Introduction
Hypoxia is a reduction in the normal level of tissue
oxygen tension and occurs in many disease processes, including
cancer. Tumor hypoxia is typically associated with poor patient
prognosis, partly because low oxygen levels reduce the
effectiveness of radiation therapy, which kills tumor cells by
generating reactive oxygen species (1). Hypoxia-inducible factor (HIF) is a
transcription factor found in mammalian cells cultured under
reduced oxygen tension and plays a key role in the cellular
response to hypoxia. HIF regulates the transcription of several
genes involved in biological processes, such as angiogenesis, cell
proliferation and survival, glucose metabolism, pH regulation and
apoptosis (2). Angiogenesis is a
crucial regulator of tumor growth and metastases (3). Tumor angiogenesis is regulated by the
production of angiogenic stimulators, including vascular
endothelial growth factor (VEGF), which is a key regulatory factor
in the prognosis of various cancers. Several studies have shown
that transforming growth factor-β1 (TGF-β1) is involved in
angiogenesis, leading to tumor progression (4). TGF-β1 signaling has been shown in
concert with HIF-1α to regulate VEGFA expression (5).
Cancer stem cells (CSCs) typically represent a small
fraction of tumor cells that have the ability to self-renew and
differentiate into many more mature cancer cells (6). HIF stabilization in hypoxic tumor
cells may promote the adoption of stem cell properties, including
self-renewal and multipotency, by stimulating the expression or
activity of Oct4, Notch, and other critical signaling pathways.
Oct4 has been shown to function in a complex with Nanog and Sox2 to
activate and repress genes controlling stem cell identity and
differentiation (7). KLF4 is
highly expressed in CSC-enriched populations in mouse primary
mammary tumors and breast cancer cell lines (8). Indeed, CSCs have been shown to be
more radioresistant than non-stem cancer cells and are, therefore,
believed to be responsible for treatment failure and tumor
recurrence (9).
Among females, breast cancer ranks first at age
20–59 years (10). In recent
years, the encouraging trend towards earlier detection and the
increasing use of systemic adjuvant treatment have improved
survival rates; however, nearly half of the breast cancer patients
treated for localized disease develop metastasis (11). A lower incidence of breast cancer
is associated with the high consumption of phytoestrogens, which
are biologically active plant-derived phenolic compounds that
structurally mimic the mammalian estrogen 17β-estradiol (12). Basic and preclinical research have
focused on resveratrol [RES
(trans-3,4′,5-trihydroxystilbene)], a naturally occurring
polyphenol enriched in grapes and red wine (13). Resveratrol has recently been shown
to function as a cancer chemoprevention agent, an anti-mutagenic
agent, and an anti-initiative agent (14).
This study demonstrated that the resveratrol analog
HS-1793 inhibits angiogenesis via the regulation of hypoxia and
displays hypoxia-induced CSC properties in FM3A mouse breast cancer
cells.
Materials and methods
Preparation of HS-1793
To obtain HS-1793, the stilbene double bond present
in resveratrol was substituted with a naphthalene ring, as
previously described (15). A
stock solution was generated in absolute ethanol at 50 mM, and the
working dilutions were generated directly in culture media. The
control vehicle was culture media containing amounts of ethanol
equivalent to those present in HS-1793.
Cell culture conditions
FM3A (murine breast cancer cells) originated from
the mammary gland of the C3H/He mouse were grown in RPMI-1640
(Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS;
Hyclone, Logan, UT, USA), 100 U/ml penicillin/streptomycin (Gibco),
in a humidified atmosphere containing 5% CO2 and 37°C.
Exponentially growing cells (~70–80% confluence) in complete medium
were pretreated for 2 h with different concentrations of HS-1793,
followed by exposure to normoxic (5% CO2 at 37°C) or
hypoxic conditions (1% O2 at 37°C) in an Anaerobic Glove
Box (Forma Scientific, Marietta, OH, USA) for treatment.
Environmental hypoxic conditions (1% O2) were achieved
in an airtight humidified chamber continuously flushed with a gas
mixture containing 5% CO2 and 95% N2.
HS-1793 treatment and assessment of cell
viability
HS-1793 was dissolved in EtOH and stored at −80°C
until use. FM3A cells were plated onto 60-mm dishes with
1×105 cells for 24 h, and the cells were treated with
HS-1793 (0, 1.3, 2.5, 5, 10 and 20 μM) under normoxic or hypoxic
conditions. The cells were harvested at 12 or 24 h after treatment
and counted using the ADAM automatic cell counter (Digital Bio.,
Korea). The relative cell count was determined as follows: final
count of treated cultures/final count of control cultures ×
100.
Hypoxia detection in tumor cells
To validate the efficiency of hypoxic treatment, a
hypoxia detection kit (Hypoxyprobe-1; Chemicon, Temecula, CA, USA)
was used to identify 2-nitro-imidazole adducts formed in cultured
FM3A cells under hypoxia. FM3A cells were cultured on 24-well
plates at a density of 1×105 cells/well for 24 h; then,
the cells were treated with HS-1793 for 2 h, followed by incubation
under normoxic or hypoxic conditions for 12 h. After treatment with
100 μM pimonidazole hydrochloride (1-[(2-hydroxo-3-piperidinyl)
propyl]-2-nitroimidazole hydrochloride) under hypoxic conditions
for 4 h, single-cell suspensions were prepared with trypsin and
spun in clean, fat-free glass slides using a cytocentrifuge.
Cytocentrifuged cells were fixed for 30 min in 4% paraformaldehyde
(PFA). After washing with phosphate-buffered saline (PBS), the
cells were permeabilized with PBS containing 0.5% Triton X-100 for
10 min. Next, the cells were incubated for 30 min at room
temperature with FITC-conjugated antibody (1:50) against
pimonidazole adducts. Hoechst 33342 was used as a nuclear
counterstaining agent. The cells were imaged under Nikon Eclipse
TS100 fluorescence microscopy (Nikon, Tokyo, Japan).
