Introduction
Ovarian cancer is one of the common gynecologic
cancers. Several studies demonstrate that accumulation of genetic
alteration causes aberrant gene expression and is associated with
cancer progression (1). Genetic
alteration contributes to the ovarian cancer progression and is
associated with the ovarian cancer incidence (2,3). The
alteration of tumor suppressor gene or oncogene, such as BRCA1 and
NDN, was observed in ovarian cancer cells (4,5).
CD90, also called as Thy-1, is a glycoprotein and
mediates the T cell activation and neurite growth (6,7).
CD90 is enriched in activated endothelial cells and is attracted to
melanoma cells by the integrin and syndecan (8–10).
CD90 has been defined as a marker for cancer stem cell (CSC) in
gastric, lung, esophageal and liver cancer. CD90+
gastric cancer stem cells possess the highest tumor initiation
ability and inhibition of ERBB2 signaling by trastuzumab reducing
tumorigenicity in vivo (11). Identification of lung cancer stem
cell characterized by CD90 phenotype that contributes to the higher
cell proliferation and stem cell marker Sox2 and Oct4 expression
was demonstrated in A549 and H446 lung cancer cell lines (12). CD90-positive tumor-initiating cell
population has aggressive tumor progression in esophageal cancer
(13). The CD90+ cells
isolated from liver cancer cell lines and clinical tumor samples
are responsible for tumor formation (14). Compared to the
CD90−CXCR4+ liver cancer cells, the
CD90+CXCR4+ cells have higher stemness
properties of sphere-forming ability and promote cancer metastasis
(15). Additionally, the
expression of stem cell markers, including CD133, CD90 and EpCAM,
are increased in the hepatocellular carcinoma tissues with bile
duct tumor thrombi compared to the tissues without bile duct tumor
thrombi (16). In our previous
study, CD90 was shown to play an oncogenic role in liver cancer
progression via the signal axis of CD90-integrin-AMPK-CD133 and
targeting CD90 and its downstream molecules may be employed as
therapeutic targets (17).
Notably, CD90 was decreased in nasopharyngeal carcinoma cell lines
and metastatic tumor tissues. CD90 has been demonstrated to
suppress myofibroblastic differentiation and the idiopathic
pulmonary fibrosis tissue harbors the highly epigenetic changes of
CD90 in fibroblastic foci (18).
Furthermore, the expression of CD90 is not detected in ovarian
cancer cells with tumorigenic ability and the introduction of CD90
into the tumorigenic clone reduces the tumor formation (19,20).
However, the mechanism by which CD90 inhibits ovarian cancer cell
growth is still largely unclear.
A previous study demonstrated that CD90 inhibits the
growth of astrocytes, which provides nutrition and structural
support to nerve cells. In contrast, CD90 does not affect the
growth of Schwann cells, which is the component of myelin sheath
(21). These data indicate that
bidirectional signal transduction attributes to the interaction
between neuron and microglia. Integrin family plays important roles
in functions and signals of CD90 in various cells. CD90 interacts
with β3 or αvβ3 integrin, thereby regulating the signaling
transduction in astrocyte and neuronal cells. The trans
interaction between CD90 on neuronal cells and integrin on
astrocyte contributes to cell adhesion (22). The CAD neuron-like cell
co-incubated with integrin αvβ3-Fc fusion protein has reduced
neurite extension and the αvβ3 integrin-derived growth inhibition
is triggered by the interaction between CD90 and Src (23). The cis interaction between
CD90 and αvβ5 integrin are reported to prevent the activation of
TGF-β1 and myofibro-blast differentiation (24), which in turn, is a promoter of
cancer formation. Our previous study indicates that there is a
cis interaction between CD90 and β3 integrin in liver cancer
and the cis interaction between these two molecules is
required for the CD90-induced CD133 upregulation. Although CD90 has
been demonstrated to play a potential tumor suppressor role in
ovarian cancer, the mechanism by which CD90 inhibits ovarian cancer
cell growth is unclear. The present study aimed to determine the
relationship between integrin and CD90 in ovarian cancer cells and
determine whether CD90 plays a tumor suppressor role in ovarian
cancer cells via β3 integrin.
Our analyses from online database reveal that CD90
is downregulated in ovarian cancer tissues and the gene copy number
of CD90 is correlated with the mRNA expression. Overexpression of
CD90 decreases the anchorage-independent growth in vitro and
tumor formation in vivo. The expression of CD90 promotes
anoikis and inhibits stemness properties. Furthermore, CD90 reduces
the CD133 expression, and the reduction of CD133 and
anchorage-independent growth ability are rescued by the β3 integrin
shRNA.
Materials and methods
Cell culture
The SKOV3 cells and stable transfectants expressing
wild-type or mutant CD90 were maintained in Dulbecco's minimum
essential medium (DMEM; HyClone Laboratories, Logan, UT, USA)
consisting of 10% fetal bovine serum (FBS), 100 units/ml penicillin
and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C
in 5% CO2.
Oncomine database analysis
The mRNA expression of CD90 in ovarian cancer was
identified from Oncomine database as the criteria of P-value
<1E-4, fold change >3, and gene ranking in the top 10%
(https://www.oncomine.org/resource/login.html)
(25,26). The CD90 mRNA expression was
identified from Yoshihara ovarian dataset.
Kaplan-Meier plotter database
analysis
The correlation between CD90 expression and
post-progression survival in ovarian cancer was analyzed by
Kaplan-Meier plotter database (http://kmplot.com/analysis/) (27). The hazard ratio with 95% confidence
intervals and log-rank P-value was also computed.
cBioPortal database analysis
The correlation between DNA copy number and mRNA
expression of CD90 in ovarian cancer is identified by cBioPortal
database (http://www.cbio-portal.org/)
(28,29).
Establishment of CD90 transfectants
SKOV3 cells were transfected with CD90 expression
vector using TurboFect (Thermo Fisher Scientific, Waltham, MA, USA)
according to the manufacturer's instruction and the resistant
transfected cells were selected with G418 at the concentration of 1
mg/ml. The expression of CD90 in SKOV3 stable transfectants was
determined by RT-PCR and flow cytometry.
Flow cytometry
The trypsinized transfectants were incubated with
anti-CD90-PE antibody (eBioscience, Inc., San Diego, CA, USA) on
ice for 20 min and are resuspended in phosphate-buffered saline
containing 1% BSA and 200 nM EDTA. The cells were analyzed using a
FACSCalibur (BD Biosciences, San Jose, CA, USA).
MTT assay
The cell proliferation of the transfectants was
analyzed by MTT assay. The cells were seeded into the 24-well
plates at the density of 2×104/well. The MTT reagent was
incubated for 4 h and the cell viability was determined at the
absorption wavelength of 590 nm.
Colony formation assay
Colony formation was performed by seeding cells at
concentrations of 5×102/well in 6-well plates. The
colonies were stained with 2% methylene blue and counted after
incubation for 10 days.
Soft agar assay
The anchorage-independent growth ability of the
transfectants was analyzed by soft agar colony assay. Five thousand
cells were prepared in 1 ml of 0.3% agar in DMEM supplemented with
10% FBS and layered onto 1.5 ml 0.6% agar in DMEM containing 10%
FBS. The colonies were incubated for 3 weeks and stained with 0.2%
crystal violet.
Tumorigenicity in NOD/SCID mice
The NOD/SCID mice were obtained from the Animal
Center of the National Cheng Kung University (Tainan, Taiwan) and
the experiments were approved by the Institutional Animal Care and
Use Committee of NCKU. The stable transfectants were injected
subcutaneously into NOD/SCID mice at the density of
1×107.
