Introduction
Glioblastoma multiforme (GBM) is the most frequently
occurring malignant intracranial tumor in adults, with aggressive,
invasive, angiogenic and destructive features. It is recognized as
diffuse astrocytoma grade IV by the World Health Organization
(1,2). GBM accounts for 12–15% of all primary
brain tumors and 50–60% of all astrocytic tumors (3). Despite the wide range of GBM
treatments, including surgery, radiotherapy, and chemotherapy, less
than 10% of patients survive over 3 years after the diagnosis
(4,5). Thus, effective therapeutic
interventions are urgently needed to improve the poor prognosis of
GBM.
As one of the four members of the ErbB family,
epidermal growth factor receptor (EGFR) tyrosine kinase has been
identified as an important factor for cancer cell growth,
proliferation, invasion and metastasis (6–8). It
is reported that EGFR gene is generally amplified and/or
overexpressed in high-grade glioblastoma multiforme, with a
frequency of approximately 50% (9). Gefitinib, a specific small molecule
inhibitor of EGFR, has been shown to exert therapeutic effect on
these highly aggressive brain tumors via inhibiting proliferation
and inducing apoptosis of tumor cells in human glioblastoma
multiforme (10,11). However, multiple studies have
demonstrated that as a single agent for the therapy of human
glioblastoma multiforme, gefitinib is limited due to its frequent
drug resistance and the serious cytotoxicity in clinical trials
(12–15).
β-Elemene
(1-methyl-1-vinyl-2,4-diisopropenyl-cyclohexane), a novel
anticancer agent, is the major active component extracted from the
traditional Chinese medicinal plant Curcuma wenyujin. It has
been shown to be effective against a variety of tumors in
vitro and in vivo such as lung carcinoma, breast,
leukemia, ovarian cancer and glioblastoma multiforme (16–20).
The significant effect of β-elemene is mainly caused by its ability
to pass through the blood-brain barrier (BBB) and reverse the
resistance of glioblastoma multiforme to other drugs such as
cisplatin (21,22). In addition, the specific mechanism
could be inhibiting the growth and DNA synthesis of multiple types
of tumor cells, which result in the apoptosis or suppressed growth
of tumor without severe side-effects (23,24).
Moreover, our previous studies have demonstrated
that β-elemene inhibited the growth of GBM cells through a p38
MAPK-dependent signaling pathway (20), deactivated the Raf/MEK/ERK pathway
in GBM cells by impairing formation of the Hsp90/Raf-1 complex
(25), and significantly inhibited
the repair of DNA damage in GBM cells in combination with radiation
or temozolomide (TMZ) via interfering with the ATM, AKT and ERK
signaling pathways (26).
In the present study, we found that β-elemene has a
sensitization effect on gefitinib in human GBM cells with low
toxicity and few side-effects. β-Elemene enhanced the inhibition of
proliferation and survival of GBM cells through the EGFR signaling
pathway, affected the activities of downstream related proteins AKT
and ERK. In addition, the combination of β-elemene and gefitinib
enhanced apoptosis and autophagy in GBM cells compared with
gefitinib monotherapy. These results suggested that β-elemene might
be considered as a valuable agent to enhance the chemotherapy
effect during glioblastoma multiforme treatment. The synergistic
therapy of β-elemene and gefitinib shows potential as a new
therapeutic strategy against human glioblastoma multiforme.
Materials and methods
Cell lines and culture
The human glioblastoma multiform cell lines U251,
U87-MG were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). These cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) with 10%
fetal bovine serum (FBS; Gibco), 100 units/ml of penicillin and 100
μg/ml streptomycin, and incubated at 37°C in a humidified
atmosphere with 5% CO2.
Reagents and antibodies
β-Elemene (99.2% purity) which was obtained from the
National Institutes for Food and Drug Control (NIFDC; Beijing,
China) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich,
St. Louis, MO, USA) at 20 mg/ml as a stock solution. Gefitinib was
purchased from Selleck Chemicals (Houston, TX, USA) and dissolved
in DMSO at 100 mM as a stock solution. The antibodies against EGFR,
phospho-EGFR (Tyr1068), AKT, phospho-AKT (ser473), ERK,
phospho-ERK, PARP, Cleaved PARP (Asp214), caspase-3, cleaved
caspase-3, LC-3, Beclin1, Atg5, Atg16L and GAPDH were purchased
from Cell Signaling Technology (Danvers, MA, USA). Anti-rabbit as
the secondary antibody also was purchased from Cell Signaling
Technology.
Cell viability assay
The viability of the cells was detected using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Human glioblastoma multiform cell lines U251 and U87-MG were
seeded at a density of 6×103 cells/well in 96-well
plates, respectively, incubated overnight and exposed to the
indicated concentrations of gefitinib (30 μM) and β-elemene (40
μg/ml) for 24 h. Thereafter, 10 μl of MTT solution (5 mg/ml) was
added to each well before the GBM cells were incubated for another
4 h at 37°C. After removal of the culture medium, the GBM cells
were lysed in 150 μl of dimethyl sulfoxide (DMSO), and the optical
density (OD) was measured at 490 nm with an absorbance reader
(Perkin-Elmer, Waltham, MA, USA). The concentration required to
inhibit cell growth by 50% (IC50) was calculated from
survival curves.
Colony formation assay
The proliferation ability of cells was detected by
colony formation assay. Human glioblastoma multiform cell lines
U251 and U87-MG were seeded in 6-well plates and counted at
1×103/well supplemented with fresh medium cultured for
24 h. Then, the GBM cells were exposed to indicated concentrations
of gefitinib (30 μM) and β-elemene (40 μg/ml) and incubated for an
additional 15 days. To visualize and count cell colonies, the GBM
cells were fixed and stained with crystal violet. Clusters of
>50 cells were counted as a colony.
Western blot assay
The human glioblastoma multiform cell lines U251 and
U87-MG were treated with gefitinib (30 μM) and β-elemene (40 μg/ml)
for 24 h, then, extracted with the RIPA buffer added the proteinase
inhibitor (PMSF, 1 mg/ml) on ice which including 25 mM Tris-HCl (pH
7.6), 150 mM Nacl, 1% Triton X-100, 1% sodium deoxycholate and 0.1%
SDS. After centrifugation at 14,000 × g at 4°C for 20 min, the
protein concentration was determined by the BCA assay kit (Thermo
Fisher Scientific, Waltham, MA, USA). The proteins resolved by
8–12% SDS-PAGE, transferred to polyvinylidene difluoride membrane
(PVDF; Amersham) and blocked with 5% skim milk in TBST buffer 2 h.