Western blot analysis
Protein samples were separated by 10% SDS-PAGE and
transferred to a polyvinylidene difluoride membrane. The membrane
was allowed to react with anti-HIF-1α (1:200; Novus, USA),
anti-VEGF (1:500; Santa Cruz Biotechnology, CA, USA), anti-OCT4
(1:200; Santa Cruz Biotechnology), anti-KLF4 (1:500; Abcam, UK),
anti-SOX (1:200; Santa Cruz) or anti-GAPDH (1:2,000; Santa Cruz
Biotechnology). Immunostaining with antibodies was performed using
the Super-Signal West Pico enhanced chemiluminescence substrate,
and detection was performed using LAS-3000PLUS (Fuji Photo Film
Co., Kanagawa, Japan).
Cell migration assay
The cells were grown to confluence in 6-well plates
for 2 days, and a scrape in the form of a cross was created through
the confluent monolayers with a plastic pipette tip. The cells were
treated with HS-1793 for 2 h, followed by incubation under normoxic
or hypoxic conditions for 24 h. Several wounded areas were marked
for orientation, observed, and then imaged using a Nikon Eclipse
TS100 microscope (Nikon) at 24 h after the scratch.
Annexin-V binding assay
FM3A cells were pretreated with 2.5 μM HS-1793 for 2
h, followed by incubation under hypoxic conditions. After 12 h,
these cells were exposed to γ-IR (0, 2, 4 Gy) using a 137Cs source
(BioBeam 8000, STS, Braunschweig, Germany) and cultured under
hypoxic conditions for 12 h. The cells were harvested and then
washed with PBS. The pellets were resuspended in 1X Annexin V
binding buffer (BD Biosciences, Bedford, MA, USA) at a
concentration of 1×106 cells/ml. Next, 100 μl of the
solution was transferred to a 5-ml culture tube, and 5 μl of
Annexin V-FITC and 5 μl 7-AAD were added. The cells were then
gently vortexed and incubated for 15 min at RT (25°C) in the dark.
Next, 400 μl of 1X binding buffer was added to each tube, and the
samples were analyzed by flow cytometry (BD FACSAria, USA) within 1
h.
Animal studies
All experiments were performed on 6-week-old C3H/He
female mice obtained from Central Lab. Animal Inc. (Seoul, Korea).
The animals were raised under SPF conditions at the Korea Institute
of Toxicology, Hospital of Dong-A University according to Good
Laboratory Practices OECD guidelines. All animal procedures were
performed according to approved protocols (approval no.
DIACUC-09-24) from the Institutional Animal Care and Use Committee
(IACUC) of Dong-A University and in accordance with recommendations
for the proper use and care of laboratory animals. FM3A cells in
logarithmic growth phase were used to establish a breast tumor
model. Xenografts were generated by subcutaneously injecting
2×106 tumor cells in 50 μl of PBS into the flank
adjacent to the right hind limb of 7-week-old female C3H/He mice.
When the tumor grew to a size of ~500 mm3, HS-1793 (1.5
mg/kg) was administered as an intraperitoneal (i.p.) single
injection for the determination of the hypoxic status and perfusion
level in the tumor tissue. When the tumor grew to a size of ~40
mm3 (~10 days), the mice were stratified into groups of
8–10 animals with equal mean tumor volumes, and HS-1793 (0.1, 0.5
and 1.5 mg/kg) was administered by i.p. injection twice a week for
30 days. The tumor sizes were measured with calipers once weekly.
The tumor volume was calculated as follows: (width)2 ×
length × 0.52. After 4 weeks, the mice were euthanized to obtain
serum samples and tumor tissues.
ELISA
The serum VEGF concentrations from animals bearing
xenografts were determined. The blood was collected from the
abdominal vein of tumor-bearing or normal mice at 40 days after the
inoculation of tumor cells. Serum samples were obtained by
centrifugation at 3,000 rpm for 10 min and stored at −20°C until
further analysis. The VEGF concentrations were determined using a
quantitative enzyme-linked immunosorbent assay (ELISA, R&D
Systems, Minneapolis, MN, USA). The amount of VEGF immunoreactivity
was calculated using recombinant mouse VEGF standards present on
each microtiter plate. Optical densities were determined at 550 nm
using a microtiter plate spectrophotometer (Beckman Coulter
detection platform, USA).
Real-time PCR quantification
Total cellular RNA was isolated from tumor tissue
using TRIzol reagent (Invitrogen) and used for reverse
transcription. Complementary DNA (cDNA) was synthesized from 1 μg
of total RNA from each sample using a Maxime RT PreMix (iNtRON)
according to the manufacturer’s instructions and used as a template
for TaqMan™ real-time quantitative PCR. The relative mRNA levels
were quantified using fluorescent TaqMan® technology.
PCR primers and probes specific for murine VEGF (assay ID: mM
01281449_m1) and HIF-1α (assay ID: mM 00468869_m1) were obtained as
TaqMan Gene Expression arrays (Applied Biosystems, Germany).
Glyceraldehyde-3-A phosphate-dehydrogenase (GAPDH) (assay ID: mM
99999915_g1) was used as an internal control. PCR amplifications of
HIF-1α, VEGF and GADPH were performed in 20-μl volumes and in
separate reactions. The mix contained 1 μl of the 20X ready-to-use
primer and probe mix for HIF-1α, VEGF or GAPDH, 2 μl of the
undiluted cDNA, and 10 μl of the 2X TaqMan Universal Master Mix
(Applied Biosystems). The probe was labeled at the 5′-end with the
reporter molecule 6-carboxy-fluorescein (FMA) and at the 3′-end
with a non-fluorescent quencher and MGB. PCR assays were performed
using a CFX96 Real-Time PCR Detection system (Bio-Rad, Mississauga,
ON, Canada). The PCR was initiated with a 2-min incubation at 50°C
and then an initial 10-min denaturation at 95°C, followed by a
total of 50 cycles of 15-sec denaturation at 95°C, and 1 min of
annealing and elongation at 60°C. For each sample, ΔΔCt (crossing
point) values were calculated as the Ct of the target gene minus
the Ct of the GAPDH gene. Gene expression was derived according to
the 2−ΔΔCt method; changes in gene expression are
expressed relative to the basal condition.
