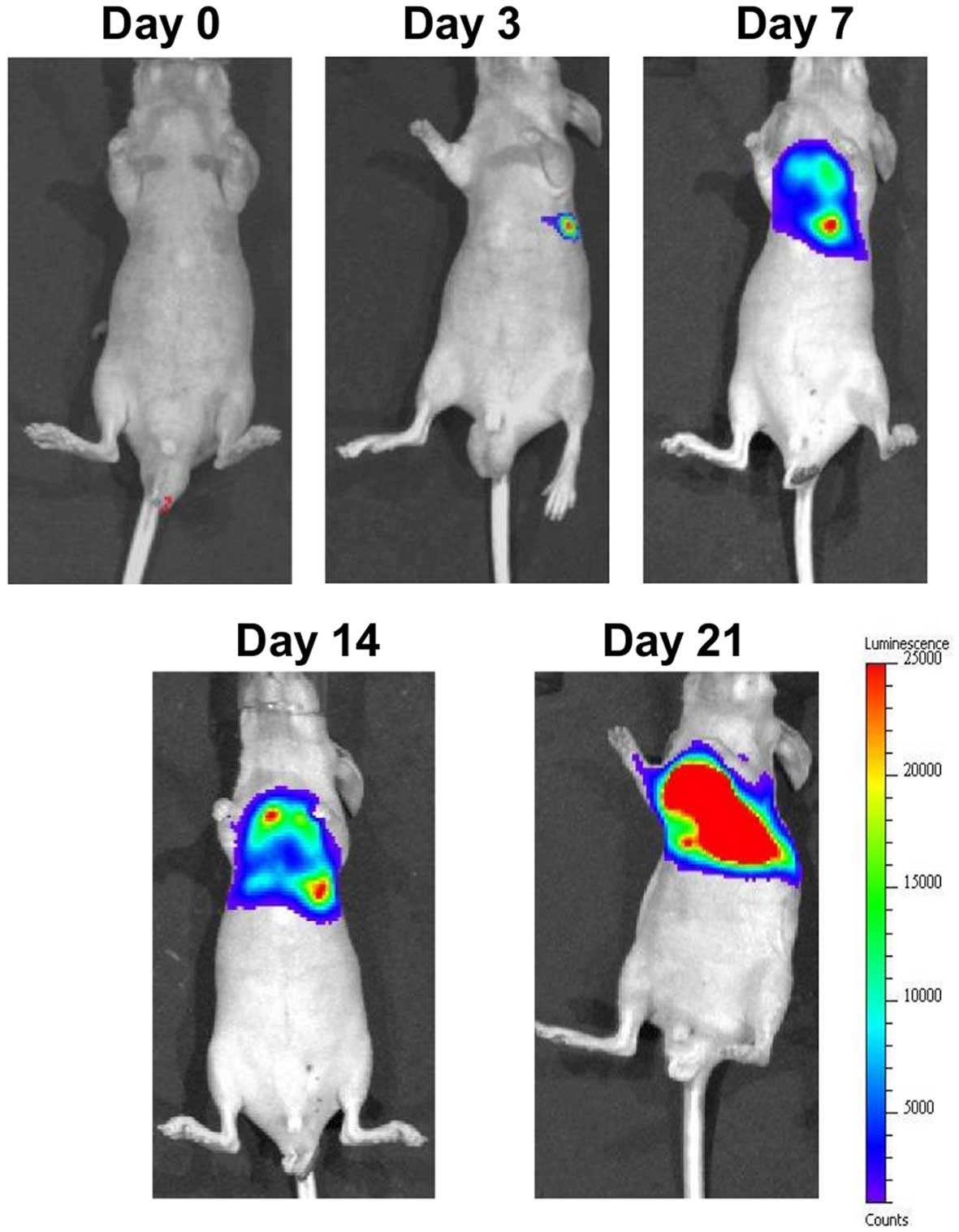
To examine the effect of the combined treatment of
TS shRNA and PMX on tumor growth in vivo, we implanted
MSTO-211H-Luc into the left thoracic cavity of nude mice and
treatment was started at day 7 post-tumor inoculation; the time at
which thoracic tumors were visible (Fig. 3). At 72 h after the last treatment
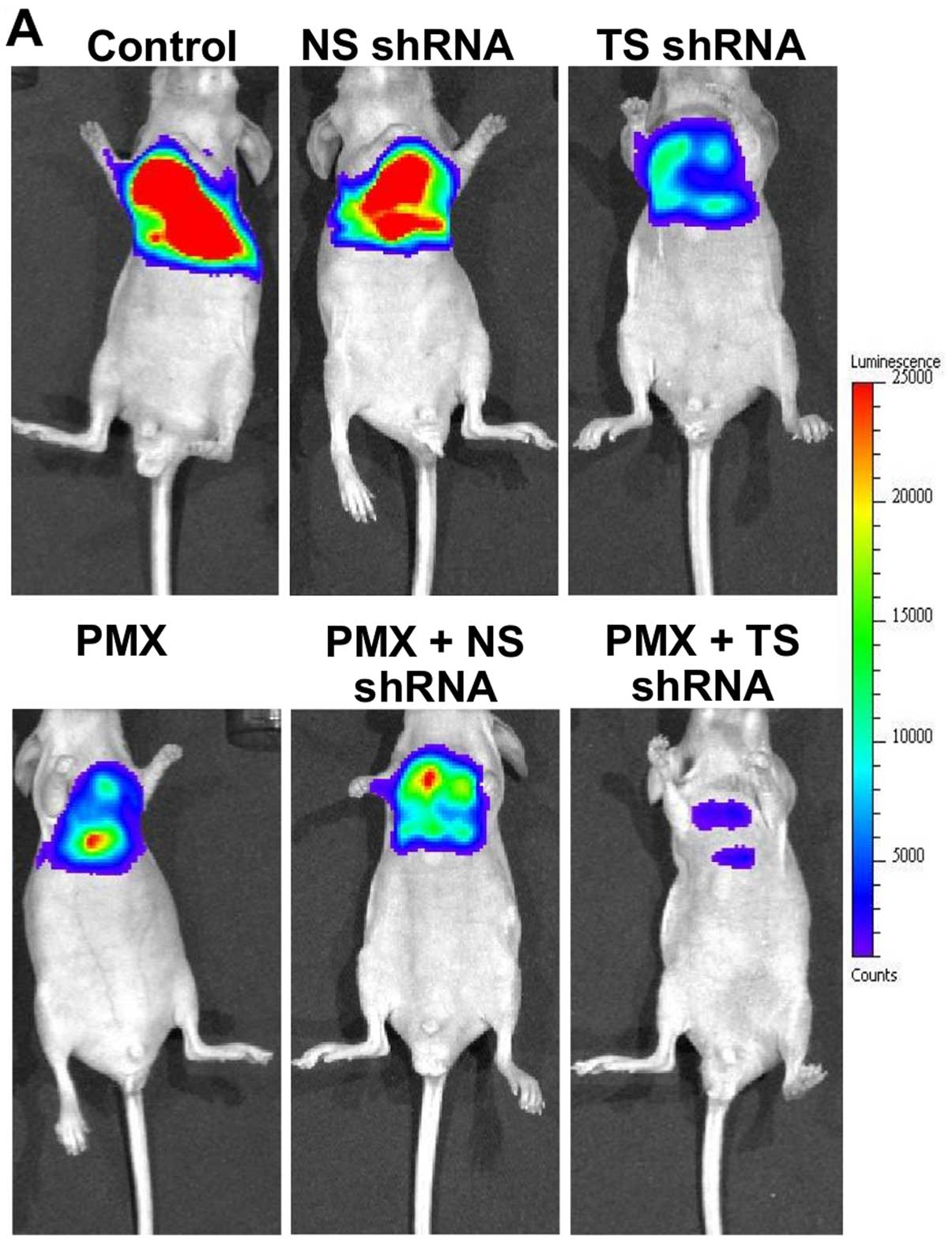
(on day 21), the bioluminescence related to tumor progression was
monitored by IVIS (Fig. 4). TS
shRNA alone and PMX alone and a combination of NS shRNA plus PMX
all exerted a moderate degree of inhibitory effect on the
development of thoracic tumors, as indicated by a slight reduction
in tumor burden (Fig. 4A), and,
consequently, a slight decrease in photon intensity, compared with
the control group (Fig. 4B). On
the contrary, treatment with a combined therapy of TS shRNA and PMX
significantly inhibited the production of thoracic tumors, which
was indicated by a remarkable reduction in tumor burden (Fig. 4A) and a substantial decrease in the
photon intensity, compared with the other treated groups
(p<0.001) (Fig. 4B). The dose
and schedule of the combined treatment of TS-shRNA and PMX were
well tolerated, as determined by the absence of a significant
amount of weight loss or other signs of acute or delayed toxicity
(data not shown).
Mouse survival was evaluated up to 45
days after a xenograft injection of MSTO-211H-Luc
The survival of treated mice is illustrated in
Fig. 4C, and the mean survival
time [MST (day)] and percentage of increased life span [ILS (%)]
are summarized in Table I. There
was no significant difference in survival between the control and
the NS shRNA-treated groups (Fig.