The membranes were then incubated with specific primary antibodies
overnight at 4°C, followed by treatment with HRP-conjugated
secondary antibodies. The protein bands were detected by ChemiDoc™
XRS+ imaging system (Bio-Rad Laboratories, Hercules, CA, USA).
Fluorescence microscopy
The human glioblastoma multiform cell line U251 was
seeded in each well of 24-well plates with poly-L-lysine-coated
coverslips and treated with gefitinib (30 μM) and β-elemene (40
μg/ml) for 24 h. Cells on coverslips were washed with
phosphate-buffered saline (PBS) 3 times, fixed with 4%
paraformaldehyde (PFA) solution in PBS at room temperature for 15
min. After washing the samples with PBS 3 times, the cells were
treated with 2% Triton X-100 5 min and blocked with 5% bovine serum
albumin (BSA) for 30 min. The slides were incubated with anti-LC3
antibody at a 1:100 for 30 min and then labeled with fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit IgG for 20 min.
The cells nuclei were stained with 4′,6-diamidino-2-phenylindole
(DAPI; Molecular Probes-Invitrogen), and immunofluorescent images
were examined under a fluorescence microscope (Olympus, Tokyo,
Japan).
Transmission electron microscopy
The human glioblastoma multiform cell line U87-MG
was treated with gefitinib (30 μM) and β-elemene (40 μg/ml) for 24
h before washed and collected by trypsinization. Then, the cells
were fixed with 2.5% glutaraldehyde for 30 min, post-fixed in 2%
osmium tetroxide for 2 h. Cells were then dehydrated by ethanol and
polymerized by epoxy resin. Finally, the embedded, sectioned,
double stained with uranyl acetate and lead citrate samples were
analysed using a JEM-1200EX transmission electron microscope (JEOL,
Tokyo, Japan).
Statistical analysis
In the present study each experiment was performed
at least three times. Statistical analysis was conducted by the
Student’s t-test and the group differences were considered
significant at P<0.05. The analysis were performed by SPSS 13.0
software.
Results
β-Elemene increases the inhibitory
effects of gefitinib on the proliferation and survival of GBM
cells
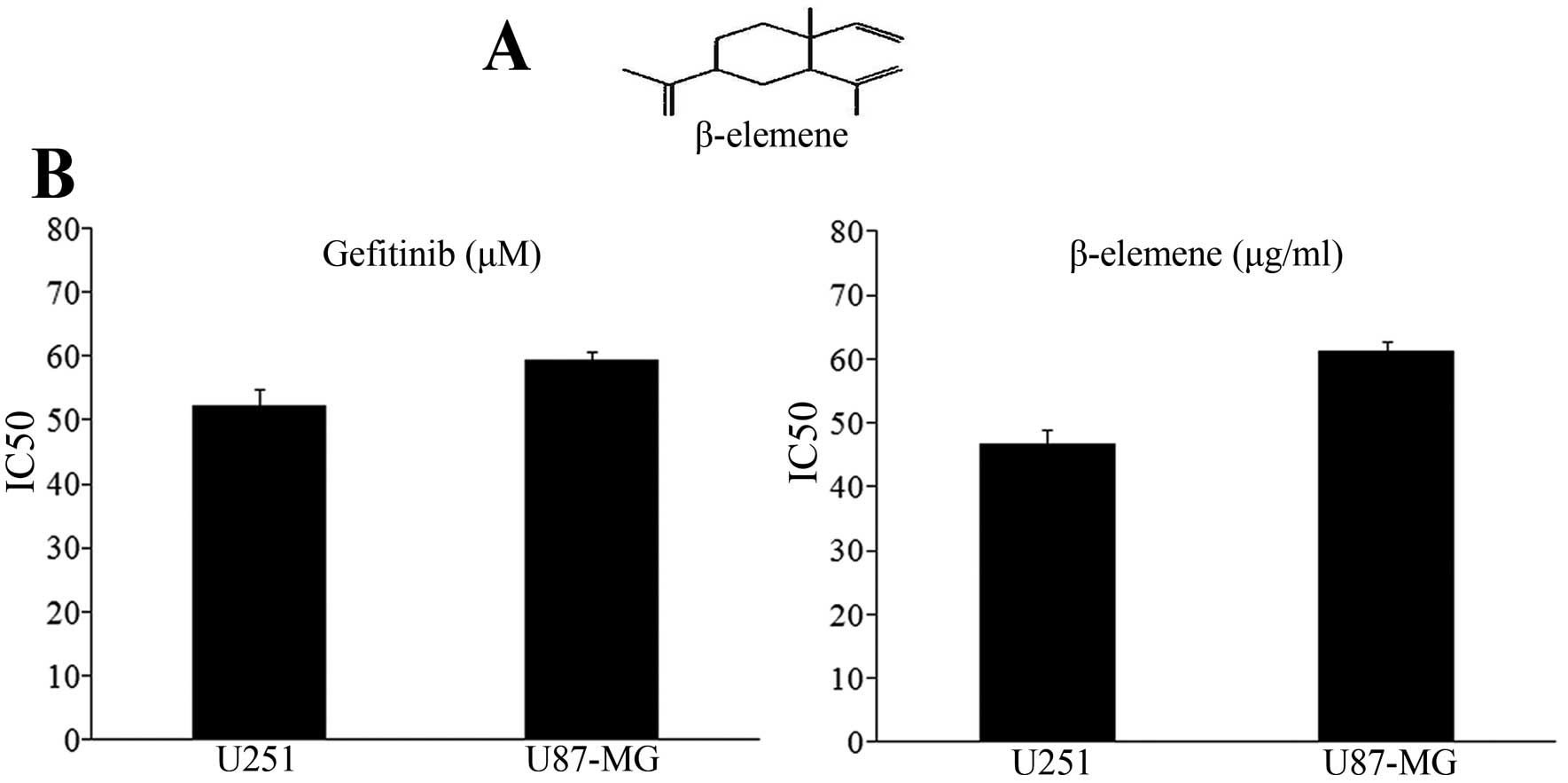
The structure of β-elemene is shown in Fig. 1A, and the value of median
inhibitory concentration (IC50) in human glioblastoma
multiforme cell lines was calculated by the growth inhibition
curves shown in Fig. 1B. The
IC50 doses for gefitinib at 52.24 μM for U251 and 59.38
μM for U87-MG. The IC50 value for β-elemene is 46.72
μg/ml for U251 and 61.33 μg/ml for U87-MG. By using the MTT assay,
we tested the effects of gefitinib alone, β-elemene alone or
combination on proliferation of glioblastoma multiforme cells.