Anoikis assay
Anoikis was analyzed by plating the cells at the
density of 5×105 into the polyhema-coated dish for 24 h.
The cells were harvested and the apoptosis was confirmed by Annexin
V and PI staining according to the manufacturer's instruction.
Western blot analysis
Antibodies against CD133 (Abcam, Cambridge, UK),
CD44 (Abcam), EpCAM (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), mTOR (Epitomics, Burlingame, CA, USA), CD24 (Epitomics), CD13
(Epitomics), PARP (Cell Signaling Technology, Beverly, MA, USA),
phosphor-AMPK (Cell Signaling Technology), AMPK (Cell Signaling
Technology) and β-actin (Chemicon, Inc., Pittsburgh, PA, USA) were
used in western blotting. Cell extracts were harvested in RIPA
lysis buffer and the protein concentration was determined with
Micro BCA™ protein assay kit (Millipore, Billerica, MA, USA).
Protein extracts (35 μg) were fractionated by SDS-PAGE and
transferred to polyvinylidene fluoride membranes (Amersham
Biosciences, Piscataway, NJ, USA) using a transfer apparatus
according to the manufacturer's instruction (Hoefer Pharmacia
Biotech, San Francisco, CA, USA). After incubation with 5% non-fat
milk, the membranes were incubated with specific primary antibody
and horseradish peroxidase-conjugated secondary antibody. The
membranes were then probed with ECL western blotting detection
system (Millipore) and visualized with the BioSpectrum AC imaging
system.
Sphere formation assay
The 5×103 transfectants were plated in
the ultra-low attachment plates (Corning Incorporated, Corning, NY,
USA) in DMEM consisting 50 ng/ml HGF, 50 ng/ml EGF, 1% bovine serum
albumin and 1% FBS. The cells were incubated for 14 days and then
were counted under light microscope.
ALDH assay
ALDH activity was performed with the
Aldefluor® kit (StemCell Technologies, Durham, NC, USA)
according to the manufacturer's instructions. The cells were
suspended in the Aldefluor assay buffer containing ALDH substrate,
with or without the ALDH inhibitor (diethylami-nobenzaldehyde,
DEAB), and were followed by incubation at 37°C for 30 min. The ALDH
activity was detected with the FACSCalibur.
Quantitative real-time reverse
transcription-PCR
Total RNA was extracted with TRIzol (MDBio, Taipei,
Taiwan) and the cDNA was synthesized using M-MLV transcriptase
(Promega, Madison, MI, USA) according to the manufacturer's
instruction. The real-time PCR was performed using KAPA™ Probe Fast
qPCR kit (Kapa Biosystems, Wilmington, MA, USA) on an Applied
Biosystems StepOnePlus™ Real-Time PCR systems. The CD133 primers
were 5′-aaggcatatgaatccaa aattga-3′ (sense) and
5′-ccaccagaggcatcagaataa-3′ (antisense); the CD24 primers were
5′-atgggcagagcaatggtg-3′ (sense) and 5′-tggaataaatctgcgtgggta-3′
(antisense); the EpCAM primers were 5′-agttggtgcacaaaatactgtcat-3′
(sense) and 5′-ctcccaagtt ttgagccatt-3′ (antisense); the CD13
primers were 5′-catccatcag a gatggcagac-3′ (sense) and
5′-tgctgaagagatcgttctgg-3′ (anti-sense); the HPRT primers were
5′-tgatagatccattcctatgactgt aga-3′ (sense) and
5′-caagacattctttccagttaaagttg-3′ (antisense). The reactions were
incubated at 95°C for 10 min, followed by 40 cycles of denaturation
at 95°C for 15 sec and annealing and extension at 60°C for 1
min.
β3 integrin-specific inhibition by
shRNA
The shRNA was obtained from the National RNAi Core
Facility (Academia Sinica, Taipei, Taiwan). The β3 integrin shRNA
targeting sequences is GATGCAGTGAATTGTACCTAT. The production of
lentiviral particles containing β3 integrin shRNA was conducted
according to the protocol provided from the National RNAi Core
Facility.
Tissue array staining
Formalin-fixed, paraffin-embedded ovarian tumor
tissue array was purchased from Super BioChips (Seoul, Korea). The
tissue array was deparaffinized with xylene and rehydrated in grade
ethanol, and the antigen retrieval was performed by autoclaving in
target retrieval buffer (Dako, Carpinteria, CA, USA).
Immunohistochemical staining was performed using a monoclonal
rabbit anti-CD90 antibody (clone EPR3133; Abcam) and a monoclonal
mouse anti-integrin β3 antibody (Clone SAP; Millipore). The color
was developed with 3-amino-9-ethylcarbazole (Dako). The sections
were subsequently counterstained with the Mayer's hematoxylin. The
lesions were assessed by a medical doctor (H.P.H.).
Statistical analysis
The statistical analyses were performed using
GraphPad Prism version 4 (GraphPad Software, Inc., La Jolla, CA,
USA). The analysis was performed using unpaired t-test, one-way
ANOVA analysis and two-way ANOVA analysis, respectively and the
data are reported as mean ± standard deviation.
Results
CD90 is underexpressed in ovarian cancer
tissues
In order to elucidate the CD90 expression in
physiological situation, the CD90 expression in ovarian tumor
tissues was analyzed using Oncomine database. Oncomine database
analysis of ovarican cancer tissue vs. normal tissue showed that
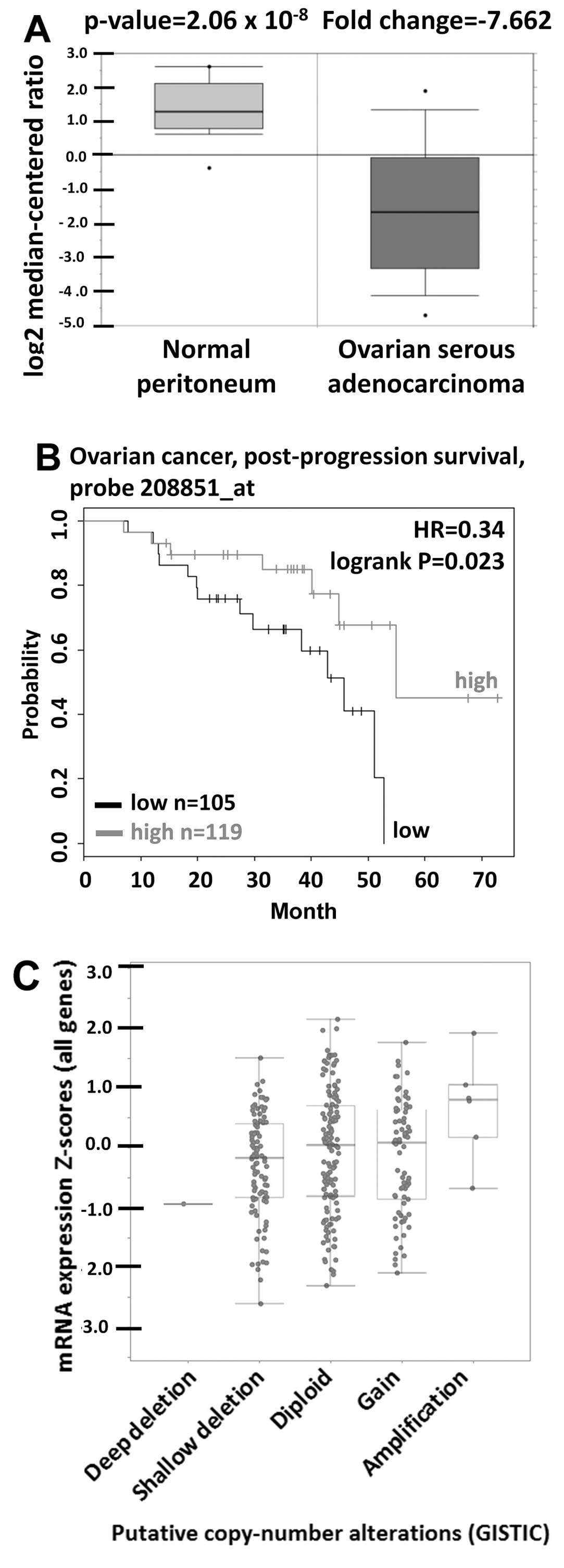
CD90 was significantly decreased in tumor tissues (Fig. 1A). The prognostic value of CD90 in
ovarian cancer was analyzed using Kaplan-Meier plotter and revealed
that low level of CD90 was correlated with poor survival rate
(Fig. 1B). The gene copy number is
important for several biological processes in normal cells and the
alterations of gene copy number are associated with cancer
initiation (30). We therefore
studied whether the variations of gene copy number are involved in
altered mRNA expression levels in ovarian cancer. The analysis from
cBio-Portal database showed that the increase of DNA copy number
from deletion, diploid, to amplification causes the increase in
mRNA expression, indicating that DNA copy number is correlated with
the mRNA expression (Fig. 1C).