4C). Treatment with either as single agents slightly prolonged
the survival, compared with the control group [ILS (%) were 20.0
and 26.6%, respectively], whereas, the combined treatment of TS
shRNA lipoplex or PMX showed a significant survival advantage; 50%
of the mice (3 out of 6) became long-term survivors (>45 days)
(Fig. 4C), compared with other
treated groups.
 | Table ISummary of median survival time (MST)
and percent increased life span [ILS (%)] of tumor-bearing mice
after treatment with PMX and/or shRNAs. |
Table I
Summary of median survival time (MST)
and percent increased life span [ILS (%)] of tumor-bearing mice
after treatment with PMX and/or shRNAs.
| Formulation | MST (days) | ILS (%) |
|---|
| Control (9% sucrose
solution) | 22.5 | - |
| NS shRNA
lipoplex | 24.0 | 6.6 |
| TS shRNA
lipoplex | 28.5 | 26.6 |
| PMX | 27.0 | 20.0 |
| PMX + NS shRNA
lipoplex | 27.5 | 22.2 |
| PMX + TS shRNA
lipoplex | >39 | >75.0 |
In vivo gene silencing efficacy of the
combined therapy of TS-shRNA and PMX in an orthotopic implantation
model
To gain further insights into the in vivo
gene silencing efficacy of TS shRNA alone or combined with PMX, the
TS mRNA levels were measured quantitatively via RT-PCR in the
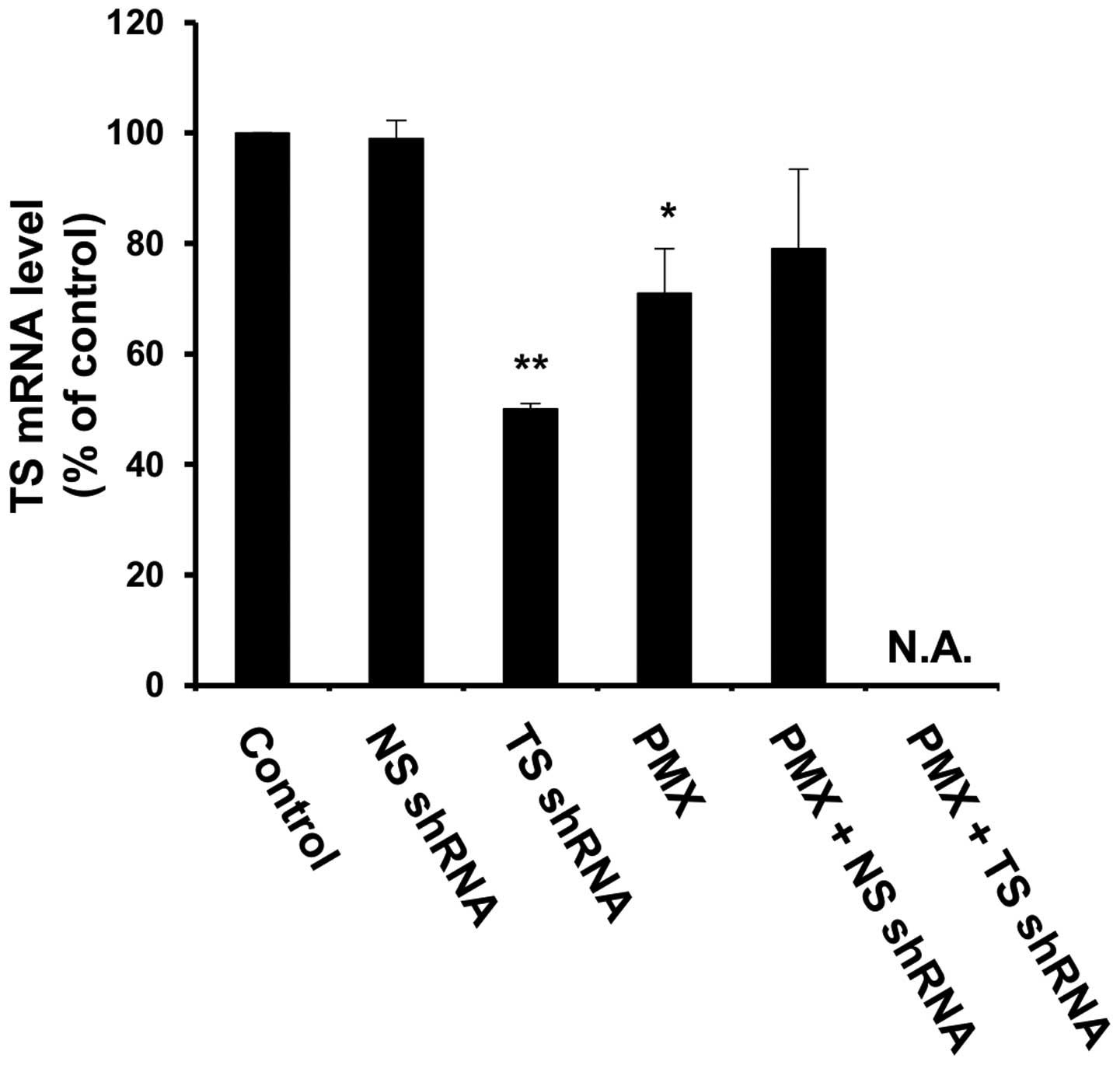
thoracic tumors 72 h after the last treatment (on day 21). Compared
with untreated (control) mice or mice treated with NS shRNA
lipoplex, a significant reduction in the TS mRNA level was observed
in mice treated with either, reaching approximately 30 and 50%,
respectively (Fig. 5).
