Introduction
Head and neck cancer is the sixth most frequent
cancer worldwide (1) and over 90%
of head and neck cancer occurs in the oral cavity (2). Oral squamous cell carcinoma (OSCC) is
a disease in the epithelial neoplasm with increased prevalence
responsible for most malignant lesions in the head and neck. This
disease represents only ~3% of all malignancies of the human body
(3), it can lead to invasion and
metastasis to cause adverse complication for the prognosis and
treatment with chemotherapy of patients. The inhibition of cancer
cell metastasis is one of the critical steps to therapy and
research in human cancer (4). The
identification of molecular pathways involved in cancer cell
metastasis plays an important role for anticancer function of
pharmacologic compounds (5).
Tumor metastasis is one of the major causes of
morbidity and mortality in cancer patients. Molecular mechanisms of
cancer cell metastasis are involved a series of molecular events
such as the tumor cell-induced extracellular matrix (ECM) which
provides biochemical and mechanical barriers (6), ECM component binding and angiogenesis
are involved in cancer cell metastatic processes, degradation of
basement membrane of ECM is a critical step in the processes of
tumor metastasis (7,8). Matrix metalloproteinases (MMPs) play
a critical role in ECM degradation and these enzymes are considered
to be essential factors involved in tumor metastasis such as MMP-2
and -9 (8,9) and MMP-9 is considered to be one of
the critical MMPs involved in cancer invasion in breast cancer
(10,11). The inhibition of MMP-2 and/or MMP-9
expression, or their upstream regulatory pathways could be one of
the treatment options for patients with cancer.
Nuclear factor-κB (NF-κB) signaling pathway plays an
important role in cancer cell escape from anticancer drug induced
cell apoptosis and resistance of chemotherapy in many human cancer
cells including head and neck cancer (12–16).
NF-κB is restricted by the inhibitory protein IκB and after IκB is
phosphorylated, NF-κB is liberated which enter the nucleus to
regulate gene expression involved in cell proliferation, cell
survival and apoptosis (17,18).
NF-κB is associated with cancer cell invasion and metastasis in
various cancers (19–22).
Akt is involved in the progression of metastasis
through various signaling mechanisms such as NF-κB, which is a
direct target of Akt (23).
Activator protein-1 (AP-1), NF-κB via mitogen-activated protein
kinase (MAPK) or phosphatidylinositol 3-kinase (PI3K)/Akt pathways
led to regulation of the expression of the MMPs gene (24,25).
MMPs and their regulatory pathways were the targets for anticancer
drugs and chemotherapeutic agents (26). The aim of the present study was to
investigate the molecular mechanisms of K87 suppressing the
migration and invasion of human oral cancer SCC-4 cells. This is
the first study that the anti-metastatic activity of K87 involves
NF-κB and MMP-2/9 inhibition in human oral cancer SCC-4 cells in
vitro.
Materials and methods
Chemicals and reagents
K87, a synthetic compound from furfuryl alcohol by
photoxidation (27) was obtained
from the laboratory of Dr Y.-H. Kuo, and the chemical structure is
shown in Fig. 1. Dimethyl
sulfoxide (DMSO) and propidium iodide (PI) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). All primary and secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The enhanced chemiluminescence (ECL)
detection system was obtained from Amersham Life Science, Inc.
(Arlington Heights, IL, USA).
Cell culture
Human oral cancer SCC-4 cells were purchased from
the Food Industry Research and Development Institute (Hsinchu,
Taiwan). Cells were cultured in DMEM:F12 medium (Gibco-Life
Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum
and antibiotics (100 units/ml penicillin and 100 μg/ml
streptomycin) (Sigma-Aldrich) on a 75-cm2 tissue culture
flasks in a humidified atmosphere of 5% CO2 at 37°C
(28,29).
Cell viability assays
SCC-4 cells (1×105 cells/well) were
placed in a 12-well plate for 24 h and were incubated with K87 (0,
1, 2.5, 5, 10, 15 and 20 μM) or 0.5% DMSO as a vehicle control for
48 h. Cells were stained with PI (5 μg/ml) and then viability was
immediately measured using flow cytometer (FACSCalibur; BD
Biosciences, San Jose, CA, USA) assay as previously described
(28).
Wound healing assay
Cells (1.5×105 cells/well) were placed in
a 6-well plate and incubated at 37°C for 24 h. The confluent cells
were scratched with a 200 μl pipette tip, followed with PBS
washing, and then treated with K87 (0, 1 and 2.5 μM) in a
serum-free medium for 12 and 24 h. Cells were examined and
photographed under a fluorescence microscope (Axio Observer A1;
Carl Zeiss, Oberkochen, Germany). The total number of cells
migrated into the scratched area was calculated (30).
Cell migration and invasion assays
For cell migration assay, Transwell (BD Biosciences,
Franklin Lakes, NJ, USA) cell culture chambers (8 mm pore size;
Millipore, Billerica, MA, USA) were coated with collagen and SCC-4
cells (5×104 cells/well) were maintained in serum-free
DMEM:F12 medium overnight, while cells were trypsinized and
resuspended in serum-free DMEM:F12 medium. In the upper chamber of
the Transwell insert, cells were placed and incubated with 0.5%
DMSO or K87 (1 and 2.5 μM). A total of 90% DMEM:F12 medium
containing 10% FBS were maintained in the lower chamber. All cells
were incubated for 24 or 48 h. The migrative cells in the lower
surface of the filter were fixed with 4% formaldehyde in PBS and
were stained with 2% crystal violet. Cell number were counted and
photographed under a light microscope at ×200. Cell invasion assay
was done as the cell migration assay except the filter membrane was
coated with Matrigel from a BioCoat Matrigel invasion kit not for
collagen as previously described (30,31).