Determination of the hypoxic status and
perfusion level in tumor tissue
As markers of hypoxia, pimonidazole hydrochloride
(1-[(2-hydroxo-3-piperidinyl)propyl]-2-nitroimidazole
hydrochloride) (16,17) and CCI-103F
(1-(2-hydroxy-3-hexafluoroisopropoxy-propyl)-2-nitroimidazole)
(18) were used. Pimonidazole
hydrochloride (Hypoxyprobe-1 Plus kit; Chemicon) and CCI-103F
(Hypoxyprobe F6 kit; Chemicon) are bioreductive chemical probes
with an immunorecognizable side chain. The addition of the first
electron in the bioreductive activation is reversibly inhibited by
oxygen, resulting in futile cycling with a half maximal
pO2 of inhibition of ~3 mM Hg and with complete
inhibition occurring at ~10 mM Hg (17). Pimonidazole hydrochloride was
dissolved in saline, and CCI-103F was dissolved in 10% dimethyl
sulfoxide (DMSO) and 90% peanut oil. To determine the effect of
HS-1793 on vessel functionality, the double-fluorescent dye
technique based on the perfusion markers, Hoechst 33342
(Sigma-Aldrich) and DiOC7 (Invitrogen) were used. Hoechst 33342 and
DiOC7 are removed very rapidly from the circulation (half-time of 2
min) and are very stable once bound to DNA. Thus, Hoechst 33342 and
DiOC7 specifically label the nuclei and mitochondria of endothelial
cells, respectively, and those of the cells adjacent to the vessel
walls, thereby delineating the perfused vessels (19,20).
Hoechst 33342 and DiOC7 were dissolved in saline.
For the single-injection efficacy of HS-1793
concerning the change in tumor hypoxia, pimonidazole (60 mg/kg) was
administered for 4 h 15 min, HS-1793 (1.5 mg/kg) was administered
for 2 h 15 min, and CCI-103F (60 mg/kg) was administered for 2 h
before animal euthanasia (Fig.
2A). With regard to the single-injection efficacy of HS-1793
and the change in tumor perfusion, Hoechst 33342 (15 mg/kg) was
administered for 26 min, HS-1793 (1.5 mg/kg) was administered for
16 min, and DiOC7 (1 mg/kg) was administered for 1 min before
animal euthanasia (Fig. 2B). For
the 30-day repeat injection efficacy of HS-1793 (0.5, 1 and 1.5
mg/kg), pimonidazole hydrochloride was injected intravenously
(i.v.) into the tail vein at a dose of 60 mg/kg, and Hoechst 33342
was injected i.v. at a dose of 15 mg/kg in a total volume of 0.1
ml. Pimonidazole hydrochloride was administered for 2 h, and H33342
was administered for 1 min before animal euthanasia. After animal
euthanasia, the tumor specimens were removed and frozen in OCT
mounting medium (Sakura Finetek, Torrance, CA, USA). Next,
consecutive 4-μm-thick frozen sections were cut on a Shandon
Cryotome FSE (Thermo Fisher, Waltham, MA, USA). The sections were
then stored at −80°C until staining.
IHC staining and fluorescence microscopy
in tumor tissues
After thawing, the sections were fixed in cold
acetone (4°C) for 30 min. Between all consecutive steps of the
staining procedure, the sections were rinsed once for 5 min in 1X
Tris-buffered saline with 0.1% Tween-20 (TBS-T). Unless otherwise
stated, all antibodies were diluted in PBS with 1% bovine serum
albumin (BSA). The sections on slides were treated with rabbit
anti-CD31 polyclonal antibody (1:100; Abcam, ab28364) or mouse
anti-HIF-1α polyclonal antibody (1:100; Abcam, ab1) as a primary
antibody overnight at 4°C. The sections were washed and then
treated with a secondary antibody using the Envision Detection kit
(Dako, K5007). Pimonidazole and CCI-103F are reductively activated
in hypoxic cells and forms stable adducts with thiol groups in
proteins, peptides, and amino acids. FITC-MAb1
(fluorescein-conjugated mouse IgG1 monoclonal antibody clone) and
rabbit anti-CCI-103F antisera (PAbF6) bind to these adducts,
allowing their detection by immunochemical means. The tumor
sections were rehydrated and preincubated for 15 min with 1% BSA,
immediately followed by incubation with FITC-conjugated
anti-pimonidazole antibody (1:50) for 30 min and rabbit
anti-CCI-103F antisera (PAbF6) (1:50) for 1 h at room temperature,
respectively. For CCI-103F staining, the sections were washed and
then treated with Texas Red Anti-Rabbit IgG (Vector, TI-100)
(1:100) for 1 h at room temperature. Finally, the tumor sections
were mounted with Malinol (Muto Pure Co.) and covered with a cover
slip. The tumor sections were stored at 4°C and scanned within 1–2
days after staining. The different fluorescence excitation and
emission properties of the Hoechst 33342 and DiOC7 dyes allow for
the detection of temporal and spatial fluctuations in perfusion.
Examinations were performed under a Nikon Eclipse 80i microscope
(Nikon) equipped with Image Pro Plus 7.0 software (Media
Cybernetics, Silver Spring, MD, USA).
Analysis of hypoxia and the perfused
vasculature
The tumor sections were quantitatively analyzed
using a semiautomatic method based on a computerized digital image
analysis system. A high-resolution intensified solid-state camera
on a fluorescence microscope (Nikon Eclipse 80i, Japan) with a
computer-controlled motorized stepping stage was used. Each tumor
cross-section was sequentially scanned at ×40 magnification using
different filters for the Hoechst 33342 (blue), FITC (green), and
Texas Red signals. After each scan, one composite digital image was
reconstructed from the individual microscopic fields. The entire
scanning procedure thus yielded three composite images from each
tumor section that was analyzed with Image Pro Plus 7.0 software
(Media Cybernetics). The figures were prepared using Adobe
Photoshop 7.0. depending on the structures and markers visualized,
different composite images were obtained. For the qualitative
analysis of changes caused by oxygen-modifying intervention, both
hypoxic and perfusion markers were stained on the same tissue. This
process resulted in composite binary images showing the hypoxic
markers CCI-103F (Texas Red) and pimonidazole (FITC) and the
perfusion markers Hoechst 33342 (blue) and DiOC7 (green). We
quantified the staining in two ways: the thresholds were applied to
the pimonidazole and CCI-103F images to derive the fractions of the
tumor section that were positive for each marker. Threshold values
are inevitably arbitrary and subject to the possibility that a
different threshold value would yield different results. To
investigate this possibility, we exported the image histograms into
Excel and generated positive fractions for each threshold
value.