After treatment with either 30 μM gefitinib, 40 μg/ml β-elemene, or
combination of these two drugs for 24 h, the viability of cells
demonstrated that β-elemene markedly increased the
gefitinib-induced inhibition of cell growth in both U251 and U87-MG
cells (Fig. 2A). For the colony
formation assay, after the treatment with indicated concentrations
of gefitinib and β-elemene for 15 days, the numbers of the colonies
were fewer and the sizes were smaller in the combination treatment
group when compared with the group treated with gefitinib alone or
the control (Fig. 2B).
Quantification of colony formation is shown in Fig. 2C. It was further confirmed that
β-elemene enhanced the efficacy of gefitinib on inhibiting cell
viability and proliferation in human glioblastoma multiforme cell
lines U251 and U87-MG.
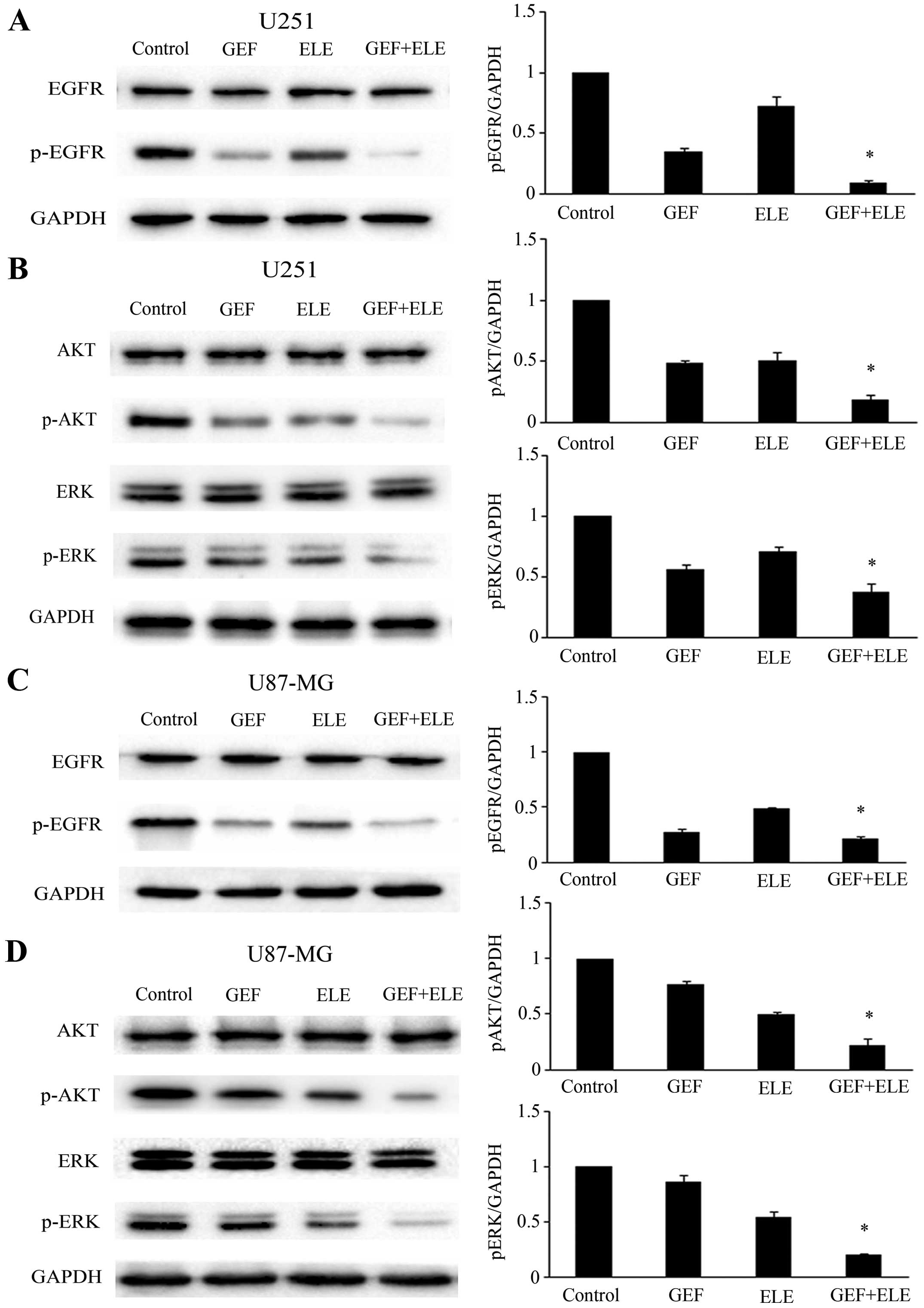
β-Elemene enhances the sensitivity of GBM
cells to gefitinib via downregulating the activation of EGFR, AKT
and ERK signalin
Human GBM cell lines U251 and U87-MG were treated
with either 30 μM gefitinib or 40 μg/ml β-elemene, or co-treatment
for 24 h. Western blot analysis showed that although gefitinib
played a significant role in the expression of p-EGFR protein
levels, combination of gefitinib and β-elemene can suppress the
activation of p-EGFR more obviously (Fig. 3A and C). We tested the downstream
EGFR signaling pathways that mediated the effect of β-elemene and
gefitinib on cell growth. We found that the combination of
gefitinib and β-elemene group has more obvious effect on decreasing
the phosphorylation of AKT and ERK than the monotherapy group
(Fig. 3B and D).
