Introduction
Meningiomas are tumours of neoplastic arachnoid cap
cells which can arise from the dura at any site. This is, however,
most commonly the skull vault, from the skull base and at sites of
dural reflections, whereas those which arise from the spine account
for only 10% of meningiomas. In addition, they are the most
frequently encountered benign, non-glial, neoplasms within the
skull, accounting for nearly a quarter of all primary intracranial
tumours (1) and have an estimated
annual incidence of 2–7 per 100,000 women and 1–5 per 100,000 men
(2). Meningiomas are typically
benign tumours but have a broad spectrum of clinical
characteristics and histologically distinct subsets which are
associated with high risk of recurrence, even after complete
resection. According to the WHO classification, benign meningiomas
(Grade I) have a low risk of recurrence and aggressive growth with
22 subtypes, including meningothelial, fibroblastic, transitional
and psammomatous meningiomas. Atypical meningiomas (Grade II) are
more likely to recur whereas anaplastic meningiomas (Grade III)
have the greatest likelihood of recurrence and/or have more
aggressive behaviour (3).
Although histologically benign meningiomas differ in
their patterns of invasion from atypical meningiomas, they can
still invade the dura, dural sinuses, skull and extracranial
compartments because of their ability to extend into mesenchymal
tissues. They are not, however, considered to be atypical or
malignant. In contrast, brain invasion is associated with
recurrence and mortality rates similar to atypical meningiomas,
even if the neoplasm appears to be otherwise completely benign
(4).
A critical step of tumour progression and recurrence
is the infiltrative invasion into the contiguous tissue.
Metalloproteinases (MMPs) are a family of 23 structurally related,
zinc-dependent endopeptidases in man. For many years, some of the
MMPs have been widely implicated as mediators of invasion and
angiogenesis with a role in degradation of the extracellular matrix
(ECM). It is now evident that the functions of MMPs are much more
complex than was initially thought since they also have other roles
such as regulation of cell adhesion (5), and control of apoptosis via release
of death or survival factors. MMPs are also known to regulate the
bioavailability and/or activity of growth factors by mediating
receptor turnover or by cleaving matrix proteins associated with
growth factors. EMMPRIN (extracellular matrix metalloproteinase
inducer), which is also known as CD147, basigin or M6, is thought
to induce tumour invasion by activating production of various MMPs.
The ability of MMP-9 to trigger the release of vascular endothelial
growth factor (VEGF) which regulates angiogenesis and vascular
permeability is also well documented (6). Additionally, the epidermal growth
factor receptor (EGFR) family of membrane-bound receptor tyrosine
kinases which comprise 4 structurally related receptors, (EGFR,
HER2, HER3/ErbB3 and HER4/ErbB4), plays an important role in tumour
invasion (7).
The expression of some MMPs has been well studied in
gliomas (8–12) and meningiomas (13–19)
particularly in relation to invasion or recurrence. Closely related
to the MMPs is the ADAM (a disintegrin and metalloproteinase)
family which has also been implicated in cancer, particularly via
ADAM-mediated activation of EGFR signalling (20). ADAM8 and ADAM19 have also been
reported to be overexpressed in various brain tumours (21) as well as ADAM10 in gliomas
(22) and have been implicated in
local invasion.
RECK (reversion-inducing cysteine-rich protein with
kazal motifs) is a tumour and metastatic suppressor protein which
is a negative regulator of MMPs and has been implicated in the
regulation of both tumour invasion and angiogenesis. The four
members of the tissue inhibitors of matrix metalloproteinases
(TIMPs) family which were previously thought to be endogenous
inhibitors of MMPs alone are now known to inhibit several ADAMs and
to be involved independently in modulation of various biological
activities including cell proliferation, migration, invasion,
angiogenesis and apoptosis (23).
Although there are several reports of elevated
levels of some ADAMs in gliomas, there is a lack of studies for the
co-expression of these with MMPs, TIMPs, various growth factors and
their receptors in benign meningiomas. The aim of this study was to
characterize RNA expression of the MMP family, 4 TIMPs, RECK as
well as 8 ADAMs and selective growth factors and/or receptors
implicated as modulators of invasion or angiogenesis as well as
predictors of recurrence. Quantitative real-time PCR (qPCR) was
used for this evaluation in 17 low grade meningiomas (12 cultured
cells and 5 tissue samples) of different subtypes, including
fibroblastic, meningothelial and transitional.
Materials and methods
Clinical samples
Seventeen low grade human meningiomas were studied
for their profiles of MMPs, TIMPs, ADAMs and various growth factors
and/or receptors (Table I). The
surgical specimens used in this study were from 11 females and 6
males, between the age of 34 and 75, with a mean age of 54. There
were different subtypes of low grade meningiomas within this group
of patients, generally Grade I except one with brain invasion
(Grade II). The tumour site for the samples included frontal and
occipital lobes as well as olfactory groove, cerebellopontine
angle, tentorium and spinal.
 | Table IClinical data, histological
classification and anatomical site of 17 meningiomas analysed. |
Table I
Clinical data, histological
classification and anatomical site of 17 meningiomas analysed.
| No | Meningioma
designation | Source | Age (years) | Gender | Anatomical
site | Histological
subtype | WHO grade |
|---|
| 1 | 0071/01
(P4) | KCH | 38 | Female | R cerebellopontine
angle | Fibroblastic | I |
| 2 | 0076/01
(P3) | KCH | 53 | Female | Occipital | Fibroblastic | I |
| 3 |
0089/01(P4) | KCH | 54 | Female | Olfactory
groove | Fibroblastic | I |
| 4 | 0196/01
(P3) | KCH | 44 | Male | R sphenoid
wing |
Meningiothelial-with brain invasion | II |
| 5 | 0250/01
(P3) | KCH | 60 | Female | Bifrontal | Meningioma-with
hemangiopericytic like pattern | I |
| 6 | 0366/01
(P3) | KCH | 49 | Female | Frontal | Meningioma NOS | I |
| 7 | 1177/00
(P5) | KCH | 45 | Female | Subfrontal | Transitional | I |
| 8 | 0191/01
(P5) | KCH | 69 | Female | Spinal | Psammomatous | I |
| 9 | 0208/01
(P3) | KCH | 51 | Female | Tentorium |
Fibroblastic-recurrent | I |
| 10 | 0263/01
(P3) | KCH | 43 | Male | Olfactory
groove |
Meningiothelial | I |
| 11 | 0460/01
(P3) | KCH | 70 | Female | Spinal | Psammomatous | I |
| 12 | 0461/01
(P2) | KCH | 34 | Male | Frontal |
Meningiothelial | I |
| 13 | BT3/01 | CA | 52 | Male | R dura frontal |
Meningiothelial | I |
| 14 | BT72 | CA | 75 | Male | L dura olfactory
groove | Transitional | I |
| 15 | BT159 | CA | 37 | Female | L dura
tentorium |
Meningiothelial | I |
| 16 | BT294 | CA | 74 | Male | R Parietal
occipital | Transitional | I |
| 17 | BT301 | CA | 71 | Female | L Frontal |
Meningiothelial | I |
The first twelve samples used (numbered 1–12,
Table I) were cultured cells at
low passages (between P2 and P6). They were obtained from patients
undergoing surgery, under local Ethical permission (LREC No
00-173), from the Department of Neurosurgery, King’s College
Hospital, London in 2000 and 2001. The tumour was diagnosed,
according to the World Health Organisation criteria by a
neuropathologist. Cells were cultured as monolayers in small
plastic culture flasks (Marathon) at 37°C, 5% CO2 in a
standard humidified incubator. Cells were routinely maintained in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with
antibiotics/antimycotic at the final concentration of 100 IU
penicillin, 100 μg amphotericin per ml and 10% fetal calf serum
(FCS; Sigma-Aldrich).
