Introduction
Breast cancer is the most frequent malignancy and
most common cause of cancer-related death in women worldwide.
Breast cancer is highly metastatic to bone where it drives bone
turnover causing bone damage. Breast cancer bone metastasis occurs
in 70–80% of patients with advanced breast cancer (1–4),
leading to severe pathological bone fractures, pain, hypercalcemia,
and spinal cord and nerve-compression syndromes (3,5),
which are a common cause of morbidity and mortality. Tumor invasion
into bone tissues is associated with osteoclast and osteoblast
recruitment, resulting in the liberation of growth factors from the
bone matrix, which can feed back to enhance tumor growth resulting
in the vicious cycle of bone metastasis (4,5).
Breast cancer promotes the formation of osteoclasts
through secretion of osteoporotic cytokines, such as parathyroid
hormone-related peptide, prostaglandin E2, tumor
necrosis factor-α (TNF-α), interleukins and leukemia inhibitory
factor (4,6,7).
Constitutively activated nuclear factor-κB (NF-κB) in breast cancer
cells has been shown to play a crucial role in the osteolytic bone
metastasis of breast cancer in stimulating osteoclastogenesis.
Enhanced NF-κB stimulates production of granulocyte
macrophage-colony stimulating factor (GM-CSF) in breast cancer
cells that enhance osteoclast development from monocytes (8). Moreover, breast cancer cells express
the receptor activator of NF-κB ligand (RANKL) that mediates
epithelial proliferation and carcinogenesis (7). Osteoblasts are negatively affected by
breast cancer cells as evidenced by an increase in apoptosis and a
decrease in proteins required for new bone formation (6). Breast cancer cell bone
metastasis-induced bone loss is due to both activated osteoclastic
bone resorption and suppressed osteoblastic bone formation.
Bisphosphonates or anti-RANKL antibody (denosumab) have been used
as the current standard of care for patients with bone metastasis
(9).
The regucalcin, whose gene is localized on the X
chromosome (10–12), plays a pivotal role as a suppressor
of protein of multi-signaling pathways in various types of cells
and tissues (13,14). The regucalcin gene expression is
regulated by various hormonal factors including calcium-related
process, calcium-regulating hormones, insulin, estrogen and other
steroid hormones (15). Regucalcin
is translocated from the cytoplasm to nucleus in various types of
cells and it regulates nuclear functions (16). Regucalcin has been shown to play a
role in the maintaining of intracellular calcium homeostasis and
inhibiting of various protein kinases, protein phosphatases and
protein synthesis in the cytoplasm and nuclear DNA and RNA
syntheses (13–16). Nuclear regucalcin has also been
shown to regulate the gene expression of various proteins (16). Moreover, regucalcin has been found
to suppress cell proliferation and apoptotic cell death that are
mediated through multiple signaling pathways (17,18).
Regucalcin has been proposed to play a pivotal role in maintaining
cell homeostasis and function as a suppressor protein of
intracellular signaling systems (13,14).
There is growing evidence that regucalcin is
involved in mitigating human carcinogenesis (17,19).
Regucalcin has been reported to be downregulated in human tumor
tissues in vivo (19–21).
We have demonstrated that survival in pancreatic cancer patients is
prolonged in subjects with increased regucalcin gene expression
(22). Furthermore, overexpression
of the human regucalcin gene suppresses the proliferation of human
pancreatic cancer MIA PaCa-2 cells in vitro (22). Taken together the data suggest that
regucalcin may play a potential role as a suppressor of human
carcinogenesis.
Because regucalcin has not previously been
investigated in the context of breast cancer, the present study was
undertaken to determine whether human regucalcin exhibits
anticancer effects and anti-bone metastatic activity in human
breast cancer. We report significantly improved relapse-free
survival in 44 breast cancer patients with higher regucalcin
expression. Moreover, overexpression of regucalcin was found to
exhibit anti-proliferative effects in MDA-MB-231 cells (23). Regucalcin may play a potential role
as a suppressor protein in human breast cancer.
Materials and methods
Materials
Dulbecco's modified Eagle's medium
(DMEM) with 4.5 g/l glucose, L-glutamine and sodium pyruvate and
antibiotics (penicillin and streptomycin) were purchased from
Corning Cellgro (Mediatech, Inc. Manassas, VA, USA). α-Minimum
essential medium (α-MEM) was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Fetal bovine serum (FBS) was from HyClone
Laboratories (Logan, UT, USA). Tumor necrosis factor-α (TNF-α) was
from R&D Systems (Minneapolis, MN, USA). Sodium butyrate,
roscovitine, sulforaphane, PD98059, staurosporine, Bay K 8644,
wortmannin, 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB),
caspase-3 inhibitor, Alizarin red, lypopolysaccharide (LPS) and all
other reagents were purchased from Sigma-Aldrich unless otherwise
specified. Gemcytabine was obtained from Hospira, Inc. (Lake
Forest, IL, USA). Antibodies for western blot analysis were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Gemcytabine and caspase-3 inhibitor were diluted in
phosphate-buffered saline (PBS) and other reagents were dissolved
in 100% ethanol to use in experiments.
Patient datasets
Gene expression and survival data of 87 breast
cancer patients were obtained through the Gene Expression Omnibus
(GEO) database (GSE6532) for outcome analysis (23–25).
These datasets contained gene expression data derived from the
Affymetrix U133 Plus2 platform. For microarray analysis, expression
and raw expression data (CEL files) were summarized and normalized
using the Robust Multi-array Average algorithm and the Bioconductor
package affy (http://www.bioconductor.org/packages/2.0/bioc/html/affy.html).
Breast cancer MDA-MB-231 cells
Human breast cancer MDA-MB-231 cells lack estrogen,
progesterone and human epithelial growth factor type 2 (HER2)
receptors, and are therefore considered as triple-negative
(26). They express high levels of
the epithelial growth factor receptor (EGFR) and activation of this
receptor and its downstream signaling events enhance migration,
proliferation, invasion and progression of the malignant phenotype
of these cells (26). MDA-MB-231
cells were obtained from the American Type Culture Collection
(ATCC; Rockville, MD, USA).
