Introduction
Cancer development is a multistep and complex
process involving initiation, promotion and progression. Cell
transformation into cancerous state, is one of the critical steps.
Chemoprevention has been considered as the most promising strategy,
which describes the use of natural or synthetic chemicals to
suppress, delay, or prevent the process of carcinogenesis (1). Phytochemicals are the focus of
chemoprevention studies because of their potential human acceptance
and low adverse reaction (1,2).
Flavonoids, widely present in fruits and vegetables, has gained
attention as a phytochemical that could reduce the risk of several
types of cancer, including prostate (3), colorectal (4), head and neck cancer (5). Eupatilin is a flavonol isolated from
Artemisia vulgaris. A previous study showed that eupatilin
exerts anti-inflammatory and anti-oxidative activities on gastric
mucosal damage, and promotes regeneration of damaged mucosa
(6). In recent years, several
studies demonstrated that eupatilin could inhibit tumor cell growth
and proliferation (7–10). However, the effect of eupatilin on
inhibition of cell transformation and the direct molecular
target(s) remain unclear.
The phosphatidylinositol 3-kinase (PI3K) pathway is
activated following interaction of a growth factor, cytokines, or
other environmental cues with a tyrosine kinase receptor (TKR).
PI3K plays an important role in regulation of many signaling
pathways to control cell proliferation, growth, survival, motility
and metabolism (11–14). The abnormal activation of PI3K has
been found in numerous cancers (15–18).
Akt is one of downstream kinases of PI3K pathway.
PI3K activation catalyzes the PIP2 to generate PIP3, which in turn
recruits PDK1 and Akt to bind the plasma membrane at pleckstrin
homology domains. Subsequently, Akt is phosphorylated by PDK1 at
threonine-308 (T308) or serinine-473 sites through targeting
rapamycin complex 2 (mTORC2). Akt activation can lead to
phosphorylation of various protein substrates, which are
consequently activated or inhibited. GSK3β is an important
downstream molecule of Akt, regulated by site-specific
phosphorylation and depending on different cell condition.
GSK3β activation and inhibition depend upon the
phosphorylation on Tyr216 and Ser9, respectively. Detailed analysis
revealed that p-GSK3βSer9 blocked the nuclear export and
degradation of cyclin D1, resulting in progressing to S phase from
G0/G1 (19,20).
Hence, PI3K/Akt/GSK-3β pathway regulation is a possible research
direction for cancer chemoprevention and PI3K is a potential target
of chemoprophylaxis.
In the present study, we found that eupatilin
inhibited JB6 cell proliferation and EGF-induced
anchorage-independent growth. Treated with eupatilin, downstream
kinases of PI3K phosphorylation, including Akt phosphorylation at
Ser473 and Thr308, GSK3β phosphorylation at Ser9 were inhibited,
causing cell cycle arrest at G1 phase by suppressing
cyclin D1. Furthermore, computer-docking model showed that
eupatilin was able to bind at the ATP pocket site of PI3K, which
was verified by pull down assay. Hence, this study suggests that
eupatilin is a potential chemopreventive agent in inhibition of
cell transformation by targeting PI3K.
Materials and methods
Materials
Fetal bovine serum (FBS) was purchased from
Gibco-BRL (Gaitherburg, MD, USA). Antibodies were purchased from
Santa Cruz Biotechnology (Paso Robles, CA, USA) and Cell Signaling
Technology (Beverly, MA, USA). Eupatilin was obtained from Chunqiu
(Nanjing, China). Epidermal growth factor (EGF) was purchased from
BD Biosciences (San Jose, CA, USA). Cell Counting kit-8 (CCK-8) and
BeyoECL Plus were purchased from Beyotime Institute of
Biotechnology (Shanghai, China). CNBr-Sepharose 4B was purchased
from Amersham Pharmacia Biotech (Piscataway, NJ, USA).
Cell proliferation
JB6 cells were plated at a density of 103
cells/well in 96-well plates and incubated for 6–10 h at 37°C. The
anti-proliferative effect of eupatilin was evaluated in cells
cultured with different concentrations of 0, 2.5, 5 or 10 μM and
time 24, 48, 72 or 96 h using WST-8. Briefly, 10 μl of the WST-8
solution was added to cell cultures for the designated times.
Plates were incubated for 2 h at 37°C. The absorbance was measured
at 450 nm using a microplate reader (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China).
Anchorage-independent cell growth
JB6 cells (8×103/ml) were cultured in
0.3% Eagle’s basal medium-agar containing 10% FBS. Cells were
treated with different concentrations of eupatilin (0, 2.5, 5, 10
and 20 μM) along with EGF (10 ng/ml). Plates were incubated for 16
days at 37°C. The colonies were counted under a microscope with the
help of the Image-Pro Plus computer software program.
Western blot assay
In this assay, JB6 cells were cultured in a 10-cm
dish at 37°C. At 90% confluency, cells were starved in MEM
containing 0.1% FBS for 16 h to synchronize the cell cycle into
G0 phase (21).