Gelatin zymography assay
SCC-4 cells (1.5×105 cells/well) were
seeded in 6-well plate for 24 h and then were incubated with K87
(0, 0.25, 0.5, 1 and 2.5 μM) in serum-free DMEM:F12 medium for 24
and 48 h and the conditioned medium was collected and the total
proteins measured. A total of 50 μg of protein was separated on 10%
SDS-PAGE containing 0.2% gelatin by electrophoresis. After
electrophoresis, the gels were washed with 2.5% Triton X-100 and
incubated in a reaction buffer (50 mM Tris-HCl, pH 7.5, 0.02%
NaN3, 150 mM NaCl, 10 mM CaCl2, 1 μM
ZnCl2) for 18 h at 37°C while shaking. After incubation,
the gels were stained with 0.2% Coomassie blue in 10% acetic acid
and 50% methanol (31–33). Both MMP-2/-9 gelatinolytic
activities were visualized as the presence of clear bands with a
blue (negative staining) background.
Western blotting assay
SCC-4 cells (1.5×106 cells) in 10-cm dish
were incubated with K87 (0 and 2.5 μM) for 6, 12, 24 and 48 h.
Cells were collected, washed, centrifuged and lysis buffer [40 mM
Tris-HCl (pH 7.4), 10 mM EDTA, 120 mM NaCl, 1 mM dithiothreitol,
0.1% Nonide P-40] was added to the cell pellet for 30 min. In each
sample protein was quantitated as previously described (32,34).
A total of 30 μg of protein was loaded on a gel [10% sodium dodecyl
sulphate (SDS)/polyacrylamide] for western blot analysis. The gel
was transferred onto a nitrocellulose membrane (Amersham Pharmacia
Biotech, Piscataway, NJ, USA) by electro-blotting at 300 mA for 90
min. After blocking with 5% non-fat skim milk, the membrane was
probed with primary antibodies against NF-κBp65, iNOS, COX-2,
ROCK1, Rho A, MMP-1, -2,- 7, -9, VEGF, GRB2, Ras, SOS1, PI3K, PKC,
PERK, p-PERK, IRE-1α, FAK, MEKK3, MKK7, ERK1/2, JNK1/2, p-c-JNK,
p-p38, p38, p-c-Jun, AKT, p-AKT(308), p-AKT(473), TIMP2, TIMP1 and
β-actin. After incubation, membrane was stained with secondary
antibody for enhanced chemiluminescence (Amersham Life Science) as
previously described (32,34). The antibody binding was detected by
enhanced chemiluminescene (ECL) procedures according to the
manufacturer's recommendation.
Real-time polymerase chain reaction
(PCR)
SCC-4 cells (1.5×105 cells/well) were
seeded onto 6-well culture plates and incubated with K87 (0 and 2.5
μM) for 24 h and were collected and total RNA was extracted using
the Qiagen RNeasy Mini kit (Qiagen, Valencia, CA, USA) as
previously described (35,36). Total RNA was reverse-transcribed
with High Capacity cDNA Reverse Transcription kit at 42°C for 30
min according to the standard protocol of the supplier (Applied
Biosystems, Foster City, CA, USA). All samples for quantitative PCR
were done under the following conditions: 2 min at 50°C, 10 min at
95°C and 40 cycles of 15 sec at 95°C, 1 min at 60°C using 1 ml of
the reverse-transcribed cDNA, 2X SYBR-Green PCR Master Mix (Applied
Biosystems) and 200 nM of forward (F) and reverse (R) primers,
including MMP-2: F-CCCCAGACAGGTGATCTTGAC and R-GCTTGC
GAGGGAAGAAGTTG; MMP-9: F-CGCTGGGCTTAG ATCATTCC and
R-GTGCCGGATGCCATTCAC; FAK: F-TGAATGGAACCTCGCAGTCA and R-TCCGCATGC
CTTGCTTTT; ROCK1-F-ATTCATTCCTACCCTCTACC ACTTTC and
R-TGTGGGACTTAACATGGCATCT; glyceraldehyde 3-phosphate dehydrogenase
(GAPDH): F-ACACC CACTCCTCCACCTTT and R-TAGCCAAATTCGTTGTC ATACC.
Each assay was run in triplicate on an Applied Biosystems 7300
Real-Time PCR system and expression fold-changes were derived using
comparative CT method (35,36).
Confocal laser scanning microscopy
SCC-4 cells (1.5×105 cells/well) were
maintained in 6-well chamber slides and were incubated with K87 (0
and 2.5 μM) for 24 h. All samples were fixed for 15 min with 3%
formaldehyde in PBS and labeled for immunofluorescence. Primary
antibodies against RHO A and ROCK1 were diluted 1:100 with blocking
buffer. They were washed with PBS, and were stained with secondary
FITC-conjugated goat anti-mouse IgG at 1:200 dilutions (green
fluorescence). Cell nuclei were counterstained with PI (red
fluorescence) and then were examined and photomicrographed using a
Leica TCS SP2 confocal spectral microscope as previously described
(32,37).