Regucalcin transfectants
Stable regucalcin transfectants overexpressing full
length or truncated regucalcin proteins in regucalcin in MDA-MB-231
cells were generated as follow. The cDNA encoding human regucalcin
with full length (900 bp), deleted exon 4 (684 bp), and deleted
exon 4 and 5 (552 bp) were cloned into the pBluescript vector
(27,28). The complete regucalcin coding cDNA
was cloned into the EcoRI site of the pCXN2 expression
vector (27). The resultant
plasmid was designated as regucalcin/pCXN2 (24). For transient transfection assay,
MDA-MB-231 cells were grown on 24-well plates to ~70% confluence.
Each of regucalcin (900 bp), deleted exon 4 (684 bp), and deleted
exons 4 and 5 (552 bp) and pCXN2 vector alone were transfected into
MDA-MB-231 cells using Lipofectamine reagent, according to the
manufacturer's instructions (Promega, Madison, WI, USA)
(27). After overnight incubation,
gemciticin (G418) (600 μg/ml of medium; Sigma-Aldrich) was added to
culture wells for selection and cells were cultured for 2 weeks.
After that, cells were plated at limiting dilution to isolate
stable transfectants. Multiple surviving clones were isolated,
transferred to 35-mm dishes, and grown in medium without G418. The
increase in regucalcin in transfectants was 15.5-fold of wild-type
cells. In experiments, transfectants were cultured in DMEM
containing 10% FBS and 1% penicillin and streptomycin for 1–7 days
in a water-saturated atmosphere containing 5% CO2 and
95% air at 37°C.
Cell proliferation
M DA-M B-231 wild-type cells
(1×105/ml/well) and MDA-MB-231 cells
(1×105/ml/well) transfected with regucalcin cDNAs of
either full length, deleted exon 4 or deleted exons 4 and 5 were
cultured using a 24-well plate in DMEM containing 10% FBS and 1%
penicillin and streptomycin for 1, 2, 3 or 7 days in a
water-saturated atmosphere containing 5% CO2 and 95% air
at 37°C (28,29). In separate experiments, MDA-MB-231
wild-type cells (1×105/ml/well) or full length
regucalcin transfectants were cultured in DMEM containing 10% FBS
and 1% penicillin and streptomycin in the presence of sodium
butyrate (10 and 100 μM), roscovitine (10 and 100 nM), sulphoraphan
(1 and 10 nM), dibucain (0.1 or 1 μM), Bay K 8644 (1 or 10 μM),
PD98059 (1 or 10 μM), wortmannin (0.1 or 1 μM), DRB (0.1 or 1 μM),
or gemcitabine (50 or 100 nM) for 3 days. After culture, the cells
were detached with trypsin from each culture dishes and
counted.
Cell death
MDA-MB-231 wild-type cells
(1×105/ml/well) and MDA-MB-231 cells
(1×105/ml/well) transfected with either full length,
deleted exon 4 or deleted exons 4 and 5 regucalcin cDNAs were
cultured using a 24-well plate in DMEM containing 10% FBS and 1%
penicillin and streptomycin for 5 days. Subconfluent cells were
cultured for additional 3 days in the presence or absence of LPS
(0.1 or 1 μg/ml), TNF-α (0.1 or 1 ng/ml) (30). In separate experiments, wild-type
MDA-MB-231 cells (1×105/ml/well) or transfectants were
cultured for 5 days to confluent, and then for an additional 24 h
in the presence or absence of LPS (1 ng/ml) or Bay K 8644 (10 μM)
with or without caspase-3 inhibitor (10 μM) (29). After culture, cells were detached
with trypsin from each culture dish.
Cell counting
After trypsinization of each culture dish using 0.2%
trypsin plus 0.02% EDTA in Ca2+/Mg2+-free PBS
for 2 min at 37°C, the detached cells from the dish were collected
by centrifugation (28–30). Cells were resuspended on PBS
solution and stained with eosin. Cell numbers were quantified by
counting under a microscope using a hemocytometer plate. For each
dish, we took the average of two counts. Cell number is shown as
number of cells per well.
Western blotting
MDA-MB-231 cells, which were transfected with
control vector or regucalcin cDNAs with full length, deleted exon 4
and deleted exons 4 and 5 were plated in 35-mm dishes at a density
of 1×106 cells/well in 2 ml of medium, and they were
cultured in DMEM containing 10% FBS and 1% penicillin and
streptomycin for 3 days. Cells were washed twice with ice cold PBS
and removed from the dish with a cell scraper. Recovered cells were
disrupted by sonication in 1.0 ml of ice cold PBS containing
protease and phosphatase inhibitors. The homogenate was centrifuged
for 5 min at 1,500 x g to obtain cell debris, and then the
supernatant including cytoplasm, nucleus and other cell fractions
were collected. The concentration of protein was determined using
Bradford dye reagent (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) using bovine serum albumin as a standard. Samples 30 μg of the
supernatant protein per lane were separated by SDS-PAGE and
transferred to nylon membranes for western blotting using specific
antibodies against regucalcin (including 33, 25 and 20 kDa)
(21) and other proteins (Santa
Cruz Biotechnology). Loading controls consisted of β-actin for
cytosolic proteins. A minimum of 3 blots from independent
experiments were scanned on an Epson Perfection 1660 Photo scanner,
and bands quantitated using ImageJ. Data from independent
experiments were normalized as a percentage of control before
averaging.
Animals and bone marrow cells
Female mice (CD1-Elite, wild-type, 2 months old),
which were purchased from Charles River, were housed in a
non-specific pathogen-free facility, and all procedures and
protocols were approved through the Institutional Animal Care and
Use Committee of Emory University. The femur and tibia were removed
immediately after sacrifice (31).
Bone marrow cells were isolated under sterile conditions from the
femurs and tibias.
Bone cells
The preosteoblastic cell line MC3T3-E1, clone 14
(MC3T3), was purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and cultured as previously described
(31).