The remaining five samples used were frozen biopsies
(numbers 13–17, with a prefix, BT). These were collected between
1991 and 1997 and kindly provided by Dr Peter Forsyth, University
of Calgary, Canada. Tissue was collected, under local Ethical
permission, in the operating theatre immediately after removal and
snap frozen in liquid nitrogen. The meningiomas were classified and
graded by a neuropathologist at this institution. Samples, having
been previously frozen in liquid nitrogen, were homogenized in
RNAzol, and frozen at −20°C until the RNA was isolated. Dr Robert
Nuttall carried out the qPCR at the School of Biological Sciences,
University of East Anglia, Norwich.
RNA isolation and reverse
transcription
Total RNA was isolated from cell culture and tissue
lysates according to the instructions provided with the RNAzol. RNA
was resuspended in diethyl pyrocarbonate-treated (Sigma-Aldrich)
water. The concentrations were then determined by spectrophotometry
using a GeneQuant pro RNA/DNA calculator (Amersham Pharmacia
Biotech, Little Chalfont, UK). One microgram of total RNA was
reverse transcribed using 2 μg random hexamers (Amersham Pharmacia
Biotech) and SuperScript II reverse transcriptase (Life
Technologies, Paisley, UK) according to the supplier’s
instructions. Complementary DNA copy (cDNA) was stored at −20°C
until required for the polymerase chain reaction (PCR).
Quantitative real-time PCR
Sequences for 100 nM probes and 200 nM primers used
in the present study for MMPs, TIMPs, ADAMs, TGF-α, HB-EGF, EGF-R,
erb-B2, erb-B3, VEGF-A, KDR and flt-1 have previously been
described elsewhere (9,24). 18S was TaqMan® Ribosomal
RNA Control reagents part no. 4308329 (Applied Biosystems,
Warrington, UK) and used as an endogenous control. Briefly, PCR
reactions were performed using the ABI Prism 7700 Sequence
Detection system (Applied Biosystems), using the manufacturer’s
protocol. Each reaction was performed in 25 μl and contained the
equivalent of 5 ng of reverse transcribed RNA (1 ng RNA for the 18S
analyses), 50% TaqMan 2X PCR Master Mix (Applied Biosystems), 100
nM each of the forward and reverse primer, and 200 nM of probe.
Conditions for the PCR reaction were 2 min at 50°C, 10 min at 95°C
and then 40 cycles, each consisting of 15 sec at 95°C, and 1 min at
60°C. To determine the relative RNA levels within the samples,
standard curves for the PCR reaction were prepared by using the
cDNA from one sample and making 2-fold serial dilutions covering
the range equivalent to 20-0.625 ng of RNA (for 18S analyses the
range was from 4 to 0.125 ng). These dilutions were subject to
real-time PCR as described above. Relative standard curves for
cycle threshold (CT) vs. input RNA were prepared, and
relative levels of starting RNA in each sample were determined. The
results for each target mRNA were normalized to those from 18S
ribosomal RNA from the same sample.
Statistical analysis
The RNA levels for each gene obtained from the
standard curves were corrected using the 18S rRNA levels. All data
displays and statistical analyses were undertaken at these ratios.
To determine association between gene levels in the meningiomas,
Spearman’s rank correlation co-efficients (rs) were calculated
between all genes regardless of sub-type or invasion. Rs values of
>0.7 [P<0.01, (n=17)] were considered of potential
significance. All statistical analyses were undertaken using
statistical software Minitab v.15.1 and SPSS v.15.0.