CD90 inhibits tumorigenicity in SKOV3
cells
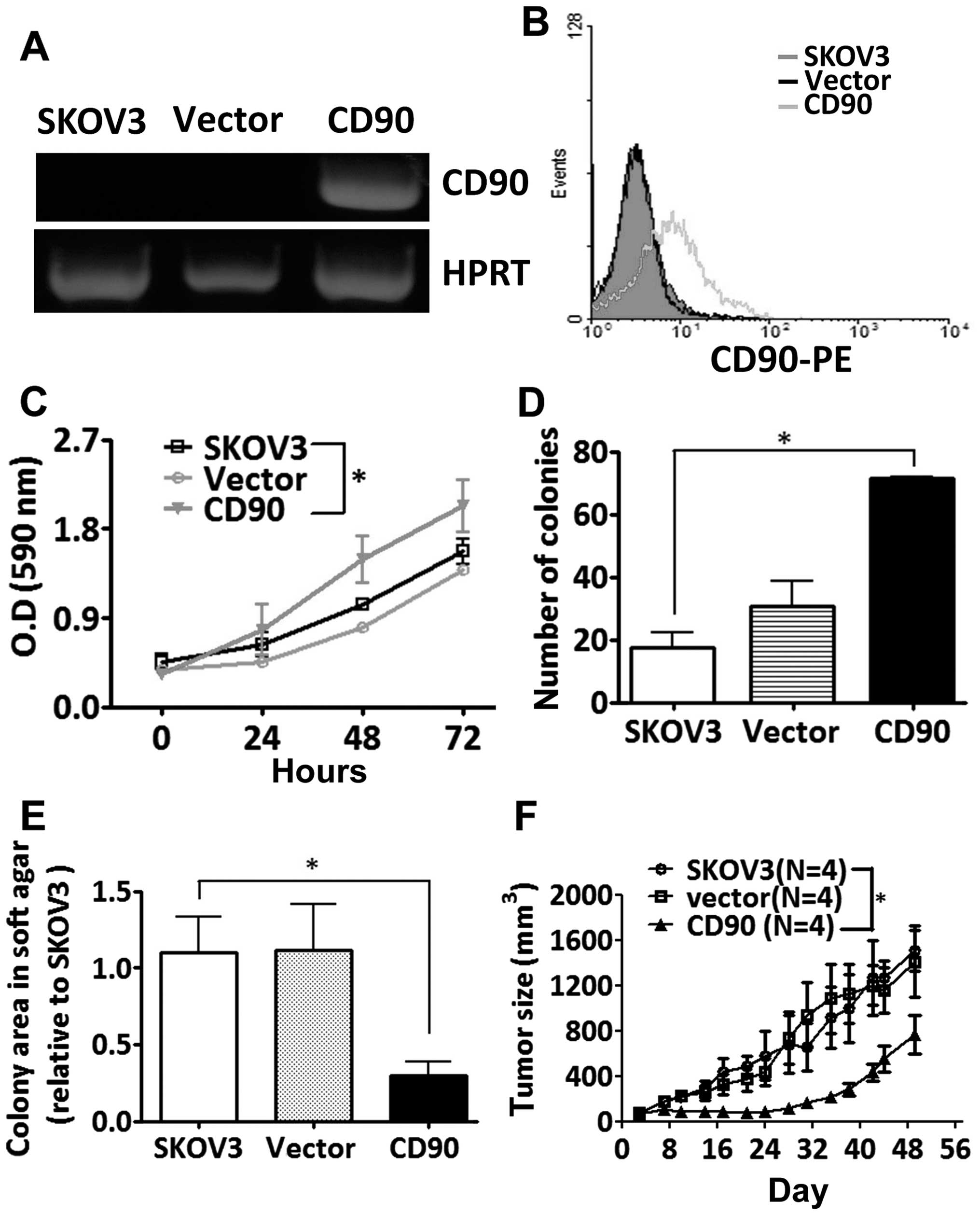
To study whether the CD90 affected ovarian cancer
cell growth, SKOV3 cells were transfected with a plasmid encoding
CD90. Ectopic expression of CD90 mRNA was analyzed by RT-PCR
analysis, and the surface expression of CD90 was detected by flow
cytometry (Fig. 2A and B). Ectopic
expression of CD90 promoted the cell proliferation by MTT and
colony formation assay (Fig. 2C and
D), but decreased anchorage-independent growth in vitro
and tumor formation in vivo (Fig. 2E and F).
