Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies worldwide and is the leading cause of cancer
death (1). It is characterized by
a poor prognosis due to a high potential for metastasis and
recurrence within a short time. The tyrosine kinase inhibitor,
sorafenib, was recently shown to prolong overall survival in
patients with advanced HCC and has become the standard drug for
first-line systemic treatment (1–3).
However, only a minority of patients show objective evidence of a
clinical response and their median overall survival remains less
than one year. These findings, and the incidence of adverse
reactions, suggest that novel treatment approaches are
required.
Immunotherapy has now been clinically validated as
an effective treatment for cancer (4–6).
Tumor antigen-specific immune targeting by cytotoxic T lymphocytes
(CTLs) may provide an additional treatment modality to eliminate
residual disease and prevent relapse in HCC patients. We have
reported that glypican-3 (GPC3), an oncofetal antigen overexpressed
in HCC, is a useful target for CTL-mediated cancer immunotherapy
and we performed clinical trials of a GPC3 peptide vaccine
(7,8). GPC3 peptide-specific CTLs were
detected in the peripheral blood of some vaccinated patients and a
very small number of the CTLs were also present in the HCC tissue,
indicating an ongoing local immune response (8). However, this tumor response was rare
and many HCC patients are unlikely to benefit from a vaccination
strategy targeting a single CTL epitope. The rarity of tumor
antigen-specific CTLs in the T cell repertoire in the thymus
suggests a requirement for a type of therapy that coordinates the
interplay between the innate and acquired immune systems in order
to eradicate HCC (9).
γδ T cells are a small subset of T lymphocytes
expressing γ- and δ-chain T cell receptor (γδ TCR); these occur at
a frequency of 0.5–10% of total T lymphocytes in peripheral blood
and play an important role in innate immune surveillance (10,11).
The most abundant circulating γδ T cells, Vγ9Vδ2 T cells, can be
activated by small non-peptide phosphorylated antigens synthesized
in the mevalonate pathway, major histocompatibility complex (MHC)
class I-related molecules A/B (MICA/B), various members of the
UL16-binding protein (ULBP) family, or poliovirus receptor (PVR,
nectin-like molecule 5, CD155) in an MHC-independent manner
(12–15).
A nitrogen-containing bisphosphonate, zoledronate
(Zol), which is widely used to prevent bone metastasis, increases
the isopentenyl pyrophosphate (IPP) mevalonate metabolite in some
tumor cells by inhibiting farnesyl pyrophosphate (FPP) synthase,
leading to γδ T cell-mediated killing of malignant tumor cells
(16–18). This process has been used in
clinical trials of γδ T cell-based immunotherapy for patients with
several cancers and was shown to be beneficial and safe, providing
a promising therapeutic strategy (19). Although in vivo and in
vitro studies indicated that Zol rendered many types of tumor
cells susceptible to γδ T cell-mediated killing, there has not been
a systematic examination of whether HCC would respond to
immunotherapy using γδ T cells and Zol. The present study
comprehensively examined the expression of γδ T cell ligands on a
variety of HCC cell lines and the effects of Zol treatment on the
responses of γδ T cells. We demonstrated that the γδ T
cell-mediated killing of all examined HCC cell lines was
significantly enhanced by Zol treatment, indicating that the
recognition of Zol-treated HCC cell lines by γδ T cells was likely
γδ T cell receptor-dependent. In addition, Zol-treated HCC cell
lines triggered γδ T cell proliferation and cytokine productions.
Our findings could contribute to the development of an
immunotherapeutic approach combining Zol with γδ T cells for the
treatment of HCC.
Materials and methods
Cytokines and chemicals
Recombinant human interleukin (IL)-2 and IL-15 were
purchased from Nipro (Osaka, Japan) and PeproTech Inc., (Rocky
Hill, NJ, USA). Zol (Zometa) was purchased from Novartis (Basel,
Switzerland). Mevastatin and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies
Anti-ULBP1 (170818), anti-ULBP2 (165903), anti-ULBP3
(166510), anti-natural killer group 2D (NKG2D) (140810), and mouse
immunoglobulin (Ig) G2a (20102) were purchased from R&D Systems
(Minneapolis, MN, USA). Anti-MICA/B (6D4), anti-CD3 (UCTH1),
anti-Nectin-2 (TX31), anti-PVR (SKII.4), anti-DNAX accessory
molecule-1 (DNAM-1) (11A8), anti-NKG2D (1D11), anti-CD27 (O323),
anti-CD45RA (H100), mouse IgG2b, κ (MPC-11) and mouse IgG1, κ
(MOPC-21) were purchased from BioLegend (San Diego, CA, USA).
Anti-TCRVγ9 (IMMU360) and anti-TCR-pan-γδ (IMMU510) were purchased
from Beckman Coulter (Fullerton, CA, USA). Anti-DNAM-1 (DX11) was
from Abcam (Cambridge, UK).
Cells
Human HCC cell lines (HLE, HLF, HuH-1, JHH5, and
JHH7) were purchased from the Health Science Research Resources
Bank (Osaka, Japan). The HepG2 and Li-7 HCC cell lines, the T2
lymphoblastoid cell line, and the K562 erythroleukemia cell line
were purchased from the RIKEN BioResource Center (Ibaraki, Japan).
The EJ1 bladder cancer cell line was provided by the Cell Resource
Center for Biomedical Research (Miyagi, Japan). The pancreatic
cancer cell line, MIAPaCa-2, was purchased from the American Type
Culture Collection (Rockville, MD, USA). All HCC cell lines, EJ1,
and MIAPaCa-2 cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich) supplemented with 100 μg/ml
L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10%
heat-inactivated fetal bovine serum (FBS; Gibco, Carlsbad, CA,
USA). T2 cells and K562 cells were cultured in Roswell Park
Memorial Institute 1640 medium (RPMI-1640; Sigma-Aldrich)
supplemented with 100 μg/ml L-glutamine, 100 U/ml penicillin, 100
μg/ml streptomycin, and 10% FBS. Phytohemagglutinin (PHA) blasts
were obtained by stimulating peripheral blood mononuclear cells
(PBMCs) with PHA (Sigma-Aldrich; 1 μg/ml) in AIM-V medium (Gibco,
Grand Island, NY, USA) supplemented with 10% human AB serum and
IL-2 (100 IU/ml). Peripheral blood mononuclear cells from healthy
donors were purchased from Cellular Technology Ltd. (Cleveland, OH,
USA).