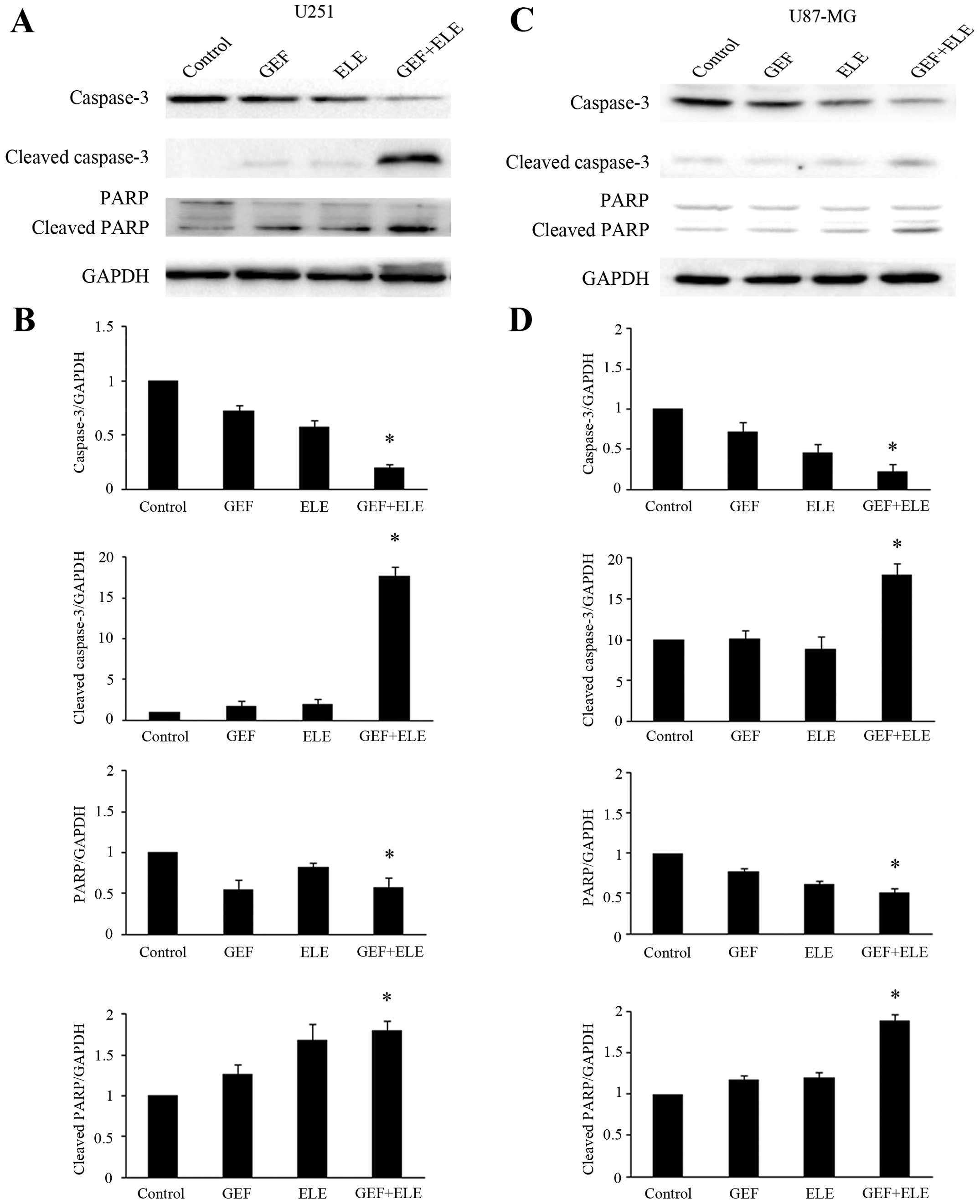
β-Elemene enhances the induction effects
of gefitinib on the apoptosis of GBM cells
Human GBM cell lines U251 and U87-MG were treated
with either 30 μM gefitinib or 40 μg/ml β-elemene, or co-treatment
for 24 h. To analyze the synergistic effect of β-elemene and
gefitinib in inducing cell apoptosis, we evaluated the activation
of caspase-3 and poly ADP-ribose polymerase (PARP) cleavage by
western blot analysis (Fig. 4A and
C). The data indicated that the co-treatment of β-elemene and
gefitinib caused significantly stronger caspase-3 and PARP cleavage
than the single medication treatment (Fig. 4B and D).
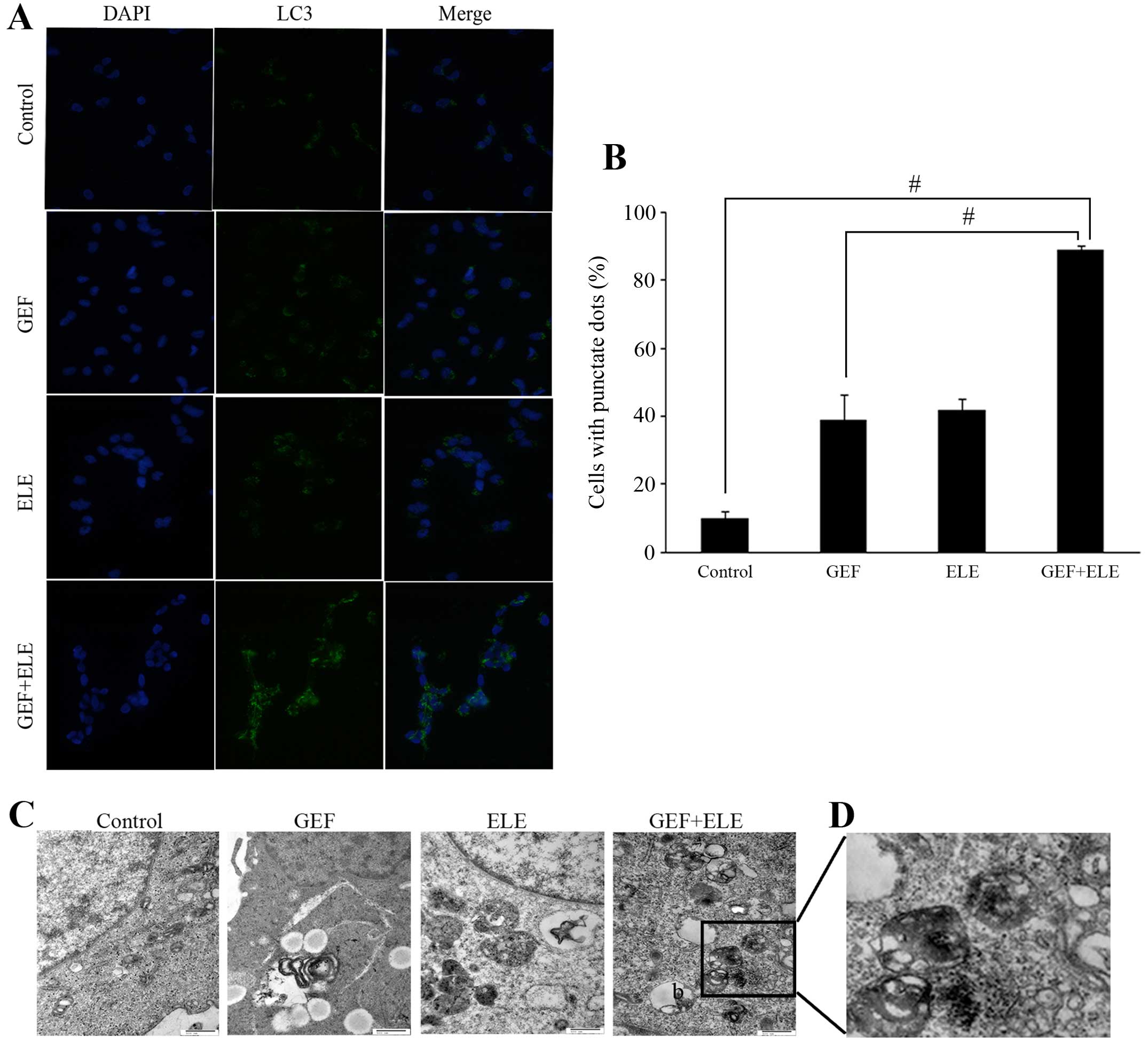
β-Elemene increases the occurrence of
autophagy induced by gefitinib in GBM cells
In addition to cell apoptosis research, we also
examined the effect of β-elemene and gefitinib on inducing
autophagy in GBM cells. Under the treatment of 30 μM gefitinib, 40
