Introduction
Gallbladder cancer (GBC) is a rare but highly
aggressive malignancy. The lack of severe symptoms makes the
diagnosis very difficult (1). Even
though there are some therapies such as cholecystectomy or radical
resection, chemotherapy, or radiotherapy (2,3),
they are not as effective as expected. The 5-year survival rate is
extremely low (4). So far there is
no systemic therapy with a satisfactory outcomes. Thus, studying
novel signal molecules involved in GBC margin and metastasis may
provide new effective therapeutic strategies.
Leptin, the product of the OB gene, is a 16 kDa
non-glycosylated peptide hormone which is synthesized almost
exclusively by adipocytes that regulates appetite and energy
expenditure at the hypothalamic level (5,6). In
recent years, accumulating evidence suggests that leptin plays an
important role in tumorigenesis, angiogenesis and metastasis of
many cancers, including breast (7), pancreatic (8), and stomach cancer (9). Previous studies have shown that
leptin could activate Janus kinase 2 (JAK2) when leptin was bound
to one form of the receptor, OB-Rb. Then JAK2 initiated downstream
signaling including members of the signal transducers and
activators of transcription (STAT) family of transcription factors
(10). However, the expression of
leptin and OB-Rb in GBCs has not been fully investigated, and the
precise role of leptin in the development and promotion of GBC
remains unknown.
In this study, we investigated the clinical
implications of leptin and OB-Rb in GBC patients. Moreover, we
explored the role of leptin and one form of its receptor OB-Rb in
GBC cells through in vitro and in vivo studies.
SOCS3/JAK2/p-STAT3 signaling pathways were also assessed and these
pathways may be involved in cell migration and metastasis by
leptin.
Materials and methods
Immunohistochemistry and evaluation
Forty paraffin-embedded specimens of normal
gallbladder tissues and 40 specimens of gallbladder cancer (GBC)
tissues were collected from January 1, 2005 to June 30, 2010 at
Department of Hepatopancreatobiliary Surgery, the Second Affiliated
Hospital of Kunming Medical University, Kunming, China. No patients
had received chemotherapy or radiotherapy before biopsy. The prior
patient's consents and approval from the Institutional Research
Ethics Committee were obtained. Rabbit anti-human polyclonal leptin
and OB-Rb antibody (Sigma, St. Louis, MO, USA) was used for
immunohistochemistry assay, which was performed following the
protocol of Universal SP kit (MXB Biotechnology, Fujian, China).
Positive staining of leptin protein is brown in the cytoplasm,
partly in the nucleus, and positive staining of OB-Rb protein is
brown in the cytomembrane. The human GBC tissue sections were
blindly examined and scored concurrently by two observers. The
intensity of the immunostaining was scored as 0 (negative), 1
(weak), 2 (moderate), or 3 (strong). Four visual fields were
selected randomly under high power lens (x400). The number of
positive cells was counted and the average positive rate was
calculated. The percentage of positive tumor cells was scored as
‘+’ (<25%), ‘++’ (26–50%), ‘+++’ (51–75%), or ‘++++’, (76–100%),
and that without any positive cells scored as ‘−’.
Cell culture
The human GBC cell sublines (GBC-SD) were obtained
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium/α-modified Eagle medium (DMEM) (Gibco Life
Technology, Gaithersburg, MD, USA) supplemented with 10% fetal
bovine serum (FBS) (Gibco Life Technology) and maintained at 37°C
in a humidified atmosphere of 5% CO2.
shRNA synthesis and transfection
Four different template oligonucleotides targeting
OB-Rb (Table II) were synthesized
by Ribobio Inc. (Guangzhou, Guangdong, China), and were annealed
and ligated into pGPH1/GFP/Neo plasmid. The shRNAs inserted vectors
were named as pGPH-GFP-s1, pGPH-GFP-s2, pGPH-GFP-s3, pGPH-GFP-s4
and pGPH-GFP-NC, respectively.
 | Table IIUni- and multivariate analyses of
factors associated with survival. |
Table II
Uni- and multivariate analyses of
factors associated with survival.
| Factor | OS | DFS |
|---|
|
|
|---|
| Univariate,
P-value | Multivariate | Univariate,
P-value | Multivariate |
|---|
|
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male vs.
female) | 0.117 | | | NA | 0.328 | | | NA |
| Age, years (≥60 vs.
