Introduction
Many studies in recent years focused on the
activities of compounds extracted from marine organisms (1). Many such compounds have been
investigated, and some have been developed into herbal medicine and
made commercially available in Japan and even all over the world
(2). In the past, we also focused
on investigating a novel polysaccharide derived from algae extract
for its biological activities (3).
The efficiency of the novel algae-extracted polysaccharide was
first reported in retinal pigment epithelial (RPE) cells, in which
the polysaccharide would protect RPE cells against oxidative damage
induced by high-glucose (3). This
efficiency of anti-oxidative damage was thought a powerful
potential to withdraw the normal cells from some unfavorable
circumstance or resist the progress of abnormal cells (cancer
cell). However, it is unclear whether this novel polysaccharide
could affect cancer cells.
Cancer continue to be the second leading cause of
death (4,5). Surgical operation, radiotherapy and
chemotherapy are the most common therapies so far, however the
side-effects produced by these procedures (especially by
chemotherapy) often bring some new problems (6,7).
Due to the limited efficacy of traditional therapy
for cancers, we sought to determine a new treatment strategy for
cancers. In this study, we for the first time investigated the
novel polysaccharide in inducing apoptosis and cell cycle arrest in
human gastric carcinoma MKN45 cells.
Apoptosis is an important cause of cell
proliferation inhibition (8).
There is compelling evidence that excessive reactive oxygen species
(ROS) production surmounts cellular antioxidant defenses,
triggering apoptosis (9). Apart
from apoptosis, cell cycle arrest is another cause of growth
inhibition (10,11). Many anticancer agents dampen
malignant growth by arresting the cell cycle at the G1, S or G2/M
phases (12). It is well known
that Jun N-terminal kinase (JNK), a member of mitogen-activated
protein kinase (MAPK) family, is associated with cell proliferation
inhibition (13,14). The activation of JNK is associated
with ROS elevation (13). p-JNK
activates downstream tumor suppressor p53, caspase-9 and -3, then
leads to apoptosis and cell cycle arrest (15).
In this study, we determined that the novel
polysaccharide derived from algae extract induced human cancer cell
(MKN45 cell) apoptosis by ROS/JNK signaling pathway and arrested
the cell cycle. Our study indicated a novel therapeutic strategy of
cancer.
Materials and methods
Preparation of the novel
polysaccharide
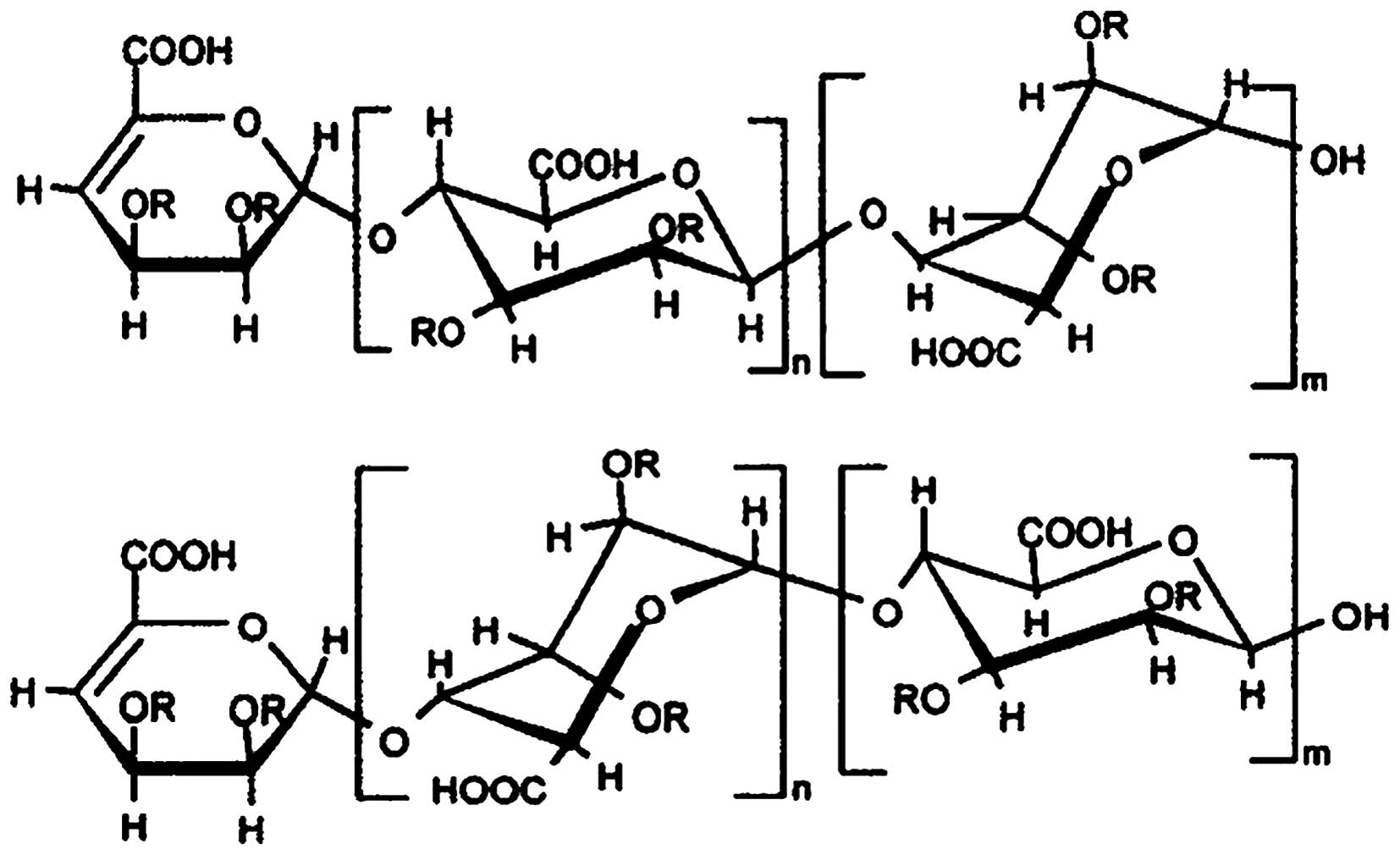
The novel polysaccharide (molecular structure was
shown as Fig. 1) derived from
algae extract was achieved by Toyo Medicine Institute (Ashikita,
