Introduction
Hepatocellular carcinoma (HCC) is the second most
common cause of cancer death worldwide, accounting for an estimated
745,000 deaths per year, representing 10% of all deaths from cancer
(1). Major risk factors for HCC
include hepatitis C virus (HCV) or hepatitis B virus (HBV)
infection, alcoholic cirrhosis and nonalcoholic fatty liver
disease. Resection, local ablation or transplantation are effective
treatments for early stage HCC. Transarterial chemoembolization
shows limited success for intermediate stage HCC without invasion
or metastasis (2). However, the
overall survival for advanced HCC is poor due to rapid tumor
progression and metastasis (3).
Therefore, it is necessary to better understand the mechanisms of
HCC metastasis and to develop new therapeutic options for advanced
or recurrent HCC.
It has been shown that expression of miRNAs is
dysregulated in all cancers (4).
miRNAs may play an oncogenic or tumor suppressive role depending on
the type of cancer. miR-199a-3p is a miRNA that displays decreased
expression in HCC (5–8). The genes that encode miR-199a-1 and
miR-199a-2 are located within introns of the DNM2 and DNM3 genes,
respectively. Previous research has shown that miR-199a-3p
regulates expression of c-MET (9,10),
mTOR (9) and PAK4 (6). We previously reported that CD44 is a
target of miR-199a-3p in HCC (11).
CD151 (Tspan24) is a member of the tetraspanin
protein family that have been linked to metastasis (12–14).
CD151 is associated with proMMP7 and proMMP9 transcription which
facilitates matrix degradation and regulates cell migration.
Several studies have demonstrated that CD151 is involved in the
regulation of pathways downstream of the hepatocyte growth factor
(HGF)/c-Met axis (15) and CD151
was remarkably overexpressed in HCC (12). High expression levels of CD151 and
integrin subunit α6 increased invasiveness of HCC cells (14) and overexpression of CD151 promoted
the expression of MMP9, which is one of the key factors in
metastasis through the PI3K/Akt/GSK-3β/Snail pathway (13). Recent studies showed that CD151
expression could be regulated by miRNAs. miR-506 suppressed CD151
in a breast cancer cell line (16)
and miR-124 inhibits invasiveness and metastatic potential of
breast cancer cells by targeting CD151 mRNA (17). In addition, miR-22 reduces cell
proliferation and invasiveness of gastric cancers by suppressing
CD151 (18).
We confirm previous findings that low miR-199a-1
expression is correlated with poor survival in HCC and that
miR-199a-3p is significantly downregulated in HCC (5–8). We
report that CD151 is a direct target of miR-199a-3p and that
reintroduction of miR-199a-3p to HCC cells strikingly suppressed
cell migration and invasion in vitro in part by targeting
CD151.
Materials and methods
Cell line and tissue specimens
The human HCC cell lines SNU-423, SNU-449,
PLC/PRF/5, HepG2, Hep3B and SK-Hep-1, were purchased from American
Type Tissue Collection (Manassas, VA, USA). SNU-423, SNU-449 cells
were cultured in RPMI-1640 medium (Invitrogen) with 10% fetal
bovine serum (Sigma). PLC/PRF/5, Hep3B, HepG2 and SK-Hep-1 were
cultured in MEM medium (Invitrogen) with 10% fetal bovine serum
(Sigma). All cell lines were successfully authenticated by the
Interdisciplinary Center for Biotechnology Research at the
University of Florida (data not shown). Eighteen paired HCC and
adjacent non-tumor liver tissues were collected from patients
during surgical resections at the Mayo Clinic (Rochester, MN, USA),
frozen in liquid nitrogen and stored at −80° until RNA and protein
were extracted. Sample collection conformed to the policies and
practices of the facility's Institutional Review Board. Patient
demographics are presented in Table
I. The TNM classification for hepatocellular carcinoma was used
for tumor stage and grade.
 | Table IPatient data. |
Table I
Patient data.
| Patient | Gender | Age | Grade | Stage | Tumor size (cm)
(largest mass) | Cirrhosis | Fibrosis stage | Etiology |
|---|
| 10 | F | 62.5 | 2 | IV | 22 | No | 0 | No known risk
factors |
| 14 | M | 89.1 | 2 | IV | 6.5 | No | 0 | Hereditary
hemochromatosis |
| 26 | M | 43.3 | 1 | I | | No | 0 | No known risk
factors |
| 33 | F | 43.2 | 2 | I | | Yes | 4 | HCV |
| 44 | F | 48.0 | 1 | I | | No | 0 | No known risk
factors |
| 69 | F | 85.0 | 1 | I | | No | 0 | No known risk
factors |
| 92 | M | 76.2 | 2 | II | | Yes | 4 | Autoimmune
hepatitis |
| 94 | F | 82.7 | 3 | II | | No | 0 | No known risk
factors |
| 97 | F | 50.8 | 2 | I | 10.5 | No | 0 | No known risk
factors |
| 99 | F | 83.3 | 3 | I | | Yes | 4 | HCV |
| 103 | F | 84.7 | 2 | II | 8 | No | 0 | Alcohol |
| 106 | M | 59.9 | 2 | I | | No | 0 | Alcohol |
| 109 | M | 80.3 | 2 | I | 6 | No | 0 | No known risk
factors |
| 306 | M | 72.3 | 2 | II | 4.3 | Yes | 4 | No known risk
factors |
| 309 | M | 81.2 | 3 | I | 5 | No | 0 | HCV probable remote
history HCB |
| 337 | M | 80.7 | 3 | II | 14.4 | Yes | 4 | Alcohol |
| 361 | M | 71.0 | 3 | I | 10.8 | No | 0 | No known risk
factors |
| 367 | M | 71.5 | 2 | II | 4 | No | 2 | HCB |
Transfection of microRNA mimic and siRNA
oligonucleotides
HCC cell lines were transfected either with 100 nM
of hsa-miR-199a-3p mimic or negative control (Ambion), or with 100
nM of CD151 siRNA or control siRNA (Thermo Scientific) using
Lipofectamine 2000 (Invitrogen) and Opti-MEM medium (Invitrogen).