Subsequently, the cells were treated with various concentrations of
eupatilin (0, 2.5, 5, 10 and 20 μM) for 2 h followed by addition of
EGF (final 10 ng/ml concentration). Cells were sonicated by the
Ultrusonic cell disrupter system and protein concentration was
determined. Total protein (40 μg) from the whole cell lysates was
separated by 10% SDS-PAGE and proteins separated in the gel were
transferred electrophoretically onto PVDF membrane (Millipore,
Billerica, MA, USA). The membranes were blocked with 5% non-fat
milk at room temperature for 2 h followed by incubated with a
1:1,000 dilution of a specific first antibody [anti-ERK1/2,
anti-p-ERK1/2, anti-Akt, anti-p-Akt (Ser473), anti-p-Akt (Thr308),
anti-GSK3β, anti-p-GSK3β (Ser9), anti-RSK2, anti-p-RSK2, anti-CREB,
anti-p-CREB were from Cell Signaling Technology; anti-cyclin D1,
anti-α-tubulin and anti-β-actin were from Santa Cruz Biotechnology]
at 4°C overnight. The next day, the membranes were washed and
incubated with their corresponding horseradish peroxidase
(HRP)-conjugated secondary antibody (1:2,000 dilution) for 2 h.
Protein bands were then developed using BeyoECL Plus on Medical
X-ray film.
Cell cycle assay
JB6 cells (I5×105) were cultured in 6-cm
dishes and allow to grow for 24 h at 37°C. Next, the cells were
starved in MEM containing 0.1% FBS for 36 h to synchronize the
cells into G0 phase. Cells were divided into two groups
followed by different concentrations of eupatilin (0, 2.5, 5, 10
and 20 μM) and (0 and 20 μM) and PI3K inhibitor LY294002 (final 10
μM concentration). Subsequently, treated with EGF (final 10 ng/ml)
and the cells were harvested after 18 h. Later, cells were fixed
with 70% ethanol at 4°C overnight. The following day 500 μl 1X PBS,
RNase A (100 μg/ml), propidium iodide (1 mg/ml) were added and
incubated at 37°C in dark for 40 min. Subsequently, cells were
analyzed by the FACSCalibur flow cytometer (BD Biosciences, San
Jose, CA, USA).
Molecular modeling
Molecular docking was performed in order to
understand the detailed binding interactions between eupatilin and
PI3K. The initial binding complex structure was constructed from
the X-ray crystal structure of human PI3K (PDB ID: 4TV3) (22) and the optimized structure of
eupatilin. Initially, the geometry of eupatilin was optimized at
the HF/6-31G* level using Gaussian 09 (23). Then, the optimized geometry of
eupatilin was used to calculate the electrostatic potential
distribution on the molecular surface at the same HF/6-31G* level.
The calculated electrostatic potential distribution was used to
determine the partial atomic charges by using the standard
restrained electrostatic potential (RESP) fitting procedure
(24). The determined RESP charges
of the eupatilin atoms were used in the following docking studies.
Briefly, eupatilin was docked into the possible active site of PI3K
by using the AutoDock 4.2 program (25). The atomic charges used for the