Unfortunately, the amount of total RNA isolated from thoracic
tumors treated with a combination treatment of TS shRNA lipoplex or
PMX was too low to determine the TS mRNA level. These results
emphasize the contribution that in vivo gene silencing of TS
shRNA made to the superior antitumor efficacy of the combined
treatment of PMX plus TS shRNA lipoplex.
In vivo biodistribution study following
intrapleural administration of shRNA lipoplex
The efficient delivery and prolonged retention of
shRNA lipoplex in the target tissues are key determinants for the
therapeutic efficacy of shRNA. Therefore, we investigated the in
vivo distribution of fluorescent-labeled lipoplex following
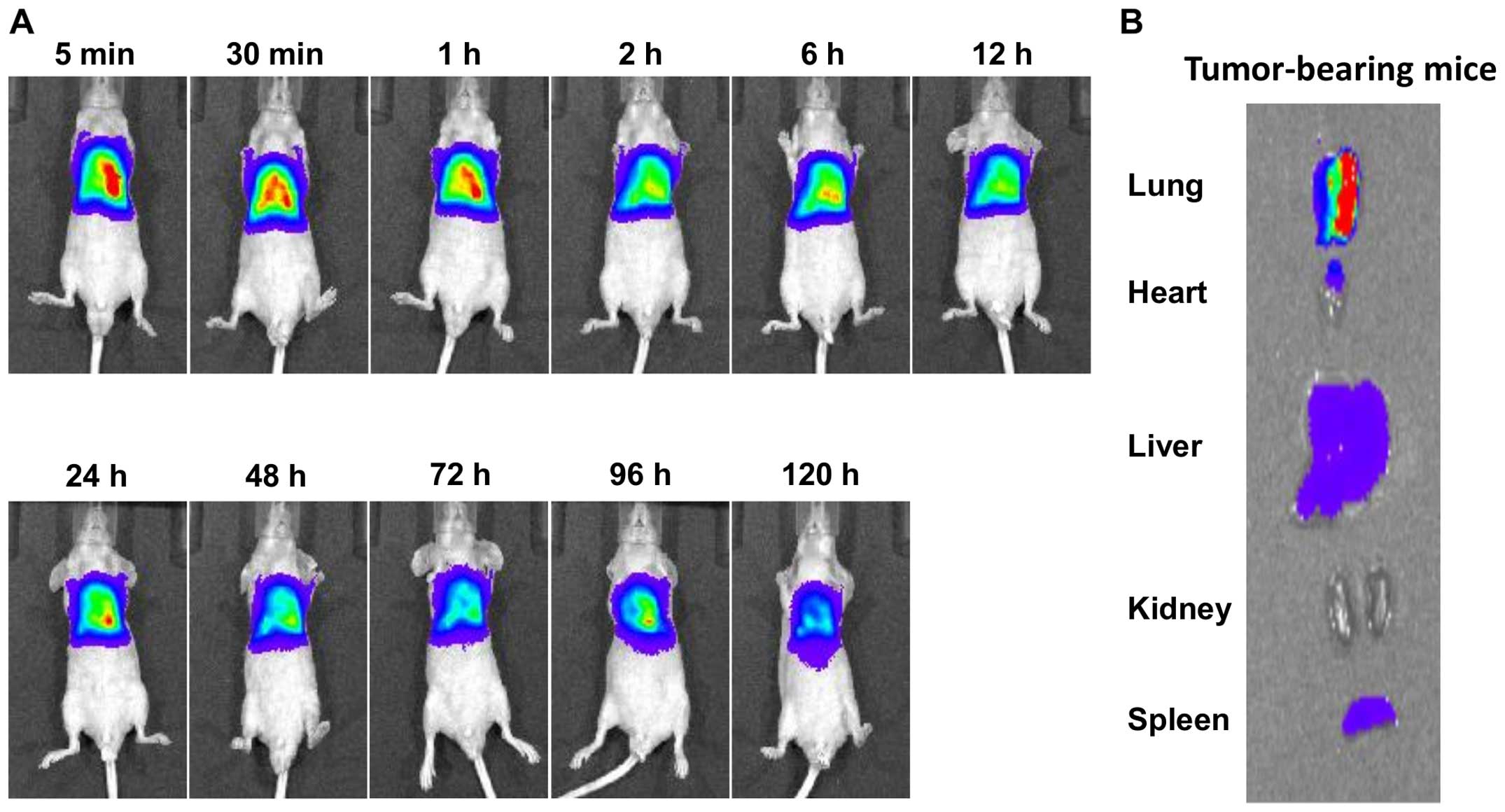
intrapleural injection in tumor-bearing mice (Fig. 6). DiR was applied into the
liposomal membrane as the hydrophobic fluorescent probe since its
near-infrared excitation and emission wavelengths could efficiently
reduce the interference of animal auto-fluorescence (29). In the mice bearing thoracic tumors,
shRNA lipoplex were retained within the pleural cavity for an
extended period (120-h post-injection) (Fig. 6A). No remarkable accumulation of
shRNA lipoplex in the major organs, particularly the liver and
spleen, was observed following the intrapleural injection of shRNA
lipoplex into orthotopic tumor-bearing mice, as presented in the
ex vivo images (Fig. 6B).
