1. Introduction
The vast majority of cancer-related deaths are
caused by metastasis, the dissemination of tumor cells from their
original sites to distant organs mainly through the blood
circulation system (1,2). Unfortunately, after decades of
exploration, our understanding of tumor metastasis is still far
from complete, let alone enough for prevention and cure.
Circulating tumor cells (CTCs) have recently been attracting great
attention due to their key role in tumor metastasis, even though
they were first discovered almost 150 years ago (3,4).
Given the value of prognosis prediction of CTCs, the US Food and
Drug Administration (FDA) has approved their clinical use in
metastatic breast, colorectal and prostate cancers (5). CTCs can be frequently and
conveniently detected and are valuable for personalized treatment.
Increasing studies have proved the value of CTC detection in a
number of different types of cancer (5–9);
however, great efforts are needed to decipher the clinical
implication underlying CTCs.
Additionally, referred to as circulating tumor
microemboli, circulating micrometastases or circulating tumor
aggregates, CTC clusters are defined as groups of tumor cells (more
than two or three cells, varied among studies) that travel together
in the bloodstream (10). Early in
the 1970s, a series of preclinical studies demonstrated that CTC
clusters had a greater predisposition of forming distal metastasis
than single CTCs (11–15). However, further studies were
stagnant for decades due to limitations of CTC cluster isolation in
human. In spite of great advances on CTCs recently, little progress
has been achieved. For instance, investigators have already
recognized that CTCs are heterogeneous, with certain subgroups of
CTCs harboring higher metastatic potential (16–18).
The prevalence and amount of CTC clusters can be
underestimated due to their short detection window and lack of
appropriate detection methods. On one hand, the life span of CTC
clusters in circulation is extremely short [much shorter than
individual CTCs, which can also exist in the circulation for only
several hours (19)] due to their
interception by small vessels. On the other hand, current
technologies are responsible for the underrating of CTC clusters
because few specialized devices exist for the detection of CTC
clusters.
The relative rarity and incompetence of existing
capture methods limit our knowledge of CTC clusters, thus many
questions are awaiting to be answered, including where CTC clusters
come from, what they consist of, and how they take advantage to
form metastases. To resolve these problems, acquirement of enough
viable CTC clusters is the key, which makes subsequent molecular,
genetic and functional experiments possible. In this review, we
discuss the valuable knowledge of CTC clusters from all relevant
aspects including discovery history, detection and isolation
methods, pathophysiological characteristics as well as their
clinical implications.
2. The discovery history of CTC
clusters
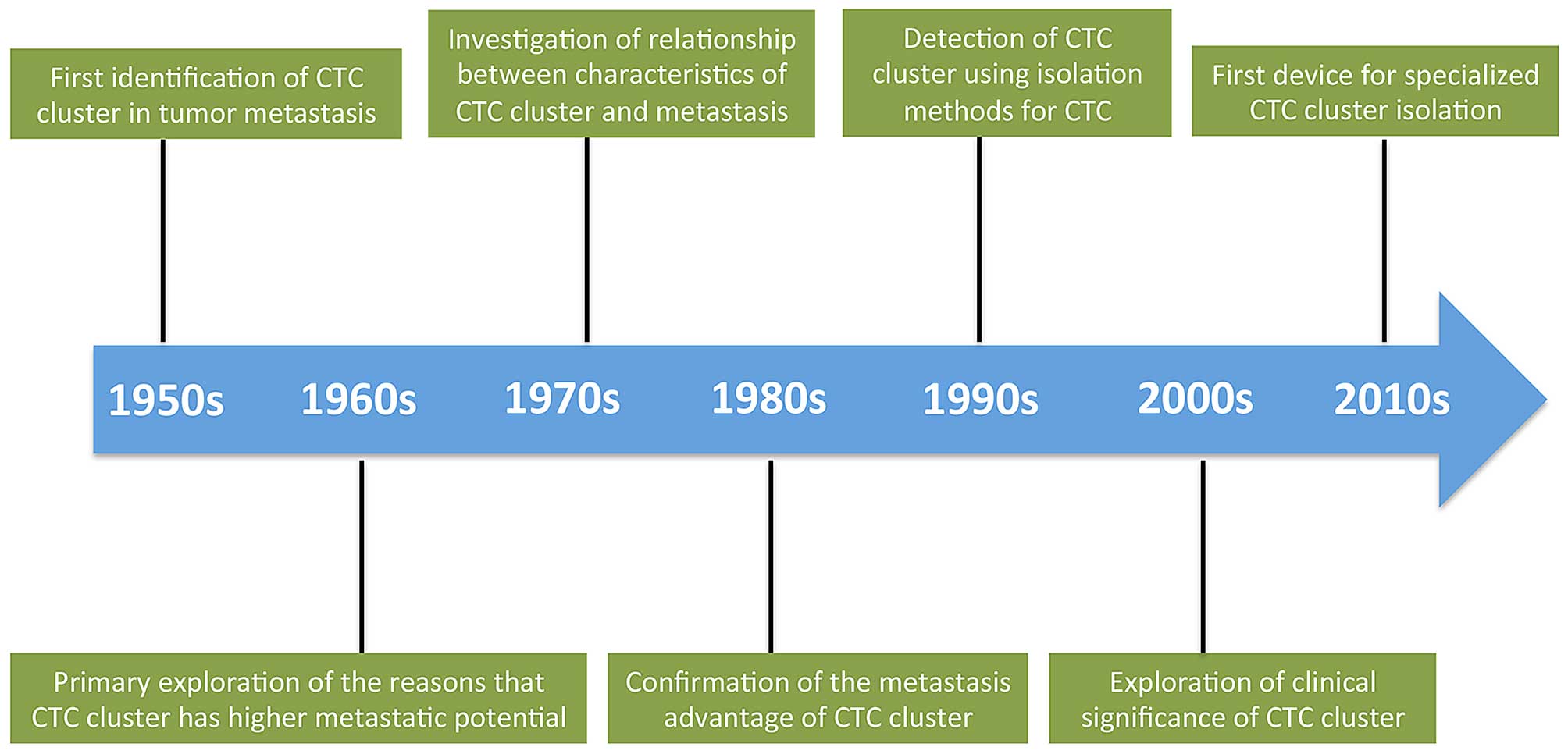
Similar to the long history of CTCs, the first
