Introduction
Lung carcinoma is one of the most common cancers in
both male and female and the leading cause of cancer related death
all around the world (1). In 2015,
there were an estimated more than 222,0000 new cases of lung cancer
accounting for 14% of all new diagnoses from cancer, and leading to
an estimated 158,040 cancer deaths, in the United States (2). Moreover, it is anticipated that the
incidence of lung cancer will keep increasing in Asian and many
developing countries with the high and increasing number of
smoking. Rates of lung cancer are mainly determined by smoking
patterns, but medical, occupational, and environmental radiation
exposures, have also been shown to increase risks of lung cancer
(3).
Lung cancer is a heterogeneous disease that has
historically been divided into two main types based on the disease
patterns and treatment strategies: small cell lung cancer (SCLC)
and non-small cell lung cancer (NSCLC) (4,5), and
NSCLC comprises approximately 80% of all lung cancers (6). Although the treatments of lung cancer
have advanced and improved greatly, the 5-year survival rate of
lung cancer patients is little improved (1). Over the last decades, the local
control for early-stage non-small cell lung cancer (NSCLC) has
dramatically improved for lung cancer patients (7,8).
However, nearly 20% of early-stage lung cancer patients are
developing distant metastasis (9,10).
In recent decades, tremendous efforts were made to understand the
occurrence and development of lung cancer, but the metastasis
mechanisms of NSCLC have not been clarified.
MicroRNAs (miRNAs) are a class of small endogenous
non-coding RNAs with approximately 18–22 nucleotides in length.
miRNAs can directly bind to the 3′ untranslated regions (3′-UTRs)
of target genes resulting in negatively regulating mRNA stability
or suppressing translation (11,12).
Increasing number of research has proven that miRNAs are involved
in various cellular and molecular processes including growth,
apoptosis, differentiation, metabolism and immunity (13,14).
In addition, many studies have confirmed that miRNAs play crucial
roles in the initiation and progression of human cancers, such as
ovarian cancer (15),
hepatocellular carcinoma (16),
breast cancer (17) and lung
carcinoma (18). miR-186 was found
deregulated in various human cancers. The expression of miR-186 was
observed significantly increased in pancreatic cancer tissues
(19). Nevertheless, recent
studies reported the expression of miR-186 was decreased in various
human malignancies and function as a tumor suppressor (20,21).
A previous study showed that miR-186 was significantly decreased in
non-small cell lung cancer (22,23).
However, the concrete mechanism of miR-186 in lung cancer
metastasis remains elusive.
In the present study, we found that miR-186 was
significantly decreased in lung cancer tissues. Overexpression of
miR-186 suppressed lung cancer cell proliferation, invasion,
migration and induced apoptosis by direct targeting of
mitogen-activated protein kinase kinase kinase 2 (MAP3K2). Our
results revealed the miR-186-MAP3K2 may represent a new potential
target for diagnosis and treatment of lung carcinoma.
Materials and methods
Clinical specimens
A total of 20 cases of NSCLC specimens (20 tumor
tissues and 20 matched adjacent normal tissues) were obtained from
the Nanfang Hospital, Southern Medical University from 2014 to
2015. All patients underwent surgery before receiving chemotherapy
and/or radiation therapy. All patients were histologically
diagnosed according to the clinicopathological criteria of the
UICC. The median age of NSCLC patients was 51.7 years (range, 49–68
years). All of the malignant tissues were from stage II–III tumors.
The study was approved by the Ethics Committee of the Nanfang
Hospital, Southern Medical University, and all volunteers provided
informed consents for the use of their specimens in this study.
Cell lines and culture condition
Primary pulmonary epithelial cells (HPAEpiC) were
purchased from ScienCell Research Laboratories (Carlsbad, CA, USA)
and cultured according to the supplier’s instructions using
alveolar epithelial cell medium (ScienCell Research Laboratories;
cat. #3201) with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA,
USA). Human lung cancer cell lines A549, 95-D, HCC827 and NCI-H1650
were obtained from Shanghai Institute of Cell Biology (Shanghai,
China). Four cancer cell lines were cultured in the RPMI-1640
medium (ATCC, Manassas, VA, USA) with 10% FBS. The media contained
100 UI/ml penicillin and 100 μg/ml streptomycin. Cells were
cultured and maintained in a humidified incubator at 37°C and
supplemented with 5% CO2.
Plasmid construction
The 3′-untranslated region (3′-UTR) of MAP3K2 was
amplified from human genomic DNA and cloned downstream of
psi-CHECK2 vector (Promega, Madison, WI, USA), and termed
MAP3K2-3′UTR-WT. Mutations of miR-186 binding sites (AUUCUUU to
CAAUCU) were introduced by QuikChange Site-Directed Mutagenesis kit
(Stratagene, La Jolla, CA, USA), and termed MAP3K2-3′UTR-MUT.
For overexpression plasmid cloning, MAP3K2 cDNA was
first reverse-transcribed from the RNA of the A549 cells using
M-MLV Reverse Transcriptase (Promega) with oligo(dT)18
primer. The coding region (1860 bp) was then amplified by PCR from
cDNA and inserted into the HindIII/BamHI site of the
pcDNA3.1 vector (Promega). The following sequences were used:
forward primer 5′-AAGCTTATGGATGATCA GCAAGC-3′ and reverse
primer 5′-GGATCCCTAGTGATA ATGCACAAACAT-3′. Cloned products
were confirmed by DNA sequencing.
Cell transfection
A549 or HCC827 (1×104) cells/well were
seeded in 96-well culture plates. After culture for 24 h, miR-186
mimics (100 mM) (Shanghai GenePharma Co., Ltd., Shanghai, China) or
MAP3K2-siRNA (100 mM) (GenePharma) or pcDNA3.1-MAP3K2 (20 μg) or
equal amount of negative control sequences and vectors were
transfected into A549 or HCC827 cells using Lipofectamine 2000
(Beyotime Institute of Biotechnology, Shanghai, China). After
cultured for different times, cells were harvested and used in
further study.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
Total RNA from tissues or 1×107 cells was
extracted using TRIzol reagent (Invitrogen Inc., Waltham, MA, USA).
