Introduction
Osteosarcoma (OS) is a prevalent primary malignancy
of bone and mainly occurs in adolescents and children (1). OS is often located in the metaphyses
of long bone where it grows rapidly, including the proximal tibia,
proximal humerus and distal femur (2,3). OS
is commonly marked by aggressive proliferation, high rate of
recurrence, and early systemic metastasis, especially the
metastasis to the lung (1–3). With surgery combined with the
treatment of chemotherapy drugs, such as cisplatin, doxorubicin and
methotrexate, a gradual improvement has been made to increase the
long-term survival rate (4).
However, the current therapeutic regimen remains undesirable and
often results in chemoresistance (5). Hence, there is an urgent clinical
need to explore new antitumor reagents for OS. The traditional
Chinese medicine, especially the herb-derived components, has
received increasing attention as a source of novel pharmacologics.
Better curative effects have been noted when herb-derived
components are combined with the traditional chemotherapy agents in
treatment for multiple cancers (6–8).
Ursolic acid (UA), one of these potential compounds,
is a pentacyclic triterpenoid. It has been identified in medical
herbs and edible plants, including loquat leaf and rosemary.
Previous studies have revealed that UA can suppress proliferation
and induce apoptosis in various tumor cells, such as prostate, lung
and pancreas (6,9,10).
Furthermore, UA has been reported to be able to inhibit tumor
progression (11), induce tumor
cell differentiation (12) and
inhibit angiogenic activity (13).
UA was also found to be chemopreventive in different animal models
(13,14), suppress tumor invasion (10), and sensitize the orthotopically
implanted pancreatic tumors to gemcitabine (6). It has been confirmed that UA can
modulate various cancer-related signals. For example, UA interferes
with DNA replication (15),
activates caspases (16) and c-Jun
N-terminal kinases (JNK) (7),
downregulates anti-apoptotic genes, such as COX-2, NO synthase and
protein tyrosine kinase (15). UA
has been shown to increase the expression of p53, while decreasing
that of NF-κB, and this effect was differentiated in tumor cells as
compared to normal cells, which did not exhibit this response to UA
(17). Moreover, UA was found to
induce cell cycle arrest at G1 phase in tumor cells (18). Recently, it was reported that UA
was effective in inducing apoptosis of MG-63 OS in vitro
(19). However, the exact
mechanism underlying these effects of UA in OS remains unknown.
It has been verified that Wnt/β-catenin signaling is
a pivotal factor in modulating proliferation, differentiation and
motility of cells (20). Aberrant
activation of Wnt/β-catenin signaling was found in a number of bone
tumors (21,22). Former studies indicated that
several ligands, receptors and co-receptors of Wnt maintain high
expression levels in OS cells, whereas Wnt inhibitors are decreased
(23,24). Therefore, a number of novel
antitumor strategies for OS have been developed by targeting the
Wnt/β-catenin signaling (22).
Although UA shows valid antitumor activities in a variety of
tumors, it still remains unclear whether the mechanism underlying
the antitumor activity of UA on OS cells is implicated with the
inhibition of Wnt/β-catenin signaling.
In the present study, we evaluated the inhibitory
effect of UA on the proliferation of human OS cells, and dissected
the possible mechanisms underlying these effects. We found that UA
could inhibit the proliferation and induce apoptosis in 143B OS
cells. The inhibitory effect of UA may be mediated by inactivating
Wnt/β-catenin signaling through upregulating p53 at least.
Materials and methods
Chemical preparations and cell lines
UA, with a purity of 98.6%, was obtained from Xi'an
Hao-Xuan Bio-Tech Co., Ltd. (Xi'an, China). The human OS cell line
143B was obtained from the American Type Culture Collection
(Manassas, VA, USA). Pifithrin-α (PFT-α) was purchased from Selleck
Chemicals (Houston, TX, USA). UA and PFT-α were dissolved with
dimethyl sulfoxide (DMSO) for experiments in vitro. For
in vivo experiments, UA was suspended in 0.4%
carboxymethylcellulose sodium. The primary antibodies rabbit
anti-human STAT3 and p-STAT3 were obtained from Abcam (Cambridge,
MA, USA), and other antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Cells were cultured with
DMEM (containing 10% FBS, 100 U/ml of penicillin and 100 μg/ml of
streptomycin). Cells were incubated in 5% CO2 and
37°C.
Cell viability assay
Cell viability was determined with Cell Counting
Kit-8 (CCK-8). In brief, 143B cells were seeded in 96-well plates
with a final density of 3×103 cells/well and incubated
for 24 h. The cells were treated with different concentrations of
UA, recombinant adenovirus or DMSO for 24, 48 and 72 h. Thereafter,
10 μl of CCK-8 (Dojindo Laboratories, Kumamoto, Japan) were added
into each well and incubated for another 4 h. The absorbance was
determined at 450 nm with a microplate reader. Each test was
conducted in triplicate.
Clonogenic assay
The clonogenic assay was employed to determine the
ability of cells in a given population to undergo unlimited
division and form colonies. This assay was carried out as described
(25). Briefly, cells were treated
with different concentrations of UA for 24 h and then replated with
2,000 cells/well into 6-well plates. Then cells were maintained up
to 14 days until colonies were formed. Plates were washed gently
with PBS and incubated with 0.25% crystal violet formalin solution
at room temperature for 20 min. Each test was conducted in
triplicate.
Flow cytometric analysis for cell cycle
and apoptosis
The 143B cells were plated into a 6-well plate. For
cell cycle assay, cells were treated with different concentrations
of UA or DMSO for 24 h. Then cells were harvested, washed with cold
(4°C) PBS, fixed with cold (4°C) 70% ethanol. Finally, cells were
suspended in 300 μl PBS, and incubated with propidium iodide (PI)
(20 mg/ml) and RNase (1 mg/ml) for 30 min. The cells were detected
with fluorescence-activated cell sorting (FACS) subsequently. The
DNA contents were analyzed with ModFit LT software. For apoptosis
analysis, cells were treated with UA for 24 h. Then the cells were
collected and washed with cold (4°C) PBS, incubated with Annexin
V-FITC/PI following the instruction of the kits (KeyGen, Nanjing,
China). Finally, the processed cells were sorted with FACS and the
data were analyzed with FlowJo. Each test was conducted in
triplicate.
