Introduction
Hepatocellular carcinoma (HCC), the most common
primary liver cancer, is one of the most prevalent malignant
diseases worldwide, and the third most common cause of
cancer-related death (1,2). Features of HCC are an aggressive
cancer with a dismal outcome largely due to metastasis and
post-surgical recurrence. HCC originates on a background of
cirrhosis, a chronic and diffuse hepatic disease, which results
from continuous liver injury and regeneration (3,4). The
majority of HCC cases are also related to chronic viral infections
including hepatitis B virus or hepatitis C virus, which induces
malignant transformation (5–8).
Hepatocarcinogenesis is a multistep process
initiated by external stimuli that leads to genetic changes in
hepatocytes or stem cells, resulting in proliferation, apoptosis,
dysplasia and neoplasia. HCC is a cancer with a poor prognosis. The
prognosis of advanced HCC remains poor in spite of the development
of novel therapeutic strategies. In recent years, improved
knowledge of the oncogenic processes and signaling pathways that
regulate tumor cell proliferation, differentiation, angiogenesis,
invasion and metastasis has led to the identification of several
potential therapeutic targets, which have driven the development of
molecularly targeted therapies (9,10).
The most effective therapeutic tool for advanced non-resectable
HCC, which can slightly improve patient survival, is based on the
multikinase inhibitor sorafenib (11,12).
New perspectives in cancer treatment have appeared recently with
the advent of the microRNAs, a novel class of non-coding small RNAs
(13).
Regucalcin, whose gene is localized on the X
chromosome (14,15), plays a potential role as a
multi-signaling pathway and transcription repressor in various
types of cells and tissues (16,17).
Regucalcin plays a pivotal role in maintaining intracellular
calcium homeostasis, inhibiting various protein kinases and protein
phosphatases, suppressing protein synthesis, DNA and RNA synthesis,
and regulating gene expression (16–20).
Moreover, regucalcin has been found to suppress cell proliferation
and apoptotic cell death mediated through various signaling factors
(19,20) and has been proposed to play a
pivotal role in maintaining cell homeostasis and functioning as a
suppressor protein of intracellular signaling systems (16,17).
Noticeably, regucalcin has been shown to be involved
in carcinogenesis (21).
Regucalcin protein expression was found to be downregulated in the
tumor tissues from mammalian and human subjects in vivo
(22), suggesting that diminished
regucalcin may play a key role in the development of
carcinogenesis. Regucalcin gene expression has been demonstrated to
be downregulated in rat hepatoma H4-II-E cells in vitro, and
enhanced proliferation was reversed by overexpression of regucalcin
(23). Moreover, we demonstrated
that prolonged overall survival with increased regucalcin
expression in pancreatic and breast cancer patients, and that
overexpression of regucalcin suppresses the proliferation of human
pancreatic cancer MIA PaCa-2 cells and breast cancer MDA-MB-231
cells in vitro (24,25).
Regucalcin may play an important role as a suppressor of
carcinogenesis.
This study was undertaken to determine the
involvement of regucalcin in human HCC, which has not been
previously investigated. Importantly, we demonstrated that
increased regucalcin gene expression is associated with prolonged
survival in HCC patients as evaluated by the analysis of gene
expression using the Gene Expression Omnibus (GEO) database
(GSE17891) in HCC derived from human patients. Moreover,
overexpression of regucalcin was found to suppress the
proliferation of human hepatoma HepG2 cells in vitro. Our
findings support the view that regucalcin may play a potential role
as a suppressor of human HCC, and that diminished expression of
regucalcin may predispose patients to development of HCC.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) with 4.5
g/l glucose, L-glutamine and sodium pyruvate and antibiotics
(penicillin and streptomycin) were purchased from Corning
(Mediatech, Inc. Manassas, VA, USA). Fetal bovine serum (FBS) was
from Hyclone (Logan, UT, USA). Lipofectamine reagent was obtained
from Promega, Madison, WI, USA). Tumor necrosis factor-α (TNF-α)
was from R&D Systems (Minneapolis, MN, USA). Sodium butyrate,
roscovitine, sulforaphane, dibucaine, PD98059, lypopolysaccharoide
(LPS), Bay K 8644, worthomannin, 5,
6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB), caspase-3
inhibitor and all other reagents were purchased from Sigma-Aldrich
(St. Louis, MO, USA) unless otherwise specified. Gemcytabine was
obtained from Hospira, Inc. (Lake Forest, IL, USA). Gemcitabine and
caspase-3 inhibitor were diluted in phosphate-buffered saline (PBS)
and other reagents were dissolved in 100% ethanol for use.
Patient datasets
A curated gene expression dataset comprising 35
normal human liver samples and 47 HCC samples were obtained though
the Gene Expression Omnibus (GEO) database (GSE45436) for analysis
of regucalcin expression (26,27).
These datasets contained gene expression data derived from the
Affymetrix U133_plus2 platform. For microarray analysis, expression
and raw expression data (CEL files) were summarized and normalized
using the Robust Multi-array Average algorithm and the Bioconductor
package affy (http://www.bioconductor.org/packages/2.0/bioc/html/affy.html).
The Spotfire Decision Site for Functional Genomics software package
(TIBCO Software, Palo Alto, CA, USA) was used for visualization of
microarray data. For protein expression analysis, a dataset of
immunohistochemistry was obtained from the Human Protein Atlas
(HPA) (www.proteinatlas.org), which is a
database of proteins in human normal tissues and cancers (28,29).
We also evaluated regucalcin expression in 3 tissues of normal
liver, especially at the site of hepatocytes, and 6 tissues of HCC
in the liver. A dataset of 2 antibodies (HPA029102 and HPA029103)
for regucalcin was used in this analysis. Outcome analysis was
based on regucalcin mRNA expression in 162 liver tissue samples
from HCC patients (GSE10143) (30). A dataset of regucalcin expression
and clinical annotation was obtained from SurvExpress (31).
Human hepatoma HepG2 cells
We used HepG2 cells (HB-8065™) cloned from human
HCC, which were obtained from the American Type Culture Collection
(ATCC; Rockville, MD, USA). This cell line was isolated from male
adolescent (15 years old) with HCC. HepG2 cells were suitable as a
transfection host.
