Introduction
Cancer cell migration, invasion, and metastasis are
preceded by the formation of pseudopodia such as lamellipodia and
filopodia. During these cellular processes, F-actin filaments
remodel into a higher order structure and then assemble an
intricate cytoskeletal network within cells (1). These dynamic three-dimensional
changes are mediated by several actin-bundling and crosslinking
proteins, and are essential for supporting filopodia at the leading
edge of migrating cells (2).
Dynamin plays an essential role in endocytosis,
participating in the membrane fission process (3–5).
Dynamin also functions in the formation of actin-rich structures,
including lamellipodia and dorsal membrane ruffles (6,7),
invadopodia (8), podosomes
(9), growth cones (10–12),
and phagocytic cups (13,14). Three dynamin isoforms exist,
namely, dynamin 1, 2, and 3 (5).
Dynamins are characterized by a GTPase domain at the N-terminus, a
bundle signaling element, a stalk domain, a
phosphoinositide-binding pleckstrin homology domain, and a proline
and arginine-rich domain at the C-terminus (PRD) (15,16).
The PRD interacts with different proteins that contain the
Src-homology-3 (SH3) domain. Of these GTPases, dynamin 2 is
ubiquitously expressed.
Cortactin, an F-actin-binding protein, was first
identified as an Src substrate (17). Cortactin also participates in
cancer cell migration, invasion, and metastasis by regulating actin
dynamics at the leading edge of migrating cells (18). Cortactin is composed of an
N-terminal acidic domain and a six-and-a-half tandem repeats
domain, which directly binds to F-actin. Cortactin also contains an
α-helix, a proline-rich region, and an SH3 domain at the
C-terminus, which interacts with the PRD of several binding
partners (19).
Both dynamin and cortactin are implicated in the
dynamics of cancer cells, including migration, invasion, and
metastasis (18). In addition, the
pharmacological inhibition of dynamin by GTPase inhibitors
suppresses specific cellular processes such as the lamellipodial
formation and invasion of human osteocarcinoma cells (20) and the growth of human prostate
adenocarcinoma cells (21).
A previous study reported that dynamin 2 binds to
cortactin (7,12). A disruption of this protein complex
can affect the shape of cancer cells (7), organization of the F-actin network
within these cells (22), and
structure of growth cones (11,12).
However, the role of the dynamin 2-cortactin complex in the
dynamics of the actin cytoskeleton in cancer cells is unclear. In
this study, we investigated whether dynamin 2 and cortactin
regulate the F-actin bundle formation in filopodia in the human
non-small cell lung carcinoma cell line H1299.
Materials and methods
Antibodies and reagents
Rabbit polyclonal anti-dynamin 1 (cat. no. PA1-660;
Thermo Fisher Scientific, Waltham, MA, USA) and anti-c-myc (cat.
no. C3956; Sigma-Aldrich, St. Louis, MO, USA) antibodies, and a
goat polyclonal anti-dynamin 2 (cat. no. sc-6400; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) antibody, were purchased. In
addition, mouse monoclonal anti-β-actin (cat. no. A5441,
Sigma-Aldrich), Dynasore (cat. no. D7693, Sigma-Aldrich),
anti-c-myc (cat. no. sc-40; Santa Cruz Biotechnology), anti-green
fluorescent protein (GFP; cat. no. sc-9996, Santa Cruz
Biotechnology), and anti-cortactin (cat. no. 05-180; EMD Millipore,
Darmstadt, Germany) antibodies were purchased. MitMAB and Dynole
34-2 were purchased from Abcam Biochemicals (Bristol, UK). Alexa
Fluor 488-conjugated anti-rabbit IgG, rhodamine-conjugated
anti-mouse IgG, and rhodamine or Alexa Fluor 488-labeled phalloidin
were obtained from Thermo Fisher Scientific. Purified rabbit
skeletal α-actinin was purchased from Cytoskeleton, Inc. (Denver,
CO, USA). Goat anti-mouse IgG- and goat anti-rabbit IgG-conjugated
gold particles were purchased from British BioCell International
(Cardiff, UK).
Cell culture
The human non-small cell lung carcinoma cell line
H1299 (Cat. no. ATCC CRL-5803; American Type Culture Collection,
Manassas, VA, USA) was cultured in Dulbecco’s modified Eagle’s
medium (DMEM, Thermo Fisher Scientific) supplemented with 10% fetal
bovine serum (FBS) at 37°C in an atmosphere of 5%
CO2.
Expression and purification of dynamin 2
and cortactin wild-types and mutants
GFP-tagged dynamin 2 cloned into pEGFP-N1 was a kind
gift from Dr Mark McNiven (Mayo Clinic, Rochester, MN, USA)
(6). His-tagged dynamin 2 produced
with the Bac-to-Bac baculovirus expression system (Thermo Fisher
Scientific) was a kind gift from Dr Hiroshi Handa (Tokyo Institute
of Technology, Tokyo, Japan) (23). The dynamin solution was
concentrated using a Centriplus YM50 (Thermo Fisher Scientific) and
stored at −80°C. The protein suspension (2–5 mg/ml protein) was
thawed at 37°C before use.
The cDNAs encoding full-length rat cortactin and its
mutants were prepared by polymerase chain reaction amplification
using specific primers (12).
Full-length cortactin or 1-450aa (Cort ΔSH3) was subcloned into the
plasmid pGEX-6p vector as BamHI-EcoRI fragments.
