Introduction
Gastric cancer is the fourth most commonly diagnosed
cancer and the second leading cause of cancer death worldwide,
especially prevalent in developing countries (1). The only potentially curative
treatment for gastric cancer is complete resection (2). However, despite aggressive surgical
intervention, more than 50% of patients undergoing radical
resection will experience disease recurrence, usually in the form
of metastatic disease (3). Thus, a
better understanding of the underlying mechanisms that promote
pathogenesis and progression of gastric cancer is urgently
needed.
ADAM17, as a sheddase, releases extracellular
domains of transmembrane proteins, which thereby modulate cell-cell
and cell environment communication (4). ADAM17 overexpression has been
demonstrated in numerous human tumors including gastric cancer
(5), and several well-designed
studies have shown correlations between the levels of ADAM17
expression and tumor progression (6,7). A
previous study found that the expression levels of ADAM17 mRNA and
protein in gastric cancer tissues are both significantly higher
than those in non-cancerous gastric mucosa (8). It is also identified that the
siRNA-targeted ADAM17 transcripts suppress deoxycholate
(DC)-induced activation of EGFR and ERK1/2, suggesting that in AGS
human gastric cancer cells, DC transactivates EGFR through M-BAR-
and ADAM/HB-EGF-dependent mechanisms (9). Another study reported that ADAM17
activated by TGF-β mediates proHB-EGF shedding to promote the
proliferation of gastric cancer cells via EGFR transactivation
(10). According to current views,
the major mechanism by which ADAM17 supports cancer development
involves shedding, and thus, activation of growth factors such as
TGF-α, HB-EGF, amphiregulin or neuregulins (11,12).
In turn, these growth factors stimulate survival, proliferation and
migration of tumor cells. Therefore, ADAM17 may be an important
molecular marker for predicting carcinogenesis, progression and
prognosis of gastric cancer.
It is reported that epithelial-mesenchymal
transition (EMT) plays a critical role in the cancer progression
and metastasis, including gastric cancer (13,14).
EMT is a process characterized by loss of cell-cell adhesion and
increase of cell motility (15).
During EMT, significant morphological transformation occurs,
including reduced expression of epithelial markers, such as
E-cadherin, and increased expression of mesenchymal markers, such
as N-cadherin and vimentin (16,17).
However, there are few reports concerning the association between
ADAM17 expression and EMT in gastric cancer.
In the present study, we examined the roles of
ADAM17 in EMT of gastric carcinoma cells and elucidated the
underlying mechanism. Our data show that ADAM17 promotes the
proliferation, migration and invasion of gastric carcinoma cells.
Importantly, ADAM17 promotes EMT probably via TGF-β/Smad signaling
in gastric carcinoma cells.
Materials and methods
Cell culture
The gastric carcinoma cell lines MGC803, MKN45,
HGC27 and BGC823 were purchased from the Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China). The cells were
cultured with Dulbecco’s modified Eagle’s medium (DMEM; HyClone
Laboratories, Beijing, China) supplemented with 10% fetal bovine
serum (FBS; Gibco, Carlsbad, CA, USA), in humidified 5%
CO2 incubator at 37°C.
Real-time PCR
Total RNA was isolated using RNAiso Plus (Takara
Bio, Shiga, Japan). Reverse transcription was performed using
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific) according to the manufacturer’s recommendations. The
SYBR-Green-based real-time PCR was then performed in triplicate
using CFX-96 sequence detection system (Bio-Rad Laboratories) and
gene expression was normalized by GAPDH. Primers are listed in
Table I. The relative fold change
in RNA expression was calculated using the 2−ΔΔCt
method.
 | Table IThe primer sequences for the GAPDH,
ADAM17, MMP-2 and MMP-9 genes used in real-time PCR
experiments. |
Table I
The primer sequences for the GAPDH,
ADAM17, MMP-2 and MMP-9 genes used in real-time PCR
experiments.
| Forward primer | Reverse primer |
|---|
| GAPDH |
5′-GGTGAAGGTCGGTGTGAACG-3′ |
5′-CTCGCTCCTGGAAGATGGTG-3′ |
| ADAM17 |
5′-AGAGCTGACCCAGATCCCAT-3′ |
5′-TACTCTCTTCCCCTCTGCCC-3′ |
| MMP-2 |
5′-CACAGGAGGAGAAGGCTGTG-3′ |
5′-GAGCTTGGGAAAGCCAGGAT-3′ |
| MMP-9 |
5′-TTCAGGGAGACGCCCATTTC-3′ |
5′-TGTAGAGTCTCTCGCTGGGG-3′ |
Plasmid construction
The ADAM17 shRNA sequence was obtained from Sigma
Company official website, which was produced by Sangon Biotech,
Co., Ltd. (Shanghai, China). The oligo sequence of ADAM17 shRNA
included: ADAM17 shRNA (F): 5′-CCG GCC TAT GTC GAT GCT GAA CAA ACT
CGA GTT TGT TCA GCA TCG ACA TAGG TTT TTG-3′ and ADAM17 shRNA (R):
5′-AAT TCA AAA ACC TAT GTC GAT GCT GAA CAA ACT CGA GTT TGT TCA GCA
TCG ACA TAG G-3′. The ADAM17 shRNA sequence was inserted into the
EcoRI and AgeI site of the pLKO.1-TRC plasmid and
ligated into the vector (Sigma-Aldrich, St. Louis, MO, USA).