docking of eupatilin were the restrained electrostatic potential
(RESP) charges. During the docking process, a conformational search
was performed using the Solis and Wets local search method
(26), and the Lamarkian genetic
algorithm (LGA) (25) was applied
to deal with the PI3K-eupatilin interactions. Among a series of
docking parameters, the grid size was set to 60 x 60 x 60 and the
grid space was the default value of 0.375 Å. The docked
enzyme-ligand complex structures were selected according to the
criteria for interacting energy combined with geometric matching
quality.
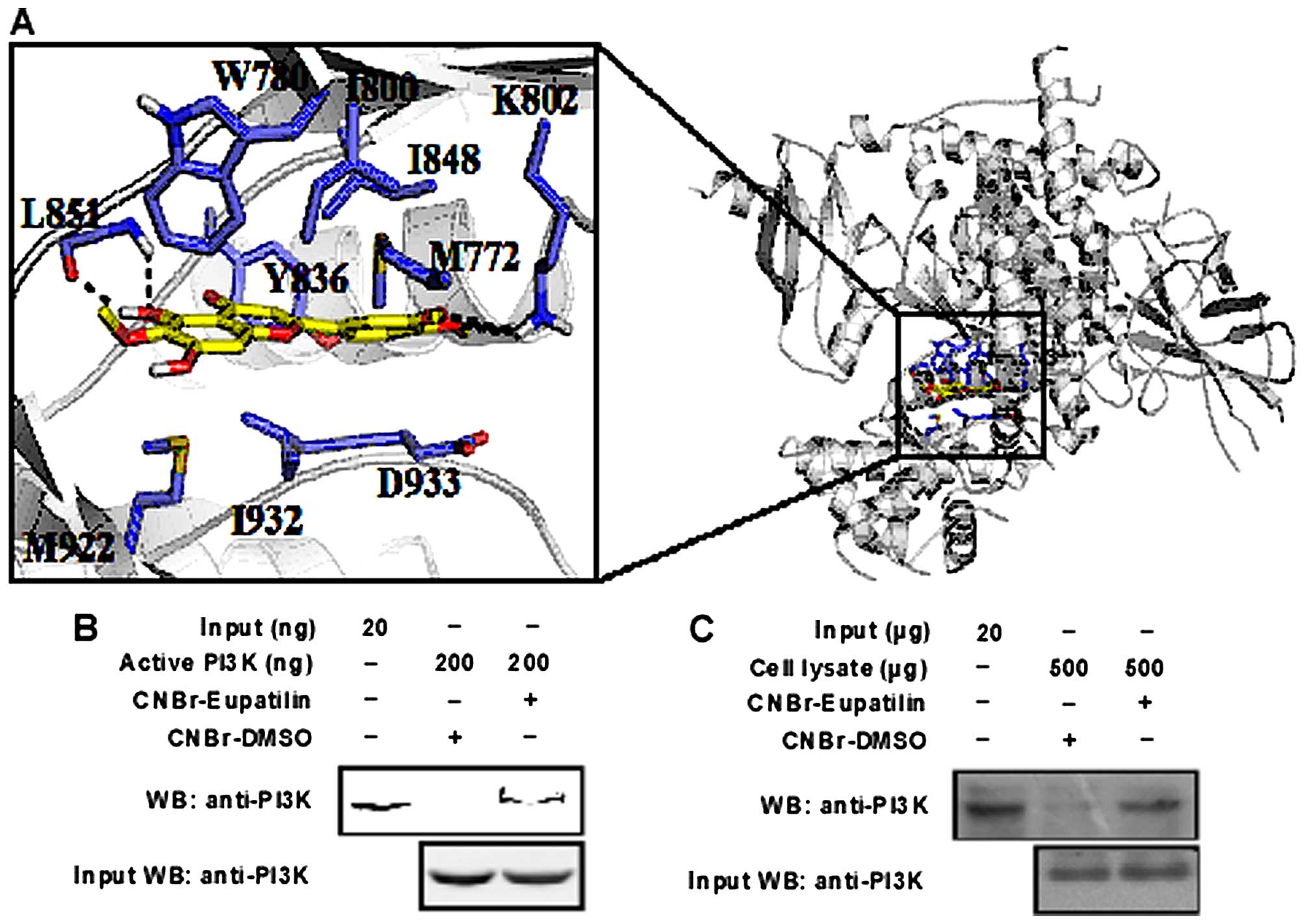
Depicted in Fig. 4A
is the constructed structure of PI3K-eupatilin binding complex.
Eupatilin fits well in the active site of PI3K and is stabilized by
extensive hydrogen bonding, π-π and van der Waals interactions. The
benzene ring sandwiched between I800, I848, M772 and D933
establishes extensive van der Waals contacts with PI3K. The two
methoxy groups from the benzene ring form hydrogen bonds with K802.
The chroman ring forms π-π stacking and van der Waals interactions
with the surrounding residues like W780, Y836, M922 and I932. In
addition, the hydroxyl group from the chroman ring forms hydrogen
bonds with the backbone of L851. The molecular modeling study
enables us to see more clearly the detailed binding interactions of
eupatilin with PI3K.
Pull down assay
Preparation of Sepharose 4B beads: the Sepharose 4B
beads (0.3 g) were washed with 30 ml of 1 mM HCl 5 min by gentle
inversion; repeated 3 times and incubated with 2 mg eupatilin or
DMSO as a control in coupling buffer (0.1 M NaHCO3, 0.5
M NaCl pH 8.3) at 4°C overnight. After washing with coupling buffer
(5 ml) the beads were incubated with 0.1 M Tris-HCl (5 ml) (pH 8.0)
buffer at 4°C overnight with rotation. Subsequently, the samples
were washed with 0.1 M acetic (pH 4.0), 0.1 M Tris-HCl and 0.5 M
NaCl (pH 8.0) and 1 ml PBS was added to resuspension. Cellular
supenatant fraction of JB6 cells (500 μg) or active PI3K with
eupatilin-Sepharose 4B (or DMSO-Sepharose 4B as a control) beads
(100 μl, 50% slurry) were incubated overnight at 4°C in reaction
buffer (150 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 50 mM
Tris pH 7.5, 1 mM dithiothreitol, 1 μg protease inhibitor mixture,
0.02 mM phenylmethyl-sulfonyl fluoride, 2 μg/ml bovine serum
albumin and 0.01% Nonidet P-40) with gentle rotation. The next day,
the beads were washed with washing buffer (150 mM NaCl, 5 mM
ethylenediaminetetraacetic acid, 50 mM Tris pH 7.5, 1 mM
dithiothreitol, 0.02 mM phenylmethylsulfonyl fluoride and 0.01%
Nonidet P-40) 5 times and the bound PI3K proteins were analyzed by
western blotting.
Statistical analysis
All quantitative data are expressed as means ±
standard deviation. The one-way analysis of variance and Student’s
t-test were used for statistical analysis by SPSS 22.0 software
(IBM, Amonk, NY, USA). Significant differences are reported at
P<0.05.