SYBR PrimeScript miRNA RT PCR kit (Takara Bio, Dalian China) was
used to detect the miR-186 expression level and results were
normalized to U6. For qRT-PCR, 2 μg of total RNA was used for
reverse-transcription PCR using M-MLV Reverse Transcriptase
(Promega). SYBR-Green Real-Time Master Mix (Toyobo Co., Ltd.,
Osaka, Japan) was used to analyze the relative expression of MAP3K2
and GAPDH was used as an internal control. All primers were
synthesized by Sangon (Sangon Biotech Co., Ltd., Shanghai, China)
(Table I). Applied Biosystems 7500
Sequence Detection system (Applied Biosystems, Foster City, CA,
USA) was used to perform qRT-PCR and data collection. miR-186 and
MAP3K2 expression levels were calculated and normalized using the
2−ΔΔCt method.
 | Table IPrimer sequences used for miRNA and
mRNA expression analysis. |
Table I
Primer sequences used for miRNA and
mRNA expression analysis.
| Name | Primer sequence
(5′-3′) |
|---|
| miR-186-RT |
CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTTTGGGCT |
| U6-RT |
CGCTTCACGAATTTGCGTGTCAT |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
| miR-186-F |
ACACTCCAGCTGGGCAGCAGCACACT |
| Universal-R |
CTCAACTGGTGTCGTGGA |
| MAP3K2-F |
CTTGCTACATGAGCGAATTGTT |
| MAP3K2-R |
AATCTGACGGGTGTATTTCCTA |
| GAPDH-F |
TGTTCGTCATGGGTGTGAA |
| GAPDH-R |
ATGGCATGGACTGTGGTCAT |
Protein extraction and western
blotting
Lung cancer tissues and cells were washed with PBS
(pH 7.4) twice and lysed in RIPA lysis and extraction buffer
(Thermo Fisher Scientific, Waltham, MA, USA) with 1% (v/v)
aprotinin (Millipore, Billerica MA, USA) and 1% (v/v) pepstatin
(Millipore) on ice for 20 min. The lysates or homogenates were
centrifuged at 12000 × g, 4°C for 15 min. Then the collected
supernatant and protein concentration was determined using a Pierce
BCA protein assay kit (Thermo Fisher Scientific). Protein samples
were separated by 12% SDS-PAGE and detected by western blot
analysis using rabbit monoclonal anti-MAP3K2 (ab33918; Abcam,
Cambridge, MA, USA) and rabbit monoclonal anti-GAPDH (ab181602;
Abcam). Goat anti-rabbit IgG H&L (ab6721; Abcam) secondary
antibody with HRP conjugated and results were developed using the
ECL technique (SuperSignal West Femto; Pierce, Rockford, IL,
USA).
Cell viability assay
The Cell Counting Kit-8 was used to estimate cell
proliferation (24). In brief,
1×104 A549 or HCC827 cells/well were seeded in 96-well
culture plates. After transfected with miR-186 mimics (100 mM)
(GenePharma) or MAP3K2-siRNA (100 mM) (GenePharma) or
pcDNA3.1-MAP3K2 (20 μg) for 24, 48 and 72 h, cells were treated
with CCK-8 solution for 1 h at room temperature. The absorbance was
measured at 450 nm using a microplate reader Thermo Plate (Rayto
Life and Analytical Science Co., Ltd., Shenzhen, China).
Apoptosis assay
The apoptosis of A549 or HCC827 cells transfected
with miR-186 mimics or MAP3K2-siRNA or pcDNA3.1-MAP3K2 (20 μg) was
tested using Annexin V-FITC/PI apoptosis detection kit (BD
Biosciences, San Jose, CA, USA) by flow cytometry as previously
described (24). All experiments
were performed in triplicate.
Invasion and migration assay
The invasion and migration ability of A549 or HCC827
cells transfected with miR-186 mimics or MAP3K2-siRNA or
pcDNA3.1-MAP3K2 (20 μg) was analyzed in 24-well Transwell chambers
(8 μm; Millipore) as previously described (25). The invading cells were fixed with
4% paraformaldehyde in PBS, stained with 0.5% crystal violet
(Sigma-Aldrich, St. Louis, MO, USA) for 15 min and counted. Invaded
or migrated cells were counted under a microscope (IX71; Olympus,
Tokyo, Japan) at ×200 magnification.
Statistical analysis
All experiments were performed in triplicate and
data were expressed as the mean ± SD. The difference among groups
was determined by one-way analysis of variance (ANOVA) analysis and
comparison between two groups was analyzed by a paired/unpaired
Student’s t-test using SPSS 18.0 program (SPSS Inc., Chicago, IL,
USA). The differences were considered statistically significant at
P<0.05.
Results
miR-186 is decreased in human lung cancer
tissues and cells
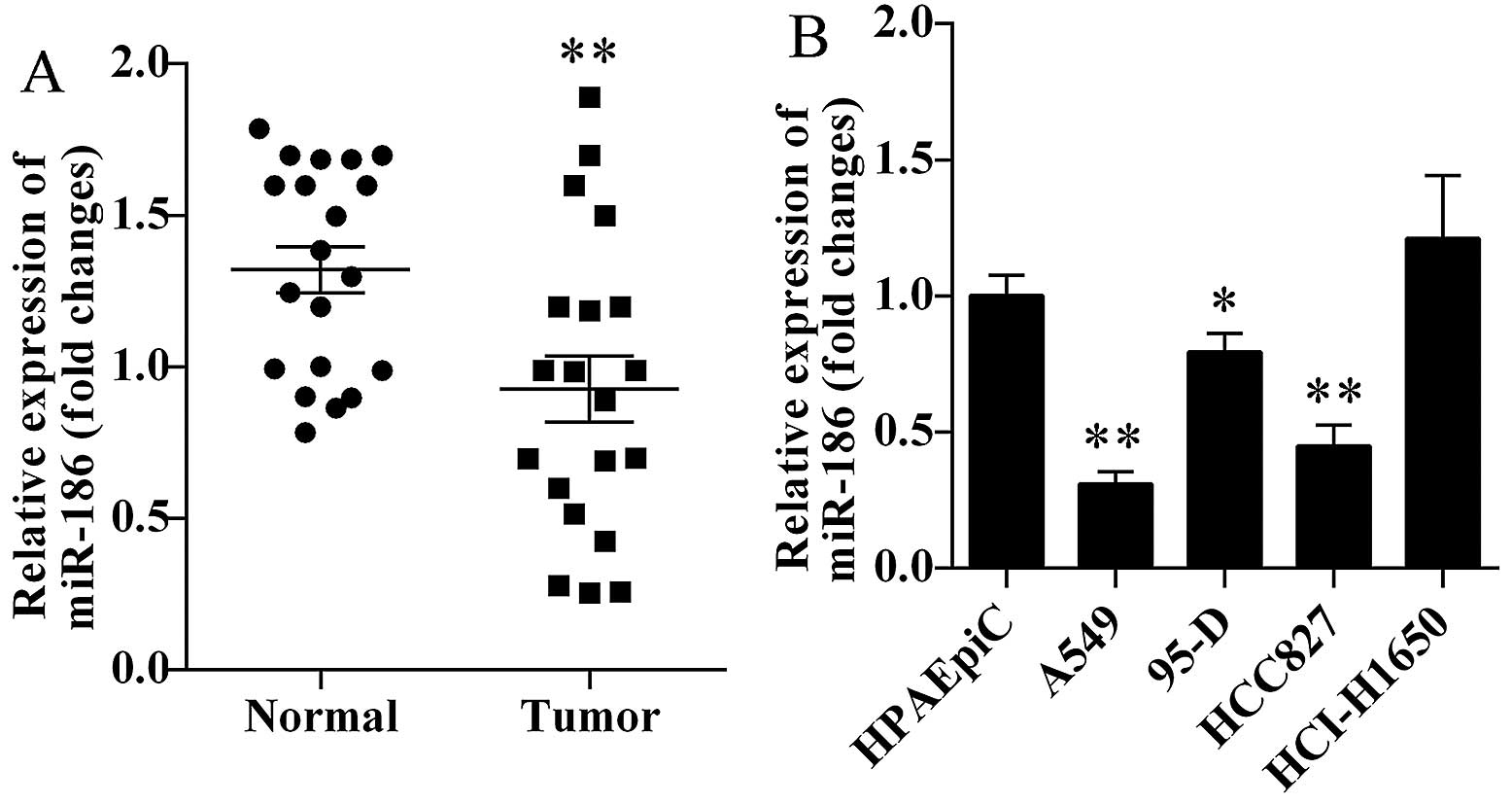
We first investigated the miR-186 expression levels
in human lung cancer tissues and corresponding normal tissues. We
found that miR-186 was markedly decreased in human lung cancer
tissues (P=0.0066; Fig. 1A).