Mineralization in co-culture of bone
marrow, preosteoblastic MC3T3 and breast cancer cells
To determine the effects of breast cancer cells on
osteoblastogenesis and mineralization of bone marrow or
preosteoblastic MC3T3, we used mineralization medium (MM)
containing ascorbic acid (100 ng/ml) and 4 mM β-glycerophosphate in
DMEM with 10% FBS and 1% penicillin and streptomycin. Bone marrow
cells (1×106 cells/1 ml/well) or preosteoblastic MC3T3
(2×105 cells/1 ml/well) were cultured for 3 days at 37°C
in a humidified 5% CO2 atmosphere, and then the cells
were co-cultured with addition of breast cancer MDA-MB-231 cells
(1×104 cells/1 ml/well) of wild-type or transfectant
using 12-well plates in α-MEM in the presence or absence of MM
containing ascorbic acid (100 ng/ml) and 4 mM β-glycerophosphate
for 18 days (31). The medium was
changed every 3 days. After culture, cells were washed with PBS and
stained with Alizarin red stain. For quantitation, 10%
cetylpyridinium chloride solution was added to each well to elute
the dye and absorbance was measured at 570 nm on a microtiter plate
reader (31).
Osteoclastogenesis in co-culture with
bone marrow cell and breast cancer cells
To determine the effects of breast cancer cells on
bone marrow osteoclastogenesis, bone marrow cells (2×105
cells/1 ml/well) were cultured in DMEM containing 10% FBS and 1%
penicillin and streptomycin using 24-well plates (1.0 ml/well)
(31). Bone marrow cells were
co-cultured in the presence of wild-type (1×104 cells/1
ml/well) or transfectant (1×104 cells/1 ml/well) with
full length of regucalcin cDNA for 3 days and then 0.5 ml of the
old medium was replaced with fresh medium, and cultures were
maintained for an additional 4 days. In other experiments, bone
marrow cells (2×105 cells/1 ml/well) were cultured for 3
days in medium and then fresh medium added. Cells were co-cultured
with MDA-MB-231 cells [wild-type (1×104 cells/1 ml/well)
or transfectants (1×104 cells/1 ml/well)] for additional
4 days (31). After culture for 7
days, the cells adherent to the 24-well plates were stained for
tartrate-resistant acid phosphatase (TRACP), a marker enzyme of
osteoclasts (32). Briefly, the
cells were washed with phosphate-buffered saline solution and fixed
with 10% neutralized formalin-phosphate (pH 7.2) for 10 min. After
the culture dishes were dried, TRACP staining was applied (32). The fixed cells were incubated for
90 min at room temperature in acetate buffer (pH 5.0) containing
naphthol AS-MX phosphate (Sigma) as a stain for the reaction
product, in the presence of 10 mM sodium tartrate. TRACP-positive
multinucleated cells (MNCs) containing three or more nuclei were
counted as osteoclast-like cells. MNCs scored were the mean ± SDM
of six cultures.
Statistical analysis
Survival curves were constructed by Kaplan-Meier
analysis and were compared with the log-rank test as performed with
IBM SPSS Statistics 18 software (IBM, Chicago, IL, USA; http://www.ibm.com). In the experiments with
MDA-MB-231 cells, statistical significance was determined using
GraphPad InStat version 3 for Windows XP (GraphPad Software, Inc.,
La Jolla, CA, USA). Multiple comparisons were performed by one-way
analysis of variance (ANOVA) with Tukey-Kramer multiple comparisons
post-test for parametric data as indicated. P<0.05 was
considered statistically significant.
Results
Survival in patients with breast
cancer
To understand the involvement of regucalcin in human
patients with breast cancer, we compared the clinical outcome
between 44 patients with higher regucalcin expression and 43
patients with lower regucalcin expression. There was a significant
difference of regucalcin expression between the two groups
(Fig. 1A). The reduction of
regucalcin expression was associated with poor prognosis in
patients with breast cancer. Breast cancer patients with the higher
regucalcin gene expression were found to have prolonged
relapse-free survival (Fig. 1B).
These findings support the view that the suppression of regucalcin
gene expression partly contributes to the development of
carcinogenesis in human breast cancer cells and leads to a worse
clinical outcome.
Generation of MDA-MB-231 cells
overexpressed with regucalcin
The cDNA-encoding human regucalcin with full length
(33 kDa), deleted exon 4 (25 kDa), and deleted exons 4 and 5 (20
kDa) was cloned into the expression vector pCXN2. Human breast
cancer MDA-MB-231 cells were transiently transfected with the pCXN2
vector or regucalcin/pCXN2 construct by lipofection. To generate
the transfectants stably overexpressing regucalcin in MDA-MB-231
cells, pCXN2 vector- or regucalcin/pCXN2-transfected MDA-MB-231
cells were cultured in neomycin-containing medium. Multiple
neomycin-resistant clones were selected, and the regucalcin content
of these clones was analyzed by immunoblotting with an
anti-regucalcin antibody. The regucalcin content in cells
transfected with regucalcin cDNA vector of full length (33 kDa) was
increased 15.5-fold as compared with that of the parental wild-type
MDA-MB-231 cells (Fig. 2A).
However, the proteins of 25 and 20 kDa were not expressed in
MDA-MB-231 cells transfected with deleted exon 4 (25 kDa) and
deleted exons 4 and 5 (20 kDa) (Fig.
2A).
 | Figure 2Overexpression of regucalcin
suppresses the proliferation in MDA-MB-231 human breast cancer
cells transfected with regucalcin cDNA vector including full
length, deleted exon 4, and deleted exons 4 and 5 in vitro.
(A) RGN content of multiple neomycin-resistant cells was analyzed
by immunoblotting with an anti-regucalcin antibody. Lane 1,
wild-type cells (designated as Wild). Lane 2, cells transfected
with RGN (deleted exons 4 and 5)/pCXN2 (designated as -4/5). Lane
3, cells transfected with RGN (deleted exon 4)/pCXN2 (designated as
-4). Lane 4, cells transfected with RGN (full length)/pCNX2
(designated as Full length). Lane 5, cells transfected with pCXN2
(designated as Mock). (B–E) Wild-type cells and transfectants were
cultured in DMEM for 1 (B), 2 (C), 3 (D) or 7 (E) days. After
culture, the number of attached cells on dish was counted. Data are
presented as mean ± SD of 2 replicate wells per data set using
different dishes and cell preparation. *P<0.001 vs.