<60) | 0.056 | | | NA | 0.070 | | | NA |
| BMI,
kg/m2 (≥30 vs. <30) | 0.584 | | | NA | 0.326 | | | NA |
| CA199, U/ml (≥35
vs. <35) | 0.002 | 3.532 | 1.485–13.406 | 0.012 | 0.004 | 3.876 | 1.514–12.711 | 0.027 |
| T classification
(T1 vs. T2) | 0.091 | | | NA | 0.242 | | | NA |
| N classification
(N0 vs. N1) | 0.093 | | | NA | 0.167 | | | NA |
| Tumor
differentiation (I/II vs. III/IV) | 0.782 | | | NA | 0.765 | | | NA |
| AJCC (I vs.
II) | 0.575 | | | NA | 0.648 | | | NA |
| Leptin expression
(low vs. high) | 0.028 | 2.271 | 1.865–18.615 | 0.001 | 0.044 | 2.874 | 1.196–19.682 | 0.003 |
| OB-Rb expression
(low vs. high) | 0.016 | 3.461 | 2.043–18.292 | 0.001 | 0.020 | 2.931 | 1.970–11.438 | 0.001 |
Transfection of shRNAs was performed using
Lipofectamine 2000 reagent (Invitrogen Co., Carlsbad, CA) according
to the manufacturer's instructions. Briefly, GBC-SD cells were
applied for OB-Rb silence, which were cultured in 6-well plates at
a density of 5×104 cells/well. Then GBC-SD cells were
subject to shR-OB-Rb and shR-Con treatment for 24, 48 and 96 h. To
evaluate the infection efficiency, cells were observed under a
fluorescence microscope and the percentage of GFP-positive cells
was counted.
Quantitative real-time PCR analysis
Total RNA from GBC-SD cells was extracted using the
TRIzol reagent (Invitrogen). Then, 2 μg of total RNA was subjected
to reverse transcription for cDNA synthesis by using MMLV (MBI
Fermentas, Euromedex, Souffelweyersheim, France). Real-time PCR was
performed with the manufacturer's (Kapa Biosystem, Hercules, CA,
USA) instructions. The primer sequences listed below were used. A
mathematical model, 2−ΔΔCT method, was used for relative
quantification in real-time PCR (11). GAPDH was used as internal control
gene to normalize the variability at mRNA expression levels.
Western blot analysis
The GBC-SD cell pellet was washed twice with
ice-cold phosphate buffered saline (PBS) and lysed with lysis
buffer (Beyotime Institute of Biotechnology, Jiangsu, China).
Protein (30 mg) was loaded and separated in 12% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and
transferred to polyvinylidene difluoride membranes (Millipore,
Bedford, MA, USA). The following antibodies were used to probe the
alterations of protein: JAK2 (Abcam, Cambridge, UK), SOCS3 (Abcam),
STAT3 and p-STAT3 (Cell Signaling Technology, Inc., Danvers, MA,
USA), MMP-3 and MMP-9 (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), VEGF-C/D (Santa Cruz Biotechnology). GAPDH (Santa Cruz
Biotechnology) was used as loading control. Signal was detected by
enhanced chemiluminescence techniques (Pierce Thermo Scientific,
Rockford, IL, USA).
Cell proliferation assay
Cells (2×104) per well were seeded into
96-well plate and incubated overnight. Then the medium was removed.
Medium (100 μl) with the final concentration of 100 nM OB-Rb shRNA
was added to each well with or without leptin (250 ng/ml). Scramble
shRNA or untreated cells were used as the control group. All groups
were in triplicate. After 24, 48, and 72 h transfection, cell
proliferation was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay (Beyotime Institute of Biotechnology).
Flow cytometry analysis
Cells (2×105) were seeded in 6-well
plates and incubated overnight to 50–60% confluence. OB-Rb shRNA
was added into the medium at a final concentration of 100 nM with
or without leptin (250 ng/ml). Scramble shRNA or untreated cells
were used as the control group. The cells were incubated with
leptin for 24 h, then treated into single cell suspension with cold
PBS. The experiment was performed following manufacturer's protocol
of Annexin V-FITC Apoptosis and Cells cycle Detection kit (Beyotime
Biotechnology, Jiangsu, China). Then, rates of apoptosis were
analyzed with FACScan system (BD Biosciences, Franklin Lakes, NJ,
USA). Each experiment was performed in triplicate,
independently.
Cell migration and invasion assay
Transwell chambers and Matrigel Invasion Chambers (8
μm pore size, Corning Inc., Corning, NY, USA) were used for cell
migration and invasion assay, respectively. GBC-SD cells
transfected with OB-Rb shRNA or scramble shRNA were treated with or
without leptin (250 ng/ml). After 24 h, cells were detached. Then
500 μl medium with 20% FBS was added into each lower chamber which
was incubated at 37°C. Incubation periods were 2 h for migration,
and 4 h for invasion. Then, the surface of the upper chamber was
swabbed with cotton-tipped applicators to remove the cells that did
not migrate. The lower membrane surface was fixed in methanol and
stained by crystal violet. Migrating cells were counted using light
microscopy (five random 100× fields per well) or a
spectrophotometer. Results were calculated from three independent
experiments.