Furthermore, we also detected the miR-186 expression levels in four
lung cancer cell lines. We found that miR-186 was significantly
decreased in A549, 95-D and HCC827 cells (Fig. 1B). Based on the above results, lung
cancer cell lines A549 and HCC827 were selected for the present
study.
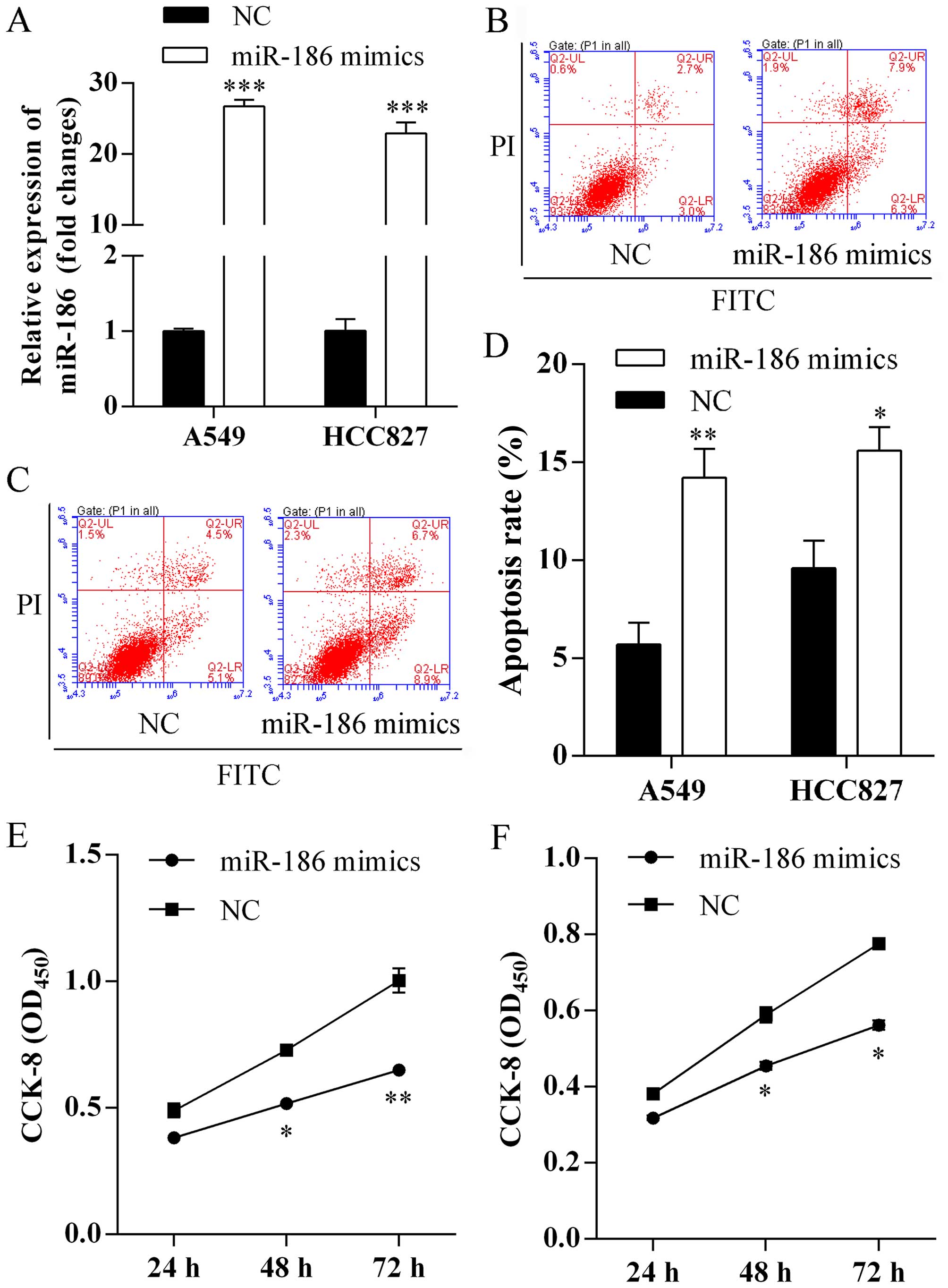
Forced expression of miR-186 induces cell
apoptosis and suppresses proliferation
To evaluate the biological functions of miR-186 on
cell apoptosis and proliferation, miR-186 mimics were first
transfected into A549 and HCC827 cells. Expression of miR-186 was
assessed using qRT-PCR analysis and a respective 26.73- and
22.92-fold increased in miR-186 mimic-transfected cells compared
with negative control (Fig. 2A).
Furthermore, we investigated miR-186 on cell apoptosis using flow
cytometric analysis. We found that more apoptotic cells were
observed in A549 (Fig. 2B) and
HCC827 (Fig. 2C) cells after
forced expression of miR-186. The apoptosis rates of A549 cells
transfected with miR-186 mimics and NC were 14.2±1.2 and 5.7±0.9%,
respectively (Fig. 2D). In
addition, the apoptosis rates of HCC827 cells transfected with
miR-186 mimics and NC were 15.6±1.1 and 9.6±1.3%, respectively
(Fig. 2D). Finally, we studied the
role of miR-186 on proliferation using CCK-8 assay. We found that
overexpression of miR-186 exhibited significantly lower
proliferation rates of A549 (Fig.
2E) and HCC827 (Fig. 2F)
cells.
