Introduction
Osteosarcoma is the most common primary malignant
bone tumor. It occurs mainly in children and adolescents with a
very high tendency for local invasion and early systemic metastases
(1,2). Generally, it is considered an
aggressive malignancy that arises from mesenchymal origin which
exhibits osteoblastic differentiation and produces malignant
osteoid matrix (2). The 10-year
overall survival rate has improved to ~65% attributed to
neoadjuvant chemotherapy and improved surgical techniques which
nevertheless remains essentially unchanged during the past two
decades (3,4). However, 10-year survival rates
continue to be unsatisfactory for patients with metastatic and
recurrent disease, respectively, 25% (5) and <20% (6). Therefore, metastasis is still a
severe challenge in osteosarcoma treatment. It is urgent to develop
novel treatment options for management of osteosarcoma, especially
for control of metastasis and recurrence.
Invasion and metastasis are important biological
characteristics of malignant tumors. In progression of tumor
invasion and metastasis, the degradation of extracellular matrix
(ECM) and the basement membrane, isolation of cells and connective
tissues, seem to be the important steps. The matrix
metalloproteinase proteins (MMPs) are demonstrated to be involved
in degradation of most extracellular matrix. They are overexpressed
in malignancies and are considered to be associated with
metastasis, invasiveness, migration and angiogenesis (7). The gelatinase, MMP-2 and MMP-9, are
the major proteases of all MMPs which contribute to migration and
invasion in osteosarcoma pathogenesis. Furthermore, MMP activities
are regulated by the tissue inhibitors of metalloproteinases
(TIMPs). Imbalance between the expression of MMPs and TIMPs is a
crucial element involved in the remodeling of the ECM exhibited in
the process of cancer invasion and metastasis (8). Therefore, the MMP-2 and MMP-9 are
targets for anticancer drugs.
Malignant bone lesions are very common in patients
with cancer. Excessive osteogenesis and osteolysis concurrence in
osteosarcoma resulting from tumor process. Receptor activator of
NF-κB (RANK) and its ligand (RANKL) play pivotal roles in the
regulation of bone remodelling to maintain bone homeostasis. One of
most important inducement of osteolysis in osteosarcoma is
overexpression of tumor-related RANKL. The over-activated RANKL
stimulates numerous osteoclastogenesis and subsequent bone
destruction and resorption (9).
Osteosaroma usually occurs in long bone cavities. The bone cortex
and periosteum restrain the tumor in cavity mesooecium as a natural
isolation. Along with the tumor progress, tumor-related activation
of MMPs and RANKL degrade the ECM and induce further bone cortex
destruction, and then the tumor cells invade into the surrounding
soft tissues with consequent metastasis.
Sinomenine
(C19H23NO4, Fig. 1A), an alkaloid isolated from the
Chinese medicinal herb, has been successfully utilized to treat
rheumatoid arthritis for centuries (10). Furthermore, sinomenine has
previously been demonstrated to have a wide range of
pharmacological effects, including anti-inflammatory effects,
immunosuppression, anti-angiogenic as well as analgesic effects.
Sinomenine has attracted great attention for its anti-neoplasm
potential. It has been demonstrated to inhibit cell proliferation
and induce apoptosis in a variety of human tumor cells (11–14).
Li et al (15) reported
that sinomenine induces vasculature normalization that contributes
to antitumor and anti-metastasis effect on breast cancer. In
addition, inhibitory effects of sinomenine on cancer invasion and
migration by repressing CD147 activity and subsequently
downregulating the expression of MMP-2, MMP-9 were also revealed
(16). Although this evidence
reveals sinomenine as a potential agent in cancer treatment,
whether sinomenine suppresses the growth of human osteosarcoma has
not been previously investigated.
In this study, we revealed the in vitro and
in vivo anti-proliferative and anti-metastatic effects of
sinomenine on human osteosarcoma cells. We found that sinomenine
effectively inhibited cell proliferation by inducing S phase cell
cycle arrest and colony formation in vitro. However,
sinomenine showed little apoptotic inducement in U2OS
and HOS cells at the dosages tested. Nevertheless, sinomenine
suppressed neovascularization via regulating the related expression
of VEGF and CD147. Additionally, sinomenine inhibited the invasion
and migration by decreasing MMP-2 and MMP-9 secretion and
activation through the CXCR4-STAT3 axis. Importantly, sinomenine
prevented osteoclastogenesis by regulating the tumor-activated
RANKL and reduced bone destruction in a mouse model. This study
suggests sinomenine as a promising anti-metastatic drug in
osteosarcoma adjuvant treatment.
Materials and methods
Cells culture
The human osteosarcoma (OS) cell lines, HOS and
U2OS were purchased form Shanghai Institute of Cell
Biology, Chinese Academy of Sciences (Shanghai, China). The human
umbilical vein endothelial cells (HUVEC) were a gift from Dr Y.Q.
Xie (Clinical Research Center, Second Affiliated Hospital of
Zhejiang University, School of Medicine). The HOS and
U2OS cells were cultivated with high-glucose Dulbecco's
modified Eagle's medium (DMEM) and RPMI-1640 medium, respectively.
Cells were incubated in 5% CO2 humidified incuator
supplemented with 10% fetal bovine serum (FBS) and 1% penicillin
and streptomycin.
Reagents and antibodies
Sinomenine were purchased from Selleck Biochemistry
(USA), and dissolved in PBS at a concentration of 500 mM and stored
at −20°C. The molecular formula of sinomenine is C19H23NO4
(Fig. 1A). Antibodies against
CXCR4, phospho-STAT3, STAT3, MMP-9, MMP-2, GAPDH, TIMP-1, TIMP-2,
CD147, RANKL, NK-κB, and phospho-NK-κB were purchased form Cell
Signaling Technology (USA) and Abcam (UK). VEGF antibody was
purchased from Santa Cruz (USA). DMEM, RPMI-1640 medium, FBS,
penicillin, streptomycin, PBS and 0.25% trypsin were purchased from
Gibco/BRL (USA). CXCR4 inhibitor, Plerixafor (AMD3100), was
purchased form Selleck (USA).
Cell viability assay
To assess the effects of sinomenine on proliferation
of osteosarcoma cells, HOS and U2OS cells were seeded in
96-well plates at 5,000 cells/well and allowed to adhere for 12 h.