It appears that orthotopic implantation of MSTO-211H cells within
the pleural cavity confined the shRNA lipoplexes to the thoracic
cavity. These results suggest that the significant prolonged
retention of shRNA lipoplex within the pleural cavity of orthotopic
tumor-bearing mice contributed to the enhanced therapeutic efficacy
of shRNA lipoplex following intrapleural administration.
Discussion
Pemetrexed (PMX) represents a mainstay in malignant
pleural mesothelioma (MPM) treatment, and resistance to its
activity is a major obstacle to successful chemotherapy for MPM.
Resistance to PMX has been attributed mainly to an increased
expression of the thymidylate synthase (TS) enzyme, a key enzyme
for DNA synthesis and repair. Many reports have investigated the
clinical relevance of high TS mRNA as a predictor of the resistance
to PMX in various cancers including MPM (30–32).
Therefore, downregulation of the TS enzyme is considered an
attractive strategy that could enhance MPM therapy with anti-folate
agents including PMX. In the present study, we proposed a novel
RNAi approach that uses a chemically synthesized short hairpin RNA
(shRNA) to downregulate the TS gene and thus restore the
chemosensitivity of PMX against human MPM (MSTO-211H) cells both
in vitro and in vivo. We demonstrated that RNAi
knockdown of the TS gene in MSTO-211H cells suppressed the
expression of TS mRNA by approximately 90% (Fig. 1), and resulted in improved
chemosensitivity to PMX in vitro (Fig. 2). In addition, intrapleural
administration of TS shRNA lipoplex in combination with PMX
significantly suppressed the growth of thoracic tumors in
orthotopic tumor-bearing mouse models (Fig. 4), which resulted in an increase in
the life span of treated mice (Table
I). These results emphasize the pivotal relevance of RNAi as an
effective tool for increasing the therapeutic efficacy of PMX
against human MPM.
RNA interference (RNAi), a sequence-specific gene
silencing mechanism induced by double-stranded RNA (dsRNA), has
emerged as a promising therapy for combating cancer (19,33,34).
The RNAi effect can be mediated with short interfering RNA (siRNA),
which is a short, double stranded RNA with 21–23 base pairs
(35), or, as recently
demonstrated, with short hairpin RNA (shRNA) (36). Although siRNA and shRNA elicit
comparable gene silencing effects in RNAi experiments, chemically
synthesized shRNA represents significant advantages over siRNA.
shRNA is produced from a single oligodeoxynucleotide that requires
less up-front oligo design and synthesis, and it uses a shorter,
high-yield transcription reaction. In addition, unlike siRNA, shRNA
does not require the annealing of 2 RNA strands, which eliminates
the potential for contamination of single-stranded RNA that can
compromise its yield and integrity (37,38).
Finally, similar to siRNA, the stability and biological activity of
shRNA can be improved via chemical modifications, along with a
reduction in innate immune responses (39).
In our orthotopic implantation model with MPM cells,
suppression of tumor progression and survival prolongation are the
most valuable parameters that can be used to evaluate therapeutic
efficiency. A potent tumor growth inhibitory effect has been
observed with the combined treatment of TS shRNA and PMX (Fig. 4), and it was accompanied by a
significant prolongation of survival (MST >39 day, ILS >75%)
(Table I). This emphasizes the
efficacy of TS shRNA in inducing potent and enduring TS gene
silencing, thereby sensitizing the mesothelioma MSTO-211H cell line
to the in vivo cytotoxic effect of PMX and leading to a
superior level of tumor growth suppression (Fig. 5) and a prolonged life span
(Fig. 4C and Table I). Furthermore, as a single agent,
either remarkably inhibited the production of thoracic tumors of
the MSTO-211H cell line, as indicated by a reduction in the tumor
burden (Fig. 4A), and,
consequently, a decrease in photon intensity (Fig. 4B). However, both treatments showed
only a marginal prolongation of survival (MST, 27 and 28.5 day,
ILS, 20 and 26.6%, respectively), compared with untreated mice
(MST, 22.5 day) (Table I),
probably as a result of aggressive re-growth of tumor cells after
cessation of treatment.