Results
Eupatilin inhibits EGF induced JB6 cells
transformation
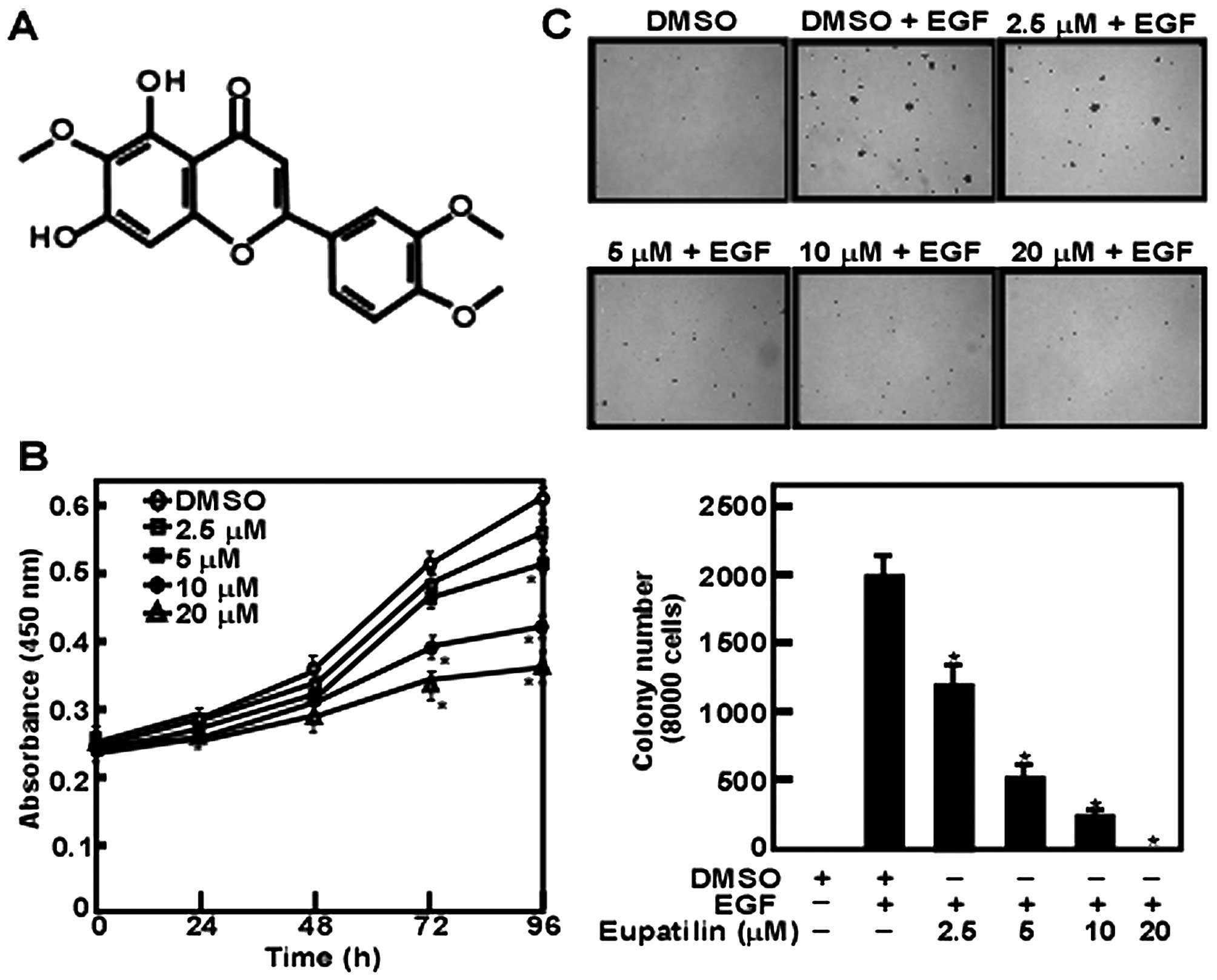
Eupatilin is an anticipative natural flavone
compound extracted from Artemisia vulgaris (Fig. 1A). Our data revealed that JB6 cell
proliferation was inhibited by eupatilin in a dose-dependent manner
with a maximal concentration at 20 μM (Fig. 1B). Furthermore, we found that JB6
cell anchorage-independent growth was affected by eupatilin. These
results showed that eupatilin inhibited EGF-induced cell colony
formation dose-dependently (Fig.
1C). However, the effect presented by various concentrations of
eupatilin was not caused by the toxicity of eupatilin.
Eupatilin inhibits the transduction of
PI3K-mediated downstream signaling pathway
To examine the mechanism of eupatilin inhibition of
cell proliferation and anchorage-independent growth, we analyzed
the role of eupatilin in activating the EGF-induced Akt and
ERK-related signaling pathway by western blotting. We found that
EGF-induced phosphorylation of Akt at Ser473 and Thr308 was
inhibited dose-dependently by eupatilin. Moreover, GSK3β at Ser9, a
downstream molecule of Akt was also downregulated by eupatilin
(Fig. 2A). However, the
ERK-related signaling pathway (ERK1/2, RSK2 and CREB) was not
affected (Fig. 2B).