recognition of CTC clusters can be dated back to 1950s (Fig. 1). In 1954, Watanabe highlighted the
role of CTC clusters in tumor metastases formation by injecting
bronchogenic carcinoma cells from jugular veins of mice, showing
that viable tumor cells in clusters formed metastases, unlike
individual cells (20). Studies in
the following two decades confirmed this result on both
melanoma-derived lung metastases and colon cancer-derived liver
metastases models (11,15,21,22)
and also illustrated that the metastases formation partially
depended on the size and concentration of CTC clusters (13,23).
Although cancer cells intercepted by vessels were seen in animal
models decades ago, the tumor cell emboli entrapped in vessels in
human were proved most recently in the microvasculature of the
lungs in the 3 out of 8 patients with metastatic breast and
cervical carcinomas (24).
Pioneers in the field also studied the relationship
between CTC clusters and the sites of metastases formation. Liotta
et al revealed that the size distribution of vessels was an
important determinant of the distribution and survival of CTC
clusters in the circulation system (14). In addition, some investigators
found that CTC clusters of different tumor cells harbored different
metastasis proclivity (25).
An attempt was also made to explore the mechanisms
of how CTC clusters possess survival and metastasis advantages.
Recent studies imply that CTC clusters have their specialized
microenvironments and are not simply an aggregation of tumor cells
(26). Interaction between tumor
cells and accessory cells was found to provide tumor cells with
survival advantages via different ways, although the detailed
mechanisms required in-depth investigation (16,27,28).
Nowadays, with the improvement of CTC cluster isolation technology,
other physical properties of CTC clusters such as density and
electromechanical characteristics have been under assessment and we
can soon expect deeper understanding of these aspects.
Despite the long history in this field, information
surrounding CTC clusters remain largely unknown. Increased efforts
are urgently required to characterize CTC clusters and fully
understand their roles in tumor metastasis, both clinically and
mechanically.
3. Methods for CTC cluster isolation,
capture and identification
Currently, very few methods have been developed for
specialized detection of CTC clusters. In most cases, CTC clusters
were incidentally observed when detecting individual CTCs. The
devices used for CTC isolation and capture are based on the
differences in physical properties (e.g., density, size,
deformability, electric charges), and biological properties (e.g.,
antigen expression) between CTCs and non-tumor cells. Currently,
limited data of CTC clusters in patients vary greatly according to
tumor type, disease stage, detection platform, and other factors
(Table I). However, these existing
platforms are not ideal for CTC cluster isolation since they
usually underestimate the amount of CTC clusters. Thus, it is
important to approach with caution when interpreting the results of
CTC clusters derived from single CTC specialized isolation
platforms.