GST-tagged cortactin W525K was generated by mutating
pGEX-6p-cortactin with the QuickChange site-directed mutagenesis
kit (Agilent Technologies, Santa Clara, CA, USA). For expression in
cells, full-length cortactin or Cort ΔSH3 was subcloned into the
pEF1 myc-His vector (Thermo Fisher Scientific) as
EcoRI-XbaI fragments. The nucleotide sequences of the
constructs were verified by DNA sequence analysis. The resulting
plasmid was transformed into the bacterial BL21 (DE3) pLysS strain
for protein expression. The expression of GST-fusion proteins was
induced by 0.1 mM isopropyl-1-thio-d-galactopyranoside at 37°C for
3–6 h in LB medium supplemented with 100 μg/ml ampicillin to
A600 = 0.8. The purification of GST-fusion proteins was
performed as previously described (24), and the cleavage of the GST with
PreScission protease was performed according to the manufacturer’s
instructions. The protein was purified on a MonoQ column
equilibrated in 20 mM Tris-HCl and 0.2 M NaCl, pH 7.7. The eluted
protein fraction (1 mg/ml protein) was stored at −80°C. For the
pull-down assay, the proteins were used without cleaving GST.
siRNA-mediated interference
Pre-annealed siRNAs for human dynamin 2 and
cortactin, and the negative control siRNA, were synthesized and
purified (Thermo Fisher Scientific). The sequences for the siRNAs
for human dynamin 2 were as follows: 5′-GGAUAUUGAGGGCAAGAAGtt-3′
(sense), 5′-CUUCUUGCCCUCAAUAUCCtt-3′ (antisense) for oligo 1;
5′-GCGAAUCGUCACCACUUACtt-3′ (sense), 5′-GUAAGUG GUGACGAUUCGCtc-3′
(antisense) for oligo 2; and 5′-GGAC UUACGACGGGAGAUCtt-3′ (sense),
5′-GAUCUCCCGU CGUAAGUCCtt-3′ (antisense) for oligo 3. The sequences
for the siRNAs for human cortactin were as follows: CCGAAUG
GAUAAGUCAGUCtt-3′ (sense), 5′-AGCUGACUUAUCCAU UCGGtc-3′ (antisense)
for oligo 1; GGUUUCGGCGGCA AAUACGtt-3′ (sense),
CGUAUUUGCCGCCGAAACCtt-3′ (antisense) for oligo2; and
CGAAUAUCAGUCGAAACUUtt-3′ (sense), AAGUUUCGACUGAUAUUCGtg-3′
(antisense) for oligo 3.
A scrambled siRNA with no significant sequence
homology to all mouse, rat, or human gene sequences was used as the
negative control. The day before transfection, the cells were
plated in 6-well plates (5×104 cells/well). One hundred
picomoles of the duplex siRNAs was transfected into the cells using
4 μl of Lipofectamine 2000 (Thermo Fisher Scientific). After 72 h,
the cells were treated differently according to experimental
design. In pilot experiments, we confirmed that all three
transfections of siRNA for dynamin 2 and cortactin were
effective.
Filopodial formation
H1299 cells were serum-starved for 16 h. Thereafter,
the cells were transfected with dynamin 2 siRNAs, cortactin siRNAs,
or the control siRNA, followed by incubation with DMEM supplemented
with 10% FBS for 45 min. For the rescue experiments, cortactin was
silenced in H1299 cells with oligo 3, and the cells were cultured
for 24 h. The cells (1×105/coverslip) were then
transfected with rat wild-type cortactin or cortactin W525K (0.25
μg each) cloned into the pIRES2-AcGFP1 expression vector (Clontech
Laboratories, Santa Clara, CA, USA). Thereafter, the cells were
stimulated with serum for 45 min, fixed, and stained with Alexa
Fluor 488 or rhodamine-labeled phalloidin for visualization of
filopodia.
Wound healing assay
H1299 cells were cultured to confluence on
glass-bottom dishes (35 mm diameter; AGC Techno Glass Co. Ltd.,
Tokyo, Japan) in DMEM supplemented with 0.2% FBS for 8 h.
Thereafter, the cell layer was wounded with a plastic pipette tip
as previously described (25). The
cells were washed with DMEM supplemented with 0.2% FBS and
incubated for 8 h in the presence of Dynasore, Dynole 34-2 or
MitMAB at the indicated concentrations. For the negative control,
cells were incubated with 1% dimethyl sulfoxide (DMSO). The cells
were visualized by Giemsa staining, followed by the acquisition of
phase contrast images from ≥20 randomly selected areas per dish.
Areas filled with migrating cells were analyzed with ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
Formation of in vitro F-actin
bundles
For the fluorescent detection of F-actin, non-muscle
actin was polymerized in F-buffer (10 mM Tris-HCl, 0.2 mM DTT, 0.2
mM CaCl2, 2 mM MgCl2, 50 mM KCl, and 0.5 mM
ATP, pH 7.5) for 1 h. Thereafter, 3.3 μM F-actin was incubated with
5 μM dynamin 1 or 2 and cortactin for 1 h, followed by an
additional 30 min with 3 μM Alexa Fluor 488-phalloidin. The samples
were spread onto glass slides and mounted, and the F-actin bundles
were observed under an epifluorescent microscope.
For the immunolocalization of dynamin and cortactin,
F-actin bundles were incubated with dynamin 1 or 2 and cortactin
for 30 min with 3 μM phalloidin to stabilize the filaments,
followed by centrifugation at 5,000 × g for 10 min. The pellet was
resuspended with 50 μl of F-buffer and then immunostained in
suspension for 30 min with 1 μl of primary antibody. The mixture
was centrifuged at 5,000 × g, and the pellet was washed with
F-buffer. The samples were incubated with secondary antibodies and
washed as previously done for the primary antibody. All steps were
performed at room temperature. The samples were spread onto glass
slides and mounted. The samples were examined under a spinning disc
confocal microscope system (CSU10, Yokogawa Electric Co., Tokyo,
Japan) combined with an inverted microscope (IX-71, Olympus Optical
Co., Ltd., Tokyo, Japan) and a CoolSNAP-HQ camera (Roper
Technologies, Sarasota, FL, USA). The confocal system was
controlled by MetaMorph software (Molecular Devices, Sunnyvale, CA,
USA). Images were processed using Adobe Photoshop CS3 or
Illustrator CS3 software.
Immunoprecipitation assay
For the immunoprecipitation assay, H1299 cells were
co-transfected with GFP-tagged dynamin 2 and either myc-tagged
cortactin or cortactin ΔSH3. The cells were lysed with 1% NP-40,
100 mM KCl, 0.5 mM EDTA, 10 mM NaF, and 20 mM HEPES/KOH, pH 7.4,
and a protease inhibitor cocktail tablet (Roche Diagnostics, Basel,
Switzerland). The protein complexes were immunoprecipitated from 1
mg of cell extract using either 5 μg of the polyclonal anti-myc
antibodies or preimmune IgG, and then visualized by western
blotting with a monoclonal anti-GFP or anti-myc antibody.