Immunofluorescence
shRNA-transfected cells (2×105) and
untreated cells were all seeded on coverslips. Cells were cultured
in a 6-well plate and incubated with leptin for 24 h, then rinsed
twice in PBS and fixed with methanol at −20°C for 20 min. Following
fixation, the coverslips were directly washed in PBS for 5 min,
followed by incubation with PBS, 0.2% Triton X-100 and 5% bovine
serum albumin for 20 min at room temperature. Following rinsing
with PBS, the cells were incubated with primary antibody at 4°C in
a humidity box. Primary antibodies included mouse anti-JAK2 (1:200;
Abcam), rabbit anti-STAT3 or p-STAT3 (1:200; CST) and rabbit
polyclonal to SOCS3 (1:200; Abcam). Coverslips were subsequently
washed 3 times with cold PBS and incubated with the corresponding
secondary antibodies (diluted to 1:500, Santa Cruz Biotechnology)
for 2 h at room temperature in the dark, humid box. DAPI staining
was then performed to identify the nuclei.
Gelatin zymography assay
Cells (2×105) transfected with OB-Rb
shRNA or scramble shRNA were seeded in a 6-well plates and
incubated for 6 h. Then leptin (250 ng/ml) was added and the cells
incubated for another 24 h. After each treatment, the cells were
washed twice with serum-free medium, and used for a zymogram
according to the protocol of Zymography kit (Genmed, Shanghai,
China).
Enzyme-linked immunosorbent assay
(ELISA)
VEGF-C and VEGF-D levels were detected using the
ELISA kit according to the manufacturer's manual (Keygentec,
Shanghai, China). Colorimetric measurement was recorded as OD 450
readings.
Xenograft model assay
All animal procedures were previously approved by
the Kunming Medical University ethics committee. Female BALB/c
nu/nu mice (4–5 weeks old, 15–18 g), from Vital River Laboratory
Animal Technology Co., Ltd. (Peking, China), were randomly assigned
into five groups as described above: control, shR-NC, leptin (1
mg/kg), shR-OB-Rb, and shR-OB-Rb + Leptin (1 mg/kg), groups.
Approximately 5×106 cells were suspended in 0.1 ml PBS
and injected subcutaneously into each mouse. The tumors were
monitored every 5 days beginning at day 5 by measuring two
perpendicular diameters with a caliper. The mice were sacrificed on
the 30th day after injection. The tumors were dissected and
weighed.
Statistical analysis
Statistical analysis was performed with SPSS
software (17.0; SPSS, Inc., Chicago, IL, USA). Values are expressed
as mean ± standard deviation (SD). The Student's t-test was used
for comparisons between groups. Categorical data were analyzed by
the chi-square or Fisher's exact tests. Correlation analysis was
performed between leptin and OB-Rb. Cumulative recurrence and
survival rates were analyzed using Kaplan-Meier's method and the
log-rank test. Cox's proportional hazards regression model was used
to analyze independent prognostic factors. Statistical significance
was defined as P-value <0.05.
Results
Expression levels of leptin and OB-Rb in
GBC tissues
To evaluate the possible roles of leptin in GBC, we
investigated the expression levels of leptin and OB-Rb in human
normal gallbladder and GBC tissues by immunohistochemistry.