Results
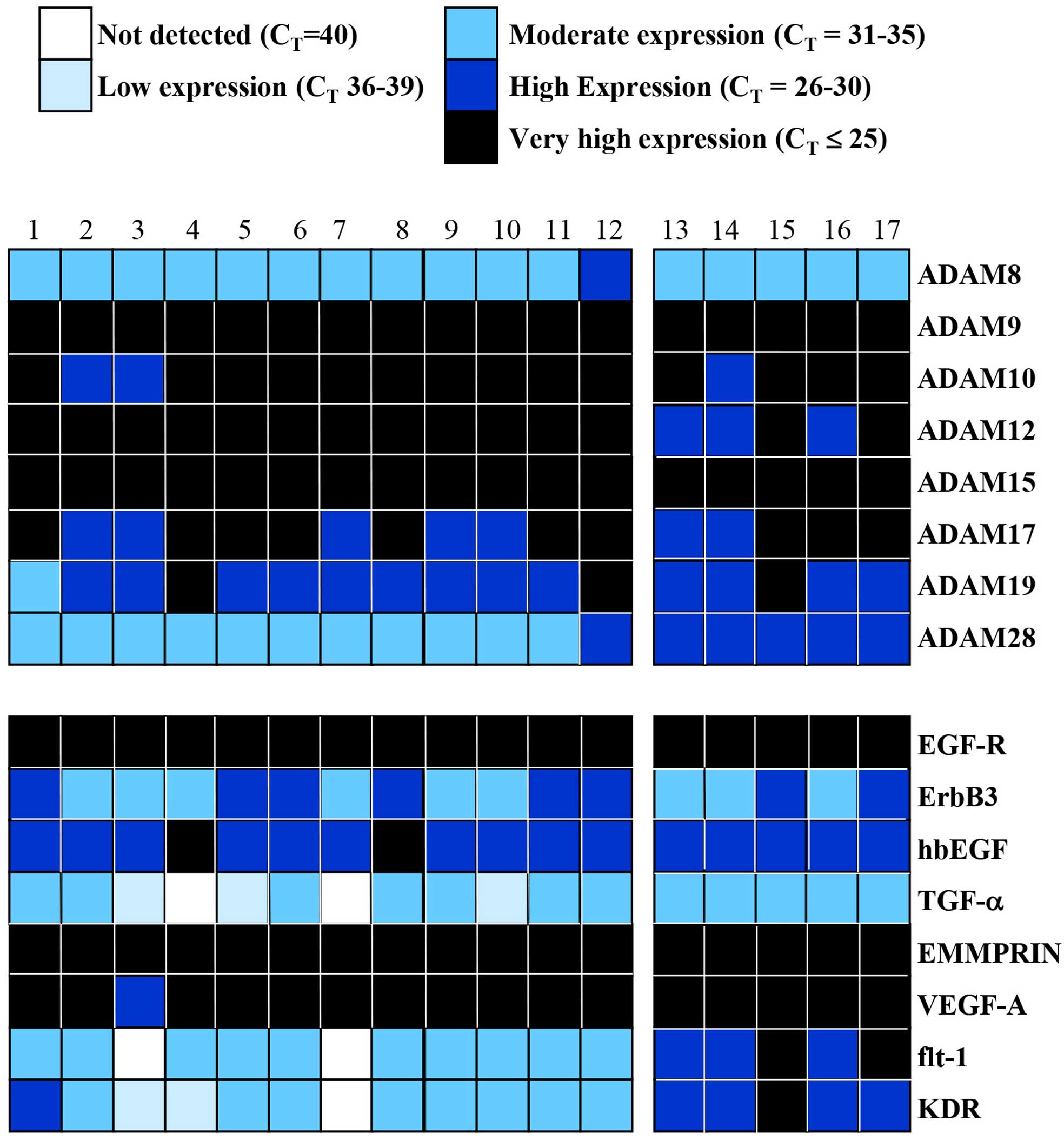
RNA levels for all human 23 MMPs, 4 TIMPs, RECK, 8
ADAMs (ADAM8, 9, 10, 12, 15, 17, 19 and 28), EMMPRIN and growth
factors and/or receptors (TGF-α, HB-EGF, EGF-R, erb-B2, erb-B3,
VEGF-A, KDR and flt-1) were profiled in a series of 17 benign
meningiomas. We first used the cycle threshold (CT) of
each gene to classify its expression as not detected
(CT=40), low (CT=36–39), moderate
(CT=31–35), high (CT=26–30) or very high
(CT=≤25) (Figs. 1 and
2).
Differential expression of MMPs and TIMPs
in 17 meningiomas
Of all the MMPs, MMP14 (membrane-type MMP or MT1
MMP) was very highly expressed in all but one sample. Similarly,
MMP2 was also very highly expressed in all the snap frozen tissue
samples (samples 13–17) and the majority of cultured cells (samples
1–12) whereas MMP9 was only highly expressed in 5 of the latter
group (Fig. 1). MMP11 and MMP19
were highly and consistently expressed in all the meningiomas
studied. Generally, expression of MMP26 and 27 was detected in a
small number of meningiomas, while some of the MMPs (MMP8, 17, 21,
23, 24 and 25) were present in most samples albeit at low or
moderate levels. There was limited or no MMP13 expression in most
samples whereas none of the meningiomas from biopsy-derived cell
cultures at low passage or snap frozen biopsy tissues expressed any
MMP20. In addition, TIMP-1, -2 and -3 were detected at very high
levels in every meningioma whereas TIMP-4 and RECK were generally
expressed at high levels (Fig.
1).
Differential expression of ADAMs and
growth factors and/or receptors in 17 meningiomas
Given the elevated levels of some ADAMs reported in
brain tumours, particularly gliomas, we quantified the RNA levels
of 8 ADAMs (ADAM8, 9, 10, 12, 15, 17, 19 and 28) in 17 meningiomas
(Fig. 2). ADAM9 and 15 were very
highly expressed in every meningioma sample in the study whereas
ADAM10, 12, 17 and 19 were either very highly or highly expressed
in them. ADAM8 was moderately expressed in all except one
meningioma.
We also analysed the RNA levels of a few members of
the EGF and VEGF families (Fig. 2)
to assess if there was any concomitant expression with MMPs, ADAMs
and TIMPs. EGF-R, EMMPRIN and VEGF-A were very highly expressed
whereas hbEGF was generally highly expressed in all the meningiomas
compared to the other EGFR receptor (ErbB3), TGF-α, and the VEGF
receptors (flt-1 or VEGFR-1 and flk/KDR or VEGFR2).
Patterns of gene expression in low
passage cultured cells compared with snap frozen biopsy tissue
samples
Close examination of the data revealed noticeable
patterns in gene expression for selected protease, growth factor
and receptor across different meningioma samples. For example, the
snap frozen biopsy tissues (samples 13–17) from the University of
Calgary showed either negligible or no expression of MMP1, 3, 10
and 12 compared to the primary cell cultures (samples 1–12) from
King’s College Hospital (Fig. 1).
The reverse pattern was apparent with ADAM28 since all the cell
cultured meningiomas, except one, expressed it moderately whereas
all the snap frozen tissue samples expressed it highly. Similarly,
flt-1 and KDR were highly expressed in the latter and mostly
moderately expressed in the former (Fig. 2).