Immunostaining and fluorescent
microscopy
H1299 cells were fixed with 4% paraformaldehyde and
stained by immunofluorescence as previously described (12).
Transmission electron microscopy
Specimens were embedded for immunoelectron
microscopy as previously described (12). In brief, H1299 cells were fixed
with cytoskeleton buffer (10 mM 2-(N-morpholino)ethanesulfonic
acid, 150 mM NaCl, 5 mM EGTA, 5 mM MgCl2, and 5 mM
glucose, pH 6.0) containing 10 μg/ml phalloidin, 0.1% Triton X-100,
and 3% formaldehyde for 1 min. The cells were then fixed for an
additional 30 min without Triton X-100, followed by washing with 5
μg/ml phalloidin in phosphate-buffered saline (PBS). After
incubation in blocking solution (10 μg/ml phalloidin, 2 mg/ml BSA,
and 100 mM glycine in PBS), the specimens were incubated with a
primary antibody diluted in blocking solution, washed with 5 μg/ml
phalloidin in PBS, incubated with goat anti-mouse or rabbit
anti-goat IgG conjugated to 10-nm gold particles, and then fixed
with 2.5% glutaraldehyde and 5 μg/ml phalloidin in PBS. The
specimens were post-fixed with 1% OsO4 in 0.1 M sodium
cacodylate buffer for 1 h, dehydrated, and embedded in EPON 812 for
ultrathin sectioning. Cross-sections were visualized under a
Hitachi H-7100 transmission electron microscope.
Determination of filopodial length
For the measurement of the filopodial length, H1299
cells were fixed and stained with rhodamine- or Alexa Fluor
488-conjugated phalloidin. Membrane protrusions supported with
F-actin bundles were defined as filopodia, and digital images were
acquired at 400–1,000× magnifications. Up to five filopodia for
each cell were randomly selected, and their lengths were measured
with ImageJ software.
Statistical analysis
Data were analyzed for statistical significance
using KaleidaGraph software (version 4.1) for the Macintosh
(Synergy Software Inc., Essex Junction, VT, USA). Analysis of
variance and Tukey’s honest significant difference post hoc test
were applied for more than two different groups, and Student’s
t-test was applied for two different groups. P-values of <0.05
and 0.001 were considered as statistically significant.
Results
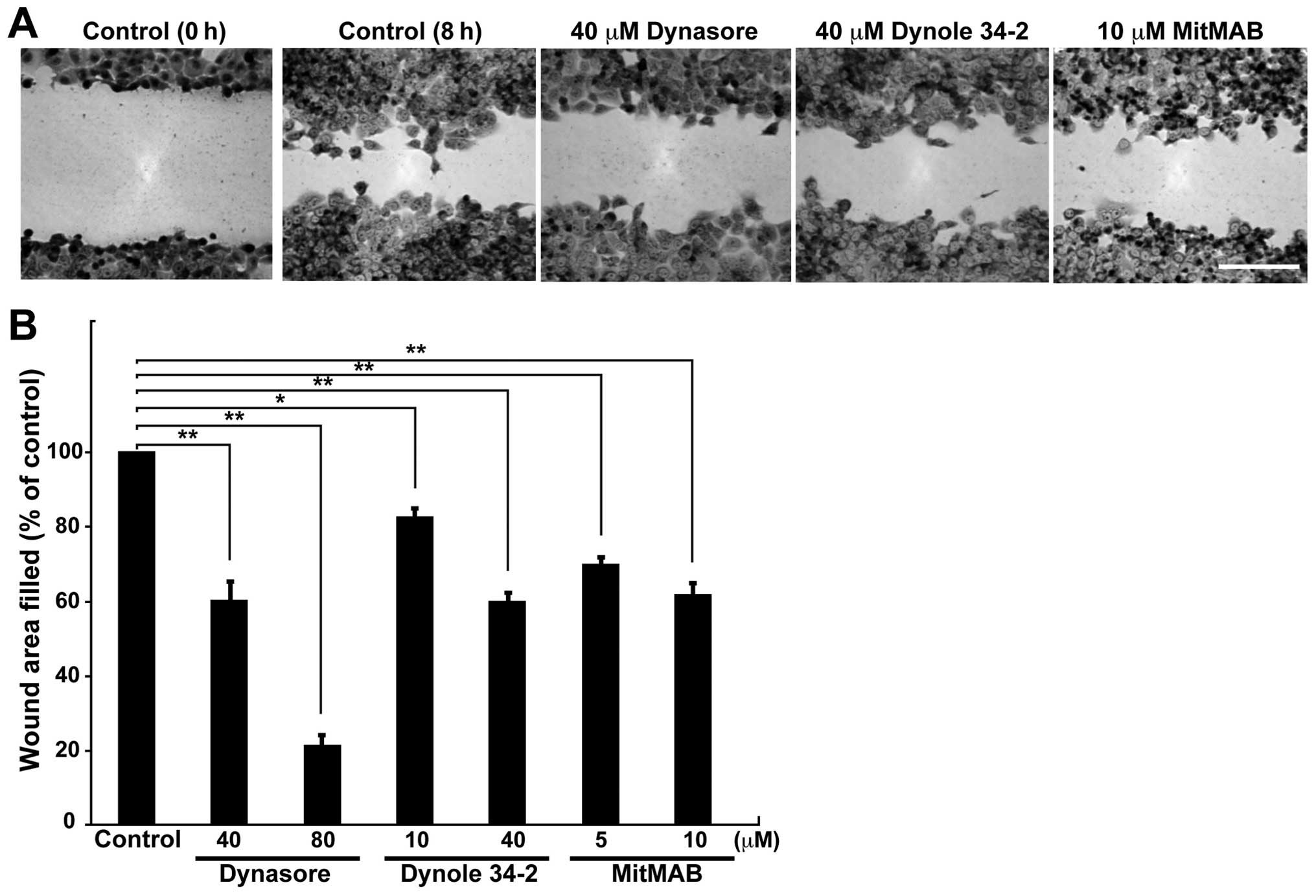
Inhibition of dynamin decreases the
migration of the human non-small cell lung carcinoma cell line
H1299
To determine whether dynamin 2 is involved in cell
migration, the effects of dynamin inhibition on cell migration were
determined by a wound healing assay. Cell migration decreased after
treatment of cells with Dynasore (26), Dynole 34-2 (27), and MitMAB (28) (Fig.
