Introduction
Lung cancer is the most common cancer with high
incidence and mortality worldwide. Approximately 80% of all lung
cancers are non-small cell lung carcinomas (NSCLC) and
adenocarcinoma is the most common type (1). Despite increased survival rate via
improved diagnosis and treatment of NSCLC, there are still many
issues to be resolved including resistance, rapid disease
recurrence and progression (2–4).
Therefore, new therapeutic strategies to overcome resistance and
improve effective treatment of NSCLC patients are urgently
required.
The endoplasmic reticulum (ER) participates in
various cellular processes, such as protein folding, lipid
biosynthesis and calcium storage. Among them precise control of
protein folding is a fundamental function of ER to maintain
cellular homeostasis (5,6). When misfolded proteins or unfolded
proteins are generated during metabolic processes or from various
stresses, polypeptide binding protein (BiP or GRP78, sensor) is
dissociated from three major ER membrane resident proteins
(mediator), inositol-requiring kinase 1 (IRE1), double-strand
RNA-activated protein kinase-like ER kinase (PERK) and activating
transcription factor 6 (ATF6) leading to activation of the unfolded
protein response (UPR) signal (6–9).
Dimerization of PERK by UPR activation increases the
phosphorylation on Ser 51 of eIF2 leading to attenuation of the
global translation initiation (10). On the contrary, eIF2
phosphorylation selectively increases the translation of
ATF4 mRNA encoding transcriptional activator of genes
involved in the UPR. One of the ATF4 target genes is CCAAT/
enhancer-binding protein homologues protein (CHOP), which is
another preferential translation target of eIF2 phosphorylation.
Increased CHOP during ER stress and UPR activation induces
expression of apoptosis-related genes including death receptors DR4
and/or DR5 (11,12). For these reasons, various types of
anticancer drugs to modulate proteins involved in the ER stress
response have been developed (13).
To develop novel cancer therapeutics to overcome
drug resistance, multi-targeting strategies has been recently
explored (14). Especially,
combination therapy targeting multiple anti-apoptotic proteins
and/or pro-apoptotic proteins has been considered effective
strategy to overcome TRAIL resistance in most types of cancer
including NSCLC and increase the anticancer capacity in treatment
of cancers (15). Various
compounds isolated from herbal plants have been studied for
combination therapy with TRAIL or conventional anticancer drugs
(16–20). Particularly, Tanshinone IIA
isolated from the dried root of Salvia miltiorrhiza Bunge
(Lamiaceae) is one of well-documented natural compounds in
terms of combination effects as well as its own anticancer
mechanism (21–25). Tanshinone IIA has shown cytotoxic
effects in several types of human cancer cells in vitro and
in vivo (26,27).
A recent study revealed that Tanshinone IIA induces
apoptosis through inhibition of JAK/STAT3 signaling in myeloid
leukemia cells (28). In the
present study, we focused on anti-tumor effects of Tanshinone IIA
to sensitize TRAIL-mediated apoptosis in human NSCLC cells through
modulation of ER stress response and apoptosis signals.
Materials and methods
Cell culture and reagents.
NSCLC cell lines were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). A549, H596,
H1299, Calu-1 and H460 cell lines were grown in RPMI-1640 medium
(HyClone Laboratories, Logan, UT, USA) containing 10%
heat-inactivated fetal bovine serum (FBS) and 1% antibiotics
(HyClone Laboratories) in a humidified atmosphere of 95% air and 5%
CO2 at 37°C, and the viability of cultured cells was
monitored by a LUNA-FLautomated cell counter (Logos Biosystems,
Anyang, Gyeonggi-do, Republic of Korea). Tanshinone IIA (M.W.=328,
T4952, ≥97% -HPLC) was purchased from Sigma Aldrich (St. Louis, MO,
USA).
MTT assay
Cytotoxicity of Tanshinone IIA, TRAIL or combination
with Tanshinone IIA and TRAIL was evaluated by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma Chemical) assay in NSCLCs. Cells were seeded in a
96-well-microplate at a density of 1×104 cells/ well and
treated with various concentrations of Tanshinone IIA. After the
indicated time, the medium was removed, and fresh medium containing
1 mg/ml MTT was added to each well. The cells were incubated at
37°C for 2 h and then an equal volume of MTT lysis buffer was added
to each well. The cells were incubated at 37°C overnight. Optical
density (OD) was measured using a microplate reader (Tecan Austria
GmbH, Grödig, Austria) at 570 nm.