Statistical analysis
The results are expressed as the mean ± standard
deviation (SD). Significant differences between the treatments and
control were evaluated by ANOVA (Dunnett’s test). A P-value
<0.05 was defined as statistically significant.
Results
HS-1793 changes the hypoxic condition in
FM3A cells
To detect the cytotoxicity of HS-1793 in FM3A cells,
we treated the cells with different concentrations of HS-1793 for
12 or 24 h under normoxic or hypoxic conditions, and we then
measured the viability of FM3A cells. In Fig. 1A and B, no marked reduction of cell
viability ≤2.5 μM HS-1793 was observed under either normoxic or
hypoxic conditions in FM3A cells at 12 or 24 h. Thus, we used 0.6,
1.3 and 2.5 μM HS-1793 in subsequent experiments. We explored
whether HS-1793 inhibits HIF-1α and VEGF expression relative to
angiogenesis under hypoxic conditions of the tumor
microenvironment. Pretreatment of FM3A cells with HS-1793 resulted
in a dose-dependent decrease in hypoxia-induced HIF-1α and VEGF
protein levels (Fig. 1C).
To investigate whether HS-1793 regulates the hypoxic
tumor microenvironment, we evaluated FM3A cells under hypoxic
conditions. The cells were treated with HS-1793 under hypoxic
conditions for 12 h and added characteristic pimonidazole adducts
of hypoxia. In Fig. 1D, the
fluorescent expression of pimonidazole was increased under hypoxic
conditions compared with that under normoxic conditions. However,
HS-1793 reduced the fluorescent expression of pimonidazole
dose-dependently.
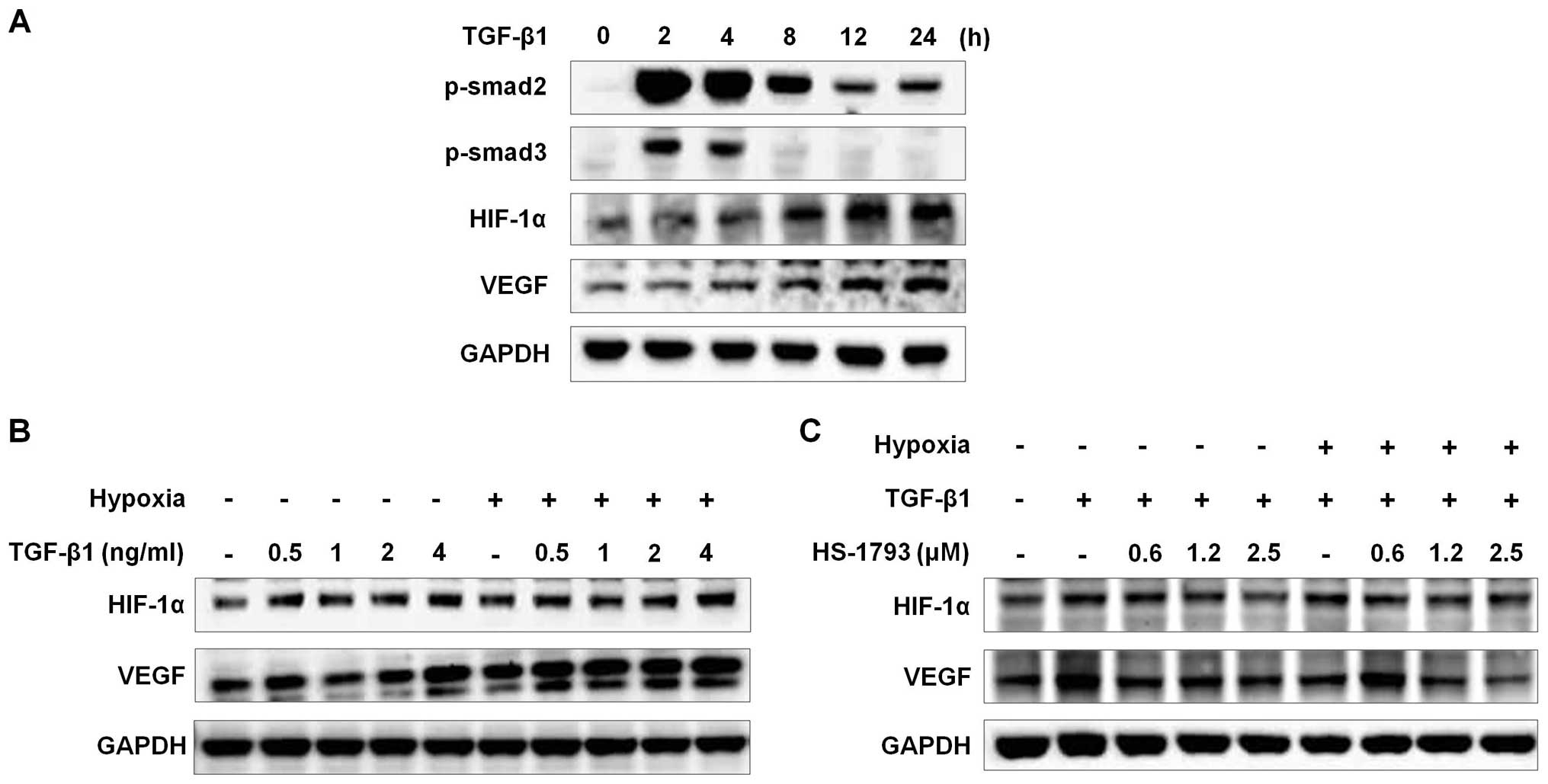
HS-1793 reduces TGF-β1-induced HIF-1α
accumulation and VEGF expression in FM3A cells
HIF-1α plays a crucial role in VEGF induction under
hypoxic conditions. To explore the role of TGF-β1 in HIF-1α and
VEGF expression under normoxic conditions, we initially evaluated
the effect of TGF-β1 on HIF-1α and VEGF expression in FM3A cells.