Lentivirus production and cell
transduction
The packaging plasmid psPAX2 and the envelope
plasmid pMD2.G were purchased from Sigma-Aldrich. PLKO.1-sh-ADAM17
was cotransfected with psPAX2 and pMD2.G into HEK293T cells using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Viruses were
harvested 48 h after transfection and viral titers were determined.
Cells were infected with 1×106 recombinant lentivirus
transduction units in the presence of 8 mg/ml polybrene
Sigma-Aldrich. Puromycin (1:10,000 dilutions) was added to cells
until the cells in the blank group were non-viable. Cells which
survived were stable infected cells.
Transient transfection
Cells were seeded in 6-well plates at a density of
4×105 cells/well. After 24 h of culture, the medium was
replaced by Opti-MEM (Invitrogen) and cultured. In total, 2 μg
plasmid was transfected using 6 μl Lipofectamine 2000 transfection
reagent (Invitrogen). After incubation for another 48 h, the
treated cells were used to investigate the effect of gene rescue
using western blot analysis or Transwell and Cell Counting kit-8
assay.
Western blotting
The cultured cells were rinsed with cold
phosphate-buffered saline (PBS) before treated with RIPA lysis
buffer at 4°C for 10 min. Then the mixture was centrifuged under
4°C at 12,000 r/min for 15 min. The supernatant was removed and the
protein concentration was measured with the BCA method.
Approximately 40 μg of protein was loaded in each lane, and
separated by 10% SDS-PAGE and then transferred to the PVDF
membrane. The membrane was blocked by 5% non-fat milk powder for 1
h at room temperature before overnight incubation with primary
antibodies 4°C, followed by the secondary antibody. The antibodies
were rabbit anti-ADAM17 (cat. no. 3976), mouse anti-β-tubulin (cat.
no. 6181), rabbit anti-N-cadherin (cat. no. 13116), rabbit
anti-E-cadherin (cat. no. 3195), rabbit anti-vimentin (cat. no.
5741), rabbit anti-Snail (cat. no. 3879), rabbit anti-TGF-β (cat.
no. 3711), rabbit anti-Smad2 (cat. no. 5339), rabbit anti-p-Smad2
(cat. no. 3108), rabbit anti-Smad3 (cat. no. 9523), rabbit
anti-p-Smad3 (cat. no. 9520) (all from Cell Signaling Technology,
Danvers, MA, USA).
Cell Counting kit-8 assay
The measurement of viable cell mass was performed
with Cell Counting kit-8 (Beyotime Institute of Biotechnology,
Shanghai China) according to the manufacturer’s instructions.
Briefly, 3,000 cells/well were seeded in a 96-well plate, grown in
an incubator (5% CO2, at 37°C). Respectively in the
first, second, third, fourth and fifth day, 10 μl CCK-8 was added
to each well, and cells were incubated at 37°C for 2 h and the
absorbance was finally determined at 490 nm.
Colony-forming assay
Transfected MGC803 and MKN45 cells were harvested,
resuspended in medium and transferred to the 6-well plate (500,
1,000 and 2,000 cells/well) for 10–14 days until large colonies
were visible. Colonies were fixed and stained with 0.05% crystal
violet for 30 min, and the number of colonies was counted or
photomicrographs were taken under phase-contrast microscope.
Wound healing assay
Cells have grown to confluence in complete cell
culture medium. At time 0 h, a scrape wound was created across the
diameter with a 10-μl pipette tip followed by extensive washes with
medium to remove dead and floating cells. The distance was recorded
at 0 and 48 h. Images were captured using an inverted microscope
equipped with a digital camera.
Migration assay and invasion assay
For assessing cell migration, 1×105 cells
in serum-free media were seeded into the Transwell inserts
(Corning) containing 8-μm permeable pores and were allowed to
migrate toward 10% FBS-containing medium. Twenty-four to 36 h
later, the migrated cells on the bottom of the insert were fixed
with 4% paraformaldehyde solution followed by crystal violet (1%)
staining. Images were taken after washing the inserts three times
with PBS. Five independent fields were counted for each Transwell
and the average numbers of cells/field were represented as graphs.
For assessing cell invasion, 1×105 cells in serum-free
medium were seeded in the Transwell inserts which had been covered
with a layer of BD Matrigel basement membrane. The cells were later
processed similarly to that of cell migration assay. Finally,
invaded cells were counted and the relative number was
calculated.
Statistical analysis
The data are presented as mean ± SD from at least
three independent experiments. All statistical analyses were
carried out using SPSS Statistics 19 software. Comparisons between
the groups were analyzed using the Student’s t-test (two groups) or
a one-way ANOVA (multiple groups). P<0.05 was considered
statistically significant.