 | Table IPrevalence and size of CTC cluster
detected in different types of cancer. |
Table I
Prevalence and size of CTC cluster
detected in different types of cancer.
| Type of cancer | Disease, stage |
Isolation/identification method | Sample | Prevalence | No. of cluster | No. of cells per
cluster | Refs. |
|---|
| Lung cancer | NSCLC, stage
III–IV | ISET/ICC | 1 ml, peripheral
blood | 0/5 (IIIA) 15/35
(IIIB/IV) | 2–39 | 3–45 | (38) |
| NSCLC, stage
I–IV | Subtraction
method/IF | Venous blood,
volume unknown | 40/78 | 1–72/mld | NA | (83) |
| NSCLC, stage
IV | NA/IF | NA | 7/14 | 4.8%a | NA | (47) |
| SCLC, extensive;
NSCLC, stage IIIB–IV | ISET (8-μm
filter)/ICC | 3 ml, peripheral
blood | 7/7 | 16–320e | NA (averagely
3.5–12f) | (10) |
| Adenocarcinoma,
squamous cell, bronchioloalveolar carcinoma | Subtraction
method/IF | 3 ml, venous
blood | 8/55 | NA | 1–3 | (49) |
| Adenocarcinoma,
squamous cell, small cell, carcinoid; stage IA–IV | EpCAM-based
microfluidic chip/IF | 5 ml for pulmonary
vein, 6–8 ml for peripheral vein | 7/32 | NA | NA (a 6-cell
cluster was shown) | (84) |
| Metastatic | HB-Chip/IF | ~4 ml, blood | 1/4 or 2/4d | NA |
2–12f | (26) |
| Metastatic | Filter/trypan
blue | 0.6 ml, blood | 3/8b | 4–18 | NA | (76) |
| NSCLC, stage
III–IV | ScreenCell Cyto
filter/ICC | 3 ml, peripheral
blood | 15/26 | 1–12 | 3–30 | (81) |
| Breast cancer | NA | Subtraction
method/IF | 3 ml, venous
blood | 0/3 or 1/3c | NA | NA | (49) |
| Metastatic | Filter/trypan
blue | 0.6 ml, blood | 5/8b | 4–20 | NA | (76) |
| Stage IV | NA/IF | NA | 13/21 | 3.4%a | NA | (47) |
| Metastatic |
Cluster-Chip/IF | 4 ml, blood | 11/27 | ~0.5/ml | 2–19 | (35) |
| Stages III and
IV | CellSearch
system/IF | 7.5 ml, blood | 20/115 | NA | NA (4-cell clusters
were reported) | (78) |
| Metastatic | CellSearch
system/IF | 7.5 ml, blood | 9/52 | 1–18 | NA (a 7-cell
cluster was shown) | (79) |
| Glioblastoma | NA | Subtraction
method/IF | 3 ml, venous
blood | 0/12 | NA | NA | (49) |
| Colorectal
cancer | Dukes stage
B-D |
Cytocentrifuge/ICC | 20 ml, antecubital
venous blood | 22/32 | 1–8 | 3–10 (excluding
doublets) | (33) |
| Metastatic | Filter/trypan
blue | 0.6 ml, blood | 4/8b | 4–27 | NA | (76) |
| Liver cancer | Non-metastatic | ISET/ICC | 3 ml, peripheral
blood | 2/44 | NA | NA (2-cell and
4-cell clusters were shown) | (41) |
| Renal cancer | Clear cell and
non-clear cell carcinomas, with or without metastases | ISET (75-μm
microfilter)/IHC | Renal venous
outflow, and 500–750 ml perfundate of kidney | 14/42 | NA | NA | (85) |
| Prostate
cancer | Non-metastatic | Immunomagnetic
method/IF | 5 ml, peripheral
blood | 8/10 | 1–17 | NA | (32) |
| Stage IV | NA/IF | NA | 11/15 | 5.3%a | NA | (47) |
| Metastatic | HB-Chip/IF | ~4 ml, blood | 1/15 or
2/15d | NA | 4–12 | (29) |
| Metastatic | Filter/trypan
blue | 0.6 ml, blood | 1/4b | 142 | NA | (76) |
| High-risk,
non-metastatic | CellSearch/IF | 7.5 ml, peripheral
blood | 1/36 | 1 | 4 | (86) |
| Metastatic
castration-resistant | Vitatex CAM
platform/IF | 2 ml, blood | 4/23 | 4–200/ml | NA (a 7-cell
cluster was shown) | (30) |
| Metastatic |
Cluster-Chip/IF | 4 ml, blood | 4/13 | ~0.28/ml | 2–8 | (35) |
| Pancreatic
cancer | Stage IV | NA/IF | NA | 4/18 | 6.2%a | NA | (47) |
| Metastatic | Filter/trypan
blue | 0.6 ml, blood | 1/3a | 3 | NA | (74) |
| Stage I–IV | CMx
platform/IF | 2 ml, peripheral
blood | 51/63 | 29.7 | NA | 87 |
| Melanoma | Metastatic |
Cluster-Chip/IF | 4 ml, blood | 6/20 | ~0.15/ml | 2–6 | (35) |
| Metastatic |
ISET/cytopathology | 10 ml, blood | 44/128 | 2–6 | NA (a cluster with
at least 45 cells was shown) | (88) |
Antibody-based methods
Antibody-based methods are the most widely used
capture techniques for CTC clusters. The antibodies are mainly
pertained to epithelial cell surface markers that are absent from
blood or stroma cells. Among these antibodies, the epithelial cell
adhesion molecule (EpCAM) is the most commonly used. In
CellSearch®, the first and only commercial CTC isolation
platform approved by the US FDA, a CTC cluster is defined as a