γδ T cells
CD3+Vγ9+ cells were isolated
using an automated cell sorter (FACS Aria II; BD Biosciences, San
Jose, CA, USA), seeded in a 96-well plate, and stimulated by PHA (1
μg/ml) in the presence of irradiated (100 Gy) allogeneic PBMCs
(8.0×104 cells/well) as feeder cells in AIM-V medium
supplemented with 10% human AB serum, IL-2 (100 IU/ml), and IL-15
(10 ng/ml).
Flow cytometry
Cell samples were treated with human γ-globulin
(Sigma-Aldrich) for 10 min in order to block Fc-receptors, stained
with the relevant fluorochrome-conjugated monoclonal antibody (mAb)
for 20 min, and washed with phosphate-buffered saline containing 2%
FBS. The stained cell samples were analyzed on a flow cytometer
(FACSCanto II; BD Bioscience) and the data were analyzed using
FlowJo software (Tree Star Inc., Ashland, OR, USA).
Cell proliferation
Cell proliferation was evaluated by a standard MTT
assay and by [3H]-thymidine incorporation assay. For the
MTT assay, cells were seeded in 96-well culture plates
(4.0×103 cells/well) and incubated with Zol. After 72 h,
MTT reagent was added directly to the medium and incubated for 4 h.
The supernatant was removed, and 200 μl of dimethyl sulfoxide was
added to each well and thoroughly mixed for 3 min. The absorbance
of the converted dye at 595 nm was measured on a Multiskan FC
microplate reader (Thermo Scientific, Vantaa, Finland). For
[3H]-thymidine incorporation assays, the cells were
cultured in 96-well culture plates (5.0×103 cells/well)
for 72 h and 37 kBq/well [3H]-thymidine was added to the
culture for the last 16 h. The incorporated radioactivity was then
measured by scintillation counting (MicroBeta2 LumiJET;
PerkinElmer, Waltham, MA, USA).
Cytotoxicity assay
Target cells were labeled with Calcein-AM (Dojindo,
Kumamoto, Japan) for 30 min at 37°C and washed three times. The
labeled cells were then incubated with effector cells at various
effector: target (E:T) ratios. Cytotoxic activity against target
cells was measured using the Terascan VPC system (Minerva Tech,
Tokyo, Japan). Fluorescent intensity was measured before and after
the 4-h incubation, and specific cytotoxic activity was calculated
using the following formula: % cytotoxicity = {1− [(average
fluorescence of the sample wells − average fluorescence of the
maximal release control wells)/(average fluorescence of the minimal
release control wells − average fluorescence of the maximal release
control wells)]} × 100%.
Cytokine measurements
The cytokine levels in the culture supernatants were
evaluated using Cytometric Bead Array Flex Sets (BD Bioscience),
according to the manufacturer's protocol. The resulting data were
analyzed using FCAP Array Software 3.0.
Small interfering RNA
Predesigned small interfering RNA (siRNA) targeting
human Nectin-2, PVR, or a negative control siRNA (Stealth
pre-designed RNAi siRNA; Invitrogen, CA, USA) were transfected into
HCC cell lines in a 12-well plate using Lipofectamine RNAiMAX
transfection reagent (Invitrogen), according to the manufacturer's
protocol.
Statistical analysis
The statistical significance of differences was
determined using one-way analysis of variance (ANOVA) with the
Bonferroni post-hoc test. P-values <0.05 were considered to
denote statistical significance.
Results
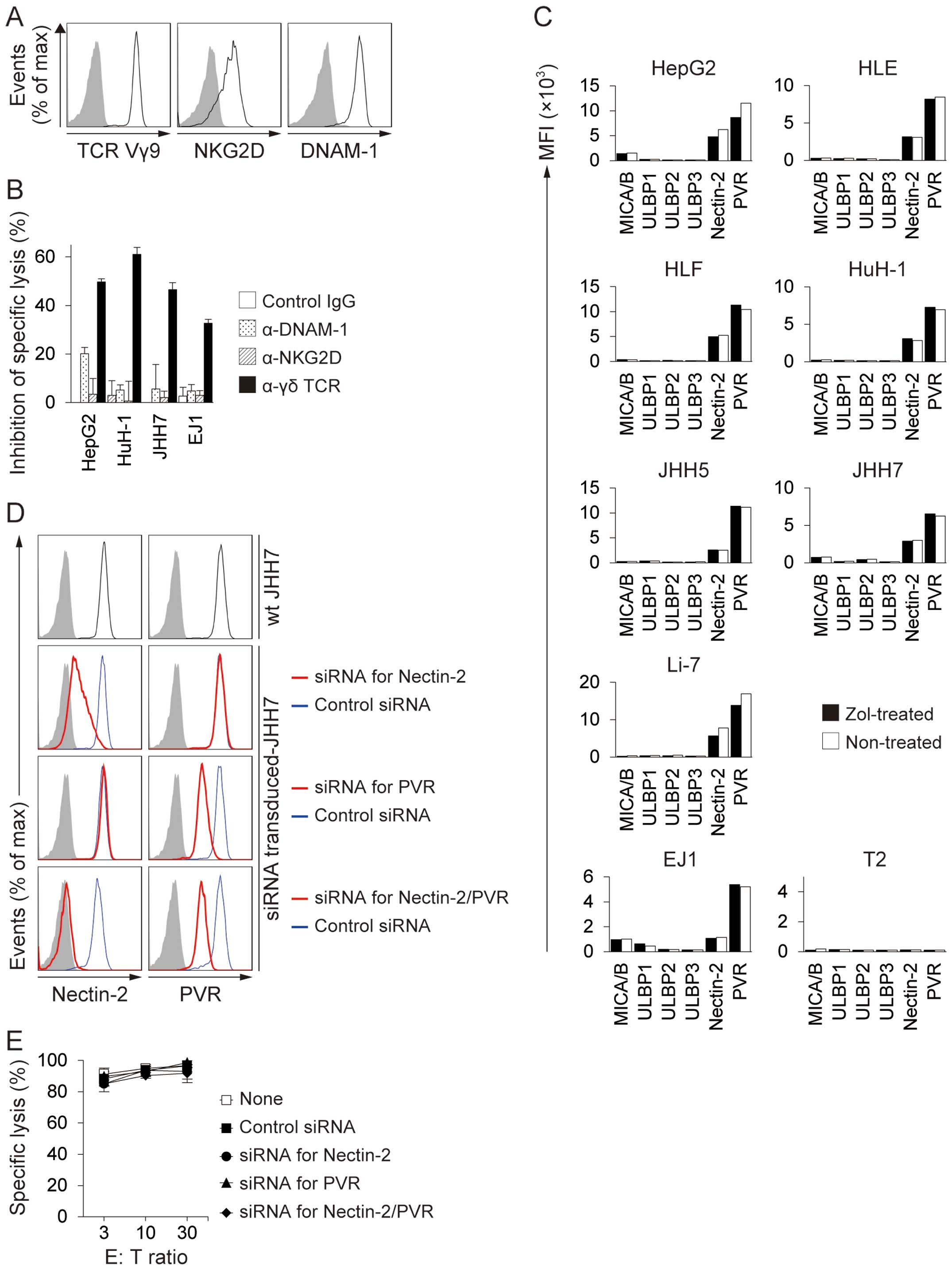
Expression of NKG2D and DNAM-1 ligands by
HCC cells
NKG2D and DNAM-1 are known to positively modulate
the cytotoxicity of γδ T cells (12,20).