Of note, an in vivo imaging study
demonstrated a prolonged retention of shRNA lipoplex within the
pleural cavity in tumor-bearing mice (Fig. 6). The electrostatic attraction
and/or binding that the positively charged surface of shRNA
lipoplex presents to the negatively charged MSTO-211H tumor cells
might tend to hold the shRNA lipoplex in the thoracic cavity
following its intrapleural administration. This prolonged retention
of shRNA lipoplex is believed to enhance its uptake by tumor cells
and to induce a potent TS gene knockdown by TS shRNA (Fig. 5), which leads to an increased
sensitivity of MSTO-211H cells to the cytotoxic effect of PMX. This
sensitizing effect of TS shRNA, in turn, contributed substantially
to the potent antitumor efficacy of PMX against MSTO-211H cells in
an orthotopic tumor mouse model (Fig.
4).
Since MPM grows locally within the thoracic cavity
and rarely displays metastasis to distant sites (24,40),
the direct administration of novel therapeutic agents such as
genetic materials should be uniquely accessible. Furthermore,
direct administration of genetic materials into the pleural cavity,
rather than systemic administration, should eliminate/alleviate the
development of severe immune reactions and/or cytokine induction
against the injected genetic materials. Many clinical trials have
revealed that the intrapleural administration of therapeutic agents
to patients with MPM is safe and well tolerated (41,42).
In our study, intrapleural administration of shRNA nanoparticles
(shRNA lipoplex) was safe and well tolerated by mice as indicated
by the absence of remarkable toxic effects (data not shown). In
addition, intrapleural administration could guarantee the effective
delivery of RNAi molecules and further reduce the adverse effects
to normal organs compared with systemic administration.
One of the major obstacles in cancer therapy is the
occurrence of cellular resistance to cancer chemotherapy (23,43).
In vitro, in vivo and clinical studies have shown
that the exposure to TS inhibitor compounds, including PMX, results
in acute induction of TS overexpression and thus the acute
development of resistance in response to TS inhibitors (30–32).
In the present study, TS gene knock-down by RNAi efficiently
suppressed the expression level of TS genes both in vitro
(Fig. 1) and in vivo
(Fig. 5), and thus restored the
chemosensitivity to PMX against MSTO-211H cells. To the best of our
knowledge, this is the first report regarding the efficacy of
chemically synthesized shRNA, delivered by cationic liposomes, in
improving the therapeutic efficacy of PMX against human MPM.
In the present study, we showed that the RNAi effect
from an intrapleural injection of shRNA designed against the TS
gene, repressed the expression of TS mRNA in human malignant
mesotheliomal MSTO-211H cells and suppressed tumor progression,
thereby prolonging the survival of mice inoculated orthotopically
with MSTO-211H cells. Our proposed combination therapy of TS shRNA
and PMX shows therapeutic promise for the treatment of MPM via the
downregulation of TS expression levels and the subsequent
prevention of the development of cellular resistance that is
observed with TS inhibitor compounds, including PMX, that are
currently used in clinical settings.
Acknowledgements
We thank Dr G.L. Scherphof for his helpful advice in
preparing this manuscript. This work was supported by the Japan
Society for the Promotion of Science, Grants-in-Aid for JSPS
Fellows and for Scientific Research (B) (15H04639), the Ministry of
Education, Culture, Sports, Science and Technology, Japan.
Abbreviations:
|
CHOL
|
cholesterol
|
|
DC-6-14
|
O,O'-ditetradecanoyl-N-(α-trimethyl
ammonioacetyl) diethanolamine chloride
|
|
DiR
|
1,1′-dioctadecyl-3,3,3′,3′-tetramethyl
indotricarbocyanine iodide
|
|
DOPC
|
dioleoylphosphatidylcholne
|
|
DOPE
|
dioleoylphosphatidylethanolamine
|
|
DHFR
|
dihydrofolate reductase
|
|
EGFR
|
epidermal growth factor receptor
|
|
FBS
|
fetal bovine serum
|
|
GAPDH
|
glyceraldehydes-3-phosphate
dehydrogenase
|
|
GARFT
|
glycinamide ribonucleotide
formyltransferase
|
|
ILS
|
increased life span
|
|
IVIS
|
in vivo imaging system
|
|
MPM
|
malignant pleural mesothelioma
|
|
MST
|
mean survival time
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
PBS
|
phosphate buffer saline
|
|
PMX
|
pemetrexed
|
|
ROI
|
region of interest
|
|
TS
|
thymidylate synthase
|
References
|
1
|
Giovannetti E, Zucali PA, Assaraf YG, Leon
LG, Smid K, Alecci C, Giancola F, Destro A, Gianoncelli L, Lorenzi
E, et al: Preclinical emergence of vandetanib as a potent
antitumour agent in mesothelioma: Molecular mechanisms underlying
its synergistic interaction with pemetrexed and carboplatin. Br J
Cancer. 105:1542–1553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostroff RM, Mehan MR, Stewart A, Ayers D,
Brody EN, Williams SA, Levin S, Black B, Harbut M, Carbone M, et
al: Early detection of malignant pleural mesothelioma in
asbestos-exposed individuals with a noninvasive proteomics-based
surveillance tool. PLoS One. 7:e460912012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kishimoto T, Ozaki S, Kato K, Nishi H and
Genba K: Malignant pleural mesothelioma in parts of Japan in
relationship to asbestos exposure. Ind Health. 42:435–439. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi H, Harada M, Maehara S and Kato
H: Localized malignant mesothelioma of the pleura. Ann Thorac
Cardiovasc Surg. 13:262–266. 2007.PubMed/NCBI
|
|
5
|
Hodgson JT, McElvenny DM, Darnton AJ,
Price MJ and Peto J: The expected burden of mesothelioma mortality
in Great Britain from 2002 to 2050. Br J Cancer. 92:587–593.