Electrophoretic mobility shift assay
(EMSA)
SCC-4 cells (1.5×106 cells/10-cm dish)
were treated with K87 (0 and 2.5 μM) for 0, 2, 4 and 8 h. After
incubation, NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce)
was used to perform nuclear extracts as previously described
(38,39) and protein concentration was
measured and biotin end-labeled oligonucleotide sequences were
50-Biotin-GATCCAGGGGACTTTCCCTAGC-30 corresponding to the consensus
site of NF-κB. A total of 5 μg of nuclear extract protein was used
for EMSA with LightShift Chemiluminescent EMSA kit based on the
manufacturer's protocol. The nuclear extracts was incubated with
Biotin end-labeled duplex DNA and the reaction mixture was
separated on 6.0% polyacrylamide gel electrophoresis, and
transferred onto nylon membranes which were subjected to
ultraviolet (UV) light cross-link for 1 min. Membrane was incubated
with blocking buffer containing stabilized streptavidin-horseradish
peroxidase conjugate (1:2,000) incubated with the substrates of the
ECL kit for 15 min. The NF-κB signals on the membranes were
detected using Chemiluminesent Nucleic Acid Detection Module
(Pierce Biotechnology).
Preparation of cytosolic and nuclear
extracts
SCC-4 cells were treated with K87 (0 and 2.5 μM) for
0, 2, 4, 6 and 8 h. Cells were washed twice with ice-cold PBS and
then immediately were suspended with the lysis buffer (0.1 mM EDTA,
1 mM DTT, 10 mM HEPES pH 7.9, 0.1 mM EDTA, 10 mM KCl, 1 mM
phenylmethylsulfonyl fluoride) for 20 min and was added 4.8 μl of
10% NP-40 to the cells and vigorously mixing for 30 sec. After
centrifugation at 13,000 × g under 4°C for 15 min, there were
divided into two parts, one was the cytosolic fractions (cytosolic
extracts) (40). The other part
was the nuclear pellets which were re-suspended in 25 μl of sample
buffer named nuclear extracts. Both proteins in the cytosolic and
nuclear extracts were quantitated and were detected by western
blotting with 10% SDS-polyacrylamide gel (PAGE) for NF-κBp105,
NF-κBp50 and NF-κBp65 protein expression level as previously
described (32,34).
Statistical analysis
All assays were performed in triplicate and data are
expressed as means ± SD. Statistically significant differences
between K87 treated and untreated (control) groups were tested by
the Student's t-test. A P<0.05 was considered to indicate
significant differences.
Results
K87 decreased the cell viability of human
oral cancer SCC-4 cells
SCC-4 cells were treated with K87 (0, 1, 2.5, 5, 10,
15 and 20 μM) for 48 h and total percentage of viable cells were
measured and the results are shown in Fig. 1B. As shown in Fig. 1B, K87 caused dose-dependent
inhibition of the growth of SCC-4 cells in the 48-h treatment, with
the various concentrations (1–20 μM) giving rise to between 18 and
56% inhibition.
K87 inhibited cell mobility, migration
and invasion and activities of MMP-2/-9 of SCC-4 cells
Results from Fig.
1B indicated that K87 at >5 μM decreased over 25% cell
viability, thus, we selected 1 and 2.5 μM for wound healing assay
and results are shown in Fig. 2A and
B. After treatment with 2.5 μM K87 for 12 and 24 h, cells
remained creviced, while untreated wounds healed better. At higher
concentrations and longer time of treatment of K87 both have higher
inhibition of cell mobility (Fig. 2A
and B). Transwell cell migration and invasion assays were used
for investigating the inhibition of K87 on cell migration and
invasion and results are present in Fig. 2C–F. K87 significantly inhibited
cell migration by 52 and 64% at 24 h and 58 and 76% at 48 h both
for 1.0 and 2.5 μM of K87 treatment (Fig. 2C and D). K87 significantly
inhibited cell invasion by 24 and 47% for 24 h, and 42 and 67% for
48 h both for 1.0 and 2.5 μM of K87 treatment (Fig. 2E and F). Inhibition of migration
and invasion in SCC-4 cells was concentration- and time-dependent.
Gelatin zymography was performed to detect the gelatinolytic
activity in conditioned media of SCC-4 cells treated by K87 and
results are shown in Fig. 2G. K87
was markedly effective in inhibiting the gelatinolytic activity of
MMP-2/-9. MMP-2/-9 activities were observed to be decreased in a
dose- and time-dependent manner.
K87 affects the expression of migration
and invasion associated proteins in SCC-4 cells
We have found that K87 suppressed cell mobility,
migration and invasion, thus, we evaluated the expression of these
molecules in SCC-4 cells. Western blot analysis revealed that K87
decreased the protein levels in NF-κBp65, COX-2, ROCK1 and Rho A
(Fig. 3A), MMP-1, -2,- 7, -9 and
VEGF (Fig. 3B), GRB2, SOS1, PI3K,
PKC, PERK and p-PERK (Fig. 3C),
FAK, MEKK3, MKK7, ERK1/2, JNK1/2, p-p38 and p38 (Fig. 3D), p-c-Jun, AKT and TIMP2 (Fig. 3E), but increased the protein levels
of iNOS (Fig. 3A), Ras and IRE-1α
(Fig. 3C), p-c-JNK (Fig. 3D), p-AKT(308), p-AKT(473) and TIMP1
(Fig. 3E). Those findings
indicated that K87 affected cell mobility, migration, invasion,
angiogenesis and metastasis associated protein in SCC-4 cells. The
expressions of majority of proteins were downregulated by K87 in a
time-dependent manner in SCC-4 cells.