Varying concentrations of sinomenine were supplemented to HOS and
U2OS cells, the cells then incubated with 5%
CO2 at 37°C for 24 and 48 h, respectively. The media
were removed and the cells added with 10% CCK-8 (Dojindo, Japan) in
100 μl DMEM or RPMI-1640 for 2 h at 37°C. The absorbance was
measured using a MR7000 microplate reader (Dynatech, NV, USA) at
450 nm. Absorbance is directly proportional to the proliferation of
cells.
Colony formation assay
The clonality of tumor cells is closely related to
tumor recurrence. In order to assess the impact of sinomenine on
the osteosarcoma cell monoclonal ability, clone formation assay was
performed. Five hundred HOS or U2OS cells were seeded in
6-well plates and treated with different concentrations on the
third day. The process was continued for 14 days or until cells had
visible colonies. The cells were fixed in 4% paraformaldehyde for
15 min and washed 2 times with PBS before staining with 0.1%
crystal violet for 10 min. The plates were photographed with ECL
ChemiDoc imaging system (Bio-Rad, USA). The results were assessed
by counting colonies using IPP software (Image Plus Pro).
Wound healing assay
The scratch migration assay is a classical method
for assessing cell migration. HOS and U2OS cells were
cultured in 6-well plates to 80% density. Scratch was made using a
100-μl pipette tip. Media was removed and the cells were treated
with varying concentrations of sinomenine in DMEM or RPMI-1640
without FBS. The width of the denuded area was measured every 6 h
under a microscope. Images were taken. The migration rate was
calculated following the equation: migration rate = (average
original width - average final width) / average original width x
100%.
Gelatin zymography assay
The gelatin zymography assay was used to evaluate
the activities of gelatinase MMP-2 and MMP-9. HOS or
U2OS cells were seeded in 6-well plates at
3×105 per well and cultivated for adherence. The cells
were treated with different concentrations for 48 h and harvested
by trypsinization. RIPA lysis buffer (Boster Biotechnology, Wuhan,
China) was supplied for cell lysis for 30 min and then centrifuged
for 15 min at 4°C. Supernatant was collected to determine the total
protein concentration using BCA Protein Assay kit (Beyotime
Biotechnology, China). Equivalent amounts of protein samples were
mixed with non-denatured loading buffer. Sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) (10%) was performed
with 0.1% gelatin at 80 V, for 2–3 h at 4°C. The gels were removed
and incubated with activation buffer (50 mM Tris-HCl, 5 mM
CaCl2, 1 μM ZnCl2, 0.02% NaN3) at
37°C for 48 h. The gels were stained with 0.05% Coomassie blue
(R-250) for 3 h and destaining in methyl alcohol, acetic acid
destaining solution until clear bands were visible, then images
were taken.
Transwell invasion assay
Transwell invasion assays were performed to assess
osteosarcoma HOS and U2OS cell migration and invasion
ability. The Martrigel (BD, USA) was applied to simulate the
extracellular matrix (ECM), and 10,000 HOS or U2OS cells
were seeded in the Transwell chamber in 200 μl non-FBS medium with
different concentrations of sinomenine and 500 μl 20% corresponding
FBS was placed in the lower 24-well plates. High serum
concentration as a chemotactic factor stimulated OS cells to break
through the Martrigel and to migrate from the chambers to the lower
wells. The penetrated cells adhered on the lower chamber membranes,
and their number were counted under three random high power fields.
Images were taken under a microscope.
ELISA of MMP-2 and MMP-9 secretion
Cells were seeded at 3×105 per 6-well
plate with suitable medium and treated with sinomenine the next
day. The cells underwent treatment for 48 h, then the supernatant
was collected for enzyme-linked immunosorbent assay (ELISA). ELISA
kit for secreted MMP-2 and MMP-9 were purchased from Boster
Institution Biochemistry (Wuhan, China). The assay was performed
following the manufacturer's instructions.
Tube formation assay
Matrigel was implanted in a 96-well plate at 100 μl
per well at 37°C overnight. HUVEC cells (2×104) or
U2OS cells then were seeded in the wells with 100 μl
1.5% FBS low-sugar DMEM or PRMI-1640 medium respectively (4
repeats). Different concentrations of sinomenine were given and the
HUVEC cells were incubated. Images were first taken under a
microscope at 3 h acquiring the tube-like structures formation, and
then every one hour. The total tube length were measured by IPP
software.
Western blot analysis
Cells were lysed in RIPA buffer (Beyotime, Shanghai,
China). Total proteins were separated by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred
to a 0.22-μm PVDF membrane (Millipore, Shanghai, China). After
blocking with 5% non-fat milk for 2 h, the membranes were incubated
overnight at 4°C with antibodies. Horseradish peroxidase
(HRP)-conjugated goat anti-mouse (1:1,500, Thermo Pierce) or goat
anti-rabbit IgG (1:1,500, Thermo Pierce) was applied as secondary
antibody for 1 h at room temperature. The immunoreactive bands were
detected using an enhanced chemiluminescent detection reagent
(Pierce) and exposured by ChemiDoc imaging system (Bio-Rad).
Human osteosarcoma orthotopic
experiment
Four-week-old, female BALB/c-nude mice were
purchased from Shanghai Laboratory Animal Center of Chinese Academy
of Sciences. The HOS cells were transfected luciferase (HOS-Luc)
for imaging in vivo. The tumors were established by
injecting 50 μl PBS containing 10×106 HOS-Luc
resuspension cells into the left tibia marrow cavity using 1 ml
injection through upper tibia tubercle from the knee. Six mice were
not injected as non-tumor negative control. At the 10th day after
HOS-Luc injection, luciferase-imaging was performed to make sure
the tumor tissue located in orthotopic tibia and the total tumor
number reached 10×107. The mice with very low luciferase
signal, or tumor tissues not located in the tibia were sacrificed.
Then the sinomenine treatment was carried out. The mice were
divided into two group randomly (6 mice each group), to receive
intraperitoneal rejection of 200 μl PBS or 150 mg/kg sinomenine.
The treatment lasted for 14 days, the body weight was determined
every 2 days. As the tumor located in the tibia cavity with an
irregular shape, it is difficult to measure the tumor volume.
Luciferase fluorescent signal was detected every week to estimate
the tumor growth. All the mice were sacrificed at day 14 after
treatment. The venous blood was collected from the mouse orbita for
blood biochemical tests. The tumor tissues were dissected and fixed
in formalin.
All the procedures involving clinical specimens were
approved by the Research Ethics Committee of the Second Affiliated
Hospital of Zhejiang University School of Medicine, China.