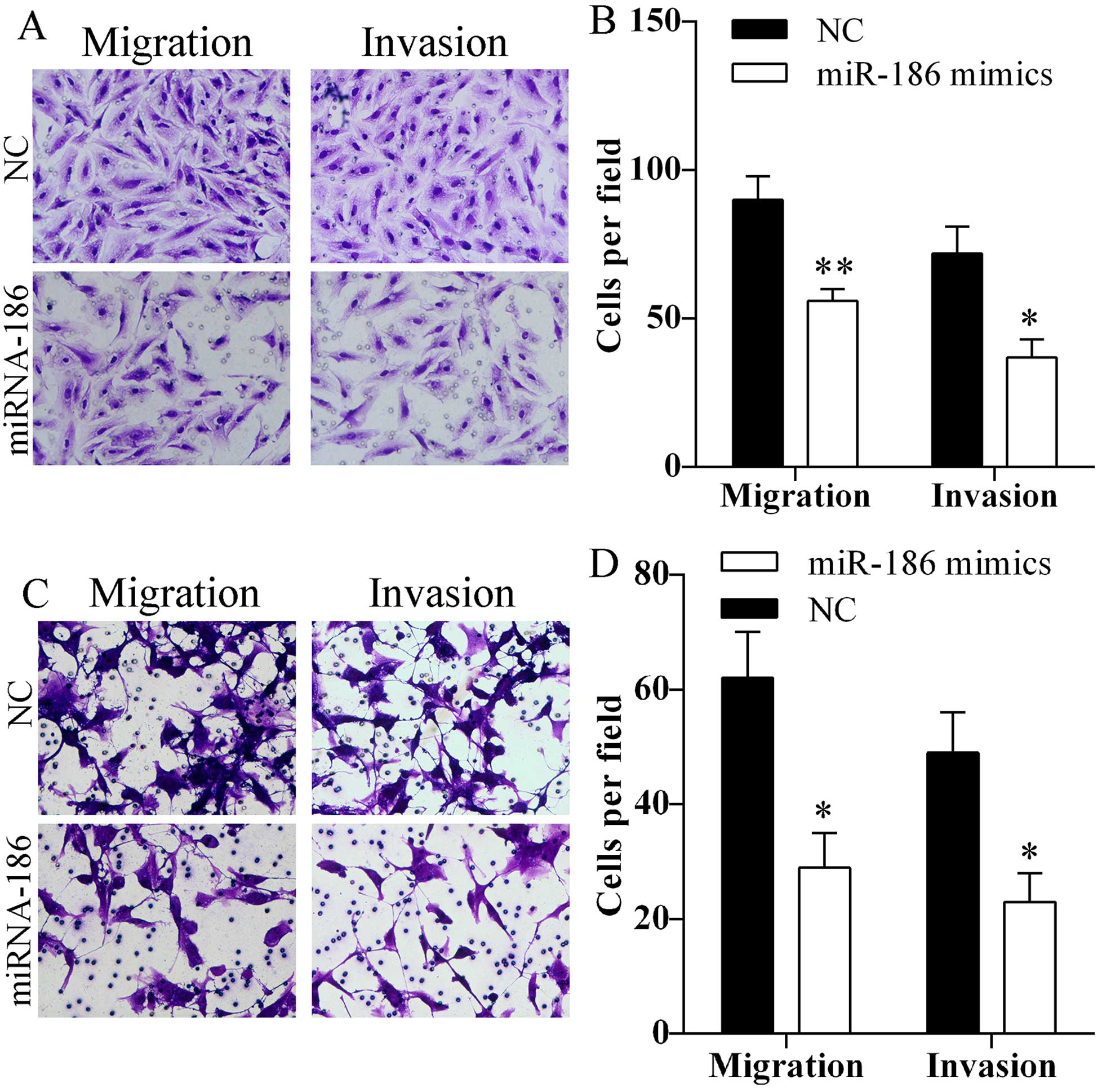
Overexpression of miR-186 inhibits cell
migration and invasion
Migration and invasion abilities of A549 and HCC827
cells after transfected with miR-186 mimics were assessed using
Transwell assay. Transfection of miR-186 mimics markedly reduced
the number of A549 (Fig. 3A and B)
and HCC827 (Fig. 3C and D) cells
that passed through the Transwell chamber. The results indicated
that miR-186 might inhibit cell migration and invasion.
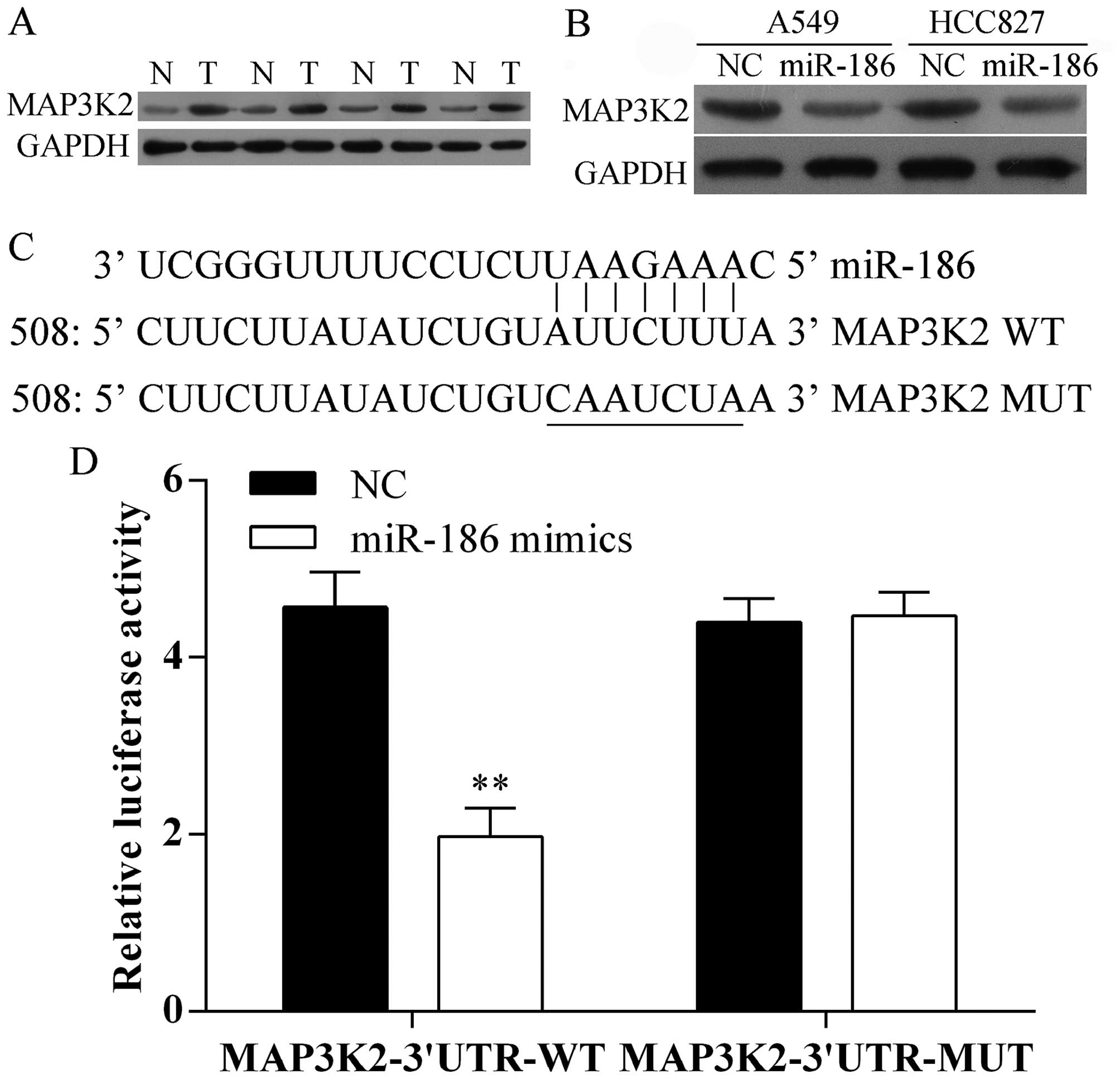
miR-186 directly targets MAP3K2
3′UTR
To evaluate the molecular mechanism by which miR-186
inhibits the proliferation and metastasis of A549 and HCC827 cells,
we first detected the protein expression level of MAP3K2 in human
lung cancer tissues and compared non-cancerous tissues using
western blot analysis. We found that MAP3K2 was increased in tumor
tissues (Fig. 4A). Furthermore,
overexpression of miR-186 suppressed the protein expression of
MAP3K2 in A549 and HCC827 cells (Fig.