Compared to normal gallbladder tissues, expression levels of leptin
were significantly upregulated in GBC tissues (P=0.000). Moreover,
OB-Rb density was significantly higher in GBC tissues than in
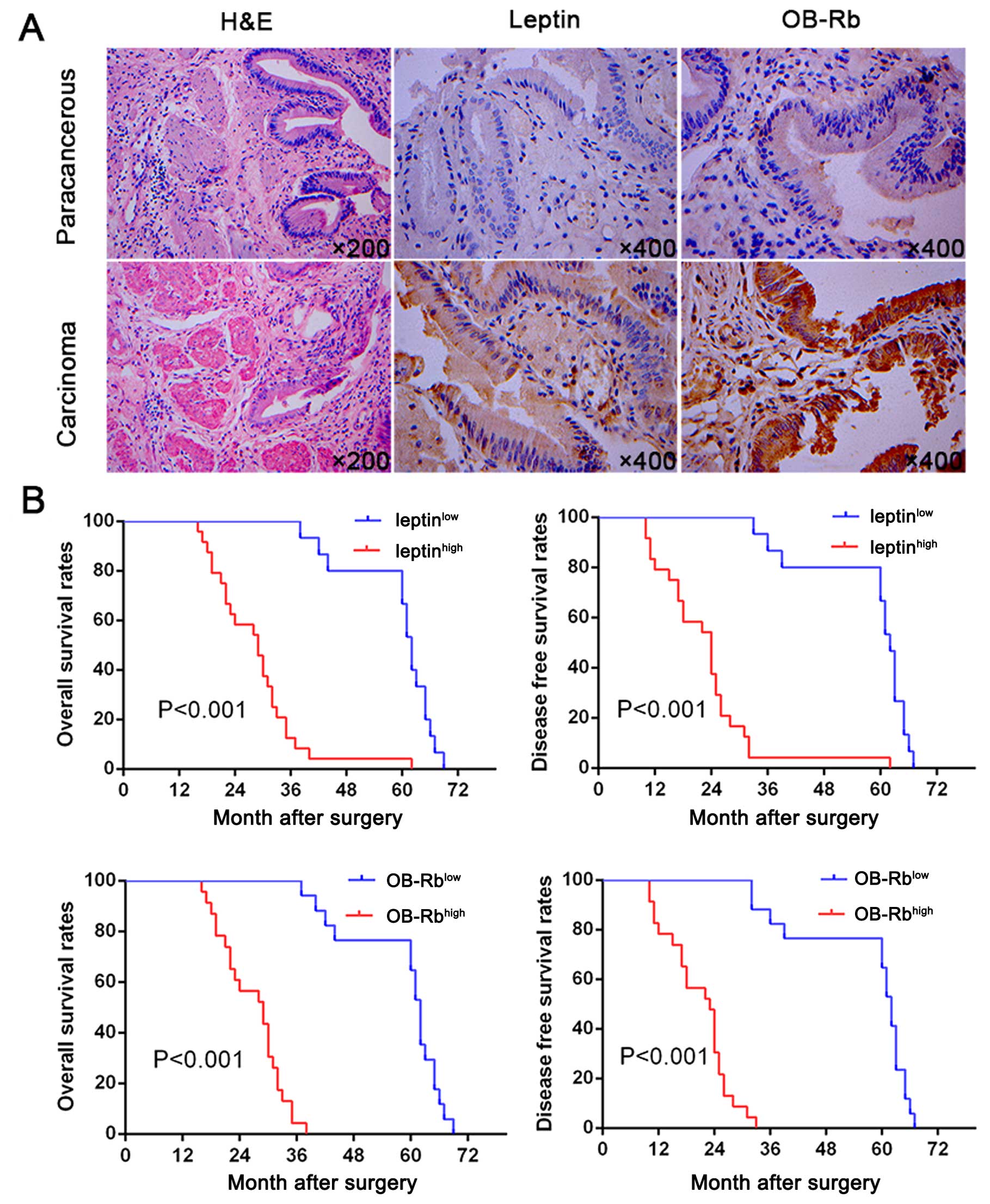
normal gallbladder tissues (P=0.001) (Fig. 1A). In addition, a scatter plot of
leptin and OB-Rb expression revealed a significantly positive
correlation between leptin and OB-Rb levels in cancerous tissues
(r=0.797, P=0.000). The characteristics of the study participants
including age, gender, BMI, T classification, N classification,
Tumor differentiation, AJCC stage are shown in Table I. Results demonstrated that GBC
patients with leptinhigh had high BMI (P<0.001),
elevated CA199 (P<0.001), high T (P=0.030), N (P=0.003)
classification and AJCC stage (P=0.001), poor differentiation
(P=0.026). Moreover, GBC patients with OB-Rbhigh had
high BMI (P<0.001), elevated CA199 (P<0.001), high T
(P=0.012), N (P=0.001) classification and AJCC stage (P<0.001),
poor differentiation (P=0.026). We then analyzed the prognostic
implication of leptin and OB-Rb expression. Importantly, we found
that patients with leptinhigh and OB-Rbhigh
expression had significantly worse prognosis than those with
leptinlow and OB-Rblow expression (Fig. 1B). Multivariate analysis identified
leptin and OB-Rb expression as an independent predictor for
disease-free survival and overall survival (OS; Table II). These results indicate that
leptin and OB-Rb is likely involved in tumorigenesis and
progression of GBC.
 | Table IClinical characteristics of 40
gallbladder carcinoma (GBC) patients and leptin and OB-Rb
expression. |
Table I
Clinical characteristics of 40
gallbladder carcinoma (GBC) patients and leptin and OB-Rb
expression.