Cells were transfected with the miRNA mimic or siRNA
oligonucleotides for 72 h prior to extraction of RNA or
protein.
RNA extraction, cDNA synthesis and
qRT-PCR
Total RNA was extracted from 18 pairs of HCC tumors
and adjacent benign liver tissue specimens. Following pulverization
in a cold mortar and pestle, total RNA was isolated from the
tissues using TRIzol reagent (Life Technologies). cDNA was
synthesized as previously described (19). Five hundred nanograms of total RNA
was used to synthesize cDNA using random primers. cDNA was analyzed
for gene expression using gene specific primers (IDT) and the
Express SYBR® GreenER qPCR super mix (Invitrogen). For
the miRNA expression analysis, cDNA primed with 100 ng of total RNA
was assayed using the TaqMan® microRNA assays (Applied
Biosystems) as described (20).
Data were normalized to 18S rRNA and the relative expression of
genes was presented using the comparative CT method.
Data were multiplied by 106 to simplify presentation.
Primer sequences are available upon request.
Dual-luciferase reporter gene assay
The full length CD151 3′UTR was cloned into the
psiCHECK-2 Vector (Promega). Three nucleotides in the binding
sequences of CD151 3′UTR was mutated by QuikChange XL Site-Directed
Mutagenesis kit (Agilent Technologies) and the mutation was
confirmed by sequencing. The luciferase reporter gene assay was
performed using the Dual-luciferase reporter assay system (Promega)
according to the manufacturer's instructions. SNU-449 cells were
plated at a density of 50,000 cells/well (24-well). Following a
24-h incubation, cells were co-transfected with miR-199a-3p mimic
or negative control oligonucleotide (50 nM) along with the WT-CD151
3′UTR or mutated CD151 3′UTR constructs using Lipofectamine 2000
(Invitrogen). Reporter gene assays were performed 48 h
post-transfection using the Dual luciferase assay system (Promega).
Renilla luciferase activity was normalized for transfection
efficiency using the corresponding Firefly luciferase activity. All
experiments were performed at least three times.
Cell proliferation assay
PLC/PRF/5, SK-Hep-1, SNU-423 and SNU-449 cells were
seeded at a density of 2,000 cells per well in 96-well culture
plates. Following a 24-h incubation period, cells were transfected
with 100 nM of CD151 siRNA or control siRNA oligonucleotides. Cell
proliferation was determined 96 h later using the WST-1 reagent
(Roche) per the manufacturer's recommendations. All experiments
were performed at least in triplicate.
Cell migration assay
A wound healing assay was performed to evaluate cell
migration in vitro. SNU-449 cells were transfected with
either 100 nM of miR-199a-3p mimic or negative control
oligonucleotides, or 100 nM of CD151 siRNA or control siRNA.
Twenty-four hours after transfection, 70 μl of the transfected
cells (3×105 cells/ml) was placed into each well of an
ibidi culture-insert (ibidi, LLC). After an overnight incubation,
the culture insert was removed to create a cell-free gap in the
monolayer. The gap closure area was photographed and analyzed by
TScratch software (21). The
percentage of the gap area closed between the beginning and end of
the experiment was calculated from at least three independent
experiments.
Matrigel invasion assay
In vitro cell invasion assays were conducted
using the CytoSelect™ 24-well cell invasion assay kit (8-μm pore
size, Cell Biolabs, Inc.). SNU-449 cells were transfected with
either 100 nM of miR-199a-3p mimic or negative control
oligonucleotides, or 100 nM of CD151 siRNA or control siRNA.
Forty-eight hours after transfection, the transfected cells were
placed into the upper chamber at a density of 1.5×105
cells per well in 1% FBS containing medium. Ten percent FBS
containing medium was placed in the lower chamber as a
chemoattractant. Cells were incubated at 37°C for 24 h and the
cells that invaded the membrane were fixed and stained. The number
of cells invading the membrane were counted in three different
fields per experiment.