Results
ADAM17 expression in gastric carcinoma
cells
First, we analyzed the ADAM17 expression in MGC803,
MKN45, HGC27 and BGC823 cells using real-time PCR and western blot
analysis. We found that ADAM17 expression, at both mRNA and protein
levels, was higher in MGC803 and MKN45 cells than that in HGC27 and
BGC823 cells (Fig. 1A and B).
Subsequently, we constructed plasmids sh-ADAM17 and pRK5M-ADAM17 to
identify the role of ADAM17 in the development of gastric cancer,
and then we examined the knockdown effect of sh-ADAM17 at both
protein and mRNA levels using sh-EGFP as a control. The mRNA and
protein levels of ADAM17 were significantly decreased in sh-ADAM17
group compared with sh-EGFP group (Fig. 1C and D). Similarly, pRK5M-vector or
pRK5M-ADAM17 were transferred into HGC27 and BGC823 cells, then
ADAM17 mRNA and protein levels were examined by real-time PCR and
western blot analysis, and the results indicated that ADAM17
expression at both mRNA and protein levels was significantly
increased in pRK5M-ADAM17 group compared with pRK5M-vector group
(Fig. 1E and F).
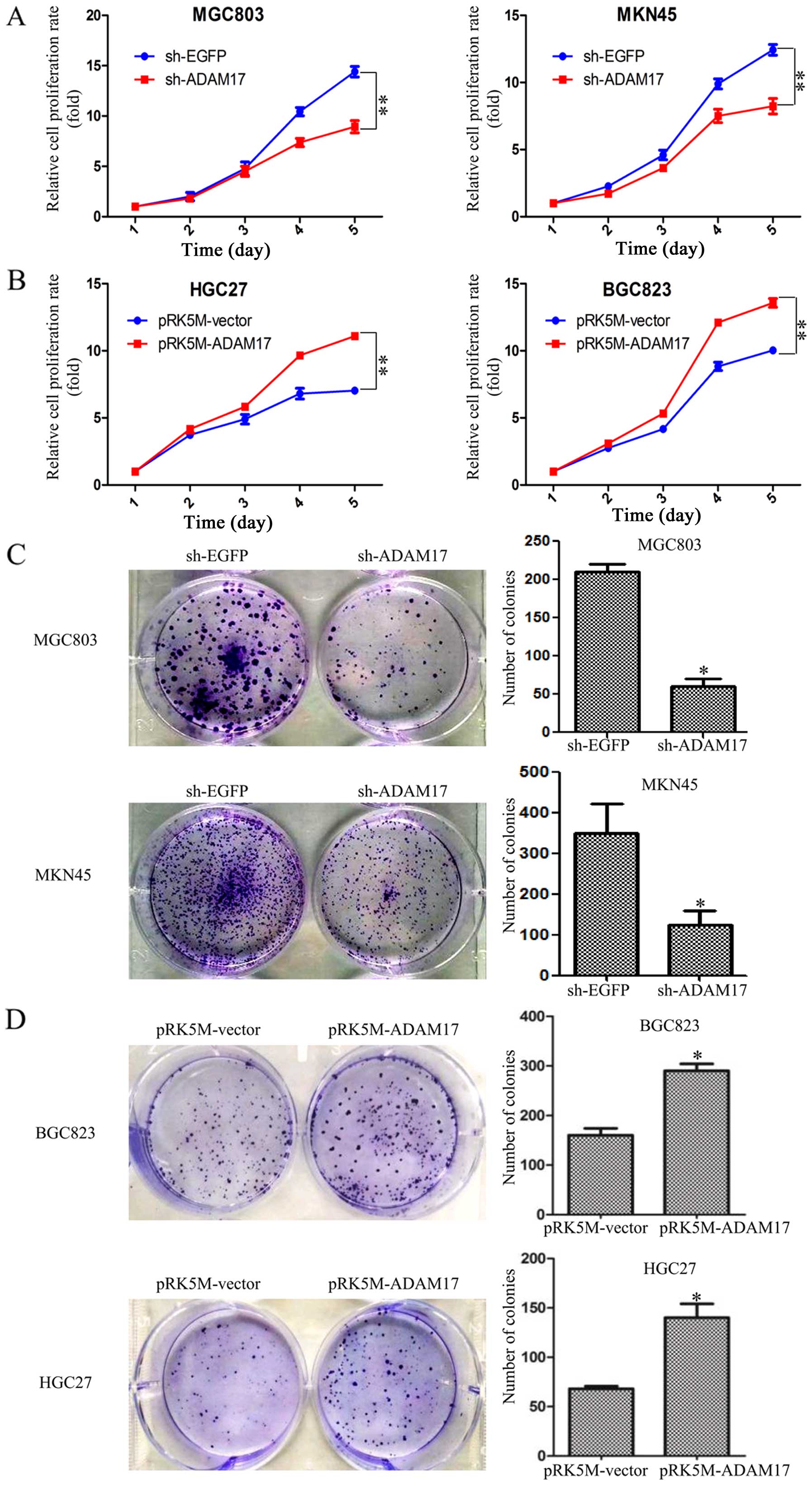
ADAM17 promotes proliferation and colony
formation in gastric carcinoma cells
To investigate the effect of ADAM17 on cell growth
in gastric carcinoma cells, we first used the CCK-8 assay to
determine the growth curves. The results indicated that ADAM17
knockdown significantly inhibited the proliferation of MGC803 and
MKN45 cells (Fig. 2A). Expectedly,
overexpression of ADAM17 promotes the ability of proliferation in
HGC27 and BGC823 cells (Fig. 2B).