 | Figure 3K87 affects the levels of associated
proteins in migration and invasion of SCC-4 cells. Cells
(1.5×105 cells/dish) were treated with K87 of 0 and 2.5
μM for 0, 6, 12, 24 and 48 h and then proteins were determined and
were used for SDS page gel electrophoresis as described in
Materials and methods. The levels of NF-κBp65, iNOS, COX-2, ROCK1
and Rho A (A), MMP-1, -2,- 7, -9 and VEGF (B), GRB2, SOS1, PI3K,
PKC, PERK, p-PERK, Ras and IRE-1α (C), FAK, MEKK3, MKK7, ERK1/2,
JNK1/2, p-c-JNK, p-p38 and p38 (D), p-c-Jun, p-AKT(308),
p-AKT(473), AKT and TIMP2 and TIMP1 (E) expression was,
respectively, estimated by western blotting as described in
Materials and methods. |
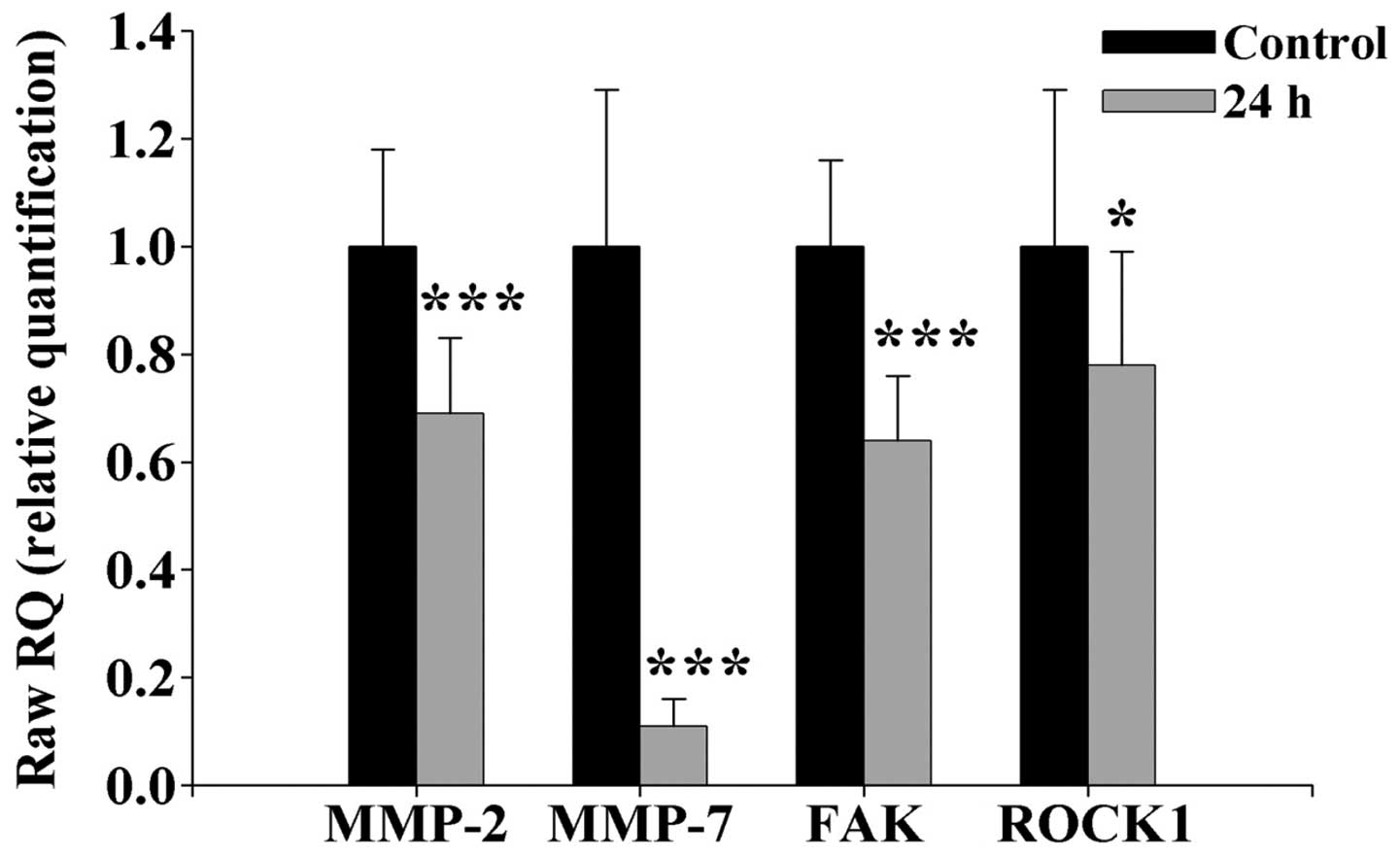
K87 downregulates the mRNA expression of
MMP-2 and -7, FAK and ROCK1 in SCC-4 cells
RT-PCR was performed for an inhibitory effect of K87
on the protein expression of MMP-2 and -7, FAK
and ROCK1 via the depressed the levels of MMP-2, and
-7, FAK and ROCK1 mRNAs expression and results
are shown in Fig. 4. K87 (2.5 μM)
significantly down-regulated MMP-2 and -7, FAK
and ROCK1 mRNA expressions by 25, 91, 72 and 17%,
respectively, in SCC-4 cells. The highest inhibition of mRNA
expression was in MMP-7. We suggested that K87 downregulated
expression of MMP-2 and -7, FAK and
ROCK1 at the transcriptional level.
K87 alters translocation of RHOA and
ROCK1 in SCC-4 cells
RHOA and ROCK1 nuclear translocation was examined
with confocal microscopy immunofluorescent imaging technique
(Fig. 5). K87 treated SCC-4 cells
were significantly decreased in the presence of RHOA (Fig. 5A) and ROCK1 (Fig. 5B) in the nuclei. The functional
consequence and the underlying mechanism of the nuclear RHOA and
ROCK1 translocation in cancer cells needs further
investigations.