Transfection of regucalcin cDNA
HepG2 cells were transfected with pCXN2 vector
expressing cDNA encoding human full length (900 bp) regucalcin
(regucalcin cDNA/pCXN2) (23). For
transient transfection assay, HepG2 cells were grown on 24-well
plates to ~70% confluence. Regucalcin cDNA/pCXN2 and empty pCXN2
vector alone were transfected into HepG2 cells using the synthetic
cationic lipid components, a Lipofectamine reagent, according to
the manufacturer’s instructions (Promega) (23). After resting overnight, Geneticin
(500–700 μg/ml G418, Sigma-Aldrich) was added to culture wells for
selection, and cells were cultured for 3 weeks. After that, cells
were plated at limiting dilution to isolate transfectants. Multiple
surviving clones were isolated, transferred to 35-mm dishes, and
grown in the medium without Geneticin. Stable expression of
regucalcin was observed in transfectants, and the clone with
protein levels of regucalcin increased by 11.2-fold as compared
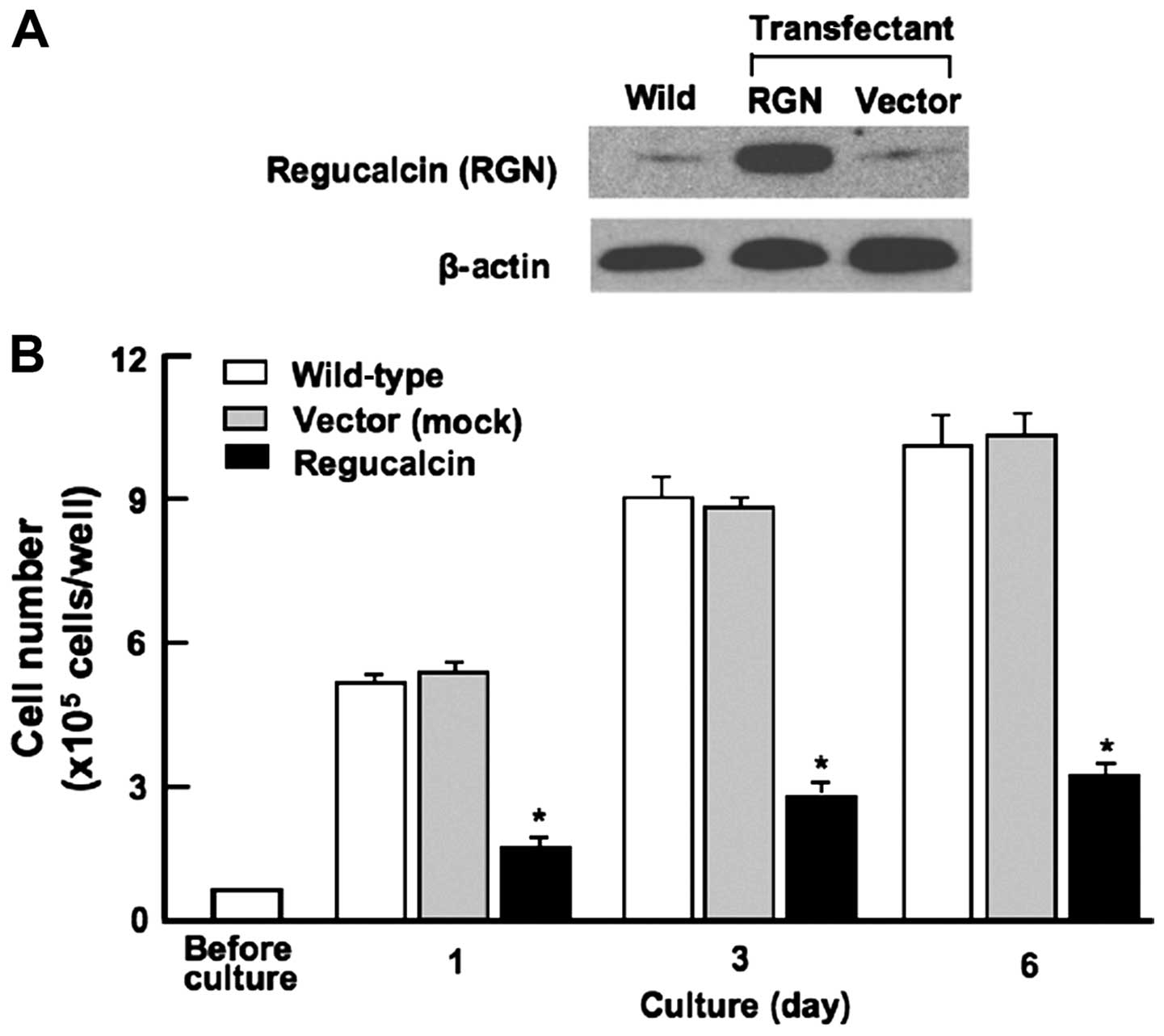
with that of wild-type cells was used in this experiment (Fig. 2A).
Western blotting
HepG2 (wild-type) cells transfected with control
vector or regucalcin cDNAs were plated in 35-mm dishes at a density
of 1×105 cells/well in 2 ml of medium, and cultured in
DMEM containing 10% FBS and 1% P/S for 3 days. Cells were washed
twice with ice-cold PBS and removed from the dish by scraping in
cell lysis buffer containing protein inhibitors. Lysed cells were
centrifuged and the pellet was homogenized by sonication in 0.1 ml
of ice-cold cell lyses buffer containing protein inhibitors. The
homogenate was centrifuged for 5 min at 1,500 g, and the protein
concentration of the supernatant was determined for western
blotting using bovine serum albumin as a standard. Samples (30 μg)
of supernatant protein per lane were separated by SDS-PAGE and
transferred to nylon membranes for western blotting using
antibodies against regucalcin (22,23)
and other proteins (Cell Signaling Technology, Danvers, MA, USA).
β-actin was used as a loading control. A minimum of 3 blots from
independent experiments were scanned on an Epson Perfection 1660
Photo scanner, and bands quantified using ImageJ. Data from
independent experiments were normalized to a percentage of control
before averaging.
Cell proliferation assays
Wild-type HepG2 cells (1×105/ml per well)
and HepG2 cells (1×105/ml per well) transfected with
regucalcin cDNA were cultured using a 24-well plate in DMEM
containing 10% FBS and 1% P/S for 1, 3 or 6 days in a
water-saturated atmosphere containing 5% CO2 and 95% air
at 37°C (32,33). In separate experiments, wild-type
HepG2 cells or transfectants were cultured in DMEM containing 10%
FBS and 1% P/S in the presence of either sodium butyrate (10 and
100 μM), roscovitine (10 and 100 nM), sulphoraphane (1 and 10 nM),
dibucaine (0.1 or 1 μM), Bay K 8644 (0.1 or 1 μM), PD98059 (1 or 10
μM), worthomannin (0.1 or 1 μM), DRB (0.1 or 1 μM), or gemcitabine
(50 or 100 nM) for 3 days. After culture, cells were detached from
each culture dishes and counted.
Cell death assay
Wild-type HepG2 cells (1×105/ml per well)
and HepG2 cells (1×105/ml per well) transfected with
regucalcin cDNA were cultured using a 24-well plate in DMEM
containing 10% FBS and 1% P/S for 4 days to confluence, and then
were cultured for additional 3 days in the presence or absence of
either LPS (0.1 or 1 μg/ml), TNF-α (0.1 or 1 ng/ml) or Bay K 8644
(0.1 or 1 μM) (34). In separate
experiments, wild-type HepG2 cells (1×105/ml per well)
or transfectants were cultured for 4 days to confluence, and then
were cultured for an additional 24 h in the presence or absence of
either LPS (1 μg/ml) or TNF-α (1 ng/ml) with or without caspase-3
inhibitor (10 μM) for 24 h (34).
After culture, cells were detached from each culture dish for
counting.
Cell counting
Cells were recovered from plates by trypsinization
of each of culture dishes using 0.2% trpysin plus 0.02% EDTA in
Ca2+/Mg2+-free PBS for 2 min at 37°C,
detached cells from dish were collected after centrifugation
(32–34). Cells were suspended on PBS solution
and stained with eosin. Cells were counted under a microscope using
a hemocytometer. For each dish, we took the average of two
quantifications.
Migration assay
We used the in vitro scratch assay method for
analysis of cell migration in vitro (35). HepG2 cells (1×105
cells/ml) of wild-type and transfectants were cultured in DMEM
containing 10% FBS and 1% P/S for 24 h using 12-well plates to
reach confluence, and then a scratch was created in the cell
monolayer. Images were captured at the beginning and at 24 or 48 h
of culture. Cells in the plate were fixed in ice-cold 95% ethanol
and stained with crystal violet (1% in PBS). After overnight
drying, the migration distance was imaged, and cells were counted
under a microscope to determine migration.