CD90 promotes anoikis and reduces
sphere-forming ability
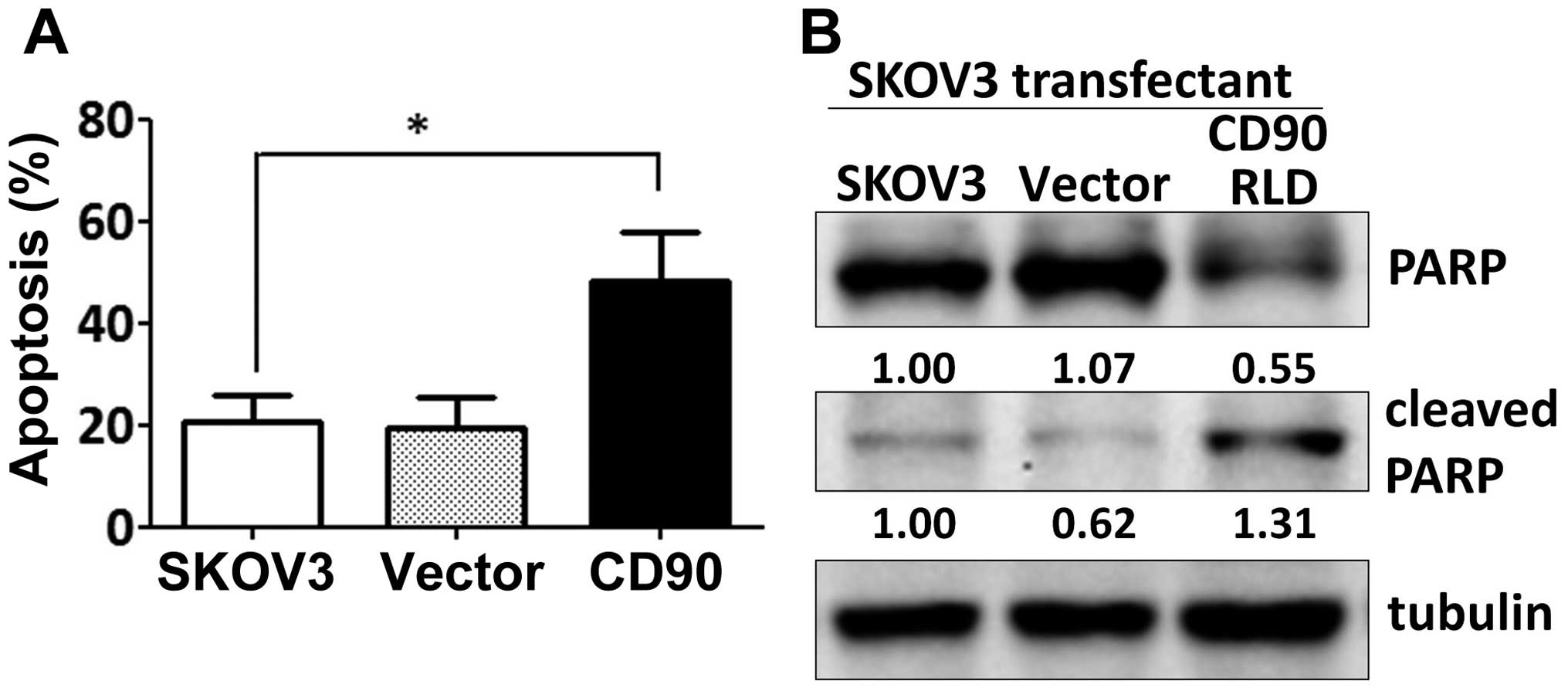
Since the effect of CD90 on anchorage-dependent
growth in vitro was not correlated with the tumor growth
in vivo, we investigated whether CD90 affected anoikis,
which is closely related with the anchorage-independent growth.
Expression of exogenous CD90 promoted cell apoptosis when SKOV3
cells were cultured on poly-HEMA-coated dish (Fig. 3A). CD90 transfectants displayed
higher level of cleaved poly(ADP-ribose) polymerase (cleaved PARP),
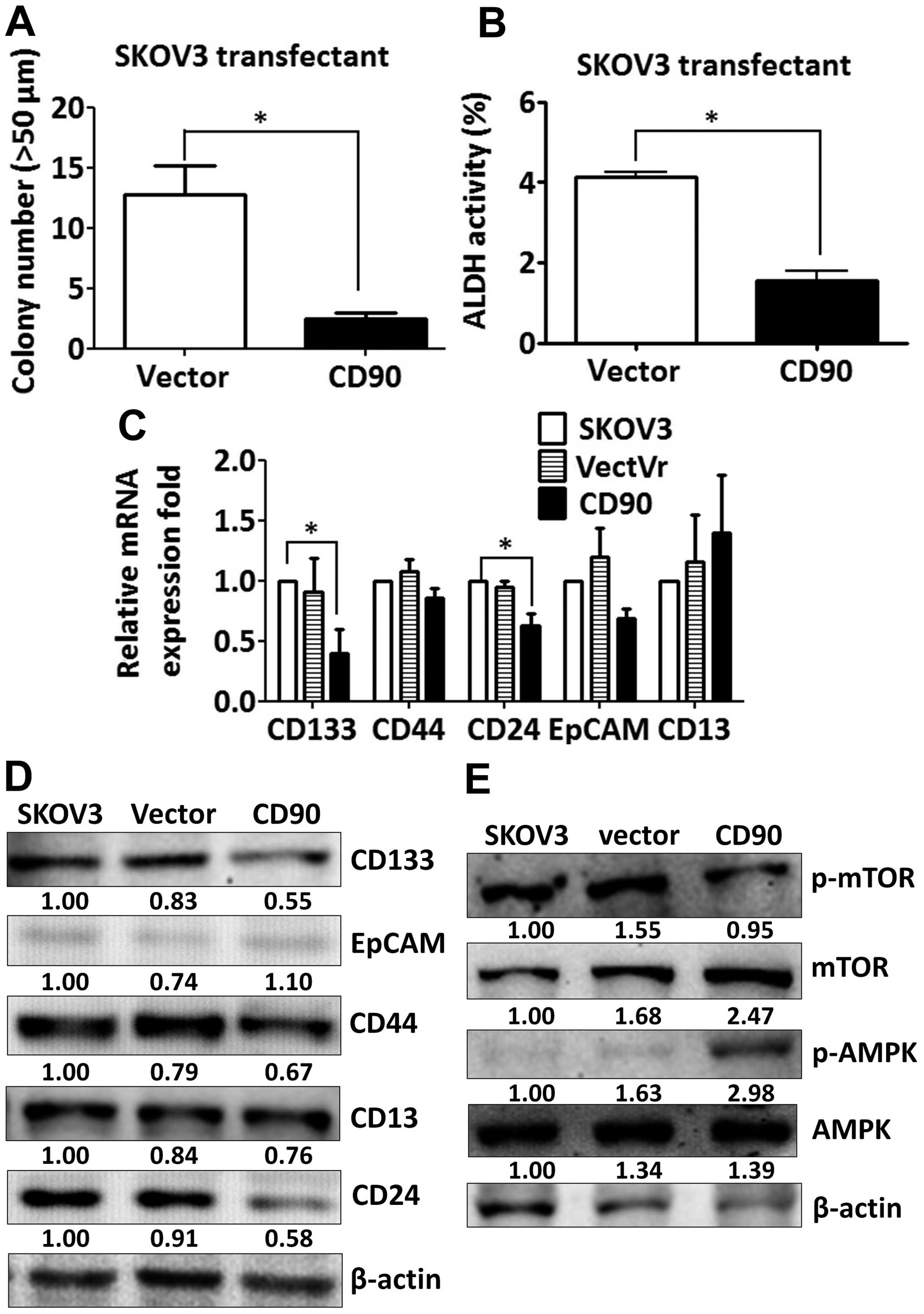
which indicates caspase-dependent activation (Fig. 3B). In addition, expression of CD90
decreased the sphere formation in ultra-low attachment culture
dishes and aldehyde dehydrogenase activity, which characterize the
subpopulation of cells with CSC properties (Fig. 4A and B). These data indicate that
the inhibition of tumor growth is correlated with the alteration of
characteristics of CSC.
CD90 decreases the expression of stem
cell markers CD133 and CD24
Since CSC markers were used to identify CSCs, we
then investigated whether ectopic expression of CD90 influences the
expression of other CSC markers. The expression of CD133 and CD24
was decreased in the SKOV3 CD90 transfectant (Fig. 4C). There was no statistically
significant difference in CD44, EpCAM and CD13 expression between
SKOV3 CD90 transfectant and the parental cell line SKOV3 (Fig. 4C). The inhibition of CD133
expression was further confirmed by western blotting (Fig. 4D). Our previous study reported that
CD90 promoted CD133 expression through the elevated mTOR
phosphorylation and decreased AMPK phosphorylation in liver cancer
(17). Next we studied whether the
mTOR and AMPK were involved in the inhibition of CD133 by ectopic
expression of CD90 in ovarian cancer. SKOV3 CD90 transfectants had
lower levels of mTOR phosphorylation and higher levels of AMPK
phosphorylation (Fig. 4E).