1A). Dynasore (80 μM) inhibited cell migration by ~80% compared
to that of control cells, whereas Dynole 34-2 and MitMAB inhibited
cell migration by 20–40% (Fig.
1B). These results indicate that dynamin is important for the
migration of H1299 cells.
Dynamin 2 colocalizes with cortactin
along F-actin bundles in filopodia of H1299 cells
Fig. 1 shows that
dynamin is involved in cell migration mediated by pseudopodia.
Thus, we investigated whether dynamin 2 participates in filopodial
formation in H1299 cells. Because cortactin functions with dynamin
1 in the bundling of F-actin, which is important for the stability
of filopodia in human neuroblastoma cell line SH-SY5Y (12), dynamin 2 and cortactin were
immunostained in serum-stimulated H1299 cells. H1299 cells formed
numerous filopodia after serum stimulation (Fig. 2A). Furthermore, dynamin 2 and
cortactin colocalized to F-actin bundles in filopodia as bright
dots (Fig. 2A). The negative
controls showed little immunoreactivity for dynamin 2 and cortactin
(Fig. 2B). In addition,
immunoelectron microscopy revealed that both proteins localized to
F-actin bundles in filopodia (Fig.
2C).
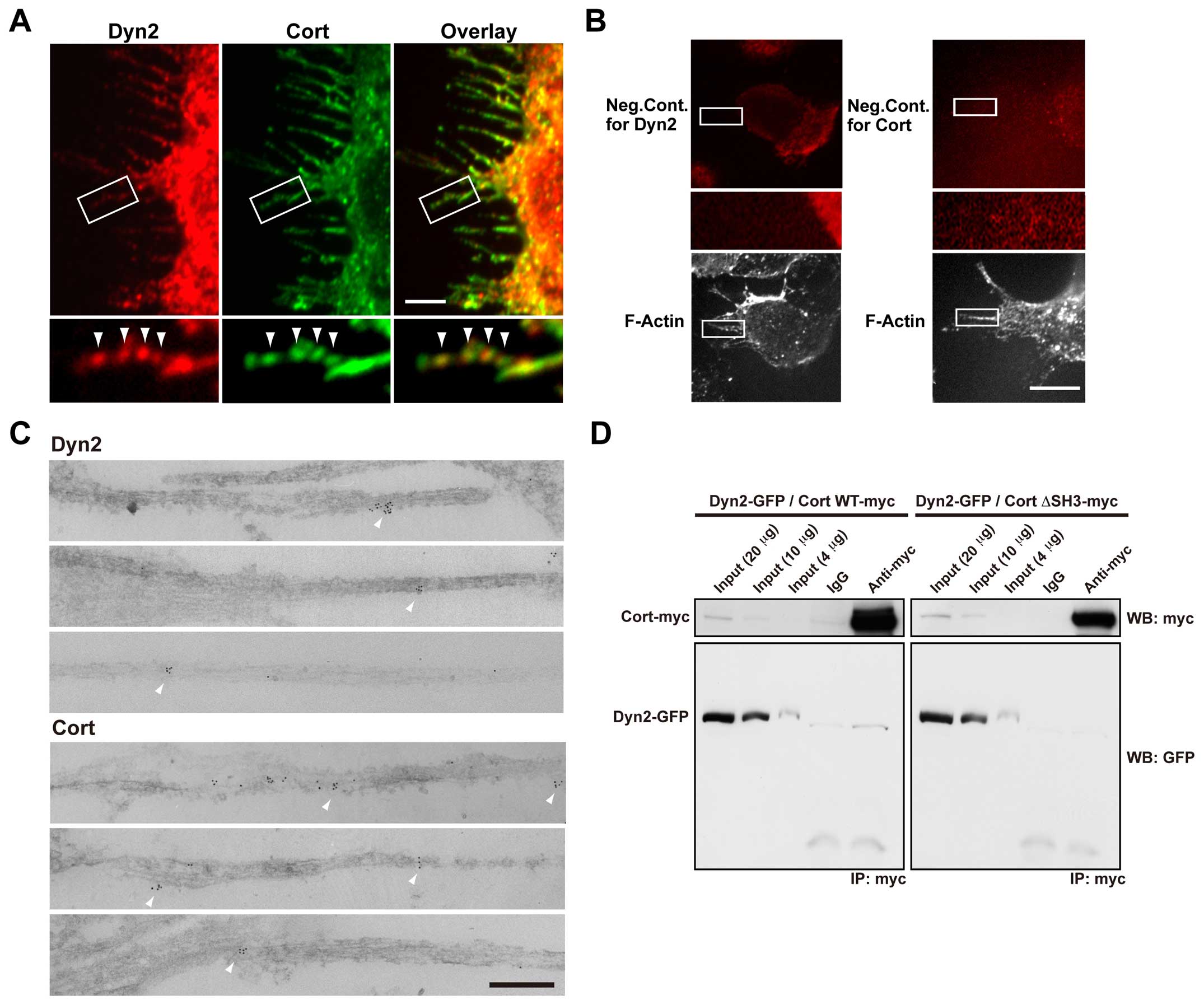 | Figure 2Dynamin 2 colocalizes with cortactin
along F-actin bundles in filopodia of serum-stimulated H1299 cells.
(A) Colocalization of dynamin 2 (Dyn2, left) and cortactin (Cort,
middle) by double-immunofluorescent staining in filopodia of
serum-stimulated H1299 cells. Boxed areas correspond to enlarged
images shown below. Dynamin 2- and cortactin-positive puncta were
present periodically along F-actin bundles in filopodia
(arrowheads). Scale bar, 5 μm (upper panels), 1.6 μm (lower
panels). (B) In the negative controls, the primary antibodies were
omitted for dynamin 2 (left) and cortactin (right). Boxed areas
correspond to enlarged images shown below. Bar, 10 μm (top and
bottom panels), 2.8 μm (middle panels). (C) Representative images
acquired by immunoelectron microscopy showing the localization of
dynamin 2 (top three panels) and cortactin (bottom three panels) in
filopodia of serum-stimulated H1299 cells. Immunoreactive dynamin 2
and cortactin were present along F-actin bundles (arrowheads).