Kumamoto, Japan). Detailed methods for the preparation of the
compound were published elsewhere (16). Briefly, the polysaccharide compound
was prepared from a type of phaeophyceae. After being extracted
with chloroform, ethyl acetate and n-butyl alcohol, the compound
was isolated by column chromatography on silica gel and Sephadex
LH-20 columns, was purified on a macroporous absorption resin
column, and then sulfonated by sulfuric acid. The structure of the
polysaccharide compound is a typical sugar chain structure made of
polymeride of disaccharide, which is rich in phenol and sulfate.
The average molecular weight of the compound was 11,680. The
molecular weight was used for calculation of molar concentration
(μM).
Cell culture
Human gastric cancer MKN45 cells expressing the
Fucci probes (MKN45-Fucci cells) were purchased from RIKEN
BioResource Center (Tokyo, Japan). MKN45 cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, Tokyo, Japan)
supplemented with 10% FBS. 100 U/ml penicillin and 100 mg/ml
streptomycin (Sigma), and were cultured in an incubator (Sanyo)
with 5% CO2 at 37°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
MKN45 cells were exposed to 100 μg/ml polysaccharide
with or without pre-treatment with NAC (5 mM) or SP600125 (5 μM).
The viability of normal cells and polysaccharide-treated cells was
determined by a colorimetric MTT assay according to the method
described previously (17).
Absorbance at 550 nm was determined by an MTP-800 micro-plate
reader (Corona Electric, Tokyo, Japan). Absorbance at 690 nm was
also measured to compensate for any interfering effects of cell
debris and the microtiter plate. Percentage of viable cell number
was calculated as: Optical density (OD) of treated sample/OD of
untreated control × 100.
Nuclear staining
MKN45 cells were plated in 6-well plates at the
density of 1×105 cells/well. After 24-h incubation, the
cells were treated with the polysaccharide at a concentration of
100 μg/ml and further incubated for another 48 h. Then the cells
were washed with PBS, fixed in 4% paraformaldehyde (Sigma) for 30
min and then stained with 20 mg/ml Hoechst 33342 for 15 min at room
temperature in the dark. Cells were then assessed by fluorescence
microscopy for morphological changes.
Fluorescent ubiquitination-based cell
cycle indicator (Fucci) system
MKN45 cells were exposed to 100 μg/ml polysaccharide
for 48 h. MKN45 cells expressing two Fucci probes: cells emit red
fluorescence (SCFSkp2) in G1/G0 phase and green
fluorescence (APCCdh1) in S/G2/M phases (18). As described (19), fluorescence and phase contrast
images were observed using an FV10i-DOC confocal laser scanning
microscope (Olympus, Tokyo, Japan) with a UPLSAPO 60× Wobjective
lens.