Immunofluorescent staining for histology
of Ki-67
The Ki-67 potein, as a marker of cell proliferation,
is used in evaluating the tumor differentiation, invasion,
metastasis and prognosis. The Ki-67 antibody was purchased from
Cell Signaling Technology (CST, USA). The dewaxed slices received
antigen retrieval and were blocked by 2% goat fetal serum for 1 h,
and then incubated with Ki-67 antibody for 2 h at room temperature.
The fluorescent secondary antibody (Alexa Fluor® 488,
ZSGB-BIO, Beijing, China) was added and incubated with the slices
for 1 h and the DAPI staining for nucleus location for 5 min. After
two PBS washes and resin mounting, the results were observed under
a fluorescence microscope and images were taken. The images were
merged using IPP software.
TRAP staining
In order to evaluate the tumor-associated osteolysis
in tibia, tartaric acid alkaline phosphatase (TRAP) stain was
performed. In brief, the dewaxed slices were washed twice with PBS,
and then the manufacturer's instructions (Keygen Biotech, Nanjing,
China) were followed. The results were observed under a microscope
after gradient alcohol dehydration and resin mounting. The images
were taken and merged by IPP software.
X-ray for orthotopic human osteosarcoma
in nude mouse tibia
X-ray treatment was implemented to estimate the
tumor-associated osteolysis in tibia. The legs with the orthotopic
human osteosarcoma in tibia cavity were resected from middle of
femur and fixed in formalin. All the samples underwent X-rays.
Tumor histology
Paraffin sections were dewaxed and hematoxylin and
eosin stained. In order to better demonstrate bone destruction by
tumor-associated osteolysis in tibia, fast green staining was
applied.
Statistical analysis
Statistical analysis was performed with SPSS 17.0
software. Statistical significance was determined using two-tailed
Student's t-test when comparing two groups. All experiments were
performed at least in triplicate and the data are presented as mean
± SD with a P-value of ≤0.05 considered as statistically
significant.
Results
Sinomenine shows little cytotoxic effect
on HOS and U2OS cells
In order to assess whether sinomenine had immediate
toxic effect on osteosarcoma cells, the osteosarcoma cell lines HOS
and U2OS were exposed to different concentrations of
sinomenine with 24 and 48 h. The cell viability was evaluated by
CCK-8 assay. Sinomenine showed slight cytotoxicity on both HOS and
U2OS cells (Fig. 1B and
C) at the concentration of 50, 100 and 400 μM, and also 1 mM in
HOS (Fig. 1B). We considered it
might be difficult to achieve blood drug concentration in the
subsequent experiment in vivo. The inhibitory effect of
sinomenine on invasion and migration in malignancy has been
reported (17). So our research
focused on the inhibition of sinomenine in invasion and migration
in osteosarcoma.
Sinomenine induces S phase arrest by
regulating cell cycle regulators
To determine whether sinomenine inhibits cell
proliferation by inducing cell cycle arrest, we detected the cell
cycle distribution in cells treated with sinomenine. The results
showed that sinomenine led to accumulation in S phase in both HOS
and U2OS cells (Fig. 2A and
B). The quantitative analysis indicated significant differences
in S phase (Fig. 2C). To elucidate
the mechanisms, western blot analysis was performed to measure the
expression of cell cycle regulated proteins. Treatment increased
the phospho-Chk2 and it might be caused by sinomenine-induced DNA
damage. The phospho-Chk2 activated the p21, which could be
stimulated by phospho-p53. The activity of Cyclin A-CDK2 complex
was inhibited by upregulated p21 which contributed to promote cell
cycle from S phase into G2/M phase (Fig. 2D and E). The data suggest that
sinomenine induces S phase arrest by altering cell cycle regulation
induced by DNA damage.
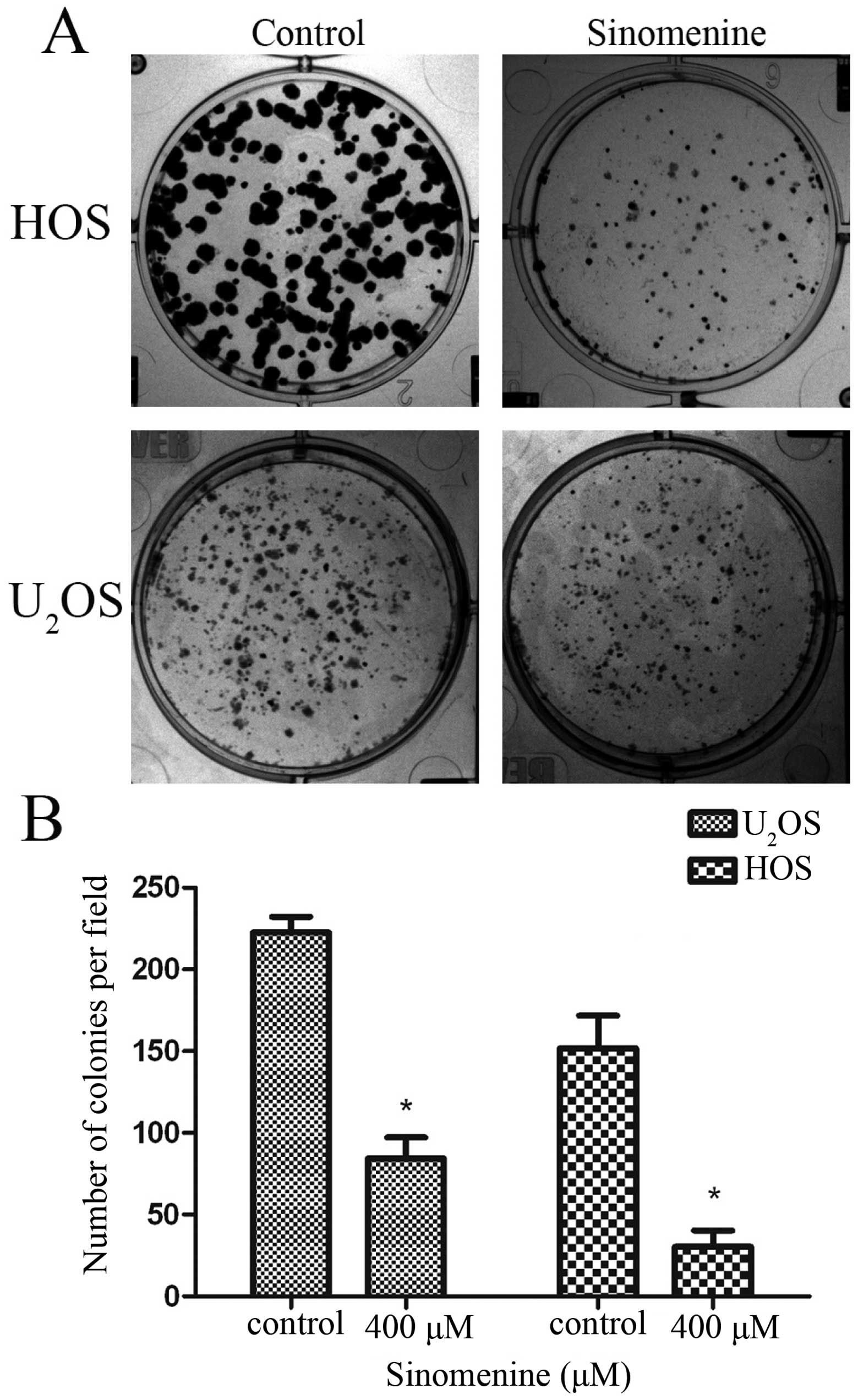
Sinomenine inhibits clone formation in
osteosarcoma cells
The residual tumor cells from chemotherapy or
operation might lead to tumor recurrence depending on the cell
monoclonal ability. The results showed that 400 μM sinomenine
inhibited the colony formation markedly in both HOS and
U2OS cells. There was an obvious decline in clone number
and size (Fig. 3). It suggests
that sinomenine inhibits the monoclonal ability in osteosarcoma
cells.