Western blotting
Whole cell lysates were prepared, and western
blotting was performed as previously described (28). In brief, total proteins from A549,
H596, H1299 and Calu-1 cells were extracted with RIPA buffer (50 mM
Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% deoxycholic acid-Na,
1 mM EDTA, 1 mM Na3VO4, 1 mM NaF) including
protease inhibitor cocktail (Roche). Primary antibodies for CHOP,
DR5, ATF4, GRP78, calnexin, PERK, PDI, IRE-1α (Cell Signaling
Technology, Beverly, MA, USA), DR4, PARP, cleaved caspase-3,
caspase-3, survivin (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), anti-β-actin (Sigma-Aldrich) and secondary antibodies coupled
to horseradish peroxidase (HRP) from Vector Laboratories Inc.
(Burlingame, CA, USA) were used for immunoblotting. Expression was
visualized by using enhanced chemiluminescence (ECL) Western
blotting detection reagent (Amersham Pharmacia, Piscataway, NJ,
USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the NSCLC cells with the
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. Total RNA (2 μg) isolated from cells
was reverse transcribed to cDNA using oligo-dT and random primers.
Reverse transcription was done using a thermal program of 50°C for
60 min and 85°C for 5 min. The cDNA was amplified by PCR using the
following specific primers:DR4(forward,5′-GGCTGAGGACAATGCTCACA-3′
and reverse, 5′-TTGCTGCTCAGAGACGAAAGTG′); DR5 (forward,
5′-GACTCTGAGACAGTGCTTCGATGA-3′ and reverse,
5′-CCATGAGGCCCAACTTCCT-3′); CHOP (forward,
5′-CAACTGCAGAGAATTCAGCTGA-3′ and reverse, 5′-AC
TGATGCTCTAGATTGTTCAT-3′); GAPDH (forward, 5′-CC
ACTCCTCCACCTTTGAC-3′ and reverse, 5′-ACCCTGTTG CTGTAGCCA-3′); XBP-1
(forward, 5′-CTGGAACAGCAA GTGGTAGA-3′ and reverse,
5′-CTGGGTCCTTCTGGGTA GAC-3′). All primers were synthesized by
Bioneer, Co. (Daejeon, Republic of Korea).
Apoptosis assay
NSCLCs were seeded in 6-well plates, treated with
indicated compounds for 24 h and stained with Annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI) kit (BioVision
Technologies Inc., Golden, CO, USA). The cells were then analyzed
using fluorescence-activated cell sorting FACSCalibur flow
cytometry.
siRNA transfection
Transfection of control or DR5 siRNA (Bioneer) was
performed using transfection reagent INTERFERin
(Polyplus-transfection, New York, NY, USA) according to the
manufacturer's protocol.
Validation of synergy between tanshinone
IIA and TRAIL
To determine the synergy between Tanshinone IIA and
TRAIL, cytotoxicity assay was performed in four NSCLS cell lines by
the gradual increase with a same constant ratio between Tanshinone
IIA and TRAIL. The values from viability assays were analyzed by
CalcuSyn software (Biosoft, Ferguson, MO, USA). The fraction of
living cells in each concentration was applied for the analysis of
synergism between Tanshinone IIA and TRAIL by CalcuSyn
software.
Statistical analysis
Data are presented as mean ± standard deviation (SD)
from at least three independent experiments in triplicate and
analyzed for statistical significance using the unpaired Student's
t-test. P<0.05 was considered statistically significant.