To investigate the effect of Zol on γδ T cell-mediated cytotoxicity
towards HCC, we first examined the expression of surface NKG2D
ligands (MICA/B and ULBP1–3) and DNAM-1 ligands (Nectin-2 and PVR)
on a variety of HCC cell lines, as compared to γδ T cell-sensitive
or non-sensitive cell lines (Fig.
1). The EJ1 bladder carcinoma cell line is known to be
sensitive to γδ T cell-mediated lysis and expressed NKG2D ligands
(MICA/B and ULBP1–3) and DNAM-1 ligands (Nectin-2 and PVR). In
contrast, T2 cells show very little sensitivity to γδ T
cell-mediated lysis and only expressed MICA/B. Although they showed
different expression patterns and levels of MICA/B and ULBP1–3
(ranging from minimal to undetectable), all HCC cell lines
consistently expressed both Nectin-2 and PVR. These results
suggested that some ligands that may activate γδ T cells were
expressed on all HCC cell lines. It also indicated that they may be
susceptible to γδ T cell-mediated lysis.
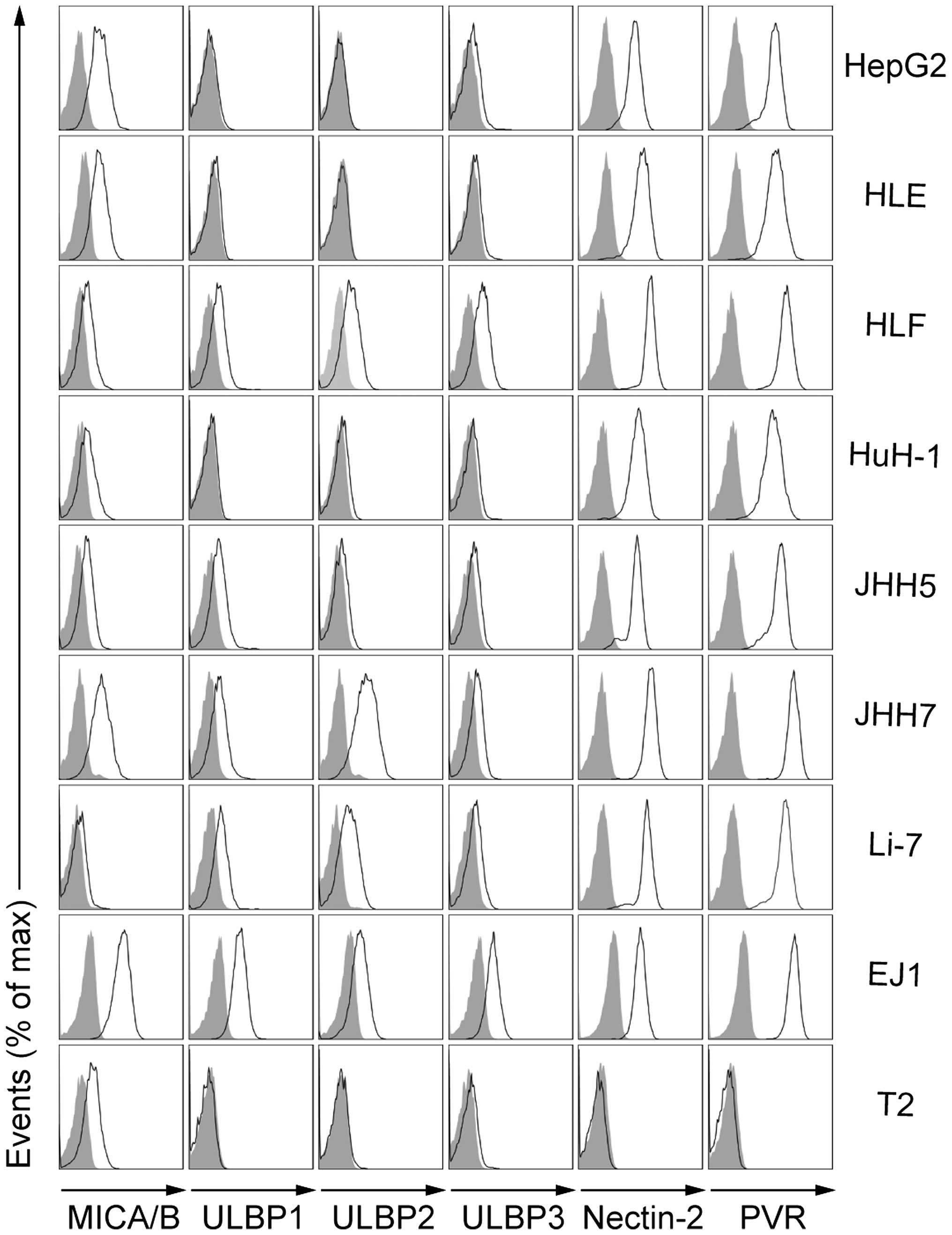 | Figure 1Expression of NKG2D and DNAM-1
ligands by HCC cell lines. Representative flow cytometry profiles
showing surface-expressed NKG2D ligands (MICA/B, ULBP1, ULBP2, and
ULBP3) and DNAM-1 ligands (Nectin-2 and PVR) on the indicated HCC
cell lines (HepG2, HLE, HLF, HuH-1, JHH5, JHH7, and Li-7). EJ1 and
T2 cell lines served as references. Open histograms represent
specific staining for the indicated molecules, while gray
histograms represent isotype control staining. |
Enhanced γδ T cell reactivity against
Zol-treated HCC cells
Zol is a potent inhibitor of FPP synthase and this
modifies the mevalonate pathway, leading to the accumulation of IPP
mevalonate metabolites. IPP acts as a phosphoantigen and activates
the expansion of human γδ T cells, which then exhibit cytotoxicity
against Zol-sensitized malignant tumor cells (21–23).