μg/ml β-elemene, and their combination in U251 for 24 h, the
localization of LC3 was evaluated by fluorescent microscopy. As
shown in Fig. 5A and B, only a few
LC3-positive puncta were observed in untreated control cells and
single medication cells, while in the cells treated with the
β-elemene and gefitinib, over 80% of cells showed LC3-positive
puncta. The formation of autophagosomes was further confirmed by
transmission electron microscopy (Fig.
5C). Upon treatment of combined 30 μM gefitinib and 40 μg/ml
β-elemene in the U87-MG cells, many, double membrane enclosed
vesicles containing engulfed organelles were observed in the
cytoplasm (Fig. 5D). With the
treatment of 30 μM gefitinib, 40 μg/ml β-elemene, and their
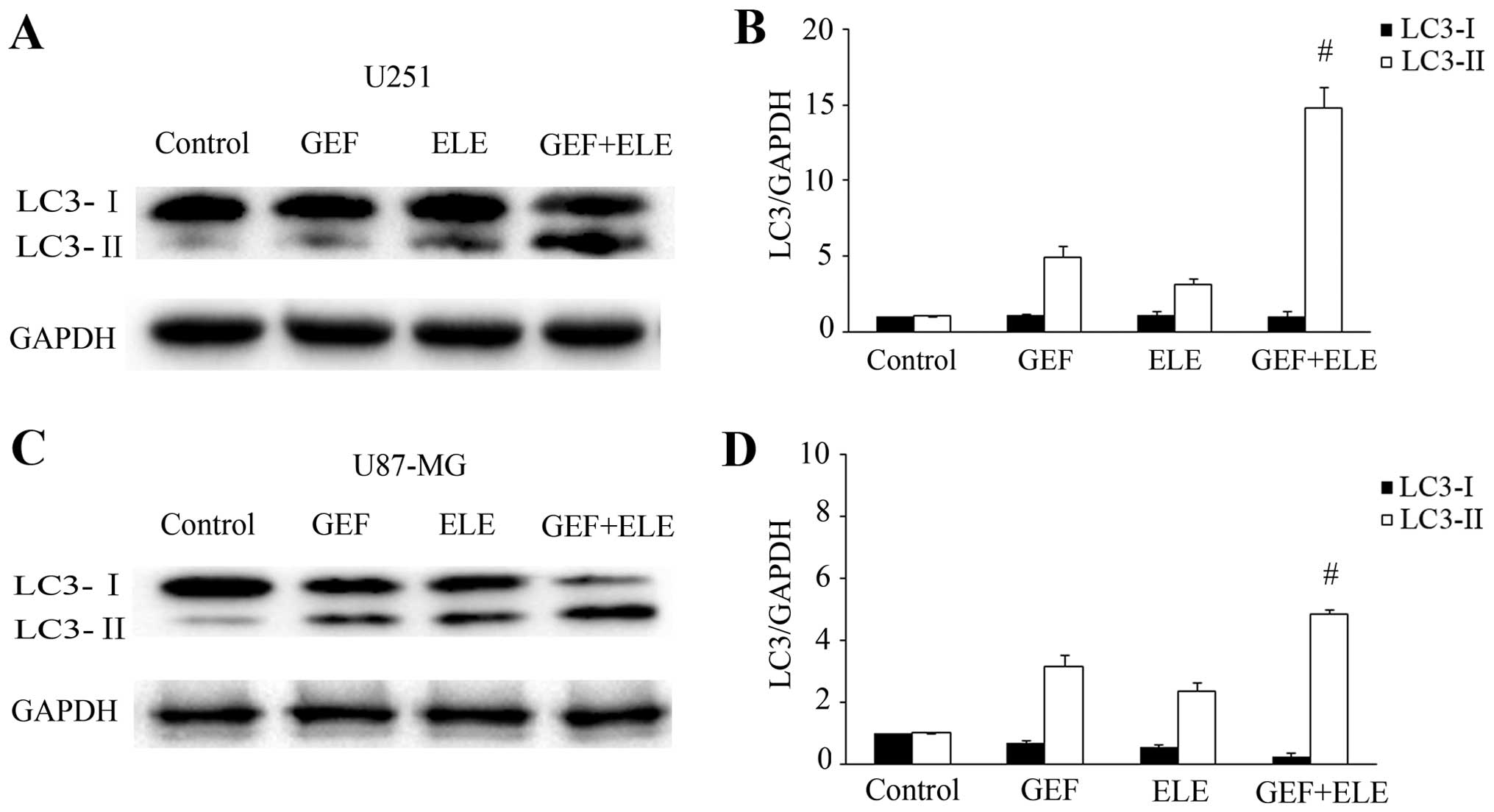
combination in human GBM cell lines U251 and U87-MG for 24 h,
levels of autophagy iconic protein LC3 (LC3-I and LC3-II) were
demonstrated by western blotting (Fig.