Results
Combination treatment of Tanshinone IIA
and TRAIL synergistically increases the cytotoxicity in
TRAIL-resistant NSCLCs
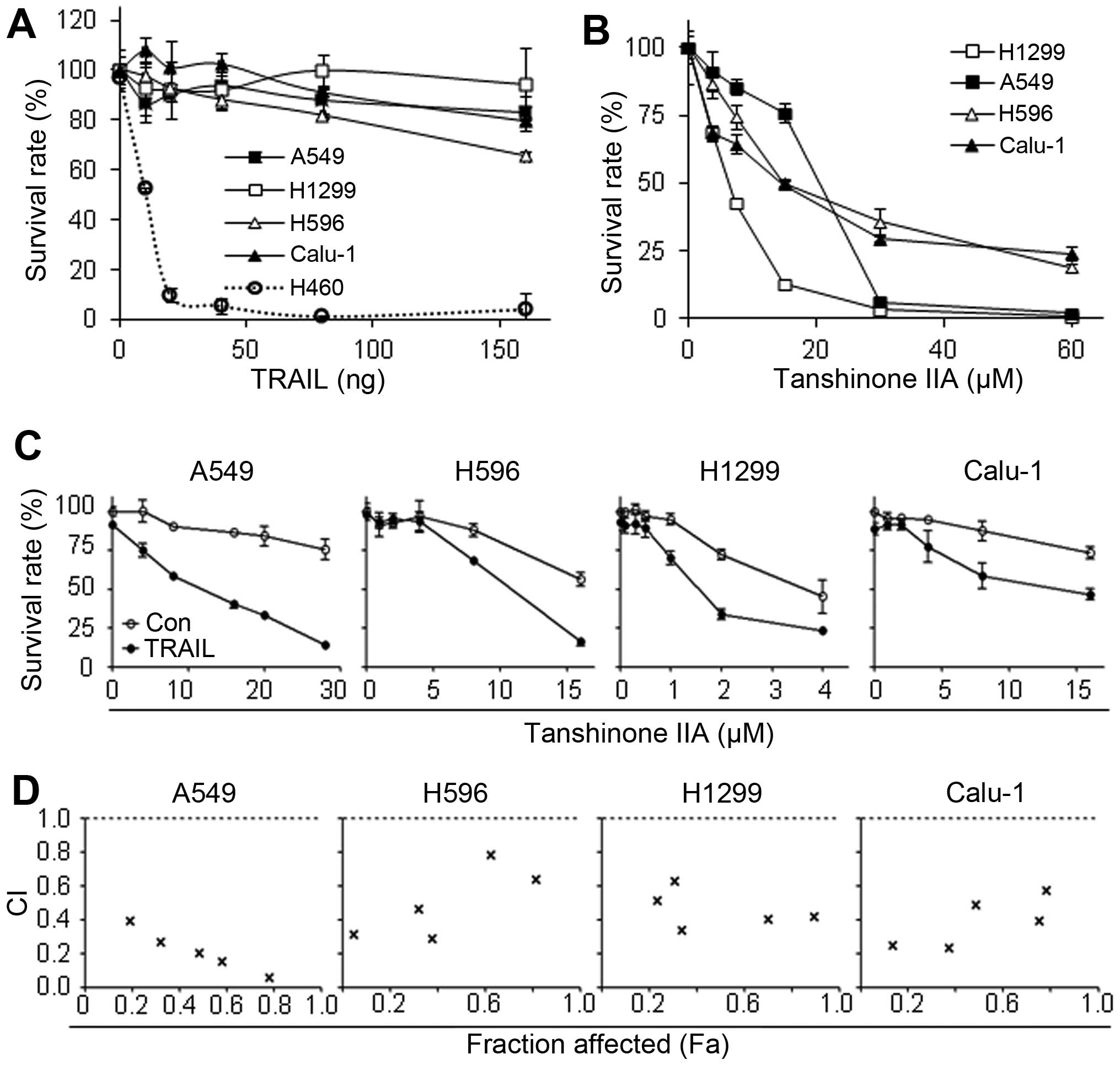
In order to investigate the possible therapeutic
effect of Tanshinone IIA and TRAIL in NSCLC, we first examined
whether single treatment of Tanshinone IIA or TRAIL affects
viability of human NSCLC cells. Consistent with previous reports
(29,30), A549, H596, H1299 and Calu-1 showed
high resistance to TRAIL, while H460 was very sensitive to TRAIL
with ~12 ng/ml IC50 (Fig.
1A). Tanshinone IIA was cytotoxic in all the tested NSCLC cell
lines with IC50 values of ~10–30 μM (Fig. 1B). Furthermore, in evaluation of
combination effect of Tanshinone IIA and TRAIL, co-treatment
synergistically decreased cell viability (Fig. 1C). Surprisingly, the combination
index (CI) values below 0.8 at all fractions affected (Fa) points,
suggesting that the combination treatment showed strong synergy in
all four tested TRAIL resistant NSCLC cell lines (Fig. 1D).
Combination treatment effectively induces
apoptosis in TRAIL-resistant NSCLCs
Canonical cell death signals through TRAIL receptors
trigger extrinsic apoptosis which occurred by extracellular death
signals (31). To verify whether
synergistic effect of Tanshinone IIA on cytotoxicity is mediated by
apoptotic cell death, we analyzed apoptosis in Tanshinone IIA and
TRAIL co-treated NSCLC cells. As shown in Fig. 2A, Tanshinone IIA/TRAIL combination
treatment intensively upregulated cleaved-caspase-3, -8 and
cleaved-PARP levels, and even combination with half dose of each
single treatment showed more increased cleaved forms indicating
induction of apoptosis. Consistently, Annexin V and PI double
staining revealed that apoptotic population was markedly increased
in co-treated NSCLC cells compared to control or single treated
A549 and H1299 cells (Fig. 2B).
These data indicate that Tanshinone IIA may be a novel TRAIL
sensitizer in NSCLC cells.