group of CTCs containing three or more cells expressing EpCAM and
cytokeratins (CKs) without expression of CD45 and has
(4′,6-diamidino-2-phenylindole)-stained nuclei (29). Blood-spiking experiments with HeLa
cells expressing histone H2B-GFP confirmed that CTC clusters were
not artifacts caused by sample manipulation (10). Although CTC clusters were rarely
captured by CellSearch in a majority of solid tumors, they were
frequently detected (25/97) in small cell lung cancer (SCLC) and
indicated poor prognosis (10). As
a representative of high-throughput microfluidic devices,
herringbone (HB)-chip was developed to improve capture efficiency
by using microvortices to increase the interaction between tumor
cells and antibodies (26). The
number of tumor cells in a CTC cluster captured by HB-chip ranged
from 4 to 12; however, CTC clusters were only found in 3 out of 19
patients with metastatic prostate or lung cancer (26). In order to further identify
invasive CTC clusters, a platform was developed to detect CTC
clusters that uptake cell-adhesion matrix, and 17% of samples were
found positive in patients with metastatic castration-resistant
prostate cancer (30).
Antibody-conjugated magnetic microbeads or
nanoparticles binding to a specific surface antigen such as CD45
were also used for isolating CTCs and CTC clusters (31–34).
After incubation, the blood sample was exposed to a non-uniform
magnetic field to isolate the labeled cells. In a study using CK
and prostate specific antigen as conjugated biomarkers in prostate
cancer, the prevalence of CTC clusters was as high as 80% (32). In another study for colorectal
cancer, the immunomagnetic labeled CK antibodies could separate CTC
clusters from the peripheral blood in 68.8% of patients (33). However, certain investigators used
a negative selection strategy to remove non-tumor cells from CTCs
and CTC clusters, and found that the prevalence of CTC clusters was
rare (31). In general, the
efficiency of immunomagnetic methods mainly depends on two factors:
i) the expression and specificity of the target antigen and the
affinity between antigens and antibodies; and ii) the efficiency of
immunomagnetic labeling process and magnetic particles.
As a by-product of CTC detection, CTC clusters are
insufficiently detected by antibody-based methods for several
reasons. Compared to single CTCs, CTC clusters have smaller
surface-area-to-volume ratios, which reduce the efficiency of
antibody capture. This situation can be more obvious in larger CTC
clusters. In addition, the integrity of CTC clusters is prone to be
impaired in devices with turbulent flow, which can result in an
anamorphic number and size of CTC clusters (35). Other limitations of antibody-based
methods for single CTC detection are also applicable to CTC
clusters, such as the limited expression of target antigens
(35).
Physical property-based methods
Physical property differences in cell density, size,
dielectric properties and mechanical plasticity, can all be
utilized to isolate CTCs and CTC clusters. For instance, size-based
isolation relies on the larger size of CTCs compared to that of
blood cells. This has been achieved by using track-etched
polycarbonate filters, which are porous membranes containing
numerous randomly distributed 8-μm-diameter, cylindrical pores
(36). Isolation by size of
epithelial tumor cells (ISET) platform seems more reasonable for
CTC cluster isolation since the difference between CTC clusters and
non-tumor cells is more significant. One study reported that 2 out
of 23 patients with primary liver cancer were positive for CTC
clusters using an ISET platform (37). Another study using a similar ISET
platform isolated CTC clusters from all patients with lung cancer
(29). As an unbiased method, ISET
was believed to be more sensitive than antibody-based methods. For
example, CTC clusters were observed in 43% of patients with
non-small cell lung cancer (NSCLC) using ISET but were completely
undetectable by CellSearch (38).