Sinomenine inhibits invasion and
migration in HOS and U2OS
We investigated the effect of sinomenine on invasion
and migration in HOS and U2OS cells by wound healing and
Transwell Matrigel assays. Treated HOS and U2OS cells
show less migration into the scratched zone (Fig. 4A and B). The migration rate
deceased from 81.13 to 62.62% in HOS and 70.72 to 49.61% in
U2OS cells (Fig. 4C and
D). The Matrigel was used to simulate the ECM in Transwell
invasion assay. Less HOS and U2OS cells digested the
Matrigel to go through the Boyden chamber membrane after 400 μM
sinomenine treatment at 24 h (Fig.
4E). The average number of invading cells reduced from 173 to
38 in HOS and 66 to 16 in U2OS cells per field at 400 μM
(Fig. 4F). Overall, these results
clearly indicate that sinomenine inhibits not only migration, but
also invasion significantly in osteosarcoma.
Anti-vasculogenic activity in
osteosarcoma of sinomenine
Angiogenesis is considered playing a central role in
tumor unrestrained growth and development of metastases (18). We tested the effet of sinomenine on
anti-vasculogenic activity. The sinomenine treated HUVECs (human
umbilical vein endothelial cells) showed lower tube formation
ability than control group dose-dependently (Fig. 5A). The relative tube length was
estimated by IPP software. Fig. 5B
shows relative tube length of treated-group reduced by 60.51%
compared to control group at the concentration of 400 μM.
Previous studies have reported that U2OS
cells have the ability to form tumor tube-like structures under
hypoxic-ischemic conditions. We investigated the inhibiting effect
of sinomenine on tumor tube-like formation in U2OS
cells. The results demonstrated that U2OS cells had the
capacity to form tube-like structures in vitro in Matrigel
and under low FBS conditions, and this can be inhibited by
sinomenine (Fig. 5C and D). The
vascular endothelial growth factor (VEGF) is considered
indispensable in tumor associated neovascularization (19). CD147, also named basigin or
emmprin, is demonstrated to induce angiogenesis via stimulation of
VEGF production. Furthermore, in tumors, CD147 most likely
stimulates matrix metalloproteinase production (20). In order to investigate the
mechanism of anti-neovascularization of sinomenine, western blot
analysis was performed to measure the expression of VEGF and CD147.
A significant decrease was observed after treatment (Fig. 5E–G).
Sinomenine inhibits the expression,
secretion and activity of MMP-2 and MMP-9
Matrix metalloproteinase (MMP) plays a crucial role
in ECM degradation in tumorigenic processes, especially the
gelatinase, MMP-2 and MMP-9. Gelatin zymography was performed to
estimate the enzymatic activity of MMP-2 and MMP-9. Supernates
collected from treated HOS and U2OS cells showed a
narrower band and indicated less gelatinase activities in a
dose-dependent manner (Fig. 6A).
The results of zymography quantitative analysis showed no
significant difference in HOS except at 400 μM concentration of
MMP-2. Whereas, marked decline was observed in U2OS
cells (Fig. 6B and C). ELISA of
supernates for MMP-2/-9 provided similar results, with a better
inhibition in U2OS than HOS cells (Fig. 6D). ELISA was performed to detect
the external secretion of MMP-2 and MMP-9 by osteosarcoma cells.
The secretion of MMP-2 in HOS and U2OS was declined from
242.62 to 148.28 ng/ml and 190.26 to 86.76 ng/ml respectively. On
the contrary, the secretion of MMP-9 in HOS and U2OS
decreased from 220.25 to 123.43 ng/ml and 162.62 to 24.61 ng/ml,
respectively (Fig. 6D). Western
blot analysis was performed to measure the protein experession of
MMP-2 and MMP-9, and, its tissue inhibitor TIMP-1 and TIMP-2
semi-quantitative gray values were calculate by IPP software. The
results showed that the expression of MMP-2 and MMP-9 had
decreased, and TIMP-1 and TIMP-2 increased correspondingly after
sinomenine treatment in both HOS and U2OS cells
(Fig. 6E and F). Overall, these
results clearly demonstrate that sinomenine inhibits not merely the
enzymatic activity of MMP-2 and MMP-9, but also the expression
resulting in reduction of the secretions.
Inhibition of CXCR4 downregulates MMP-2
and MMP-9 expression and suppresses invasion in
U2OS
It has been reported that CXCR4 signaling activates
the STAT3 pathway and contributes to invasion, metastasis and
tumorigenesis in many types of cancers. We obtained primary cells
derived from three patients suffering from osteosarcoma and the
expression of CXCR4 was assessed by western blot analysis. The
expression of CXCR4 was increased in osteosarcoma primary cells
(OS1, OS2 and OS3, Fig. 7A)
compared with hFOB cells (human fetal osteoblast). The expression
of MMP-2 and -9 was downregulated through inhibiting CXCR4 by
plerixafor, and the inhibition could be reversed by CXCR4 activator
SDF-1 (Fig. 7B). The invasion
potential also decreased when we blocked the CXCR4 pathway by its
specific inhibitor plerixafor, and the inhibition could be reversed
by CXCR4 activator SDF-1 (Fig.