Construction of recombinant
adenoviruses
Recombinant adenoviruses expressing β-catenin (AdBC)
and small interfering RNA fragments targeting β-catenin (AdsiBC)
were constructed with AdEasy system (26), respectively. AdBC was tagged with
green fluorescence protein and AdsiBC was tagged with red
fluorescence protein. The adenovirus-expressing green fluorescence
protein (AdGFP) only was used as vector control.
Western blotting
Subconfluent 143B cells were plated in a 6-well
plate and treated with pre-designated concentrations of UA or DMSO.
For total cellular protein or tissue protein, cells and tissues
were harvested and lysed using ice-cold lysis buffer at
pre-designated time-points. For subcellular fractionation, the
protein was extracted with NE-PER™ Nuclear and Cytoplasmic
Extraction Reagents (Pierce Biotechnology, Inc., Rockford, IL, USA)
based on the manufacturer's instructions. The lysates were boiled
for 10 min, subjected to SDS-PAGE separation and transferred to
polyvinylidene difluoride (PVDF) membranes. Then the membranes were
blotted with corresponding primary antibodies, followed by
incubation with HRP-labelled second antibodies. Finally, the bands
of target proteins were developed with the SuperSignal West Pico
Substrate (Pierce Biotechnology, Inc.). All assays were performed
in triplicate.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Cells were treated with indicated concentrations of
UA in T-25 culture flasks. Total RNA was extracted with TRIzol
reagents (Invitrogen, Carlsbad, CA, USA) and transcribed to cDNA
templates with RT reaction at pre-designated time-points. Then, the
cDNA templates were used to detect the expression levels of target
genes by PCR. The primer sequences are available upon request. All
assays were performed in triplicate.
Luciferase reporter assay
Cells were seeded in T-25 culture flasks and
transfected with β-catenin/TCF-4 luciferase reporter (pTOP-luc) 3
μg per flask with Lipofectamine 2000 (Invitrogen) (25,27).
The cells were replated into a 24-well plate 16 h after
transfection, and then treated with indicated concentrations of UA
or DMSO. The cell lysates were subjected to luciferase assays with
luciferase assay kit (Promega Corp., Madison, WI, USA) 24 h after
treatment. All assays were performed in triplicate.
Xenograft model of human OS
The animal experiment was approved by the
Institutional Animal Care and Use Committee (IACUC) of Chongqing
Medical University. Athymic nude mice (female, 4–6 weeks old,
5/group) were from the Animal Center of Chongqing Medical
University (Chongqing, China). The 143B cells were collected and
re-suspended in cold PBS (4°C) to 2×107 cells/ml. Then
cells in 100 μl of PBS were injected subcutaneously into the right
flanks of the nude mice. Three days after injection, the athymic
nude mice were given UA (100 and 200 mg/kg) or solvent by
intragastric administration once a day for 4 weeks. The mice were
sacrificed and the tumor samples were photographed and harvested
for histological evaluation.
Immunohistochemical staining and
histological evaluation
Retrieved tumor masses were fixed with 4%
paraformaldehyde and embedded with paraffin, respectively. Serial
sections were deparaffinized and rehydrated in a gradient fashion.
Then the slides were stained with hematoxylin and eosin (H&E)
(25). For immunohistochemical
staining, the slides were further processed for antigen retrieval,
and incubated with proliferating cell nuclear antigen (PCNA)
antibody (1:100 dilution), or Wnt/β-catenin antibody (1:50
dilution) or isotype IgG as control. Finally, the slides were
incubated with streptavidin-labelled secondary antibodies and
visualized with 3,3′-diaminobenzidine (DAB) tetrahydro-chloride
reagent (25,27).
Statistical analysis
All quantitative tests were performed in triplicate.
Statistical analyses were performed with GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA). All measurement results were
expressed as mean ± SD. Statistical significances between the two
groups were determined with Student's t-test. p<0.05 was
considered statistically significant.