We isolated γδ T cells from PBMCs and stimulated with PHA in the
presence of IL-2 and IL-15. In contrast to the γδ T cells in
freshly isolated PBMCs, which showed
CD27+CD45RA+ naïve or
CD27+CD45RA− central memory phenotypes, γδ T
cells that had been expanded for 14 days showed a
CD27−CD45RA− effector memory phenotype
(Fig. 2A). We examined the
cytotoxic activity of these expanded γδ T cells against HCC cell
lines that had been preincubated with or without Zol (Fig. 2B). In the absence of Zol
pretreatment, γδ T cells showed weak cytotoxicity against HCC cell
lines, but not against T2 cells. These weak cytotoxic activities
toward HCC cell lines were possibly mediated by DNAM-1/PVR
interactions (24). Irrespective
of Zol treatment, T2 cells do not express PVR or Nectin-2.
Accordingly, these molecules are not essential for the killing of
T2 cells by γδ T cells. It is conceivable that Zol treatment may
not induce the accumulation of IPP in T2 cells. The cytotoxicity of
γδ T cells against all HCC cell lines was remarkably enhanced
following pretreatment with 5 μM Zol. Since γδ T cells are
multifunctional inflammatory cells that produce various cytokines
and cytotoxic molecules, we next examined the production of
cytokines [IL-2, IL-4, IL-5, IL-10, IL-13, IL-17A, interferon-γ
(IFN-γ), granulocyte macrophage colony-stimulating factor (GM-CSF),
tumor necrosis factor-α (TNF-α)] and granzyme B in response to HCC
cell lines that had been preincubated with or without Zol (Fig. 2C). Most Zol-treated HCC cell lines
triggered the production of IL-4, IL-5, IL-13, IFN-γ, GM-CSF,
TNF-α, and granzyme B by γδ T cells, but not that of IL-2, IL-10,
and IL-17A. However, Zol treatment of T2 cells, which showed very
little sensitivity to γδ T cell-mediated lysis, did not stimulate
production of these cytokines. In addition, γδ T cells showed
enhanced proliferative responses in the presence of Zol-treated HCC
cell lines, as compared to the untreated cells (Fig. 2D). These results suggested that Zol
treatment of HCC cell lines considerably enhanced the cytotoxicity,
cytokine production, and proliferation of γδ T cells. The cytokine
production and proliferation of γδ T cells in the presence of
Zol-treated HCC cell lines tended to correlate with the γδ T
cell-mediated cytotoxicity.
Enhanced sensitivity of Zol-treated HCC
cell lines to γδ TCRs
γδ T cells express TCR Vγ9, NKG2D, and DNAM-1, and
can recognize target cells via interactions with individual
receptors or with a combination of receptors (Fig. 3A) (12,25,26).
To determine the contributions of TCR, NKG2D, and DNAM-1 to the
recognition of Zol-treated HCC cell lines, we performed
cytotoxicity assays in the presence of blocking mAbs for these
receptors. Zol-pretreated HCC cell lines were lysed by γδ T cells
at an E:T ratio of 30:1. The addition of anti-γδ TCR mAb markedly
inhibited the cell lysis of all HCC cell lines tested (Fig. 3B). However, the addition of