wild-type (white bar) or control vector (grey bar). One way ANOVA,
Tukey-Kramer post-test. RGN, regucalcin. |
Overexpression of regucalcin suppresses
the proliferation of MDA-MB-231 cells
To determine the effects of the over-expression of
endogenous regucalcin on the proliferation of MDA-MB-231 cells
in vitro, the cancer cells were cultured for 1, 2, 3 and 7
days. Numbers of wild-type cells were increased over time in
culture (Fig. 2B–E). This increase
was suppressed in MDA-MB-231 cells transfectanted with regucalcin
cDNA of full length (34 kDa) for 1 (Fig. 2B), 2 (Fig. 2C), 3 (Fig. 2D) and 7 (Fig. 2E) days. The proliferations of
MDA-MB-231 cells transfected with exon 4-deleted regucalcin cDNA or
the exons 4 and 5-deleted regucalcin cDNA were not significantly
suppressed with culture for 7 days as compared with that of the
transfectants with the regucalcin cDNA of full length (Fig. 2B–E). Overexpression of regucalcin
with full length was found to specifically exhibit suppressive
effects on the proliferation of MDA-MB-231 cells in
vitro.
Proliferation in MDA-MB-231 cells was determined in
the presence of various inhibitors that induce cell cycle arrest
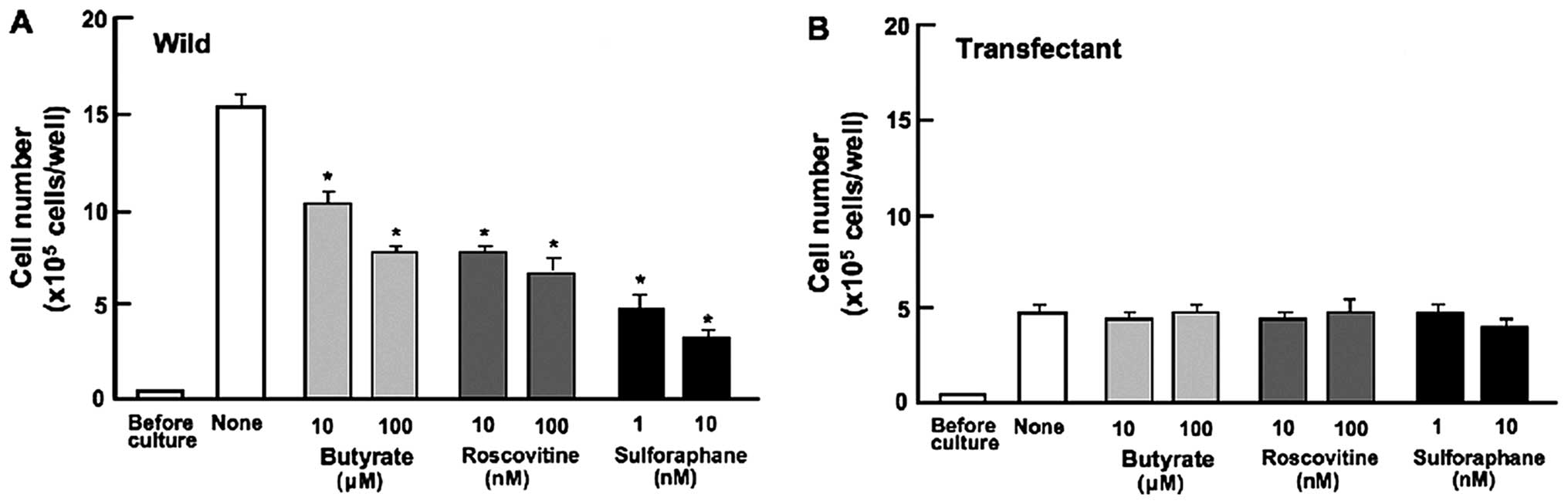
in vitro (Fig. 3).
Wild-type cells were cultured for 3 days in the presence of
butyrate (10 and 100 μM), roscovitine (10 and 100 nM) or
sulforaphane (1 and 10 nM) (28,33,34).
Cell proliferation was suppressed in the presence of these
inhibitors (Fig. 3A). Such effects
were not revealed in the transfectants (Fig. 3B). Endogenous regucalcin was
suggested to induce G1 and G2/M phase cell cycle arrest in
MDA-MB-231 cells.
Next, to determine the mechanistic characterization
for suppressive effects of regucalcin on cell proliferation, we
examined whether suppressive effects of overexpression of
regucalcin on the proliferation of MDA-MB-231 cells are modulated
by various signaling factors that suppress the proliferation.
Proliferation in MDA-MB-231 cells (wild-type) was suppressed in the
presence of dibucaine (0.1 or 1 μM), an inhibitor of
calcium/calmodulin-dependent protein kinases (28), or Bay K 8644 (0.1 or 1 μM), an
agonist of calcium entry into cells (35) (Fig.
4A). Such effects were not seen in transfectants (Fig. 4B). Likewise, the proliferation of
MDA-MB-231 cells (wild-type) was suppressed by culture with
wortmannin (0.1 or 1 μM), an inhibitor of phosphatidylinositol
3-kinase (PI3K) (36), and PD98059
(1 or 10 μM), an extracellular signal-regulated kinase (ERK)
inhibitor (37) (Fig. 4C). Suppressive effects of these
inhibitors on cell proliferation were not revealed in transfectants
(Fig. 4D). DRB is an inhibitor of
transcriptional activity with RNA polymerase II inhibition
(38). Gemcitabine is a strong
antitumor agent that induces nuclear DNA damage (39). Proliferation of MDA-MB-231 cells
was suppressed by culture with DRB (0.1 or 1 μM) or gemcitabine (50
or 100 nM) (Fig. 4E). However, the
suppressive effects of DRB, but not gemcitabine, were not
potentiated in transfectants (Fig.
4F).
 | Figure 4Suppressive effects of various
inhibitors of signaling pathways on the proliferation in MDA-MB-231
human breast cancer cells are not exhibited in the transfectants
overexpressed with regucalcin full length in vitro.