HIF-1α and VEGF expression was upregulated by TGF-β1 at the protein
level in a time-dependent manner. An increase in the HIF-1α level
was clearly detectable 12 h after treatment, indicating that TGF-β1
enhances the stability of HIF-1α proteins in FM3A cells. To explore
the signaling pathways implicated in the TGF-β1 induction of VEGF,
we investigated the involvement of the Smad pathway, a crucial
mediator of the TGF-β1 signaling cascade. In response to TGF-β1,
the levels of Smad2 and Smad 3 phosphorylation were increased in
FM3A cells (Fig. 2A). Moreover,
FM3A cells were treated with TGF-β1 under normoxic and hypoxic
conditions for 12 h. As shown in Fig.
2B, the HIF-1α and VEGF protein levels were markedly increased
by TGF-β1. However, pretreatment with HS-1793 in FM3A cells reduced
HIF-1α and VEGF expression dose-dependently (Fig. 2C). Therefore, these findings
suggest that HS-1793 inhibits angiogenesis via the regulation of
HIF-1α and VEGF expression not only under hypoxia but also under
the TGF-β1-treated condition.
HS-1793 improves the perfusion level and
hypoxic status in tumor tissues in mice
We assessed blood flow fluctuations in
HS-1793-injected mice bearing FM3A breast cancer cells using the
double-fluorescent dye method based on two perfusion markers,
Hoechst 33342 and DiOC7. Fluorescence microscopy revealed similar
intensities to Hoechst 33342 and DiOC7 in control FM3A breast
tumors. By contrast, HS-1793-treated (1.5 mg/kg) tumors showed
uniform uptake of DiOC7 dye, indicative of improved vessel
functionality. To determine whether HS-1793 leads to decreased
tumor hypoxia, mice bearing tumors were injected with the hypoxia
markers pimonidazole and CCI-103F. Pimonidazole and CCI-103F were
stained with green (FITC) and red (Texas Red) colors, respectively,
on the photomicrograph. At 2-h time point, the staining intensities
of the two hypoxia markers within the same range of a control tumor
were similar between pimonidazole and CCI-103F. The extensive
hypoxia that was present before HS-1793 treatment (1.5 mg/kg)
(hypoxic fraction determined by pimonidazole staining) was reduced
after HS-1793 treatment (1.5 mg/kg) (hypoxic fraction determined by
CCI-103F staining) (Fig. 3C). As
shown in Fig. 3D, a significant
difference between the untreated and HS-1793-treated images was
obtained over a wide range of threshold values.
 | Figure 3Changes in tumor perfusion and
hypoxia after HS-1793 treatment in FM3A tumor-bearing mice.
Experimental setup with the injection schedule for the two
sequentially administered hypoxic (A) and perfusion cell markers
(B). Composite panoramic sections (original ×40, reduced for
panoramic view) in tumors stained for both perfusion (Hoechst
33342, blue; DiOC7, green) and hypoxia markers (Pimonidazole,
green; CCI 103F, red) (C). The mean perfusion and hypoxic fractions
were exported as image histograms into Excel, and positive
fractions were generated for each threshold value (D). Composite
panoramic sections (original ×40, reduced for panoramic view) in
the tumor tissue (untreated control, 0.5, 1 and 1.5 mg/kg) captured
the Hoechst 33342-labeled cells (upper) and pimonidazole-stained
cells (down). Tissue regions of a section from a tumor isolated on
day 30 (E). The mean Hoechst and pimonidazole fraction were
exported as image histograms into Excel, and positive fractions
were generated for each threshold value (F and G).
#P<0.05, compared with the untreated control. |
The mean number of Hoechst 33342-labeled cells
represents the blood perfusion of the tumors and indirectly
represents the level of hypoxia in the tumors. The lower number of
labeled cells suggests impaired blood perfusion and a higher level
of hypoxia in the tumors. The Hoechst 33342 stain appeared as a
blue color on the photomicrograph (Fig. 3E). The percentages of Hoechst
33342-labeled cells in the untreated control and HS-1793 treated
groups (0.5/1.0/1.5 mg/kg) were 13.8 and 17.0/20.9/22.6%,
respectively (Fig. 3F). The number
of labeled cells in the HS-1793-treated group increased
significantly compared with that in the untreated control group.
This result suggests increased blood perfusion and slightly
decreased tumor hypoxia after HS-1793 treatment.
To examine the effects of HS-1793 treatment on tumor
hypoxia, the changes in the extent of the hypoxic areas in FM3A
xenografts were assessed by immunohistochemical analysis with
pimonidazole. As shown in Fig. 3E,
an obvious reduction of the hypoxic areas, that is, the
pimonidazole-positive regions, was observed in tumors treated with
HS-1793. The proportions of the hypoxic areas to the entire tumor
regions were scored as 19.2% (untreated control) and 14.0% (HS-1793
treated mouse, 1.5 mg/kg), respectively, indicating that the
proportion of the hypoxic areas after HS-1793 treatment was
significantly decreased compared with that in the untreated control
(Fig. 3G).
HS-1793 stimulates anti-angiogenesis
through HIF-1α suppression
To examine the effect of HS-1793 on HIF-1α
expression in tumor tissue, we performed immunohistochemical
analysis of HIF-1α (Fig. 4A).
Quantitative data showed that HS-1793 treatment in mice bearing
FM3A cells (1.5 mg/kg) affected HIF-1α expression (0.6%) compared
with the untreated control (1.9%; Fig.
4B). The mRNA levels of HIF-1α following treatment with HS-1793
were determined on day 30 after treatment. The results showed that
HIF-1α mRNA expression decreased significantly after HS-1793 (1.5
mg/kg) treatment compared with that in the untreated control
(Fig. 4C).