2005.PubMed/NCBI
|
|
6
|
Joshi TK and Gupta RK: Asbestos-related
morbidity in India. Int J Occup Environ Health. 9:249–253. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lucchi M, Chella A, Melfi F, Dini P,
Tibaldi C, Fontanini G and Mussi A: Four-modality therapy in
malignant pleural mesothelioma: A phase II study. J Thorac Oncol.
2:237–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stahel RA, Weder W, Lievens Y and Felip E;
ESMO Guidelines Working Group. Malignant pleural mesothelioma: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 21(Suppl 5): v126–v128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nasreen N, Khodayari N and Mohammed KA:
Advances in malignant pleural mesothelioma therapy: Targeting EphA2
a novel approach. Am J Cancer Res. 2:222–234. 2012.
|
|
10
|
Steele JP and Klabatsa A: Chemotherapy
options and new advances in malignant pleural mesothelioma. Ann
Oncol. 16:345–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ceresoli GL, Gridelli C and Santoro A:
Multidisciplinary treatment of malignant pleural mesothelioma.
Oncologist. 12:850–863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Katirtzoglou N, Gkiozos I, Makrilia N,
Tsaroucha E, Rapti A, Stratakos G, Fountzilas G and Syrigos KN:
Carboplatin plus pemetrexed as first-line treatment of patients
with malignant pleural mesothelioma: A phase II study. Clin Lung
Cancer. 11:30–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sorensen JB, Sundstrom S, Perell K and
Thielsen AK: Pemetrexed as second-line treatment in malignant
pleural mesothelioma after platinum-based first-line treatment. J
Thorac Oncol. 2:147–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanauske AR, Chen V, Paoletti P and
Niyikiza C: Pemetrexed disodium: A novel antifolate clinically
active against multiple solid tumors. Oncologist. 6:363–373. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marangolo M and Vertogen B: Pemetrexed and
malignant pleural mesothelioma. Ann Oncol. 17(Suppl 5): v103–v105.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scagliotti GV, Shin DM, Kindler HL,
Vasconcelles MJ, Keppler U, Manegold C, Burris H, Gatzemeier U,
Blatter J, Symanowski JT, et al: Phase II study of pemetrexed with
and without folic acid and vitamin B12 as front-line therapy in
malignant pleural mesothelioma. J Clin Oncol. 21:1556–1561. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozasa H, Oguri T, Uemura T, Miyazaki M,
Maeno K, Sato S and Ueda R: Significance of thymidylate synthase
for resistance to pemetrexed in lung cancer. Cancer Sci.
101:161–166. 2010. View Article : Google Scholar
|
|
18
|
Zhang D, Ochi N, Takigawa N, Tanimoto Y,
Chen Y, Ichihara E, Hotta K, Tabata M, Tanimoto M and Kiura K:
Establishment of pemetrexed-resistant non-small cell lung cancer
cell lines. Cancer Lett. 309:228–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Milhavet O, Gary DS and Mattson MP: RNA
interference in biology and medicine. Pharmacol Rev. 55:629–648.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shirasaki T, Maruya S, Mizukami H,
Kakehata S, Kurotaki H, Yagihashi S and Shinkawa H: Effects of
small interfering RNA targeting thymidylate synthase on survival of
ACC3 cells from salivary adenoid cystic carcinoma. BMC Cancer.
8:3482008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeang KT: RNAi in the regulation of
mammalian viral infections. BMC Biol. 10:582012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
John M, Constien R, Akinc A, Goldberg M,
Moon Y-A, Spranger M, Hadwiger P, Soutschek J, Vornlocher H-P,
Manoharan M, et al: Effective RNAi-mediated gene silencing without
interruption of the endogenous microRNA pathway. Nature.
449:745–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura K, Abu Lila AS, Matsunaga M, Doi
Y, Ishida T and Kiwada H: A double-modulation strategy in cancer
treatment with a chemotherapeutic agent and siRNA. Mol Ther.
19:2040–2047. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leung RK and Whittaker PA: RNA
interference: From gene silencing to gene-specific therapeutics.