Statistical analysis
Statistical significance was determined using
GraphPad InStat version 3 for Windows XP (GraphPad Software Inc.,
La Jolla, CA, USA). Multiple comparisons were performed by one-way
analysis of variance (ANOVA) with Tukey-Kramer multiple comparisons
post hoc test for parametric data as indicated. Survival curves
were constructed by Kaplan-Meier analysis and were compared with
the log-rank test. Other data were analyzed with the paired or
unpaired Student’s t-test as performed with IBM SPSS Statistics 18
software (IBM, Chicago, IL, USA http://www.ibm.com). A p-value of <0.05 was
considered statistically significant.
Results
Prolonged survival in HCC patients with
increased regucalcin gene expression
To understand the involvement of regucalcin in human
patients with HCC, we analyzed the expression levels of regucalcin
in normal liver tissues and HCC. We analyzed microarray data from
the GEO database (GSE45436) (as described in Materials and methods)
to evaluate regucalcin expression levels in 35 normal pancreas and
47 HCC patients. Overall regucalcin expression was visually reduced
in HCC patients as compared with that of tissues derived from
normal liver (Fig. 1A).
Quantitative analysis confirmed that expression of regucalcin in
HCC patients was remarkably reduced as compared with that in the
tissue of normal liver (Fig. 1B).
To confirm the reduction of regucalcin protein levels, we checked
the expression of regucalcin in 3 normal liver tissue samples and
in 6 HCC samples using the immunohistochemistry database (The Human
Protein Atlas). Results from 2 independent regucalcin antibodies
revealed that the expression of regucalcin in HCC patients was
markedly suppressed as compared to that of normal liver (Fig. 1C). Moreover, we compared the
clinical outcome between 81 HCC patients with higher regucalcin
expression and 81 HCC patients with lower regucalcin expression.
The reduction of regucalcin expression was associated with poor
prognosis in HCC patients. Higher regucalcin gene expression was
found to prolong survival in HCC patients (Fig. 1D). These findings support the
notion that suppression of regucalcin gene expression partly
contributes to the development of carcinogenesis in human HCC cells
and leads to a worse clinical outcome.
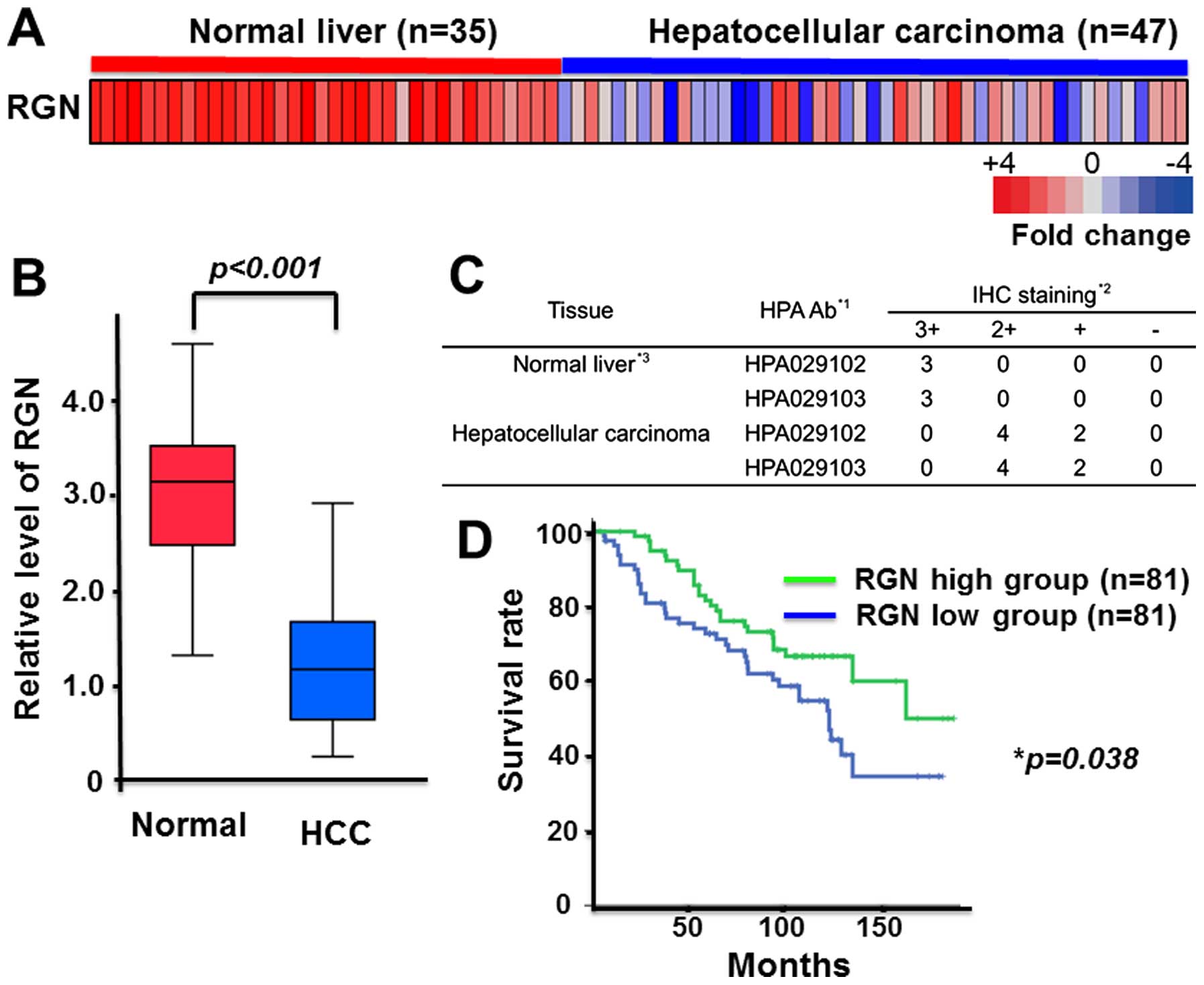 | Figure 1Prolonged survival in hepatocellular
carcinoma (HCC) patients with the higher regucalcin gene
expression. (A) Microarray expression analysis of regucalcin in 35
normal liver and 47 HCC (26,27).
Each colored square on the bottom right represents the relative
mean transcript abundance; highest expression is shown in red,
average expression in white, and lowest expression is shown in
blue. Reduced regucalcin expression weakens survival of HCC
patients. (B) Quantification of regucalcin expression in normal
liver and HCC similar to that in (A). (C) Degree of
immunohistochemical staining for regucalcin into 3 tissues of
normal liver and 6 HCC though the immunohistochemistry (IHC)
database (The Human Protein Atlas). The results of 2 antibodies are
indicated. IHC staining scores: 3+, storong; 2+, moderate; +, weak;
−, negative. (D) Survival curves for HCC, and survival was longer
in high expression as compared with low expression of regucalcin.