7C). Similar results were observed in sinomenine treatment
groups (Fig. 7B–D). The evidence
demonstrated that CXCR4 plays an important role in contributing to
invasion in osteosarcoma by regulating the expression of MMP-2 and
MMP-9. Based on the outcomes between plerixafor and sinomenine
treatment, we speculate that CXCR4 is the core site for sinomenine
therapy.
 | Figure 7CXCR4 is overexpressed in primary
osteosarcoma cells and promotes invasion in U2OS cells.
(A) We obtained primary cells derived from three patients suffering
from osteosarcoma. The splitting of tumor tissues were performed by
western blot analysis. The expression of CXCR4 in primary
osteosarcoma cells were detected. OS1, OS2, OS3, three primary
osteosarcoma cells. (B) Suppression of CXCR4 by plerixafor (CXCR4
inhibitor, 500 ng/ml, 48 h) downregulates the expression of MMP-2
and MMP-9. The downregulation can be reversed by SDF-1 (100 ng/ml,
pre-incubate for 2 h). Sinomenine treatment had similar effect to
plerixafor. The bands were quantitative analyzed by ImageJ
software. *P<0.05, versus control. NS, no
significance. #P<0.05 compare with plerixafor
treatment. (C) Cells were treated with plerixafor and sinomenine,
with or without SDF-1 simultaneously for 24 h. Then cells were
harvested for Transwell assay. Representative images are presented.
(x200 magnification). (D) Quantitative analysis of Transwell assay
is shown in the histograms. *, **P<0.05, versus
control; #P<0.05 versus sinomenine treatment;
##P<0.05 versus plerixafor treatment. NS, no
significance. Experiments were repeated three times. |
Sinomenine inhibits invasion through
CXCR4-STAT3 pathway
Sinomenine treatment inhibited expression of CXCR4
and the STAT3 phosphorylation according to western blot and
immunofluorescence analysis (Fig.
8A–C, H and I) as well as the expression of RANKL and NF-κB
(p65) phosphorylation (Fig. 8A, D and
E). The downregulated expression of CXCR4 by sinomenine was
activated via SDF-1 and subsequently upregulated the expression of
RANKL, VEGF, MMP-2 and MMP-9 (Fig. 8F
and G) which indicated CXCR4 playing a key role in sinomenine
treatment to regulate STAT3 phosphorylation and RANKL, VEGF,
MMP-2/-9 expression downstream. All these studies prove that
sinomenine inhibits invasion and metastasis through suppressing
CXCR4 and STAT3 phosphorylation and then downregulating expression
of MMP-2 and -9, VEGF and RANKL.
 | Figure 8Sinomenine inhibits invasion via
suppressing CXCR4 and phospho-STAT3 pathway, and regulates the
expression of RANKL, phospho-NK-κB. The inhibition can be reversed
by SDF-1 in U2OS. (A–E) Sinomenine downregulates
expression of CXCR4, phospho-STAT3, RANKL and phospho-NK-κB, the
expression of STAT3 and NK-κB were upregulated slightly in
U2OS cells. *P<0.05, compared with
control, NS, no significance. (F and G) The U2OS cells
were incubated with sinomenine and with or without SDF-1 (100
ng/ml, pre-incubate for 2 h before sinomenine) for 48 h, the cells
were harvested for western blot analysis. *P<0.05,
compared with control; #P<0.05, compared with
sinomenine treatment; ##P<0.01, compared with
sinomenine treatment. (H and I) Immunofluorescent staining for
U2OS cells. The 48-h sinomenine treated U2OS
cells were harvested for immunofluorescence. The cell nucleus was
labeled with DAPI (blue), the actin filament was labeled by
actin-tracker (green) for cytomembrane location. The target
proteins were stained using CXCR4 (bar, 200 μm) and p-STAT3 (bar,
20 μm) antibody, and then incubated with fluorescent secondary
antibody (Alexa Fluor 488, red). The images were merged by IPP
software. GAPDH was used as internal control. Experiments were
repeated three times. |
Sinomenine inhibits proliferation and
osteolysis destruction of osteosarcoma in vivo
In vivo effect on osteosarcoma was detected
via intraperitoneal administration of sinomenine in tumor
orthotopic mouse model at 150 mg/kg. The HOS-Luc cells were
injected in left tibia cavity. The tumor samples were harvested by
abscission from middle femoral. H&E staining and fast green
staining was carried out to observe osteolysis destruction of the
mouse tibia. The typical spindle osteosarcoma cells and visible
myxoid degeneration were observed in H&E sections. Osteosarcoma
cells infiltrated into bone cortex and marrow cavity.
Double-nuclear or multi-nuclear osteosarcoma cells infiltrated in
bone cortex inducing osteolysis were viewed under high power field
(Fig. 9C, H&E staining,
control group). The tumor cells invaded periosteum inducing
classical Codman triangle in osteosarcoma (Fig. 9C, H&E staining,
sinomenine-treatment group, high power field). All the H&E
staining results suggested that it was a high-grade malignant
tumor. The bone cortex showed serious destruction in the control
group, but the condition improved in the sinomenine treatment
group. The fast green staining showed the ossein equal to bone
cortex and also indicated improvement to the bone destruction by
sinomenine treatment (Fig. 9C,
fast green staining). TRAP staining was performed to indicate the
tumor associated osteoclastogenesis in bone and tumor tissue. The
number of osteoclast declined significantly after 150
mg/kg-sinomenine treatment (Fig.
9C, TRAP staining). The results of blood biochemistry
examination for ALP and LDH are presented (Fig. 9E).
Discussion
Osteosarcoma is the most common primary malignant
bone tumor. Although the long-term survival reaches 68%, the
prognosis of patients with recurrence and metastasis in
osteosarcoma is poor (6,21). Innovative pharmaceuticals need
further improvement of outcome in osteosarcoma, especially in
metastatic management. Sinomenine, traditional Chinese herbal
medicine, is known because of its anti-inflammatory effect on
arthritis (22–24). Substantial research has reported
that sinomenine has antitumor activity in various malignant tumors.
Li et al reported that sinomenine induces breast cancer cell
death via MAPK signal pathway and reactive oxygen species (ROS)
generation (15). Combined
chemotherapy with senomenine treatment (25–28)
has been revealed to sensitize multidrug-resistant cancer cells in
various cancers. Song et al demonstrated that sinomenine
inhibited invasion and migration in breast cancer by suppressing
NF-κB activation (17).