Results
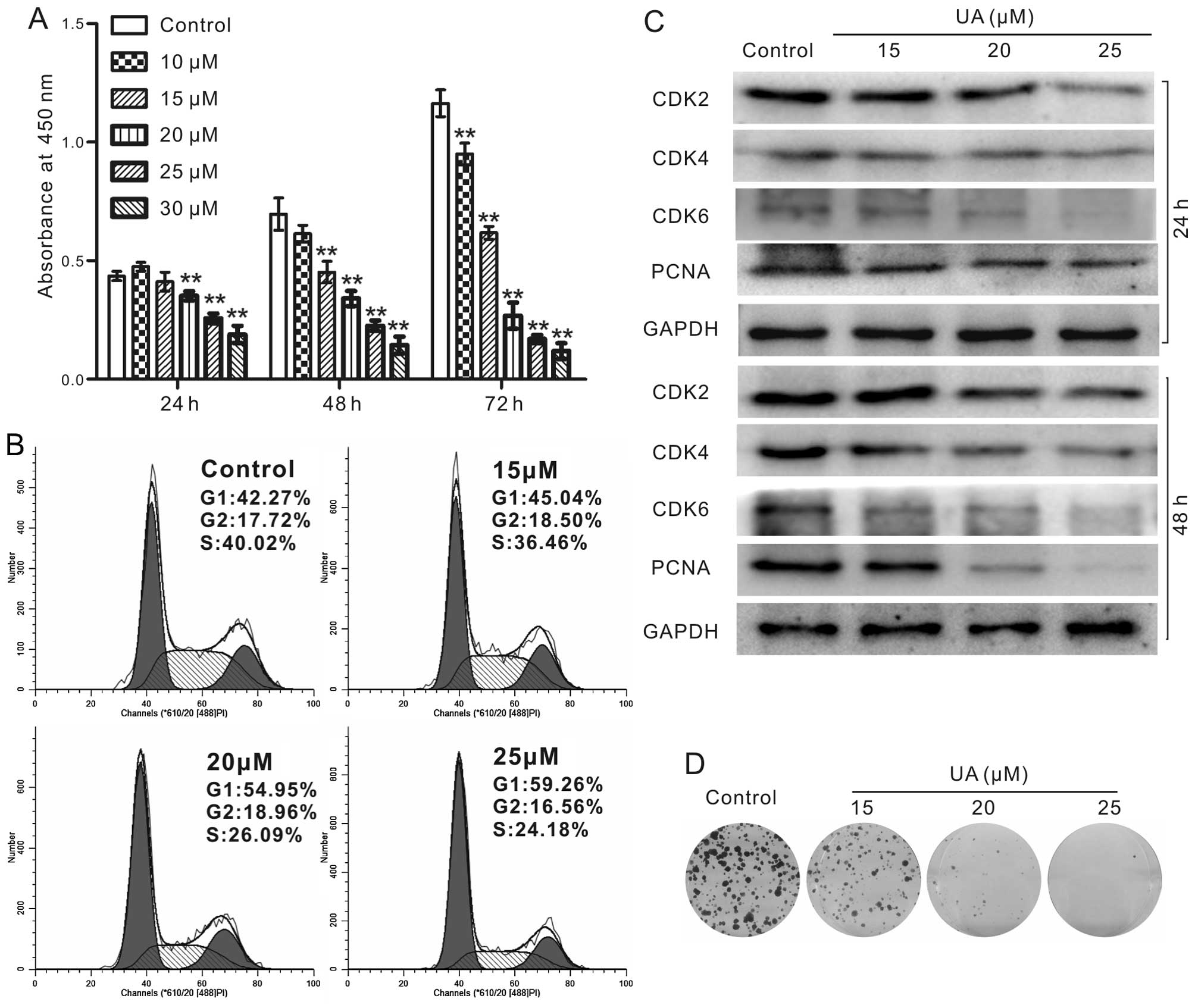
UA inhibits cell proliferation in 143B
cells
To identify whether UA may serve as an effective
chemotherapeutic reagent for human OS, the CCK-8 assay was employed
to validate the anti-proliferative effect of UA in 143B cells. We
found that the proliferation of 143B cells can be inhibited
markedly by UA in a time- and concentration-dependent manner
(Fig. 1A). Cell cycle analyses
indicated that UA induces cell cycle arrest at G1 phase in 143B
cells (Fig. 1B). We further
checked the biomarkers of G1 arrest. The results indicated that UA
inhibited the expression of cyclin-dependent kinase 2 (CDK2), CDK4
and CDK6 (Fig. 1C). Moreover, UA
effectively suppresses the protein level of PCNA (Fig. 1C), an indicator for the status of
proliferation (28). We further
checked whether UA can affect the long-term colony formation
ability in human OS cells. Our results illustrated that UA
concentration-dependently inhibits the colony formation in 143B
cells (Fig. 1D). The above results
showed that UA is capable of inhibiting cell proliferation in 143B
cells.
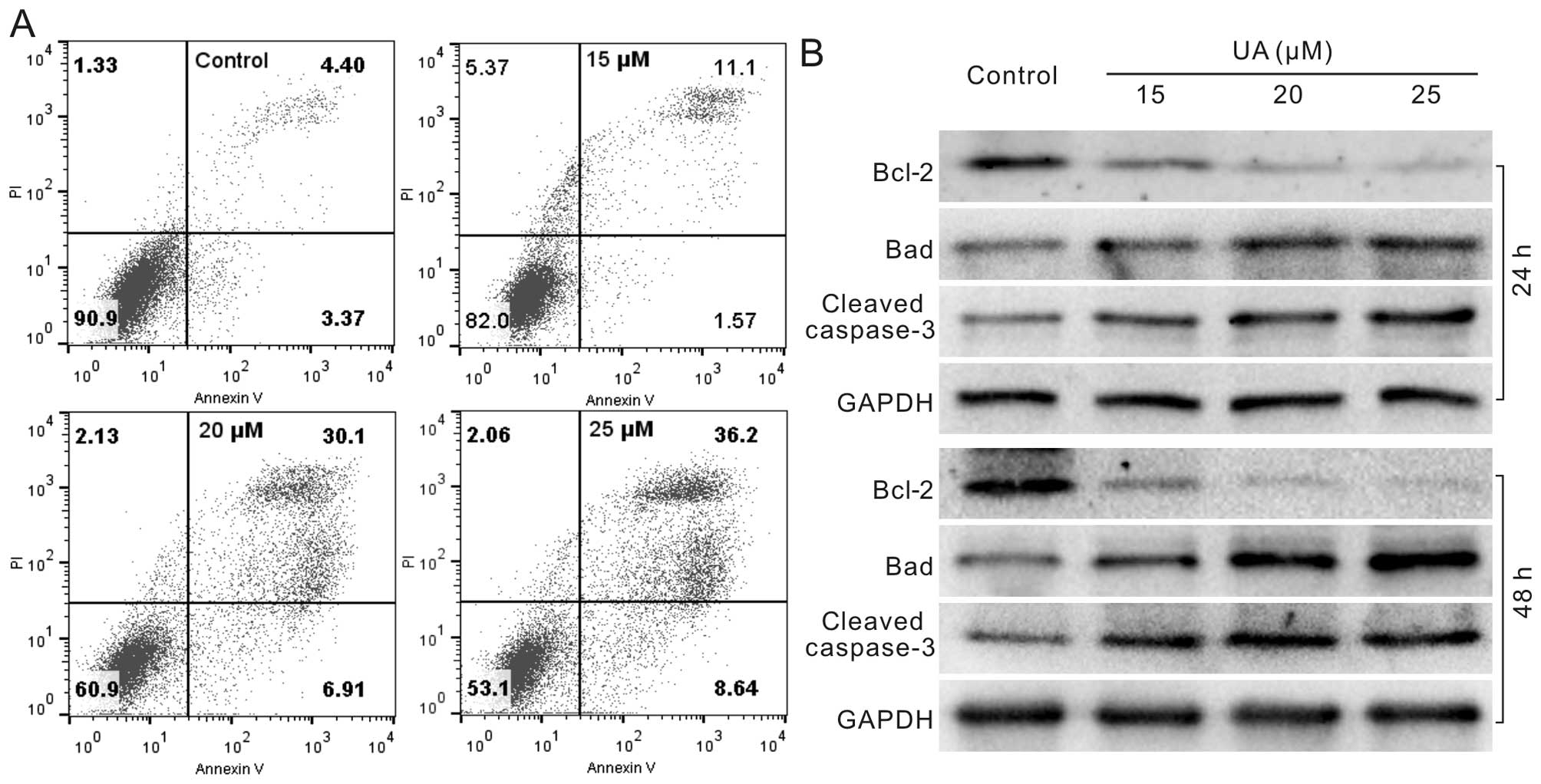
UA induces apoptosis in 143B cells
Next, we determined whether apoptosis occurs in
human OS cells with the treatment of UA. 143B cells were treated
with different concentrations of UA for 24 or 48 h. Then cells were
analyzed with flow cytometric assay or lysed for western blotting.
The results showed that UA can increase the apoptotic cell rate
(Fig. 2A), enhance the protein