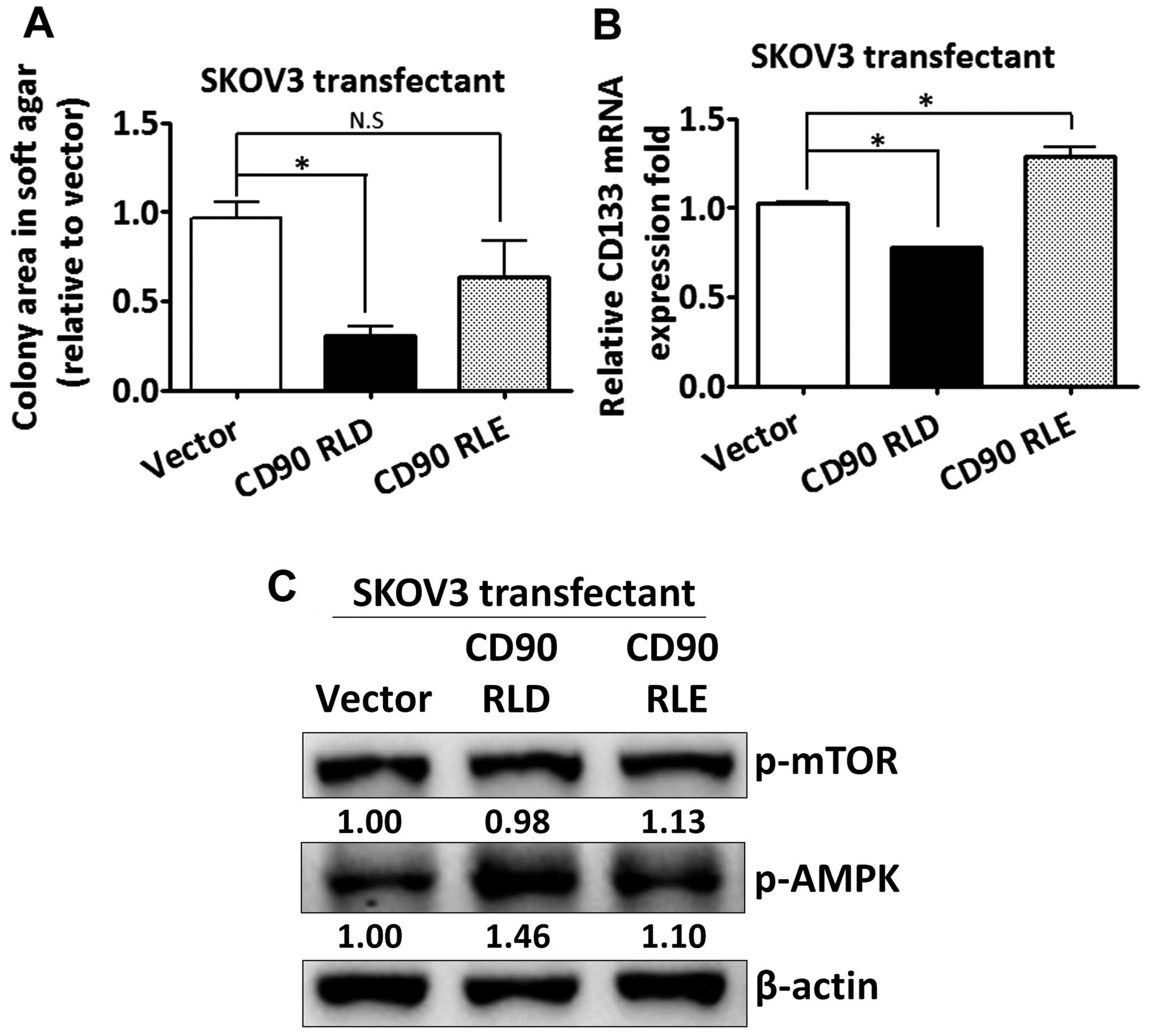
The CD90 RLD domain affects
anchorage-independent growth and CD133 expression
Given that CD90 has been shown to promote liver
cancer progression through interaction with β3 integrin with its
RLD sequence, we sought to determine whether the RLD domain of CD90
mediated the signal transduction in regulating CD133 expression in
ovarian cancer. The RLD residue of CD90 was replaced with RLE, and
the mutant CD90 was delivered into SKOV3 cells. The mutant CD90 did
not inhibit anchorage-independent growth (Fig. 5A). The inhibition of CD133 mRNA by
CD90 was rescued by the mutant CD90 (Fig. 5B). Besides, the alteration of AMPK
and mTOR phosphorylation was attenuated in SKOV3 transfectant
expressing mutant CD90 (Fig.
5C).
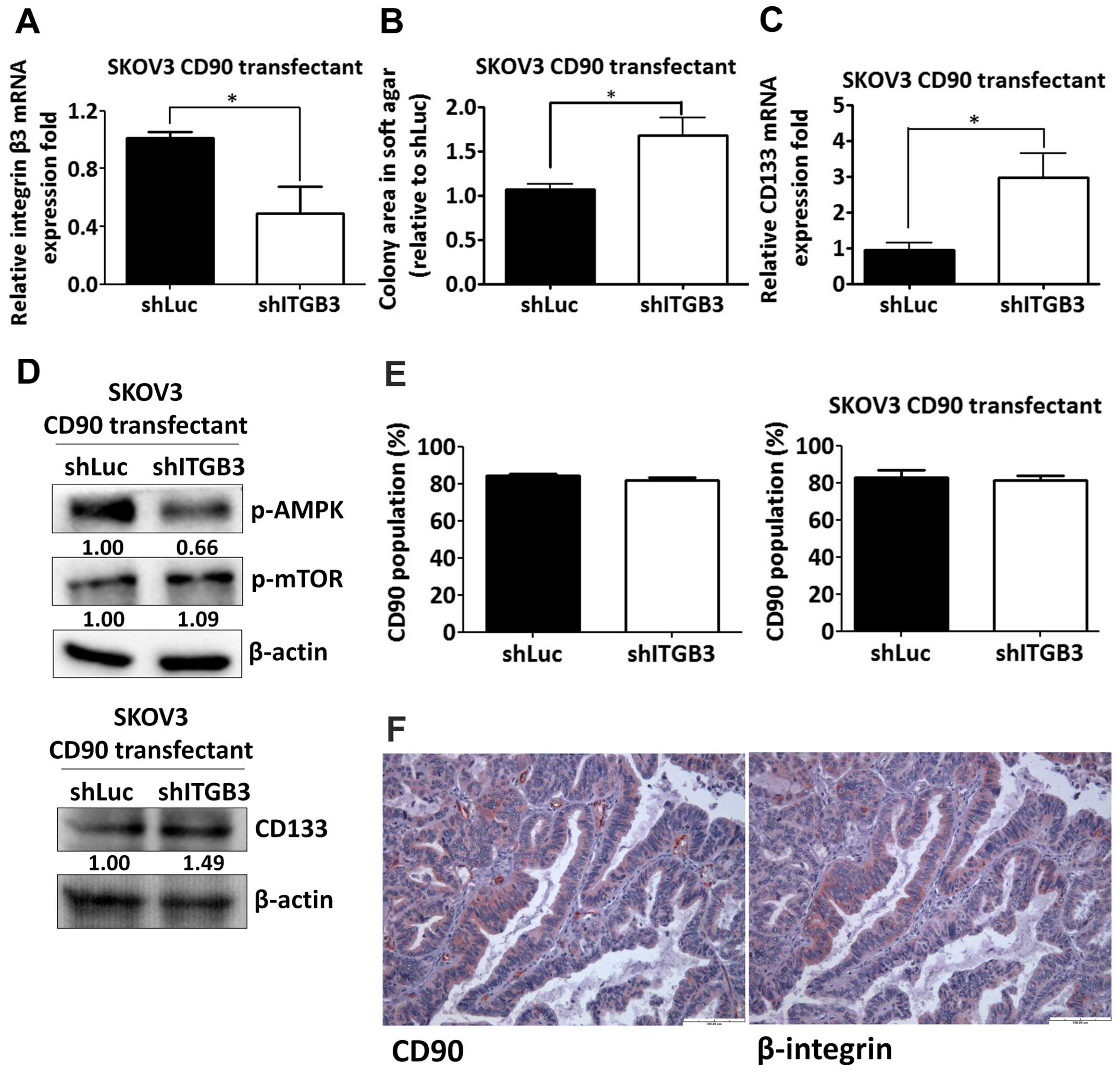
Silencing of β3 integrin increases
anchorage-independent growth and CD133 expression
Given the effect of the RLD domain of CD90 in
mediating CD133 inhibition, we next determined whether CD90
regulated its effects through β3 integrin in SKOV3 cells. The β3
integrin shRNA was delivered to SKOV3 CD90 transfectants and the
knockdown efficacy was determined by quantitative RT-PCR (Fig. 6A). The anchorage-independent growth
ability and CD133 mRNA expression was increased by β3 integrin
shRNA (Fig. 6B and C).