Detection of intracellular ROS
Intracellular accumulation of ROS was estimated
using the fluorescent dye H2-DCFDA (Life Technologies,
Tokyo, Japan), which is converted to a membrane impermeable and
highly fluorescent compound, dichlorofluorescin diacetate (DCF), in
the cell in the presence of ROS. The MKN45 cells were seeded in a
6-well plate at the density of 1×105 cells/well.
Following treatment with the polysaccharide or SP600125 (5 μM),
MKN45 cells were further incubated for 48 h. The cells were rinsed
with a serum-free medium and were incubated in 5 μM
H2-DCFDA for 60 min at 37°C. The cells were then
examined under a fluorescence microscope (C1-T-SM; Nikon, Tokyo,
Japan), collected and subjected to a fluorescence spectrophotometer
(F-2500; Hitachi, Tokyo, Japan) to detect the fluorescence of DCF
inside cells (excitation, 488 nm; emission, 521 nm) as described
(20).
Western blot analysis
Electrophoresis was performed using a vertical slab
gel with 12% polyacrylamide content according to the method
described previously (21). The
transfer of proteins from the SDS polyacrylamide gel to a membrane
was performed electrophoretically according to the method described
previously (22) with certain
modifications using a Semi Dry Electroblotter (Sartorius AG,
Goettingen, Germany) for 90 min with an electric current of 15 V.
The membrane was treated with Block Ace™ (4%) for 30 min at 22°C.
The first reaction was performed using rabbit immunoglobulin (IG) G
antibodies against JNK, p-JNK, p53, caspase-9 and -3 (Sigma) in PBS
containing 0.03% Tween-20 for 1 h at 22°C. Following washing in the
same buffer, the second reaction was performed using horseradish
peroxidase (HRP)-conjugated anti-rabbit goat IgG (20 ng/ml) for 30
min at 22°C. After washing, the enhanced chemiluminescence (ECL)
reaction was performed on the membrane using the ECL Plus Western
Blotting Detection System™ (GE Healthcare Life Sciences, UK).
Statistical analysis
Analyses were performed using SPSS version 19.0 (IBM
SPSS, Armonk, NY, USA). Student’s t-test was used and the data are
expressed as the mean ± SD. Each experiment was repeated at least 3
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
The novel polysaccharide suppresses cell
proliferation and induces apoptosis in MKN45 cells
Human gastric cancer MKN45 cells were exposed to 100
μg/ml polysaccharide and incubated for 48 h. The cell viability was
determined by MTT assay and the cell apoptosis was determined by
nuclear staining. The novel polysaccharide significantly suppressed
cell proliferation (Fig. 2A), and
induced cell apoptosis (Fig. 2B)
in MKN45 cells (P<0.01).
The novel polysaccharide arrests MKN45
cell cycle at G2/M phase
MKN45 cells were exposed to 100 μg/ml polysaccharide
and incubated for 48 h. The cell cycles were analyzed by Fucci
system. The novel polysaccharide significantly arrested the MKN45
cell cycle at G2/M phase (Fig. 3)
(P<0.01).
The novel polysaccharide induces ROS
generation in MKN45 cells
After MKN45 cells were exposed to 100 μg/ml
polysaccharide for 48 h, intracellular accumulation of ROS was
estimated using the fluorescent dye H2-DCFDA and flow
cytometry was estimated using DCFH-DA. The novel polysaccharide
significantly induced ROS generation in MKN45 cells (Fig. 4) (P<0.01).