HCC, hepatocellular carcinoma; HPA, The Human Protein Atlas; IHC,
immunohistochemistry; RGN, regucalcin. |
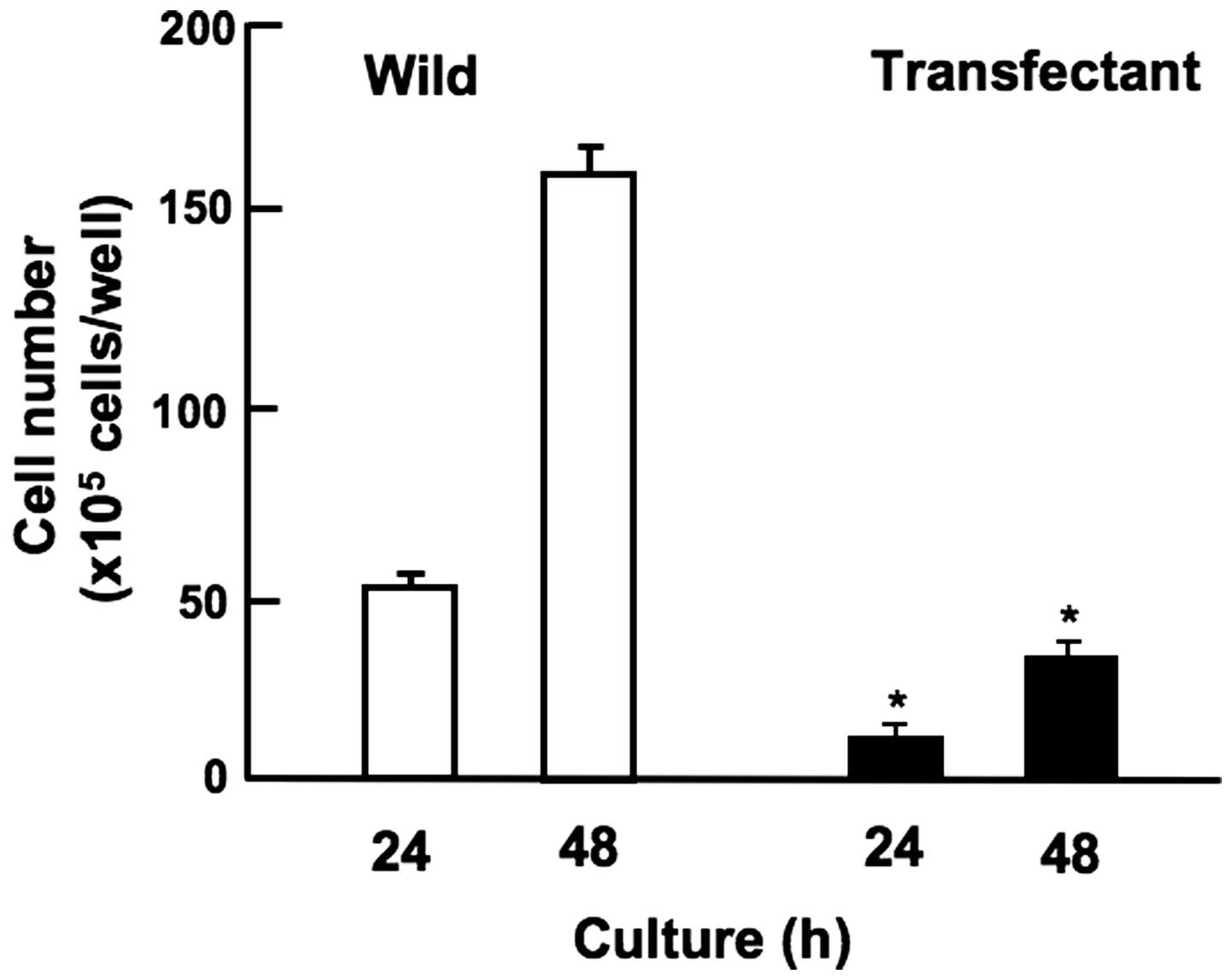
Generation of regucalcin-overexpressing
HepG2 cells
HepG2 cells were transiently transfected with empty
pCXN2 vector or full length (33-kDa protein) regucalcin/pCXN2
construct using lipofection. Regucalcin protein in transfected
cells was found to be increased by 11.2-fold as compared with that
of the parental wild-type HepG2 cells (Fig. 2A). To determine the effects of the
overexpression of endogenous regucalcin on the proliferation of
HepG2 cells in vitro, the cancer cells were cultured for 1,
3, and 6 days. Cell proliferation in culture was significantly
reduced in regucalcin-overexpressing transfectants at 1, 3, and 6
days (Fig. 2B). Thus,
overexpression of regucalcin was found to suppress the
proliferation of HepG2 cells in vitro.
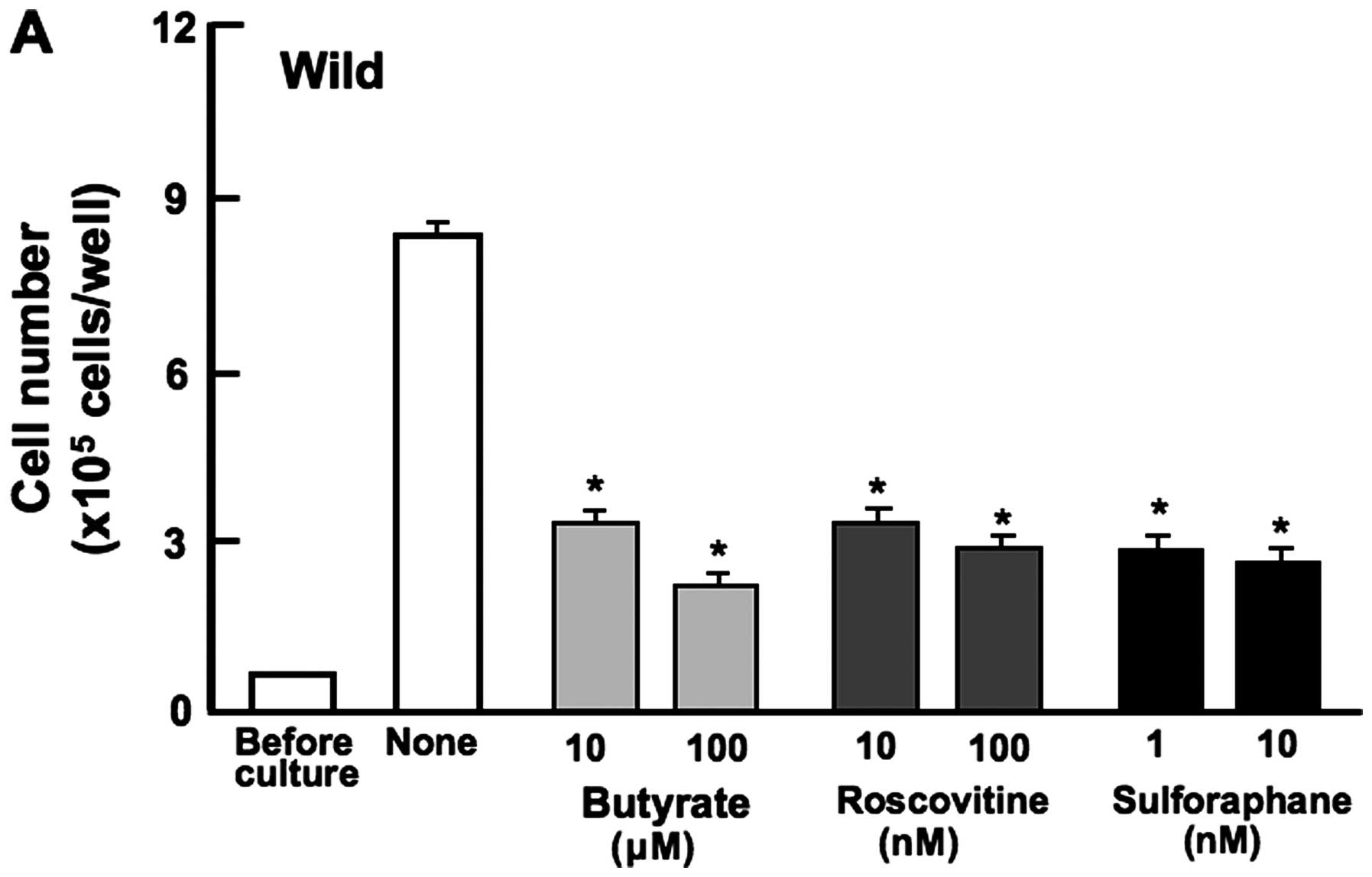
Suppressive effects of regucalcin
overexpression on HepG2 cell proliferation are related to
regulation of various signaling pathways
Proliferation in HepG2 cells was determined in the
presence of various inhibitors that induce cell cycle arrest in
vitro (Fig. 3). Wild-type
cells were cultured for 3 days in the presence of butyrate (10 and
100 μM), roscovitine (10 and 100 nM) or sulforaphane (1 and 10 nM)
(32,36,37).