To further confirm the effect of ADAM17 on proliferation of gastric
carcinoma cells, we evaluated their ability of colony formation in
the above mentioned cells. Colonies with strong, highly dense
staining and at least 50 cells per colony were counted. We found
that ADAM17 knockdown resulted in smaller colonies and lower colony
density compared to the control group in both MGC803 and MKN45
cells. The colony formation rates were 202±11 and 50±9 in sh-EGFP
and sh-ADAM17 MGC803 cells, and 331±8 and 114±7 in sh-EGFP and
sh-ADAM17 MKN45 cells (Fig. 2C).
To confirm the above results, the HGC27 and BGC823 cells were
transfected with pRK5M-vector or pRK5M-ADAM17 plasmids,
respectively. We found that ADAM17 overexpression increased the
colony sizes and densities compare to control group, and the number
of colonies (defined as ≥50 cells) was 150±10 and 300±13 in BGC823
cells, 77±12 and 153±9 in HGC27 cells, respectively (Fig. 2D). It was confirmed that ADAM17
promotes proliferation and colony formation in gastric carcinoma
cells.
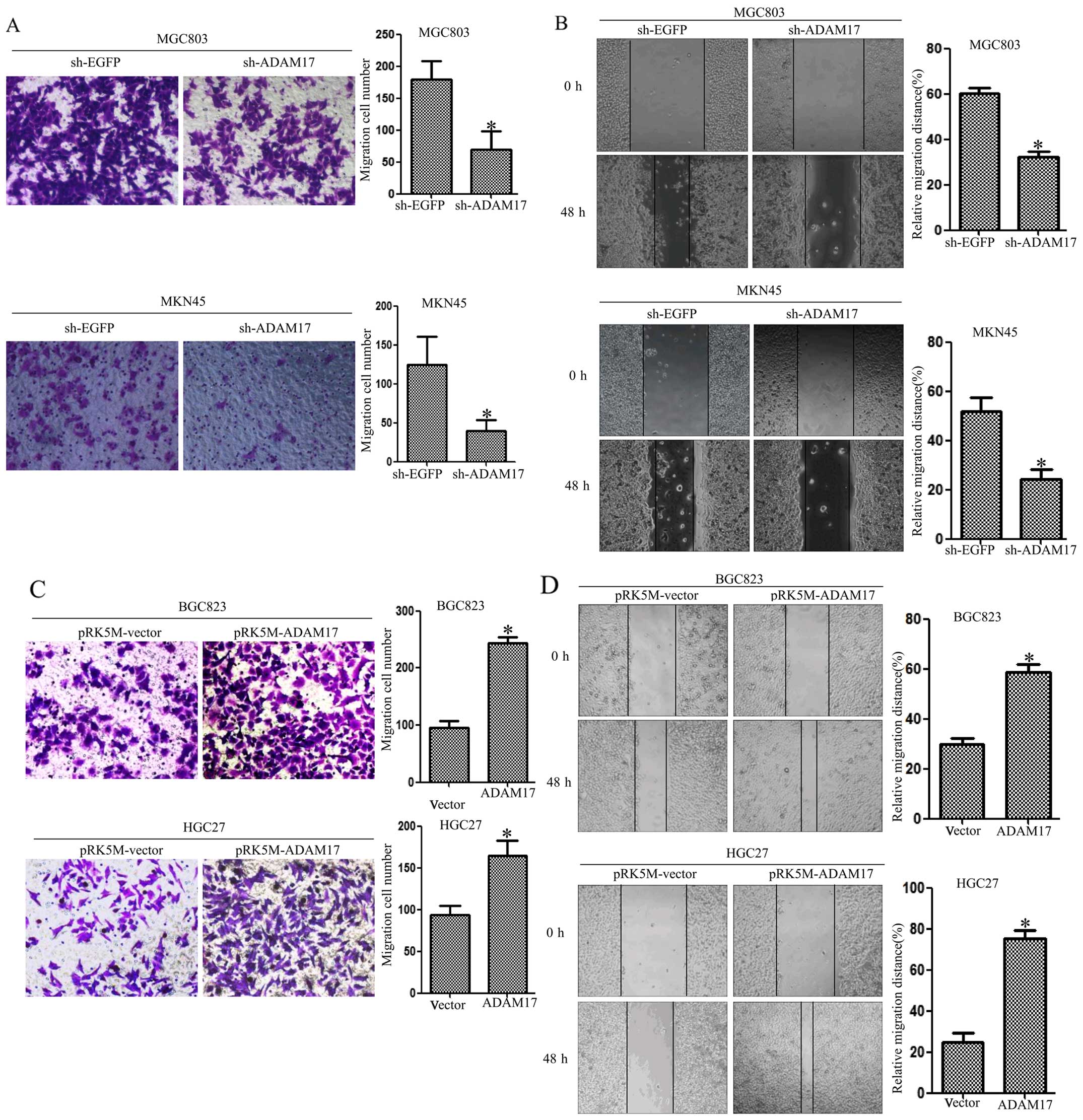
ADAM17 enhances the migration ability of
gastric carcinoma cells
Next, we examined the ability of migration by
Transwell assays and wound scratch assays. First, we examined the
migratory potential of MGC803 and MKN45 cells using Transwell
assays. We found that the migration rates were 180±15 and 60±12 in
sh-EGFP and sh-ADAM17 MGC803 cells, and 120±14 and 40±11 in sh-EGFP
and sh-ADAM17 MKN45 cells (Fig.