Scale bar, 20 nm. (D) Immunoprecipitation (IP) results
demonstrating an in vivo interaction between dynamin 2 and
cortactin. H1299 cells were co-transfected with GFP-tagged dynamin
2 (Dyn2-GFP) and either myc-tagged wild-type cortactin (Cort
WT-myc, left) or cortactin ΔSH3 (Cort ΔSH3-myc, right). The protein
complexes were immunoprecipitated using a polyclonal anti-myc
antibody or preimmune IgG (IgG), and then visualized by western
blotting (WB) with monoclonal anti-GFP or anti-myc antibodies.
Total cell lysates (4, 10 and 20 μg) were also analyzed
(input). |
These results prompted us to examine the possible
interaction of dynamin 2 and cortactin by immunoprecipitation.
Exogenously expressed dynamin 2-GFP was co-precipitated with
full-length cortactin-myc using a polyclonal anti-myc antibodies
and H1299 cell lysates (Fig. 2D,
left). Cort ΔSH3-myc, a dynamin 2 binding deficient mutant that
lacks its SH3 domain, was unable to precipitate dynamin 2 (Fig. 2D, right). Taken together, these
results illustrate that these proteins interact at F-actin bundles
in filopodia of H1299 cells.
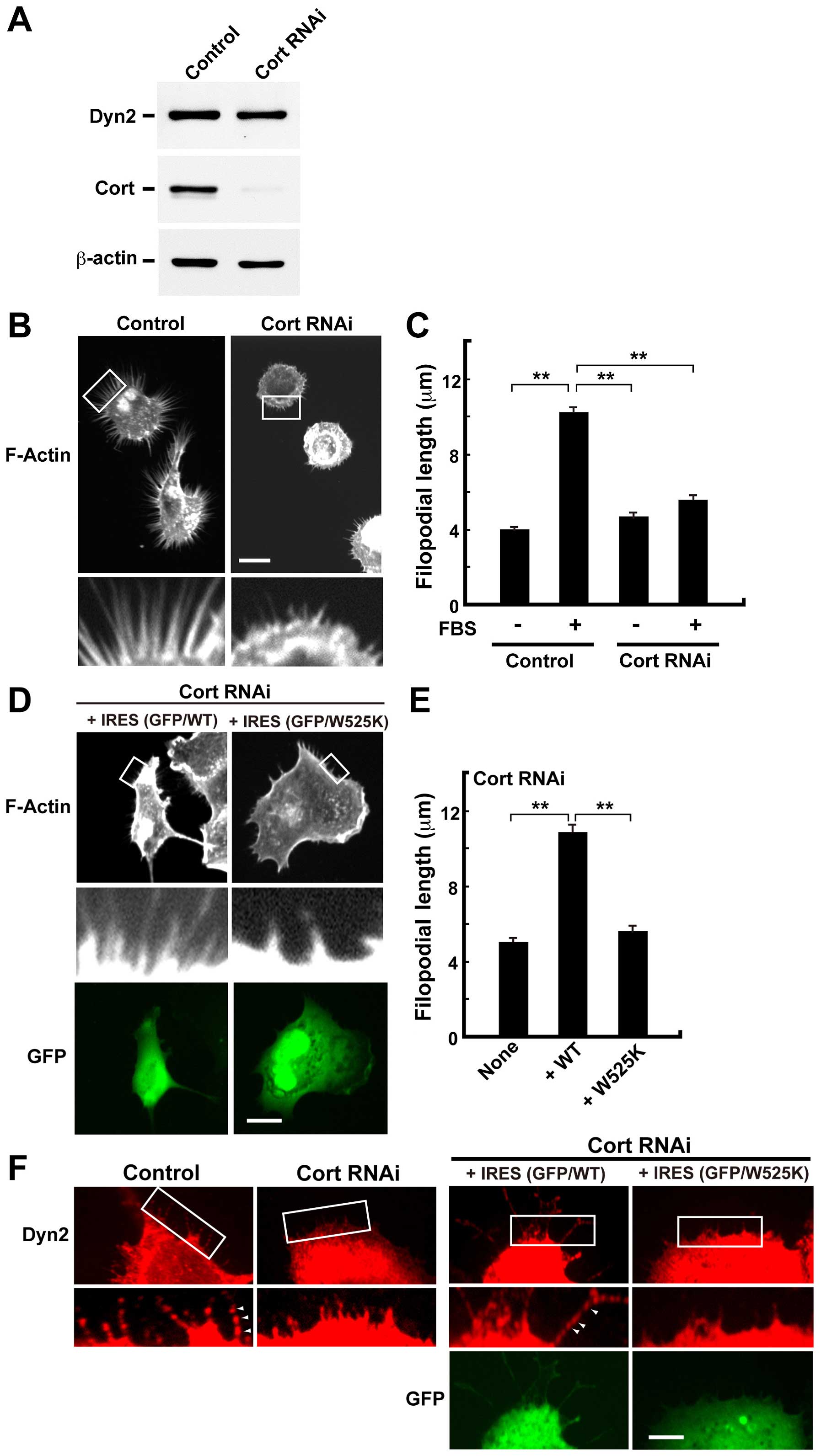
Dynamin 2 and cortactin are required for
serum-induced filopodial formation in H1299 cells
To examine the role of dynamin 2 in filopodial
formation, dynamin 2 was silenced in H1299 cells by RNAi. Compared
with the control, knockdown of dynamin 2 in H1299 cells with
specific siRNAs reduced its level by ~95% as revealed by western
blotting (Fig. 3A). Compared with
the length of filopodia in serum-stimulated control cells (10.2±0.5
μm), dynamin 2 knockdown decreased filopodial extension in silenced
cells (4.7±0.6 μm) (Fig. 3B and
C). In addition, dynasore inhibited filopodial extension
(2.4±0.08 μm). This effect was rescued after the inhibitor was
removed (8.1±2.4 μm) (Fig. 3D and
E).