Proliferation of wild-type cells was suppressed in the presence of
these inhibitors (Fig. 3A) but not
in transfectants (Fig. 3B). This
result suggested that endogenous regucalcin induces G1 and G2/M
phase cell cycle arrest in HepG2 cells.
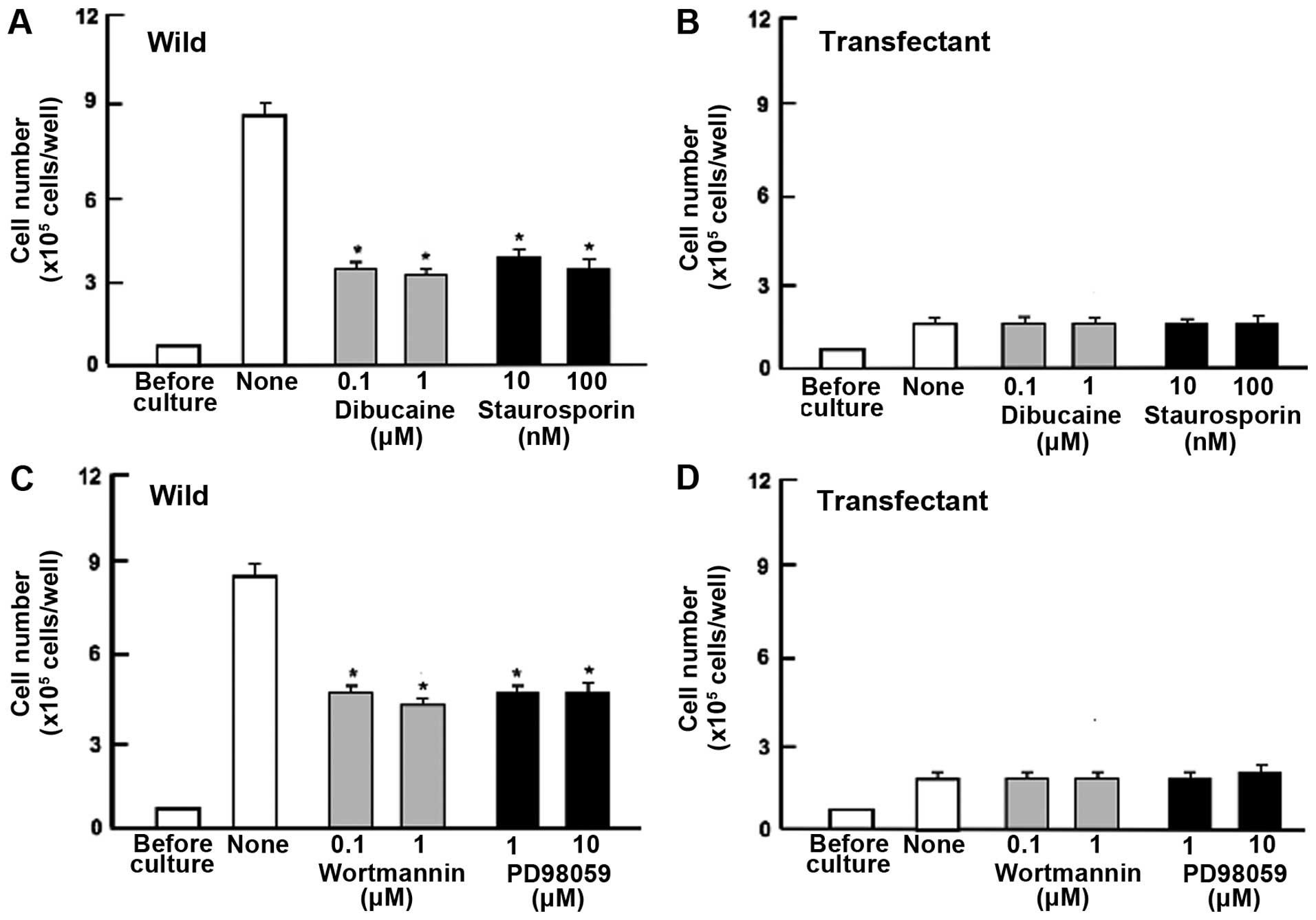
Next, to determine the mechanism of regucalcin
suppression of cell proliferation, we examined key signaling
factors that regulate proliferation. Proliferation in wild-type
cells was weakly suppressed by dibucaine (0.1 or 1 μM), an
inhibitor of calcium/calmodulin-dependent protein kinases (32), and staurosporine (10 or 100 nM), a
calcium signaling-related inhibitor (32) (Fig.
4A). Blocking these pathways had no effect on the ability of
regucalcin to suppress cell proliferation (Fig. 4B). Likewise, worthomannin (0.1 or 1
μM), an inhibitor of phosphatidylinositol 3-kinase (PI3K) (36), and PD98059 (1 or 10 μM), an
inhibitor of extracellular signal-regulated kinase (ERK) and
mitogen-activated protein kinase (MAPK) (39) (Fig.
4C) significantly inhibited cell proliferation in wild-type
cells. Suppressive effects of these inhibitors on cell
proliferation were not exhibited in transfectants (Fig. 4D). DRB is an inhibitor of
transcriptional activity with RNA polymerase II inhibition
(40). Gemcitabine is a strong
antitumor agent that induces nuclear DNA damage (41). Proliferation of HepG2 cells was
suppressed by culture with DRB (0.1 or 1 μM) or gemcitabine (50 or
100 nM) (Fig. 4E). However, these
effects were not seen in transfectants (Fig. 4F).