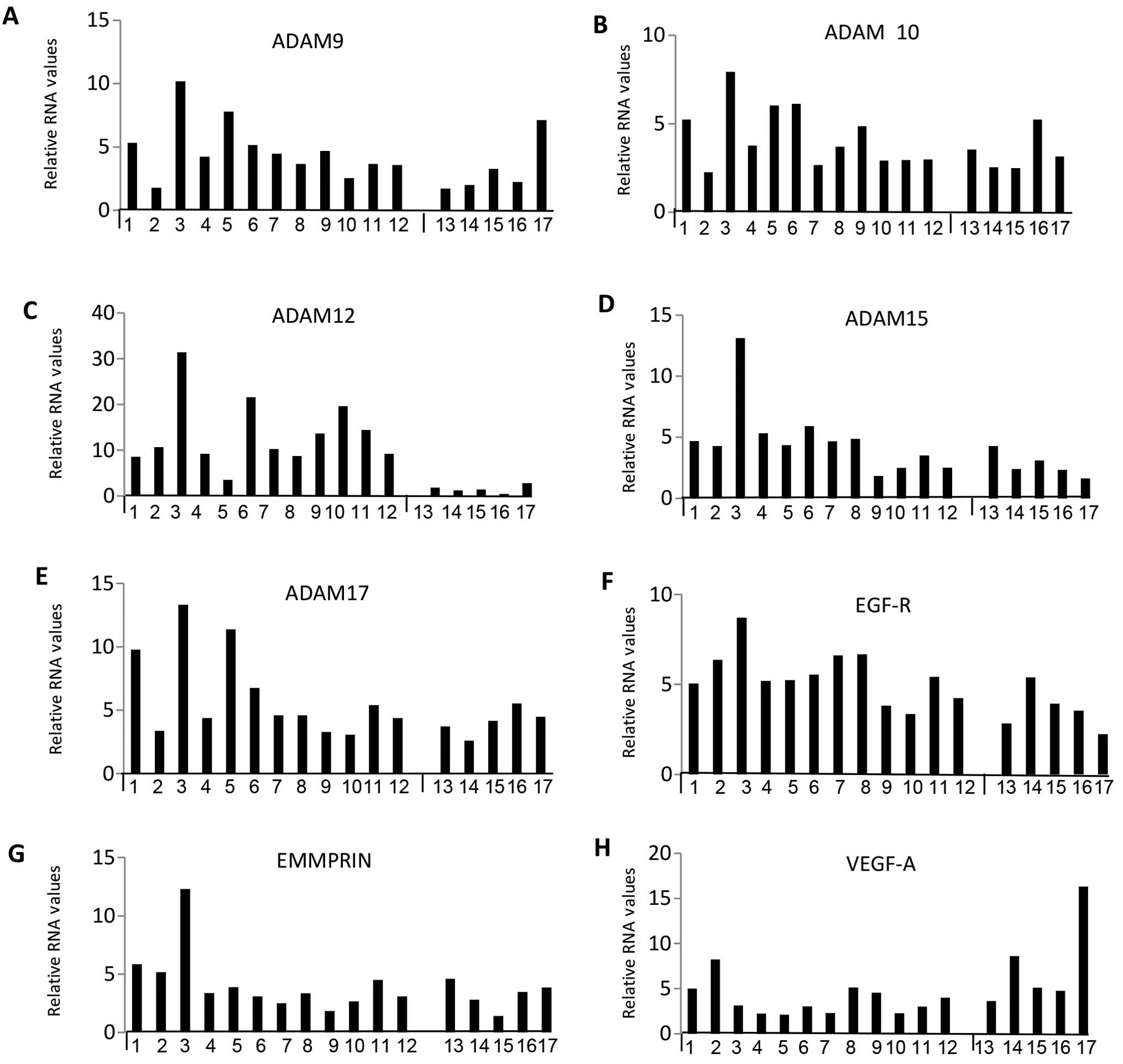
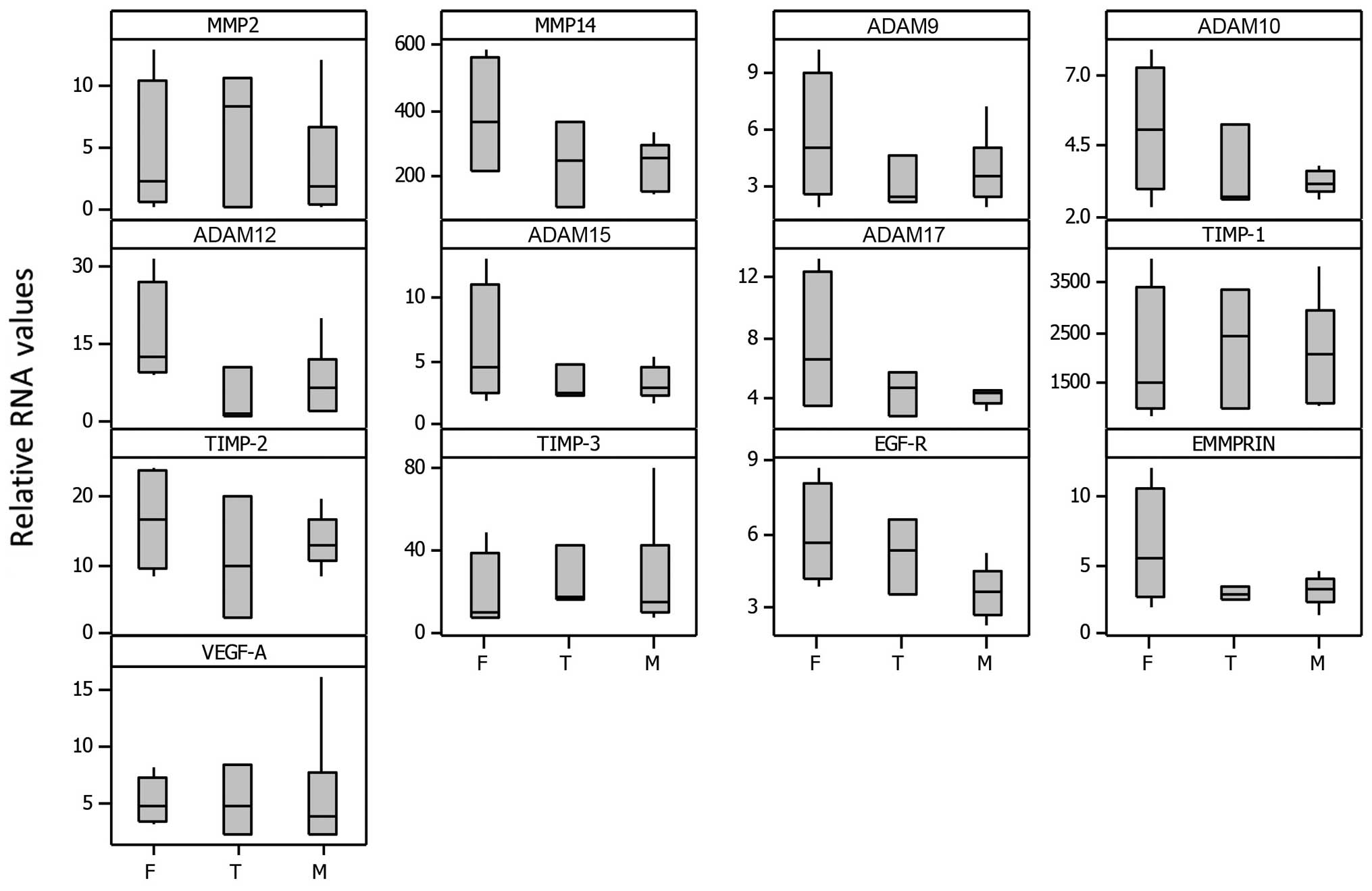
Correlations between selected 13 elevated
genes expressed in subtypes of meningiomas
Next, we statistically analysed the data to see if
there were any meaningful patterns or correlations for 13 selected
MMPs, ADAMs, growth factors and receptors ( MMP2 and 14, ADAM9, 10,
12, 15 and 17, TIMP-1, -2 and -3, EGF-R, EMMPRIN and VEGF-A) which
were very highly expressed. Figs.