Furthermore, there are studies suggested that sinome-nine inhibits
metastasis via suppressing vascularization and osteoclast formation
(29,30). Sinomenine has been reported to be
anti-neoplasmic by promoting apoptosis in several cancer lines
(28,29), but we did not find similar results
in our study in osteosarcoma. Nevertheless, significant inhibitory
effects were found on proliferation and metastasis via S phase
arrest and the functions in anti-osteolysis,
anti-neovascularization, and suppression of the secretion and
activation of MMP-2 and MMP-9 through CXCR4-STAT3 pathway in
vitro and in vivo.
Unlike previously reported (13,15),
we found a significant S phase arrest in the cell cycle, using flow
cytometry and protein level western blot analysis. Both the HOS and
U2OS cells were blocked in S phase by
sinomenine-treatment DNA damage (Fig.
2A–C). The checkpoint kinase Chk2 was activated and
phosphorylated for DNA repair which induced downstream
phosphorylation of p53 and activation of p21. The function of
cyclin A-CDK2 complex was suppressed by p21 which contributed to
block cells from S phase into G2 phase (Fig. 2D). We did not assess the cycle
regulator expression in HOS cell line because of its p53
deficiency. We repeated our experiments and speculate the different
results to be due to different cycle regulator expression in the
various malignancies.
CXCR4 is capable of directing the trafficking of
normal and malignant cells to organs that express high levels of
stromal-derived factor-1 (SDF-1), including the lymph nodes, lungs,
liver and bone. CXCR4 involvement in metastasis has been suggested
in a variety of tumors and its expression in the primary site has
been clinically correlated with tumor progression or poor survival
in osteosarcoma. It is generally recognized to be associated with
metastasis and play an essential role in cell migration and
invasion (31–35). Constitutive activation of STAT3 has
been found in a wide variety of human tumors. Aberrant STAT3
signaling promotes initiation and progression of human cancers by
either inhibiting apoptosis or inducing cell proliferation,
angiogenesis, invasion, and metastasis. It has been reported that
CXCR4 signaling activates the JAK2/STAT3 pathway in many types of
cancers (36,37). To elucidate the signaling pathways
underlying sinomenine-mediated responses in osteosarcoma cells, we
further investigated the effects of sinomenine on CXCR4 and STAT3
pathways. In this study, we found over-expression of CXCR4 in human
primary osteosarcoma. In addition, we explored the role of CXCR4
contributing to cell invasion in U2OS cells.
Downregulating the CXCR4 by its specific inhibitor plerixafor
suppressed the expression of MMP-2 and MMP-9 (Fig. 7B) while inhibited the invasion
assessed by Transwell assay (Fig.
7C). The inhibitory effect induced by CXCR4 downregulation
could be blocked by CXCR4 activator SDF-1. Our results were
compared with sinomenine treatment group (Fig. 7B and C). The sinomenine decreased
the expression of CXCR4 and MMP-2 and -9 in U2OS and the
inhibitory effect was reversed by using SDF-1 in the treatment
group. There results suggested the essential role of CXCR4 in
contributing to invasion and as acting site in sinomenine treatment
in osteosarcoma cells. The western blot analysis and
immunofluorescence staining confirmed our viewpoint (Fig. 8A and I). The sinomenine treatment
indeed suppressed the expression of CXCR4 and its downstream STAT3
phosphorylation and the MMP-2 and MMP-9. Further study revealed
that SDF-1 activated sinomenine-inhibited CXCR4, which had a
positive correlation to RANKL, VEGF and MMP-2 and -9 expression and
in Transwell assay. In addition, our results showed that sinomenine
treatment inhibited tube formation ability of HUVECs. The capacity
of U2OS cells to form tube-like structures was also
surppressed by sinomenine. Thus, strong evidence was provided that
sinomenine inhibited invasion and metastasis mainly through
CXCR4-STAT3 pathway and then regulated the expression of MMP-2,
MMP-9, RANKL and VEGF downstream in osteosarcoma.
Osteoclasts are multinucleated cells of
hematopoietic origin and are responsible for the degradation of
mineralized bone matrix. RANKL is a key cytokine for osteoclast
differentiation, survival and function (38). Activation of the NF-κB pathway is a
key factor in RANKL-induced osteoclast differentiation. Blocking
RANKL and its associated signaling cascades is a vital step for
successful treatment of some osteoclast-related diseases. Our
results showed that sinomenine inhibited the expression of RANKL
and phospho-NF-κB. In addition, an orthotopic mouse model of
osteosarcoma was used to investigate the treatment effects of
sinomenine in vivo. In X-ray examination, bone destruction
improved in sinomenine treated group comparing with the control
group (Fig. 9B). H&E staining
and fast green staining also displayed that bone cortices were
destructed more seriously in the control group (Fig. 9C). TRAP staining was performed to
examine the tumor-associated osteoclastogenesis in bone and tumor
tissue, showing that the number of osteoclasts declined
significantly after sinomenine treatment. The in vivo
results further confirmed sinomenine as a potent suppressor of
osteoclast formation and bone destruction and resorption.
The osteogenic marker ALP is highly expressed inside
normal bone and osteosarcoma cells. The serum ALP level increases
with bone destruction because the ALP is released into serum from
bone cells. Therefore, generally the high level of ALP is
considered to be associated with osteolysis and disease progress
which indicates a poor prognosis in clinic. Thus, ALP is adopted as
clinically meaningful marker in diagnosis and predicting prognosis
in osteosarcoma (39,40). Serum LDH are also known to reflect
the tumor burden and associated with poor prognosis (41). Decrease of ALP or LDH levels may be
a symptom of a positive reaction to treatment. In our study, the
levels of serum ALP increased significantly in tumor group compared
with tumor-free group, and decreased markedly after sinomenine
treatment, which reflect positive treatment effects of sinomenine.
However, in evaluation of serum LDH, we did not observe this
tendency. Contrarily, the LDH level increased in the treatment
group. This might be due to the hepatic impairment induced by
sinomenine. Combined with TRAP, H&E, and X-ray examination, we
have reason to believe that sinomenine-treatment restraints bone
destruction and osteolysis in osteosarcoma through inhibiting
RANKL-NF-κB expression and tumor-associated osteoclast activation.
The immunohistochemistry confirmed that sinomenine downregulated
the RANKL and VEGF expression by inhibiting CXCR4 in
vivo.