Tanshinone IIA-mediated cell death is
through DR5 induction, but not DR4 in TRAIL-resistant NSCLCs
Cancer cells usually acquire TRAIL resistance via
downregulation of DR4 and/or DR5 (32,33).
To confirm if Tanshinone IIA overcomes the TRAIL resistance by
modulation of death receptors, we evaluated the expression of the
proteins. Tanshinone IIA treatment significantly increased DR5, but
not DR4 in A549, H596 and H1299 cells (Fig. 3A). Since CHOP is one of major
transcriptional activators of DR5 (34,35),
we next examined the effect of Tanshinone IIA on the expression of
CHOP. As shown in Fig. 3B and C,
Tanshinone IIA transcriptionally induced CHOP and also DR5 in a
dose-dependent manner. In order to confirm the role of DR5 in the
Tanshinone IIA-mediated TRAIL sensitization of NSCLC cells, we
depleted DR5 with specific siRNA (Fig.
3D). As shown in Fig. 3E,
inhibition of DR5 restored the TRAIL and Tanshinone IIA-mediated
decrease of cell viability. These results indicated that Tanshinone
IIA significantly increased expression of DR5, leading to increase
of TRAIL sensitivity in human NSCLC cells.
Tanshinone IIA selectively induces the
PERK/ATF4 pathway of ER stress response signals causing induction
of DR5
Since CHOP is transcriptionally induced by ER stress
response signals (36), we then
examined whether Tanshinone IIA can regulate signaling molecules
involved in ER stress response in NSCLCs. As shown in Fig. 4A, Tanshinone IIA significantly
induced GRP78 which is an ER stress sensor protein. Tanshinone IIA
also activated the PERK/ATF4 signal which is one of three ER stress
inducers, but the other two pathways, ATF6 and IRE-1α signals, were
not affected at all (Fig. 4A). In
addition, Tanshinone IIA did not induce the splicing of XBP1, while
treatment of a well-known ER stress inducer thapsigargin (TG)
greatly increased the spliced form of XBP1 mRNA (Fig. 4B). Furthermore, Tanshinone IIA did
not affect the expression of other ER resident chaperons
contributing protein folding (Fig.
4A). These data indicate that Tanshi-none IIA increases DR5 and
CHOP by selective activation of the PERK/ATF4 signal.
Tanshinone IIA suppresses STAT3 and its
downstream target survivin
Because tumor cells with TRAIL resistance usually
possess increased levels of anti-apoptotic proteins (37,38),
these proteins have been regarded major targets for development of
cancer therapeutics. As shown in Fig.
5, Tanshinone IIA decreased phosphorylation of STAT3, which is
known to induce anti-apoptotic proteins including survivin, while
total STAT3 expression was not changed at all. Furthermore,
Tanshinone IIA suppressed survivin in a dose-dependent manner
(Fig. 5). However, other
anti-apoptotic proteins Bcl-xL, Bcl-2 and Mcl-1 were not affected
by Tanshinone IIA (Fig. 5A). These
results suggest that Tanshinone IIA inhibits the STAT3 pathway
leading to suppression of survivin in human NSCLC cells.
Discussion
Even though activation of the TRAIL receptor pathway
is a promising therapeutic strategy to selectively remove cancer
cells, most cancers including NSCLC have various ways to evade the
TRAIL-mediated cell death. Therefore, combination treatment
strategies to overcome TRAIL resistance have been intensively
studied. Particularly, herbal compounds targeting death receptors
are considered effective interventions to increase the TRAIL
sensitivity in tumor cells. Recent studies have proven that several
natural compounds such as cycloanthranilylproline,
cryptotanshinone, kurarinone, curcumin and rocaglamide increased
the TRAIL sensitivity in various types of cancer resistant to TRAIL
(39–43).
In the present study, we evaluated whether another
herbal compound, tanshinone IIA can overcome the TRAIL resistance
in human NSCLC cells. Consistent with previous reports (44,45),
tanshinone IIA significantly increased DR5 through CHOP induction
(Fig. 3). Furthermore, we newly
revealed that Tanshinone IIA selectively activated the PERK/ATF4
pathway (Fig. 4). These results
suggest that Tanshinone IIA induces DR5 by increasing CHOP through
selective activation of the PERK/ATF4 pathway, one of the ER stress
response signals. Reactive oxygen species (ROS), chemically
reactive molecules, play roles in regulating cell signaling and
homeostasis (46). ROS is
generated during oxygen usage in all cellular compartments, such as
the mitochondrial respiratory chain and ER (47) suggesting ROS as one of ER stress
inducers. Based on our previous report, decursin has also similar
functions, such as induction of selective ER stress via ROS
generation and TRAIL sensitivity in lung cancer cells (18). Recent report indicated that
Tanshi-none IIA induces considerable amount of ROS formation in
A549, but not H596 cells, which lack the NAD(P)H:quinone
oxidoreductase (NQO1) (48).