Pharmacol Ther. 107:222–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abu Lila AS, Matsumoto H, Doi Y, Nakamura
H, Ishida T and Kiwada H: Tumor-type-dependent vascular
permeability constitutes a potential impediment to the therapeutic
efficacy of liposomal oxaliplatin. Eur J Pharm Biopharm.
81:524–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bartlett GR: Colorimetric assay methods
for free and phosphorylated glyceric acids. J Biol Chem.
234:469–471. 1959.PubMed/NCBI
|
|
27
|
Kviecinski MR, Felipe KB, Schoenfelder T,
de Lemos Wiese LP, Rossi MH, Gonçalez E, Felicio JD, Filho DW and
Pedrosa RC: Study of the antitumor potential of Bidens pilosa
(Asteraceae) used in Brazilian folk medicine. J Ethnopharmacol.
117:69–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abu Lila AS, Kizuki S, Doi Y, Suzuki T,
Ishida T and Kiwada H: Oxaliplatin encapsulated in PEG-coated
cationic liposomes induces significant tumor growth suppression via
a dual-targeting approach in a murine solid tumor model. J Control
Release. 137:8–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma P, Brown S, Walter G, Santra S and
Moudgil B: Nanoparticles for bioimaging. Adv Colloid Interface Sci.
123–126:471–485. 2006. View Article : Google Scholar
|
|
30
|
Takezawa K, Okamoto I, Okamoto W, Takeda
M, Sakai K, Tsukioka S, Kuwata K, Yamaguchi H, Nishio K and
Nakagawa K: Thymidylate synthase as a determinant of pemetrexed
sensitivity in non-small cell lung cancer. Br J Cancer.
104:1594–1601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shintani Y, Ohta M, Hirabayashi H, Tanaka
H, Iuchi K, Nakagawa K, Maeda H, Kido T, Miyoshi S and Matsuda H:
New prognostic indicator for non-small-cell lung cancer,
quantitation of thymidylate synthase by real-time reverse
transcription polymerase chain reaction. Int J Cancer. 104:790–795.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hashimoto H, Ozeki Y, Sato M, Obara K,
Matsutani N, Nakagishi Y, Ogata T and Maehara T: Significance of
thymidylate synthase gene expression level in patients with
adenocarcinoma of the lung. Cancer. 106:1595–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang C, Li M, Chen C and Yao Q: Small
interfering RNA therapy in cancer: Mechanism, potential targets,
and clinical applications. Expert Opin Ther Targets. 12:637–645.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tabach Y, Billi AC, Hayes GD, Newman MA,
Zuk O, Gabel H, Kamath R, Yacoby K, Chapman B, Garcia SM, et al:
Identification of small RNA pathway genes using patterns of
phylogenetic conservation and divergence. Nature. 493:694–698.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen HY, Wang JM, Wang HY, Zhang Y, Liu W,
Pan L, Wang W, Chen S, Jin W and Wang L: Effect of short hairpin
RNA-induced CXCR4 silence on ovarian cancer cell. Biomed
Pharmacother. 66:549–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castanotto D and Rossi JJ: The promises
and pitfalls of RNA-interference-based therapeutics. Nature.
457:426–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Terasawa K, Shimizu K and Tsujimoto G:
Synthetic pre-miRNA-based shRNA as potent RNAi triggers. J Nucleic
Acids. 131579:20112011.
|
|
39
|
Jackson AL and Linsley PS: Recognizing and
avoiding siRNA off-target effects for target identification and
therapeutic application. Nat Rev Drug Discov. 9:57–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Iwahori K, Serada S, Fujimoto M, Nomura S,
Osaki T, Lee CM, Mizuguchi H, Takahashi T, Ripley B, Okumura M, et
al: Overexpression of SOCS3 exhibits preclinical antitumor activity
against malignant pleural mesothelioma. Int J Cancer.
129:1005–1017. 2011. View Article : Google Scholar
|
|
41
|
Sterman DH, Recio A, Carroll RG, Gillespie
CT, Haas A, Vachani A, Kapoor V, Sun J, Hodinka R, Brown JL, et al:
A phase I clinical trial of single-dose intrapleural IFN-beta gene
transfer for malignant pleural mesothelioma and metastatic pleural
effusions: High rate of antitumor immune responses. Clin Cancer
Res. 13:4456–4466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sterman DH, Recio A, Vachani A, Sun J,
Cheung L, DeLong P, Amin KM, Litzky LA, Wilson JM, Kaiser LR, et
al: Long-term follow-up of patients with malignant pleural
mesothelioma receiving high-dose adenovirus herpes simplex
thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res.
11:7444–7453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Storch K and Cordes N: Focal
adhesion-chromatin linkage controls tumor cell resistance to radio-
and chemotherapy. Chemother Res Pract. 2012:3192872012.PubMed/NCBI
|