| Leptin | | OB-Rb | |
|---|
|
| |
| |
|---|
|
Characteristics | Low (n=15) | High (n=25) | P-value | Low (n=17) | High (n=23) | P-value |
|---|
| Age |
| ≥60 | 8 | 11 | 0.567 | 11 | 8 | 0.061 |
| <60 | 7 | 14 | | 6 | 15 | |
| Gender |
| Male | 3 | 9 | 0.285 | 5 | 7 | 0.994 |
| Female | 12 | 16 | | 12 | 16 | |
| BMI,
kg/m2 |
| <30 | 12 | 1 | <0.001 | 13 | 0 | <0.001 |
| ≥30 | 3 | 24 | | 4 | 23 | |
| CA199, U/ml |
| <35 | 9 | 1 | <0.001 | 10 | 0 | <0.001 |
| ≥35 | 6 | 24 | | 7 | 23 | |
| T classification
(1) |
| T1b/T2 | 14 | 15 | 0.030 | 16 | 13 | 0.012 |
| T3 | 1 | 10 | | 1 | 10 | |
| N classification
(1) |
| N0 | 15 | 14 | 0.003 | 17 | 12 | 0.001 |
| N1 | 0 | 11 | | 0 | 11 | |
| Tumor
differentiation |
| I/II | 13 | 13 | 0.026 | 13 | 13 | 0.191 |
| III/IV | 2 | 12 | | 4 | 10 | |
| AJCC (1) |
| I | 14 | 10 | 0.001 | 16 | 8 | <0.001 |
| II | 1 | 15 | | 1 | 15 | |
Involvement of OB-Rb receptor in
leptin-mediated growth, migration and invasion of GBC-SD cells
Leptin exhibits its effects on cancer through
interaction with specific leptin receptors (OB-Rb and OB-Rs)
(12). In this study, we examined
the effect of leptin on GBC-SD cell growth, migration and invasion.
The result from MTT assay showed leptin (40 ng/ml) significantly
increased the proliferation of GBC-SD cells after 24-h incubation
compared with basal values, which were significantly inhibited by
shR-OB-Rb transfection (Fig.
2A).
Leptin was able to promote GBC-SD cell migration,
which was significantly suppressed in GBC-SD cell transfected with
OB-Rb shRNA at 24 h compared with the control group as shown in
Fig. 2B. Transwell matrix
penetration assay showed that leptin treatment increased the mean
of GBC-SD cell invasive number, which could be repressed by
transfection with OB-Rb shRNA (Fig.
2C). Flow cytometry was used to analyze cell apoptosis in
GBC-SD treated with leptin or OB-Rb shRNA for 24 h. As shown in
Fig. 2D–G, leptin treatment or
OB-Rb knockdown significantly induced G2/M-phase cell cycle arrest,
and decreased cells number of G0/G1 and S-phase. OB-Rb knockdown
significantly induced apoptosis in GBC-SD cell line, compared with
the other groups (P<0.05).
Leptin promoting growth and metastasis
were retarded by OB-Rb RNAi
In vivo, the volumes and weight of xenograft
tumors removed from nude mice which were injected with leptin and
shR-NC were both higher than the other groups. In addition, they
were retarded apparently in Leptin + shR-OB-Rb group after 30 days.
As shown in Fig. 2H–J, the growth
of xenograft tumors in shR-OB-Rb group obviously less than the
other groups.
Signaling pathways of JAK2/STAT3/SOCS3
were involved in leptin stimulation
The leptin action was by signaling via JAK2 and
phosphorylation of STAT3 or other pathways such as SOCS3 (13,14).
In GBC-SD cells, we found that leptin increased JAK2 expression
levels and STAT3 phosphorylation, and decreased SOCS3 expression
levels. Such an effect was blocked by shR-OB-Rb treatment (Fig. 3A and B). The immunofluorescence
experiments also confirmed similar results (Fig. 3C). These results indicate that the
JAK2/STAT3/SOCS3 pathway is involved in leptin-induced migration of
human GBC cells.