We also examined the effects of cortactin knockdown
by RNAi on filopodial formation. Compared with the control,
knockdown of cortactin reduced its level by ~95% as revealed by
western blotting (Fig. 4A).
Compared with the length of filopodia in control cells (10.2±0.39
μm), cortactin knockdown also decreased filopodial extension after
serum-stimulation (5.6±0.17 μm) (Fig.
4B and C). The inhibition of filopodial formation in
cortactin-silenced cells was rescued by exogenous expression of
wild-type cortactin (10.8±0.54 μm) but not by cortactin W525K, a
binding-defective mutant of dynamin 2 (29) (Fig. 4D
and E). In addition, the punctate-like localization of dynamin
2 along F-actin bundles reappeared in wild-type cortactin
expressing cells (Fig. 4F, right).
These results indicate that dynamin 2 and cortactin are required
for filopodial formation.
F-actin bundling by the dynamin
2-cortactin complex stabilizes F-actin
The effects of dynamin 2 and cortactin on the
formation of F-actin bundles were examined in vitro. In this
experiment, preformed F-actin were incubated with or without
cortactin and dynamin 2 in the presence of GTP. F-actin alone
appeared as uniform filaments (Fig.
5A, actin alone). The addition of dynamin 2 to F-actin
filaments did not cause any visible change in their distribution
(Fig. 5A, + Dyn2). However,
F-actin incubated with wild-type cortactin, cortactin W525K or
cortactin ΔSH3 often formed small clusters (Fig. 5A, + Cort WT, + Cort W525K, or +
Cort ΔSH3), consistent with a previously published report (12). The presence of both dynamin 2 and
wild-type cortactin resulted in the formation of long and thick
F-actin bundles (Fig. 5A, + Dyn2 +
Cort WT), which were similar to those formed by dynamin 1 and
cortactin (Fig. 5A, + Dyn1 + Cort
WT). On the other hand, the long and thick F-actin bundles were
much less evident in the presence of dynamin 2 and cortactin W525K
or ΔSH3 (Fig. 5A, + Dyn2 + Cort
W525K or + Dyn2 + Cort ΔSH3).
To localize dynamin 2 and cortactin to F-actin
bundles, the preformed F-actin bundles were used for
immunofluores-cent staining. Dynamin 2 and cortactin colocalized as
bright dots along F-actin bundles (Fig. 5B, left). The localization of
dynamin 2 and cortactin was similar to that of the dynamin
1-cortactin complex (Fig. 5B,
right) (12).
Lastly, we examined whether actin bundling by the
dynamin 2-cortactin complex can affect F-actin stability. To
address this, the depolymerization kinetics of preformed
pyrene-labeled F-actin were examined after the solution was diluted
10-fold with buffer. In the presence of dynamin 2 and cortactin,
the rate of depolymerization by dilution decreased to a level
comparable to that induced by α-actinin, an actin-crosslinking
protein, indicating that dynamin 2 and cortactin stabilize F-actin
bundles (Fig. 5C). These results
indicate that the dynamin 2-cortactin complex stabilizes F-actin
bundles in filopodia prior to cell migration.
Discussion
The involvement of dynamin in the dynamics of cancer
cells such as cell migration, invasion, and metastasis has been
reported (18). However, the
precise role of dynamin in these cellular processes is not entirely
clear. We recently reported that actin bundling by the dynamin
1-cortactin complex is crucial for neurite extension in developing
neurons (12). In this study, we
examined the possibility that a similar F-actin-bundling mechanism
is involved in the migration of H1299 cells, a human non-small cell
lung carcinoma cell line.
We showed that cortactin and dynamin 2 mostly
colocalized along F-actin bundles in filopodia of serum-stimulated
H1299 cells (Fig. 2).
Pharmacological inhibition of dynamin 2 by Dynasore, Dynole 34-2 or
MitMAB decreased cell migration (Fig.
1) and filopodial formation (Fig.
3). Furthermore, filopodia were shorter in dynamin 2- and
cortactin-depleted cells than in control cells (Figs. 3 and 4). In cortactin-silenced cells, the
exogenous expression of wild-type cortactin rescued the
punctate-like localization of dynamin 2 and filopodial formation
(Fig. 4). Both dynamin 2 and
cortactin bundled F-actin, and these proteins increased F-actin
stability (Fig. 5). These results
indicate that dynamin 2 and cortactin participate in cancer cell
migration by stabilizing F-actin bundles in filopodia.