3A). To test the effects of ADAM17 knockdown on cell motility,
we performed wound scratch assays. For these assays, a scrape wound
was created on confluent cultures of MGC803 and MKN45 cells
expressing either sh-EGFP or sh-ADAM17. MGC803 and MKN45 cells
expressing sh-ADAM17 displayed reduced motility in comparison to
MGC803 and MKN45 cells expressing sh-EGFP (Fig. 3B). To confirm the above results,
HGC27 cells and BGC823 cells were transfected with pRK5M-vector or
pRK5M-ADAM17 plasmids. Conversely, overexpression of ADAM17
promoted the ability of migration in HGC27 and BGC823 cells
(Fig. 3C and D). These data
suggest that ADAM17 promotes the ability of migration in gastric
carcinoma cells.
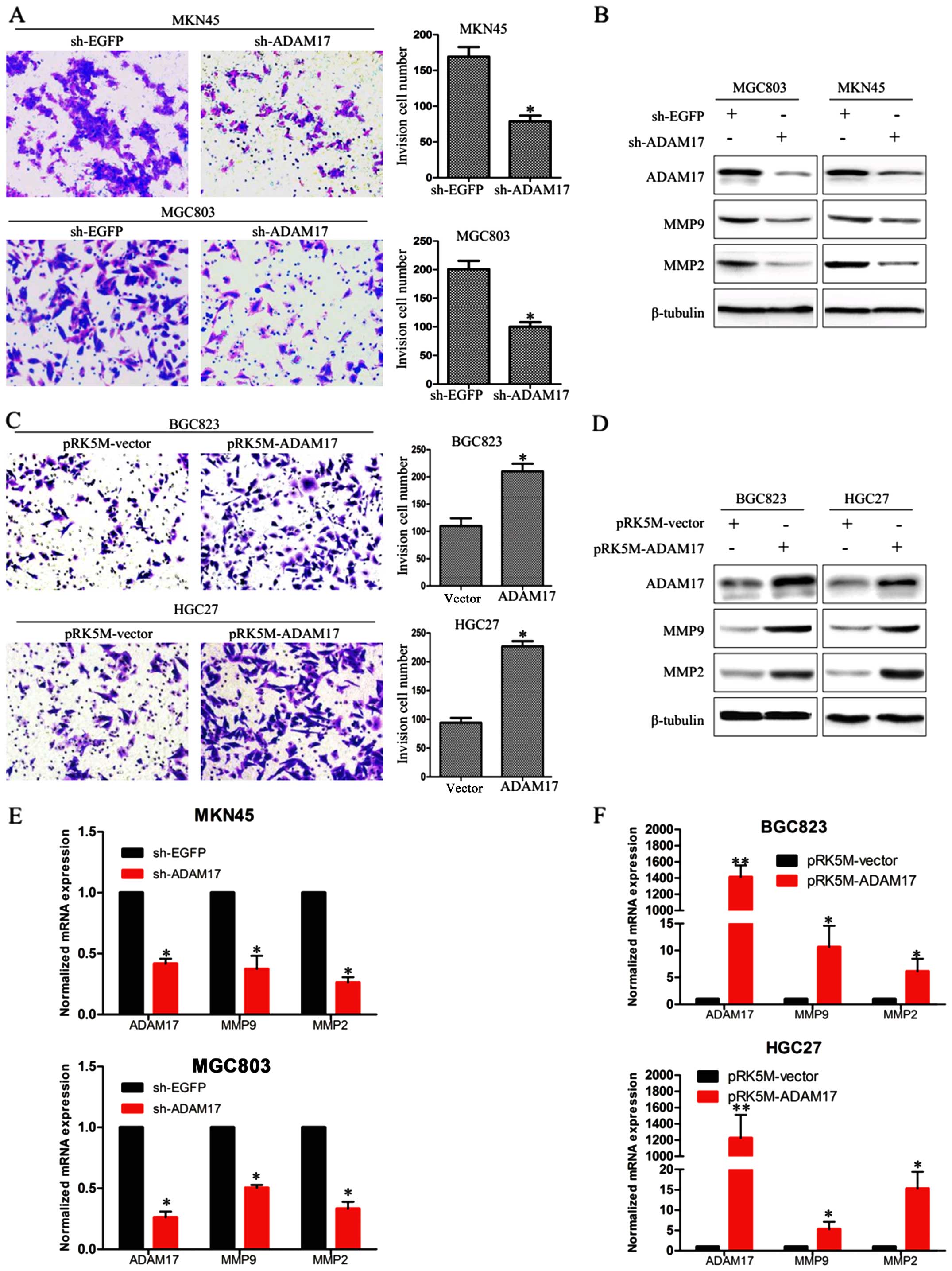
ADAM17 promotes cell invasion in gastric
carcinoma cells
Then, we examined the effect of ADAM17 on the
invasion ability of gastric carcinoma cells using BD Matrigel
invasion assays. We transfected MGC803 and MKN45 cells with sh-EGFP
or sh-ADAM17 plasmids, and HGC27 and BGC823 cells with pRK5M-vector
or pRK5M-ADAM17 plasmids for 72 h. The number of invasive cells
were 170±12 and 74±9 in sh-EGFP and sh-ADAM17 MKN45 cells, and
200±6 and 98±8 in sh-EGFP and sh-ADAM17 MGC803 cells (Fig. 4A), indicating that knockdown of
ADAM17 obviously inhibited the invasion ability of MGC803 and MKN45
cells. Moreover, the number of invasive cells were 105±9 and 200±5
in vector and pRK5M-ADAM17 BGC823 cells, and 98±11 and 210±8 in
vector and pRK5M-ADAM17 HGC27 cells, suggesting that upregulation
of ADAM17 significantly enhanced the invasion ability of BGC823 and
HGC27 cells (Fig. 4C). Also,
ADAM17 knockdown was conduced to downregulate the MMP-2 and MMP-9
at both mRNA and protein levels in MGC803 and MKN45 cells (Fig. 4B and E), while ADAM17
overexpression upregulated the expression of MMP-2 and MMP-9 in
BGC823 andHGC27 cells (Fig. 4D and
F). The above data suggest that ADAM17 promotes the invasion
ability of gastric cancer cells.
ADAM17 promotes EMT via TGF-β/Smad
signaling in gastric carcinoma cells
The EMT is deemed to be associated with the ability
of migration and invasion in cancer cells. Therefore, we detected
EMT markers at the protein level by western blotting. Our data
suggested that ADAM17 knockdown resulted in downregulation of
vimentin, Snail, N-cadherin and upregulation of E-cadherin in