CD90-induced phosphorylation of mTOR and AMPK were attenuated after
silencing β3 integrin expression (Fig.
6D). In addition, β3 integrin shRNA did not alter the CD90
expression in either SKOV3 stable CD90 transfectants or SKOV3 cells
transiently transfected with CD90 by flow cytometric analysis
(Fig. 6E). To further verify the
physiological role of CD90 and β3 integrin on tumor cells, we
exploited the tissue array to analyze the CD90 and β3 integrin
expression by immunohistochemistry and found that both expression
of CD90 and β3 integrin were observed in tumor tissues (Fig. 6F), indicating the physiological
interaction between CD90 and β3 integrin on tumor cells.
Discussion
In the present study, we demonstrated that CD90 is
decreased in ovarian cancer tissue and the lower expression level
of CD90 predicts poor prognosis. CD90 functions as a tumor
suppressor gene in ovarian cancer and growth inhibition is
associated with the characteristic of anoikis and stemness
properties, including sphere-forming ability and ALDH activity. We
found decreased expression of CD133 in the CD90 transfectants
compared to the parental SKOV3 cells. Signaling analyses reveal
that AMPK and mTOR are correlated with the inhibition of CD133.
Furthermore, the mutant CD90 attenuates the signaling transduction
of AMPK/mTOR, CD133 expression and the anchorage-independent growth
ability. The inhibition of CD133 expression and
anchorage-independent growth by CD90 is restored by β3 integrin
shRNA.
In the present study, we examined the effect of β3
integrin shRNA on anchorage-independent growth, CD133 expression
and mTOR/AMPK signal molecules in the transfectant expressing
ectopic CD90 (Fig. 6B–D), and
found that β3 integrin shRNA attenuated CD90-induced phenomena,
indicating that β3 integrin is associated with CD90-mediated
phenotype alteration. Previous study showed that β3 integrin
downregulates SKOV3 cell growth (31), indicating that β3 integrin may
restore the anchorage-independent growth in ovarian cancer cells in
a CD90-independent manner. Nevertheless, mutation of the β3
integrin binding domain in CD90 significantly attenuated its
ability on CD133 expression and anchorage-independent growth
(Fig. 5). Furthermore, β3 integrin
shRNA did not alter CD90 expression in either SKOV3 stable CD90
transfectants or SKOV3 cells transiently transfected with CD90
(Fig. 6E). Altogether, the
inhibitory effect of CD90 on CD133 expression and
anchorage-independent growth may be regulated directly through the
activation of β3 integrin.
In this study, we clarified the role of CD90 in
SKOV3 cell line, which is an adenocarcinoma ovarian cancer cell.
Serous adenocarcinoma is the common type of ovarian cancer and the
high-grade serous adenocarcinoma arises from elsewhere in the
peritoneal cavity. In addition, we identified CD90 expression from
microarray datasets in Oncomine database as the criteria of P-value
<1E-4, fold change >3, and gene ranking in the top 10% in
Gene Summary View and only one dataset from Yoshihara was
identified. Therefore, we identified the CD90 expression from this
dataset comparing the normal peritoneum and ovarian serous
adenocarcinoma in Fig. 1A. We
further checked the other six datasets under the criteria of
P<0.05 and found that CD90 was increased in different types of
cancer tissues, including carcinoma, papillary carcinoma,
cystadenocarcinoma, clear cell adenocarcinoma and serous
adenocarcinoma (Table I),
indicating that CD90 expression has tissue-specific expression
pattern. It is interesting to note that the expression of CD90 in
serous adenocarcinoma was decreased in Yoshihara's dataset, but
increased in Adib's and Hendrix's datasets. The clinical cancer
tissues from Adib's dataset were identified from College Hospitals
NHS Trust located in UK. The samples from Hendrix's dataset were
derived from the University of Michigan Health System and the Johns
Hopkins Hospital located in USA. By contrast, Yoshihara obtained
the clinical tissues from the Department of Obstetrics and
Gynecology of Niigata University located in Japan. A previous study
showed that Asian ovarian cancer patient has good disease-specific
survival compared to Caucasian ovarian cancer patient, and the
disease-specific survival of Asian immigrants was higher than U.S
born Asians (32), indicating the
important effect of genetic and environmental factors on tumor
development.
 | Table ICD90 expression in ovarian cancer
from Oncomine database. |
Table I
CD90 expression in ovarian cancer
from Oncomine database.
| Dataset | Cancer subtype | Fold change | P-value | Gene rank (Top
%) |
|---|
| Yoshihara | Peritoneum vs.
ovarian serous adenocarcinoma | −7.662 | 2.06E-08 | 7 |
| Hendrix | Ovary vs. ovarian
serous adenocarcinoma | 1.265 | 0.015 | 29 |
| Adib | Ovary vs. ovarian
serous adenocarcinoma | 1.539 | 0.014 | 10 |
| Bonome | Ovarian surface
epithelium vs. ovarian carcinoma | 2.608 | 4.78E-09 | 7 |
| Welsh | Ovary vs. ovarian
serous surface papillary carcinoma | 2.026 | 0.016 | 32 |
| TCGA | Ovary vs. ovarian
serous cystadenocarcinoma | 2.615 | 0.005 | 31 |
| Lu | Ovarian surface
epithelium vs. ovarian clear cell adenocarcinoma | 1.546 | 0.033 | 18 |
Oncomine database indicated that CD90 expression is
increased in liver cancer, but decreased in ovarian cancer tissue
as the criteria of P-value <1E-4, fold change >3, and gene
ranking in the top 10% in Gene Summary View. The differential CD90
expression between liver and ovarian cancer tissues is in
accordance with the finding that oncogenic role of CD90 in liver
cancer in our previous study and the tumor suppressor role of CD90
in ovarian cancer in the present study. In addition, the results of
this study describing the effects of CD90 on CD133 expression and
mTOR and AMPK phosphorylation in ovarian cancer are opposite to the
results of our previous study in liver cancer (17). These data indicate that CD90 play
different roles in different cancers.