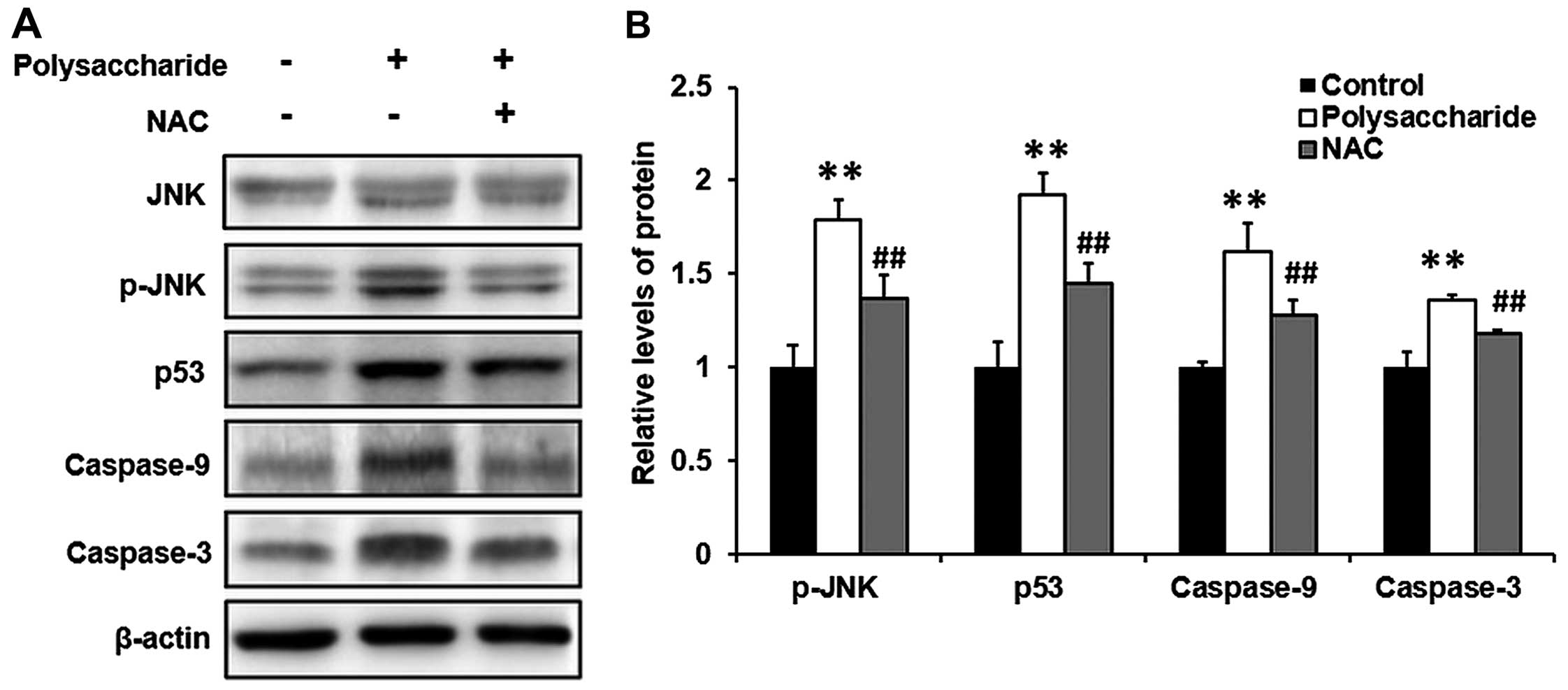
The novel polysaccharide induces the
phosphorylation of p-JNK, p53, caspase-9 and -3 in MKN45 cells
MKN45 cells were exposed to 100 μg/ml polysaccharide
and incubated for 48 h. The expression levels of p-JNK, p53,
caspase-9 and -3 were determined by western blot analysis. The
novel polysaccharide significantly induced the phosphorylation of
p-JNK, p53, caspase-9 and -3 in MKN45 cells (Fig. 5) (P<0.01).
NAC and SP600125 inhibit the effects of
the novel polysaccharide on cell proliferation and apoptosis in
MKN45 cells
MKN45 cells were pretreated with NAC (5 mM) (the
inhibitor for ROS) or SP600125 (5 μM) (the inhibitor for JNK) for 1
h prior to the polysaccharide (100 μg/ml) treatment. Then the cells
were further incubated for 48 h. The cell viability and cell
apoptosis were determined by MTT assay and nuclear staining,
respectively. The novel polysaccharide significantly suppressed
MKN45 cell proliferation and induced cell apoptosis. Pretreatment
with NAC or SP600125 significantly increased the cell proliferation
(Fig. 2A) and prevented cell
apoptosis (Fig. 2B) in MKN45 cells
(P<0.01).
NAC and SP600125 prevent the novel
polysaccharide-induced MKN45 cell cycle arrest
MKN45 cells were pretreated with NAC (5 mM) (the
inhibitor for ROS) or SP600125 (5 μM) (the inhibitor for JNK) for 1
h prior to the polysaccharide (100 μg/ml) treatment. Then the cells
were further incubated for 48 h. The cell cycles were determined by
the Fucci system. The novel polysaccharide significantly arrested
the MKN45 cell cycle at G2/M phase. Pretreatment with NAC or
SP600125 prevented the polysaccharide-induced cell cycle arrest
significantly (Fig. 3)
(P<0.01).
NAC prevents the novel
polysaccharide-induced phosphorylation of p-JNK, p53, caspase-9 and
-3 in MKN45 cells
To further determine the potential signaling pathway
involved in the process, MKN45 cells were pretreated with NAC (5
mM) (an inhibitor for ROS) for 1 h before the polysaccharide (100
μg/ml) treatment. Then the cells were further incubated for 48 h.