4B). Moreover, we predicted that the MAP3K2 is one of the
target genes of miR-186 in human using the predication tool
TargetScan and miRanda. miR-186 has one predictive target site in
the MAP3K2 3′ UTR (Fig. 4C). In
addition, the luciferase activity of MAP3K2 3′UTR-WT was
significantly decreased upon overexpressed by miR-186 in A549
cells, whereas, its mutant type was not changed (Fig. 4D). Taken together, our data
indicated that MAP3K2 is a direct target gene of miR-186.
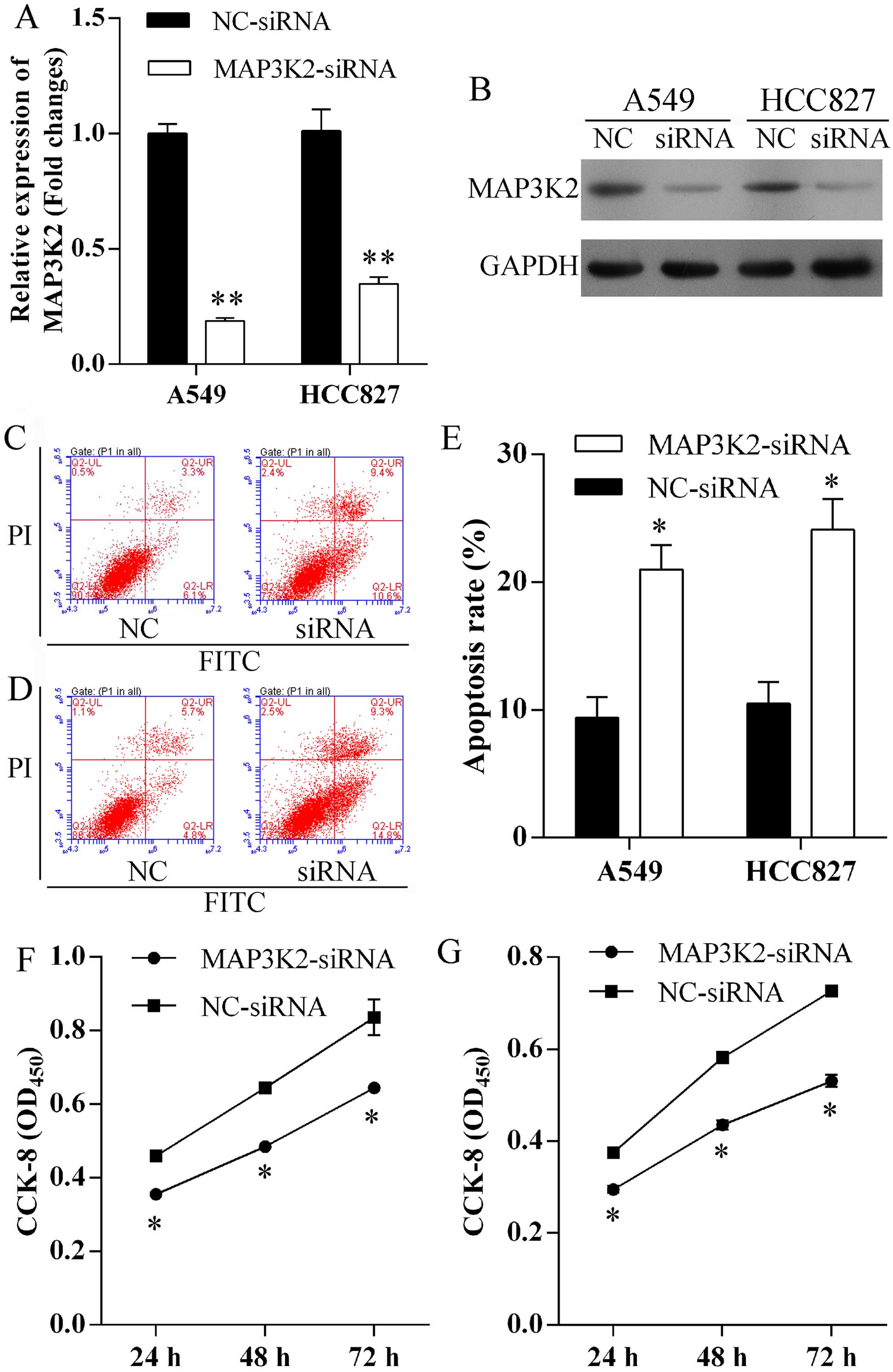
Silence MAP3K2 expression induces cell
apoptosis and suppresses proliferation
To evaluate the role of MAP3K2 in miR-186-induced
cell apoptosis and proliferation, MAP3K2-siRNA was transfected into
A549 and HCC827 cells to specifically knock down MAP3K2 expression.
MAP3K2 expression level was remarkably repressed at mRNA (Fig. 5A) and protein (Fig. 5B) level in A549 and HCC827 cells
after MAP3K2-siRNA transfection. Furthermore, increased number of
apoptotic cells were observed in A549 (Fig. 5C) and HCC827 (Fig. 5D) cells following repressed MAP3K2
expression. The apoptosis rates of A549 cells transfected with
MAP3K2-siRNA and NC-siRNA were 20.0±1.4 and 9.4±1.2%, respectively
(Fig. 5E). In addition, the
apoptosis rates of HCC827 cells transfected with MAP3K2-siRNA and
NC-siRNA were 24.1±1.9 and 10.5±1.3%, respectively (Fig. 5E). Finally, we found that the
knock-down of MAP3K2 exhibited significantly lower proliferation
rates of A549 (Fig. 5F) and HCC827
(Fig. 5G) cells.