Generally, the ISET platform holds a few advantages: i) it retains
the natural status of CTC clusters without the binding of
antibodies or nanoparticles; ii) it allows direct filtration of
peripheral blood without preprocessing; iii) it can maintain the
integrity of CTC clusters; iv) it is cost-friendly compared to
antibody-based methods.
Physical property-based methods of CTC cluster
isolation can be also combined with and strengthened by
microfluidic technology (39–44).
By taking advantage of centrifugal forces which can facilitate CTC
and CTC cluster separation, a two-inlet, two-outlet spiral
microchannel with a total length of 10 cm was designed (45). This device is able to continuously
collect viable CTCs and CTC clusters allowing simple coupling with
a convectional 96-well plate for subsequent biological assays, and
CTC clusters with size of 50–100 μm in patients were successfully
detected (45). Harouaka et
al developed a new flexible micro spring array (FMSA) device
for enrichment of viable CTC clusters according to their sizes. The
FMSA device was based on flexible structures at micro scale that
minimized cell damage and could preserve cell viability while
maximizing throughput to allow for rapid enrichment directly from
blood samples without sample preprocessing. CTC clusters with 2–20
tumor cells were detected in patients with breast, lung, and
colorectal cancer using the FMSA device (46). Extraordinarily, the first attempt
for specific isolation of CTC clusters was achieved in 2015. The
Cluster-Chip, based on microfluidic and antigen-independent
technologies, is able to isolate CTC clusters through specialized
bifurcating traps under low-shear stress conditions that preserve
their integrity. Even two-cell clusters can be efficiently captured
using this technique (35). The
chip comprises of a set of triangular pillars and captures CTC
clusters by relying on the strength of cell-to-cell junctions as
clusters flow through the pillars at physiological speed. This
model is designed to exclude two-cell clumps with a loosened
combination, which may occur in incidentally attached cells.
Cluster-Chip was able to find CTC clusters in 30–40% of patients
with metastatic breast, prostate cancer or melanoma (35).
Additional innovative detection
strategies for CTC clusters
Some additional approaches have been developed to
detect CTC clusters by taking advantage of the physical and
biological properties of epithelial cells. High-resolution imaging
combined with enrichment methods was used to isolate CTC clusters.
A group of investigators separated CK-positive, CD45-negative CTC
clusters, which were then analyzed by a hematopathologist. In their
report, CTC clusters were detected in 93, 54, 50 and 22% of
patients with prostate cancer, breast cancer, NSCLC, and pancreatic
cancer, respectively (47).
Another study reported a novel integrated cellular and molecular
approach of subtraction enrichment and immunostaining-fluorescencen
in situ hybridization (48). The integrated platform depleted
white blood cells and red blood cells and established an
expeditious detection of non-hypotonic damaged and
non-hematopoietic CTC clusters, regardless of CKs or EpCAM
expression or size variation. This platform was able to efficiently
detect, isolate, and characterize CTC clusters from various types
of cancer including lung cancer, glioma and melanoma (48).
Special methods for CTC cluster
identification
Theoretically, it is difficult to judge whether an
individual cell is a tumor cell or not. This dilemma also exists in
the identification of CTC clusters. Most researchers prefer to use
CKs as tumor markers. Some investigators adapted fluorescence in
situ hybridization with the centromere of chromosome 8 (CEP8),
since more than 2 hybridization signals of CEP8 indicates
chromosomal variation and the cell is expected to be malignant
(49). Aptamers specifically
selected from postoperative tumor tissue were also used for tumor
cell identification, and were able to detect more CTC clusters than
CKs (50).
4. Biological significance of CTC cluster
and its role in tumor metastasis
Beyond the enumeration of CTC clusters, their
molecular characterization offers insights into their origins and
metastatic potential. It may also provide clues to their evolution
during the course of cancer treatment and the mechanisms of
treatment resistance relevant to CTC clusters. However, CTC
clusters with different sizes or components have distinct
biological and physical characteristics. Specifically, King et
al systematically studied the physical features of CTC clusters
that consisted of 2–5 tumor cells, which provided a straightforward
view of the enhanced metastatic potential of CTC clusters (51).