anti-NKG2D or anti-DNAM-1 mAbs only produced marginal inhibition of
cell lysis. Zol treatment was not associated with any significant
change in the expression of NKG2D or DNAM-1 ligands in any of the
HCC cell lines tested (Fig. 3C).
In addition, siRNA-mediated Nectin-2 and PVR knockdown had no
effect on their susceptibility to γδ T cell-mediated lysis
(Fig. 3D and E). These results
indicated that γδ TCRs were important for the recognition of
Zol-treated HCC cells.
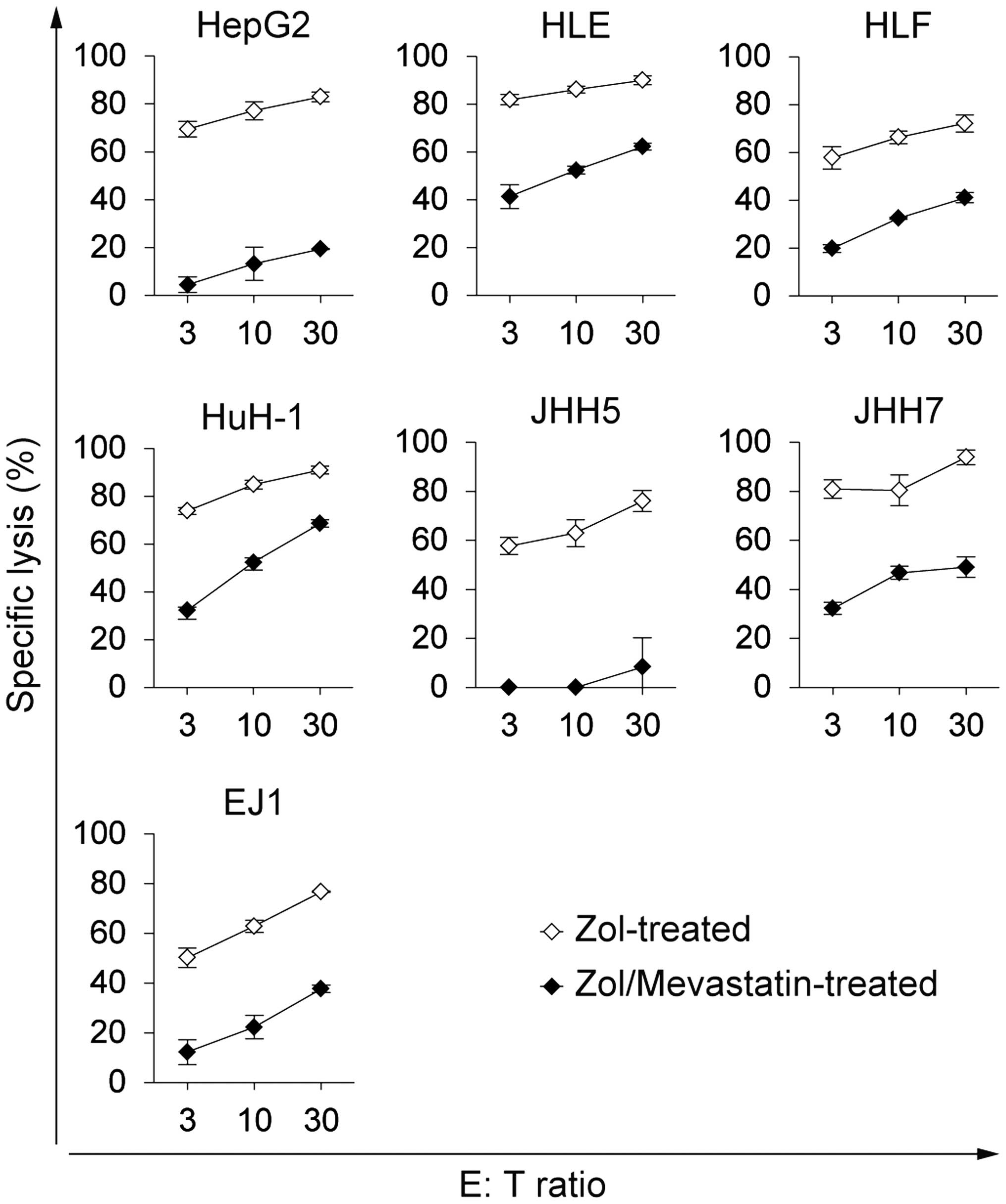
Inhibition of γδ T cell recognition of
Zol-treated HCC cell lines by mevastatin
The cytotoxicity of γδ T cells towards Zol-treated
HCC cells decreased in the presence of mevastatin, which blocked
IPP synthesis (Fig. 4). This
finding suggests that increased levels of mevalonate pathway
metabolites, such as IPP, are likely to be the ligands responsible
for the enhanced susceptibility of HCC cells to γδ T cell-mediated
killing.
γδ T cells exert specific killing
activity against Zol-treated HCC cell lines without damaging
Zol-untreated cells
To investigate the non-specific killing of target
cells by activated γδ T cells, γδ T cells were co-cultured with
Zol-treated HepG2 cells and with an equal number of
Calcein-AM-labeled, Zol-untreated cells, and the killing activity
against Zol-untreated cells was determined (Fig. 5). Activation of γδ T cells by
Zol-treated HepG2 cells did not produce any cytotoxic effects on
Zol-untreated HepG2, T2, or PHA blasts. In contrast, Zol-treated
HepG2 cells were effectively killed by activated γδ T cells. Cells
that were not susceptible to Zol, such as T2 and PHA blasts, were
not killed, regardless of Zol treatment. These results suggested
that activated γδ T cells specifically killed Zol-treated HCC
cells, without affecting the Zol-untreated cells.
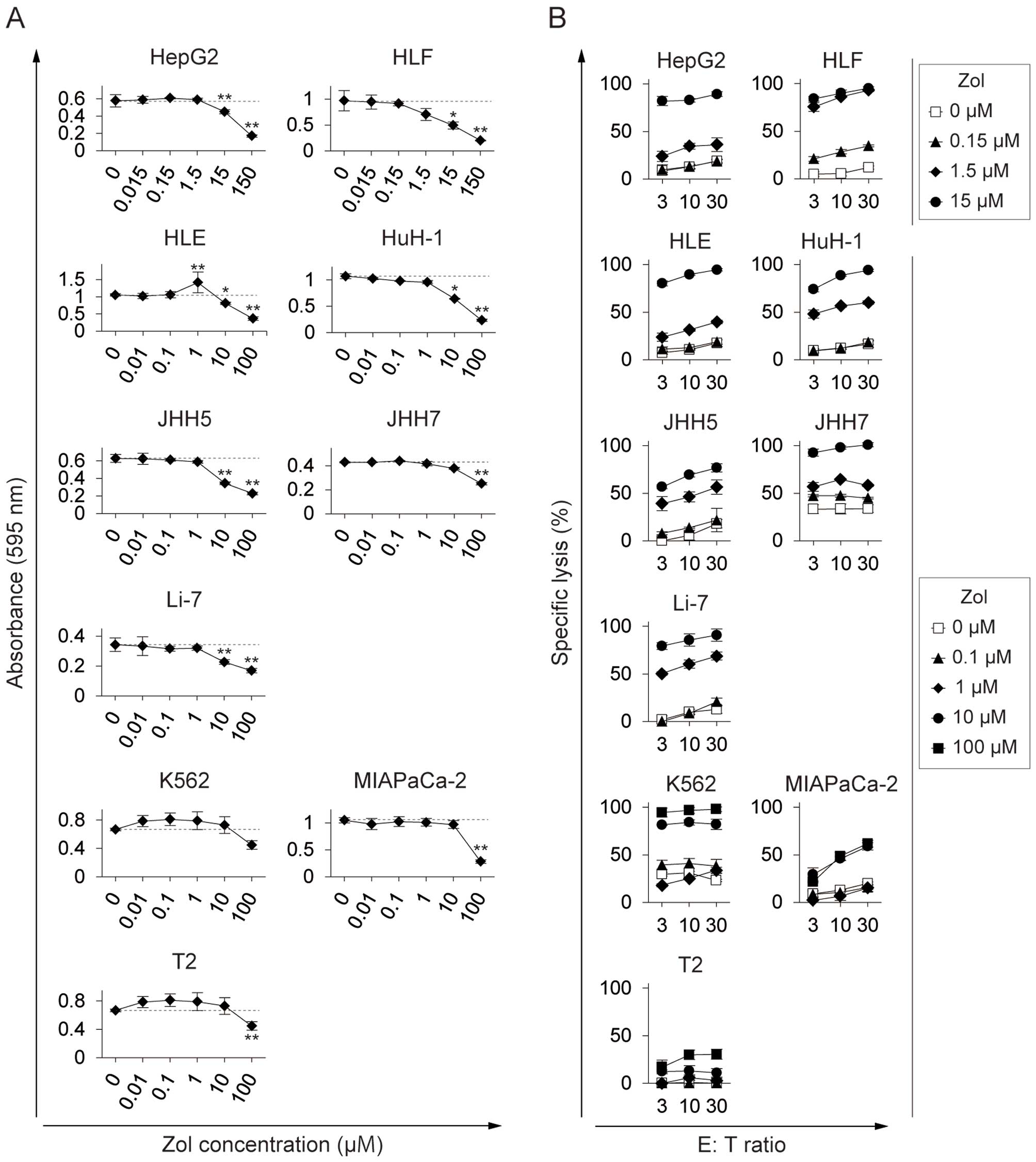
Lower Zol concentration enhances HCC
susceptibility to γδ T cell killing than that required to inhibit
HCC proliferation
Zol exerts direct antitumor effects on HCC cells,
inhibiting cell proliferation, migration, and adhesion (27). We found striking differences
between the concentration of Zol required to sensitize HCC cells to
γδ T cell-mediated killing and that required to directly inhibit
HCC proliferation. Zol consistently inhibited the proliferation of
all HCC cell lines at concentrations >10–15 μM (Fig. 6A). In contrast, Zol consistently
enhanced HCC susceptibility to γδ T cell killing at concentrations
above 1.0–1.5 μM (Fig. 6B). In
addition, the concentration of Zol required for these effects in
HCCs was one or two orders of magnitude lower than those required
in K562, MIAPaCa-2, and T2 cells. This indicated that Zol treatment
may serve to potentiate the effector functions of γδ T cells in
many patients with HCC at a dose below that required to directly
inhibit tumor proliferation.