Protein extraction and
immunoblotting
Cell protein lysates in RIPA buffer (Sigma) were
separated on NuPAGE 4–12% Bis-Tris gels (Novex) and
electrophoretically transferred to polyvinylidene difluoride
membranes (Roche). The blotting was performed for CD151 (ab33315,
Abcam). β-actin (Abcam) or GAPDH (sc-32233, Santa Cruz
Biotechnology) were used as loading controls. Secondary horseradish
peroxidase antibody was detected using the ECL Western Blotting
Analysis system (Amersham Biosciences).
Statistical analysis
The matched samples were compared using paired
t-tests and samples subjected to different treatments were compared
using a Student's 2-sample t-tests. A p<0.05 was considered
significant. The Cancer Genome Atlas (TCGA) microRNA-seq expression
data and patients' clinical information (n=141) were downloaded
through the TCGA data portal. Patients were dichotomized into two
groups (high and low) according to the median expression of
miR-199-1 or miR-199-2. The probabilities of 5-year survival
between groups were compared by using the Kaplan-Meier method and
log-rank test. Data analysis was performed using SAS 9.4 (SAS, Inc;
Cary, NC, USA). For the analysis of miR-199-1 expression in paired
benign and HCC, data from 49 pairs of benign and HCC were used.
This represents all of the paired specimen data for HCC on the TCGA
data portal.
Results
CD151 is a target of miR-199a-3p in
HCC
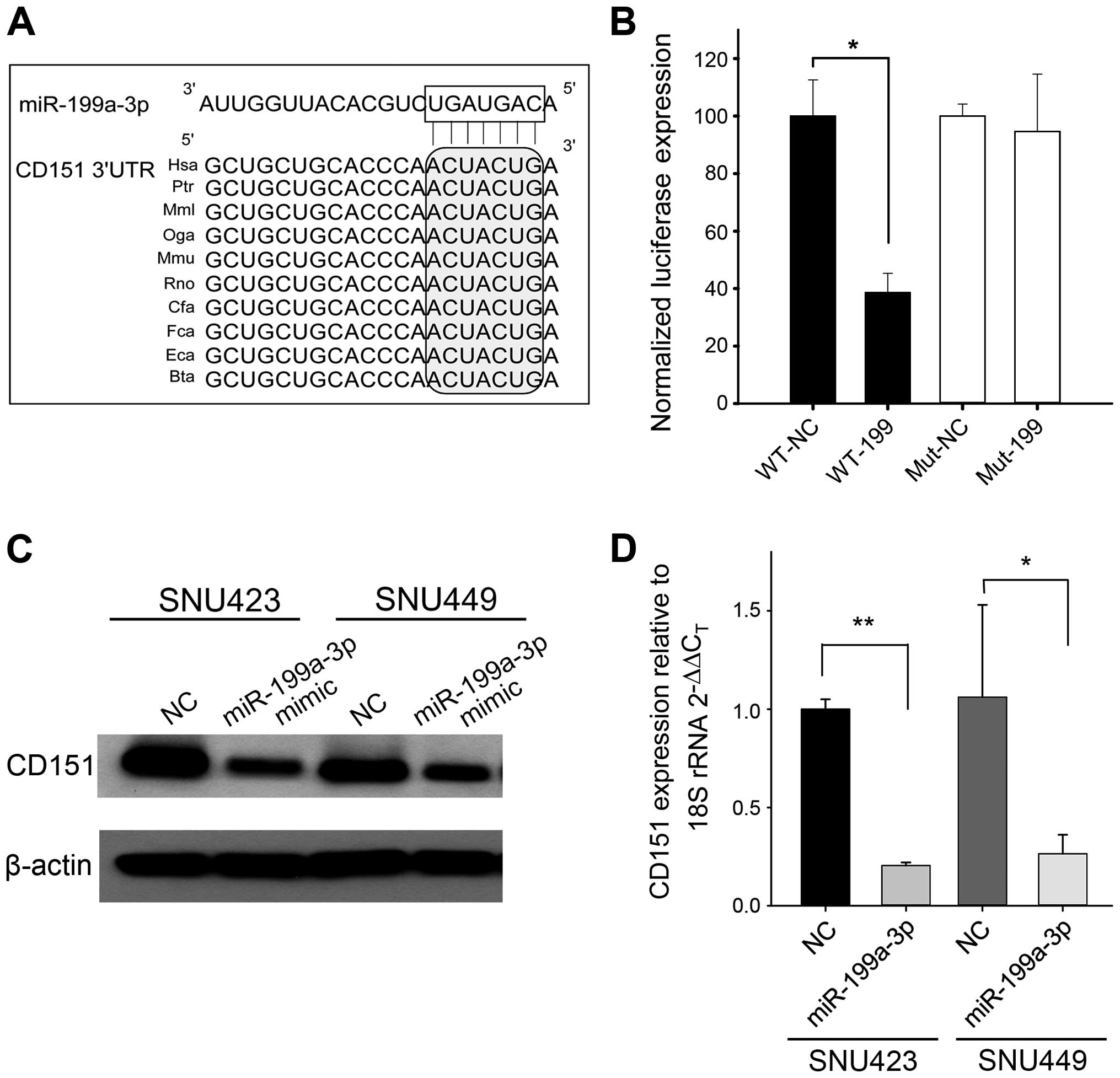
To better understand the role of miR-199a-3p in HCC,
we searched potential miR-199a-3p targets using the TargetScan
algorithm. A putative miR-199a-3p binding site within the CD151
3′UTR was highly conserved (Fig.