The phosphorylation of JNK and p-JNK were determined by western
blot analysis. The novel polysaccharide significantly induced the
p-JNK phosphorylation, and the novel polysaccharide-induced
phosphorylation of p-JNK was significantly prevented by the
pretreatment with NAC (Fig. 5)
(P<0.01).
SP600125 does not affect the novel
polysaccharide-induce ROS generation in MKN45 cells
MKN45 cells were pretreated with SP600125 (5 μM) (an
inhibitor for JNK) for 1 h prior to the polysaccharide (100 μg/ml)
treatment. Then the cells were further incubated for 48 h.
Intracellular accumulation of ROS was estimated using the
fluorescent dye H2-DCFDA and flow cytometry using
DCFH-DA. The novel polysaccharide significantly induced ROS
generation in MKN45 cells (P<0.01), however, pretreatment with
SP600125 did not affect the polysaccharide-induce ROS generation in
MKN45 cells (Fig. 4). These data
suggested that ROS is upstream of JNK.
Discussion
In recent years, as elevating of therapeutic quality
of cancer chemotherapy was recognized, researchers focused on the
application of marine organisms in molecular treatment for diseases
(1,23). Many such marine organism
extracted-compounds have been investigated, and some have been
developed into herbal medicine and made commercially available in
Japan and even all over the world (2). In the past, we focused on
investigating a novel polysaccharide derived from algae extract for
its biological activities (3)
(molecular structure is shown in Fig.
1). In this study, we investigated the activity of the novel
polysaccharide on cancer cell apoptosis and cell cycle arrest. The
pre-experiments were performed in a dose-dependent manner (1, 10,
100 and 1,000 μg/ml) and a time-dependent manner (12, 24 and 48 h).
We found significant difference when the concentration reached 100
μg/ml and the treatment-time reached 48 h, so we decided to use 100
μg/ml of polysaccharide and 48 h of treatment for our
experiments.
Cancer is the principal enemy for human life and
health (4), a leading cause of
mortality worldwide (24).
Chemotherapy and radiotherapy are the most common therapy in the
patients when surgical operation is not possible, however the
side-effects are hard to bear and always bring suffering,
physically and psychologically (6,7). In
consideration of the unwanted side-effects (25), it is crucial to develop new
treatment strategies for cancers.
Proliferation and apoptosis are very important
physiological processes for cancer cells (26). Apoptosis is an important cause of
cell proliferation inhibition (8).
Apoptosis (programmed cell death), is an essential mechanism
through which many types of chemotherapeutic agents inhibit tumor
growth (27). This study
demonstrated that the novel polysaccharide suppressed cell
proliferation (Fig. 2A) and
induced apoptosis (Fig. 2B) in
MKN45 cells. Apart from apoptosis, cell cycle arrest is another
cause of growth inhibition (10,11).
Many anticancer agents dampen malignant growth by arresting the
cell cycle at the G1, S or G2/M phases (12). Deregulation of cell cycle has been
linked with cancer initiation and progression (28). Arresting the cell cycle is an
effective method to regulate cell cycle progression, and contribute
to malignant cell proliferation (11). In this study, by using the Fucci
system, we confirmed these theories and showed that the novel
polysaccharide arrested MKN45 cells at G2/M phase (Fig. 3). These results suggested the
potential of the novel polysaccharide as an anti-cancer agent.
ROS, which is the byproduct of normal cellular
oxidative processes, has been suggested to regulate the process
involved in the initiation of apoptotic signaling (29) and has been implicated in several
oncogenic pathways. There is compelling evidence that ROS
production surmounts cellular antioxidant defenses, triggering
apoptosis (9), and cancer cells
are more sensitive to rapid increases in ROS levels than normal
cells. ROS-mediated cytotoxicity has also been identified as an
important mechanism in some anticancer agents (30). Accumulating evidence indicates that
many anticancer agents destroy tumor cells by raising the level of
ROS above a toxic threshold (31).
Oncogenic transformation elevates basal ROS levels significantly so
that any further acute increases can trigger reactivation of the
apoptotic program in cancer cells (32). To investigate whether the novel
polysaccharide-induced MKN45 cell apoptosis and cell cycle arrest
is promoted through an increase in ROS production, we measured ROS
levels. Our results showed that the novel polysaccharide induced
ROS generation in MKN45 cells significantly (Fig. 4).
It is well known that JNK, a member of
mitogen-activated protein kinase (MAPK) family, is associated with
cell proliferation inhibition (13,14).