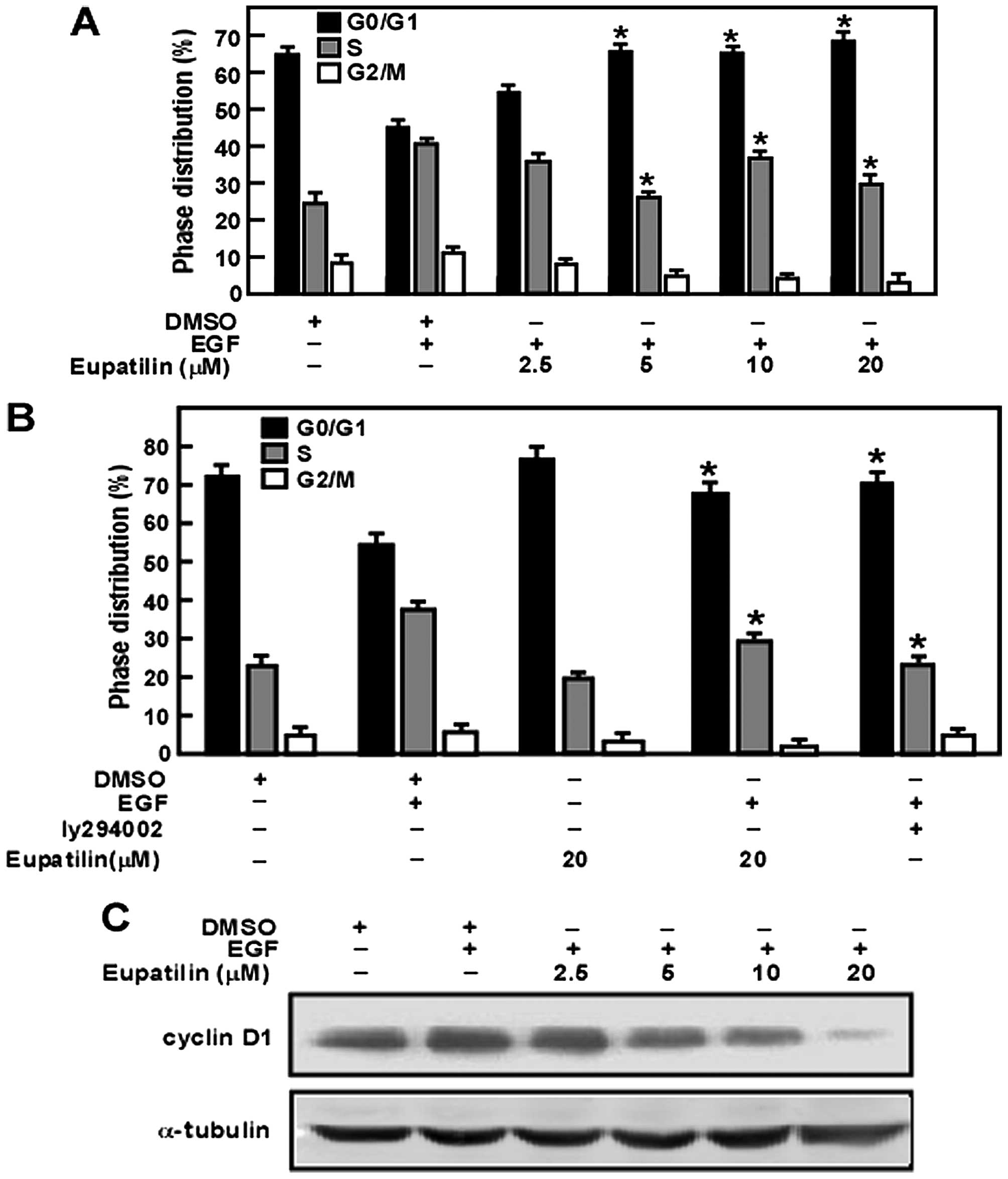
Eupatilin triggers cell cycle arrest in
G0/G1 phase by inhibiting the activity of
cyclin D1
To reveal the mechanism of eupatilin in inhibiting
JB6 cell proliferation, we analyzed the effect of eupatilin on the
cell cycle. Our results showed that eupatilin arrested cells in
G0/G1 phase in a dose-dependent manner.
Moreover, cyclin D1 is required for the G1/S transition
(27). Thus, these studies
indicated that eupatilin might downregulate the expression of
cyclin D1 leading to arrest of the cell cycle in
G0/G1 phase (Fig. 3A). Furthermore, eupatilin had a
similar function as PI3K inhibitor LY294002 (28) (Fig.
3B). Additionally, our results revealed that eupatilin affected
downregulation of the expression of cyclin D1
concentration-dependently (Fig.
3C).