6A and C). In addition, level of LC3-II was more significantly
increased in the combination group of gefitinib and β-elemene
(Fig. 6B and D). These data
indicated that the potentiation of β-elemene in gefitinib treatment
not only resulted in apoptosis but also induced autophagy.
Effects of β-elemene and gefitinib
combined on autophagy related proteins in GBM cells
There are many autophagy-regulatory genes playing
important roles in autophagy reaction, such as Becline 1, Atg5 and
Atg16L. Human GBM cell lines U251 and U87-MG were treated with
either 30 μM gefitinib or 40 μg/ml β-elemene, or co-treatment for
24 h. Western blot analysis showed that the level of Becline 1
protein in U251 cells was upregulated (Fig. 7A and B), whereas the expression of
Beclin1 in U87-MG and other Atg proteins such as Atg5 and Atg16L
were not significantly changed in U251 and U87-MG cells (Fig. 7C and D). A possibility is that
β-elemene may affect autophagic flux rather than induce
autophagy.
Discussion
EGFR signaling pathway involved in cancer
development and prognosis opened avenues for targeted therapies,
which made treatment more tumor-specific (27). However, EGFR-targeted therapeutics
has been limited in clinical trials because of drug resistance and
the severity of the various associated side-effects of EGFR
tyrosine kinase inhibitor gefitinib (12,14).
Comparatively, β-elemene, as a traditional anti-cancer Chinese
herbal medicine, has been approved by the State Food and Drug
Administration of China for the treatment of malignant effusion and
some solid tumors. It also exhibited a wide range of anticancer
effects with low toxicity and few side-effects in vitro and
in vivo (20,28). Numerous basic studies have
concluded that β-elemene has strong antitumor activity on human GBM
cell lines, as well as on rats and glioblastoma-bearing nude mice
by inhibiting cell proliferation, inducing tumor cell apoptosis,
and arresting cell cycle processes (29–31).
Simultaneously, β-elemene is able to activate the p38 MAPK
signaling pathway and inactivate RAF/MEK/ERK signaling pathway to
induce cell cycle G0/G1 phase arrest of GBM cells (20). In addition, they are associated
with therapeutic resistance of GBM cells. The data presented here
provide evidence that the combination medication of β-elemene and
gefitinib efficacy is obviously more effective than gefitinib
alone.
As key downstream proteins of EGFR signaling
pathway, AKT and ERK participated in many fundamental cellular
processes such as increasing the ability of survival, migration and
invasion of tumor cells (32). It
has been reported that berberine induced senescence of GBM cells by
downregulating the EGFR-MEK-ERK signaling pathway (33). Although current research related to
β-elemene and EGFR is still not clear, some reports have shown that
β-elemene could inhibit GBM cells growth by altering the activities
of AKT and ERK (26,34). The concentrations of β-elemene used
in this study were consistent with or even lower than reported by
others demonstrating substantial growth inhibition of different
types of cancer cells. The IC50 value of U87-MG GBM
cells showed stronger resistance than the U251 cells to either
gefitinib or β-elemene treatment. The experimental group combining
gefitinib and β-elemene significantly reduced the activities not
only the phosphorylation of AKT and ERK but also the EGFR. The
above indicated that β-elemene and gefitinib inhibited the
proliferation and survival of GBM via inhibiting the activation of
AKT and ERK which are related to the downstream EGFR signaling
pathways, and β-elemene in combination with gefitinib produced a
synergistic effect against human GBM cells.
Apoptosis is a special biological character of
programmed cell death. Several findings have shown that β-elemene
markedly promoted cisplatin-induced apoptotic cell death, decreased
the expression level of the Bcl-2 protein, increased cytochrome
c release, activated poly ADP-ribose polymerase and
caspase-3, -7 and -9 in prostate cancer cells. Concurrently, the
apoptotic percentage of prostate cancer cells was increased by
β-elemene in a dose- and time-dependent manner (35,36).
In addition, β-elemene could induce apoptosis and arrest non-small
cell lung cancer cells at the G2/M phase (37). Furthermore, previous studies have
demonstrated that β-elemene induced apoptosis in human glioma cells
through inhibiting the molecular complex Hsp90/Raf-1 and
upregulating Bax, Fas/FasL and downregulating Bcl-2 (25,38).
In the present study, we found that the changes in apoptosis
related proteins are not obvious after the separate treatment of
β-elemene and gefitinib. However, the combination treatment of
β-elemene and gefitinib induced apoptosis in GBM cells through
mitochondrial apoptotic pathway, this is also supported by evidence
including the release of activated PARP and caspase-3.
Based on more comprehensive study of β-elemene in
killing tumor cells especially research on the mechanism by which
β-elemene induces autophagy of GBM cells, our experiments examined
the activity of both apoptosis and autophagy in the human GBM cells
subjected to combination drug therapy of β-elemene and gefitinib.
Autophagy is another key cellular process known to promote
occurrence of cell death. It is an intracellular degradation
process in eukaryotic cells induced by stress, organelles within
double membraned autophagosomes via degrading cytoplasmic
components to maintain cell homeostasis (39,40).
β-Elemene is associated with occurrence of autophagy in other tumor
cells. Several studies have suggested that β-elemene significantly
induced the conversion of LC3-I into LC3-II as well as the
formation of autolysosomes, indicating the activation of autophagy.
The derivatives of β-elemene were able to suppress the
proliferation of SGC-7901 and HeLa cells by inhibiting mTOR
activity and inducing autophagy (17,41).
In the present study, a robust autophagy was observed among the
cells treated with combination of β-elemene and gefitinib to
enhance gefitinib induced apoptosis, which was verified by the
increase of punctate LC3 and the morphologic changes. Western
blotting showed that the combination of β-elemene and gefitinib
indeed induced the conversion of LC3-II from LC3-I, and these
specific changes of LC3 have been characterized as an
autophagosomal marker in mammalian autophagy. Accumulated evidence
suggested that the induction of autophagy is associated with the
upregulation of certain Atg proteins. For example, Thyagarajan
et al (42) reported that
triterpene-induced autophagy is accompanied by the upregulation of
Beclin 1. Notably, neither β-elemene nor combination gefitinib and
β-elemene induced autophagy with significantly alternating the
levels of Atg proteins except for the expression level of the
Beclin1 protein in our study. We speculate that it may be related
to the type of tumor cells, malignant degree and concentration of
specificity drug treatment. Another possibility is that β-elemene
may affect autophagic flux rather than induce autophagy. Under the
combined treatment of gefitinib and β-elemene, the activity of
Beclin1 protein is significantly upregulated comparing with
gefitinib monotherapy in the human GBM cells.