Origins of CTC clusters
The origins of CTC clusters and the way their
integrity is preserved are both interesting to note. CTC clusters
can directly be derived from primary tumors or from the
aggregation/proliferation of single CTCs. Current evidence acquired
from breast cancer remains limited, and excludes intravascular
aggregation of CTCs as a main cause of CTC clusters (16). In parallel with this, we designed
an in vitro platform to mimic blood stream, and the results
showed that the aggregation/proliferation of single CTCs was
impossible because of the sheer force of blood (unpublished data).
However, the aggregation and proliferation were still possible when
CTCs were located in inflammatory sites or when small vessels
intercepted CTCs.
Differently originated CTC clusters may have
different formation mechanisms and possess different biological
characteristics. The molecules responsible for tumor cell
aggregation within a CTC cluster are currently under investigation
and could be good targets for treatment. At least in breast cancer,
plakoglobin and keratin 14 that are both associated with desmosomes
and hemidesmosomes have been found to be critical for CTC cluster
formation. Inhibition of these proteins reduced CTC cluster
formation and distal metastases (16,52).
Interactions between Thomsen-Friedenreich glycoantigen and
galectin-3 also take part in breast cancer cell aggregation
(53). In addition, some
pro-inflammatory cytokines such as interleukin-6 and tumor necrosis
factor-α could also promote tumor cell growth as clusters and
induce adhesive recruitment in the circulation system (54). For patients of metastatic stage,
the existence of CTC clusters from metastases is possible and makes
things more complicated. The dynamic change of CTC clusters in the
circulation system is also mysterious because it is difficult to
monitor CTC clusters in vivo. In the future, more
investigations are warranted to solve these specific problems, and
should then be directed towards finding targeted therapeutic
approaches that can be performed to lower the risk of CTC clusters
and improve cancer treatments.
Internal characteristics of CTC
clusters
Some studies have demonstrated that CTC clusters are
comprised of a number of tumor cells with or without non-tumor
cells such as mesenchymal cells, epithelial cells, pericytes,
immune cells, platelets, and cancer-associated fibroblasts
(16,27,55–62).
These non-malignant components contribute to the survival and
metastatic advantages of CTC clusters in various ways. For
instance, the presence of heterotypic tumor-derived stromal cells
(e.g., fibroblasts, endothelial or tumor-infiltrated myeloid cells)
increased the viability of tumor cells within CTC clusters, and
facilitated metastases formation (27). However, when the cancer-associated
fibroblasts were partially depleted, a significantly decreased
number of metastases was observed (27). Another study using tumor cell and
endothelial cell co-culture in a mouse model found that tumor cells
were more potent in promoting angiogenesis in the presence of
endothelial cells and resulted in increased numbers and larger size
of metastases (28). Promotion of
metastasis was also associated with the interplay between tumor
cells and non-tumor cells such as platelets and leukocytes within
CTC clusters (56,57,59,63).
Platelets in the CTC clusters are believed to protect tumor cells
from blood shear damage and immune attacks by physically shielding
other complex influences via paracrine signaling and direct contact
(64). Furthermore, some undefined
cells such as CK-positive dendritic-like cells were also found in
CTC clusters, but the nature and significance were unknown
(33).
CTC clusters also show mesenchymal traits (63). The epithelial-mesenchymal
transition (EMT) status of tumor cells within a CTC cluster is
convincing, and is more obvious than that of single CTC (29). For example, at least one third of
tumor cells in the clusters were CK negative in colorectal cancer
(33). EpCAM and CK were both
heterogeneously expressed in CTC clusters derived from NSCLC
patients (29). Specifically, a
majority of isolated CTC clusters derived from metastatic NSCLC
patients harbored dual epithelial-mesenchymal phenotype, suggesting
that EMT is a relevant process for metastasis caused by CTC
clusters (65). Several hypotheses
have been proposed to explain the EMT status in CTC clusters. For
instance, the expression of mesenchymal markers in a CTC cluster
could result from proliferation of a single cell that had undergone
EMT, or from the mesenchymal transformation of a pre-existing CTC
cluster in the circulation system (63).