Consistently, we also detected ER stress response signals in
Tanshinone IIA-treated A549 cells, but not in H596 (data not
shown), indicating that ER stress induction by Tanshinone IIA may
be mediated by ROS generation. However, more detailed molecular
mechanisms how Tanshinone IIA induces synergistic cytotoxic effect
with TRAIL in H596 cells should be further elucidated.
Because most tumors are resistant to TRAIL,
targeting the main factors associated with the TRAIL resistance is
a common strategy to effectively increase the TRAIL sensitivity in
treating cancer. For example, inhibition of cell survival factors
including survivin and/or induction of anti-apoptotic factors have
been used for overcoming the TRAIL resistance (49–55).
STAT3 is a well-known transcription factor regulating genes
encoding proteins involved in cell survival including survivin and
Bcl-2. Major function of STAT3 is to convey signals from the cell
surface to the nucleus on activation by cytokines and growth
factors (56,57). Phosphorylation of STAT3 by
activation of receptor tyrosine kinases (RTKs), such as EGFR, leads
to its dimerization and then translocation to the nucleus. Numerous
studies have proved that hyperphosphorylated STAT3 are widely
detected in various types of human tumor specimens and is required
for malignant transformation of cultured cells (58–62).
In this regard, diverse novel STAT3 inhibitors have been developed
(60,63). Tanshinone IIA significantly
downregulated phosphorylation of STAT3, probably leading to
suppression of survivin in human NSCLC cells (Fig. 5).
Taken together, these results clearly demonstrate
that Tanshinone IIA selectively induces the ER stress,
PERK/ATF4/CHOP pathway leading to significant induction of DR5 and
reduces survivin via inactivating STAT3. Furthermore, combination
treatment of Tanshinone IIA and TRAIL showed synergistic induction
of apoptotic cell death in human NSCLC cells resistant to TRAIL
(Fig. 5B), suggesting that
Tanshinone IIA is a promising TRAIL sensitizer in NSCLCs and that
combination of Tanshinone IIA with TRAIL would be a good
therapeutic strategy for treating NSCLCs.
Acknowledgements
The present study was supported by the research
grants (NRF-2014R1A1A2057918, NRF-2014R1A1A2056230 and
NRF-2015R1A4A1042399) from the National Research Foundation of
Korea.
Abbreviations:
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
NSCLC
|
non-small cell lung cancer
|
|
TR
|
TRAIL-resistant
|
|
DR5
|
death receptor 5
|
|
DR4
|
death receptor 4
|
|
CHOP
|
CCAAT/enhancer-binding protein
homologues protein
|
|
ER
|
endoplasmic reticulum
|
|
PERK
|
pancreatic ER kinase
|
|
ATF4
|
activating transcription factor 4
|
|
ROS
|
reactive oxygen species
|
|
UPR
|
unfolded protein response
|
|
IRE1
|
inositol-requiring kinase 1
|
|
BiP
|
polypeptide binding protein
|
|
eIF2
|
eukaryotic initiation factor 2
|
References
|
1
|
Mitsudomi T: Advances in target therapy
for lung cancer. Jpn J Clin Oncol. 40:101–106. 2010. View Article : Google Scholar
|
|
2
|
Sun JM, Choi YL, Ji JH, Ahn JS, Kim KM,
Han J, Ahn MJ and Park K: Small-cell lung cancer detection in
never-smokers: Clinical characteristics and multigene mutation
profiling using targeted next-generation sequencing. Ann Oncol.
26:161–166. 2015. View Article : Google Scholar
|
|
3
|
Xu C, Zhou Q and Wu YL: Can EGFR-TKIs be
used in first line treatment for advanced non-small cell lung
cancer based on selection according to clinical factors? - A
literature-based meta-analysis. J Hematol Oncol. 5:622012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng DJ, Yu GH, Gao JF and Gu JD:
Concomitant EGFR inhibitors combined with radiation for treatment
of non-small cell lung carcinoma. Asian Pac J Cancer Prev.
14:4485–4494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schubert U, Antón LC, Gibbs J, Norbury CC,
Yewdell JW and Bennink JR: Rapid degradation of a large fraction of
newly synthesized proteins by proteasomes. Nature. 404:770–774.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yewdell JW and Nicchitta CV: The DRiP
hypothesis decennial: Support, controversy, refinement and
extension. Trends Immunol. 27:368–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schröder M and Kaufman RJ: ER stress and
the unfolded protein response. Mutat Res. 569:29–63. 2005.