Wild-type cells (A, C and E) or transfectants (B, D and F)
overexpressed with regucalcin of full length were cultured in the
absence or presence of dibucaine (0.1 or 1 μM), Bay K 8644 (0.1 or
1 μM), wortmannin (0.1 or 1 μM), PD98059 (1 or 10 μM), DRB (0.1 or
1 μM), or gemcitabine (50 or 100 nM) for 3 days. After culture, the
number of attached cells on the dish was counted. Data are
presented as mean ± SD of 2 replicate wells per data set using
different dishes and cell preparation. *P<0.001 vs.
control (none; white bar). One way ANOVA, Tukey-Kramer post-test.
Wild, wild-type cells. |
Overexpression of regucalcin protects
cell death in MDA-MB-231 cells
To determine the effects of the overexpression of
regucalcin on cell death in MDA-MB-231 cells, the cells were
cultured for 5 days to reach subconfluency. Subconfluent cells were
cultured for an additional 24 h. Number of wild-type cells was
decreased in the presence of LPS (0.1 or 1 μg/ml) or TNF-α (0.1 or
1 ng/ml), which is known to induce apoptotic cell death (30) (Fig.
5A). Such effects were not exhibited in transfectants
overexpressing regucalcin full length (Fig. 5B). In addition, stimulatory effects
of LPS (0.1 or 1 μg/ml) or TNF-α (0.1 or 1 ng/ml) on apoptotic cell
death were exhibited in MDA-MB-231 cells transfected with the
regucalcin cDNA deleted with the exon 4 or with the exons 4 and 5
(Fig. 5C and D). Thus,
overexpression of regucalcin with full length was found to
specifically protect cell death induced by LPS or TNF-α in
MDA-MB-231 cells.
To determine whether the preventive effects of
regucalcin on cell death are involved in caspase-3, MDA-MB-231
wild-type cells and transfectants (with full length of regucalcin)
were cultured for 5 days to subconfluency, and then the cells were
additionally cultured in the presence of LPS (1 μg/ml) or Bay K
8644 (1 μM) with or without caspase-3 inhibitors (10 μM) for 24 h
(Fig. 5E and F). Stimulatory
effects of LPS or Bay K 8644 on cell death were completely
prevented in the presence of caspase-3 inhibitor (Fig. 5E). LPS- or Bay K 8644-induced cell
death was not seen in transfectants in the presence or absence of
caspase-3 inhibitor (Fig. 5F).
Thus, overexpression of regucalcin prevents cell death due to
decreasing the activity of caspase-3 that activates nuclear DNA
fragmentation, which induces apoptosis of cells.
Changes in various protein levels related
to cell signaling
It was examined whether overexpression of regucalcin
regulates protein levels related to cell signalings in MDA-MB-231
cells in vitro using westren blot analysis (Fig. 6). Protein levels of Akt,
phospho-Akt, MAPK, phospho-MAPK, SAPK/JNK, and phospho-SAPK/JNK
were decreased by overexpression of regucalcin (Fig. 6A). These results suggested that
over-expression of regucalcin suppresses signaling pathways that
are related to activation of EGFR in MDA-MB-231 cells. Moreover,
overexpression of regucalcin increased protein level of p53, a
tumor suppressor protein, and it decreased K-ras, c-fos and c-jun,
an oncogene, in MDA-MB-231 cells (Fig.
6B). Notably, overexpression of regucalcin was found to
decrease protein levels of β-catenin, a transcription factor
related to Wnt signaling, and p65 related to NF-κB signaling
(Fig. 6B). In addition,
overexpression of regucalcin decreased protein levels of caspase-3
and cleaved caspase-3 (Fig.
6C).
Overexpression of regucalcin suppresses
the differentiation of bone marrow cells co-cultured with
MDA-MB-231 cells
To determine an involvement of regucalcin in the
bone metastasis of MDA-MB-231 cells, we examined changes in
mineralizations in bone marrow osteoblasts or of the osteoblastic
cell line MC3T3 co-cultured with MDA-MB-231 cells in vitro
(Fig. 7). Bone marrow cells were
cultured in the presence or absence of mineralization medium (MM)
(Fig. 7A). After 3 days, bone
marrow cells were co-cultured with addition of MDA-MB-231 cells
(wild-type) or transfectants for 18 days that revealed
mineralization. Mineralization in bone marrow cells was suppressed
by co-culture with MDA-MB-231 cells. This suppression was prevented
in the presence of transfectants (Fig.
7A). Next, preosteoblastic MC3T3 cells were cultured for 3
days, and then the cells were co-cultured with addition of
MDA-MB-231 cells (wild-type) or transfectants in medium containing
MM for additional 18 days in vitro (Fig. 7B). Co-culture with MDA-MB-231 cells
suppressed mineralization in preosteoblastic MC3T3 cells. This
suppresstion was not exhibited in the case of transfectants
(Fig. 7B).
Moreover, we examined the effects of overexpression
of regucalcin on osteoclastogenesis in vitro (Fig. 8). Mouse bone marrow cells were
co-cultured in the presence or absence of MDA-MB-231 cells
(wild-type) or transfectants overexpressed with regucalcin of full
length for 7 days (Fig. 8A).
Osteoclastogenesis in bone marrow cells was markedly enhanced with
MDA-MB-231 cells (wild-type). However, such an effect was not seen
in the case of transfectants (Fig.
8A). Next, bone marrow cells were cultured for 3 days, and then
MDA-MB-231 cells (wild-type) or transfectants were seeded on bone
marrow cells, and those cells were cultured for additional 4 days
(Fig. 8B). Overexpression of
regucalcin markedly suppressed osteoclastogenesis enhanced by
co-culture with MDA-MB-231 cells (Fig.
8B).
Discussion
The present study demonstrates that relapse-free
survival was prolonged in the breast cancer patients with increased
regucalcin gene expression, and that overexpression of regucalcin
with full length (33 kDa) suppresses the proliferation and bone
cell effect in culture of MDA-MB-231 human breast cancer cells
in vitro model. These findings may support the view that
regucalcin is involved as a suppressive factor in human breast
cancer.
Alternatively spliced variants with the deleted exon
4 (25 kDa) and deleted exons 4 and 5 (20 kDa) of the regucalcin
cDNA have been shown to be present in various types of human cells
and tissues, although their protein levels were extremely low
(21). MDA-MB-231 cells
transfected with these cDNA vectors did not exhibit significant
suppressive effects on the proliferation and apoptotic cell death.