shR-OB-Rb downregulated MMP-3/9 activity
and expression of VEGF-C/D increased by leptin
Numerous studies have mechanistically associated the
invasive and metastasis ability of cancer cells with expression of
VEGF factors (15) and activation
of MMP family (16). To understand
the mechanism by which leptin promoted the invasiveness and
migration of GBC-SD cells, we investigated the expression of
VEGF-C/D and activity of MMP-9 in GBC-SD cells treated with leptin
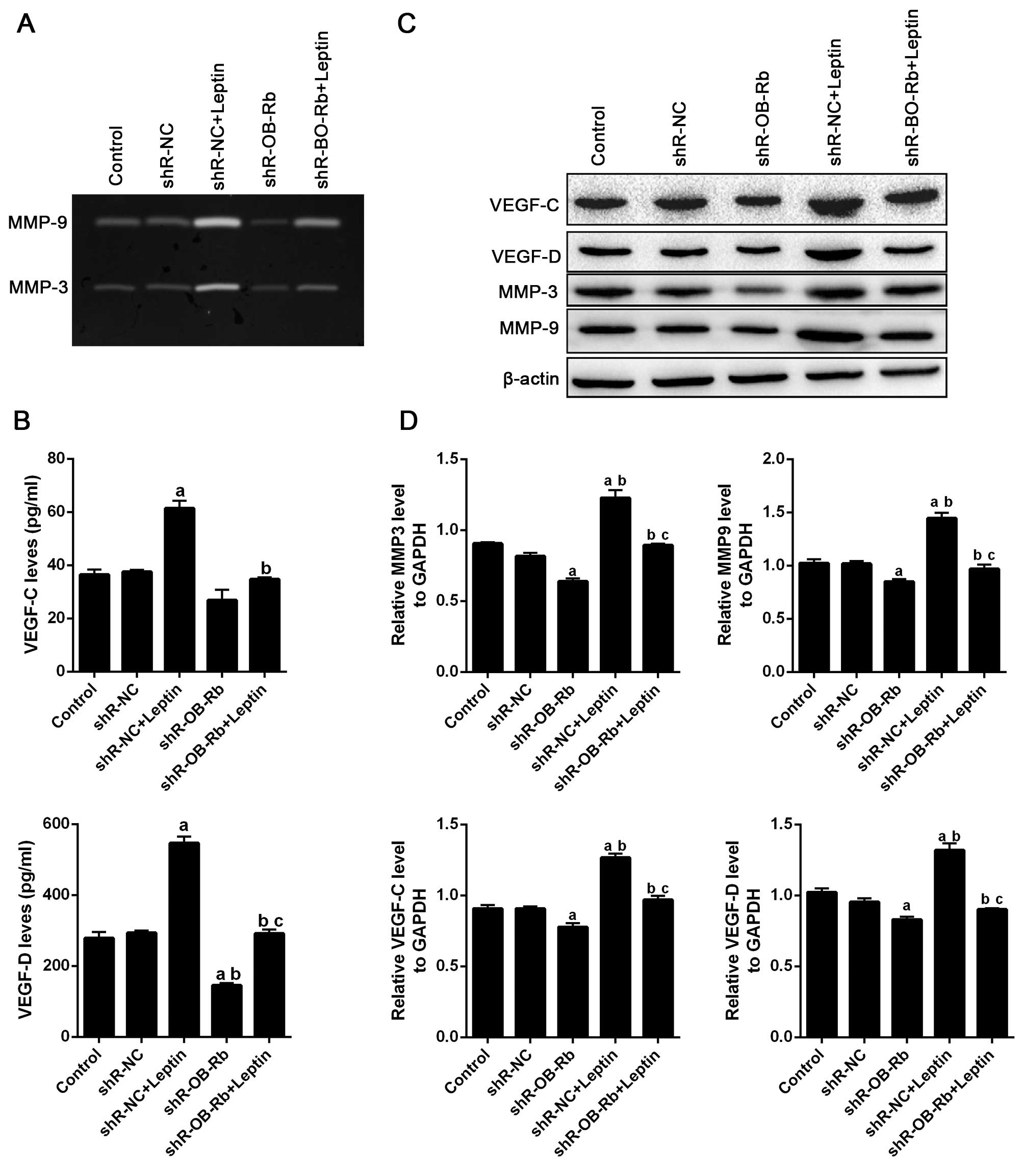
and/or transfected with shR-OB-Rb. As shown in Fig. 4A, gelatin zymography assay results
show leptin increases MMP-3 and MMP-9 activity in GBC-SD cells,
which are attenuated by transfecting shR-OB-Rb. ELISA also
confirmed similar result in expression of VEGF-C and VEGF-D
(Fig. 4B). Then we detected
activity of MMP-3/9 and expression of VEGF-C/D in vivo,
which were confirmed by western blot (Fig. 4C and D) and immunofluorescence
(Fig. 4E–J) analysis. Taken
together, these data suggested that leptin upregulated VEGF-C/D
levels and activated MMP-3/9 in vivo and in
vitro.