level of Bad and cleaved caspase-3, and reduces the level of Bcl-2
concentration-dependently (Fig.
2B). According to the above results, UA can induce apoptosis in
OS cells.
UA inhibits the growth of OS tumor in
nude mice
We next assessed the antitumor activity of UA in
vivo with a well-established xenograft OS model (27). The results showed that tumor masses
in UA-treated group are smaller than those in control group, and UA
inhibits the tumor growth significantly compared with control group
(Fig. 3A and B). Subsequently,
histologic assay was conducted to evaluate the xenograft samples.
H&E staining results revealed that more necrotic cells occur in
UA-treated groups than that of the control group (Fig. 3C). Furthermore, the expression of
PCNA was markedly decreased in UA-treated groups (Fig. 3D), which was consistent with our
data in vitro. In addition, we evaluated tumor tissue at
molecular level and found that p53 was strongly elevated by UA,
while β-catenin, NF-κB and the phosphorylation of STAT3 were
decreased (Fig. 3E). These data
implied that UA may suppress the growth of OS via β-catenin and
inflammatory signaling. Collectively, these in vivo results
supported that UA may be a potential antitumor reagent for human
OS.
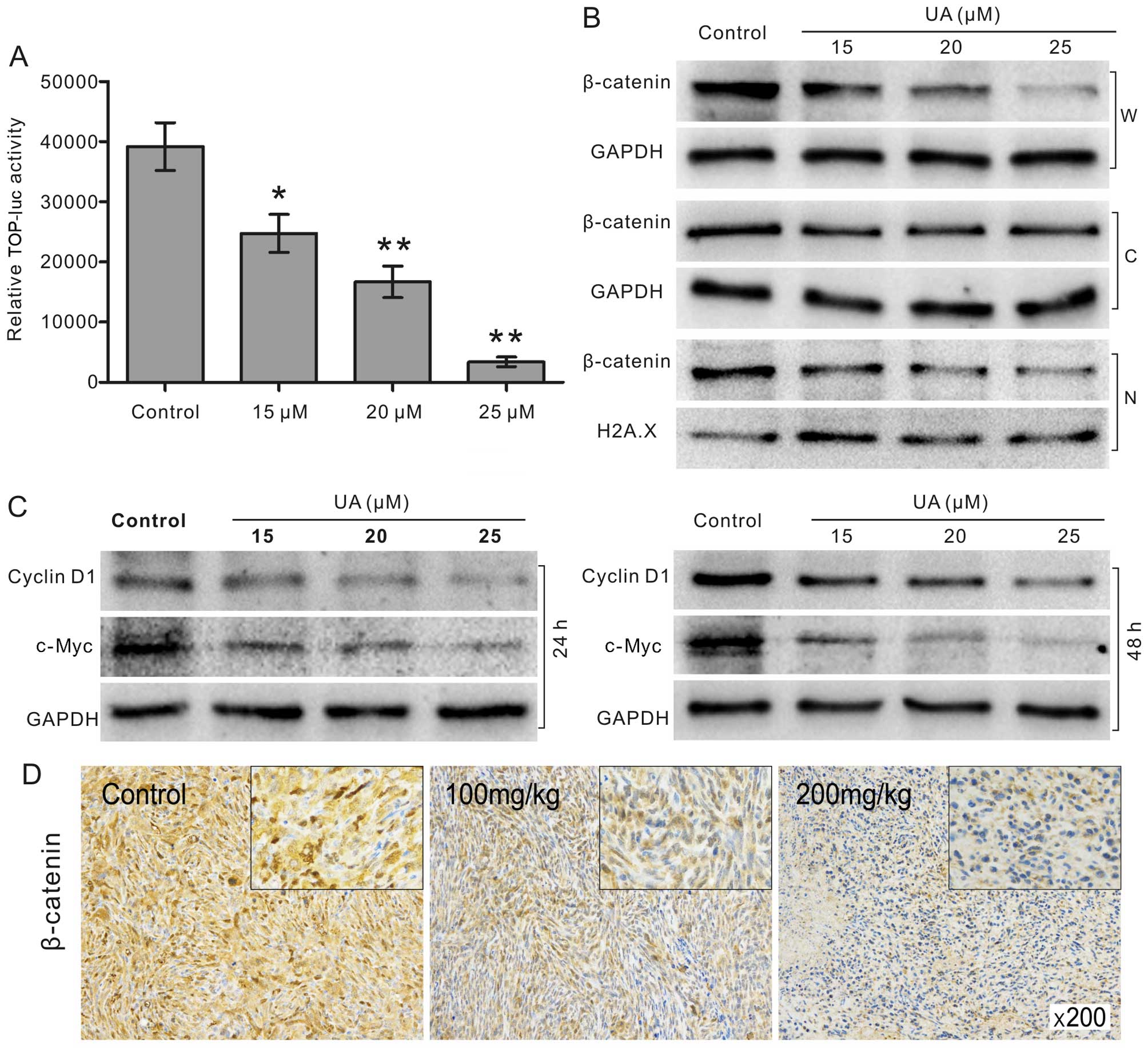
UA suppresses Wnt/β-catenin signaling in
143B cells
Cell proliferation is well regulated by multiple
signaling pathways. With luciferase reporter assay, we found that
the transcriptional activity of β-catenin/TCF-4 reporter was
effectively reduced by UA (Fig.