3 and 4 show the relative RNA
levels for individual samples while Fig. 5 represents box plots for select
genes grouped according to meningioma subtype (fibroblastic,
transitional and meningothelial). Relative RNA expression was
normalized to 18S rRNA levels and is presented relative to normal
brain sample. Although the sample size was small, [F (fibroblastic)
n=4, T (transitional) n=3 and M (meningothelial) n=6], some trends
were evident, in that the fibroblastic subtype generally showed
more expression compared with the meningothelial whereas the
transitional showed intermediate expression, particularly for
MMP14, ADAM9, 10, 12, 15 and 17, TIMP-2, EGFR and EMMPRIN. Overall,
the fibroblastic meningiomas displayed the highest median levels
for 10 of the 13 selected gene expressions as well as having the
maximum expression in 11 out of 13 of them.
Spearman rank analysis showed that the RNA levels
had significant correlations (Rs >0.7), between some of the
MMPs, TIMPs, growth factors and their receptors in the meningiomas
(Table II). The data revealed
that the levels of MMP12 correlated positively with MMP1, 3, 10,
TIMP4 and RECK, whereas levels of ADAM12 correlated positively with
MMP1, 3, 10, 12, 16, TIMP4 and RECK but negatively with that of
KDR. The levels of ADAM17 correlated with levels of ADAM9 and 10
only; levels of ADAM15 were positively correlated with EGFR and
negatively with levels of flt-1. ADAM28 levels were positively
correlated with both VEGF receptors (flt-1 and KDR).
 | Table IIInter-correlation (Spearman’s rank)
of MMPs, ADAMs, TIMPs and growth factors/receptors in a series of
meningiomas. |
Table II
Inter-correlation (Spearman’s rank)
of MMPs, ADAMs, TIMPs and growth factors/receptors in a series of
meningiomas.
| MMP1 | MMP2 | MMP3 | MMP8 | MMP10 | MMP11 | MMP12 | MMP15 | MMP16 | MMP25 | MMP26 | ADAM9 | ADAM10 | ADAM12 | ADAM15 | ADAM28 | EGFR | Flt-1 |
|---|
| MMP3 | 0.92a | | | | | | | | | | | | | | | | | |
| MMP9 | | | | 0.76a | | | | | | | | | | | | | | |
| MMP10 | 0.84a | | 0.90a | | | | | | | | | | | | | | | |
| MMP12 | 0.89b | | 0.93a | | 0.82a | | | | | | | | | | | | | |
| MMP13 | 0.68b | | | | | | | | | | | | | | | | | |
| MMP14 | | 0.68b | | | | | | | | | | | | | | | | |
| MMP16 | | | | | 0.75a | | | | | | | | | | | | | |
| MMP19 | | | | | | 0.65b | | | | | | | | | | | | |
| MMP27 | | | | | | | | | | | 0.72a | | | | | | | |
| ADAM12 | 0.83a | | 0.86a | | 0.85a | | 0.86a | | 0.68b | | | | | | | | | |
| ADAM17 | | | | | | | | | | | | 0.69b | 0.70b | | | | | |
| TIMP-1 | | | | | | | | | | 0.72a | | | | | | 0.83a | | |
| TIMP-3 | | | | | | | | 0.79a | | | | | | | | | | |
| TIMP-4 | 0.80a | | 0.73b | | 0.78a | | 0.75a | | | | | | | 0.67b | | | | |
| RECK | 0.71b | | | | | | 0.69b | | | | | | | 0.67b | | | | |
| EGFR | 0.65b | | | | | | | | | | | | | | 0.72a | | | |
| hbEGF | | | | | | | | | | | | | | | | | 0.66b | |
| Flt-1 | | 0.67b | | | | | | | | | | | | | −0.71a | 0.72a | | |
| KDR | | | | | | | | | | | | | | −0.71a | | 0.68b | | 0.72a |
Discussion
Most benign meningiomas grow slowly by expansion,
leading to compression of adjacent structures. Others vary in their
biological characteristics, such as invasion, in that some of these
neoplasms tend to invade brain parenchyma and bone because of their
ability to extend into mesenchymal tissues. Recurrence is usually
seen in classic benign meningiomas due to incomplete resection in
contrast to atypical and anaplastic meningiomas, which show
increased rate of proliferation and cord like cellular invasion
into underlying brain parenchyma. An important biological feature
of recurrence is invasion which is mediated by a cohort of
proteases including MMPs and ADAMs for the degradation of a variety
of extracellular matrix (ECM) macromolecules.
Over the last two decades, different techniques
(such as western and northern blots, zymography and
immunohistochemistry) have been used to show expression of a few
MMPs and their endogenous inhibitors, TIMPs, in brain tumours.
However, with the advent of molecular technology, particularly
qPCR, comprehensive studies for the gene expression of a battery of
MMPs, ADAMs, TIMPs and growth factors has become possible allowing
identification of different correlations and patterns associated
with various biological features of these extrinsic brain tumours.
Indeed, the discovery of ADAMs and changes in proposed roles of
MMPs and TIMPs generally suggests that they may contribute to
different stages of tumour progression by regulating cell
proliferation, invasion, angiogenesis and apoptosis as they are
known to regulate growth factor activities as well as integrin
function (21,25–29).
In this study, low passage biopsy-derived cultured
cells (samples 1–12 from King’s College Hospital) were used since
our research interest has been primarily on in vitro studies
of tumour cell invasion. However, snap frozen biopsy tissues
(samples 13–17 from University of Calgary) were included for
comparative purposes.
Ubiquitous expression of a battery of MMPs has been
reported in the literature in benign meningiomas, some of which
have been associated with the potential to invade. The high or
moderate expression of MMP1 and MMP12 seen in the cultures cells
(samples 1–12) is consistent with the finding of other workers who
have implicated them in invasion of meningiomas (30) and gliomas (31), respectively. Other mediators of
invasion which are well documented include MMP2, 9 and 14. Notably,
of the 23 MMPs studied, MMP14 was expressed very highly in the vast
majority of meningiomas, whereas MMP9 was expressed highly in only
5 of the low passage cultured cells. MMP2 was generally very highly
or highly expressed. This finding is consistent with previous
reports suggesting their association with the potential to invade,
especially increased levels of MMP2 and MMP9 in Grade 1 meningiomas
as prognostic or predictive factors of recurrence (16,32).