Various apoptotic stimuli can rapidly activate MAPKs, which include
p-JNK (33). The activation of JNK
is associated with ROS elevation (13). p-JNK activates a downstream tumor
suppressor (15). p53, caspase-9
and -3, then leads to apoptosis and cell cycle arrest. p53, a tumor
suppressor, is well known for coordinating apoptosis to preserve
genomic stability and prevent tumor formation (34). p53 can induce the expression of
several factors involved in apoptosis, such as caspase-9 and -3
(35). The activation of caspase-9
and -3 damage the cell structure and cause functional disorder by
proteolysis (36). In addition to
cell cycle arrest, p53 also triggers cell cycle arrest to provide
time for self-mediated apoptosis through transcriptional activation
of cyclin-dependent kinase inhibitor (37). p-JNK activated p53, caspase-9 and
-3, and finally leads to apoptosis and cell cycle arrest (15). Our study showed that the novel
polysaccharide induced the phosphorylation of p-JNK, p53, caspase-9
and -3 (Fig. 5), suggesting the
involvement of p-JNK, p53, caspase-9 and -3 in this process.
To better understand the mechanism, we used NAC, an
inhibitor for ROS, and SP600125, an inhibitor for JNK. Pretreatment
with NAC or SP600125 significantly increased the cell proliferation
inhibited by the polysaccharide (Fig.
2A), prevented the polysaccharide-induced cell apoptosis
(Fig. 2B) and cell cycle arrest
significantly (Fig. 3) in MKN45
cells. Furthermore, we found that pretreatment with NAC prevented
the protein phosphorylation induced by the novel polysaccharide
(Fig. 5), however, pretreatment
with SP600125 did not affect the generation of ROS (Fig. 4), suggesting that the novel
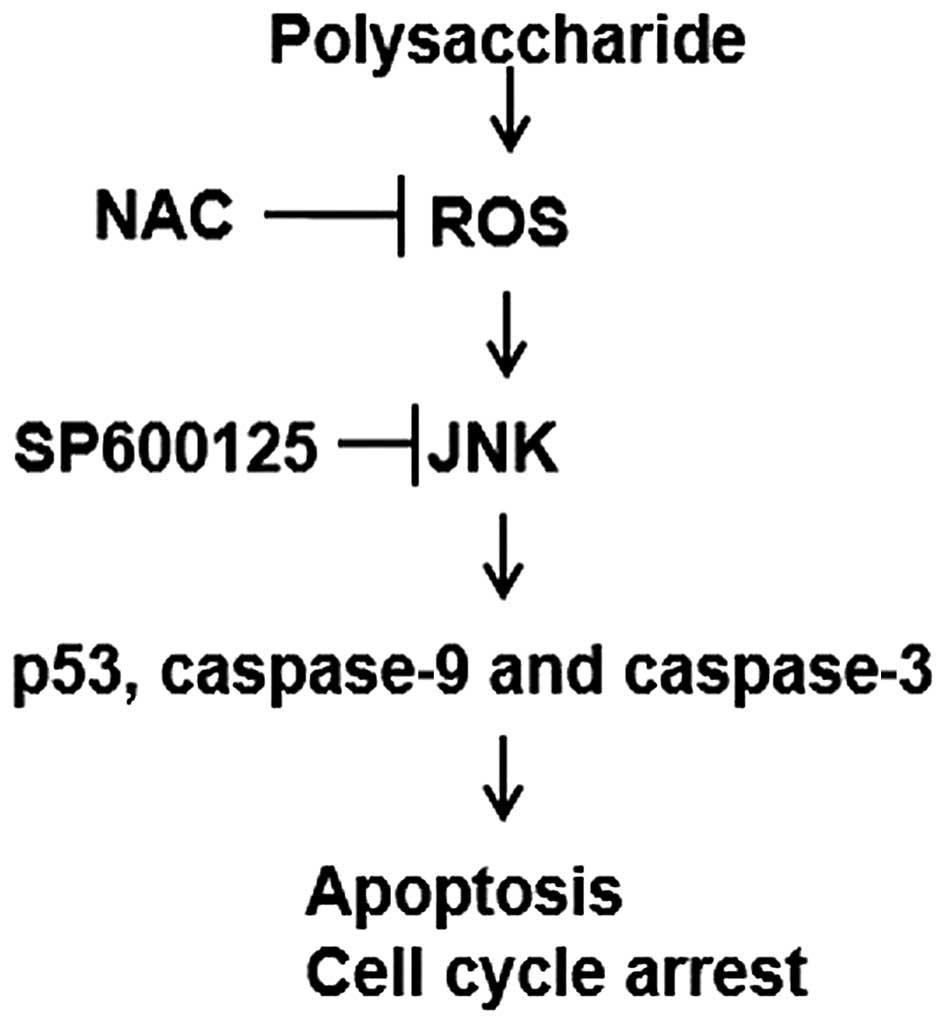
polysaccharide induces apoptosis and cell cycle arrest in MKN45
cells via ROS/JNK signaling pathway, and ROS is upstream of
JNK.