There are still deficiencies in our experiment. We
only verified the CXCR4-STAT3 pathway in U2OS cells, but
not in HOS cells considering its P53 gene loss and atypical signal
regulation in vitro. It might be better to complete it in
HOS cells. The orthotopic tumor histology sections were made in
tibia transection not in shaft position. It most probably missed
the typical osteolysis area in tibia cortex. It would be better to
make the sections in tibia axle. We found the mouse blood
biochemistry of LDH increased after sinomenine treatment. We
speculated it might be induced by drug hepatotoxicity. We did not
harvest the mice liver for further examination, and we did not meet
the inhibitory effect on tumor growth in osteosarcoma through
sinomenine treatment. Tumor cells seeded in marrow cavity also
developed in tibia around soft tissues in treatment group even if
under low bone destruction and osteolysis condition. It indicates a
complicated progress in osteosarcoma development, the bone cortex
is destroyed not only by tumor-associated osteoclastogenesis, but
also direct aggression by osteosarcoma cells. There might be a
better outcome if higher doses were given or sinomenine combined
with other agents in osteosarcoma therapy.
In conclusion, this study is the first to
demonstrate that sinomenine can effectively inhibit the
proliferation by inducing S phase arrest, and suppresses metastasis
in osteosarcoma via downregulating CXCR4-STAT3 signal pathway and
then restraining the RANKL-mediated bone destruction stimulated by
osteoclastogenesis and VEGF-related neovascularization in
osteosarcoma. The above results suggest that sinomenine may be a
promising adjuvant agent for metastatic control in
osteosarcoma.
Acknowledgements
This study was supported in part by grants from the
National Natural Science Foundation of China (no. 81172547). We
thank Dr Y.-Q. Xie (Department of Clinical Research Centre,
Zhejiang, China) for the gift of HUVEC.
References
|
1
|
Quaye AA, Raskin KA, Ecker JL and Leffert
LR: Management of a parturient with high-grade osteosarcoma of the
proximal femur: A multidisciplinary approach. Int J Obstet Anesth.
19:340–342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raymond AK and Jaffe N: Osteosarcoma
multidisciplinary approach to the management from the pathologist's
perspective. Cancer Treat Res. 152:63–84. 2009. View Article : Google Scholar
|
|
3
|
Weiss A, Gill J, Goldberg J, Lagmay J,
Spraker-Perlman H, Venkatramani R and Reed D: Advances in therapy
for pediatric sarcomas. Curr Oncol Rep. 16:3952014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudawara I, Aoki Y, Ueda T, Araki N, Naka
N, Nakanishi H, Matsumine A, Ieguchi M, Mori S, Myoui A, et al:
Neoadjuvant and adjuvant chemotherapy with high-dose ifosfamide,
doxorubicin, cisplatin and high-dose methotrexate in non-metastatic
osteosarcoma of the extremities: A phase II trial in Japan. J
Chemother. 25:41–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kager L, Zoubek A, Pötschger U, Kastner U,
Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M,
Winkelmann W, et al; Cooperative German-Austrian-Swiss Osteosarcoma
Study Group. Primary metastatic osteosarcoma: Presentation and
outcome of patients treated on neoadjuvant Cooperative Osteosarcoma
Study Group protocols. J Clin Oncol. 21:2011–2018. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kempf-Bielack B, Bielack SS, Jürgens H,
Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G,
Kabisch H, et al: Osteosarcoma relapse after combined modality
therapy: An analysis of unselected patients in the Cooperative
Osteosarcoma Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Birkedal-Hansen H, Moore WG, Bodden MK,
Windsor LJ, Birkedal-Hansen B, DeCarlo A and Engler JA: Matrix
metalloproteinases: A review. Crit Rev Oral Biol Med. 4:197–250.
1993.PubMed/NCBI
|
|
8
|
Nabeshima K, Iwasaki H, Koga K, Hojo H,
Suzumiya J and Kikuchi M: Emmprin (basigin/CD147): Matrix
metalloproteinase modulator and multifunctional cell recognition
molecule that plays a critical role in cancer progression. Pathol
Int. 56:359–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clézardin P: The role of
RANK/RANKL/osteoprotegerin (OPG) triad in cancer-induced bone
diseases: Physiopathology and clinical implications. Bull Cancer.
98:837–846. 2011.In French.
|
|
10
|
Yamasaki H: Pharmacology of sinomenine, an
anti-rheumatic alkaloid from Sinomenium acutum. Acta Med Okayama.
30:1–20. 1976.PubMed/NCBI
|
|
11
|
Zhou L, Luan H, Liu Q, Jiang T, Liang H,
Dong X and Shang H: Activation of PI3K/Akt and ERK signaling
pathways antagonized sinomenine-induced lung cancer cell apoptosis.
Mol Med Rep. 5:1256–1260. 2012.PubMed/NCBI
|
|
12
|
Li XJ, Yue PY, Ha WY, Wong DY, Tin MM,
Wang PX, Wong RN and Liu L: Effect of sinomenine on gene expression
of the IL-1 beta-activated human synovial sarcoma. Life Sci.
79:665–673. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu XL, Zeng J, Chen YL, He PM, Wen MX, Ren
MD, Hu YN, Lu GF and He S: Sinomenine hydrochloride inhibits human
hepatocellular carcinoma cell growth in vitro and in vivo:
Involvement of cell cycle arrest and apoptosis induction. Int J
Oncol. 42:229–238. 2013.
|
|
14
|
Lv Y, Li C, Li S and Hao Z: Sinomenine
inhibits proliferation of SGC-7901 gastric adenocarcinoma cells via
suppression of cyclooxygenase-2 expression. Oncol Lett. 2:741–745.
2011.
|
|
15
|
Li X, Wang K, Ren Y, Zhang L, Tang XJ,
Zhang HM, Zhao CQ, Liu PJ, Zhang JM and He JJ: MAPK signaling
mediates sinomenine hydrochloride-induced human breast cancer cell
death via both reactive oxygen species-dependent and -independent
pathways: An in vitro and in vivo study. Cell Death Dis.
5:e13562014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ou YQ, Chen LH, Li XJ, Lin ZB and Li WD:
Sinomenine influences capacity for invasion and migration in
activated human monocytic THP-1 cells by inhibiting the expression
of MMP-2, MMP-9, and CD147. Acta Pharmacol Sin. 30:435–441. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song L, Liu D, Zhao Y, He J, Kang H, Dai
Z, Wang X, Zhang S and Zan Y: Sinomenine inhibits breast cancer
cell invasion and migration by suppressing NF-κB activation
mediated by IL-4/ miR-324-5p/CUEDC2 axis. Biochem Biophys Res
Commun. 464:705–710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Q, Zhu Y, Deng Z, Long H, Zhang S and
Chen X: VEGF and EMMPRIN expression correlates with survival of
patients with osteosarcoma. Surg Oncol. 20:13–19. 2011. View Article : Google Scholar
|
|
19
|
Benayoun Y, Petellat F, Leclerc O, et al:
Current treatments for corneal neovascularization. J Fr Ophtalmol.