View Article : Google Scholar
|
|
8
|
van Anken E and Braakman I: Endoplasmic
reticulum stress and the making of a professional secretory cell.
Crit Rev Biochem Mol Biol. 40:269–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van der Vlies D, Makkinje M, Jansens A,
Braakman I, Verkleij AJ, Wirtz KW and Post JA: Oxidation of ER
resident proteins upon oxidative stress: Effects of altering
cellular redox/antioxidant status and implications for protein
maturation. Antioxid Redox Signal. 5:381–387. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi Y, Vattem KM, Sood R, An J, Liang J,
Stramm L and Wek RC: Identification and characterization of
pancreatic eukaryotic initiation factor 2 alpha-subunit kinase,
PEK, involved in translational control. Mol Cell Biol.
18:7499–7509. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wek RC and Cavener DR: Translational
control and the unfolded protein response. Antioxid Redox Signal.
9:2357–2371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L, Su L and Liu X: PKCδ regulates death
receptor 5 expression induced by PS-341 through ATF4-ATF3/CHOP axis
in human lung cancer cells. Mol Cancer Ther. 11:2174–2182. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yadav RK, Chae SW, Kim HR and Chae HJ:
Endoplasmic reticulum stress and cancer. J Cancer Prev. 19:75–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yap TA, Omlin A and de Bono JS:
Development of therapeutic combinations targeting major cancer
signaling pathways. J Clin Oncol. 31:1592–1605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang S: The promise of cancer therapeutics
targeting the TNF-related apoptosis-inducing ligand and TRAIL
receptor pathway. Oncogene. 27:6207–6215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frew AJ, Lindemann RK, Martin BP, Clarke
CJ, Sharkey J, Anthony DA, Banks KM, Haynes NM, Gangatirkar P,
Stanley K, et al: Combination therapy of established cancer using a
histone deacetylase inhibitor and a TRAIL receptor agonist. Proc
Natl Acad Sci USA. 105:11317–11322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hellwig CT and Rehm M: TRAIL signaling and
synergy mechanisms used in TRAIL-based combination therapies. Mol
Cancer Ther. 11:3–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim J, Yun M, Kim EO, Jung DB, Won G, Kim
B, Jung JH and Kim SH: Generation of ROS by Decursin selectively
induces the ER stress pathway components ATF4/PERK leading to the
synergistic enhancement of TRAIL-induced apoptosis. Br J Pharmacol.
Dec 11–2015. View Article : Google Scholar : Epub ahead of
print.
|
|
19
|
Matsuzaki H, Schmied BM, Ulrich A, Standop
J, Schneider MB, Batra SK, Picha KS and Pour PM: Combination of
tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and
actinomycin D induces apoptosis even in TRAIL-resistant human
pancreatic cancer cells. Clin Cancer Res. 7:407–414.
2001.PubMed/NCBI
|
|
20
|
Park D, Ha IJ, Park SY, Choi M, Lim SL,
Kim SH, Lee JH, Ahn KS, Yun M and Lee SG: Morusin induces TRAIL
sensitization by regulating EGFR and DR5 in human glioblastoma
cells. J Nat Prod. Feb 1–2016.Epub ahead of print. View Article : Google Scholar
|
|
21
|
Chiu SC, Huang SY, Chang SF, Chen SP, Chen
CC, Lin TH, Liu HH, Tsai TH, Lee SS, Pang CY, et al: Potential
therapeutic roles of tanshinone IIA in human bladder cancer cells.
Int J Mol Sci. 15:15622–15637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
23
|
Jiao JW and Wen F: Tanshinone IIA acts via
p38 MAPK to induce apoptosis and the down-regulation of ERCC1 and
lung-resistance protein in cisplatin-resistant ovarian cancer
cells. Oncol Rep. 25:781–788. 2011.
|
|
24
|
Su CC and Lin YH: Tanshinone IIA inhibits
human breast cancer cells through increased Bax to Bcl-xL ratios.
Int J Mol Med. 22:357–361. 2008.PubMed/NCBI
|
|
25
|
Tseng PY, Lu WC, Hsieh MJ, Chien SY and
Chen MK: Tanshinone IIA induces apoptosis in human oral cancer KB
cells through a mitochondria-dependent pathway. BioMed Res Int.
2014:5405162014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeon YJ, Kim JS, Hwang GH, Wu Z, Han HJ,
Park SH, Chang W, Kim LK, Lee YM, Liu KH, et al: Inhibition of
cytochrome P450 2J2 by tanshinone IIA induces apoptotic cell death
in hepatocellular carcinoma HepG2 cells. Eur J Pharmacol.