There are some reports on the anti-angiogenic
activity of resveratrol (21). In
this study, we examined the anti-angiogenic activity of the
resveratrol analog HS-1793 and found that HS-1793 significantly
decreased microvessel density (MVD; Fig. 4D). MVD, that is, the percentage of
endothelial cells associated with pericytes, is used as a
quantitative measure of vascular maturation. Quantitative analysis
of pericyte coverage showed 8.29% MVD staining in HS-1793-treated
(1.5 mg/kg) FM3A tumors compared with control FM3A tumors (15.68%;
Fig. 4E).
The VEGF peptide concentration in mouse serum was
significantly higher in untreated control mice (446 pg/ml) than in
tumor-free mice (187 pg/ml) but was significantly reduced
(P<0.05) in HS-1793-treated mice in a dose-dependent manner
(Fig. 4F). Therefore, these
findings suggest that HS-1793 inhibits angiogenesis via the
downregulation of HIF-1α and VEGF expression under hypoxic
conditions of the tumor microenvironment.
To determine the effect of HS-1793 treatment (0.5,
1.0 and 1.5 mg/kg) twice a week for 30 days on tumor growth,
changes in the tumor volume were calculated for a period of 30 days
following treatment. The tumor volume in the HS-1793-treated mouse
group (1.0 and 1.5 mg/kg) was greatly decreased after HS-1793
treatment compared with the untreated control (22). These results indicate that HS-1793
delays tumor growth via the improvement of hypoxia in murine breast
cancer.
HS-1793 inhibits hypoxia-induced cancer
stem cell properties, including migration and radio-resistance of
FM3A cells
Hypoxia increases the number of cells that express
CSC markers in bulk populations (23–27).
Interestingly, our data showed that HS-1793 decreased the hypoxia
induced expression of OCT4, KLF4 and SOX2 protein (Fig. 5A). In other experiments, we
investigated the effects of HS-1793 on cell migration under hypoxic
conditions. As shown in Fig. 5B,
there was minimum migration of FM3A cells under normoxic
conditions, while we observed higher levels of FM3A cell migration
under hypoxic conditions. However, HS-1793 inhibited
hypoxia-induced cell migration in a dose-dependent manner.
Accordingly, these results suggest that HS-1793 effectively
inhibits the CSC properties of FM3A cells induced by hypoxia. The
combinatorial effect of HS-1793 (2.5 μM) and radiation (2 or 4 Gy)
in hypoxic FM3A cells was evaluated by the Annexin V/PE apoptosis
assay after 12 h of treatment. The 7-AAD-positive cells that also
bound Annexin V were defined as late apoptotic cells (Annexin
V+, 7-AAD+). In Fig. 5C, Annexin V/PE-positive cells were
decreased by radiation treatment under hypoxic conditions compared
with that under normoxic conditions. However, pretreatment with
HS-1793 increased the percentage of late apoptotic cells
(4.6±0.38%) compared with the percentage of apoptotic cells in the
radiation-treatment (2.5±0.15%) groups at 1% O2
(hypoxia). These data suggest that the combinatorial treatment of
HS-1793 and radiation increases the therapeutic effect in hypoxic
FM3A cells.
Discussion
Breast cancer remains a common and frequently fatal
disease among women. Generally, the process of tumor progression is
characterized by rapid cellular growth accompanied by alterations
in the microenvironment of tumor cells (28). Critical changes in the cellular
microenvironment occur under hypoxic conditions, where oxygen
supply is inadequate (29). Breast
carcinomas usually support their growth by stimulating blood vessel
development (angiogenesis). Blood flow within these new vessels is
often chaotic, causing periods of hypoxia followed by
reperfusion.
Aggressive and metastatic cancer phenotypes that are
associated with resistance to radiation therapy, chemotherapy, and
a poor treatment outcome can be generated as a result of the
hypoxic environment within the tumor (30). Hypoxia detected in the central
regions of solid tumors is a leading cause of angiogenesis, a
fundamental determinant of malignant tumor progression, by
activation of angiogenic factors (31). A key factor in this process is
HIF-1. The overexpression of HIF-1α protein has been shown in many
human cancers and their metastases and is closely associated with a
more aggressive tumor phenotype, including an advanced tumor grade,
an increased vascularity, increased resistance to
chemo/radiotherapies, and tumor progression (32). The most characterized HIF-regulated
gene is VEGF, which is involved in regulating endothelial cell
proliferation and blood vessel formation in both normal and cancer
cells (33). TGF-β1 is involved in
blood vessel formation, and one of its oncogenic functions is to
promote tumor angiogenesis, which is associated with its ability to
induce VEGF expression (34).
Hypoxia, TGF-β1 and VEGF are important factors of the tumor
microenvironment that regulate cancer progression and metastasis
(35). Recently, resveratrol
remarkably inhibited hypoxia-induced HIF-1α accumulation and VEGF
expression in both human tongue squamous cell carcinomas (SCC-9)
and hepatoma (HepG2) cells and dramatically suppressed
hypoxia-stimulated invasiveness of SCC-9 cells in vitro
(36).
The in vitro and in vivo findings from
this study suggest that the resveratrol analog HS-1793 modulates
the hypoxic status and perfusion levels in mouse breast cancer FM3A
cells. Our data show that HS-1793 decreased HIF-1α and VEGF
expression under hypoxic conditions in FM3A cells (Fig. 1). FM3A cells were treated with
TGF-β1 under normal and hypoxic conditions, and HIF-1α and VEGF
protein levels were markedly increased by TGF-β1. However,
pretreatment with HS-1793 reduced HIF-1α and VEGF expression in
FM3A cells dose-dependently (Fig.