Discussion
Our results indicate that leptin and OB-Rb are
expressed at a high level in GBC patients, compare with normal
gallbladder tissue. In addition, a high level of leptin and OB-Rb
is a poor prognostic marker for GBC patient. Furthermore, this
study shows for the first time that leptin and OB-Rb mediates
migration of human gallbladder cancer (GBC) cells. We show that
in vitro: i) leptin stimulates growth and migration of
GBC-SD cells; ii) the enhancement of GBC-SD cell growth by leptin
is associated with G2/M cell cycle arrest, iii) activity of MMP-3/9
and expression VEGF-C/D is determined. iv) JAK2/STAT3/SOCS3 pathway
is involved in this process. Moreover, in vivo v) genetic
ablation of leptin-mediated signaling enhanced cancer growth in an
animal model of GBC. Moreover, these effect could all be attenuated
by OB-Rb receptor shRNA.
Several studies have shown strong epidemiologic
evidence suggesting the existence of a close link between obesity,
a clinical condition characterized by high levels of circulating
leptin (17) and a multitude of
cancers, such as prostate (18),
mammary (19), endometrial
(20), hepatocellular (21), colon (22), pancreatic (23), adenocarcinoma of esophagus
(24), and cholangiocarcinoma
(25). In this study, consistent
data show that leptin enhanced progression of GBC. Leptin is
usually related to binding with its receptor OB-Rb, which belongs
to the cytokine receptor superfamily (26). It has been reported that human
cancer cells expressed OB-Rb and other OB-Rs leptin receptors
(27). However, the role of OB-Rb
in human GBC is mostly unknown. In this setting, we found that cell
migration and integrin upregulation induced by leptin were
attenuated by OB-Rb knockdown. Upon leptin binding, OB-Rb could
activate JAK2, which in turn phosphorylated tyrosine residues in
the receptor tails, leading to the recruitment and activation of
STAT-3 (28). The leptin receptor,
through the activation of JAK2, was also able to downregulate SOCS3
proteins and stimulate the downstream signaling pathway (13,29).
Herein, we used the OB-Rb shRNA to determine its role and found
that it inhibited leptin-induced migration and JAK2/STAT-3/SOCS3
upregulation, indicating the possible involvement of OB-Rb in
leptin-induced cell growth and migration in GBC-SD cells.
Human MMPs, also known as collagenase, are a matrix
metalloproteinase originally identified in breast carcinomas
(30). Recent studies have
revealed that this enzyme was also produced by a variety of
malignant tumors (31). In all of
the cases, the expression of MMPs was associated with aggressive
tumors. GBC is known to have a high potential for invasion and
metastasis. In the present study, we compared the expression levels
of MMP-3 and MMP-9 in GBC cells with and without leptin and/or
OB-Rb shRNA treatment. We found MMP-3/9 was activated by treating
with leptin, and downregulated by OB-Rb interruption suggesting
that leptin's regulation of MMPs is in a tissue-specific
manner.
In addition, cell migration, and metastatic
colonization must be successful with angiogenesis, which is
essential for metastasized tumors in distant sites (32). Thus, we detected VEGF-C/D, which is
the most important mediator of tumor angiogenesis, inducing
formation of new blood vessels (33). Our study indicated that leptin
could increase VEGF-C/D expression in vivo and in
vitro. However, further research is necessary to clarify the
mechanism underlying this process. Considering previous studies and
the results described above, leptin and its receptor OB-Rb could be
a potential therapeutic target for GBC.
In summary, our data showed that leptin and its
receptor OB-Rb may be implicate in growth and metastasis of
gall-bladder carcinoma, which may involve the regulation of MMPs
and VEGF family through SOCS3/JAK2/STAT3 pathways. Regulation of
leptin and its receptor OB-Rb could serve as a promising
intervention strategy for gene therapy of gallbladder
carcinoma.
Acknowledgements
This work is supported by grants from the Basic
Research for Application Fund of Yunnan China (nos. 2012FB050 and
2011FZ124). The work is also funded by National Natural Science
Foundation of China (83160360).