K87 affects NF-κB DNA binding activity
and protein expressions in SCC-4 cells
Western blotting showed that K87 inhibited the
expression of NF-κB in SCC-4 cells (Fig. 3A). Thus, for further understanding
the NF-κB binding, we used EMSA assay and the results are shown in
Fig. 6A. K87 suppressed the
nuclear activation of NF-κB and these effects are time-dependent.
Cells were treated with K87 (2.5 μM) for 2, 4 and 8 h, then
harvested for cytosolic and nuclear extracts for western blotting
assay and results are shown in Fig. 6B
and C. IKK, p-IKK and IκB (Fig.
6B), NF-κBp105, NF-κBp50 and NF-κBp65 protein expression
(Fig. 6C) was decreased in
agreement with the results from EMSA assay which shown that K87
suppressed the nuclear activation of NF-κB (Fig. 6A).
Discussion
Oral cancer is highly prevalent among the human
population and is one of the most common cancers in males in
Taiwan. Surgery, radiotherapy and chemotherapy has improved
survival rates in the early stage of this disease, but the local
recurrences and distant metastases are still a serious problem for
oral cancer patients. Currently, novel therapeutic strategies and
new drugs for defeating oral cancer cell metastases are essential.
Cancer metastasis is a multistep process including cell mobility,
migration, invasion, intravasation, entry into blood or lymphatic
vessel, extravasation, and developing new tumors in other organs
(41) that make it more difficult
to treat patient, and account for the main causes of death in
cancer patients with treatment failure.
We investigated the pharmacological activity of K87
on SCC-4 cell mobility, migration and invasion. Our findings
demonstrated that: i) K87 decreased total cell viability (Fig. 1B), suppressed cell mobility
(Fig. 2A and B), migration and
invasion (Fig. 2C and D) and
inhibited MMP-2 and -9 activities (Fig. 2G); ii) K87 inhibited cell migration
and invasion associated protein expression (Fig. 3E); iii) K87 downregulates the mRNA
expression of MMP-2 and -7, FAK and
ROCK1 (Fig. 4); iv) K87
alters translocation of RHOA and ROCK1 in SCC-4 cells (Fig. 5); v) K87 affects NF-κB DNA binding
activity in SCC-4 cells in vitro (Fig. 6A) and inhibited the expression of
NF-κB proteins (Fig. 6B and C) in
SCC-4 cells.
K87 is a synthesized chemical and not known to
exhibit anticancer property by inhibiting cancer cell growth,
migration and invasion. Our studies investigated that K87
significantly suppressed the mobility, migration and invasion of
SCC-4 cells, which did not result from cell growth arrest, because
we selected low concentrations of K87 (0–2.5 μM) throughout the
experiments. Our findings indicated that K87 treatment strongly
attenuated the cell migration and invasion of SCC-4 cells through
downregulation of MMP-2, -7, FAK and
ROCK1 mRNA (Fig. 4) and
protein levels and MMP-2 and -9 activities, as well as inhibition
of NF-κB. MMPs play an important role in degradation of matrix
barriers surrounding the tumor, for tumor growth, invasion,
angiogenesis and metastasis (42–45).
When undergoing tumor growth and invasion, both MMP-2 and MMP-9 are
involved in the initial breakdown of collagen and basement membrane
components (38), thus, the
inhibition of MMP expression and activity that may lead to
suppressed tumor invasion and migration. K87 inhibited MMP-2 and -9
protein expression (Fig. 3B) and
MMP-2 mRNA expression (Fig.
4) that may lead to suppress migration and invasion of SCC-4
cells. MMP-9 is abundantly expressed in breast cancer cells and is
associated with cell invasion and metastasis (46). MMP-2/-9 are the potential targets
for anti-metastatic drug function (47). K87 suppressed the activities of
MMP-2 and -9 (Fig. 2G), inhibited
the mRNA (Fig. 4) and protein
expression (Fig. 3B) of FAK in
SCC-4 cells. FAK was reported to be involved with MMP-9 production
in cholangiocarcinoma (48).
Three major MAPKs (p38, JNK and ERK1/2) are the
signal transducers involved in cell survival, apoptosis and cell
differentiation (49). K87
inhibited the protein expression of ERK1/2, JNK1/2 and p38
(Fig. 3D) which indicated a role
for the MAPK signaling pathway in the regulation of cell migration
and invasion of SCC-4 cells. K87 suppressed the protein expression
of PI3K and Akt in SCC-4 cells (Fig.
3C and E). The PI3K/Akt signaling pathway is implicated in cell
migration and invasion (50,51)
especially in the metastasis of prostate cancer CaP cells (52). The PI3K-AKT signaling pathways are
involved in MMPs for UPA gene regulation, cell survival and cell
invasion (53,54).
NF-κB plays an important role in the cell
proliferation, apoptosis, carcinogenesis, invasion and metastasis
(55). The transcriptions of
MMP-2/-9 genes are regulated by upstream regulatory factors
including NF-κB p65 and c-Jun (56,57).
Constitutively active PI3K and NF-κB signaling pathways (58) and blocking PI3K/AKT and NF-κB
signaling pathways led to decrease breast cancer cell migration
(59). We have found the
inhibition of MM-2/-9 activities (Fig.
2G) and protein expression of NF-κBp105, NF-κBp50 and NF-κBp65
(Fig. 6B and C) and NF-κB binding
DNA (Fig. 6A) in SCC-4 cells. MMPs
are regulated primarily through NF-κB at the level of transcription
through PI3K/Akt pathway (60,61).