Dynamin assembles at the neck of deeply invaginated
endocytic pits (30). Upon GTP
hydrolysis, however, dynamin undergoes a conformational change,
resulting in the fission of endocytic pits and release of endocytic
vesicles (31–33). In addition, dynamin 1 forms a
ring-like complex with cortactin, which switches from an open to a
closed state upon GTP hydrolysis. This change promotes the bundling
of F-actin filaments (12). The
mechanism of actin bundling mediated by the dynamin 2-cortactin
complex is similar to that of the dynamin 1-cortactin complex,
because dynamin 2 and cortactin also facilitated the formation of
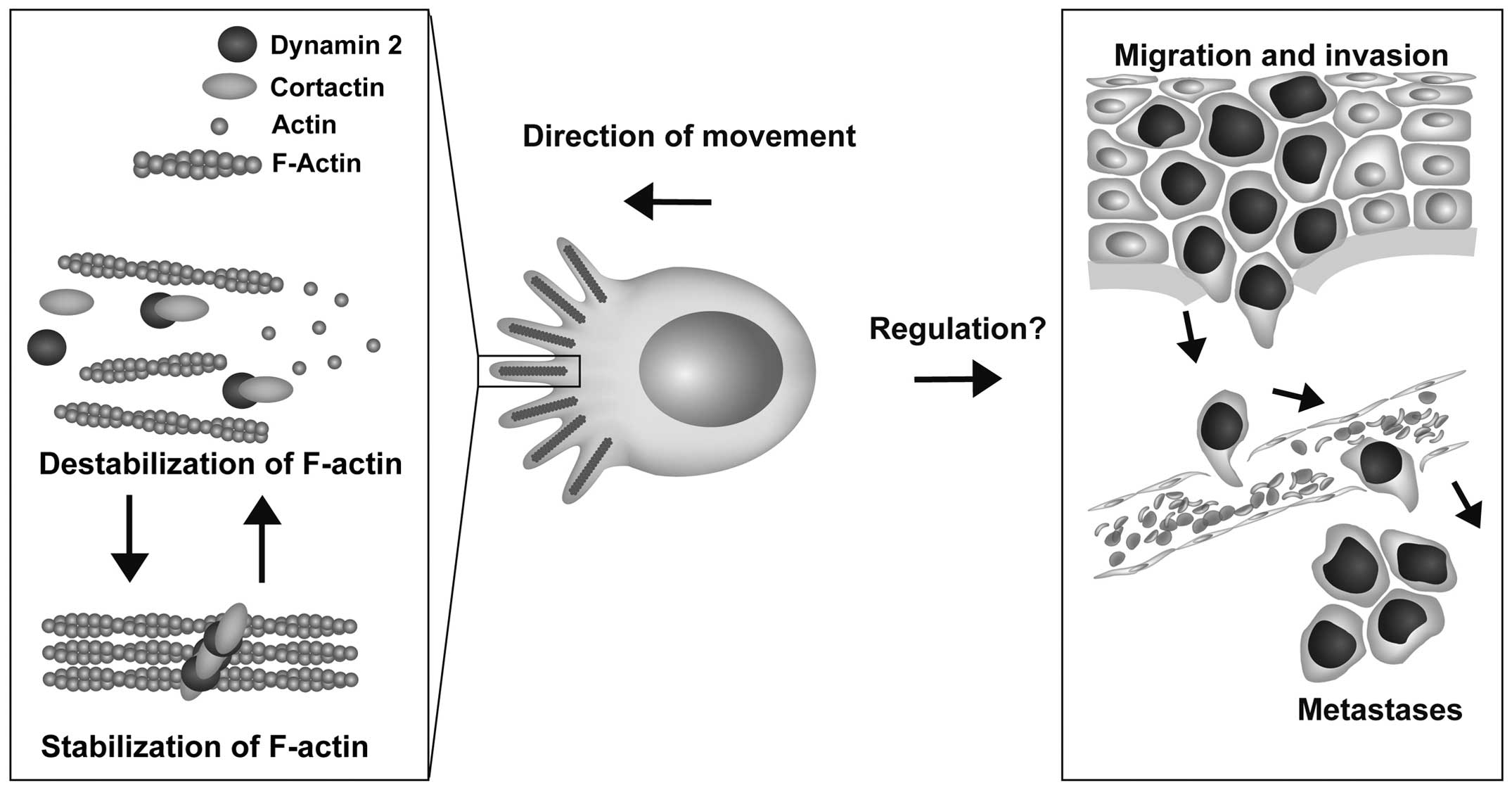
long and thick F-actin bundles to which they colocalized (Fig. 5). This mechanochemical property may
be critical for the formation of F-actin bundles in filopodia of
other cell types as well (Fig. 6).
Additional studies are needed to determine the precise
mechanism.
Dynamin associates with tumorigenesis, particularly
tumor cell migration and invasion. For example, increased dynamin 2
expression potentiates the migration and invasion of pancreatic
ductal cancer cells (25), and
tyrosine phosphorylated dynamin 2 promotes the growth and
invasiveness of glioblastomas (34). Thus, the involvement of dynamin in
the formation of F-actin bundles might promote cancer
malignancy.
In conclusion, we showed that dynamin 2 and
cortactin participate in the formation of F-actin bundles, which
stabilize filopodia in migrating cancer cells. Taken together,
these results suggest that dynamin might be a potential molecular
target for anticancer therapy.
Acknowledgements
The authors thank Yuki Masuoka, Dr Shun-AI Li, and
Nana Okazaki for technical assistance. This study was supported in
part by grants from the Ministry of Education, Science, Sports, and
Culture of Japan (grant no. 26670201 to H.Y.; grant no. 15K1533007
to K.T.), the Astellas Foundation for Research on Metabolic
Disorders (to H.Y.), and the Japan Foundation for Applied
Enzymology (to H.Y.).
References
|
1
|
Arjonen A, Kaukonen R and Ivaska J:
Filopodia and adhesion in cancer cell motility. Cell Adhes Migr.
5:421–430. 2011. View Article : Google Scholar
|
|
2
|
Ridley AJ: Life at the leading edge. Cell.
145:1012–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takei K, Slepnev VI, Haucke V and De
Camilli P: Functional partnership between amphiphysin and dynamin
in clathrin-mediated endocytosis. Nat Cell Biol. 1:33–39.
1999.PubMed/NCBI
|
|
4
|
Mettlen M, Pucadyil T, Ramachandran R and
Schmid SL: Dissecting dynamin’s role in clathrin-mediated
endocytosis. Biochem Soc Trans. 37:1022–1026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Praefcke GJ and McMahon HT: The dynamin
superfamily: Universal membrane tubulation and fission molecules?
Nat Rev Mol Cell Biol. 5:133–147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao H, Garcia F and McNiven MA:
Differential distribution of dynamin isoforms in mammalian cells.
Mol Biol Cell. 9:2595–2609. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McNiven MA, Kim L, Krueger EW, Orth JD,
Cao H and Wong TW: Regulated interactions between dynamin and the
actin-binding protein cortactin modulate cell shape. J Cell Biol.
151:187–198. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baldassarre M, Pompeo A, Beznoussenko G,
Castaldi C, Cortellino S, McNiven MA, Luini A and Buccione R:
Dynamin participates in focal extracellular matrix degradation by
invasive cells. Mol Biol Cell. 14:1074–1084. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ochoa GC, Slepnev VI, Neff L, Ringstad N,
Takei K, Daniell L, Kim W, Cao H, McNiven M, Baron R, et al: A
functional link between dynamin and the actin cytoskeleton at
podosomes. J Cell Biol. 150:377–389. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Torre E, McNiven MA and Urrutia R: Dynamin
1 antisense oligonucleotide treatment prevents neurite formation in
cultured hippocampal neurons. J Biol Chem. 269:32411–32417.