1A). A luciferase reporter assay was used to confirm the
binding of miR-199a-3p to the CD151 3′UTR. Luciferase expression
was reduced by >50% by miR-199a-3p mimic (Fig. 1B). The luciferase expression was
not reduced substantially when the miR-199a-3p binding site on the
CD151 3′UTR was mutated (Fig. 1B).
To further investigate if miR-199a-3p functionally regulates CD151,
SNU-423 and SNU-449 cells were transfected with miR-199a-3p mimic
or negative control oligonucleotides. Western blotting experiments
showed that the protein expression of CD151 was reduced by 60% in
SNU-423 and by 43% in SNU-449 cells compared to control (Fig. 1C). qRT-PCR showed that miR-199a-3p
mimic reduced CD151 mRNA in these cell lines (Fig. 1D). Together these data indicate
that CD151 is a direct target of miR-199a-3p and that the
miR-199a-3p binding enhances CD151 mRNA degradation.
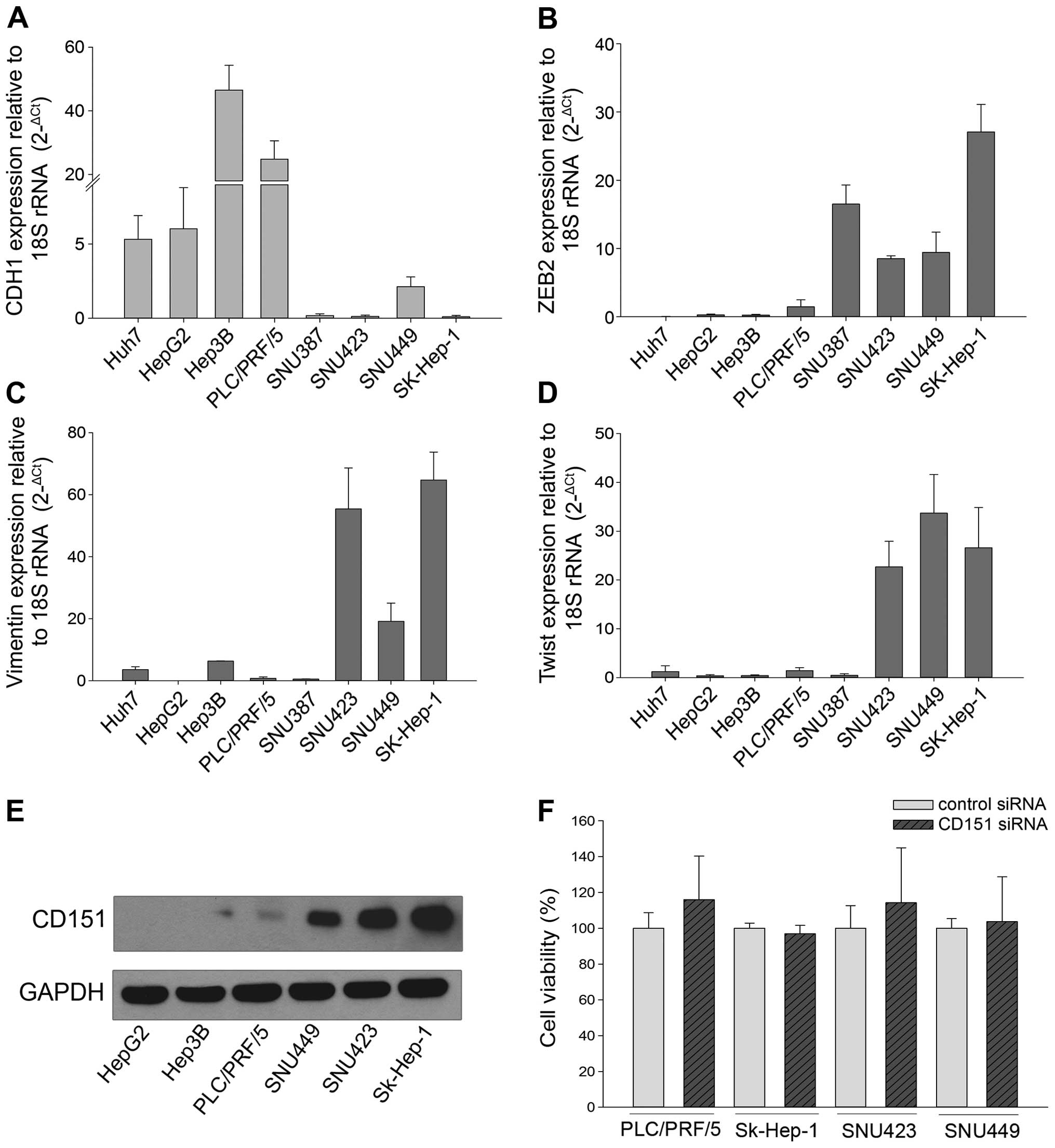
Attenuation of CD151 does not reduce HCC
proliferation
CD151 protein as well as several
epithelial-mesenchymal transition (EMT) markers were examined in
HCC cell lines. HepG2, Hep3B and PLC/PRF/5 express high levels of
CDH1, an epithelial marker whereas SNU-449, SNU-423 and SK-Hep-1
express high levels of the mesenchymal markers VIM, ZEB2 and TWIST
(Fig. 2A–D). Interestingly, CD151
protein was overexpressed in the mesenchymal-like cell lines
(SNU-449, SNU-423 and SK-Hep-1) compared to cell lines expressing
epithelial genes (HepG2, Hep3B and PLC/PRF/5) (Fig. 2E), suggesting CD151 is associated
with the mesenchymal phenotype. To determine if a direct
relationship exists between mesenchymal markers and CD151, the
expression of epithelial (CDH1) and mesenchymal (VIM, CDH2 and
ZEB1) was measured in SNU-423 cells following siRNA knockdown of
CD151. Knockdown of CD151 by ≤80% did not significantly alter the
expression of CDH1, VIM, CDH2 or ZEB1 (data not shown).