In addition, the proteins, which corresponded to these variants,
were not expressed in MDA-MB-231 cells transfected with regucalcin
cDNA of above variants. Thus, overexpression of regucalcin with
full length was found to specifically exhibit suppressive effects
on the proliferation and death in MDA-MB-231 cells in
vitro.
Suppressive effects of regucalcin overexpression on
the proliferation of MDA-MB-231 cells were not exhibited in the
presence of butyrate, roscovitine or sulphoraphan that induce cell
cycle arrest. Roscovitine is a potent and selective inhibitor of
the cyclin-dependent kinase cdc2, cdk2m and cdk5 (33). Sulforaphane induces G2/M phase cell
cycle arrest (34). Butyrate
induces inhibition of G1 progression (28). Regucalcin was suggested to induce
G1 and G2/M phase cell cycle arrest in MDA-MB-231 cells.
To determine the mechanistic characterization of the
suppressive effects of regucalcin on cell proliferation, we used
various inhibitors that regulate intracellular signaling processes.
Suppressive effects of regucalcin overexpression on the
proliferation in MDA-MB-231 cells were not potentiated in the
presence of TNF-α, an enhancer of NF-κB signaling (40), Bay K 8644, an agonist of
Ca2+ entry in cells (35), PD98059, an inhibitor of
ERK/mitogen-activated protein (MAP) kinase signaling pathway
(36,37), and wortmannin, an inhibitor of
PI3/Akt signaling pathway (36),
which suppressed the proliferation of wild-type cells. Regucalcin
may exhibit suppressive effects on the proliferation by inhibiting
various intracellular signaling pathways in MDA-MB-231 cells. We
confirmed that the protein levels of Akt, phospho-Akt, MAPK,
phospho-MAPK, SAPK/JNK and phospho-SAPK/JNK were decreased by
overexpression of regucalcin. Thus, regucalcin may suppress
signaling pathways related to EGFR in breast cancer cells.
Moreover, overexpression of regucalcin suppressed protein levels of
β-catenin and NF-κB p65, which are transcription factors related to
cell signaling. These proteins are known to constitutively
expressed in breast cancer cells (8). Regucalcin may reveal suppressive
effects on transcription activity related to β-catenin and NF-κB
signalings.
Moreover, suppressive effects of regucalcin
overexpression on cell proliferation were not potentiated in the
presence of DRB, an inhibitor of transcriptional activity with RNA
polymerase II inhibition (38).
Regucalcin has been shown to suppress transcriptional activity in
the nucleus of MDA-MB-231 cells (15). Thus molecular mechanism showed
similarity to the action of regucalcin in cloned normal rat kidney
proximal epithelial cells and cloned rat hepatoma H4-II-E cells
in vitro (28,29). Suppressive effects of regucalcin on
the proliferation were independent on the death in MDA-MB-231
cells, since regucalcin prevents cell death induced by various
stimulatory factors.
Gemcitabine is an antitumor agent that induces
nuclear DNA damage (39). This
agent is known to suppress cell proliferation and stimulate
apoptotic cell death in various types of cancer cells (39). Suppressive effects of regucalcin
overexpression on the proliferation were furthermore suppressed in
the presence of gemcitabine in MDA-MB-231 cells, suggesting that
regucalcin partly acts via different pathways in the action mode of
gemcitabine.
Overexpression of regucalcin has been shown to
prevent apoptotic cell death induced by various stimulatory factors
including TNF-α, LPS, thapasigargin, and Bay K 8644 in cloned
normal rat kidney proximal epithelial cells and cloned rat hepatoma
H4-II-E cells in vitro (29,30).
Overexpression of regucalcin was found to suppress death induced by
various stimulatory factors in MDA-MB-231 cells in vitro.
This effect was not exhibited in the presence of caspase-3
inhibitor. In addition, overexpression of regucalcin decreased
protein levels of caspase-3 and cleaved caspase-3. Regucalcin may
prevent cell death through the mechanism by which it decreases the
activity of caspase-3 that activates nuclear DNA fragmentation and
induces apoptosis. Regucalcin may directly inhibit caspase-3
activity. Regucalcin has also been shown to directly inhibit
calcium-activated endonuclease in rat liver nucleus in vitro
(41).
Bone marrow mesenchymal stem cells are multipotent
cells, which among other cell lineages give rise to adipocytes and
osteoblasts (42,43). This occurs through cross talk
between complex signaling pathways including those derived from
bone morphogenic proteins, winglesstype MMTV integration site (Wnt)
proteins, hedgehogs, delta/jagged proteins, transcriptional
regulators including peroxisome proliferators-activated
receptor-gamma (PPARγ) and runt-related transcription factor 2
(Runx2) and MAPK/ERK signaling pathway (42–45).
We determined whether overexpression of regucalcin exhibits
suppressive effects on bone metastasis activity of MDA-MB-231 cells
using co-culture system with bone marrow cells in vitro.
This in vitro model may be a useful tool in estimation of
bone metastasis activity in vitro (31). Osteoblastic mineralization in mouse
bone marrow cells was markedly suppressed after co-culture with
MDA-MB-231 cells in vitro. Such an effect was also observed
in the case of preosteoblastic MC3T3 cells in vitro. Thus,
MDA-MB-231 cells were confirmed to directly suppress osteoblastic
mineralization in vitro. TNF-α, which is produced in breast
cancer cells (3,6,7),
suppresses osteoblastic mineralization that is mediated through
activation of NF-κB signaling (40). MDA-MB-231 cell-induced suppression
of osteoblastic mineralization may be partly related to TNF-α,
which is produced by breast cancer cells. Moreover, overexpression
of regucalcin was found to prevent the suppression of osteoblastic
mineralization in bone marrow cells and preosteoblastic MC3T3
cells, which were induced by co-culture with MDA-MB-231 cells.
Regucalcin may prevent suppression of osteoblastic mineralization
induced by TNF-α in preosteoblastic MC3T3 through suppressing of
TNF-α-induced activation NF-κB signaling in preosteoblastic MC3T3
in vitro.
Osteoclasts are differentiated from hematopoietic
precursors of the monocyte/macrophage lineage by stimulation with a
TNF family cytokine, RANKL and macrophage-colony stimulating factor
(46). Osteoclastogenesis in mouse
bone marrow culture in the absence of bone resorbing-factors was
enhanced by co-culture with MDA-MB-231 cells in vitro.