According to the mechanism described in Fig. 6, the novel polysaccharide derived
from algae extract induces the generation of ROS, then enhances the
phosphorylation of JNK, and activates the downstream cascades p53,
caspase-9 and -3, finally suppresses cancer cell proliferation,
induces cell apoptosis and arrests the cell cycles. The potential
of the novel polysaccharide in cancer cells is expected to provide
insight into the development of novel clinical treatments for
cancers.
References
|
1
|
Wang Y, Nie M, Lu Y, Wang R, Li J, Yang B,
Xia M, Zhang H and Li X: Fucoidan exerts protective effects against
diabetic nephropathy related to spontaneous diabetes through the
NF-κB signaling pathway in vivo and in vitro. Int J Mol Med.
35:1067–1073. 2015.PubMed/NCBI
|
|
2
|
Abdjul DB, Yamazaki H, Kanno S, Takahashi
O, Kirikoshi R, Ukai K and Namikoshi M: Structures and biological
evaluations of agelasines isolated from the Okinawan marine sponge
Agelas nakamurai. J Nat Prod. 78:1428–1433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie P, Fujii I, Zhao J, Shinohara M and
Matsukura M: A novel polysaccharide compound derived from algae
extracts protects retinal pigment epithelial cells from high
glucose-induced oxidative damage in vitro. Biol Pharm Bull.
35:1447–1453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu S, Powers S, Zhu W and Hannun YA:
Substantial contribution of extrinsic risk factors to cancer
development. Nature. 529:43–47. 2016. View Article : Google Scholar :
|
|
5
|
Xu J, Kochanek KD, Murphy SL and
Tejada-Vera B: Deaths: Final data for 2007. Natl Vital Stat Rep.
58:1–19. 2010.PubMed/NCBI
|
|
6
|
Iwamoto T: Clinical application of drug
delivery systems in cancer chemotherapy: Review of the efficacy and
side effects of approved drugs. Biol Pharm Bull. 36:715–718. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meirow D and Nugent D: The effects of
radiotherapy and chemotherapy on female reproduction. Hum Reprod
Update. 7:535–543. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pal D, Sharma U, Singh SK, Kakkar N and
Prasad R: Over-expression of telomere binding factors (TRF1 &
TRF2) in renal cell carcinoma and their inhibition by using SiRNA
induce apoptosis, reduce cell proliferation and migration in vitro.
PLoS One. 10:e01156512015. View Article : Google Scholar
|
|
9
|
Myatt SS, Brosens JJ and Lam EW: Sense and
sensitivity: FOXO and ROS in cancer development and treatment.
Antioxid Redox Signal. 14:675–687. 2011. View Article : Google Scholar
|
|
10
|
Tian X, Yang N, Li B, Zhang J, Xu X, Yue
R, Li H, Chen L, Shen Y and Zhang W: Inhibition of HL-60 cell
growth via cell cycle arrest and apoptosis induction by a
cycloartane-labdane heterodimer from Pseudolarix amabilis. Org
Biomol Chem. 14:2618–2624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Snoek BC, de Wilt LH, Jansen G and Peters
GJ: Role of E3 ubiquitin ligases in lung cancer. World J Clin
Oncol. 4:58–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gamet-Payrastre L, Li P, Lumeau S, Cassar
G, Dupont MA, Chevolleau S, Gasc N, Tulliez J and Tercé F:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|
|
13
|
Uchakina ON, Ban H and McKallip RJ:
Targeting hyaluronic acid production for the treatment of leukemia:
Treatment with 4-methylumbelliferone leads to induction of
MAPK-mediated apoptosis in K562 leukemia. Leuk Res. 37:1294–1301.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng L, Yang J, Chen H, Ma B, Pan K, Su C,
Xu F and Zhang J: Knockdown of TMEM16A suppressed MAPK and
inhibited cell proliferation and migration in hepatocellular
carcinoma. Onco Targets Ther. 9:325–333. 2016.PubMed/NCBI
|
|
15
|
Leber B, Geng F, Kale J and Andrews DW:
Drugs targeting Bcl-2 family members as an emerging strategy in
cancer. Expert Rev Mol Med. 12:e282010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang D, Fujii I, Lin C, Ito K, Guan H,
Zhao J, Shinohara M and Matsukura M: The stimulatory activities of
polysaccharide compounds derived from algae extracts on insulin
secretion in vitro. Biol Pharm Bull. 31:921–924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan Z, Feng W, Hong J, Zheng Q, Shuai J
and Ge Y: p38MAPK and ERK promote nitric oxide production in
cultured human retinal pigmented epithelial cells induced by high
concentration glucose. Nitric Oxide. 20:9–15. 2009. View Article : Google Scholar
|
|
18
|
Sakaue-Sawano A, Kurokawa H, Morimura T,
Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi
H, et al: Visualizing spatiotemporal dynamics of multicellular
cell-cycle progression. Cell. 132:487–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roccio M, Hahnewald S, Perny M and Senn P:
Cell cycle reactivation of cochlear progenitor cells in neonatal
FUCCI mice by a GSK3 small molecule inhibitor. Sci Rep.