764:480–488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Munagala R, Aqil F, Jeyabalan J and Gupta
RC: Tanshinone IIA inhibits viral oncogene expression leading to
apoptosis and inhibition of cervical cancer. Cancer Lett.
356:536–546. 2015. View Article : Google Scholar
|
|
28
|
Jung JH, Kwon TR, Jeong SJ, Kim EO, Sohn
EJ, Yun M and Kim SH: Apoptosis induced by Tanshinone IIA and
crypto-tanshinone is mediated by distinct JAK/STAT3/5 and SHP1/2
signaling in chronic myeloid leukemia K562 cells. Evid Based
Complement Alternat Med. 2013:8056392013. View Article : Google Scholar
|
|
29
|
Jin CY, Moon DO, Lee JD, Heo MS, Choi YH,
Lee CM, Park YM and Kim GY: Sulforaphane sensitizes tumor necrosis
factor-related apoptosis-inducing ligand-mediated apoptosis through
downregulation of ERK and Akt in lung adenocarcinoma A549 cells.
Carcinogenesis. 28:1058–1066. 2007. View Article : Google Scholar
|
|
30
|
La Monica S, Galetti M, Alfieri RR,
Cavazzoni A, Ardizzoni A, Tiseo M, Capelletti M, Goldoni M,
Tagliaferri S, Mutti A, et al: Everolimus restores gefitinib
sensitivity in resistant non-small cell lung cancer cell lines.
Biochem Pharmacol. 78:460–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wallach D, Varfolomeev EE, Malinin NL,
Goltsev YV, Kovalenko AV and Boldin MP: Tumor necrosis factor
receptor and Fas signaling mechanisms. Annu Rev Immunol.
17:331–367. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ozören N, Fisher MJ, Kim K, Liu CX, Genin
A, Shifman Y, Dicker DT, Spinner NB, Lisitsyn NA and El-Deiry WS:
Homozygous deletion of the death receptor DR4 gene in a
naso-pharyngeal cancer cell line is associated with TRAIL
resistance. Int J Oncol. 16:917–925. 2000.
|
|
33
|
Wang S and El-Deiry WS: Inducible
silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor
xenograft growth and confers resistance to chemotherapeutic agent
5-fluorouracil. Cancer Res. 64:6666–6672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JY, Jung KH, Morgan MJ, Kang YR, Lee
HS, Koo GB, Hong SS, Kwon SW and Kim YS: Sensitization of
TRAIL-induced cell death by 20(S)-ginsenoside Rg3 via CHOP-mediated
DR5 upregulation in human hepatocellular carcinoma cells. Mol
Cancer Ther. 12:274–285. 2013. View Article : Google Scholar
|
|
35
|
Oh YT, Liu X, Yue P, Kang S, Chen J,
Taunton J, Khuri FR and Sun SY: ERK/ribosomal S6 kinase (RSK)
signaling positively regulates death receptor 5 expression through
co-activation of CHOP and Elk1. J Biol Chem. 285:41310–41319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaguchi H and Wang HG: CHOP is involved
in endoplasmic reticulum stress-induced apoptosis by enhancing DR5
expression in human carcinoma cells. J Biol Chem. 279:45495–45502.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ackler S, Mitten MJ, Chen J, Clarin J,
Foster K, Jin S, Phillips DC, Schlessinger S, Wang B, Leverson JD,
et al: Navitoclax (ABT-263) and bendamustine ± rituximab induce
enhanced killing of non-Hodgkin's lymphoma tumours in vivo. Br J
Pharmacol. 167:881–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang G, Zhan Y, Wang H and Li W: ABT-263
sensitizes TRAIL-resistant hepatocarcinoma cells by downregulating
the Bcl-2 family of anti-apoptotic protein. Cancer Chemother
Pharmacol. 69:799–805. 2012. View Article : Google Scholar
|
|
39
|
Bleumink M, Köhler R, Giaisi M, Proksch P,
Krammer PH and Li-Weber M: Rocaglamide breaks TRAIL resistance in
HTLV-1-associated adult T-cell leukemia/lymphoma by translational
suppression of c-FLIP expression. Cell Death Differ. 18:362–370.