NF-κB is constitutively activated through a PI3K-dependent
activation of IKK (62,63). The K87-mediated suppression of
NF-κB in SCC-4 cells probably offers a molecular basis for its
ability to inhibit cell migration and invasion.
The present study showed that K87 inhibited cell
mobility, invasion and migration of SCC-4 cells by regulating the
activities of MMP-2 and -9. K87 inhibited the MAPK (p38, ERK and
JNK) signaling pathway by reducing AKT/PI3K, and NF-κBp65 leading
to MMP-2/-9 downregulation as summarized in Fig. 7. Based on these observations, we
suggested that K87 inhibited the migration and invasion of SCC-4
cells via the inhibition of MAPK and NF-κB signaling pathways.
Acknowledgements
The present study was supported by the grants
CMU103-ASIA-01 by the China Medical University, Taichung, in part
by the Taiwan Ministry of Health and the Welfare Clinical Trial and
Research Center of Excellence (MOHW105-TDU-B-212-133019) and by the
CMU under the Aim for Top University Plan of the Ministry of
Education, Taiwan. The experiments and data analysis were performed
in part through the use of the Medical Research Core Facilities
Center, Office of Research & Development at China Medical
University, Taichung, Taiwan.
References
|
1
|
Deshpande AM and Wong DT: Molecular
mechanisms of head and neck cancer. Expert Rev Anticancer Ther.
8:799–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsantoulis PK, Kastrinakis NG, Tourvas AD,
Laskaris G and Gorgoulis VG: Advances in the biology of oral
cancer. Oral Oncol. 43:523–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan S, Tang QL, Lin YJ, Chen WL, Li JS,
Huang ZQ, Yang ZH, Wang YY, Zhang DM, Wang HJ, et al: A review of
clinical and histological parameters associated with contralateral
neck metastases in oral squamous cell carcinoma. Int J Oral Sci.
3:180–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan N and Mukhtar H: Cancer and
metastasis: Prevention and treatment by green tea. Cancer
Metastasis Rev. 29:435–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ying H, Biroc SL, Li WW, Alicke B, Xuan
JA, Pagila R, Ohashi Y, Okada T, Kamata Y and Dinter H: The Rho
kinase inhibitor fasudil inhibits tumor progression in human and
rat tumor models. Mol Cancer Ther. 5:2158–2164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woessner JF Jr: Matrix metalloproteinases
and their inhibitors in connective tissue remodeling. FASEB J.
5:2145–2154. 1991.PubMed/NCBI
|
|
7
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Velinov N, Poptodorov G, Gabrovski N and
Gabrovski S: The role of matrixmetalloproteinases in the tumor
growth and metastasis. Khirurgiia (Sofiia). 44–49. 2010.(In
Bulgarian).
|
|
9
|
Ram M, Sherer Y and Shoenfeld Y: Matrix
metalloproteinase-9 and autoimmune diseases. J Clin Immunol.
26:299–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: A tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itoh Y and Nagase H: Matrix
metalloproteinases in cancer. Essays Biochem. 38:21–36. 2002.
View Article : Google Scholar
|
|
12
|
Helbig G, Christopherson KW II,
Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE and
Nakshatri H: NF-kappaB promotes breast cancer cell migration and
metastasis by inducing the expression of the chemokine receptor
CXCR4. J Biol Chem. 278:21631–21638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koyama S: Differential expression of
intracellular apoptotic signaling molecules in tumor and
tumor-infiltrating lymphocytes during development of invasion
and/or metastasis of gastric carcinoma. Dig Dis Sci. 48:2290–2300.
2003. View Article : Google Scholar
|
|
14
|
Pai SI and Westra WH: Molecular pathology
of head and neck cancer: Implications for diagnosis, prognosis, and
treatment. Annu Rev Pathol. 4:49–70. 2009. View Article : Google Scholar
|
|
15
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang CY, Mayo MW and Baldwin AS Jr: TNF-
and cancer therapy-induced apoptosis: Potentiation by inhibition of
NF-kappaB. Science. 274:784–787. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McNulty SE, Tohidian NB and Meyskens FL
Jr: RelA, p50 and inhibitor of kappa B alpha are elevated in human
metastatic melanoma cells and respond aberrantly to ultraviolet
light B. Pigment Cell Res. 14:456–465. 2001. View Article : Google Scholar
|
|
20
|
Park BK, Zhang H, Zeng Q, Dai J, Keller
ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, et al: NF-kappaB in
breast cancer cells promotes osteolytic bone metastasis by inducing
osteoclastogenesis via GM-CSF. Nat Med. 13:62–69. 2007. View Article : Google Scholar
|
|
21
|
Sasaki N, Morisaki T, Hashizume K, Yao T,
Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka
M, et al: Nuclear factor-kappaB p65 (RelA) transcription factor is
constitutively activated in human gastric carcinoma tissue. Clin
Cancer Res. 7:4136–4142. 2001.PubMed/NCBI
|
|
22
|
Shishodia S and Aggarwal BB: Nuclear
factor-kappaB activation mediates cellular transformation,
proliferation, invasion angiogenesis and metastasis of cancer.