Moreover, in another study, the prognostic value of MMP9 in the
risk of recurrence of meningiomas was investigated by analysing its
expression in a series of meningiomas of different histological
type and grade. It was expressed highly in 64% of them and was
significantly associated with histological grade (18).
MMP28 (Epilysin) was highly or moderately expressed
in every meningioma in the present study. Very recently one study
suggested that significant elevation of MMP28 levels in
glioblastoma patients may predict unfavorable overall survival
(12). High expressions of MMP11
and 19 seen in all the meningiomas in this study are consistent
with the findings of previous reports using RT-PCR, western
blotting and immunohistochemistry which indicated that they
correlated with the WHO-grading of human malignant gliomas
(11). Indeed, MMP19 has also been
reported to be a facilitator of invasion in gliomas (33). This further confirms the notion
that benign meningiomas express some of these MMPs as they have
potential to invade like malignant gliomas. In contrast, MMP20 was
not detected in any of the meningiomas, similar to previous
findings with various cancers including de novo glioblastoma
multiforme (9) and 7 established
GBM cell lines (11).
Often results based on different methodology are not
comparable leading to dissimilar and contradictory conclusions.
Nonetheless, a recent study has reviewed MMP expression by
different methods in glioma cell cultures, established cell lines
and biopsy tissue samples and suggested that there is a correlation
for the expression of MMP1, 2, 7, 9, 11, 14, 15 and 25 with tumour
grade. Furthermore, MMP3, 8, 10, 13, 16, 17, 20, 21, 23, 26, 27 and
28 may not have a major role in tumorigenesis as some of these have
limited or no expression (34).
The tissue inhibitors of MMPs, TIMP-1, -2 and -3
were also very highly expressed in all 17 meningiomas studied
whereas TIMP-4 and RECK (a negative regulator of MMPs) were only
highly expressed generally. Although TIMP-1 level is thought to
correlate positively with histological grades of glioma, TIMP-4 has
a negative correlation. TIMP-3 has the broadest spectrum for
inhibition as it also inhibits several members of the ADAM family
(35). TIMPs are now believed to
be multifunctional proteins with some biological activities, which
may be partially due to MMP inhibition but may also be independent
of MMPs, such as modulation of cell proliferation, invasion,
anti-angiogenesis, pro- and anti-apoptosis (23). RECK (reversion-inducing
cysteine-rich protein) has been implicated in the regulation of
both tumour invasion and angiogenesis by inhibiting activities of
MMPs 2, 9 and 14. It has also been shown that downregulation of the
RECK gene is critical for invasion in T98G, a human glioblastoma
cell line (36).
Our results also showed that all 8 ADAMs were
expressed moderately, highly or very highly (Fig. 2). Of these, ADAMs 9, 10, 12, 15 and
17 were very highly expressed whereas ADAM8 mRNA levels were
moderately expressed in almost all the meningiomas. Due to lack of
reports of ADAMs on meningiomas in the literature, comparison was
only possible with studies on gliomas. Western blotting, RT-PCR and
immunohistochemical studies have shown high expression of ADAM8 and
ADAM19 genes in astrocytomas with a role in invasion (21). Overexpression of ADAM12 detected by
RT-PCR in glioblastomas is thought to imply a role in proliferation
through shedding of heparin-binding epidermal growth factor
(37). It has been suggested that
both ADAMs 10 and 17 modulate tumour progression through their
influence on distinct cellular pathways. They also regulate the
activation of the EGFR tyrosine kinase family in the shedding of
EGFR ligands (38).
The RNA levels of a few growth factors and their
receptors were also profiled in this study to determine if there
was any correlation between their expression with that of MMPs,
ADAMs and TIMPs. Several reports have documented that
overexpression of epidermal growth factor receptors correlate with
grade of malignancy in glioma. Elevated levels of EGFR observed in
every meningioma in the present study, is consistent with that
demonstrated in a large cohort of meningiomas in which the highest
degree of its expression was in benign meningiomas. In addition,
they compared immunohistochemistry results to malignant
meningiomas, concluding that the expression is inversely correlated
to tumour grade and may serve as a potential therapeutic target
with selective EGFR inhibitors (39).
The cytokine VEGF was originally described as
vascular permeability factor and it functions as a positive
regulator of angiogenesis by promoting migration, proliferation and
tube formation of endothelial cells. The elevated levels of VEGF
seen in this study confirm earlier reports not only on benign
meningiomas, but atypical and malignant ones as well (40). It has been suggested that the
increased ratio of the pro-angiogenesis factor VEGF to the
anti-angiogenic factor SEMASA (which is expressed in human
meningiomas association with low microvessel density) is a negative
predictor of recurrence in these neoplasms (41).
EMMPRIN, the extracellular MMP inducer, was also
very highly expressed in all the meningiomas. It is thought to
induce tumour invasion by activating MMP production (e.g. MMP1, 2
and 15) and modulating cell-substrate adhesion processes. Recent
reports of it have suggested positive correlation of EMMPRIN
expression with WHO grades of both gliomas and meningiomas
(42).
Overall, our results show a differential
co-expression of the 44 genes studied, 13 of which were very highly
expressed in every meningioma investigated. These include 2 of 23
MMPs (MMP2 and 14), 3 of 4 TIMPs (TIMP-1, -2 and -3), 5 of 8 ADAMs
(ADAM9, 10, 12, 15 and 17), the growth factor receptor, EGF-R, the
cell surface bound MMP regulator, EMMPRIN and the regulator of
angiogenesis and vascular permeability, VEGF-A (Figs. 1 and 2). Normal controls for comparison have
already been reported in our previous study on gene profiles in
human cancer cells, including gliomas (9). However, distinct differences in gene
expression patterns were consistent for both types of samples
(biopsy derived cultured cells and snap frozen tumours). MMP1, 3,
10 and 12 were either absent or had low expression in the snap
frozen biopsy samples but often highly expressed in biopsy-derived
cultured cells (Figs. 1Figure 2Figure 3–4). Nevertheless, the reverse pattern was
seen with ADAM28, vascular endothelial growth factor receptors,
Flt-1 (VEGFR-1) and KDR/Flk-1 (Kinase insert domain receptor or
VEGFR-2) which were highly expressed in the snap frozen biopsy
samples. This might reflect either differences in gene expression
due to the in vitro culture conditions or the effect of the
stroma in the expression of specific genes in the snap frozen
samples.