MGC803 and MKN45 cells (Fig. 5A).
In contrast, ADAM17 overexpression led to upregulation of vimentin,
Snail, N-cadherin and downregulation of E-cadherin in BGC823 and
HGC27 cells (Fig. 5B). It is
confirmed that ADAM17 promotes EMT in gastric carcinoma cells. As
TGF-β/Smad signaling is closely related to EMT in cancer, we
investigated the effects of ADAM17 on the classic TGF-β/Smad
signaling. We found that ADAM17 knockdown downregulated TGF-β,
p-Smad2/3 in MGC803 and MKN45 cells (Fig. 5C), while ADAM17 overexpression
resulted in upregulation of TGF-β, p-Smad2/3 in BGC823 and HGC27
cells (Fig. 5D). Knocking down or
overexpressing ADAM17 had no influence on total Smad2/3 protein.
These data suggest that ADAM17 promotes EMT in gastric carcinoma
cells via TGF-β/Smad signaling.
Discussion
It has been reported that ADAM17 may function as an
oncogene to promote cancer cell growth (18). ADAM17 expression is significantly
increased in different types of cancers, including gastric cancer
(19–22). In this study, we identify that
ADAM17 promotes proliferation, migration and invasion in gastric
cancer cells. Importantly, we found that ADAM17 promotes EMT
probably via TGF-β/Smad signaling pathway in gastric cancer. These
findings suggest that ADAM17 could represent a novel anticancer
strategy.
Epithelial-mesenchymal transition (EMT) is a
critical cellular process in cancer metastasis, during which
epithelial polarized cells become motile mesenchymal cells
(23). The process of EMT consists
of three major steps in cancer: i) loss of cell-cell junctions and
a decrease in the epithelial marker E-cadherin; ii) acquisition of
the mesenchymal marker N-cadherin; and iii) cytoskeleton
rearrangement intended for invasive properties. Additionally, these
changes are paralleled with secretion of matrix
metalloproteinase-2/-9 (MMP-2/-9) and focal adhesion kinase (FAK)
(24). MMP-2/-9, proteolytic
enzymes that degrade and modify the extracellular matrix (ECM), act
directly on cell surface molecules and activate EMT (25). In the present study, we find that
ADAM17 overexpression elevates the expression of MMP-2 and MMP-9,
while ADAM17 knockdown downregulates the expression of MMP-2 and
MMP-9. Therefore, we identified that ADAM17 elevates the expression
of MMP-2 and MMP-9 thereby accelerating EMT.