Cancer Treat Res. 119:139–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liptay S, Weber CK, Ludwig L, Wagner M,
Adler G and Schmid RM: Mitogenic and antiapoptotic role of
constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J
Cancer. 105:735–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aguirre Ghiso JA, Alonso DF, Farías EF,
Gomez DE and de Kier Joffè EB: Deregulation of the signaling
pathways controlling urokinase production. Its relationship with
the invasive phenotype. Eur J Biochem. 263:295–304. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.
|
|
26
|
Mannello F, Tonti G and Papa S: Matrix
metalloproteinase inhibitors as anticancer therapeutics. Curr
Cancer Drug Targets. 5:285–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu FS, Huang AC, Yang JS, Yu CS, Lu CC,
Chiang JH, Chiu CF and Chung JG: Safrole induces cell death in
human tongue squamous cancer SCC-4 cells through
mitochondria-dependent caspase activation cascade apoptotic
signaling pathways. Environ Toxicol. 27:433–444. 2012. View Article : Google Scholar
|
|
28
|
Jao HY, Yu FS, Yu CS, Chang SJ, Liu KC,
Liao CL, Ji BC, Bau DT and Chung JG: Suppression of the migration
and invasion is mediated by triptolide in B16F10 mouse melanoma
cells through the NF-kappaB-dependent pathway. Environ Toxicol. Sep
29–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang YM, Velmurugan BK, Kuo WW, Chen YS,
Ho TJ, Tsai CT, Ye CX, Tsai CH, Tsai FJ and Huang CY: Inhibitory
effect of alpinate Oxyphyllae fructus extracts on Ang II-induced
cardiac pathological remodeling-related pathways in H9c2
cardiomyoblast cells. Biomedicine. 3:148–152. 2013. View Article : Google Scholar
|
|
30
|
Lai KC, Hsu SC, Kuo CL, Ip SW, Yang JS,
Hsu YM, Huang HY, Wu SH and Chung JG: Phenethyl isothiocyanate
inhibited tumor migration and invasion via suppressing multiple
signal transduction pathways in human colon cancer HT29 cells. J
Agric Food Chem. 58:11148–11155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lai WW, Hsu SC, Chueh FS, Chen YY, Yang
JS, Lin JP, Lien JC, Tsai CH and Chung JG: Quercetin inhibits
migration and invasion of SAS human oral cancer cells through
inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling
pathways. Anticancer Res. 33:1941–1950. 2013.PubMed/NCBI
|
|
32
|
Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL,
Lin JP, Ma YS, Wu CC and Chung JG: Curcumin inhibits the migration
and invasion of human A549 lung cancer cells through the inhibition
of matrix metalloproteinase-2 and -9 and Vascular Endothelial
Growth Factor (VEGF). Cancer Lett. 285:127–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin MC, Tsai SY, Wang FY, Liu FH, Syu JN
and Tang FY: Leptin induces cell invasion and the upregulation of
matrilysin in human colon cancer cells. Biomedicine. 3:174–180.
2013. View Article : Google Scholar
|
|
34
|
Lin HJ, Su CC, Lu HF, Yang JS, Hsu SC, Ip
SW, Wu JJ, Li YC, Ho CC, Wu CC, et al: Curcumin blocks migration
and invasion of mouse-rat hybrid retina ganglion cells (N18)
through the inhibition of MMP-2, -9, FAK, Rho A and Rock-1 gene
expression. Oncol Rep. 23:665–670. 2010.PubMed/NCBI
|
|
35
|
Liu KC, Huang AC, Wu PP, Lin HY, Chueh FS,
Yang JS, Lu CC, Chiang JH, Meng M and Chung JG: Gallic acid
suppresses the migration and invasion of PC-3 human prostate cancer
cells via inhibition of matrix metalloproteinase-2 and -9 signaling
pathways. Oncol Rep. 26:177–184. 2011.PubMed/NCBI
|
|
36
|
Lu KW, Chen JC, Lai TY, Yang JS, Weng SW,
Ma YS, Lu PJ, Weng JR, Chueh FS, Wood WG, et al: Gypenosides
inhibits migration and invasion of human oral cancer SAS cells
through the inhibition of matrix metalloproteinase-2 -9 and
urokinase-plasminogen by ERK1/2 and NF-kappa B signaling pathways.
Hum Exp Toxicol. 30:406–415. 2011. View Article : Google Scholar
|
|
37
|
Lu HF, Tung WL, Yang JS, Huang FM, Lee CS,
Huang YP, Liao WY, Chen YL and Chung JG: In vitro suppression of
growth of murine WEHI-3 leukemia cells and in vivo promotion of
phagocytosis in a leukemia mice model by indole-3-carbinol. J Agric
Food Chem. 60:7634–7643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Q, Han Y, Wang C, Shan S, Wang Y, Zhang
J and Ren T: MicroRNA-125b promotes tumor metastasis through
targeting tumor protein 53-induced nuclear protein 1 in patients
with non-small-cell lung cancer. Cancer Cell Int. 15:842015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu SH, Hsiao YT, Kuo CL, Yu FS, Hsu SC, Wu
PP, Chen JC, Hsia TC, Liu HC, Hsu WH, et al: Bufalin inhibits
NCI-H460 human lung cancer cell metastasis in vitro by inhibiting
MAPKs, MMPs, and NF-κB pathways. Am J Chin Med. 43:1247–1264. 2015.
View Article : Google Scholar
|
|
40
|
Lin ML, Chung JG, Lu YC, Yang CY and Chen
SS: Rhein inhibits invasion and migration of human nasopharyngeal
carcinoma cells in vitro by down-regulation of matrix
metalloproteinases-9 and vascular endothelial growth factor. Oral
Oncol. 45:531–537. 2009. View Article : Google Scholar
|
|
41
|
Köhrmann A, Kammerer U, Kapp M, Dietl J
and Anacker J: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer and breast cancer cell lines: New
findings and review of the literature. BMC Cancer. 9:1882009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Folgueras AR, Pendás AM, Sánchez LM and
López-Otín C: Matrix metalloproteinases in cancer: From new
functions to improved inhibition strategies. Int J Dev Biol.