References
|
1
|
Dwivedi AN, Jain S and Dixit R: Gall
bladder carcinoma: Aggressive malignancy with protean loco-regional
and distant spread. World J Clin Cases. 3:231–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boutros C, Gary M, Baldwin K and
Somasundar P: Gallbladder cancer: Past, present and an uncertain
future. Surg Oncol. 21:e183–e191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caldow Pilgrim CH, Groeschl RT, Quebbeman
EJ and Gamblin TC: Recent advances in systemic therapies and
radiotherapy for gallbladder cancer. Surg Oncol. 22:61–67. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halaas JL, Gajiwala KS, Maffei M, Cohen
SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK and Friedman JM:
Weight-reducing effects of the plasma protein encoded by the obese
gene. Science. 269:543–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vona-Davis L and Rose DP: Adipokines as
endocrine, paracrine, and autocrine factors in breast cancer risk
and progression. Endocr Relat Cancer. 14:189–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Surmacz E: Obesity hormone leptin: A new
target in breast cancer? Breast Cancer Res. 9:3012007.PubMed/NCBI
|
|
8
|
Fan Y, Gan Y, Shen Y, Cai X, Song Y, Zhao
F, Yao M, Gu J and Tu H: Leptin signaling enhances cell invasion
and promotes the metastasis of human pancreatic cancer via
increasing MMP-13 production. Oncotarget. 6:16120–16134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong Z, Xu X, Du L, Yang Y, Cheng H, Zhang
X, Li Z, Wang L, Li J, Liu H, et al: Leptin-mediated regulation of
MT1-MMP localization is KIF1B dependent and enhances gastric cancer
cell invasion. Carcinogenesis. 34:974–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks
AS and Myers MG Jr: Regulation of Jak kinases by intracellular
leptin receptor sequences. J Biol Chem. 277:41547–41555. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
12
|
Barone I, Catalano S, Gelsomino L, Marsico
S, Giordano C, Panza S, Bonofiglio D, Bossi G, Covington KR, Fuqua
SA, et al: Leptin mediates tumor-stromal interactions that promote
the invasive growth of breast cancer cells. Cancer Res.
72:1416–1427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frühbeck G: Intracellular signalling
pathways activated by leptin. Biochem J. 393:7–20. 2006. View Article : Google Scholar :
|
|
14
|
Yang R and Barouch LA: Leptin signaling
and obesity: Cardiovascular consequences. Circ Res. 101:545–559.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong Y, Chippada-Venkata UD and Oh WK:
Roles of matrix metalloproteinases and their natural inhibitors in
prostate cancer progression. Cancers (Basel). 6:1298–1327. 2014.
View Article : Google Scholar
|
|
17
|
Garofalo C and Surmacz E: Leptin and
cancer. J Cell Physiol. 207:12–22. 2006. View Article : Google Scholar
|
|
18
|
Saglam K, Aydur E, Yilmaz M and Göktaş S:
Leptin influences cellular differentiation and progression in
prostate cancer. J Urol. 169:1308–1311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'brien SN, Welter BH and Price TM:
Presence of leptin in breast cell lines and breast tumors. Biochem
Biophys Res Commun. 259:695–698. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petridou E, Belechri M, Dessypris N,
Koukoulomatis P, Diakomanolis E, Spanos E and Trichopoulos D:
Leptin and body mass index in relation to endometrial cancer risk.
Ann Nutr Metab. 46:147–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saxena NK, Sharma D, Ding X, Lin S, Marra
F, Merlin D and Anania FA: Concomitant activation of the JAK/STAT,
PI3K/AKT, and ERK signaling is involved in leptin-mediated
promotion of invasion and migration of hepatocellular carcinoma
cells. Cancer Res. 67:2497–2507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bahceci M, Tuzcu A, Akkus M, Yaldiz M and
Ozbay A: The effect of high-fat diet on the development of obesity
and serum leptin level in rats. Eat Weight Disord. 4:128–132. 1999.
View Article : Google Scholar
|
|
23
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Somasundar P, Riggs D, Jackson B,
Vona-Davis L and McFadden DW: Leptin stimulates esophageal
adenocarcinoma growth by nonapoptotic mechanisms. Am J Surg.
186:575–578. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fava G, Alpini G, Rychlicki C, Saccomanno
S, DeMorrow S, Trozzi L, Candelaresi C, Venter J, Di Sario A,
Marzioni M, et al: Leptin enhances cholangiocarcinoma cell growth.
Cancer Res. 68:6752–6761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang W-H, Liu S-C, Tsai C-H, Fong YC, Wang
SJ, Chang YS and Tang CH: Leptin induces IL-6 expression through
OBRl receptor signaling pathway in human synovial fibroblasts. PLoS
One. 8:e755512013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Otvos L Jr and Surmacz E: Targeting the
leptin receptor: A potential new mode of treatment for breast
cancer. Expert Rev Anticancer Ther. 11:1147–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oh HK, Choi YS, Yang Y-I, Kim J-H, Leung
PC and Choi J-H: Leptin receptor is induced in endometriosis and
leptin stimulates the growth of endometriotic epithelial cells
through the JAK2/STAT3 and ERK pathways. Mol Hum Reprod.
19:160–168. 2013. View Article : Google Scholar
|
|
29
|
Benomar Y, Roy AF, Aubourg A, Djiane J and
Taouis M: Cross down-regulation of leptin and insulin receptor
expression and signalling in a human neuronal cell line. Biochem J.
388:929–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation- and
metastasis-sustaining neovasculature. Matrix Biol. 44–46:94–112.
2015. View Article : Google Scholar
|
|
32
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|