4A). Given that the stabilization and nuclear translocation of
β-catenin are critical events in the activation of Wnt/β-catenin
signaling (27), we employed
western blotting assay to check whether UA can decrease the level
of β-catenin in the whole cell, cytoplasm, and nucleus. The results
indicated that UA decreases the protein level of β-catenin not only
in the nucleus, but also in the cytoplasm and the whole cells
(Fig. 4B). Moreover, we checked
the level of downstream targets in Wnt/β-catenin signaling. The
results showed that the expression of c-Myc and cyclin D1 were both
decreased by UA concentration-dependently (Fig. 4C). The immunohistochemical results
showed that β-catenin positive cells were reduced with UA treatment
dose-dependently (Fig. 4D). These
results suggested that the anti-proliferative effects of UA in OS
cells may be associated with the suppression of Wnt/β-catenin
signaling.
Wnt/β-catenin partly mediates the
anti-proliferative effect of UA in 143B cells
To investigate the role of Wnt/β-catenin signaling
in the anti-proliferative effect of UA in 143B cells, we employed
recombinant adenovirus to mediate the exogenous expression or
knockdown for β-catenin. With CCK-8 assay, we found that exogenous
expression of β-catenin attenuated the anti-proliferative effects
of UA, while knockdown of β-catenin enhanced this function of UA in
143B cells (Fig. 5A). FACS
analysis results indicated that overexpression of β-catenin
attenuated the G1 phase arrest induced by UA in 143B cells. On the
contrary, β-catenin knockdown augmented UA-induced G1 phase arrest
(Fig. 5B). Thus, our data
indicated that UA may exert its antitumor effects in OS cells by
partly inactivating Wnt/β-catenin signaling.
UA inactivates Wnt/β-catenin signaling
through upregulating p53 in 143B cells
Although inactivation of Wnt/β-catenin signaling
partly mediates the anti-proliferative effects of UA in 143B cells,
the mechanism on how UA regulates Wnt/β-catenin signaling remains
unknown. With further research, we discovered that UA upregulated
the mRNA level of p53 (Fig. 6A),
increased the protein expression level of p53 and reduced the
expression of MDM2 time- and concentration-dependently (Fig. 6B). A previous study demonstrated
that overexpression of p53 downregulates β-catenin in human and
mouse cells (29). Therefore, we
hypothesized that UA-induced inactivation of Wnt/β-catenin
signaling may be mediated through the activation of p53. With
western blotting assay, we found that the effects of UA on
β-catenin, c-Myc and cyclin D1 were partly reversed by p53
inhibitor (PFT-α) (Fig. 6C).
Furthermore, the results of CCK-8 assay also showed that PFT-α can
partly attenuate the anti-proliferative effects of UA in 143B cells
(Fig. 6D), which is similar with
the effects of exogenous expression of β-catenin on
anti-proliferative effects of UA (Fig.
5A). Our data suggested that the inactivation of Wnt/β-catenin
signaling induced by UA may be mediated by upregulating p53 in OS
cells.
Discussion
In this study, we demonstrated that UA may be a
potential anti-proliferative drug for OS cells in vivo and
in vitro. Mechanistically, we discovered that the anticancer
activities of UA may be partly mediated by suppression of
Wnt/β-catenin signaling through upregulating p53 at least.
OS is one of the common malignants, which accounts
for the primary OS-induced mortalities. Although surgical and
medical advances have been made during the past decades, the
overall survival rate of patients with OS remains 60–65% (30). The present drugs used for OS
chemotherapy are mainly the same as that used in 1980s, such as
doxorubicin, etoposide, cisplatin, ifosfamide and high-dose
methotrexate (31). Therefore, it
is urgent to explore more efficient drugs or treatment regiments
for OS.
Herb-derived component is becoming increasingly
important in tumor therapies. For example, curcumin, sinomenine and
oldenlandia were all identified to be effective anti-osteosarcoma
drugs (32–34). UA was identified in wax coating of
apples 100 years ago. Nowadays, UA can be extracted from many
medical herbs and edible plants (35). It shows multiple pharmacological
functions, such as inhibition of tumor progression, induction of
cell differentiation, inhibition of angiogenic activity and control
of oxidants (35). For cancer, it
has been documented that UA can induce apoptosis in prostatic
cancer cells (36), inhibit the
proliferation of pancreatic cancer, increase the antitumor
potential of gemcitabine (6),
inhibit colorectal cancer angiogenesis (13), and chemoprevent the genesis,
metastasis and invasion of tumor in different animal models
(10,14). Recently, it was reported that UA
was effective in inducing apoptosis in MG-63 OS in vitro
(19). Accordingly, our data also
showed that UA inhibits proliferation time- and
concentration-dependently in 143B OS cells (Fig. 1); in addition, UA also induces
apoptosis in 143B cells by activating caspase-3 and modulating the
proteins associated with survival, such as Bad and Bcl-2 (Fig. 2). With further analysis, we proved
that UA is able to inhibit the growth of OS tumor in vivo
(Fig. 3). This evidence supported
the conclusion that UA may be a promising natural compound for
tumor therapy, such as OS at least.