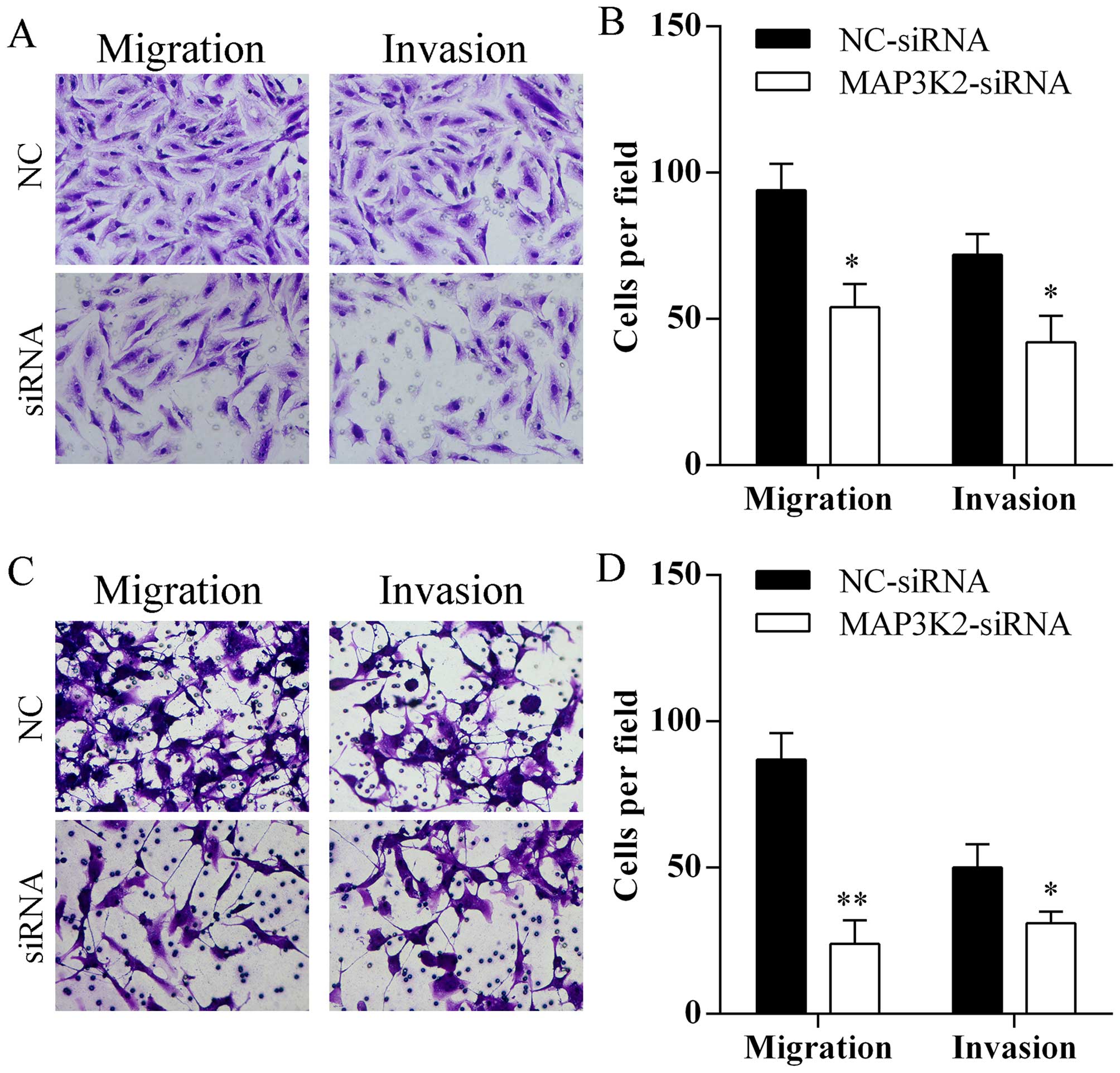
Silencing MAP3K2 expression inhibits cell
migration and invasion
Transfection of MAP3K2-siRNA markedly reduced the
number of A549 (Fig. 6A and B) and
HCC827 (Fig. 6C and D) cells that
passed through the Transwell chamber.
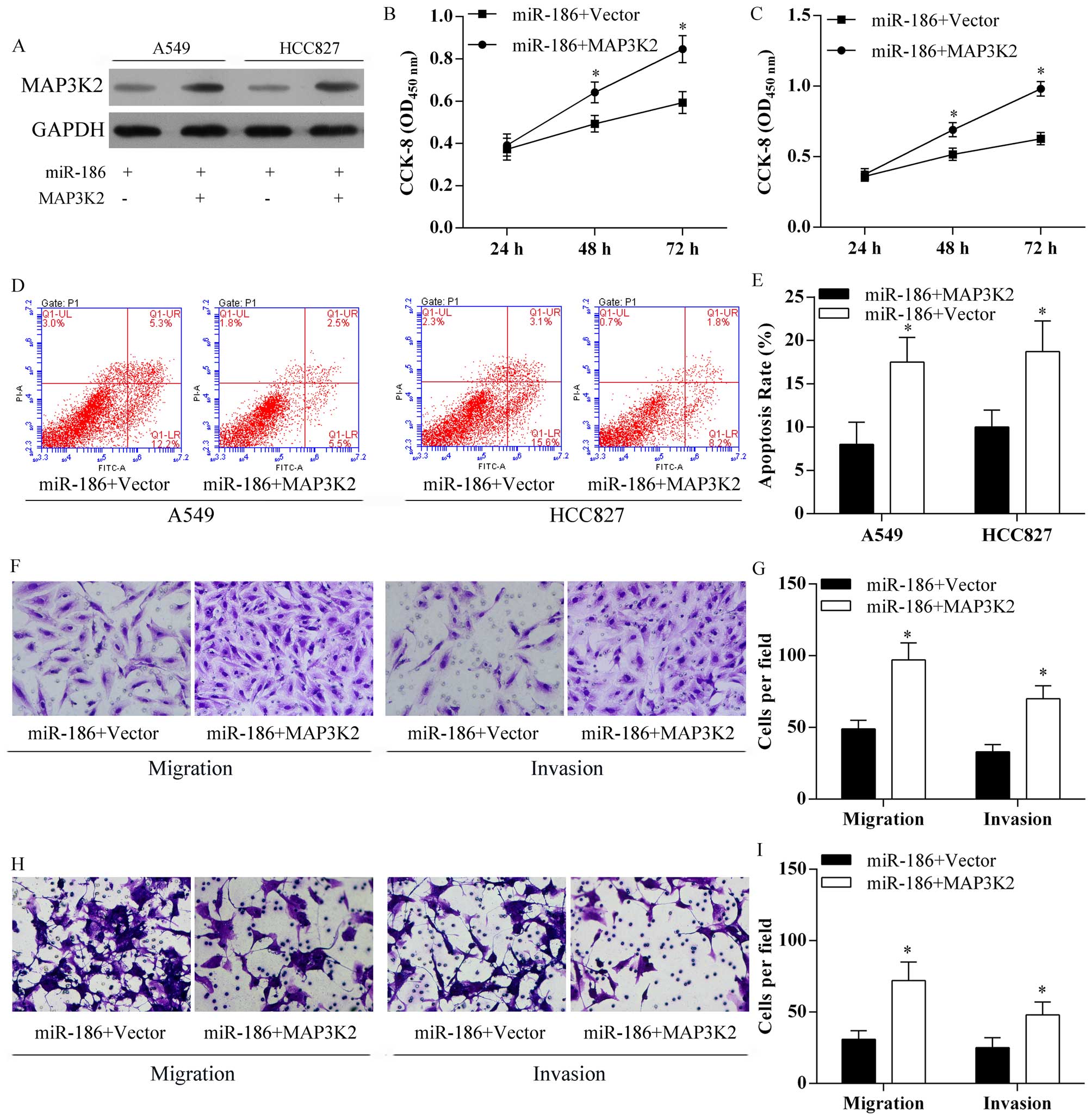
MAP3K2 ameliorated the inhibitory effect
of miR-186 on cell proliferation and metastasis
For further study, MAP3K2 was overexpressed after
transfection of pcDNA3.1-MAP3K2 vectors in miR-186 A549 and HCC827
cells (Fig. 7A). In addition,
overexpression of MAP3K2 in A549 (Fig.