It is noteworthy that due to the limited number of
samples in the study, the data obtained for correlations must be
treated with caution. Nonetheless, co-expression pattern analysis,
using Spearman’s rank, in the present study showed several positive
correlations between MMPs, ADAMs, TIMPs and growth factors
(Table II). In particular, ADAM12
correlated positively with a number of MMPs (MMP1, 3, 10, 12 and
16), TIMP4 and RECK but negatively with the VEGF receptor, KDR.
Interestingly, the histological subtype analysis correlations
(Fig. 5) confirmed our previous
findings on MMP-2 and -9 in 18 cell cultures of meningiomas using
gelatin zymography (16). With the
use of RT-PCR, in this study, we found that the fibroblastic
subtype generally showed the highest expression compared to the
meningothelial whereas the transitional showed intermediate
expression for MMP14, ADAM9, 10, 12, 15 and 17, TIMP-2, EGFR and
EMMPRIN.
The presence of brain invasion is considered to
predict aggressive clinical behaviour and recurrence. After
complete resection, the recurrence rate in meningiomas, is
estimated to be 10–32% within 10 years. Although factors for
recurrence are not well understood in benign meningiomas, high
levels of MMP9 and VEGF expression have been proposed. It was
possible to follow up the 12 patients from King’s College Hospital
(Table I) over a period of 15
years, to evaluate if the overexpression of any of the genes in
this study was related to clinical outcome. Most patients were
regularly followed up and showed no sign of recurrence over a
period of 8–15 years. At the time of surgical resection in 2001,
patient no. 3 was diagnosed with brain-invasive benign meningioma
(Grade II) but follow-up was only possible for 1 year. There was no
sign of recurrence then. Patient no. 5 was the only one in the
study who had recurrence in 2001, having had undergone surgery
previously in 1995. She was free from further recurrence until the
most recent follow-up in 2010. It was only patient no. 12 who had a
second surgery for recurrence after 14 years in 2015. It would have
been interesting to find possible links for recurrence with the
gene expression in these patients but the data did not show any
predictive indicators.
In conclusion, this study provides new clues about
the molecular mechanisms implicated in this poorly characterized
tumour and identifies several potential targets for therapeutic
intervention. Unlike the positive correlations seen between MMPs
(e.g. MMP14) implicated in invasion and increased malignancy in
glioma, this study implies that benign meningiomas may have the
potential to invade or recur and permit angiogenesis. Moreover,
elevated levels of TIMPs-1, -2 and -3 may regulate MMP proteolysis
and also inhibit apoptosis. Taken together, the data suggest that
within the tumour environment, elevated levels of some MMPs, ADAMs,
TIMPs and RECK may indicate that these meningiomas, although benign
by definition, have the potential to invade and recur. The 13
selected elevated gene expressions may serve as potential targets
for therapeutic intervention. We can also postulate that our
findings may support the notion that expression for MMP14, ADAM9,
10, 12, 15 and 17, TIMP-2, EGFR and EMMPRIN reflects the
histological subtype of meningioma. Further studies include the
characterising the functional role (and elucidating molecular
mechanisms implicated) for the pattern of co-expression of MMPs,
ADAMs, TIMPs and growth factors in a larger cohort of invasive and
recurrent meningiomas.
Acknowledgements
The present study was financially supported by the
Royal College of Surgeons of England for Mr. Andrew J Martin’s
Surgical Research Fellowship. The authors are also grateful to Dr
Robert Nuttall for his valuable assistance.
References
|
1
|
Perry A, Louis DN, Scheithauer BW, Budka H
and Von Deimling A: Meningioma. WHO Classifications of Tumours of
the Central Nervous System. 4th edition. Louis DN, Ohgaki H,
Wiestler OD and Cavenee WK: International Agency for Research on
Cancer; Lyon: pp. 164–172. 2007
|
|
2
|
Love S, Louis DN and Ellison DW:
Greenfield’s Neuropathology. 8th edition. Hodder Arnold; London:
2008
|
|
3
|
Louis DN, Ohgaki H, Wiestler OD and
Cavenee WK: World Health Organisation Classification of tumours of
the central nervous system. IARC Press; Lyon: 2007
|
|
4
|
Perry A, Scheithauer BW, Stafford SL,
Lohse CM and Wollan PC: ‘Malignancy’ in meningiomas: A
clinicopathologic study of 116 patients, with grading implications.
Cancer. 85:2046–2056. 1999.PubMed/NCBI
|
|
5
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roy R, Zhang B and Moses MA: Making the
cut: Protease-mediated regulation of angiogenesis. Exp Cell Res.
312:608–622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel R and Leung HY: Targeting the
EGFR-family for therapy: Biological challenges and clinical
perspective. Curr Pharm Des. 18:2672–2679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Meter TE, Rooprai HK, Kibble MM,
Fillmore HL, Broaddus WC and Pilkington GJ: The role of matrix
metalloproteinase genes in glioma invasion: Co-dependent and
interactive proteolysis. J Neurooncol. 53:213–235. 2001. View Article : Google Scholar
|
|
9
|
Nuttall RK, Pennington CJ, Taplin J, Wheal
A, Yong VW, Forsyth PA and Edwards DR: Elevated membrane-type
matrix metalloproteinases in gliomas revealed by profiling
proteases and inhibitors in human cancer cells. Mol Cancer Res.
1:333–345. 2003.PubMed/NCBI
|
|
10
|
Stojic J, Hagemann C, Haas S, Herbold C,
Kühnel S, Gerngras S, Roggendorf W, Roosen K and Vince GH:
Expression of matrix metalloproteinases MMP-1, MMP-11 and MMP-19 is
correlated with the WHO-grading of human malignant gliomas.
Neurosci Res. 60:40–49. 2008. View Article : Google Scholar
|
|
11
|
Hagemann C, Anacker J, Haas S, Riesner D,
Schömig B, Ernestus R-I and Vince GH: Comparative expression
pattern of Matrix-Metalloproteinases in human glioblastoma
cell-lines and primary cultures. BMC Res Notes. 3:293–302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Zhang K, Chen X, Zhao C and Sun Z:
Epilysin is overexpressed in glioblastoma and related to clinical
outcome of patients. Med Oncol. 32:3632015. View Article : Google Scholar
|
|
13
|
Kirches E, Grunewald J, von Bossanyi P,
Szibor R, Plate I, Krüger S, Warich-Kirches M and Dietzmann K:
Expression of matrix metalloproteinases in a series of 12
meningiomas. Clin Neuropathol. 20:26–30. 2001.PubMed/NCBI
|
|
14
|
Nordqvist AC, Smurawa H and Mathiesen T:
Expression of matrix metalloproteinases 2 and 9 in meningiomas
associated with different degrees of brain invasiveness and edema.