As reported, UA is a multi-target natural product
(37), the antitumor effects of UA
may be mediated by inactivating Wnt/β-catenin, PI3K/Akt, MAPK and
NF-κB signaling (12,38,39).
Considering OS, the anticancer activity of UA may be associated
with upregulating caspase and activating ERK, JNK, and p38 MAPK
signaling (19). However, the
exact mechanism underlying the antitumor effects of UA in OS still
remains unclear. Wnt/β-catenin signaling is involved in the
processes of maintenance of homeostasis and development by
regulating cell proliferation, differentiation, migration and
apoptosis, as well as keeping stem cells under pluripotent state
(40). The aberrant activation of
Wnt/β-catenin signaling was implicated with tumorigenic, metastasis
and invasion of a variety of cancers (41), including OS. When Wnt/β-catenin
signaling is activated, β-catenin accumulates in the cytoplasm and
then translocates into the nucleus, where it regulates the
expression of downstream target genes to regulate the growth and
survival of cells (22).
Therefore, many antitumor drugs target Wnt/β-catenin signaling
(27,42,43).
A previous study has proved that accumulation of β-catenin in
nuclear and/or cytoplasm occurred in OS cells, and the accumulation
may be associated with the pathogenesis of OS (44). As Wnt/β-catenin signaling is a
target of UA, we speculated that the anticancer activity of UA in
143B cells may be also associated with it. In the present study, we
found that UA can inhibit the transcriptional activity of pTOP-luc
reporter in 143B cells (Fig. 4A),
as well as the expression of β-catenin in cytoplasm and nucleus
in vitro and in vivo (Fig. 4B and D). It is noteworthy that
c-Myc and cyclin D1 are downstream targets of Wnt/β-catenin
(45). We found that UA can reduce
the expression of c-Myc and cyclin D1 (Fig. 4C). All this evidence indicates that
UA can inhibit Wnt/β-catenin signaling in 143B OS cells. Our
results further demonstrated that exogenous expression of β-catenin
attenuates the effects of anti-proliferation and cell cycle arrest
induced by UA in 143B cells, while knockdown of β-catenin enhances
these functions of UA (Fig. 5).
Thus, the antitumor activities of UA in 143B OS cells may be
mediated by inactivating Wnt/β-catenin signaling, but this finding
alone does not reveal how Wnt/β-catenin signaling is modulated and
thus additional experiments need to be conducted to elucidate the
inhibitory mechanism.
p53, a well-known tumor suppressor, is a cell cycle
regulator with a transient half-life (46). The function of p53 is regulated by
enhancing its transcription and post-translational stabilization to
escape ubiquitin-dependent degradation (47). An earlier study reported that UA
can induce apoptosis in SW480 cells by increasing p53 (48). Moreover, Wnt/β-catenin signaling
can be downregulated by p53 (29,49).
To make sure that p53 is involved in the UA-induced cell growth
inhibition and apoptosis, we analyzed the effect of UA on the
expression level of p53 in 143B cells. The results showed that both
mRNA and protein level of p53 are increased by UA (Fig. 6A and B). Although Wnt/β-catenin
signaling is tightly modulated by the Axin/APC/GSK3β complex
(50), the level of β-catenin can
also be negatively regulated by p53 (29,49).
Furthermore, the downregulation of β-catenin induced by p53 was
accompanied with the inhibition of its transcription potential
(49). So we employed PFT-α, a p53
inhibitor, to determine whether p53 mediates the inhibition of
Wnt/β-catenin signaling induced by UA. PFT-α was verified to
effectively enhance the expression of β-catenin in gastric
adenocarcinoma cells (51).
However, a converse observation that PFT-α decreases the protein
level of β-catenin in WB-F344 cells was reported in another study
(52). These findings suggested
that the effects of PFT-α on β-catenin may be cell type-specific.
Our results indicated that PFT-α can effectively upregulate the
expression of β-catenin, as well as the targets of Wnt/β-catenin
signaling in 143B OS cells (Fig.
6C). We further analyzed the effect of p53 inactivation by
PFT-α on cell proliferation in 143B OS cells, and found that PFT-α
promotes the growth of 143B cells and attenuates the
anti-proliferative effects of UA. Hence, the inhibitory effects of
UA on Wnt/β-catenin signaling may be mediated by upregulating p53
partly in 143B cells.
Taken together, our data suggested that UA can be
used as an effective chemotherapy agent for human OS. The
anti-tumor activity of UA on OS may be mediated by inactivating
Wnt/β-catenin signaling through upregulating p53. However, the
exact molecular mechanisms through which UA upregulates p53 need to
be further investigated.
Acknowledgements
We thank Dr Tong-Chuan He (University of Chicago,
IL, USA) for providing recombinant adenoviruses and pTOP-luc
plasmid. This study was supported by a research grant from the
National Natural Science Foundation of China (grant nos. NSFC
81372120 and 81572226 to Bai-Cheng He).