Eupatilin specifically binds with
PI3K
PI3K plays a decisive role in tumorigenesis
(15,16) through Akt phosphorylation. We
supposed that PI3K might be a molecular target of eupatilin based
on our western blotting data. We tested this idea by constructing a
computer docking model, which showed that eupatilin was able to
bind at the ATP binding pocket of p110, a catalytic subunit of PI3K
(Fig. 4A). Subsequently, we
verified the binding of eupatilin with PI3K by pull down assay. We
found that eupatilin-Sepharose 4B beads can pull down PI3K, while
Sepharose 4B beads could not be bind singly (Fig. 4B). Moreover, we confirmed in cell
lysates that eupatilin could bind with endogenous PI3K (Fig. 4C).
Discussion
Cell transformation is a critical characteristic of
carcinogenesis. In the present study, we used mouse epidermal JB6
cells, which are ideal in research on the molecular mechanisms of
neoplastic transformation (29–32).
EGF or TPA was used to make the normal cells to transform into
cancer cells (33) through
activating some signaling pathways, involved in cell proliferation,
survival, motility and metabolism. EGF-induced cells produced
moderate size, tumorigenic, anchorage-independent colonies in soft
agar assay (34), which is not
possible in normal cells. These cancer promoters strongly activate
PI3K/Akt, and MAPK signaling pathways which have direct role in
carcinogenesis (35). Thus, it is
an important strategy of cancer chemoprevention to identify a
molecular target which activates the signaling pathways in cell
transformation phase for novel anticancer molecules.
Flavonoids are well known as promising
chemopreventive agents against human cancers. Eupatilin, a dietary
flavone compound, has anti-ulcer, anti-inflammatory and cell cycle
regulator inhibitory effects (6,8,36).
Accumulating evidence shows that eupatilin has antitumor function
against the different types of cancer, including gastric and
endometrial cancer (36). In the
present study, we found that eupatilin could inhibit JB6 cell
proliferation and growth in a dose-dependent manner (Fig. 1B). Moreover, anchorage-independent
colony formation was decreased with the increase of eupatilin
concentration in anchorage-independent cell growth assay (Fig. 1C). These data suggested that
eupatilin has cellular targets in EGF-induced JB6 cell
transformation.
PI3K/Akt signaling pathway plays a pivotal role
(37) in many biological processes
such as regulation of cell survival, cell growth (38), apoptosis (39) and cell migration. Missense
mutations in PI3K were detected in the colon, brain, breast and
stomach cancer, leading to promotion of cell proliferation and
tumorigenesis (12,40–42).
Overexpression of PI3K/Akt signaling pathways activated the cell
cycle dependence protein kinase (CDK) (43) following phosphorylation of GSK-3β
at Ser9, leading to inhibition of GSK-3β activity and increasing
cyclin D1 expression with the promotion of G1 period
development. Thus, activated PI3K pathway has a role in promoting
carcinogenesis (44–47). GSK3β is a complex kinase, acting
either as a tumor promoter or suppressor in different types of
cancer (48). The different sites
of phosphorylation decides the activation of GSK3β. GSK3β
phosphorylation at Tyr216 or Ser9 causes activation or inhibition
state, respectively. Ma et al (49) showed that tumor promoters of EGF
and TPA induce strong phosphorylation of GSK3β at Ser9 in JB6
P+ cells, accompanied by increasing
anchorage-independent cell growth. Overexpression of S9A mutant in
JB6 cells, leads to inactivation of GSK3β phosphorylation at Ser9
and it becomes less sensitive to EGF induced pGSK3β (Ser9) with the
upregulation of cyclin D1. These results indicated that the cells
were more resistant to the negative regulation of GK3β (49). In the present study, we showed that
EGF induced the activation of PI3K/Akt/GSK-3β signaling pathway in
JB6 cells. It also increased the phosphorylation of Akt at Ser473,
Thr308 and GSK-3β at Ser9 compared with the control (Fig. 2A). Moreover, EGF treatment promotes
the cyclin D1 expression (Fig. 3C)
and increases cell percentage of S phase (Fig. 3A). However, we showed that
eupatilin effectively reduced phosphorylation of Akt at Ser473 and
Thr308 induced by EGF in a dose-dependent manner. Similarly, the
phosphorylation of GSK3β (Ser9) was attenuated by eupatilin
treatment (Fig. 2A). Eupatilin
also decreased the expression level of cyclin D1 (Fig. 3C) and arrest the cell cycle arrest
at G1 phase (Fig. 3A).
The results indicated that eupatilin could suppress EGF-induced JB6
cell transformation mediated through the PI3K/Akt/GSK3β pathway.
Hence, we speculated that PI3K might be a molecular target of
eupatilin. The idea was primarily verified by computer docking
models, showing that eupatilin could strongly bind at ATP binding
pocket of P110, a catalytic subunit of PI3K (Fig. 4A). Subsequently, we confirmed our
hypothesis by pull-down assay with eupatilin-conjugated beads in
vitro (Fig. 4B) and ex
vivo (Fig. 4C).
In conclusion, eupatilin significantly contributes
to inhibition of EGF-induced JB6 cell transformation through
directly targeting PI3K. Thus, eupatilin is a potential
chemopreventive agent which may provide some insights into
prevention or therapy for tumorigenesis caused by aberrant PI3K
signaling pathway.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81372269, 81472324 and
81572812) and the Science Foundation of Henan Education Department
(no. 13HASTIT022).