1994.PubMed/NCBI
|
|
11
|
Kurklinsky S, Chen J and McNiven MA:
Growth cone morphology and spreading are regulated by a
dynamin-cortactin complex at point contacts in hippocampal neurons.
J Neurochem. 117:48–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamada H, Abe T, Satoh A, Okazaki N, Tago
S, Kobayashi K, Yoshida Y, Oda Y, Watanabe M, Tomizawa K, et al:
Stabilization of actin bundles by a dynamin 1/cortactin ring
complex is necessary for growth cone filopodia. J Neurosci.
33:4514–4526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gold ES, Underhill DM, Morrissette NS, Guo
J, McNiven MA and Aderem A: Dynamin 2 is required for phagocytosis
in macrophages. J Exp Med. 190:1849–1856. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Otsuka A, Abe T, Watanabe M, Yagisawa H,
Takei K and Yamada H: Dynamin 2 is required for actin assembly in
phagocytosis in Sertoli cells. Biochem Biophys Res Commun.
378:478–482. 2009. View Article : Google Scholar
|
|
15
|
Faelber K, Posor Y, Gao S, Held M, Roske
Y, Schulze D, Haucke V, Noé F and Daumke O: Crystal structure of
nucleotide-free dynamin. Nature. 477:556–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ford MG, Jenni S and Nunnari J: The
crystal structure of dynamin. Nature. 477:561–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu H, Reynolds AB, Kanner SB, Vines RR and
Parsons JT: Identification and characterization of a novel
cytoskeleton-associated pp60src substrate. Mol Cell Biol.
11:5113–5124. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
MacGrath SM and Koleske AJ: Cortactin in
cell migration and cancer at a glance. J Cell Sci. 125:1621–1626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ammer AG and Weed SA: Cortactin branches
out: Roles in regulating protrusive actin dynamics. Cell Motil
Cytoskeleton. 65:687–707. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamada H, Abe T, Li SA, Masuoka Y, Isoda
M, Watanabe M, Nasu Y, Kumon H, Asai A and Takei K: Dynasore, a
dynamin inhibitor, suppresses lamellipodia formation and cancer
cell invasion by destabilizing actin filaments. Biochem Biophys Res
Commun. 390:1142–1148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada H, Abe T, Li SA, Tago S, Huang P,
Watanabe M, Ikeda S, Ogo N, Asai A and Takei K:
N′-[4-(dipropylamino)benzylidene]-2-hydroxybenzohydrazide is a
dynamin GTPase inhibitor that suppresses cancer cell migration and
invasion by inhibiting actin polymerization. Biochem Biophys Res
Commun. 443:511–517. 2014. View Article : Google Scholar
|
|
22
|
Mooren OL, Kotova TI, Moore AJ and Schafer
DA: Dynamin2 GTPase and cortactin remodel actin filaments. J Biol
Chem. 284:23995–24005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Masaike Y, Takagi T, Hirota M, Yamada J,
Ishihara S, Yung TM, Inoue T, Sawa C, Sagara H, Sakamoto S, et al:
Identification of dynamin-2-mediated endocytosis as a new target of
osteoporosis drugs, bisphosphonates. Mol Pharmacol. 77:262–269.
2010. View Article : Google Scholar
|
|
24
|
Slepnev VI, Ochoa GC, Butler MH and De
Camilli P: Tandem arrangement of the clathrin and AP-2 binding
domains in amphiphysin 1 and disruption of clathrin coat function
by amphiphysin fragments comprising these sites. J Biol Chem.
275:17583–17589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eppinga RD, Krueger EW, Weller SG, Zhang
L, Cao H and McNiven MA: Increased expression of the large GTPase
dynamin 2 potentiates metastatic migration and invasion of
pancreatic ductal carcinoma. Oncogene. 31:1228–1241. 2012.
View Article : Google Scholar
|
|
26
|
Macia E, Ehrlich M, Massol R, Boucrot E,
Brunner C and Kirchhausen T: Dynasore, a cell-permeable inhibitor
of dynamin. Dev Cell. 10:839–850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hill TA, Gordon CP, McGeachie AB,
Venn-Brown B, Odell LR, Chau N, Quan A, Mariana A, Sakoff JA,
Chircop M, et al: Inhibition of dynamin mediated endocytosis by the
dynoles--synthesis and functional activity of a family of indoles.
J Med Chem. 52:3762–3773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quan A, McGeachie AB, Keating DJ, van Dam
EM, Rusak J, Chau N, Malladi CS, Chen C, McCluskey A, Cousin MA, et
al: Myristyl trimethyl ammonium bromide and octadecyl trimethyl
ammonium bromide are surface-active small molecule dynamin
inhibitors that block endocytosis mediated by dynamin I or dynamin
II. Mol Pharmacol. 72:1425–1439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schafer DA, Weed SA, Binns D, Karginov AV,
Parsons JT and Cooper JA: Dynamin 2 and cortactin regulate actin
assembly and filament organization. Curr Biol. 12:1852–1857. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takei K, McPherson PS, Schmid SL and De
Camilli P: Tubular membrane invaginations coated by dynamin rings
are induced by GTP-gamma S in nerve terminals. Nature. 374:186–190.
1995. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sweitzer SM and Hinshaw JE: Dynamin
undergoes a GTP-dependent conformational change causing
vesiculation. Cell. 93:1021–1029. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takei K, Haucke V, Slepnev V, Farsad K,
Salazar M, Chen H and De Camilli P: Generation of coated
intermediates of clathrin-mediated endocytosis on protein-free
liposomes. Cell. 94:131–141. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roux A, Uyhazi K, Frost A and De Camilli
P: GTP-dependent twisting of dynamin implicates constriction and
tension in membrane fission. Nature. 441:528–531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng H, Liu KW, Guo P, Zhang P, Cheng T,
McNiven MA, Johnson GR, Hu B and Cheng SY: Dynamin 2 mediates
PDGFRα-SHP-2-promoted glioblastoma growth and invasion. Oncogene.
31:2691–2702. 2012. View Article : Google Scholar
|