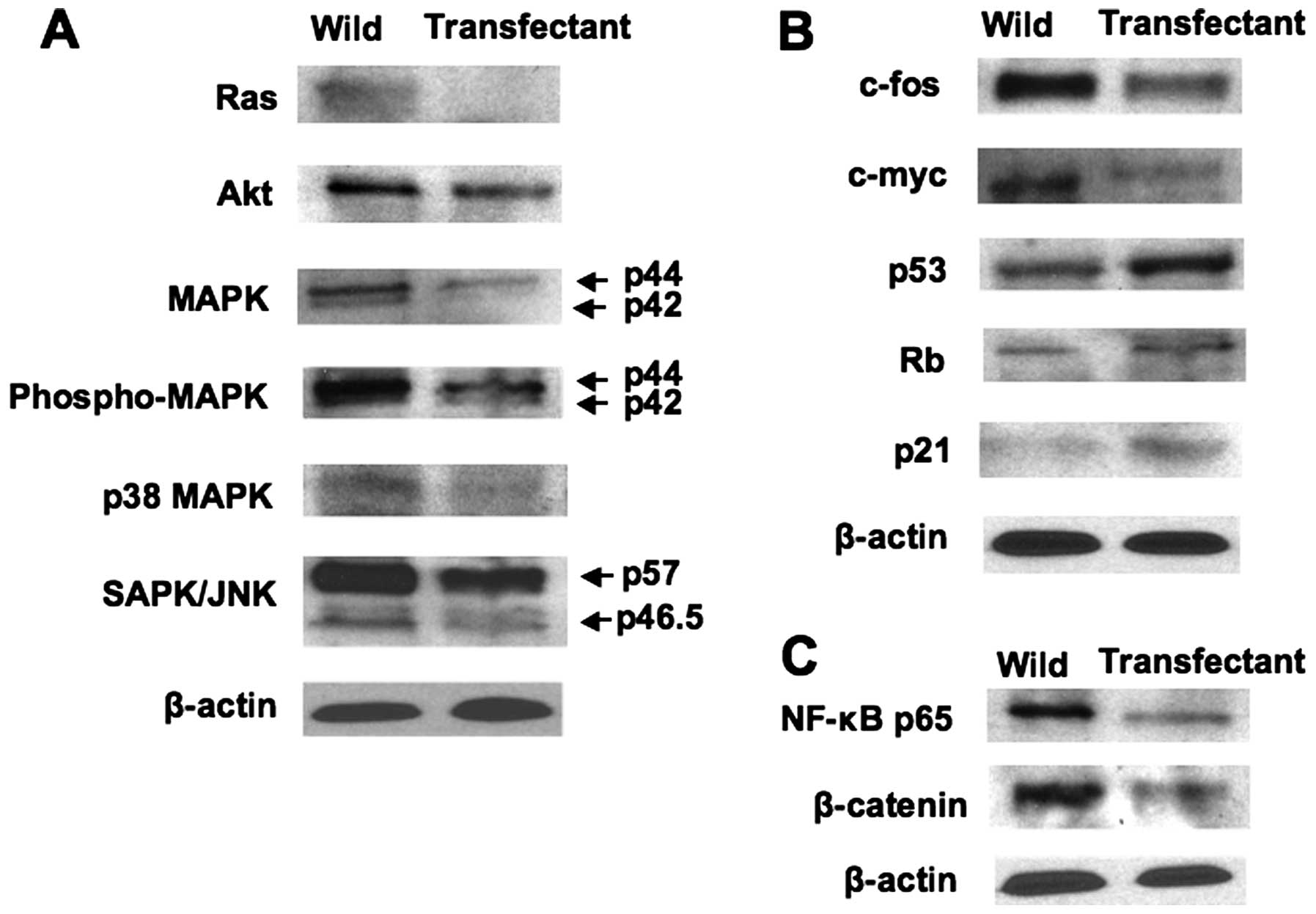
Overexpression of regucalcin was found to regulate
the expression of key proteins related to signaling pathways and
transcription activity using western blot analysis. Protein levels
of Ras, Akt, MAPK, phospho MAPK, p38-MAPK, and SAPK/JNK were
decreased by overexpression of regucalcin (Fig. 5A). These results suggested that
overexpression of regucalcin suppresses signaling pathways that are
related to activation of ras in HepG2 cells. In addition,
overexpression of regucalcin decreased protein levels of
c-fos and c-myc in HepG2 cells (Fig. 5B). Protein levels of p53 and
Rb, tumor suppressors, and p21, cell cycle regulator, were
increased by overexpression of regucalcin (Fig. 5B). Moreover, overexpression of
regucalcin suppressed protein levels of transcription factor NF-κB
p65 and β-catenin in HepG2 cells (Fig.
5C).
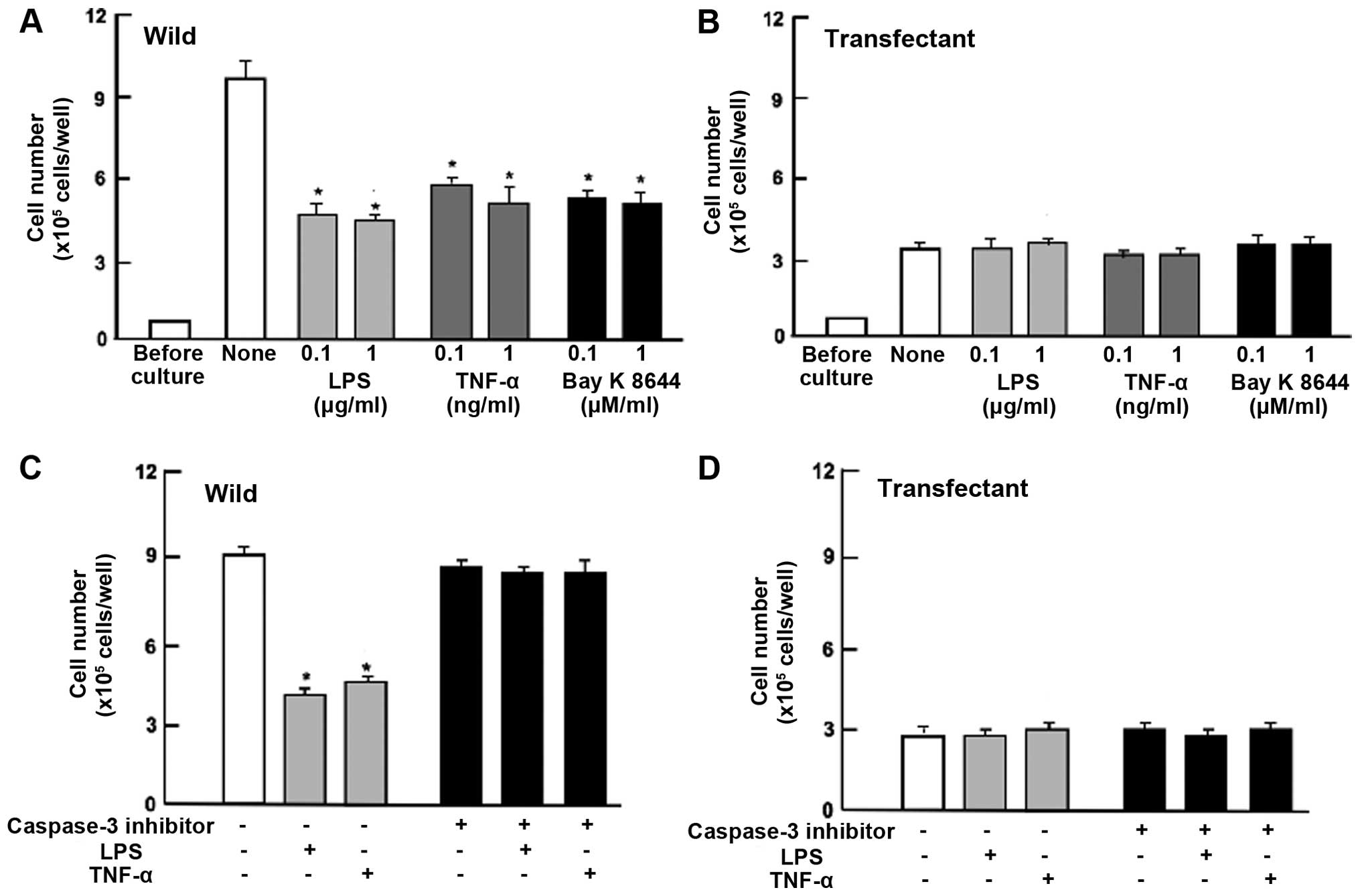
Overexpression of regucalcin prevents
cell death induced by various stimulatory factors
To determine the effects of the overexpression of
endogenous regucalcin on cell death in HepG2 cells, the cells were
cultured for 5 days to confluence and then for an additional 24 h.
The number of wild-type cells was decreased in the presence of LPS
(0.1 or 1 μg/ml), TNF-α (0.1 or 1 ng/ml) or Bay K 8644 (0.1 and 1
μM), which is known to induce apoptotic cell death (20,34)
(Fig. 6A). Such effects were not
seen in the regucalcin-overexpressing HepG2 cells that did not
exhibit a significant effect on the death (Fig. 6B). Death of HepG2 cells (wild-type)
was also induced once the cells were cultured in the presence of
TNF-α (1 ng/ml) or Bay K 8644 (1 μM) for 24 and 72 h (Fig. 6A). Such effects were not seen in
the transfectants with regucalcin of full length (Fig. 6B).