To determine if CD151 regulates cell proliferation,
we transfected four different HCC cell lines with CD151 or control
siRNA. Knockdown of CD151 by >80% failed to reduce cell
proliferation in either CD151-negative (PLC/PRF/5) or
CD151-positive (SK-Hep-1, SNU-423 and SNU-449) cells (Fig. 2F). These results suggest that CD151
is not involved in regulating HCC cellular proliferation.
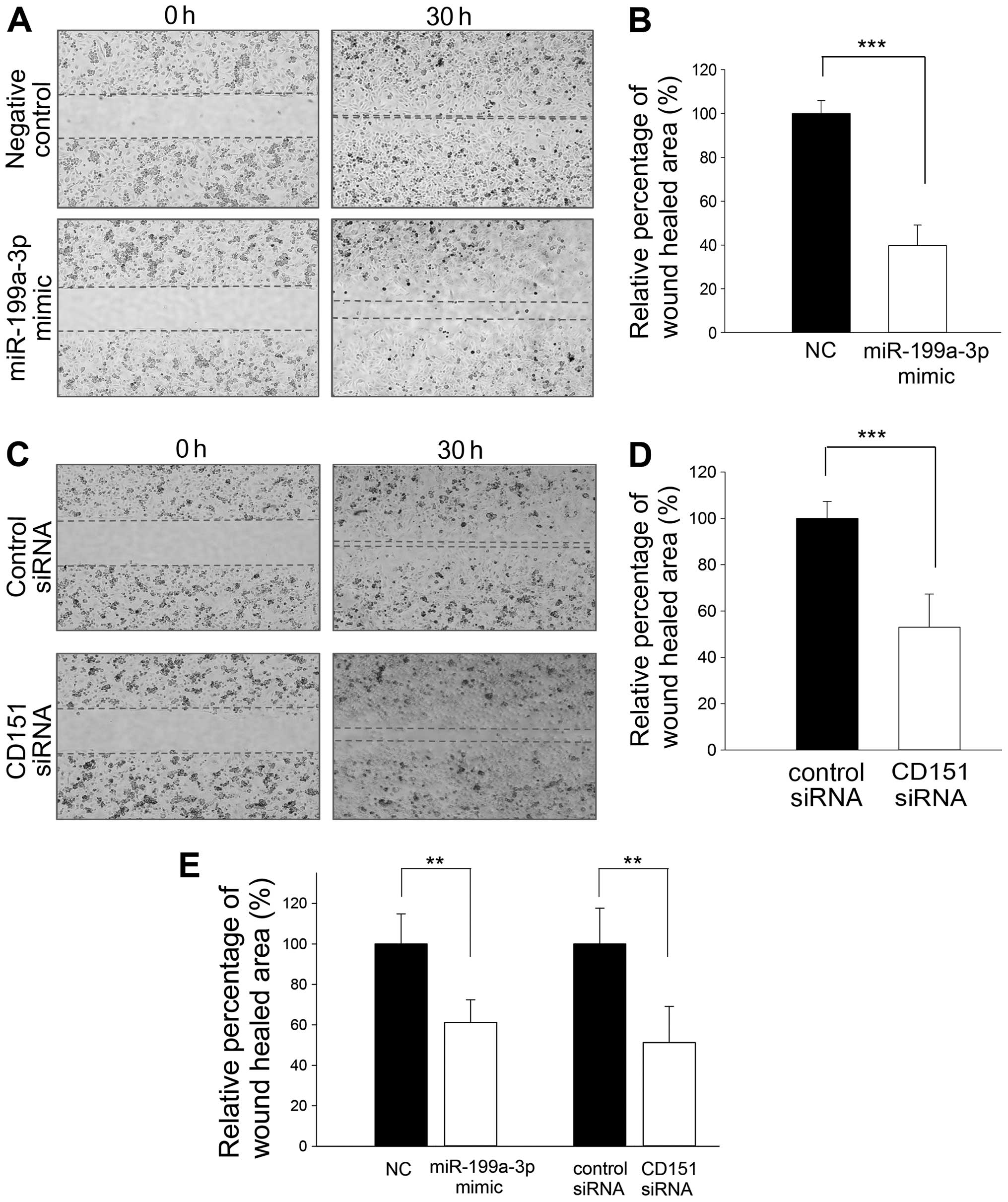
In vitro cell migration and invasion is
inhibited by miR-199a-3p through targeting CD151
Next we determined whether miR-199a-3p mimic could
inhibit in vitro cell migration and invasion under
conditions of CD151 suppression. CD151-positive SNU-449 and SNU-423
cells were transfected with miR-199a-3p mimic under the identical
conditions shown to suppress CD151 mRNA and protein expression. As
a control, wound healing and invasion assays were performed
following transfection with CD151 or control siRNA. Compared to
control oligonucleotide, wound healing was decreased in SNU-449
cells after transfection of miR-199a-3p mimic (Fig. 3A and B). Wound healing was also
significantly reduced after CD151 siRNA transfection compared to
control siRNA (Fig. 3C and D). We
also repeated the wound healing experiment in a second cell line
(SNU-423 cells) with similar results (Fig. 3E). In addition, the number of
invading SNU-449 cells was strikingly reduced after transfection
with miR-199a-3p mimic (Fig. 4A and
B). Cell invasiveness was also significantly suppressed after
CD151 siRNA transfection (Fig. 4C and
D). The results were reproduced in SNU-423 cells (Fig. 4E). These data suggest that
suppression of CD151 expression by miR-199a-3p mimic can reduce
cell migration and invasion in vitro.
miR-199a-3p and CD151 expression
inversely correlates in HCC specimens
The expression of miR-199a-3p was measured in 18
pairs of human HCC tissues and adjacent benign tissues by qRT-PCR.
miR-199a-3p was significantly downregulated in the HCC tissues
compared to the adjacent benign liver (p<0.0001, paired t-test,
Fig. 5A), confirming previous
results (5–8). We also evaluated the expression of
miR-199a-1 and miR-199a-2 in 49 pairs of tumor and adjacent benign
from the TCGA data set. miR-199a-1 and miR-199a-2 are two isogenic
genes encoding miR-199a-3p. The expression of miR-199a-1 (Fig. 5B, p<0.0001) and miR-199a-2