5:178862015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rastogi RP, Singh SP, Häder DP and Sinha
RP: Detection of reactive oxygen species (ROS) by the
oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in
the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys
Res Commun. 397:603–607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sasaki A, Arawaka S, Sato H and Kato T:
Sensitive western blotting for detection of endogenous
Ser129-phosphorylated α-synuclein in intracellular and
extracellular spaces. Sci Rep. 5:142112015. View Article : Google Scholar
|
|
23
|
Ho JW, Leung YK and Chan CP: Herbal
medicine in the treatment of cancer. Curr Med Chem Anticancer
Agents. 2:209–214. 2002. View Article : Google Scholar
|
|
24
|
Lacroix M1, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection, and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar
|
|
25
|
Messaoudi K, Clavreul A and Lagarce F:
Toward an effective strategy in glioblastoma treatment. Part I:
Resistance mechanisms and strategies to overcome resistance of
glioblastoma to temozolomide. Drug Discov Today. 20:899–905. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng R, Li Z, Lin Z, Wang Y, Wang W, Hu B,
Wang X, Zhang J, Wang Y, Zhou R, et al: The HSP90 inhibitor 17-PAG
effectively inhibits the proliferation and migration of
androgen-independent prostate cancer cells. Am J Cancer Res.
5:3198–3209. 2015.PubMed/NCBI
|
|
27
|
Cooper WA, Kohonen-Corish MR, Zhuang L,
McCaughan B, Kennedy C, Screaton G, Sutherland RL and Lee CS: Role
and prognostic significance of tumor necrosis factor-related
apoptosis-inducing ligand death receptor DR5 in nonsmall-cell lung
cancer and precursor lesions. Cancer. 113:135–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Drexler HG: Review of alterations of the
cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18
and p19 in human leukemia-lymphoma cells. Leukemia. 12:845–859.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dewaele M, Maes H and Agostinis P:
ROS-mediated mechanisms of autophagy stimulation and their
relevance in cancer therapy. Autophagy. 6:838–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kirshner JR, He S, Balasubramanyam V,
Kepros J, Yang CY, Zhang M, Du Z, Barsoum J and Bertin J:
Elesclomol induces cancer cell apoptosis through oxidative stress.
Mol Cancer Ther. 7:2319–2327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
You BR and Park WH: Zebularine-induced
apoptosis in Calu-6 lung cancer cells is influenced by ROS and GSH
level changes. Tumour Biol. 34:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kong D, Zheng T, Zhang M, Wang D, Du S, Li
X, Fang J and Cao X: Static mechanical stress induces apoptosis in
rat endplate chondrocytes through MAPK and mitochondria-dependent
caspase activation signaling pathways. PLoS One. 8:e694032013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
An HK, Kim KS, Lee JW, Park MH, Moon HI,
Park SJ, Baik JS, Kim CH and Lee YC: Mimulone-induced autophagy
through p53-mediated AMPK/mTOR pathway increases caspase-mediated
apoptotic cell death in A549 human lung cancer cells. PLoS One.
9:e1146072014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Q, Su L, Liu N, Zhang L, Xu W and
Fang H: Cyclin dependent kinase 1 inhibitors: A review of recent
progress. Curr Med Chem. 18:2025–2043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee K, Hart MR, Briehl MM, Mazar AP and
Tome ME: The copper chelator ATN-224 induces caspase-independent
cell death in diffuse large B cell lymphoma. Int J Oncol.
45:439–447. 2014.PubMed/NCBI
|
|
37
|
Lane DP: Cancer. p53, guardian of the
genome. Nature. 358:15–16. 1992. View
Article : Google Scholar : PubMed/NCBI
|