References
|
1
|
Cheng S, Zhang X, Huang N, Qiu Q, Jin Y
and Jiang D: Down-regulation of S100A9 inhibits osteosarcoma cell
growth through inactivating MAPK and NF-κB signaling pathways. BMC
Cancer. 16:253. 2016. View Article : Google Scholar
|
|
2
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteo-sarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kempf-Bielack B, Bielack SS, Jürgens H,
Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G,
Kabisch H, et al: Osteosarcoma relapse after combined modality
therapy: An analysis of unselected patients in the Cooperative
Osteosarcoma Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ando K, Heymann M-F, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar
|
|
5
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: Current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prasad S, Yadav VR, Sung B, Gupta SC,
Tyagi AK and Aggarwal BB: Ursolic acid inhibits the growth of human
pancreatic cancer and enhances the antitumor potential of
gemcitabine in an orthotopic mouse model through suppression of the
inflammatory microenvironment. Oncotarget. 7:13182–13196.
2016.PubMed/NCBI
|
|
7
|
Pu F, Chen F, Lin S, Chen S, Zhang Z, Wang
B and Shao Z: The synergistic anticancer effect of cisplatin
combined with Oldenlandia diffusa in osteosarcoma MG-63 cell line
in vitro. Onco Targets Ther. 9:255–263. 2016.PubMed/NCBI
|
|
8
|
Chen S, Wang Z, Huang Y, O'Barr SA, Wong
RA, Yeung S and Chow MSS: Ginseng and anticancer drug combination
to improve cancer chemotherapy: A critical review. Evid Based
Complement Alternat Med. 2014:1689402014.PubMed/NCBI
|
|
9
|
Zhang Y, Kong C, Zeng Y, Wang L, Li Z,
Wang H, Xu C and Sun Y: Ursolic acid induces PC-3 cell apoptosis
via activation of JNK and inhibition of Akt pathways in vitro. Mol
Carcinog. 49:374–385. 2010.PubMed/NCBI
|
|
10
|
Huang CY, Lin CY, Tsai CW and Yin MC:
Inhibition of cell proliferation, invasion and migration by ursolic
acid in human lung cancer cell lines. Toxicol In Vitro.
25:1274–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shanmugam MK, Ong TH, Kumar AP, Lun CK, Ho
PC, Wong PTH, Hui KM and Sethi G: Ursolic acid inhibits the
initiation, progression of prostate cancer and prolongs the
survival of TRAMP mice by modulating pro-inflammatory pathways.
PLoS One. 7:e324762012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng L, Zhang R, Tang F, Li C, Xing Y-Y
and Xi T: Ursolic acid induces U937 cells differentiation by
PI3K/Akt pathway activation. Chin J Nat Med. 12:15–19.
2014.PubMed/NCBI
|
|
13
|
Lin J, Chen Y, Wei L, Hong Z, Sferra TJ
and Peng J: Ursolic acid inhibits colorectal cancer angiogenesis
through suppression of multiple signaling pathways. Int J Oncol.
43:1666–1674. 2013.PubMed/NCBI
|
|
14
|
Gayathri R, Priya DKD, Gunassekaran GR and
Sakthisekaran D: Ursolic acid attenuates oxidative stress-mediated
hepatocellular carcinoma induction by diethylnitrosamine in male
Wistar rats. Asian Pac J Cancer Prev. 10:933–938. 2009.
|
|
15
|
Choi BM, Park R, Pae HO, Yoo JC, Kim YC,
Jun CD, Jung BH, Oh GS, So HS, Kim YM, et al: Cyclic adenosine
monophosphate inhibits ursolic acid-induced apoptosis via
activation of protein kinase A in human leukaemic HL-60 cells.
Pharmacol Toxicol. 86:53–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harmand PO, Duval R, Delage C and Simon A:
Ursolic acid induces apoptosis through mitochondrial intrinsic
pathway and caspase-3 activation in M4Beu melanoma cells. Int J
Cancer. 114:1–11. 2005. View Article : Google Scholar
|
|
17
|
Lee YH, Kumar NC and Glickman RD:
Modulation of photo-chemical damage in normal and malignant cells
by naturally occurring compounds. Photochem Photobiol.
88:1385–1395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee YH, Wang E, Kumar N and Glickman RD:
Ursolic acid differentially modulates apoptosis in skin melanoma
and retinal pigment epithelial cells exposed to UV-VIS broadband
radiation. Apoptosis. 19:816–828. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu CC, Cheng CH, Lee YH, Chang IL, Chen
HY, Hsieh CP and Chueh PJ: Ursolic acid triggers apoptosis in human
osteosarcoma cells via caspase activation and the ERK1/2 MAPK
pathway. J Agric Food Chem. 64:4220–4226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Behrens J and Lustig B: The Wnt connection
to tumorigenesis. Int J Dev Biol. 48:477–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen C, Zhao M, Tian A, Zhang X, Yao Z and
Ma X: Aberrant activation of Wnt/β-catenin signaling drives
proliferation of bone sarcoma cells. Oncotarget. 6:17570–17583.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian J, He H and Lei G: Wnt/β-catenin
pathway in bone cancers. Tumour Biol. 35:9439–9445. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoang BH, Kubo T, Healey JH, Sowers R,
Mazza B, Yang R, Huvos AG, Meyers PA and Gorlick R: Expression of
LDL receptor-related protein 5 (LRP5) as a novel marker for disease
progression in high-grade osteosarcoma. Int J Cancer. 109:106–111.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kansara M, Tsang M, Kodjabachian L, Sims
NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons
PJ, et al: Wnt inhibitory factor 1 is epigenetically silenced in
human osteosarcoma, and targeted disruption accelerates
osteosarcomagenesis in mice. J Clin Invest. 119:837–851. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He BC, Gao JL, Luo X, Luo J, Shen J, Wang
L, Zhou Q, Wang YT, Luu HH, Haydon RC, et al: Ginsenoside Rg3
inhibits colorectal tumor growth through the downregulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011. View Article : Google Scholar
|
|
26
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Liu YZ, Zhang RX, Wang X, Meng ZJ,
Huang J, Wu K, Luo JY, Zuo GW, Chen L, et al: Oridonin inhibits the
proliferation of human osteosarcoma cells by suppressing
Wnt/β-catenin signaling. Int J Oncol. 45:795–803. 2014.PubMed/NCBI
|
|
28
|
Park HR and Park YK: Expression of p53
protein, PCNA, and Ki-67 in osteosarcomas of bone. J Korean Med
Sci. 10:360–367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sadot E, Geiger B, Oren M and Ben-Ze'ev A:
Down-regulation of beta-catenin by activated p53. Mol Cell Biol.