Taken together, this study provides the first
evidence that β-elemene could enhance the efficacy of gefitinib to
inhibit the proliferation and survival of GBM cells along with
downregulating the activity of EGFR, AKT and ERK. In addition, the
antitumor effect of β-elemene could strengthen the ability of
gefitinib to induce apoptosis of GBM cells. More importantly, the
EGFR signaling pathway was inhibited by the combination of
β-elemene and gefitinib, which not only led to activation of a
protective autophagy but also significantly enhanced the
apoptosis-inducing ability. This combination treatment scheme might
be a new efficacious strategy for the treatment of primary brain
tumors in the future.
Acknowledgements
The present study was supported by the National
Science Foundation of China (no. 81202964) and the Liaoning
Province Natural Science Foundation of China (no. 2013023043). We
also thank all our colleagues in our research group for their
generous support.
Abbreviations:
|
GBM
|
glioblastoma multiforme
|
|
EGFR
|
epidermal growth factor receptor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
BBB
|
blood-brain barrier
|
|
TMZ
|
temozolomide
|
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eimer S, Belaud-Rotureau M-A, Airiau K,
Jeanneteau M, Laharanne E, Véron N, Vital A, Loiseau H, Merlio JP
and Belloc F: Autophagy inhibition cooperates with erlotinib to
induce glioblastoma cell death. Cancer Biol Ther. 11:1017–1027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gudinaviciene I, Pranys D and Juozaityte
E: Impact of morphology and biology on the prognosis of patients
with gliomas. Medicina (Kaunas). 40:112–120. 2004.
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al; European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group. Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fine HA, Dear KB, Loeffler JS, Black PM
and Canellos GP: Meta-analysis of radiation therapy with and
without adjuvant chemotherapy for malignant gliomas in adults.
Cancer. 71:2585–2597. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Appert-Collin A, Hubert P, Crémel G and
Bennasroune A: Role of ErbB receptors in cancer cell migration and
invasion. Front Pharmacol. 6:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lund-Johansen M, Bjerkvig R, Humphrey PA,
Bigner SH, Bigner DD and Laerum OD: Effect of epidermal growth
factor on glioma cell growth, migration, and invasion in vitro.
Cancer Res. 50:6039–6044. 1990.PubMed/NCBI
|
|
8
|
Nishikawa R, Ji XD, Harmon RC, Lazar CS,
Gill GN, Cavenee WK and Huang HJ: A mutant epidermal growth factor
receptor common in human glioma confers enhanced tumorigenicity.
Proc Natl Acad Sci USA. 91:7727–7731. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li B, Chang CM, Yuan M, McKenna WG and Shu
HK: Resistance to small molecule inhibitors of epidermal growth
factor receptor in malignant gliomas. Cancer Res. 63:7443–7450.
2003.PubMed/NCBI
|
|
10
|
Chang CY, Kuan YH, Ou YC, Li JR, Wu CC,
Pan PH, Chen WY, Huang HY and Chen CJ: Autophagy contributes to
gefitinib-induced glioma cell growth inhibition. Exp Cell Res.
327:102–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang CY, Shen CC, Su HL and Chen CJ:
Gefitinib induces apoptosis in human glioma cells by targeting Bad
phosphorylation. J Neurooncol. 105:507–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rich JN, Reardon DA, Peery T, Dowell JM,
Quinn JA, Penne KL, Wikstrand CJ, Van Duyn LB, Dancey JE, McLendon
RE, et al: Phase II trial of gefitinib in recurrent glioblastoma. J
Clin Oncol. 22:133–142. 2004. View Article : Google Scholar
|
|
13
|
Agarwal S, Sane R, Gallardo JL, Ohlfest JR
and Elmquist WF: Distribution of gefitinib to the brain is limited
by P-glycoprotein (ABCB1) and breast cancer resistance protein
(ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 334:147–155.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao L, Yang G, Shi Y, Su C and Chang J:
Co-delivery of Gefitinib and chloroquine by chitosan nanoparticles
for overcoming the drug acquired resistance. J Nanobiotechnology.
13:572015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou ZZ, Nie PP, Li YW, Hou BX, Rui-Li, Shi
XP, Ma ZK, Han BW and Luo XY: Synergistic induction of apoptosis by
salinomycin and gefitinib through lysosomal and mitochondrial
dependent pathway overcomes gefitinib resistance in colorectal
cancer. Oncotarget. Oct 5–2015.(Epub ahead of print). View Article : Google Scholar
|
|
16
|
Wang G, Li X, Huang F, Zhao J, Ding H,
Cunningham C, Coad JE, Flynn DC, Reed E and Li QQ: Antitumor effect
of beta-elemene in non-small-cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding XF, Shen M, Xu LY, Dong JH and Chen
G: 13,14-bis(cis-3,5-dimethyl-1-piperazinyl)-β-elemene, a novel
β-elemene derivative, shows potent antitumor activities via
inhibition of mTOR in human breast cancer cells. Oncol Lett.
5:1554–1558. 2013.PubMed/NCBI
|
|
18
|
Yu Z, Wang R, Xu L, Xie S, Dong J and Jing
Y: β-Elemene piperazine derivatives induce apoptosis in human
leukemia cells through downregulation of c-FLIP and generation of
ROS. PLoS One. 6:e158432011. View Article : Google Scholar
|
|
19
|
Li X, Wang G, Zhao J, Ding H, Cunningham
C, Chen F, Flynn DC, Reed E and Li QQ: Antiproliferative effect of
beta-elemene in chemoresistant ovarian carcinoma cells is mediated
through arrest of the cell cycle at the G2-M phase. Cell Mol Life
Sci. 62:894–904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ
and Xu YH: Anti-tumor effect of beta-elemene in glioblastoma cells
depends on p38 MAPK activation. Cancer Lett. 264:127–134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu XS, Xie T, Lin J, Fan HZ, Huang-Fu HJ,
Ni LF and Yan HF: An investigation of the ability of elemene to
pass through the blood-brain barrier and its effect on brain
carcinomas. J Pharm Pharmacol. 61:1653–1656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alvarez-García V, González A,
Alonso-González C, Martínez-Campa C and Cos S: Regulation of
vascular endothelial growth factor by melatonin in human breast
cancer cells. J Pineal Res. 54:373–380. 2013.
|
|
23
|
Lu X, Wang Y, Luo H, Qiu W, Han H, Chen X
and Yang L: β-elemene inhibits the proliferation of T24 bladder
carcinoma cells through upregulation of the expression of Smad4.