J Neurosurg. 95:839–844. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rooprai HK, Van Meter TE, Robinson SD,
King A, Rucklidge GJ and Pilkington GJ: Expression of MMP-2 and -9
in short-term cultures of meningioma: Influence of histological
subtype. Int J Mol Med. 12:977–981. 2003.PubMed/NCBI
|
|
16
|
Okada M, Miyake K, Matsumoto Y, Kawai N,
Kunishio K and Nagao S: Matrix metalloproteinase-2 and matrix
metalloproteinase-9 expressions correlate with the recurrence of
intracranial meningiomas. J Neurooncol. 66:29–37. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
von Randow AJU, Schindler S and Tews DS:
Expression of extracellular matrix-degrading proteins in classic,
atypical, and anaplastic meningiomas. Pathol Res Pract.
202:365–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barresi V, Vitarelli E, Tuccari G and
Barresi G: MMP-9 expression in meningiomas: A prognostic marker for
recurrence risk? J Neurooncol. 102:189–196. 2011. View Article : Google Scholar
|
|
19
|
Barresi V, Alafaci C, Caffo M, Barresi G
and Tuccari G: Clinicopathological characteristics, hormone
receptor status and matrix metallo-proteinase-9 (MMP-9)
immunohistochemical expression in spinal meningiomas. Pathol Res
Pract. 208:350–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wildeboer D, Naus S, Amy Sang QX, Bartsch
JW and Pagenstecher A: Metalloproteinase disintegrins ADAM8 and
ADAM19 are highly regulated in human primary brain tumors and their
expression levels and activities are associated with invasiveness.
J Neuropathol Exp Neurol. 65:516–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu M, Qiu BO, Xiong W, Chen D and Wu A:
Expression of a-disintegrin and metalloproteinase 10 correlates
with grade of malignancy in human glioma. Oncol Lett. 9:2157–2162.
2015.PubMed/NCBI
|
|
23
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): An ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toft-Hansen H, Nuttall RK, Edwards DR and
Owens T: Key metalloproteinases are expressed by specific cell
types in experimental autoimmune encephalomyelitis. J Immunol.
173:5209–5218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clark IM, Swingler TE, Sampieri CL and
Edwards DR: The regulation of matrix metalloproteinases and their
inhibitors. Int J Biochem Cell Biol. 40:1362–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murphy G and Nagase H: Localizing matrix
metalloproteinase activities in the pericellular environment. FEBS
J. 278:2–15. 2011. View Article : Google Scholar
|
|
27
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rocks N, Paulissen G, El Hour M, Quesada
F, Crahay C, Gueders M, Foidart JM, Noel A and Cataldo D: Emerging
roles of ADAM and ADAMTS metalloproteinases in cancer. Biochimie.
90:369–379. 2008. View Article : Google Scholar
|
|
29
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
30
|
Nagashima G, Fujimoto T, Suzuki R, Asai J,
Itokawa H and Noda M: Dural invasion of meningioma: A histological
and immunohistochemical study. Brain Tumor Pathol. 23:13–17. 2006.
View Article : Google Scholar
|
|
31
|
Sarkar S, Nuttall RK, Liu S, Edwards DR
and Yong VW: Tenascin-C stimulates glioma cell invasion through
matrix metalloproteinase-12. Cancer Res. 66:11771–11780. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okuducu AF, Zils U, Michaelis SA, Mawrin C
and von Deimling A: Increased expression of avian erythroblastosis
virus E26 oncogene homolog 1 in World Health Organization grade 1
meningiomas is associated with an elevated risk of recurrence and
is correlated with the expression of its target genes matrix
metalloproteinase-2 and MMP-9. Cancer. 107:1365–1372. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lettau I, Hattermann K, Held-Feindt J,
Brauer R, Sedlacek R and Mentlein R: Matrix metalloproteinase-19 is
highly expressed in astroglial tumors and promotes invasion of
glioma cells. J Neuropathol Exp Neurol. 69:215–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hagemann C, Anacker J, Ernestus RI and
Vince GH: A complete compilation of matrix metalloproteinase
expression in human malignant gliomas. World J Clin Oncol. 3:67–79.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagase H and Murphy G: Tailoring TIMPs for
selective metalloproteinase inhibition. The Cancer Degradome.
Edwards D, Hoyer-Hansen G, Blasi F and Sloane BF: Springer Science;
New York: pp. 787–810. 2008, View Article : Google Scholar
|
|
36
|
Silveira Corrêa TC, Massaro RR, Brohem CA,
Taboga SR, Lamers ML, Santos MF and Maria-Engler SS: RECK-mediated
inhibition of glioma migration and invasion. J Cell Biochem.
110:52–61. 2010.PubMed/NCBI
|
|
37
|
Kodama T, Ikeda E, Okada A, Ohtsuka T,
Shimoda M, Shiomi T, Yoshida K, Nakada M, Ohuchi E and Okada Y:
ADAM12 is selectively overexpressed in human glioblastomas and is
associated with glioblastoma cell proliferation and shedding of
heparin-binding epidermal growth factor. Am J Pathol.
165:1743–1753. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Saftig P and Reiss K: The ‘A Disintegrin
And Metalloproteases’ ADAM10 and ADAM17: Novel drug targets with
therapeutic potential? Eur J Cell Biol. 90:527–535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wernicke AG, Dicker AP, Whiton M, Ivanidze
J, Hyslop T, Hammond EH, Perry A, Andrews DW and Kenyon L:
Assessment of Epidermal Growth Factor Receptor (EGFR) expression in
human meningioma. Radiat Oncol. 5:46–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pistolesi S, Boldrini L, Gisfredi S, De
Ieso K, Camacci T, Caniglia M, Lupi G, Leocata P, Basolo F,
Pingitore R, et al: Angiogenesis in intracranial meningiomas:
Immunohistochemical and molecular study. Neuropathol Appl
Neurobiol. 30:118–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barresi V and Tuccari G: Increased ratio
of vascular endothelial growth factor to semaphorin3A is a negative
prognostic factor in human meningiomas. Neuropathology. 30:537–546.
2010.PubMed/NCBI
|
|
42
|
Tsai WC, Chen Y, Huang LC, Lee HS, Ma HI,
Huang SM, Sytwu HK and Hueng DY: EMMPRIN expression positively
correlates with WHO grades of astrocytomas and meningiomas. J
Neurooncol. 114:281–290. 2013. View Article : Google Scholar : PubMed/NCBI
|