It has been reported firmly that TGF-β signaling
pathway plays crucial roles in regulating malignancy initiation,
progression and metastasis, including gastric cancer (26). The effects of TGF-β on migration
and invasion are associated with changes in ECM components,
including collagen (27),
fibronectin (28), laminin
(29), MMP-2 and MMP-9 (30,31).
ADAM17 is also involved in proteolytical digestion of collagen IV
of the ECM and the release from the cell surface of several
integral proteins, which suggest that ADAM17 affects the invasive
activity of a variety of cancers (32,33).
Therefore, there may be a possible link between ADAM17 and TGF-β.
In this study, we presented clear evidence that ADAM17 induced
expression of the TGF-β, and increased phosphorylated Smad2/3 while
the total Smad2/3 expression was relatively unchanged. In the TGF-β
signaling pathway, TGF-β receptor kinases phosphorylated Smad2 and
Smad3 in the C terminal residue, resulting in forming a complex
with Smad4, which plays the role of a common mediator, and the
nuclear translocation to regulate gene expression leading to the
stimulation of EMT (34,35). Hence, it is confirmed that ADAM17
promotes EMT probably via TGF-β/Smad signaling in gastric carcinoma
cells.
In conclusion, ADAM17 promotes proliferation,
migration and invasion in gastric carcinoma cells. Importantly, the
results detail a mechanism of ADAM17-mediated EMT through
upregulating TGF-β/Smad signaling pathway. These findings suggest
that ADAM17 might be an important therapeutic target candidate in
gastric cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (81472333 and
81372718) and the Natural Science Foundation of Jiangsu Province
(BK20131247).
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Feng Y, Gao Y and Hou R: Clinical
benefits of combined chemotherapy with S-1, oxaliplatin, and
docetaxel in advanced gastric cancer patients with palliative
surgery. Onco Targets Ther. 9:1269–1273. 2016.PubMed/NCBI
|
|
3
|
Pecqueux M, Fritzmann J, Adamu M, Thorlund
K, Kahlert C, Reißfelder C, Weitz J and Rahbari NN: Free
intraperitoneal tumor cells and outcome in gastric cancer patients:
A systematic review and meta-analysis. Oncotarget. 6:35564–35578.
2015.PubMed/NCBI
|
|
4
|
Xu P and Derynck R: Direct activation of
TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF
receptor-dependent cell proliferation. Mol Cell. 37:551–566. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang TC, Zhu WG, Huang MD, Fan RH and
Chen XF: Prognostic value of ADAM17 in human gastric cancer. Med
Oncol. 29:2684–2690. 2012. View Article : Google Scholar
|
|
6
|
Kenny PA and Bissell MJ: Targeting
TACE-dependent EGFR ligand shedding in breast cancer. J Clin
Invest. 117:337–345. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szalad A, Katakowski M, Zheng X, Jiang F
and Chopp M: Transcription factor Sp1 induces ADAM17 and
contributes to tumor cell invasiveness under hypoxia. J Exp Clin
Cancer Res. 28:1292009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshimura T, Tomita T, Dixon MF, Axon AT,
Robinson PA and Crabtree JE: ADAMs (a disintegrin and
metalloproteinase) messenger RNA expression in Helicobacter
pylori-infected, normal, and neoplastic gastric mucosa. J Infect
Dis. 185:332–340. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasuda H, Hirata S, Inoue K, Mashima H,
Ohnishi H and Yoshiba M: Involvement of membrane-type bile acid
receptor M-BAR/TGR5 in bile acid-induced activation of epidermal
growth factor receptor and mitogen-activated protein kinases in
gastric carcinoma cells. Biochem Biophys Res Commun. 354:154–159.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ebi M, Kataoka H, Shimura T, Kubota E,
Hirata Y, Mizushima T, Mizoshita T, Tanaka M, Mabuchi M, Tsukamoto
H, et al: TGFβ induces proHB-EGF shedding and EGFR transactivation
through ADAM activation in gastric cancer cells. Biochem Biophys
Res Commun. 402:449–454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tape CJ, Willems SH, Dombernowsky SL,
Stanley PL, Fogarasi M, Ouwehand W, McCafferty J and Murphy G:
Cross-domain inhibition of TACE ectodomain. Proc Natl Acad Sci USA.
108:5578–5583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Richards FM, Tape CJ, Jodrell DI and
Murphy G: Anti-tumour effects of a specific anti-ADAM17 antibody in
an ovarian cancer model in vivo. PLoS One. 7:e405972012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang L, Wu RL and Xu AM:
Epithelial-mesenchymal transition in gastric cancer. Am J Transl
Res. 7:2141–2158. 2015.
|
|
15
|
Savagner P: Epithelial-mesenchymal
transitions: From cell plasticity to concept elasticity. Curr Top
Dev Biol. 112:273–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2014. View Article : Google Scholar
|
|
18
|
Duffy MJ, Mullooly M, O’Donovan N, Sukor
S, Crown J, Pierce A and McGowan PM: The ADAMs family of proteases:
New biomarkers and therapeutic targets for cancer? Clin Proteomics.