To determine whether the preventive effects of
regucalcin on cell death are mediated via caspase-3, confluent
wild-type cells and transfectants were additionally cultured in the
presence of LPS (1 μg/ml) or TNF-α (1 ng/ml) with or without
caspase-3 inhibitors (10 μM) for 24 h (Fig. 6C and D). Effects of LPS or TNF-α on
cell death were completely prevented in the presence of caspase-3
inhibitor (Fig. 6C). LPS- or
TNF-α-induced cell death was not seen in transfectants that were
cultured with or without caspase-3 inhibitor (Fig. 6D). These results suggest that
endogenous regucalcin prevents cell death due to decreasing the
activity of caspase-3 that activates nuclear DNA fragmentation,
inducing apoptotic cell death. Thus, suppressive effects of
regucalcin overexpression on the proliferation of HepG2 cells were
independent of its effect on cell death.
Overexpression of regucalcin suppresses
cell migration
Overexpression of regucalcin suppressed migration of
human pancreatic cancer HepG2 cells in vitro (Fig. 7). HepG2 cells of wild-type and
transfectants were cultured for 24 h using 12-well plates to reach
subconfluence, and then a scratch was created in a cell monolayer.
Cells migrating into the space of the scratch were stained and
counted. Overexpression of regucalcin prevented migration of cells
into the scratch as compared with that of wild-type cells (Fig. 7).
Discussion
Using HCC microarray analysis of the GEO database
(GSE15471) this study demonstrates overall regucalcin expression is
reduced in human HCC patients as compared with that of normal human
liver tissues. Furtheremore, patient survival is prolonged with
increased regucalcin gene expression while suppressed regucalcin
expression was found to be associated with poor prognosis in HCC
patients. These findings support the view that suppressed
regucalcin gene expression partly contributes to the development or
aggressiveness of carcinogenesis in human HCC and leads to a worse
clinical outcome.
Overexpression of regucalcin was demonstrated to
exhibit anticancer effects in HepG2 cells from human HCC by
suppressing the proliferation of cancer cells in vitro. This
effect of regucalcin was independent of cell death. In addition,
overexpression of regucalcin was found to suppress the migration of
HepG2 cells using the scratch assay in vitro, supporting
anticancer effects of regucalcin in vitro. Our findings may
support the view that regucalcin plays a potential role as a
suppressor protein in human HCC cells.
Suppressive effects of regucalcin overexpression on
the proliferation of HepG2 cells were not altered in the presence
of butyrate, roscovitine or sulphoraphane that induce cell-cycle
arrest. Roscovitine is a potent and selective inhibitor of the
cyclin-dependent kinase cdc2, cdk2m and cdk5 (34). Sulforaphane induces G2/M phase cell
cycle arrest (37). Butyrate
induces an inhibition of G1 progression (32). Endogenous regucalcin appears to
induce G1 and G2/M phase cell cycle arrest in HepG2 cells. Similar
data have been reported in cloned rat hepatoma H4-II-E cells
(32) and normal rat kidney
proximal tubular epithelial NRK52E cells (33).
To determine a mechanistic cause of the suppressive
effects of regucalcin overexpression on cell proliferation, we used
various inhibitors that regulate intracellular signaling pathways.
Suppressive effects of regucalcin overexpression on the
proliferation in HepG2 cells were not altered in the presence of
dibucaine, an inhibitor of calcium/calmodulin-dependent protein
kinases (32), worthomannin, an
inhibitor of PI3/Akt signaling pathway (38), and PD98059, an inhibitor of ERK/MAP
kinase-related to signaling pathway (39). Regucalcin was shown to exhibit
suppressive effects on the proliferation by inhibiting various
intracellular signaling pathways, which are related to
Ca2+-dependent kinases, PI3/Akt, and ERK/MAPK in HepG2
cells. Results with western blot analysis confirmed that
overexpression of regucalcin led to decrease in protein levels that
were involved in the signaling of Ras, Akt and MAPK in HepG2
cells.
In addition, suppressive effects of regucalcin
overexpression on cell proliferation were not changed in the
presence of DRB, an inhibitor of transcriptional activity with RNA
polymerase II inhibition (40).
Regucalcin was observed to suppress transcriptional activity in
HepG2 cells and to bind to DNA and regulate nuclear gene expression
(42). Regucalcin may thus
regulate transcriptional activity in HepG2 cells.
Gemcitabine is well known as an antitumor agent that
induces nuclear DNA damage, and it is used clinically for the
therapy of human pancreatic cancer (41). This agent suppresses cell
proliferation and stimulates apoptotic cell death in various types
of cancer cells. Suppressive effects of regucalcin overexpression
on the proliferation were not potentiated in the presence of
gemcitabine in HepG2 cells, suggesting that regucalcin partly acts
on pathways related to the action mode of gemcitabine. Regucalcin
has been shown to inhibit DNA and RNA synthesis in isolated rat
liver nuclei (17–19).
Overexpression of regucalcin was demonstrated to
increase the gene expression of p53 and Rb, a suppressor of tumor,
and p21, an inhibitor of cell cycle, and suppress the gene
expressions of ras, c-jun and c-myc, oncogenes, in cloned rat
hepatoma H4II-E cells in vitro (42). Overexpression of regucalcin was
also found to increase the protein levels of p-53, Rb and p21, and
decrease the protein levels of ras, c-fos and c-myc in HepG2 cells
in vitro. These results may support the view that regucalcin
partly mediates a suppressive effect on the proliferation of HepG2
cells by regulating the expression of tumor suppressor proteins and
oncogenes. Interestingly, overexpression of regucalcin was found to
decrease NF-κB p65 and β-catenin in HepG2 cells. Regucalcin may
suppress signaling process and transcription activity related to
NF-κB and β-catenin in HepG2 cells. This effect may partly
contribute in the revelation of anticancer effects of regucalcin in
HCC.
Overexpression of regucalcin was found to prevent
cell death induced by various stimulatory factors including LPS,
TNF-α, and Bay K 8644 in HepG2 cells in vitro, supporting
the view that the suppressive effects of regucalcin on the
proliferation were not based on cell death. Preventive effect of
regucalcin on cell death was not enhanced in the presence of
caspase-3 inhibitor. Regucalcin was suggested to prevent cell death
through the mechanism by which it increases caspase-3 activity that
activates nuclear DNA fragmentation that induces apoptosis of
cells. It is possible that regucalcin directly inhibits caspase-3.
In addition, overexpression of regucalcin has been shown to
decrease the protein levels of caspase-3 and clevaged caspase-3 in
human pancreatic cancer MIA PaCa-2 cells (24) and breast cancer MDA-MB-231 cells
(25). Regucalcin has also been
shown to directly inhibit calcium-activated endonuclease in
isolated rat liver nucleus in vitro (43) and to suppress nitric oxide
synthetase activity and to regulate the gene expression of various
proteins that are involved in apoptosis in cloned rat hepatoma
H4-II-E cells in vitro (34).
In conclusion, this study demonstrates that
prolonged survival is associated with increased regucalcin gene
expression in HCC patients, and that the overexpression of
regucalcin suppresses the proliferation enhanced through various
signaling pathways in human hepatoma HepG2 cells in vitro.
Our findings may support the view that endogenous regucalcin plays
a potential role as a suppressor protein in the development of
hepatocarcinogenesis. Overexpression of regucalcin may constitute a
novel therapeutic approach to treating HCC.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dragani TA: Risk of HCC: Genetic
heterogeneity and complex genetics. J Hepatol. 52:252–257. 2010.
View Article : Google Scholar
|
|
5
|
Lee YH and Yun Y: HBx protein of hepatitis
B virus activates Jak1-STAT signaling. J Biol Chem.