7B) and HCC827 (Fig. 7C)
overexpressing miR-186 cells significantly decreased the inhibitory
effect of miR-186 on lung cancer cell proliferation (Fig. 7B and C). Moreover, overexpression
of MAP3K2 in A549 and HCC827 overexpressing miR-186 cells
significantly reduced miR-186 induced cell apoptosis (Fig. 7D and E). Moreover, overexpression
of MAP3K2 in A549 (Fig. 7F and G)
and HCC827 (Fig. H and I)
overexpressing miR-186 cells significantly decreased the inhibitory
effect of miR-186 on lung cancer cell migration and invasion
(Fig. 7F–I).
Discussion
In recent years, increased number of studies found
that miRNAs played critical roles in fundamental cellular processes
(26,27), and an increasingly recognized that
miRNAs are abnormally expressed and involved in a variety of human
cancers (16,18,24,28).
The expression of miR-186 has been found disordered in different
human cancers. Zhang et al (19) found that miR-186 was significantly
upregulated in pancreatic cancer and might play a critical role in
diagnosis and prognosis of pancreatic cancer. Whereas, miR-186 was
discovered decreased in lung adenocarcinoma and correlated with
patient survival (22,23). Furthermore, tumor-suppressive
effects of miR-186 in medulloblastomas (29), endometrial (20) and prostate cancer (21) were also observed. However, little
is known of the potential role of miR-186 on growth and metastasis
of human non-small cell lung cancer.
In the present study, we first analyzed the
expression of miR-186 in 20 lung cancer tissues and four cancer
cell lines. Results showed that miR-186 was significantly decreased
in cancer tissues and cell lines. The results were similar with
previous research (22,23). To understand the biological role of
miR-186 in lung cancer, the impacts of miR-186 on cell
proliferation, metastasis and apoptosis were analyzed using CCK-8
assay, Transwell assay and flow cytometry apoptosis assay,
respectively. Here, we found that overexpression of miR-186 induced
A549 and HCC827 cell apoptosis and suppressed cell proliferation.
Also, upregulation of miR-186 inhibited A549 and HCC827 cell
migration and invasion. These findings suggested miR-186 played
important roles in occurrence and metastasis of human non-small
cell lung cancer and might be a useful diagnostic and predictive
biomarker.
Subsequently, to understand the mechanism of miR-186
in regulating cell growth and metastasis, we demonstrated that
mitogen-activated protein kinase kinase kinase 2 (MAP3K2) is a
direct target of miR-186 in human lung cancer. MAP3K2, a member of
serine/threonine protein kinase family in MAPK signaling pathway,
is able to activate c-Jun N-terminal kinase (JNK) and ERK5 and
preferentially activates other kinases involved in the MAP kinase
signaling pathway (30,31). MAP3K2 was found to have important
roles in epidermal growth factor (EGF), T-cell receptor and
fibroblast growth factor 2 (FGF-2) signaling pathways in MAP3K2
knockout mice (30,32,33).
A previous study found that MAP3K2 is able to discriminate tumor
from normal cells (34) and MAP3K2
contributed to growth of human hepatocellular carcinoma (35), and miR-520b suppressed hepatoma
cell proliferation by targeting MEKK2 (MAP3K2) (35), suggesting that MAP3K2 may play
important roles in the development of human cancer. In the present
study, we found that MAP3K2 protein was significantly increased in
human lung cancer tissues and cell lines. Furthermore, luciferase
assays and western blot analysis confirmed that miR-186 directly
targets MAP3K2 in lung cancer. In addition, knockdown of MAP3K2 by
RNA interference assay markedly reduced the expression of MAP3K2
and suppressed lung cancer cell proliferation, migration and
invasion and induced cell apoptosis. All the results indicate that
decreased expression of miR-186 promotes cell proliferation and
suppresses cell apoptosis capacity of human lung cancer through the
MAP3K2-mediated signal pathway.
In conclusion, we demonstrated that miR-186 is
decreased in human lung cancer tissues and cell lines, which
targets MAP3K2 directly. miR-186 mimics transfection and knockdown
of MAP3K2 inhibited cell proliferative and metastasis of A549 and
HCC827 cells and promoted cell apoptosis. Based on the above, the
present study may represent a novel indicator of poor prognosis in
human lung cancer, and miR-186-MAP3K2 may represent a potential
therapeutic target for diagnosis and therapy of human lung
cancer.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith RA, Manassaram-Baptiste D, Brooks D,
Doroshenk M, Fedewa S, Saslow D, Brawley OW and Wender R: Cancer
screening in the United States, 2015: A review of current American
cancer society guidelines and current issues in cancer screening.
CA Cancer J Clin. 65:30–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Novaes FT, Cataneo DC, Ruiz RL Jr,
Defaveri J, Michelin OC and Cataneo AJ: Lung cancer: histology,
staging, treatment and survival. J Bras Pneumol. 34:595–600. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Youlden DR, Cramb SM and Baade PD: The
International Epidemiology of lung cancer: Geographical
distribution and secular trends. J Thorac Oncol. 3:819–831. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson JL, Pillai S and Chellappan SP:
Genetic and biochemical alterations in non-small cell lung cancer.