2). When the tumor grew to a size of ~500 mm3, to
determine the acute injection efficacy of HS-1793, hypoxia markers
(pimonidazole/CCI-103F) and perfusion markers (Hoechst 33342/DiOC7)
were used to evaluate changes in tumor hypoxia and perfusion. The
results indicated a reduced hypoxic status and enhanced perfusion
in the tumor mass. To evaluate the therapeutic effect of HS-1793,
mouse breast cancer cells (FM3A) in the logarithmic growth phase
were inoculated subcutaneously on the right flank of female C3H/He
mice, and the effects of intraperitoneal injection of HS-1793 on
HIF-1α and VEGF expression, microvessel density, the change in the
hypoxic status and perfusion level, and tumor growth were
determined after 4 weeks of treatment. The higher level of serum
VEGF in tumor-bearing mice was dose-dependently diminished in the
HS-1793-treated groups, a result that was in accordance with the
result of HIF-1α expression in the tumor tissue. The tumor tissue
of the HS-1793-treated group showed weak expression in
immunofluorescent staining of pimonidazole, which specifically
binds to thiol-containing proteins in hypoxic cells, and
immunohistochemical staining of the endothelial cell marker CD31.
However, Hoechst 33342-labeled cells, which represent the blood
perfusion of tumor tissue, were markedly increased in the
HS-1793-treated groups (Figs. 3
and 4). Finally, HS-1793 delays
tumor growth via the improvement of hypoxia in murine breast cancer
(22).
CSCs account for a minor fraction of tumor
populations; however, those cells might be particularly involved in
tumor initiation, proliferation, or metastatic process. HIF-1 has
been implicated in the maintenance of CSCs (37,38),
and knockdown experiments have concluded that HIFs are required for
CSC survival and tumor progression (39). In this study, HS-1793 effectively
inhibited hypoxia-induced CSC properties, including the migration
of tumor cells under hypoxic conditions in FM3A cells (Fig. 5A and B). Radiation therapy is an
effective modality for the treatment of many tumors (40). However, the dose-limiting normal
tissue toxicity and radio-resistant tumors are still linked to
life-threatening radiation treatment failure (41). The enhanced level of HIFs in tumors
can be correlated with the level of oxygen within the tumor and has
been shown to correlate with tumor radiation resistance (42,43).
IR-induced apoptosis was suppressed under hypoxic conditions
compared with that under normoxic conditions. However, HS-1793
enhanced IR-induced apoptosis in hypoxic FM3A cells (Fig. 5C).
In conclusion, this study demonstrated that the
resveratrol analog HS-1793 exerts anti-metastatic effects by the
inhibition of cell migration via the reduction of HIF-1α and VEGF
expression, and HS-1793 inhibits hypoxia-induced cancer stem cell
properties and enhances IR-induced apoptosis in hypoxic mouse
breast tumor FM3A cells. The results of this study demonstrate that
HS-1793 increases the therapeutic efficacy of radiation in hypoxic
cells through the modulation of the hypoxic condition.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (DIRAMS) grant funded by the Korea government
(MSIP) (50590-2016) and the [Basic Science Research Program]
through the National Research Foundation of Korea (NRF) (NRF-2013
M2A2A 7043665) funded by the Ministry of Science, ICT & Future
Planning.
References
|
1
|
Keith B and Simon MC: Hypoxia-inducible
factors, stem cells, and cancer. Cell. 129:465–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rich J, Borton A and Wang X: Transforming
growth factor-beta signaling in cancer. Microsc Res Tech.
52:363–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sánchez-Elsner T, Botella LM, Velasco B,
Corbí A, Attisano L and Bernabéu C: Synergistic cooperation between
hypoxia and transforming growth factor-beta pathways on human
vascular endothelial growth factor gene expression. J Biol Chem.
276:38527–38535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boyer LA, Lee TI, Cole MF, Johnstone SE,
Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG,
et al: Core transcriptional regulatory circuitry in human embryonic
stem cells. Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu F, Li J, Chen H, Fu J, Ray S, Huang S,
Zheng H and Ai W: Kruppel-like factor 4 (KLF4) is required for
maintenance of breast cancer stem cells and for cell migration and
invasion. Oncogene. 30:2161–2172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baumann M, Krause M and Hill R: Exploring
the role of cancer stem cells in radioresistance. Nat Rev Cancer.
8:545–554. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeSantis C, Siegel R and Jemal A: Breast
cancer facts and figures 2007–2008. Amer Can Soc. 1–32. 2008.
|
|
12
|
Limer JL and Speirs V: Phytooestrogens and
breast cancer chemoprevention. Breast Cancer Res. 6:119–127. 2004.
View Article : Google Scholar :
|
|
13
|
Privat C, Telo JP, Bernardes-Genisson V,
Vieira A, Souchard JP and Nepveu F: Antioxidant properties of
trans-epsilon-viniferin as compared to stilbene derivatives in
aqueous and nonaqueous media. J Agric Food Chem. 50:1213–1217.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong AN, Yu R, Hebbar V, Chen C, Owuor E,
Hu R, Ee R and Mandlekar S: Signal transduction events elicited by
cancer prevention compounds. Mutat Res. 480–481:231–241. 2001.
View Article : Google Scholar
|
|
15
|
Jeong SH, Jo WS, Song S, Suh H, Seol SY,
Leem SH, Kwon TK and Yoo YH: A novel resveratrol derivative,
HS1793, overcomes the resistance conferred by Bcl-2 in human
leukemic U937 cells. Biochem Pharmacol. 77:1337–1347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raleigh JA, Miller GG, Franko AJ, Koch CJ,
Fuciarelli AF and Kelly DA: Fluorescence immunohistochemical
detection of hypoxic cells in spheroids and tumours. Br J Cancer.