Integrin functions as heterodimer and is generated
from at least 18 α and 8 β subunits to form 24 distinct receptors.
The distinct integrin heterodimer binds to different extra-cellular
matrix components to regulate diverse biological responses
(33). Integrin mediates the
signal transduction during carcinogenesis and it has multiple
functions to regulate cancer progression within different tumors.
The expression of αvβ3 and αvβ5 integrin is highly expressed in
breast cancer and is associated with the increased tumor size and
bone metastasis (34,35). In contrast, the α2β1 integrin is
decreased in the breast cancer tissue and the restoration of α2β1
integrin inhibits the tumorigenic ability (36). The overexpression of β3 integrin
decreases the tumor weight and metastasis in ovarian cancer cells
(31), and the expression of β3
integrin is associated with the good prognosis in ovarian cancer
(37). Integrin has been
demonstrated to correlate with the cancer stem cell growth and the
α6 integrin is co-expressed with conventional CSC markers in
glioblastoma stem cells (38,39).
The α1 and β5 integrin promote CD133+ prostate cancer
stem cell (PCSC) differentiation (40). These studies suggest that CD90 may
play a tumor suppressor role in ovarian cancer at least in part
mediated via β3 integrin.
Tumor suppressor gene is functional for growth
inhibition. It is interesting to note that CD90 inhibited the
tumorigenic ability in vitro and in vivo, but
increased the cell proliferation in SKOV3 cells. We further found
that CD90 decreased the stemness properties of ALDH activity and
sphere formation ability, indicating that CD90 is critical for the
characteristics of stem cells. Previous studies show that the
balance between proliferation and quiescence regulation is
important for stem cells, and mesenchymal stem cell decreases the
proliferation for the long-term self-renewal preservation (41,42).
Horsley et al (43) found
that NFATc1 contributes to the balance between quiescence and
proliferation of skin stem cells and regulates the quiescence stage
by repressing CDK4 expression. In addition, the cancer stem-like
cells are thought to have the slow-cycling phenotype (44). Consequently, we propose that
CD90-mediated proliferation may occur through the alteration of CSC
property. Previous studies have demonstrated that mTOR signal
pathway is involved in cancer stem cell growth and targeting mTOR
by inhibitor can be used as cancer therapy (45,46).
AMPK, which is a downstream signal molecule of mTOR, has been
demonstrated to regulate drug resistance and cancer stem cell
growth (47). In our previous
study, CD90 promoted CD133 expression through the signal axis of
mTOR and AMPK, thereby inducing liver tumor formation (17). Therefore, we suggest that CD90
triggered the same signal axis, but activated the opposite
phosphorylation of mTOR and AMPK for liver and ovarian cancer
development.
In conclusion, CD90 is underexpressed in ovarian
cancer and CD90 overexpression decreases tumor growth via β3
integrin. The β3 integrin suppression by shRNA is able to restore
the CD90-regulated tumor inhibition. The present study provides new
insight into the contribution of CD90 in ovarian cancer, implying
the application of therapy in the future.
References
|
1
|
Sadikovic B, Al-Romaih K, Squire JA and
Zielenska M: Cause and consequences of genetic and epigenetic
alterations in human cancer. Curr Genomics. 9:394–408. 2008.
View Article : Google Scholar
|
|
2
|
Claus EB, Risch N and Thompson WD: Genetic
analysis of breast cancer in the cancer and steroid hormone study.
Am J Hum Genet. 48:232–242. 1991.PubMed/NCBI
|
|
3
|
Easton DF, Bishop DT, Ford D and Crockford
GP; The Breast Cancer Linkage Consortium. Genetic linkage analysis
in familial breast and ovarian cancer: Results from 214 families.
Am J Hum Genet. 52:678–701. 1993.PubMed/NCBI
|
|
4
|
Welcsh PL and King MC: BRCA1 and BRCA2 and
the genetics of breast and ovarian cancer. Hum Mol Genet.
10:705–713. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang H, Das P, Yu Y, Mao W, Wang Y,
Baggerly K, Wang Y, Marquez RT, Bedi A, Liu J, et al: NDN is an
imprinted tumor suppressor gene that is downregulated in ovarian
cancers through genetic and epigenetic mechanisms. Oncotarget.
7:3018–3032. 2016.
|
|
6
|
Bellio M, Leal LM, Scharfstein J and Dos
Reis GA: Interactions between CD3 and Thy1 T cell activation
pathways: Blockade of CD3-mediated T lymphocyte activation induced
by immobilized anti-Thy1 antibodies. Cell Immunol. 135:534–540.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Avalos AM, Valdivia AD, Muñoz N,
Herrera-Molina R, Tapia JC, Lavandero S, Chiong M, Burridge K,
Schneider P, Quest AF, et al: Neuronal Thy-1 induces astrocyte
adhesion by engaging syndecan-4 in a cooperative interaction with
alphav-beta3 integrin that activates PKCalpha and RhoA. J Cell Sci.
122:3462–3471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schubert K, Gutknecht D, Köberle M,
Anderegg U and Saalbach A: Melanoma cells use Thy-1 (CD90) on
endothelial cells for metastasis formation. Am J Pathol.
182:266–276. 2013. View Article : Google Scholar
|
|
9
|
Fiore VF, Ju L, Chen Y, Zhu C and Barker
TH: Dynamic catch of a Thy-1-α5β1+syndecan-4
trimolecular complex. Nat Commun. 5:48862014. View Article : Google Scholar
|
|
10
|
Kong M, Muñoz N, Valdivia A, Alvarez A,
Herrera-Molina R, Cárdenas A, Schneider P, Burridge K, Quest AF and
Leyton L: Thy-1-mediated cell-cell contact induces astrocyte
migration through the engagement of αVβ3
integrin and syndecan-4. Biochim Biophys Acta. 1833:1409–1420.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang J, Zhang Y, Chuai S, Wang Z, Zheng
D, Xu F, Zhang Y, Li C, Liang Y and Chen Z: Trastuzumab (herceptin)
targets gastric cancer stem cells characterized by CD90 phenotype.
Oncogene. 31:671–682. 2012. View Article : Google Scholar
|
|
12
|
Yan X, Luo H, Zhou X, Zhu B, Wang Y and
Bian X: Identification of CD90 as a marker for lung cancer stem
cells in A549 and H446 cell lines. Oncol Rep. 30:2733–2740.
2013.PubMed/NCBI
|
|
13
|
Tang KH, Dai YD, Tong M, Chan YP, Kwan PS,
Fu L, Qin YR, Tsao SW, Lung HL, Lung ML, et al: A CD90+
tumor-initiating cell population with an aggressive signature and
metastatic capacity in esophageal cancer. Cancer Res. 73:2322–2332.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai
P, Chu PW, Lam CT, Poon RT and Fan ST: Significance of
CD90+ cancer stem cells in human liver cancer. Cancer
Cell. 13:153–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu L, Zhang W, Wang J and Liu R: Evidence
of CD90+CXCR4+ cells as circulating tumor
stem cells in hepatocellular carcinoma. Tumour Biol. 36:5353–5360.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pang YB, Zhong JH, Luo XL, Ou C, Guo Z,
Xiang BD, Peng NF and Li LQ: Clinicopathological characteristics
and liver stem cell marker expression in hepatocellular carcinoma
involving bile duct tumor thrombi. Tumour Biol. 37:5879–5884. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen WC, Chang YS, Hsu HP, Yen MC, Huang
HL, Cho CY, Wang CY, Weng TY, Lai PT, Chen CS, et al: Therapeutics
targeting CD90-integrin-AMPK-CD133 signal axis in liver cancer.