8:92011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu B, Sha L, Wang Y, Xu W, Yu Y, Feng F,
Sun C and Xia L: Diagnostic and prognostic value of a disintegrin
and metalloproteinase-17 in patients with gliomas. Oncol Lett.
8:2616–2620. 2014.PubMed/NCBI
|
|
20
|
Cai M, Wang Z, Zhang J, Zhou H, Jin L, Bai
R and Weng Y: Adam17, a target of mir-326, promotes EMT-induced
cells invasion in lung adenocarcinoma. Cell Physiol Biochem.
36:1175–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Schaeybroeck S, Kalimutho M, Dunne PD,
Carson R, Allen W, Jithesh PV, Redmond KL, Sasazuki T, Shirasawa S,
Blayney J, et al: ADAM17-dependent c-MET-STAT3 signaling mediates
resistance to MEK inhibitors in KRAS mutant colorectal cancer. Cell
Rep. 7:1940–1955. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shou ZX, Jin X and Zhao ZS: Upregulated
expression of ADAM17 is a prognostic marker for patients with
gastric cancer. Ann Surg. 256:1014–1022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tennakoon AH, Izawa T, Kuwamura M and
Yamate J: Pathogenesis of type 2 epithelial to mesenchymal
transition (EMT) in renal and hepatic fibrosis. J Clin Med.
5:52015. View Article : Google Scholar
|
|
24
|
Kim YJ, Choi WI, Jeon BN, Choi KC, Kim K,
Kim TJ, Ham J, Jang HJ, Kang KS and Ko H: Stereospecific effects of
ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial-mesenchymal
transition and suppresses lung cancer migration, invasion and
anoikis resistance. Toxicology. 322:23–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cichon MA and Radisky DC: ROS-induced
epithelial-mesenchymal transition in mammary epithelial cells is
mediated by NF-κB-dependent activation of Snail. Oncotarget.
5:2827–2838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Q, Zheng X, Chen L, Xu B, Yang X,
Jiang J and Wu C: Smad2/3/4 pathway contributes to TGF-beta-induced
MiRNA-181b Expression to promote gastric cancer metastasis by
targeting Timp3. Cell Physiol Biochem. 39:453–466. 2016. View Article : Google Scholar
|
|
27
|
Zimmerman KA, Xing D, Pallero MA, Lu A,
Ikawa M, Black L, Hoyt KL, Kabarowski JH, Michalak M and
Murphy-Ullrich JE: Calreticulin regulates neointima formation and
collagen deposition following carotid artery ligation. J Vasc Res.
52:306–320. 2015. View Article : Google Scholar
|
|
28
|
Huang P, Zhang Y, Jiang T and Zhang N:
Effects of p38 MAPK signaling pathway and aldose reductase on
transforming growth factor-beta1 induced expression of fibronectin
in cultured human mesangial cells. Zhonghua Bing Li Xue Za Zhi.
44:778–782. 2015.(In Chinese).
|
|
29
|
Tennant BR, Chen J, Shih AZ, Luciani DS
and Hoffman BG: Myt3 mediates laminin-V/integrin-β1-induced
islet-cell migration via Tgfbi. Mol Endocrinol. 29:1254–1268. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirunsai M, Srikuea R and Yimlamai T: Heat
stress promotes extracellular matrix remodelling via TGF-beta1 and
MMP-2/TIMP-2 modulation in tenotomised soleus and plantaris
muscles. Int J Hyperthermia. 31:336–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao J, Cheng Q, Ye P, Yang G, Liu S, Ao
Q, Liu Y and Hu Y: Atorvastatin improves pathological changes in
the aged kidney by upregulating peroxisome proliferator-activated
receptor expression and reducing matrix metalloproteinase-9 and
transforming growth factor-β1 levels. Exp Gerontol. 74:37–42. 2016.
View Article : Google Scholar
|
|
32
|
Weskamp G, Mendelson K, Swendeman S, Le
Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K,
et al: Pathological neovascularization is reduced by inactivation
of ADAM17 in endothelial cells but not in pericytes. Circ Res.
106:932–940. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu Y, Jiang F, Zheng X, Katakowski M,
Buller B, To SS and Chopp M: TGF-β1 promotes motility and
invasiveness of glioma cells through activation of ADAM17. Oncol
Rep. 25:1329–1335. 2011.PubMed/NCBI
|
|
34
|
Song J: EMT or apoptosis: A decision for
TGF-beta. Cell Res. 17:289–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang Y, Zhu F, Zhang H, Chen D, Zhang X,
Gao Q and Li Y: Conditional ablation of TGF-β signaling inhibits
tumor progression and invasion in an induced mouse bladder cancer
model. Sci Rep. 6:294792016. View Article : Google Scholar
|