References
|
1
|
DiMarco-Crook C and Xiao H: Diet-based
strategies for cancer chemoprevention: The role of combination
regimens using dietary bioactive components. Annu Rev Food Sci
Technol. 6:505–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mukhtar H and Ahmad N: Cancer
chemoprevention: Future holds in multiple agents. Toxicol Appl
Pharmacol. 158:207–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adhami VM, Ahmad N and Mukhtar H:
Molecular targets for green tea in prostate cancer prevention. J
Nutr. 133(Suppl): 2417S–2424S. 2003.PubMed/NCBI
|
|
4
|
Ogawa K, Hara T, Shimizu M, Nagano J, Ohno
T, Hoshi M, Ito H, Tsurumi H, Saito K, Seishima M, et al:
(−)-Epigallocatechin gallate inhibits the expression of indoleamine
2,3-dioxygenase in human colorectal cancer cells. Oncol Lett.
4:546–550. 2012.
|
|
5
|
Kim JW, Amin AR and Shin DM:
Chemoprevention of head and neck cancer with green tea polyphenols.
Cancer Prev Res (Phila). 3:900–909. 2010. View Article : Google Scholar
|
|
6
|
Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim
YB, Surh YJ, Cho SW, Lee KM and Hahm KB: Oxidative stress is more
important than acid in the pathogenesis of reflux oesophagitis in
rats. Gut. 49:364–371. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheong JH, Hong SY, Zheng Y and Noh SH:
Eupatilin inhibits gastric cancer cell growth by blocking
STAT3-mediated VEGF expression. J Gastric Cancer. 11:16–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho JH, Lee JG, Yang YI, Kim JH, Ahn JH,
Baek NI, Lee KT and Choi JH: Eupatilin, a dietary flavonoid,
induces G2/M cell cycle arrest in human endometrial cancer cells.
Food Chem Toxicol. 49:1737–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim MJ, Kim DH, Na HK, Oh TY, Shin CY and
Surh YJ: Eupatilin, a pharmacologically active flavone derived from
Artemisia plants, induces apoptosis in human gastric cancer (AGS)
cells. J Environ Pathol Toxicol Oncol. 24:261–269. 2005. View Article : Google Scholar
|
|
10
|
Park BB, Yoon J, Kim E, Choi J, Won Y,
Choi J and Lee YY: Inhibitory effects of eupatilin on tumor
invasion of human gastric cancer MKN-1 cells. Tumour Biol.
34:875–885. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vanhaesebroeck B, Guillermet-Guibert J,
Graupera M and Bilanges B: The emerging mechanisms of
isoform-specific PI3K signalling. Nat Rev Mol Cell Biol.
11:329–341. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2015. View Article : Google Scholar :
|
|
15
|
Shukla S and Gupta S: Apigenin-induced
cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and
loss of cyclin D1 associated retinoblastoma dephosphorylation in
human prostate cancer cells. Cell Cycle. 6:1102–1114. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gershtein ES, Scherbakov AM, Shatskaya VA,
Kushlinsky NE and Krasil’nikov MA: Phosphatidylinositol
3-kinase/AKT signalling pathway components in human breast cancer:
Clinicopathological correlations. Anticancer Res. 27:1777–1782.
2007.PubMed/NCBI
|
|
17
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leis H, Segrelles C, Ruiz S, Santos M and
Paramio JM: Expression, localization, and activity of glycogen
synthase kinase 3beta during mouse skin tumorigenesis. Mol
Carcinog. 35:180–185. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diehl JA, Cheng M, Roussel MF and Sherr
CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis
and subcellular localization. Genes Dev. 12:3499–3511. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmad N, Feyes DK, Nieminen AL, Agarwal R
and Mukhtar H: Green tea constituent epigallocatechin-3-gallate and
induction of apoptosis and cell cycle arrest in human carcinoma
cells. J Natl Cancer Inst. 89:1881–1886. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen P, Deng YL, Bergqvist S, Falk MD, Liu
W, Timofeevski S and Brooun A: Engineering of an isolated p110α
subunit of PI3Kα permits crystallization and provides a platform
for structure-based drug design. Protein Sci. 23:1332–1340. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frisch MJ, Trucks MJ, Schlegel HB,
Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci
B and Petersson GA: Gaussian 09, Revision A. 02. Gaussian. Inc.;
Wallingford, CT: 2009
|
|
24
|
Bayly C, Cieplak P, Cornell WD and Kollman
PA: A well-behaved electrostatic potential based method using
charge restraints for deriving atomic charges: the RESP model. J
Phys Chem. 97:10269–10280. 1993. View Article : Google Scholar
|
|
25
|
Morris GM, Goodsell DS, Halliday RS, Huey
R, Hart WE, Belew RK and Olson AJ: Automated docking using a
Lamarckian Genetic Algorithm and empirical binding free energy
function. J Comput Chem. 19:1639–1662. 1998. View Article : Google Scholar
|
|
26
|
Solis FJ and Wets R: Minimization by
random search techniques. Math Oper Res. 6:19–30. 1981. View Article : Google Scholar
|
|
27
|
Demidenko ZN and Blagosklonny MV: Growth
stimulation leads to cellular senescence when the cell cycle is
blocked. Cell Cycle. 7:3355–3361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gong C, Liao H, Wang J, Lin Y, Qi J, Qin
L, Tian LQ and Guo FJ: LY294002 induces G0/G1 cell cycle arrest and
apoptosis of cancer stem-like cells from human osteosarcoma via
down-regulation of PI3K activity. Asian Pac J Cancer Prev.