Oncotarget. 6:42923–42937. 2015.PubMed/NCBI
|
|
18
|
Lung HL, Bangarusamy DK, Xie D, Cheung AK,
Cheng Y, Kumaran MK, Miller L, Liu ET, Guan XY, Sham JS, et al:
THY1 is a candidate tumour suppressor gene with decreased
expression in metastatic nasopharyngeal carcinoma. Oncogene.
24:6525–6532. 2005.PubMed/NCBI
|
|
19
|
Abeysinghe HR, Pollock SJ, Guckert NL,
Veyberman Y, Keng P, Halterman M, Federoff HJ, Rosenblatt JP and
Wang N: The role of the THY1 gene in human ovarian cancer
suppression based on transfection studies. Cancer Genet Cytogenet.
149:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abeysinghe HR, Cao Q, Xu J, Pollock S,
Veyberman Y, Guckert NL, Keng P and Wang N: THY1 expression is
associated with tumor suppression of human ovarian cancer. Cancer
Genet Cytogenet. 143:125–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tiveron MC, Barboni E, Pliego Rivero FB,
Gormley AM, Seeley PJ, Grosveld F and Morris R: Selective
inhibition of neurite outgrowth on mature astrocytes by Thy-1
glycoprotein. Nature. 355:745–748. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hermosilla T, Muñoz D, Herrera-Molina R,
Valdivia A, Muñoz N, Nham SU, Schneider P, Burridge K, Quest AF and
Leyton L: Direct Thy-1/alphaVbeta3 integrin interaction mediates
neuron to astrocyte communication. Biochim Biophys Acta.
1783:1111–1120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herrera-Molina R, Frischknecht R,
Maldonado H, Seidenbecher CI, Gundelfinger ED, Hetz C, Aylwin ML,
Schneider P, Quest AF and Leyton L: Astrocytic
αVβ3 integrin inhibits neurite outgrowth and
promotes retraction of neuronal processes by clustering Thy-1. PLoS
One. 7:e342952012. View Article : Google Scholar
|
|
24
|
Zhou Y, Hagood JS, Lu B, Merryman WD and
Murphy-Ullrich JE: Thy-1-integrin alphav beta5 interactions inhibit
lung fibroblast contraction-induced latent transforming growth
factor-beta1 activation and myofibroblast differentiation. J Biol
Chem. 285:22382–22393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar
|
|
28
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBio-Portal. Sci Signal. 6:pl12013. View Article : Google Scholar
|
|
29
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rothenberg SM and Settleman J: Discovering
tumor suppressor genes through genome-wide copy number analysis.
Curr Genomics. 11:297–310. 2010. View Article : Google Scholar :
|
|
31
|
Chen J, Zhang J, Zhao Y, Li J and Fu M:
Integrin beta3 down-regulates invasive features of ovarian cancer
cells in SKOV3 cell subclones. J Cancer Res Clin Oncol.
135:909–917. 2009. View Article : Google Scholar
|
|
32
|
Fuh KC, Shin JY, Kapp DS, Brooks RA, Ueda
S, Urban RR, Chen LM and Chan JK: Survival differences of Asian and
Caucasian epithelial ovarian cancer patients in the United States.
Gynecol Oncol. 136:491–497. 2015. View Article : Google Scholar
|
|
33
|
Bouvard D, Pouwels J, De Franceschi N and
Ivaska J: Integrin inactivators: Balancing cellular functions in
vitro and in vivo. Nat Rev Mol Cell Biol. 14:430–442. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takayama S, Ishii S, Ikeda T, Masamura S,
Doi M and Kitajima M: The relationship between bone metastasis from
human breast cancer and integrin αvβ3
expression. Anticancer Res. 25(1A): 79–83. 2005.PubMed/NCBI
|
|
35
|
Sloan EK, Pouliot N, Stanley KL, Chia J,
Moseley JM, Hards DK and Anderson RL: Tumor-specific expression of
alphavbeta3 integrin promotes spontaneous metastasis of breast
cancer to bone. Breast Cancer Res. 8:R202006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zutter MM, Santoro SA, Staatz WD and Tsung
YL: Re-expression of the alpha 2 beta 1 integrin abrogates the
malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci
USA. 92:7411–7415. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaur S, Kenny HA, Jagadeeswaran S,
Zillhardt MR, Montag AG, Kistner E, Yamada SD, Mitra AK and Lengyel
E: β3-integrin expression on tumor cells inhibits tumor
progression, reduces metastasis, and is associated with a favorable
prognosis in patients with ovarian cancer. Am J Pathol.
175:2184–2196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hongo K, Tanaka J, Tsuno NH, Kawai K,
Nishikawa T, Shuno Y, Sasaki K, Kaneko M, Hiyoshi M, Sunami E, et
al: CD133(−) cells, derived from a single human colon cancer cell
line, are more resistant to 5-fluorouracil (FU) than CD133(+)
cells, dependent on the β1-integrin signaling. J Surg Res.
175:278–288. 2012. View Article : Google Scholar
|
|
39
|
Lathia JD, Gallagher J, Heddleston JM,
Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE,
Hjelmeland AB, et al: Integrin alpha 6 regulates glioblastoma stem
cells. Cell Stem Cell. 6:421–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rentala S, Yalavarthy PD and Mangamoori
LN: Alpha1 and beta1 integrins enhance the homing and
differentiation of cultured prostate cancer stem cells. Asian J
Androl. 12:548–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pattappa G, Thorpe SD, Jegard NC, Heywood
HK, de Bruijn JD and Lee DA: Continuous and uninterrupted oxygen
tension influences the colony formation and oxidative metabolism of
human mesenchymal stem cells. Tissue Eng Part C Methods. 19:68–79.
2013. View Article : Google Scholar
|
|
42
|
Ito K and Suda T: Metabolic requirements
for the maintenance of self-renewing stem cells. Nat Rev Mol Cell
Biol. 15:243–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Horsley V, Aliprantis AO, Polak L,
Glimcher LH and Fuchs E: NFATc1 balances quiescence and
proliferation of skin stem cells. Cell. 132:299–310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moore N and Lyle S: Quiescent,
slow-cycling stem cell populations in cancer: A review of the
evidence and discussion of significance. J Oncol. 2011:3960762011.
View Article : Google Scholar
|
|
45
|
Kolev VN, Wright QG, Vidal CM, Ring JE,
Shapiro IM, Ricono J, Weaver DT, Padval MV, Pachter JA and Xu Q:
PI3K/ mTOR dual inhibitor VS-5584 preferentially targets cancer
stem cells. Cancer Res. 75:446–455. 2015. View Article : Google Scholar
|
|
46
|
Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M,
Wang XA, Zhang F, Jiang L, Zhang Y, et al: Fibronectin promotes
cell proliferation and invasion through mTOR signaling pathway
activation in gallbladder cancer. Cancer Lett. 360:141–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Z, Liu P, Chen Q, Deng S, Liu X, Situ
H, Zhong S, Hann S and Lin Y: Targeting AMPK signaling pathway to
overcome drug resistance for cancer therapy. Curr Drug Targets.
17:853–864. 2016. View Article : Google Scholar
|