Breast cancer cells are known to produce RANKL, which plays a
pivotal role in formation from preosteoclastic cells to mature
osteoclasts (7). Stimulatory
effects of MDA-MB-231 cells on osteoclastogenesis in bone marrow
culture may be due to RANKL, which may be produced in breast cancer
cells. Overexpression of regucalcin was found to suppress
osteoclastogenesis in bone marrow cell culture enhanced by
co-culture with MDA-MB-231 cells in vitro. This suppressive
effect may be related by antagonizing activation of NF-κB signaling
induced by RANKL. Overexpressed regucalcin may suppress the
activation of NF-κB signaling process in MDA-MB-231 cells in
vitro.
Importantly, overexpression of regucalcin was found
to decrease protein levels of β-catenin, a transcription factor
related to Wnt signaling, and p65 involved in NF-κB signaling in
MDA-MB-231 cells (47,48). Regucalcin may exhibit potential
suppressive effects on signaling process related to β-catenin and
NF-κB that are transcription factors in MDA-MB-231 cells. Such
effects of regucalcin may be related to suppression of metastatic
bone activity in MDA-MB-231 breast cancer cells, although further
mechanism remains to be elucidated.
Regucalcin gene expression has been shown to be
depressed in human breast cancer tissues as compared with that in
normal tissues (20). This
suggests that downregulated regucalcin gene expression is involved
in carcinogenesis of breast cells and that its cell function is
disordered. Our findings support the view that suppressed
regucalcin gene expression may lead to disturbance of the functions
of breast cells and development to carcinogenesis, since regucalcin
plays a pivotal role as a suppressor protein in intracellular
signaling processes in various types of cells and tissues (13–17).
Overexpression of regucalcin may play a potential role in the
prevention and therapy of breast cancer.
In conclusion, the present study demonstrates that
the relapse-free survival is prolonged in the breast cancer
patients with increased regucalcin gene expression, and that
overexpression of regucalcin exhibits anti-proliferation and
anti-bone metastatic activity in MDA-MB-231 human breast cancer
bone metastatic cells in vitro. Overexpression of the
regucalcin gene may be a new useful tool in the prevention and
therapy in breast cancer bone metastasis in vivo.
References
|
1
|
Boyce BF, Yoneda T and Guise TA: Factors
regulating the growth of metastatic cancer in bone. Endocr Relat
Cancer. 6:333–347. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akhtari M, Mansuri J, Newman KA, Guise TM
and Seth P: Biology of breast cancer bone metastasis. Cancer Biol
Ther. 7:3–9. 2008. View Article : Google Scholar
|
|
5
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YC, Sosnoski DM and Mastro AM: Breast
cancer metastasis to the bone: Mechanisms of bone loss. Breast
Cancer Res. 12:2152010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonzalez-Suarez E, Jacob AP, Jones J,
Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D and
Dougall WC: RANK ligand mediates progestin-induced mammary
epithelial proliferation and carcinogenesis. Nature. 468:103–107.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park BK, Zhang H, Zeng Q, Dai J, Keller
ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, et al: NF-kappaB in
breast cancer cells promotes osteolytic bone metastasis by inducing
osteoclastogenesis via GM-CSF. Nat Med. 13:62–69. 2007. View Article : Google Scholar
|
|
9
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimokawa N and Yamaguchi M: Molecular
cloning and sequencing of the cDNA coding for a calcium-binding
protein regucalcin from rat liver. FEBS Lett. 327:251–255. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimokawa N, Matsuda Y and Yamaguchi M:
Genomic cloning and chromosomal assignment of rat regucalcin gene.
Mol Cell Biochem. 151:157–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiselton DL, McDowall J, Brandau O,
Ramser J, d'Esposito F, Bhattacharya SS, Ross MT, Hardcastle AJ and
Meindl A: An integrated, functionally annotated gene map of the
DXS8026-ELK1 interval on human Xp11.3-Xp11.23: Potential hotspot
for neurogenetic disorders. Genomics. 79:560–572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamaguchi M: Role of regucalcin in
maintaining cell homeostasis and function (Review). Int J Mol Med.
15:371–389. 2005.PubMed/NCBI
|
|
14
|
Yamaguchi M: Regucalcin and cell
regulation: Role as a suppressor in cell signaling. Mol Cell
Biochem. 353:101–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi M: The transcriptional
regulation of regucalcin gene expression. Mol Cell Biochem.
346:147–171. 2011. View Article : Google Scholar
|
|
16
|
Yamaguchi M: Role of regucalcin in cell
nuclear regulation: Involvement as a transcription factor. Cell
Tissue Res. 354:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamaguchi M: Suppressive role of
regucalcin in liver cell proliferation: Involvement in
carcinogenesis. Cell Prolif. 46:243–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaguchi M: The anti-apoptotic effect of
regucalcin is mediated through multisignaling pathways. Apoptosis.
18:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi M: Involvement of regucalcin as
a suppressor protein in human carcinogenesis: Insight into the gene
therapy. J Cancer Res Clin Oncol. 141:1333–1341. 2015. View Article : Google Scholar
|
|
20
|
Maia C, Santos C, Schmitt F and Socorro S:
Regucalcin is under-expressed in human breast and prostate cancers:
Effect of sex steroid hormones. J Cell Biochem. 107:667–676. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murata T and Yamaguchi M: Alternatively
spliced variants of the regucalcin gene in various human normal and
tumor tissues. Int J Mol Med. 34:1141–1146. 2014.PubMed/NCBI
|
|
22
|
Yamaguchi M, Osuka S, Weitzmann MN,
El-Rayes BF, Shoji M and Murata T: Prolonged survival in pancreatic
cancer patients with increased regucalcin gene expression:
Overexpression of regucalcin suppresses the proliferation in human
pancreatic cancer MIA PaCa-2 cells in vitro. Int J Oncol.
48:1955–1964. 2016.PubMed/NCBI
|
|
23
|
Loi S, Haibe-Kains B, Desmedt C, Lallemand
F, Tutt AM, Gillet C, Ellis P, Harris A, Bergh J, Foekens JA, et
al: Definition of clinically distinct molecular subtypes in
estrogen receptor-positive breast carcinomas through genomic grade.