Biochem Res Int. 2012:9404052012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ginsberg RJ and Rubinstein LV: Randomized
trial of lobectomy versus limited resection for T1 N0 non-small
cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg.
60:615–622; discussion 622–613. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Timmerman R, Paulus R, Galvin J, Michalski
J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone
D, et al: Stereotactic body radiation therapy for inoperable early
stage lung cancer. JAMA. 303:1070–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chi A, Liao Z, Nguyen NP, Xu J, Stea B and
Komaki R: Systemic review of the patterns of failure following
stereotactic body radiation therapy in early-stage non-small-cell
lung cancer: clinical implications. Radiother Oncol. 94:1–11. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martini N, Bains MS, Burt ME, Zakowski MF,
McCormack P, Rusch VW and Ginsberg RJ: Incidence of local
recurrence and second primary tumors in resected stage I lung
cancer. J Thorac Cardiovasc Surg. 109:120–129. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP and Chen CZ: Micromanagers of
gene expression: The potentially widespread influence of metazoan
microRNAs. Nat Rev Genet. 5:396–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar
|
|
14
|
Kim VN: MicroRNA biogenesis: Coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Lu Z, Unruh AK, Ivan C, Baggerly
KA, Calin GA, Li Z, Bast RC Jr and Le XF: Clinically relevant
microRNAs in ovarian cancer. Mol Cancer Res. 13:393–401. 2015.
View Article : Google Scholar :
|
|
16
|
Zhao N, Sun H, Sun B, Zhu D, Zhao X, Wang
Y, Gu Q, Dong X, Liu F, Zhang Y, et al: miR-27a-3p suppresses tumor
metastasis and VM by down-regulating VE-cadherin expression and
inhibiting EMT: An essential role for Twist-1 in HCC. Sci Rep.
6:230912016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomes BC, Santos B, Rueff J and Rodrigues
AS: Methods for studying MicroRNA expression and their targets in
formalin-fixed, paraffin-embedded (FFPE) breast cancer tissues.
Methods Mol Biol. 1395:189–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim G, An HJ, Lee MJ, Song JY, Jeong JY,
Lee JH and Jeong HC: Hsa-miR-1246 and hsa-miR-1290 are associated
with stemness and invasiveness of non-small cell lung cancer. Lung
Cancer. 91:15–22. 2016. View Article : Google Scholar
|
|
19
|
Zhang Y, Li M, Wang H, Fisher WE, Lin PH,
Yao Q and Chen C: Profiling of 95 microRNAs in pancreatic cancer
cell lines and surgical specimens by real-time PCR analysis. World
J Surg. 33:698–709. 2009. View Article : Google Scholar
|
|
20
|
Myatt SS, Wang J, Monteiro LJ, Christian
M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S and Lam EW:
Definition of microRNAs that repress expression of the tumor
suppressor gene FOXO1 in endometrial cancer. Cancer Res.
70:367–377. 2010. View Article : Google Scholar
|
|
21
|
Erdmann K, Kaulke K, Thomae C, Huebner D,
Sergon M, Froehner M, Wirth MP and Fuessel S: Elevated expression
of prostate cancer-associated genes is linked to down-regulation of
microRNAs. BMC Cancer. 14:82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai J, Wu J, Zhang H, Fang L, Huang Y,
Yang Y, Zhu X, Li R and Li M: miR-186 downregulation correlates
with poor survival in lung adenocarcinoma, where it interferes with
cell-cycle regulation. Cancer Res. 73:756–766. 2013. View Article : Google Scholar
|
|
23
|
Cui G, Cui M, Li Y, Liang Y, Li W, Guo H
and Zhao S: MiR-186 targets ROCK1 to suppress the growth and
metastasis of NSCLC cells. Tumour Biol. 35:8933–8937. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruan WD, Wang P, Feng S, Xue Y and Zhang
B: MicroRNA-497 inhibits cell proliferation, migration, and
invasion by targeting AMOT in human osteosarcoma cells. Onco
Targets Ther. 9:303–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Liu G, Li Q, Wang F, Xie F, Zhai
R, Guo Y, Chen T, Zhang N, Ni W, et al: Mucin1 promotes the
migration and invasion of hepatocellular carcinoma cells via
JNK-mediated phosphorylation of Smad2 at the C-terminal and linker
regions. Oncotarget. 6:19264–19278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian L, Fang YX, Xue JL and Chen JZ: Four
microRNAs promote prostate cell proliferation with regulation of
PTEN and its downstream signals in vitro. PLoS One. 8:e758852013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hauser B, Zhao Y, Pang X, Ling Z, Myers E,
Wang P, Califano J and Gu X: Functions of MiRNA-128 on the
regulation of head and neck squamous cell carcinoma growth and
apoptosis. PLoS One. 10:e01163212015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lv SQ, Kim YH, Giulio F, Shalaby T,
Nobusawa S, Yang H, Zhou Z, Grotzer M and Ohgaki H: Genetic
alterations in microRNAs in medulloblastomas. Brain Pathol.
22:230–239. 2012. View Article : Google Scholar
|
|
30
|
Su B, Cheng J, Yang J and Guo Z: MEKK2 is
required for T-cell receptor signals in JNK activation and
interleukin-2 gene expression. J Biol Chem. 276:14784–14790. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chayama K, Papst PJ, Garrington TP, Pratt
JC, Ishizuka T, Webb S, Ganiatsas S, Zon LI, Sun W, Johnson GL, et
al: Role of MEKK2-MEK5 in the regulation of TNF-alpha gene
expression and MEKK2-MKK7 in the activation of c-Jun N-terminal
kinase in mast cells. Proc Natl Acad Sci USA. 98:4599–4604. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schaefer BC, Ware MF, Marrack P, Fanger
GR, Kappler JW, Johnson GL and Monks CR: Live cell fluorescence
imaging of T cell MEKK2: Redistribution and activation in response
to antigen stimulation of the T cell receptor. Immunity.
11:411–421. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun W, Wei X, Kesavan K, Garrington TP,
Fan R, Mei J, Anderson SM, Gelfand EW and Johnson GL: MEK kinase 2
and the adaptor protein Lad regulate extracellular signal-regulated
kinase 5 activation by epidermal growth factor via Src. Mol Cell
Biol. 23:2298–2308. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cazares LH1, Troyer D, Mendrinos S, Lance
RA, Nyalwidhe JO, Beydoun HA, Clements MA, Drake RR and Semmes OJ:
Imaging mass spectrometry of a specific fragment of
mitogen-activated protein kinase/extracellular signal-regulated
kinase kinase kinase 2 discriminates cancer from uninvolved
prostate tissue. Clin Cancer Res. 15:5541–5551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang W, Kong G, Zhang J, Wang T, Ye L and
Zhang X: MicroRNA-520b inhibits growth of hepatoma cells by
targeting MEKK2 and cyclin D1. PLoS One. 7:e314502012. View Article : Google Scholar : PubMed/NCBI
|