2011. View Article : Google Scholar :
|
|
40
|
Hasegawa H, Yamada Y, Komiyama K, Hayashi
M, Ishibashi M, Sunazuka T, Izuhara T, Sugahara K, Tsuruda K,
Masuda M, et al: A novel natural compound, a
cycloanthranilylproline derivative (Fuligocandin B), sensitizes
leukemia cells to apoptosis induced by tumor necrosis factor
related apoptosis-inducing ligand (TRAIL) through
15-deoxy-Delta12,14 prostaglandin J2
production. Blood. 110:1664–1674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park S, Cho DH, Andera L, Suh N and Kim I:
Curcumin enhances TRAIL-induced apoptosis of breast cancer cells by
regulating apoptosis-related proteins. Mol Cell Biochem. 383:39–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Seo OW, Kim JH, Lee KS, Lee KS, Kim JH,
Won MH, Ha KS, Kwon YG and Kim YM: Kurarinone promotes
TRAIL-induced apoptosis by inhibiting NF-κB-dependent cFLIP
expression in HeLa cells. Exp Mol Med. 44:653–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tse AK, Chow KY, Cao HH, Cheng CY, Kwan
HY, Yu H, Zhu GY, Wu YC, Fong WF and Yu ZL: The herbal compound
cryptotanshinone restores sensitivity in cancer cells that are
resistant to the tumor necrosis factor-related apoptosis-inducing
ligand. J Biol Chem. 288:29923–29933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang CC, Kuan CP, Lin JY, Lai JS and Ho
TF: Tanshinone IIA facilitates TRAIL sensitization by up-regulating
DR5 through the ROS-JNK-CHOP signaling Axis in human ovarian
carcinoma cell lines. Chem Res Toxicol. 28:1574–1583. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin JY, Ke YM, Lai JS and Ho TF:
Tanshinone IIA enhances the effects of TRAIL by downregulating
survivin in human ovarian carcinoma cells. Phytomedicine.
22:929–938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Devasagayam TP, Tilak JC, Boloor KK, Sane
KS, Ghaskadbi SS and Lele RD: Free radicals and antioxidants in
human health: Current status and future prospects. J Assoc
Physicians India. 52:794–804. 2004.
|
|
47
|
Stadtman ER: Importance of individuality
in oxidative stress and aging. Free Radic Biol Med. 33:597–604.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Y, Zhu J and Zhang W: Antitumor
effect of traditional Chinese herbal medicines against lung cancer.
Anticancer Drugs. 25:983–991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gillissen B, Wendt J, Richter A, Richter
A, Müer A, Overkamp T, Gebhardt N, Preissner R, Belka C, Dörken B,
et al: Endogenous Bak inhibitors Mcl-1 and Bcl-xL: Differential
impact on TRAIL resistance in Bax-deficient carcinoma. J Cell Biol.
188:851–862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Johnson TR, Stone K, Nikrad M, Yeh T, Zong
WX, Thompson CB, Nesterov A and Kraft AS: The proteasome inhibitor
PS-341 overcomes TRAIL resistance in Bax and caspase 9-negative or
Bcl-xL overexpressing cells. Oncogene. 22:4953–4963. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lemke J, von Karstedt S, Abd El Hay M,
Conti A, Arce F, Montinaro A, Papenfuss K, El-Bahrawy MA and
Walczak H: Selective CDK9 inhibition overcomes TRAIL resistance by
concomitant suppression of cFlip and Mcl-1. Cell Death Differ.
21:491–502. 2014. View Article : Google Scholar :
|
|
52
|
Lirdprapamongkol K, Sakurai H, Abdelhamed
S, Yokoyama S, Athikomkulchai S, Viriyaroj A, Awale S, Ruchirawat
S, Svasti J and Saiki I: Chrysin overcomes TRAIL resistance of
cancer cells through Mcl-1 downregulation by inhibiting STAT3
phosphorylation. Int J Oncol. 43:329–337. 2013.PubMed/NCBI
|
|
53
|
Siegelin MD, Gaiser T, Habel A and
Siegelin Y: Daidzein overcomes TRAIL-resistance in malignant glioma
cells by modulating the expression of the intrinsic apoptotic
inhibitor, bcl-2. Neurosci Lett. 454:223–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA,
Lim JH, Kwon TK and Choi KS: Monensin, a polyether ionophore
antibiotic, overcomes TRAIL resistance in glioma cells via
endoplasmic reticulum stress, DR5 upregulation and c-FLIP
downregulation. Carcinogenesis. 34:1918–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zang F, Wei X, Leng X, Yu M and Sun B:
C-FLIP(L) contributes to TRAIL resistance in HER2-positive breast
cancer. Biochem Biophys Res Commun. 450:267–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Buettner R, Mora LB and Jove R: Activated
STAT signaling in human tumors provides novel molecular targets for
therapeutic intervention. Clin Cancer Res. 8:945–954.
2002.PubMed/NCBI
|
|
61
|
Song JI and Grandis JR: STAT signaling in
head and neck cancer. Oncogene. 19:2489–2495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|