J Clin Oncol. 25:1239–1246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loi S, Haibe-Kains B, Desmedt C, Wirapati
P, Lallemand F, Tutt AM, Gillet C, Ellis P, Ryder K, Reid JF, et
al: Predicting prognosis using molecular profiling in estrogen
receptor-positive breast cancer treated with tamoxifen. BMC
Genomics. 9:2392008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loi S, Haibe-Kains B, Majjaj S, Lallemand
F, Durbecq V, Larsimont D, Gonzalez-Angulo AM, Pusztai L, Symmans
WF, Bardelli A, et al: PIK3CA mutations associated with gene
signature of low mTORC1 signaling and better outcomes in estrogen
receptor-positive breast cancer. Proc Natl Acad Sci USA.
107:10208–10213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoneda T, Williams PJ, Hiraga T, Niewolna
M and Nishimura R: A bone-seeking clone exhibits different
biological properties from the MDA-MB-231 parental human breast
cancer cells and a brain-seeking clone in vivo and in vitro. J Bone
Miner Res. 16:1486–1495. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Misawa H, Inagaki S and Yamaguchi M:
Suppression of cell proliferation and deoxyribonucleic acid
synthesis in the cloned rat hepatoma H4-II-E cells overexpressing
regucalcin. J Cell Biochem. 84:143–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa T, Sawada N and Yamaguchi M:
Overexpression of regucalcin suppresses cell proliferation of
cloned normal rat kidney proximal tubular epithelial NRK52E cells.
Int J Mol Med. 16:637–643. 2005.PubMed/NCBI
|
|
30
|
Izumi T and Yamaguchi M: Overexpression of
regucalcin suppresses cell death in cloned rat hepatoma H4-II-E
cells induced by tumor necrosis factor-alpha or thapsigargin. J
Cell Biochem. 92:296–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamaguchi M, Zhu S, Weitzmann MN, Snyder
JP and Shoji M: Curcumin analog UBS109 prevents bone marrow
osteoblastogenesis and osteoclastogenesis disordered by co-culture
with breast cancer MDA-MB-231 bone metastatic cells in vitro. Mol
Cell Biochem. 401:1–10. 2015. View Article : Google Scholar
|
|
32
|
Minkin C: Bone acid phosphatase:
Tartrate-resistant acid phosphatase as a marker osteoclast
function. Calcif Tissue Int. 34:285–290. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meijer L, Borgne A, Mulner O, Chong JP,
Blow JJ, Inagaki N, Inagaki M, Delcros JG and Moulinoux JP:
Biochemical and cellular effects of roscovitine, a potent and
selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and
cdk5. Eur J Biochem. 243:527–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh SV, Herman-Antosiewicz A, Singh AV,
Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L and Baskaran R:
Sulforaphane-induced G2/M phase cell cycle arrest involves
checkpoint kinase 2-mediated phosphorylation of cell division cycle
25C. J Biol Chem. 279:25813–25822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cano-Abad MF, Villarroya M, García AG,
Gabilan NH and López MG: Calcium entry through L-type calcium
channels causes mitochondrial disruption and chromaffin cell death.
J Biol Chem. 276:39695–39704. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Serrano-Nascimento C, da Silva Teixeira S,
Nicola JP, Nachbar RT, Masini-Repiso AM and Nunes MT: The acute
inhibitory effect of iodide excess on sodium/iodide symporter
expression and activity involves the PI3K/Akt signaling pathway.
Endocrinology. 155:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen QW, Edvinsson L and Xu CB: Role of
ERK/MAPK in endothelin receptor signaling in human aortic smooth
muscle cells. BMC Cell Biol. 10:522009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Palangat M, Grass JA, Langelier MF,
Coulombe B and Landick R: The RPB2 flap loop of human RNA
polymerase II is dispensable for transcription initiation and
elongation. Mol Cell Biol. 31:3312–3325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancer. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Li A, Strait K, Zhang H, Nanes MS
and Weitzmann MN: Endogenous TNFalpha lowers maximum peak bone mass
and inhibits osteoblastic Smad activation through NF-kappaB. J Bone
Miner Res. 22:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamaguchi M and Sakurai T: Inhibitory
effect of calcium-binding protein regucalcin on
Ca2+-activated DNA fragmentation in rat liver nuclei.
FEBS Lett. 279:281–284. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gharibi B, Abraham AA, Ham J and Evans BA:
Adenosine receptor subtype expression and activation influence the
differentiation of mesenchymal stem cells to osteoblasts and
adipocytes. J Bone Miner Res. 26:2112–2124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Muruganandan S, Roman AA and Sinal CJ:
Adipocyte differentiation of bone marrow-derived mesenchymal stem
cells: Cross talk with the osteoblastogenic program. Cell Mol Life
Sci. 66:236–253. 2009. View Article : Google Scholar
|
|
44
|
Wu L, Cai X, Dong H, Jing W, Huang Y, Yang
X, Wu Y and Lin Y: Serum regulates adipogenesis of mesenchymal stem
cells via MEK/ERK-dependent PPARgamma expression and
phosphorylation. J Cell Mol Med. 14:922–932. 2010. View Article : Google Scholar
|
|
45
|
Laudes M: Role of WNT signalling in the
determination of human mesenchymal stem cells into preadipocytes. J
Mol Endocrinol. 46:R65–R72. 2011.PubMed/NCBI
|
|
46
|
Zaidi M, Blair HC, Moonga BS, Abe E and
Huang CL: Osteoclastogenesis, bone resorption, and osteoclast-based
therapeutics. J Bone Miner Res. 18:599–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu J, Prosperi JR, Choudhury N, Olopade OI
and Goss KH: β-Catenin is required for the tumorigenic behavior of
triple-negative breast cancer cells. PLoS One. 10:e01170972015.
View Article : Google Scholar
|
|
48
|
Li L, Zhao F, Lu J, Li T, Yang H, Wu C and
Liu Y: Notch-1 signaling promotes the malignant features of human
breast cancer through NF-κB activation. PLoS One. 9:e959122014.
View Article : Google Scholar
|