(p<0.0001, not shown) was reduced in the tumor compared to the
adjacent benign tissue. Next, we investigated the correlation
between miR-199a gene expression and survival by analyzing data
from the TCGA database. HCC patients with high miR-199a-1
expression had better survival compared to those with low
miR-199a-1 levels (p<0.05, Fig.
5C). While there was good separation between survival and
miR-199a-2 expression, the correlation was not significant (p=0.3,
data not shown).
CD151 mRNA (qRT-PCR) and protein (western blotting)
were also examined in paired specimens of HCC and adjacent benign
liver. CD151 mRNA was upregulated in HCC tissues compared to paired
benign tissues (p<0.001, paired t-test, Fig. 6A). Expression of CD151 was also
increased in the paired HCC tissues from the TCGA data (data not
shown). Moreover, CD151 protein expression was strongly
overexpressed in HCC tissues (Fig.
6B). Finally, using Pearson correlation analysis, we found a
strong inverse correlation between CD151 mRNA and miR-199a-3p
expression in HCC (Fig. 6C).
Discussion
Several studies have reported reduced expression of
miR-199a in HCC (6–8,22,23).
We confirm these results in a new cohort of HCC patients (Fig. 5A) and in the TCGA data set
(Fig. 5B). We show that low
expression of miR-199a-1 (Fig. 5C)
correlates with poor survival in HCC, confirming the data of
Fornari et al (9).
Furthermore, we report that miR-199a-3p regulates CD151. CD151 is a
tetraspanin protein family member and has been implicated in HCC
invasion and migration (12–14).
The role of CD151 in HCC invasion and metastasis is believed to
rely on its ability to form complexes with laminin-binding integrin
receptors (α6β1, α6β4 and α3β1) (14,24)
as well as regulate cell-cell and cell-matrix interactions
(25). CD151 was previously
associated with the mesenchymal phenotype in HCC (14). Knockdown of CD151 in CD151-positive
HCCLM3 cells, or overexpression of CD151 in CD151-negative HepG2
cells, altered the mesenchymal phenotype when these cells were
cultured along with laminin 5 (14). Induction of EMT occurred through
hyperactivation of the PI3K-Akt-Snail and PTEN feedback pathway
(14). Successful knockdown of
CD151 did not alter the expression of EMT markers, however our
cells were not cultured along with laminin 5 as previously shown
(14).