Discussion
The anti-HCC potential of γδ T cells and the
mechanisms involved in their cytotoxic activity have been well
characterized previously (24,27–30).
However, most of these studies have investigated primary HCC cells
or only investigated a limited number of HCC cell lines, such as
HepG2 and HuH-7. In the present study, we used a variety of HCC
cell lines and found in vitro evidence supporting the roles
of γδ T cells and Zol in immune surveillance against HCCs.
Some HCC cell lines are known to be targets for γδ T
cells, either spontaneously or after Zol treatment (28,29).
HCC cells may show impaired regulation of the mevalonate pathway
and the endogenous mevalonate metabolite, IPP, can be recognized by
γδ TCRs (31). The interaction
between the costimulatory receptor, NKG2D, and its ligands is
considered to play a role in triggering γδ T cell cytotoxicity
(14,20,31).
DNAM-1 has also been implicated in the cytotoxicity of these cells
via a specific interaction with PVR, expressed by HCC cell lines
(12,24). Following Zol treatment, all HCC
cell lines showed enhanced susceptibility to γδ T cell-mediated
killing (Fig. 2B), in the absence
of any significant change in the expression of NKG2D and DNAM-1
ligands (Fig. 3C). It is
conceivable that although NKG2D and DNAM-1 may exert additive
effects on γδ T cell responses, treatment of HCC cells with Zol
further increases intracellular accumulation of IPP, which
generally enhances their susceptibility to γδ T cell-mediated
killing. This indicated that treatment of HCC patients with Zol
could improve the in vivo antitumor efficacy.
Zol was previously reported to directly inhibit HCC
proliferation and migration at concentrations >10 μM in
vitro (27). This was
consistent with the present analysis of a wide variety of HCC cell
lines, where Zol consistently inhibited their proliferation at
concentrations >10–15 μM (Fig.
6A). The Zol concentration required to enhance HCC cell
susceptibility to γδ T cell-mediated killing was on average one
order of magnitude lower than this (Fig. 6B), indicating that γδ T
cell-mediated HCC clearance may occur in vivo at safer doses
than those required to directly inhibit HCC proliferation.
It is noteworthy that the Zol concentration required
for γδ T cell killing in HCC cells was lower than those required in
other cancer cell lines. It is conceivable that Zol-resistant
cancer cells may have a high frequency of FPP synthase somatic
mutations, leading to the reduction of upstream IPP accumulation.
In contrast, Zol-susceptible cancer cells such as HCCs may have
fewer FPP synthase mutations, thereby promoting the accumulation of
IPP. It is also possible that there is a difference in the rate of
Zol metabolism in these cancer cell types. Zol may be rapidly taken
up through fluid-phase endocytosis, particularly in HCCs, resulting
in intracellular Zol levels that are capable of activating γδ T
cells (32). However, further
investigation is required to define the molecular mechanism that
determines susceptibility to γδ T cell-mediated killing.
Our established γδ T cell lines, which were expanded
in the presence of IL-2 and IL-15, produced Th1 and Th2 cytokines,
but not Th17 cytokines (Fig. 2C).
This finding is in agreement with a previous report showing that
IL-2 and IL-15 stimulation of naïve γδ T cells resulted in the
differentiation of producers of IFN-γ, but not IL-17 (33). Recent studies indicated that
IL-17-producing γδ T cells induce tumor-promoting effects by
facilitating angiogenesis in patients with colorectal cancer
(18,34). Although our established γδ T cells
hardly produced any IL-17 in vitro, they may produce IL-17
and behave differently in the presence of high inflammatory
cytokine levels, such as those found in the tumor microenvironment
(35). Therefore, targeting the
inflammatory cytokines responsible for IL-17 production (such as
IL-1β, IL-6, transforming growth factor-β, and IL-23) in
situ may potentiate γδ T cell-based immunotherapy.
γδ T cells showed proliferative responses in the
presence of Zol-sensitized HCC cell lines in vitro (Fig. 2D), implying that they may also
proliferate on encountering Zol-sensitized HCC cells in
vivo. This may be beneficial as it could potentially increase
the number of effector cells in the tumor microenvironment.
There is evidence that Vδ1- or Vδ2-TCR-expressing T
cells in HCC tissues represent a functionally suppressed phenotype
(CD27+CD45−) (30). However, Vδ2-TCR expressing T cells
are present in HCC tissues more predominantly than
Vδ1-TCR-expressing T cells, which implies that Zol treatment of HCC
may activate the Vδ2-TCR-expressing T cells and change them to a
CD27−CD45− effector memory phenotype that
play an important role in the eradication of HCCs.
All of the HCC cell lines used in this study
expressed the DNAM-1 ligands, PVR and Nectin-2, indicating that
many HCC cell lines were susceptible to DNAM-1-mediated killing
(24). T cell immunoreceptor with
Ig and ITIM domains (TIGIT) is an inhibitory molecule that was
recently identified and found to be expressed on natural killer
cells and CTLs; this inhibits DNAM-1 homodimerization and thus
reduces DNAM-1-mediated killing activity (36). A recent clinical trial using
anti-TIGIT and anti-programmed cell death-1 (PD-1) indicated a good
clinical efficacy in melanoma patients (37). We have already identified increased
expression of TIGIT and PD-1 in activated γδ T cells (data not
shown) and co-administration of mAbs targeting these proteins may
enhance the efficacy of γδ T cell-based immunotherapy. Novel
regimens combining these mAbs and Zol are currently under
investigation.