48:411–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar :
|
|
45
|
Schütz A, Schneidenbach D, Aust G,
Tannapfel A, Steinert M and Wittekind C: Differential expression
and activity status of MMP-1, MMP-2 and MMP-9 in tumor and stromal
cells of squamous cell carcinomas of the lung. Tumour Biol.
23:179–184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu X, Zhu Y, Yan H, Liu B, Li Y, Zhou Q
and Xu K: Isothiocyanates induce oxidative stress and suppress the
metastasis potential of human non-small cell lung cancer cells. BMC
Cancer. 10:2692010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weng CJ and Yen GC: The in vitro and in
vivo experimental evidences disclose the chemopreventive effects of
Ganoderma lucidum on cancer invasion and metastasis. Clin Exp
Metastasis. 27:361–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rodríguez-Berriguete G, Fraile B,
Martínez-Onsurbe P, Olmedilla G, Paniagua R and Royuela M: MAP
Kinases and Prostate Cancer. J Signal Transduct. 2012:1691702012.
View Article : Google Scholar
|
|
49
|
Yu T, Wu Y, Helman JI, Wen Y, Wang C and
Li L: CXCR4 promotes oral squamous cell carcinoma migration and
invasion through inducing expression of MMP-9 and MMP-13 via the
ERK signaling pathway. Mol Cancer Res. 9:161–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Conley-LaComb MK, Saliganan A, Kandagatla
P, Chen YQ, Cher ML and Chinni SR: PTEN loss mediated Akt
activation promotes prostate tumor growth and metastasis via
CXCL12/CXCR4 signaling. Mol Cancer. 12:852013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA
and Chen JH: Inhibitory effects of andrographolide on migration and
invasion in human non-small cell lung cancer A549 cells via
down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol.
632:23–32. 2010. View Article : Google Scholar
|
|
52
|
Yang SF, Chen MK, Hsieh YS, Yang JS,
Zavras AI, Hsieh YH, Su SC, Kao TY, Chen PN and Chu SC:
Antimetastatic effects of Terminalia catappa L. on oral cancer via
a down-regulation of metastasis-associated proteases. Food Chem
Toxicol. 48:1052–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim D, Kim S, Koh H, Yoon SO, Chung AS,
Cho KS and Chung J: Akt/PKB promotes cancer cell invasion via
increased motility and metalloproteinase production. FASEB J.
15:1953–1962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Prabhu L, Mundade R, Korc M, Loehrer PJ
and Lu T: Critical role of NF-κB in pancreatic cancer. Oncotarget.
5:10969–10975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vayalil PK and Katiyar SK: Treatment of
epigallocatechin-3-gal-late inhibits matrix metalloproteinases-2
and -9 via inhibition of activation of mitogen-activated protein
kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145
cells. Prostate. 59:33–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cheung LW, Leung PC and Wong AS:
Gonadotropin-releasing hormone promotes ovarian cancer cell
invasiveness through c-Jun NH2-terminal kinase-mediated activation
of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Res.
66:10902–10910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sweeney C, Li L, Shanmugam R,
Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M,
Nakshatri H and Cheng L: Nuclear factor-kappaB is constitutively
activated in prostate cancer in vitro and is overexpressed in
prostatic intraepithelial neoplasia and adenocarcinoma of the
prostate. Clin Cancer Res. 10:5501–5507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Price JT, Tiganis T, Agarwal A, Djakiew D
and Thompson EW: Epidermal growth factor promotes MDA-MB-231 breast
cancer cell migration through a phosphatidylinositol 3′-kinase and
phospholipase C-dependent mechanism. Cancer Res. 59:5475–5478.
1999.PubMed/NCBI
|
|
59
|
Noma N, Asagiri M, Takeiri M, Ohmae S,
Takemoto K, Iwaisako K, Minato N, Maeda-Yamamoto M, Simizu S and
Umezawa K: Inhibition of MMP-2-mediated mast cell invasion by NF-κB
inhibitor DHMEQ in mast cells. Int Arch Allergy Immunol. 166:84–90.
2015. View Article : Google Scholar
|
|
60
|
Arlt A, Gehrz A, Müerköster S, Vorndamm J,
Kruse ML, Fölsch UR and Schäfer H: Role of NF-kappaB and Akt/PI3K
in the resistance of pancreatic carcinoma cell lines against
gemcitabine-induced cell death. Oncogene. 22:3243–3251. 2003.
View Article : Google Scholar
|
|
61
|
Chen PS, Shih YW, Huang HC and Cheng HW:
Diosgenin, a steroidal saponin, inhibits migration and invasion of
human prostate cancer PC-3 cells by reducing matrix
metalloproteinases expression. PLoS One. 6:e201642011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Eccles SA, Box GM, Court WJ, Bone EA,
Thomas W and Brown PD: Control of lymphatic and hematogenous
metastasis of a rat mammary carcinoma by the matrix
metalloproteinase inhibitor batimastat (BB-94). Cancer Res.
56:2815–2822. 1996.PubMed/NCBI
|
|
63
|
Wang W, Abbruzzese JL, Evans DB, Larry L,
Cleary KR and Chiao PJ: The nuclear factor-kappa B RelA
transcription factor is constitutively activated in human
pancreatic adenocarcinoma cells. Clin Cancer Res. 5:119–127.
1999.PubMed/NCBI
|