We report that CD151 mRNA and protein are increased
in HCC patients. Liver cirrhosis is one predisposing factor to HCC.
We compared the expression of miR-199a-3p and CD151 in cirrhotic
and non-cirrhotic HCC patients to determine if a relationship
exists. Thirty-six percent (%) of the patients in our data set had
cirrhosis (Table I), however there
was no correlation between CD151 or miR-199a-3p and cirrhosis (data
not shown). Also no correlation existed between miR-199a-3p and
CD151 in the TCGA data set (data not shown). The regulation of
CD151 by miR-199a-3p was further confirmed in HCC patients by the
strong negative correlation between these two RNAs (Fig. 6C). While validating additional,
putative target genes was beyond the scope of this study, it is of
interest to note that miRBase predicts miR-199a-3p to regulate both
integrin α6 (ITGA6) as well as integrin α3 (ITGA3). Thus,
miR-199a-3p may play a key role in regulating two of the three
factors involved in the CD151, integrin α3/6 and laminin 5
interaction, a complex that was reported to be critical for
invasion and metastasis in HCC (14).
In addition to CD151, miR-199a-3p has been shown to
regulate other tumor and metastasis promoting genes in HCC
including c-met (9,10), mTOR (9) and CD44 (11). The reduced invasion and migration
reported cannot be accounted solely by miR-199a-3p suppressing
CD151 and the miRNA is likely suppressing known (i.e., c-met, mTOR
and CD44) as well as unknown metastasis-related target genes. Since
reduced miR-199a-3p expression in HCC would result in increased
levels of these cancer promoting genes, it is conceivable that
treating advanced HCC using miR-199a-3p oligo mimics could be used
as a treatment for a disease that has very few treatment options.
Treating HCC with miRNA mimics has great potential as it is well
known that oligonucleotides accumulate in highly perfused organs
such as the liver (26). In fact,
miR-34 mimic is currently in phase I trial for the treatment of HCC
(clinicaltrials.gov).
We previously showed that miR-199a-3p reduced the
proliferation of CD44-positive HCC cell but not in CD44-negative
HCC cells by reducing CD44 protein levels (11). We report here that miR-199a-3p
regulation of CD151 results in decreased invasion and migration,
but CD151 does not directly regulate cell growth since siRNA
knockdown of CD151 did not affect the proliferation of HCC cells.
While CD44 is known to regulate cell adhesion and cell-cell
interactions, it is also a well-known marker of stemness. In
addition to its role in regulating metastasis-related mRNAs,
miR-199a-3p also regulates HCV (27) and HBV replication (28). These findings, coupled with those
reported herein, emphasize the critical role that a single
deregulated miRNA may have on the cancer phenotype. Reduced
miR-199a-3p could influence oncogenesis at various stages of
development. Increased HCV and HBV replication may occur early on
by reduced miR-199a-3p (28)
followed by promoting proliferation in a CD44-dependent manner and
increasing invasion and metastasis at later stages.
In conclusion, we showed that CD151 is involved in
regulation of in vitro invasion and migration but not
proliferation of HCC cell lines. miR-199a-3p, a miRNA that is
significantly reduced in HCC, directly targets CD151. These data
further implicate miR-199a-3p in the progression of HCC and
suggests that oligonucleotide therapy using a miR-199a-3p mimic may
be effective for treating advanced HCC.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Raoul JL, Sangro B, Forner A, Mazzaferro
V, Piscaglia F, Bolondi L and Lencioni R: Evolving strategies for
the management of intermediate-stage hepatocellular carcinoma:
Available evidence and expert opinion on the use of transarterial
chemoembolization. Cancer Treat Rev. 37:212–220. 2011. View Article : Google Scholar
|
|
3
|
Aravalli RN, Steer CJ and Cressman EN:
Molecular mechanisms of hepatocellular carcinoma. Hepatology.
48:2047–2063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gramantieri L, Ferracin M, Fornari F,
Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E,
Grazi GL, et al: Cyclin G1 is a target of miR-122a, a microRNA
frequently down-regulated in human hepatocellular carcinoma. Cancer
Res. 67:6092–6099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong
Q, Qin L, Wu X, Zheng Y, Yang Y, et al: Identification of miRNomes
in human liver and hepatocellular carcinoma reveals miR-199a/b-3p
as therapeutic target for hepatocellular carcinoma. Cancer Cell.
19:232–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang J, Gusev Y, Aderca I, Mettler TA,
Nagorney DM, Brackett DJ, Roberts LR and Schmittgen TD: Association
of MicroRNA expression in hepatocellular carcinomas with hepatitis
infection, cirrhosis, and patient survival. Clin Cancer Res.