Whether CTC clusters are of oligoclonal/polyclonal
or monoclonal origin is also under investigation. In breast and
pancreatic cancers, oligoclonal/polyclonal rather than monoclonal
CTC clusters were observed by assessing lung metastases in a mouse
model (16,66). Although CTC clusters have up to
100-fold increased metastatic potential compared to individual CTCs
(67), it remains uncertain
whether the tumor cells within a CTC cluster have identical
metastatic potentials. Intravenous injections of CTC clusters
comprised of two cell lines with distinct metastatic potentials
resulted in lung metastases with the metastatic unique cell line
karyotype (68). This finding
suggested that the presence of metastatic cells did not change the
inability of non-metastatic cells to form a distant organ, and
implied that different tumor cells within the same cluster
maintained their own metastatic potential (68). Accordingly, the metastatic
potential of a CTC cluster may be dependent on the most malignant
tumor cells. However, contradictory results were provided by
another study, in which two cell lines that harbored different
metastatic potential were mixed and injected into the flank of nude
mice (58). Intriguingly, in
addition to the polyclonal primary tumor at the inoculation site,
the CTC clusters and almost 90% of the lung metastases were also
found to be polyclonal (58). When
the cell lines were injected respectively, merely 10% of the
metastases arose from the less metastatic cell line, suggesting
that tumor cells with lower metastatic potential acquired higher
metastatic capability when cooperating with other cell lines in the
CTC clusters (58).
In summary, clear evidence has shown eminent
heterogeneity in CTC clusters and their marked complex
compositions. Future studies should pay close attention to the
dynamic change of the composition of CTC clusters, crosstalk
between different compositions, and their roles in metastasis.
Metastasis-associated features of CTC
clusters
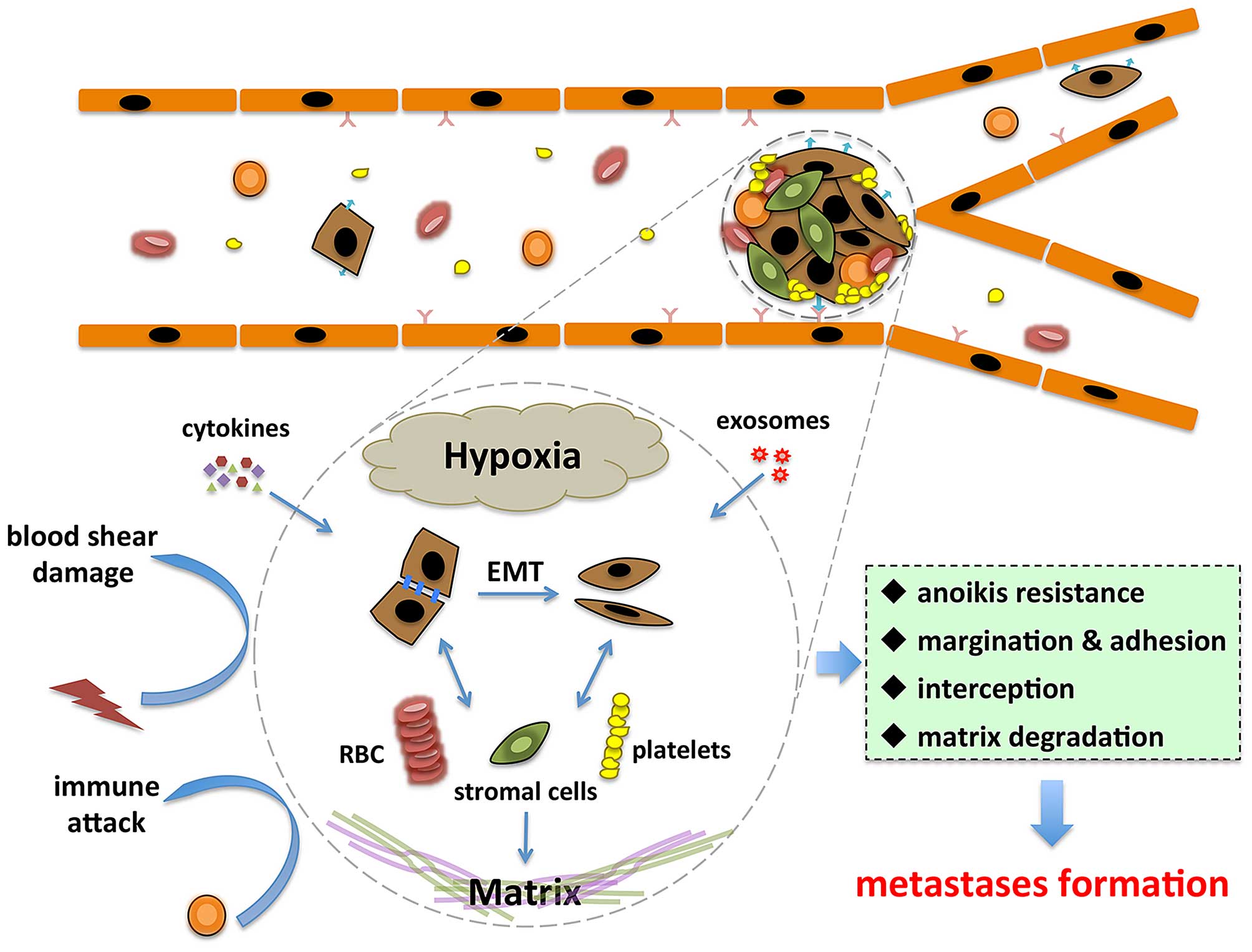
Current studies have partially elucidated the
reasons for CTC clusters to have higher potential of metastasis
(Fig. 2). First, tumor cells
within CTC clusters showed prolonged survival and decreased
apoptosis (29). Mechanically, CTC
clusters prevent tumor cells from anoikis through interaction
between circulating galectin-3 and cancer-associated mucin1 (MUC1),
also promoting the formation and survival of CTC clusters in the
circulation system (69). The
elevated galectin-3 in the circulation also facilitate tumor cancer
adhesion to endothelial cells by interacting with MUC1 on tumor
cell surface (70,71). These findings deepen our
understanding of the molecular mechanisms of CTC cluster
dissemination and suggests that interference of this interaction
may provide a novel therapeutic approach for preventing metastasis.
Moreover, accessory cells such as fibroblasts, endothelial cells,
platelets, and immune cells can form a niche (27,72,73),
which is beneficial for tumor cells in CTC clusters when
facilitating metastasis (27,73,74).
For instance, platelets induce tumor cells to undergo EMT via
TGF-β/Smad and NF-κB pathways by secretion of TGF-β and direct