Recent studies have indicated that many
immunologically relevant tumor antigens are neoantigens derived
from point mutation of normal genes, which trigger potent antitumor
immune responses (38). It is
conceivable that the initial killing process triggered by γδ T
cells may disseminate neoantigens that are subsequently taken up
and cross-presented by DCs, inducing further CTL-mediated immune
responses. Moreover, activated γδ T cells acquire a professional
antigen-presenting cell function to express high levels of
costimulatory molecules such as CD80 and CD86, as well as molecules
associated with lymph node homing (39,40).
This function may further boost the generation of a potent and
long-lasting immune response.
In conclusion, many human HCC cells are highly
sensitive to Zol and are most likely to produce phosphoantigens,
such as IPP, which trigger γδ T cell-mediated antitumor responses.
The in vitro data resulting from this study may foster the
development of therapies targeting γδ T cells for the treatment of
HCC patients, which can be optimized by Zol. It will be important
to gather additional therapeutic evidence in vivo using
appropriate animal models.
Acknowledgements
The authors would like to thank Dr Ryo Abe for
helpful discussions. This study was supported in part by the
National Cancer Center Research and Development Fund (25-A-7), the
Ministry of Education, Culture, Sports, Science and Technology of
Japan (MEXT 25861253, 26462078, 26861133, 15H06881), the Practical
Research for Innovative Cancer Control from Japan Agency for
Medical Research and development (AMED 15ck0196002h0103), and the
Program for Development of Innovative Research on Cancer
Therapeutics (P-Direct) from AMED (15cm0106115h0002).
Abbreviations:
|
CTL
|
cytotoxic T lymphocyte
|
|
DNAM-1
|
DNAX accessory molecule-1
|
|
FPP
|
farnesyl pyrophosphate
|
|
GM-CSF
|
granulocyte macrophage
colony-stimulating factor
|
|
GPC3
|
glypican-3
|
|
HCC
|
hepatocellular carcinoma
|
|
IL
|
interleukin
|
|
Ig
|
immunoglobulin
|
|
IFN-γ
|
interferon-γ
|
|
IPP
|
isopentenyl pyrophosphate
|
|
MHC
|
major histocompatibility complex
|
|
MICA/B
|
MHC class I chain-related A/B
|
|
mAb
|
monoclonal antibody
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)
-2,5-diphenyltetrazolium bromide
|
|
NKG2D
|
natural killer group 2D
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
PHA
|
phytohemagglutinin
|
|
PD-1
|
programmed cell death-1
|
|
PVR
|
poliovirus receptor
|
|
TCR
|
T cell receptor
|
|
TIGIT
|
T cell immunoreceptor with Ig and ITIM
domains
|
|
TNF-α
|
tumor necrosis factor-α
|
|
ULBP
|
UL 16-binding protein
|
|
Zol
|
zoledronate
|
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar
|
|
3
|
Kelley RK: Adjuvant sorafenib for liver
cancer: Wrong stage, wrong dose. Lancet Oncol. 16:1279–1281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahoney KM, Rennert PD and Freeman GJ:
Combination cancer immunotherapy and new immunomodulatory targets.
Nat Rev Drug Discov. 14:561–584. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosenberg SA and Restifo NP: Adoptive cell
transfer as personalized immunotherapy for human cancer. Science.
348:62–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayashi E, Motomura Y, Shirakawa H,
Yoshikawa T, Oba N, Nishinakagawa S, Mizuguchi Y, Kojima T, Nomura
K and Nakatsura T: Detection of glypican-3-specific CTLs in chronic
hepatitis and liver cirrhosis. Oncol Rep. 22:149–154. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sawada Y, Yoshikawa T, Nobuoka D,
Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K,
Konishi M, et al: Phase I trial of a glypican-3-derived peptide
vaccine for advanced hepatocellular carcinoma: Immunologic evidence
and potential for improving overall survival. Clin Cancer Res.
18:3686–3696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshikawa T, Takahara M, Tomiyama M, Nieda
M, Maekawa R and Nakatsura T: Large-scale expansion of γδ T cells
and peptide-specific cytotoxic T cells using zoledronate for
adoptive immunotherapy. Int J Oncol. 45:1847–1856. 2014.PubMed/NCBI
|
|
10
|
Bonneville M, O'Brien RL and Born WK:
Gammadelta T cell effector functions: A blend of innate programming
and acquired plasticity. Nat Rev Immunol. 10:467–478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Acker HH, Anguille S, Van Tendeloo VF
and Lion E: Empowering gamma delta T cells with antitumor immunity
by dendritic cell-based immunotherapy. OncoImmunology.
4:e10215382015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gertner-Dardenne J, Castellano R,
Mamessier E, Garbit S, Kochbati E, Etienne A, Charbonnier A,
Collette Y, Vey N and Olive D: Human Vγ9Vδ2 T cells specifically
recognize and kill acute myeloid leukemic blasts. J Immunol.
188:4701–4708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gober HJ, Kistowska M, Angman L, Jenö P,
Mori L and De Libero G: Human T cell receptor gammadelta cells
recognize endogenous mevalonate metabolites in tumor cells. J Exp
Med. 197:163–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong Y, Cao W, Xi X, Ma C, Cui L and He W:
The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces
cytotoxicity to tumor cells through both TCRgammadelta and NKG2D.
Blood. 114:310–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nedellec S, Sabourin C, Bonneville M and
Scotet E: NKG2D costimulates human V gamma 9V delta 2 T cell
antitumor cytotoxicity through protein kinase C theta-dependent
modulation of early TCR-induced calcium and transduction signals. J
Immunol. 185:55–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benzaïd I, Mönkkönen H, Bonnelye E,
Mönkkönen J and Clézardin P: In vivo phosphoantigen levels in
bisphosphonate-treated human breast tumors trigger Vγ9Vδ2 T-cell
antitumor cytotoxicity through ICAM-1 engagement. Clin Cancer Res.
18:6249–6259. 2012. View Article : Google Scholar
|
|
17
|
Benzaïd I, Mönkkönen H, Stresing V,
Bonnelye E, Green J, Mönkkönen J, Touraine JL and Clézardin P: High
phosphoantigen levels in bisphosphonate-treated human breast tumors
promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo.