56:395–400. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rijken PF, Bernsen HJ, Peters JP, Hodgkiss
RJ, Raleigh JA and van der Kogel AJ: Spatial relationship between
hypoxia and the (perfused) vascular network in a human glioma
xenograft: A quantitative multi-parameter analysis. Int J Radiat
Oncol Biol Phys. 48:571–582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raleigh JA, Franko AJ, Treiber EO, Lunt JA
and Allen PS: Covalent binding of a fluorinated 2-nitroimidazole to
EMT-6 tumors in Balb/C mice: Detection by F-19 nuclear magnetic
resonance at 2.35 T. Int J Radiat Oncol Biol Phys. 12:1243–1245.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bernsen HJ, Rijken PF, Hagemeier NE and
van der Kogel AJ: A quantitative analysis of vascularization and
perfusion of human glioma xenografts at different implantation
sites. Microvasc Res. 57:244–257. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Durand RE and Raleigh JA: Identification
of nonproliferating but viable hypoxic tumor cells in vivo. Cancer
Res. 58:3547–3550. 1998.PubMed/NCBI
|
|
21
|
Garvin S, Ollinger K and Dabrosin C:
Resveratrol induces apoptosis and inhibits angiogenesis in human
breast cancer xenografts in vivo. Cancer Lett. 231:113–122. 2006.
View Article : Google Scholar
|
|
22
|
Jeong MH, Yang KM, Choi YJ, Kim SD, Yoo
YH, Seo SY, Lee SH, Ryu SR, Lee CM, Suh H, et al: Resveratrol
analog, HS-1793 enhance anti-tumor immunity by reducing the
CD4+CD25+ regulatory T cells in FM3A tumor
bearing mice. Int Immunopharmacol. 14:328–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jögi A, Øra I, Nilsson H, Lindeheim A,
Makino Y, Poellinger L, Axelson H and Påhlman S: Hypoxia alters
gene expression in human neuroblastoma cells toward an immature and
neural crest-like phenotype. Proc Natl Acad Sci USA. 99:7021–7026.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tavaluc RT, Hart LS, Dicker DT and
El-Deiry WS: Effects of low confluency, serum starvation and
hypoxia on the side population of cancer cell lines. Cell Cycle.
6:2554–2562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giuntoli S, Rovida E, Gozzini A, Barbetti
V, Cipolleschi MG, Olivotto M and Dello Sbarba P: Severe hypoxia
defines heterogeneity and selects highly immature progenitors
within clonal erythroleukemia cells. Stem Cells. 25:1119–1125.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Das B, Tsuchida R, Malkin D, Koren G,
Baruchel S and Yeger H: Hypoxia enhances tumor stemness by
increasing the invasive and tumorigenic side population fraction.
Stem Cells. 26:1818–1830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCord AM, Jamal M, Shankavaram UT, Lang
FF, Camphausen K and Tofilon PJ: Physiologic oxygen concentration
enhances the stem-like properties of CD133+ human
glioblastoma cells in vitro. Mol Cancer Res. 7:489–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vaupel P: The role of hypoxia-induced
factors in tumor progression. Oncologist. 9(Suppl 5): 10–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ryan HE, Poloni M, McNulty W, Elson D,
Gassmann M, Arbeit JM and Johnson RS: Hypoxia-inducible
factor-1alpha is a positive factor in solid tumor growth. Cancer
Res. 60:4010–4015. 2000.PubMed/NCBI
|
|
30
|
Brizel DM, Scully SP, Harrelson JM,
Layfield LJ, Bean JM, Prosnitz LR and Dewhirst MW: Tumor
oxygenation predicts for the likelihood of distant metastases in
human soft tissue sarcoma. Cancer Res. 56:941–943. 1996.PubMed/NCBI
|
|
31
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zagzag D, Zhong H, Scalzitti JM, Laughner
E, Simons JW and Semenza GL: Expression of hypoxia-inducible factor
1alpha in brain tumors: Association with angiogenesis, invasion,
and progression. Cancer. 88:2606–2618. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brown NS, Jones A, Fujiyama C, Harris AL
and Bicknell R: Thymidine phosphorylase induces carcinoma cell
oxidative stress and promotes secretion of angiogenic factors.
Cancer Res. 60:6298–6302. 2000.PubMed/NCBI
|
|
34
|
Benckert C, Jonas S, Cramer T, Von
Marschall Z, Schäfer G, Peters M, Wagner K, Radke C, Wiedenmann B,
Neuhaus P, et al: Transforming growth factor beta 1 stimulates
vascular endothelial growth factor gene transcription in human
cholangiocellular carcinoma cells. Cancer Res. 63:1083–1092.
2003.PubMed/NCBI
|
|
35
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J
and Le AD: Resveratrol inhibits hypoxia-induced accumulation of
hypoxia-inducible factor-1alpha and VEGF expression in human tongue
squamous cell carcinoma and hepatoma cells. Mol Cancer Ther.
4:1465–1474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Conley SJ, Gheordunescu E, Kakarala P,
Newman B, Korkaya H, Heath AN, Clouthier SG and Wicha MS:
Antiangiogenic agents increase breast cancer stem cells via the
generation of tumor hypoxia. Proc Natl Acad Sci USA. 109:2784–2789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schwab LP, Peacock DL, Majumdar D, Ingels
JF, Jensen LC, Smith KD, Cushing RC and Seagroves TN:
Hypoxia-inducible factor 1α promotes primary tumor growth and
tumor-initiating cell activity in breast cancer. Breast Cancer Res.
14:R62012. View Article : Google Scholar
|
|
39
|
Li Z, Bao S, Wu Q, Wang H, Eyler C,
Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al:
Hypoxia-inducible factors regulate tumorigenic capacity of glioma
stem cells. Cancer Cell. 15:501–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rosen EM, Fan S, Rockwell S and Goldberg
ID: The molecular and cellular basis of radiosensitivity:
Implications for understanding how normal tissues and tumors
respond to therapeutic radiation. Cancer Invest. 17:56–72. 1999.
View Article : Google Scholar
|
|
41
|
Camphausen K and Tofilon PJ: Combining
radiation and molecular targeting in cancer therapy. Cancer Biol
Ther. 3:247–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moeller BJ, Cao Y, Li CY and Dewhirst MW:
Radiation activates HIF-1 to regulate vascular radiosensitivity in
tumors: Role of reoxygenation, free radicals, and stress granules.
Cancer Cell. 5:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dewhirst MW, Cao Y and Moeller B: Cycling
hypoxia and free radicals regulate angiogenesis and radiotherapy
response. Nat Rev Cancer. 8:425–437. 2008. View Article : Google Scholar : PubMed/NCBI
|