38:996–1008. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan L, Zucker S and Toole BP: Roles of the
multifunctional glycoprotein, emmprin (basigin; CD147), in tumour
progression. Thromb Haemost. 93:199–204. 2005.PubMed/NCBI
|
|
21
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W,
Gebhardt M, Goorin AM, et al: Osteosarcoma: A randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J
Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen DP, Wong CK, Leung PC, Fung KP, Lau
CB, Lau CP, Li EK, Tam LS and Lam CW: Anti-inflammatory activities
of Chinese herbal medicine sinomenine and Liang Miao San on tumor
necrosis factor-α-activated human fibroblast-like synoviocytes in
rheumatoid arthritis. J Ethnopharmacol. 137:457–468. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qian L, Xu Z, Zhang W, Wilson B, Hong JS
and Flood PM: Sinomenine, a natural dextrorotatory morphinan
analog, is anti-inflammatory and neuroprotective through inhibition
of microglial NADPH oxidase. J Neuroinflammation. 4:232007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Q, Luo J, Zhu Q, Li Y and Yin S:
Synthesis and anti-inflammatory activities investigation of
sinomenine derivatives on ring C. Nat Prod Res. 20:1015–1023. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z, Duan ZJ, Chang JY, Zhang ZF, Chu R,
Li YL, Dai KH, Mo GQ and Chang QY: Sinomenine sensitizes
multidrug-resistant colon cancer cells (Caco-2) to doxorubicin by
downregulation of MDR-1 expression. PLoS One. 9:e985602014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao F, Yang Z, Lu X, Guo X and Dong W:
Sinomenine sensitizes gastric cancer cells to 5-fluorouracil in
vitro and in vivo. Oncol Lett. 6:1604–1610. 2013.PubMed/NCBI
|
|
27
|
Chen Y, Zhang L, Lu X, Wu K, Zeng J, Gao
Y, Shi Q, Wang X, Chang LS and He D: Sinomenine reverses multidrug
resistance in bladder cancer cells via P-glycoprotein-dependent and
independent manners. Pharmazie. 69:48–54. 2014.PubMed/NCBI
|
|
28
|
Zhang JX, Yang ZR, Wu DD, Song J, Guo XF,
Wang J and Dong WG: Suppressive effect of sinomenine combined with
5-fluorouracil on colon carcinoma cell growth. Asian Pac J Cancer
Prev. 15:6737–6743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Ren Y, Tang X, Wang K, Liu Y,
Zhang L, Li X, Liu P, Zhao C and He J: Vascular normalization
induced by sinomenine hydrochloride results in suppressed mammary
tumor growth and metastasis. Sci Rep. 5:88882015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, He L, Hu Y, Duan H, Li X, Tan S, Zou
M, Gu C, Zeng X, Yu L, et al: Sinomenine suppresses osteoclast
formation and Mycobacterium tuberculosis H37Ra-induced bone loss by
modulating RANKL signaling pathways. PLoS One. 8:e742742013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruffini PA, Morandi P, Cabioglu N,
Altundag K and Cristofanilli M: Manipulating the
chemokine-chemokine receptor network to treat cancer. Cancer.
109:2392–2404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Micucci C, Matacchione G, Valli D, Orciari
S and Catalano A: HIF2α is involved in the expansion of
CXCR4-positive cancer stem-like cells in renal cell carcinoma. Br J
Cancer. 113:1178–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu JY, Shi HF, Gao XL, Ma QQ and Zhang B:
Effect of CXCR4 pretreated with ultrasound-exposed microbubbles on
accelerating homing of bone marrow mesenchymal stem cells to
ischemic myocardium in AMI rats. Asian Pac J Trop Med. 8:766–771.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sand LG, Scotlandi K, Berghuis D,
Snaar-Jagalska BE, Picci P, Schmidt T, Szuhai K and Hogendoorn PC:
CXCL14, CXCR7 expression and CXCR4 splice variant ratio associate
with survival and metastases in Ewing sarcoma patients. Eur J
Cancer. 51:2624–2633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han AR, Lee JY, Kim HJ, Min WS, Park G and
Kim SH: A CXCR4 antagonist leads to tumor suppression by activation
of immune cells in a leukemia-induced microenvironment. Oncol Rep.
34:2880–2888. 2015.PubMed/NCBI
|
|
36
|
Liu X, Xiao Q, Bai X, Yu Z, Sun M, Zhao H,
Mi X, Wang E, Yao W, Jin F, et al: Activation of STAT3 is involved
in malignancy mediated by CXCL12-CXCR4 signaling in human breast
cancer. Oncol Rep. 32:2760–2768. 2014.PubMed/NCBI
|
|
37
|
Shen HB, Gu ZQ, Jian K and Qi J:
CXCR4-mediated Stat3 activation is essential for CXCL12-induced
cell invasion in bladder cancer. Tumour Biol. 34:1839–1845. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanada R, Hanada T, Sigl V, Schramek D and
Penninger JM: RANKL/RANK - beyond bones. J Mol Med (Berl).
89:647–656. 2011. View Article : Google Scholar
|
|
39
|
Li C, Shi X, Zhou G, Liu X, Wu S and Zhao
J: The canonical Wnt-beta-catenin pathway in development and
chemotherapy of osteosarcoma. Front Biosci (Landmark Ed).
18:1384–1391. 2013. View
Article : Google Scholar
|
|
40
|
Moore AS, Dernell WS, Ogilvie GK, Kristal
O, Elmslie R, Kitchell B, Susaneck S, Rosenthal R, Klein MK,
Obradovich J, et al: Doxorubicin and BAY 12-9566 for the treatment
of osteosarcoma in dogs: A randomized, double-blind,
placebo-controlled study. J Vet Intern Med. 21:783–790. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Durnali A, Alkis N, Cangur S, Yukruk FA,
Inal A, Tokluoglu S, Seker MM, Bal O, Akman T, Inanc M, et al:
Prognostic factors for teenage and adult patients with high-grade
osteosarcoma: An analysis of 240 patients. Med Oncol. 30:6242013.
View Article : Google Scholar : PubMed/NCBI
|