21:6768–6781. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Serra M and Hattinger CM: The
pharmacogenomics of osteosarcoma. Pharmacogenomics J. May
31–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hattinger CM, Fanelli M, Tavanti E, Vella
S, Ferrari S, Picci P and Serra M: Advances in emerging drugs for
osteosarcoma. Expert Opin Emerg Drugs. 20:495–514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen P, Wang H, Yang F, Chen H, He W and
Wang J: Curcumin promotes osteosarcoma cell death by activating
miR-125a/ERRα signal pathway. J Cell Biochem. May 27–2016.(Epub
ahead of print). View Article : Google Scholar
|
|
33
|
Siegel HJ and Pressey JG: Current concepts
on the surgical and medical management of osteosarcoma. Expert Rev
Anticancer Ther. 8:1257–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie T, Ren HY, Lin HQ, Mao JP, Zhu T, Wang
SD and Ye ZM: Sinomenine prevents metastasis of human osteosarcoma
cells via S phase arrest and suppression of tumor-related
neovascularization and osteolysis through the CXCR4-STAT3 pathway.
Int J Oncol. 48:2098–2112. 2016.PubMed/NCBI
|
|
35
|
Kashyap D, Tuli HS and Sharma AK: Ursolic
acid (UA): A metabolite with promising therapeutic potential. Life
Sci. 146:201–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng Y, Lin ZM, Ge N, Zhang DL, Huang J
and Kong F: Ursolic acid induces apoptosis of prostate cancer cells
via the PI3K/Akt/mTOR pathway. Am J Chin Med. 43:1471–1486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu BJ, Wang YQ, Wei XJ, Rong LQ, Wei D,
Yan CM, Wang DJ and Sun JY: Expression of WNT-5a and ROR2
correlates with disease severity in osteosarcoma. Mol Med Rep.
5:1033–1036. 2012.PubMed/NCBI
|
|
38
|
Park JH, Kwon HY, Sohn EJ, Kim KA, Kim B,
Jeong SJ, Song JH, Koo JS and Kim SH: Inhibition of Wnt/β-catenin
signaling mediates ursolic acid-induced apoptosis in PC-3 prostate
cancer cells. Pharmacol Rep. 65:1366–1374. 2013. View Article : Google Scholar
|
|
39
|
Li J, Liang X and Yang X: Ursolic acid
inhibits growth and induces apoptosis in gemcitabine-resistant
human pancreatic cancer via the JNK and PI3K/Akt/NF-κB pathways.
Oncol Rep. 28:501–510. 2012.PubMed/NCBI
|
|
40
|
Kahn M: Can we safely target the WNT
pathway? Nat Rev Drug Discov. 13:513–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsieh HY, Shen CH, Lin R-I, Feng YM, Huang
SY, Wang YH, Wu SF, Hsu CD and Chan MW: Cyproheptadine exhibits
antitumor activity in urothelial carcinoma cells by targeting GSK3β
to suppress mTOR and β-catenin signaling pathways. Cancer Lett.
370:56–65. 2016. View Article : Google Scholar
|
|
43
|
Vilchez V, Turcios L, Zaytseva Y, Stewart
R, Lee EY, Maynard E, Shah MB, Daily MF, Tzeng CW, Davenport D, et
al: Cancer stem cell marker expression alone and in combination
with microvascular invasion predicts poor prognosis in patients
undergoing transplantation for hepatocellular carcinoma. Am J Surg.
212:238–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haydon RC, Deyrup A, Ishikawa A, Heck R,
Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, et al:
Cytoplasmic and/or nuclear accumulation of the beta-catenin protein
is a frequent event in human osteosarcoma. Int J Cancer.
102:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang WG, Chen SJ, He JS, Li JS and Zang
XF: The tumor suppressive role of RASSF1A in osteosarcoma through
the Wnt signaling pathway. Tumour Biol. 37:8869–8877. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hasty P and Christy BA: p53 as an
intervention target for cancer and aging. Pathobiol Aging Age Relat
Dis. 3:32013.
|
|
47
|
Bansal N, Kadamb R, Mittal S, Vig L,
Sharma R, Dwarakanath BS and Saluja D: Tumor suppressor protein p53
recruits human Sin3B/HDAC1 complex for down-regulation of its
target promoters in response to genotoxic stress. PLoS One.
6:e261562011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nam H and Kim M-M: Ursolic acid induces
apoptosis of SW480 cells via p53 activation. Food Chem Toxicol.
62:579–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Levina E, Oren M and Ben-Ze'ev A:
Downregulation of beta-catenin by p53 involves changes in the rate
of beta-catenin phosphorylation and Axin dynamics. Oncogene.
23:4444–4453. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mishra R: Glycogen synthase kinase 3 beta:
Can it be a target for oral cancer. Mol Cancer. 9:1442010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fuentes RG, Toume K, Arai MA, Sadhu SK,
Ahmed F and Ishibashi M: Scopadulciol, isolated from Scoparia
dulcis, induces β-catenin degradation and overcomes tumor necrosis
factor-related apoptosis ligand resistance in AGS human gastric
adenocarcinoma cells. J Nat Prod. 78:864–872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xie BS, He XX, Ai ZL and Yao SK:
Involvement of β-catenin in matrine-induced autophagy and apoptosis
in WB-F344 cells. Mol Med Rep. 9:2547–2553. 2014.PubMed/NCBI
|