Cancer Res. 71:4562–4572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rei M, Pennington DJ and Silva-Santos B:
The emerging Protumor role of γδ T lymphocytes: Implications for
cancer immunotherapy. Cancer Res. 75:798–802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fisher JP, Heuijerjans J, Yan M,
Gustafsson K and Anderson J: γδ T cells for cancer immunotherapy: A
systematic review of clinical trials. OncoImmunology. 3:e275722014.
View Article : Google Scholar
|
|
20
|
Corvaisier M, Moreau-Aubry A, Diez E,
Bennouna J, Mosnier JF, Scotet E, Bonneville M and Jotereau F: V
gamma 9V delta 2 T cell response to colon carcinoma cells. J
Immunol. 175:5481–5488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Carlo E, Bocca P, Emionite L, Cilli M,
Cipollone G, Morandi F, Raffaghello L, Pistoia V and Prigione I:
Mechanisms of the antitumor activity of human Vγ9Vδ2 T cells in
combination with zoledronic acid in a preclinical model of
neuroblastoma. Mol Ther. 21:1034–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Idrees AS, Sugie T, Inoue C, Murata-Hirai
K, Okamura H, Morita CT, Minato N, Toi M and Tanaka Y: Comparison
of γδ T cell responses and farnesyl diphosphate synthase inhibition
in tumor cells pretreated with zoledronic acid. Cancer Sci.
104:536–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugie T, Murata-Hirai K, Iwasaki M, Morita
CT, Li W, Okamura H, Minato N, Toi M and Tanaka Y: Zoledronic
acid-induced expansion of γδ T cells from early-stage breast cancer
patients: Effect of IL-18 on helper NK cells. Cancer Immunol
Immunother. 62:677–687. 2013. View Article : Google Scholar :
|
|
24
|
Toutirais O, Cabillic F, Le Friec G, Salot
S, Loyer P, Le Gallo M, Desille M, de La Pintière CT, Daniel P,
Bouet F, et al: DNAX accessory molecule-1 (CD226) promotes human
hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur
J Immunol. 39:1361–1368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deniger DC, Maiti SN, Mi T, Switzer KC,
Ramachandran V, Hurton LV, Ang S, Olivares S, Rabinovich BA, Huls
MH, et al: Activating and propagating polyclonal gamma delta T
cells with broad specificity for malignancies. Clin Cancer Res.
20:5708–5719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Viey E, Fromont G, Escudier B, Morel Y, Da
Rocha S, Chouaib S and Caignard A: Phosphostim-activated gamma
delta T cells kill autologous metastatic renal cell carcinoma. J
Immunol. 174:1338–1347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Honda Y, Takahashi S, Zhang Y, Ono A,
Murakami E, Shi N, Kawaoka T, Miki D, Tsuge M, Hiraga N, et al:
Effects of bisphosphonate zoledronic acid in hepatocellular
carcinoma, depending on mevalonate pathway. J Gastroenterol
Hepatol. 30:619–627. 2015. View Article : Google Scholar
|
|
28
|
Bouet-Toussaint F, Cabillic F, Toutirais
O, Le Gallo M, Thomas de la Pintière C, Daniel P, Genetet N,
Meunier B, Dupont-Bierre E, Boudjema K, et al: Vgamma9Vdelta2 T
cell-mediated recognition of human solid tumors. Potential for
immunotherapy of hepatocellular and colorectal carcinomas. Cancer
Immunol Immunother. 57:531–539. 2008. View Article : Google Scholar
|
|
29
|
Cabillic F, Toutirais O, Lavoué V, de La
Pintière CT, Daniel P, Rioux-Leclerc N, Turlin B, Mönkkönen H,
Mönkkönen J, Boudjema K, et al: Aminobisphosphonate-pretreated
dendritic cells trigger successful Vgamma9Vdelta2 T cell
amplification for immunotherapy in advanced cancer patients. Cancer
Immunol Immunother. 59:1611–1619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yi Y, He HW, Wang JX, Cai XY, Li YW, Zhou
J, Cheng YF, Jin JJ, Fan J and Qiu SJ: The functional impairment of
HCC-infiltrating γδ T cells, partially mediated by regulatory T
cells in a TGFβ- and IL-10-dependent manner. J Hepatol. 58:977–983.
2013. View Article : Google Scholar
|
|
31
|
Li J, Herold MJ, Kimmel B, Müller I,
Rincon-Orozco B, Kunzmann V and Herrmann T: Reduced expression of
the mevalonate pathway enzyme farnesyl pyrophosphate synthase
unveils recognition of tumor cells by Vgamma9Vdelta2 T cells. J
Immunol. 182:8118–8124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thompson K, Rogers MJ, Coxon FP and
Crockett JC: Cytosolic entry of bisphosphonate drugs requires
acidification of vesicles after fluid-phase endocytosis. Mol
Pharmacol. 69:1624–1632. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ribot JC, Ribeiro ST, Correia DV, Sousa AE
and Silva-Santos B: Human γδ thymocytes are functionally immature
and differentiate into cytotoxic type 1 effector T cells upon
IL-2/IL-15 signaling. J Immunol. 192:2237–2243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang
Z, Wang C, Zhang Z, Xia W, et al: γδT17 cells promote the
accumulation and expansion of myeloid-derived suppressor cells in
human colorectal cancer. Immunity. 40:785–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caccamo N, La Mendola C, Orlando V,
Meraviglia S, Todaro M, Stassi G, Sireci G, Fournié JJ and Dieli F:
Differentiation, phenotype, and function of
interleukin-17-producing human Vγ9Vδ2 T cells. Blood. 118:129–138.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnston RJ, Comps-Agrar L, Hackney J, Yu
X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al:
The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T
cell effector function. Cancer Cell. 26:923–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chauvin JM, Pagliano O, Fourcade J, Sun Z,
Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, et al:
TIGIT and PD-1 impair tumor antigen-specific CD8+ T
cells in melanoma patients. J Clin Invest. 125:2046–2058. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brandes M, Willimann K and Moser B:
Professional antigen-presentation function by human gammadelta T
Cells. Science. 309:264–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moser B and Eberl M: γδ T-APCs: A novel
tool for immunotherapy? Cell Mol Life Sci. 68:2443–2452. 2011.
View Article : Google Scholar : PubMed/NCBI
|