273:25510–25515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andrisani OM and Barnabas S: The
transcriptional function of the hepatitis B virus X protein and its
role in hepatocarcinogenesis (Review). Int J Oncol. 15:373–379.
1999.PubMed/NCBI
|
|
7
|
Benn J and Schneider RJ: Hepatitis B virus
HBx protein activates Ras-GTP complex formation and establishes a
Ras, Raf, MAP kinase signaling cascade. Proc Natl Acad Sci USA.
91:10350–10354. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cha MY, Kim CM, Park YM and Ryu WS:
Hepatitis B virus X protein is essential for the activation of
Wnt/beta-catenin signaling in hepatoma cells. Hepatology.
39:1683–1693. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin JW and Chung Y-H: Molecular targeted
therapy for hepatocellular carcinoma: Current and future. World J
Gastroenterol. 19:6144–6155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singal AG, Marrero JA and Singal AG:
Recent advances in the treatment of hepatocellular carcinoma. Curr
Opin Gastroenterol. 26:189–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al; SHARP Investigators Study Group. Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Callegari E, Elamin BK, Sabbioni S,
Gramantieri L and Negrini M: Role of microRNAs in hepatocellular
carcinoma: A clinical perspective. Onco Targets Ther. 6:1167–1178.
2013.PubMed/NCBI
|
|
14
|
Shimokawa N and Yamaguchi M: Molecular
cloning and sequencing of the cDNA coding for a calcium-binding
protein regucalcin from rat liver. FEBS Lett. 327:251–255. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi M: The transcriptional
regulation of regucalcin gene expression. Mol Cell Biochem.
346:147–171. 2011. View Article : Google Scholar
|
|
16
|
Yamaguchi M: Role of regucalcin in
maintaining cell homeostasis and function (Review). Int J Mol Med.
15:371–389. 2005.PubMed/NCBI
|
|
17
|
Yamaguchi M: Regucalcin and cell
regulation: Role as a suppressor protein in signal transduction.
Mol Cell Biochem. 353:101–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaguchi M: Role of regucalcin in cell
nuclear regulation: Involvement as a transcription factor. Cell
Tissue Res. 354:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi M: Suppressive role of
regucalcin in liver cell proliferation: Involvement in
carcinogenesis. Cell Prolif. 46:243–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaguchi M: The anti-apoptotic effect of
regucalcin is mediated through multisignaling pathways. Apoptosis.
18:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaguchi M: Involvement of regucalcin as
a suppressor protein in human carcinogenesis: Insight into the gene
therapy. J Cancer Res Clin Oncol. 141:1333–1341. 2015. View Article : Google Scholar
|
|
22
|
Murata T and Yamaguchi M: Alternatively
spliced variants of the regucalcin gene in various human normal and
tumor tissues. Int J Mol Med. 34:1141–1146. 2014.PubMed/NCBI
|
|
23
|
Misawa H, Inagaki S and Yamaguchi M:
Suppression of cell proliferation and deoxyribonucleic acid
synthesis in the cloned rat hepatoma H4-II-E cells overexpressing
regucalcin. J Cell Biochem. 84:143–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi M, Osuka S, Weitzmann MN,
El-Rayes BF, Shoji M and Murata T: Prolonged survival in pancreatic
cancer patients with increased regucalcin gene expression:
Overexpression of regucalcin suppresses the proliferation in human
pancreatic cancer MIA PaCa-2 cells in vitro. Int J Oncol.
48:1955–1964. 2016.PubMed/NCBI
|
|
25
|
Yamaguchi M, Osuka S, Weitzmann MN, Shoji
M and Murata T: Increased regucalcin gene expression extends
survival in breast cancer patients: Overexpression of regucalcin
suppresses the proliferation and metastatic bone activity in
MDA-MB-231 human breast cancer cells in vitro. Int J Oncol.
49:812–822. 2016.PubMed/NCBI
|
|
26
|
Shangguan H, Tan S-Y and Zhang J-R:
Bioinformatics analysis of gene expression profiles in
hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 19:2054–2061.
2015.PubMed/NCBI
|
|
27
|
Wang H-W, Hsieh TH, Huang SY, Chau GY,
Tung CY, Su CW and Wu JC: Forfeited hepatogenesis program and
increased embryonic stem cell traits in young hepatocellular
carcinoma (HCC) comparing to elderly HCC. BMC Genomics. 14:7362013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uhlen M, Oksvold P, Fagerberg L, Lundberg
E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S,
et al: Towards a knowledge-based Human Protein Atlas. Nat
Biotechnol. 28:1248–1250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoshida Y, Villanueva A, Kobayashi M, Peix
J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, et
al: Gene expression in fixed tissues and outcome in hepatocellular
carcinoma. N Engl J Med. 359:1995–2004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aguirre-Gamboa R, Gomez-Rueda H,
Martínez-Ledesma E, Martínez-Torteya A, Chacolla-Huaringa R,
Rodriguez-Barrientos A, Tamez-Peña JG and Treviño V: SurvExpress:
An online biomarker validation tool and database for cancer gene
expression data using survival analysis. PLoS One. 8:e742502013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakagawa T, Sawada N and Yamaguchi M:
Overexpression of regucalcin suppresses cell proliferation of
cloned normal rat kidney proximal tubular epithelial NRK52E cells.
Int J Mol Med. 16:637–643. 2005.PubMed/NCBI
|
|
34
|
Izumi T and Yamaguchi M: Overexpression of
regucalcin suppresses cell death in cloned rat hepatoma H4-II-E
cells induced by tumor necrosis factor-alpha or thapsigargin. J
Cell Biochem. 92:296–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang C-C, Park AY and Guan J-L: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meijer L, Borgne A, Mulner O, Chong JP,
Blow JJ, Inagaki N, Inagaki M, Delcros JG and Moulinoux JP:
Biochemical and cellular effects of roscovitine, a potent and
selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and
cdk5. Eur J Biochem. 243:527–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Singh SV, Herman-Antosiewicz A, Singh AV,
Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L and Baskaran R:
Sulforaphane-induced G2/M phase cell cycle arrest involves
checkpoint kinase 2-mediated phosphorylation of cell division cycle
25C. J Biol Chem. 279:25813–25822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Serrano-Nascimento C, da Silva Teixeira S,
Nicola JP, Nachbar RT, Masini-Repiso AM and Nunes MT: The acute
inhibitory effect of iodide excess on sodium/iodide symporter
expression and activity involves the PI3K/Akt signaling pathway.
Endocrinology. 155:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen S, Wang Y, Ruan W, Wang X and Pan C:
Reversing multidrug resistance in hepatocellular carcinoma cells by
inhibiting extracellular signal-regulated kinase/mitogen-activated
protein kinase signaling pathway activity. Oncol Lett. 8:2333–2339.
2014.PubMed/NCBI
|
|
40
|
Palangat M, Grass JA, Langelier MF,
Coulombe B and Landick R: The RPB2 flap loop of human RNA
polymerase II is dispensable for transcription initiation and
elongation. Mol Cell Biol. 31:3312–3325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancer. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsurusaki Y and Yamaguchi M: Role of
regucalcin in liver nuclear function: Binding of regucalcin to
nuclear protein or DNA and modulation of tumor-related gene
expression. Int J Mol Med. 14:277–281. 2004.PubMed/NCBI
|
|
43
|
Yamaguchi M and Sakurai T: Inhibitory
effect of calcium-binding protein regucalcin on
Ca2+-activated DNA fragmentation in rat liver nuclei.
FEBS Lett. 279:281–284. 1991. View Article : Google Scholar : PubMed/NCBI
|