13:3103–3107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bode AM and Dong Z: Molecular and cellular
targets. Mol Carcinog. 45:422–430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong Z, Crawford HC, Lavrovsky V, Taub D,
Watts R, Matrisian LM and Colburn NH: A dominant negative mutant of
jun blocking 12-O-tetradecanoylphorbol-13-acetate-induced invasion
in mouse keratinocytes. Mol Carcinog. 19:204–212. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang C, Ma WY, Young MR, Colburn N and
Dong Z: Shortage of mitogen-activated protein kinase is responsible
for resistance to AP-1 transactivation and transformation in mouse
JB6 cells. Proc Natl Acad Sci USA. 95:156–161. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nomura M, Ichimatsu D, Moritani S, Koyama
I, Dong Z, Yokogawa K and Miyamoto K: Inhibition of epidermal
growth factor-induced cell transformation and Akt activation by
caffeine. Mol Carcinog. 44:67–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu Z, Ghosh S, Wang Z and Hunter T:
Downregulation of caveolin-1 function by EGF leads to the loss of
E-cadherin, increased transcriptional activity of beta-catenin, and
enhanced tumor cell invasion. Cancer Cell. 4:499–515. 2003.
View Article : Google Scholar
|
|
34
|
Mooradian DL and Diglio CA: Effects of
epidermal growth factor and transforming growth factor-beta 1 on
rat heart endothelial cell anchorage-dependent and -independent
growth. Exp Cell Res. 186:122–129. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee CJ, Jang JH, Lee JY, Lee MH, Li Y, Ryu
HW, Choi KI, Dong Z, Lee HS, Oh SR, et al: Aschantin targeting on
the kinase domain of mammalian target of rapamycin suppresses
epidermal growth factor-induced neoplastic cell transformation.
Carcinogenesis. 36:1223–1234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lim JC, Park SY, Nam Y, Nguyen TT and Sohn
UD: The protective effect of eupatilin against hydrogen
peroxide-induced injury involving 5-lipoxygenase in feline
esophageal epithelial cells. Korean J Physiol Pharmacol.
16:313–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu K, Park C, Li S, Lee KW, Liu H, He L,
Soung NK, Ahn JS, Bode AM, Dong Z, et al: Aloe-emodin suppresses
prostate cancer by targeting the mTOR complex 2. Carcinogenesis.
33:1406–1411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao N, Zhang Z, Jiang BH and Shi X: Role
of PI3K/AKT/mTOR signaling in the cell cycle progression of human
prostate cancer. Biochem Biophys Res Commun. 310:1124–1132. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yap TA, Garrett MD, Walton MI, Raynaud F,
de Bono JS and Workman P: Targeting the PI3K-AKT-mTOR pathway:
Progress, pitfalls, and promises. Curr Opin Pharmacol. 8:393–412.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bachman KE, Argani P, Samuels Y, Silliman
N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C, et al:
The PIK3CA gene is mutated with high frequency in human breast
cancers. Cancer Biol Ther. 3:772–775. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Broderick DK, Di C, Parrett TJ, Samuels
YR, Cummins JM, McLendon RE, Fults DW, Velculescu VE, Bigner DD and
Yan H: Mutations of PIK3CA in anaplastic oligodendrogliomas,
high-grade astrocytomas, and medulloblastomas. Cancer Res.
64:5048–5050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Samuels Y, Wang Z, Bardelli A, Silliman N,
Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin YJ, Lee JH, Kim YM, Oh GT and Lee H:
Macrophage inhibitory cytokine-1 stimulates proliferation of human
umbilical vein endothelial cells by up-regulating cyclins D1 and E
through the PI3K/Akt-, ERK-, and JNK-dependent AP-1 and E2F
activation signaling pathways. Cell Signal. 24:1485–1495. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sarker D and Workman P: Pharmacodynamic
biomarkers for molecular cancer therapeutics. Adv Cancer Res.
96:213–268. 2007. View Article : Google Scholar
|
|
45
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Patel S: Exploring novel therapeutic
targets in GIST: Focus on the PI3K/Akt/mTOR pathway. Curr Oncol
Rep. 15:386–395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mishra R: Glycogen synthase kinase 3 beta:
Can it be a target for oral cancer. Mol Cancer. 9:1442010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ma C, Wang J, Gao Y, Gao TW, Chen G, Bower
KA, Odetallah M, Ding M, Ke Z and Luo J: The role of glycogen
synthase kinase 3beta in the transformation of epidermal cells.
Cancer Res. 67:7756–7764. 2007. View Article : Google Scholar : PubMed/NCBI
|