14:419–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murakami Y, Yasuda T, Saigo K, Urashima T,
Toyoda H, Okanoue T and Shimotohno K: Comprehensive analysis of
microRNA expression patterns in hepatocellular carcinoma and
non-tumorous tissues. Oncogene. 25:2537–2545. 2006. View Article : Google Scholar
|
|
9
|
Fornari F, Milazzo M, Chieco P, Negrini M,
Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L and Gramantieri
L: MiR-199a-3p regulates mTOR and c-Met to influence the
doxo-rubicin sensitivity of human hepatocarcinoma cells. Cancer
Res. 70:5184–5193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim S, Lee UJ, Kim MN, Lee EJ, Kim JY, Lee
MY, Choung S, Kim YJ and Choi YC: MicroRNA miR-199a*
regulates the MET proto-oncogene and the downstream extracellular
signal-regulated kinase 2 (ERK2). J Biol Chem. 283:18158–18166.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henry JC, Park JK, Jiang J, Kim JH,
Nagorney DM, Roberts LR, Banerjee S and Schmittgen TD: miR-199a-3p
targets CD44 and reduces proliferation of CD44 positive
hepatocellular carcinoma cell lines. Biochem Biophys Res Commun.
403:120–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu
MY, Xu Y, Song ZJ, Wang ZJ, Wu JC, et al: Role of overexpression of
CD151 and/or c-Met in predicting prognosis of hepatocellular
carcinoma. Hepatology. 49:491–503. 2009. View Article : Google Scholar
|
|
13
|
Shi GM, Ke AW, Zhou J, Wang XY, Xu Y, Ding
ZB, Devbhandari RP, Huang XY, Qiu SJ, Shi YH, et al: CD151
modulates expression of matrix metalloproteinase 9 and promotes
neoangiogenesis and progression of hepatocellular carcinoma.
Hepatology. 52:183–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ke AW, Shi GM, Zhou J, Huang XY, Shi YH,
Ding ZB, Wang XY, Devbhandari RP and Fan J: CD151 amplifies
signaling by integrin alpha6beta1 to PI3K and induces the
epithelial-mesenchymal transition in HCC cells. Gastroenterology.
140:1629–1641. e152011. View Article : Google Scholar
|
|
15
|
Klosek SK, Nakashiro K, Hara S, Shintani
S, Hasegawa H and Hamakawa H: CD151 forms a functional complex with
c-Met in human salivary gland cancer cells. Biochem Biophys Res
Commun. 336:408–416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arora H, Qureshi R and Park WY: miR-506
regulates epithelial mesenchymal transition in breast cancer cell
lines. PLoS One. 8:e642732013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han ZB, Yang Z, Chi Y, Zhang L, Wang Y, Ji
Y, Wang J, Zhao H and Han ZC: MicroRNA-124 suppresses breast cancer
cell growth and motility by targeting CD151. Cell Physiol Biochem.
31:823–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Yu H, Lu X, Zhang P, Wang M and Hu
Y: MiR-22 suppresses the proliferation and invasion of gastric
cancer cells by inhibiting CD151. Biochem Biophys Res Commun.
445:175–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tirmenstein MA, Nicholls-Grzemski FA,
Schmittgen TD, Zakrajsek BA and Fariss MW: Characterization of
nitric oxide production following isolation of rat hepatocytes.
Toxicol Sci. 53:56–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gebäck T, Schulz MM, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009.PubMed/NCBI
|
|
22
|
Duan Q, Wang X, Gong W, Ni L, Chen C, He
X, Chen F, Yang L, Wang P and Wang DW: ER stress negatively
modulates the expression of the miR-199a/214 cluster to regulates
tumor survival and progression in human hepatocellular cancer. PLoS
One. 7:e315182012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi KQ, Lin Z, Chen XJ, Song M, Wang YQ,
Cai YJ, Yang NB, Zheng MH, Dong JZ, Zhang L, et al: Hepatocellular
carcinoma associated microRNA expression signature: Integrated
bioinformatics analysis, experimental validation and clinical
significance. Oncotarget. 6:25093–25108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sadej R, Grudowska A, Turczyk L, Kordek R
and Romanska HM: CD151 in cancer progression and metastasis: A
complex scenario. Lab Invest. 94:41–51. 2014. View Article : Google Scholar
|
|
25
|
Johnson JL, Winterwood N, DeMali KA and
Stipp CS: Tetraspanin CD151 regulates RhoA activation and the
dynamic stability of carcinoma cell-cell contacts. J Cell Sci.
122:2263–2273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
DeLong RK, Nolting A, Fisher M, Chen Q,
Wickstrom E, Kligshteyn M, Demirdji S, Caruthers M and Juliano RL:
Comparative pharmacokinetics, tissue distribution, and tumor
accumulation of phosphorothioate, phosphorodithioate, and
methylphosphonate oligonucleotides in nude mice. Antisense Nucleic
Acid Drug Dev. 7:71–77. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murakami Y, Aly HH, Tajima A, Inoue I and
Shimotohno K: Regulation of the hepatitis C virus genome
replication by miR-199a. J Hepatol. 50:453–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang GL, Li YX, Zheng SQ, Liu M, Li X and
Tang H: Suppression of hepatitis B virus replication by
microRNA-199a-3p and microRNA-210. Antiviral Res. 88:169–175. 2010.
View Article : Google Scholar : PubMed/NCBI
|