Mol Med Rep. 7:513–518. 2013.
|
|
24
|
Liu J, Zhang Y, Qu J, Xu L, Hou K, Zhang
J, Qu X and Liu Y: β-Elemene-induced autophagy protects human
gastric cancer cells from undergoing apoptosis. BMC Cancer.
11:1832011. View Article : Google Scholar
|
|
25
|
Zhao YS, Zhu TZ, Chen YW, Yao YQ, Wu CM,
Wei ZQ, Wang W and Xu YH: β-elemene inhibits Hsp90/Raf-1 molecular
complex inducing apoptosis of glioblastoma cells. J Neurooncol.
107:307–314. 2012. View Article : Google Scholar
|
|
26
|
Liu S, Zhou L, Zhao Y and Yuan Y:
β-elemene enhances both radiosensitivity and chemosensitivity of
glioblastoma cells through the inhibition of the ATM signaling
pathway. Oncol Rep. 34:943–951. 2015.PubMed/NCBI
|
|
27
|
Zhao Q, Kretschmer N, Bauer R and Efferth
T: Shikonin and its derivatives inhibit the epidermal growth factor
receptor signaling and synergistically kill glioblastoma cells in
combination with erlotinib. International journal of cancer Int J
Cancer. 137:1446–1456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu T, Li X, Luo L, Wang X, Li Z, Xie P,
Gao X, Song Z, Su J and Liang G: Reversion of malignant phenotypes
of human glioblastoma cells by β-elemene through β-catenin-mediated
regulation of stemness-, differentiation- and
epithelial-to-mesenchymal transition-related molecules. J Transl
Med. 13:3562015. View Article : Google Scholar
|
|
29
|
Sun Y, Liu G, Zhang Y, Zhu H, Ren Y and
Shen YM: Synthesis and in vitro anti-proliferative activity of
beta-elemene monosubstituted derivatives in HeLa cells mediated
through arrest of cell cycle at the G1 phase. Bioorg Med Chem.
17:1118–1124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu Z, Wang R, Xu L, Dong J and Jing Y:
N-(beta-Elemene-13-yl) tryptophan methyl ester induces apoptosis in
human leukemia cells and synergizes with arsenic trioxide through a
hydrogen peroxide dependent pathway. Cancer Lett. 269:165–173.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao YQ, Xu YH, Lu J, Zhou HY and Wang YZ:
Effect of p38 MAPK on elemene-induced cell cycle arrest in C6
glioblastoma cells. Zhonghua Yi Xue Za Zhi. 88:56–58. 2008.(In
Chinese). PubMed/NCBI
|
|
32
|
Zhou J, Du T, Li B, Rong Y, Verkhratsky A
and Peng L: Crosstalk between MAPK/ERK and PI3K/AKT signal pathways
during brain ischemia/reperfusion. ASN Neuro. 7:72015. View Article : Google Scholar
|
|
33
|
Liu Q, Xu X, Zhao M, Wei Z, Li X, Zhang X,
Liu Z, Gong Y and Shao C: Berberine induces senescence of human
glioblastoma cells by downregulating the EGFR-MEK-ERK signaling
pathway. Mol Cancer Ther. 14:355–363. 2015. View Article : Google Scholar
|
|
34
|
Zhan YH, Liu J, Qu XJ, Hou KZ, Wang KF,
Liu YP and Wu B: β-Elemene induces apoptosis in human renal-cell
carcinoma 786-0 cells through inhibition of MAPK/ERK and PI3K/ Akt/
mTOR signalling pathways. Asian Pac J Cancer Prev. 13:2739–2744.
2012. View Article : Google Scholar
|
|
35
|
Li QQ, Wang G, Reed E, Huang L and Cuff
CF: Evaluation of cisplatin in combination with β-elemene as a
regimen for prostate cancer chemotherapy. Basic Clin Pharmacol
Toxicol. 107:868–876. 2010.PubMed/NCBI
|
|
36
|
Li QQ, Wang G, Huang F, Banda M and Reed
E: Antineoplastic effect of beta-elemene on prostate cancer cells
and other types of solid tumour cells. J Pharm Pharmacol.
62:1018–1027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang CF, Fan L, Tian M, Qi XS, Liu JX,
Feng JB, Du SS, Su X and Wang YY: Radiosensitizing effect of
schinifoline from Zanthoxylum schinifolium Sieb et Zucc on human
non-small cell lung cancer A549 cells: A preliminary in vitro
investigation. Molecules. 19:20128–20138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li CL, Chang L, Guo L, Zhao D, Liu HB,
Wang QS, Zhang P, Du WZ, Liu X, Zhang HT, et al: β-elemene induces
caspase-dependent apoptosis in human glioma cells in vitro through
the upregulation of Bax and Fas/ FasL and downregulation of Bcl-2.
Asian Pac J Cancer Prev. 15:10407–10412. 2014. View Article : Google Scholar
|
|
39
|
Shi JM, Bai LL, Zhang DM, Yiu A, Yin ZQ,
Han WL, Liu JS, Li Y, Fu DY and Ye WC: Saxifragifolin D induces the
interplay between apoptosis and autophagy in breast cancer cells
through ROS-dependent endoplasmic reticulum stress. Biochem
Pharmacol. 85:913–926. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guan C, Liu W, Yue Y, Jin H, Wang X and
Wang XJ: Inhibitory effect of β-elemene on human breast cancer
cells. Int J Clin Exp Pathol. 7:3948–3956. 2014.
|
|
42
|
Thyagarajan A, Jedinak A, Nguyen H, Terry
C, Baldridge LA, Jiang J and Sliva D: Triterpenes from Ganoderma
Lucidum induce autophagy in colon cancer through the inhibition of
p38 mitogen-activated kinase (p38 MAPK). Nutr Cancer. 62:630–640.
2010. View Article : Google Scholar : PubMed/NCBI
|