interaction with tumor cells (72).
Second, the physical specialty of CTC clusters
allows for a greater likelihood of it residing in distant organs.
Microvasculature of viscera can retain large CTCs, thus it can
retain CTC clusters more easily (24). Compared to single CTCs, CTC
clusters have larger sizes and a slower travelling velocity, making
them easier to be intercepted by small vessels (75). In traditional concepts, interaction
of tumor cells with vascular endothelial cells makes up the premise
of the extravasation of tumor cells. From a physical biology
standpoint, it has been reported that CTC clusters tended to
undergo margination, rotation, and adherence to endothelium via
interaction with E-selectin (51).
CTC clusters have a much lower rolling velocity than individual
CTCs, and are susceptible for margination and attachment to
vascular wall even in vessels with diameters not small enough to
intercept clusters (51). In
addition to sphere-like clusters, various appearances of CTC
clusters associated with the collective migration of tumor cells
were observed (29). Most
noticeably, CTC clusters with linear or triangular geometry showed
no difference in velocity along the vessel (51). These clusters also provide a
special microenvironment for the tumor cells within them, and this
microenvironment varies according to different sizes and
constitutions of the clusters. CTC clusters with bigger sizes have
significant hypoxic microenvironments compared to those with
smaller sizes and single CTCs (76).
In addition, cytokines such as interleukin-6 and
tumor necrosis factor-α can induce a positive feedback loop,
leading to the aggregation of subsequent tumor cells and adhesion
of tumor cells to endothelium (54). Alternatively, intravascular
metastatic formation has also been proposed in certain cases in
order to better understand tumor metastasis (49,53,77).
CTC clusters seem more likely to take this strategy due to their
large size and survival advantage in vessels. However, this
assumption is challenged by a recent study. By using microfluidic
devices and zebrafish models, Au et al discovered that CTC
clusters containing ≤20 cells were able to pass through
capillary-sized vessels by reorganizing into single-file chain-like
geometries (67) Astonishingly,
the shape of these CTC clusters is highly plastic, and the cells
are able to easily reorganize again into a sphere-like cluster
after having traversed the capillaries (67). From another view, these findings
provide us with a better understanding of CTC cluster-based
metastasis.
5. Clinical application of CTC clusters
As a minimally invasive technique with potential
roles in diagnosis, decision-making, treatment assessment, and
relapse monitoring in patients, CTCs have been involved in many
preclinical studies and clinical trials. Particularly,
investigators have explored the clinical implications of CTC
clusters (Table II). The presence
of CTC clusters in patients is a harbinger of bad prognosis, and
certain studies have even identified an association between CTC
clusters and survival. One such study enrolled 97 patients with
SCLC and found that 32% of patients were positive for CTC clusters,
and the presence of CTC clusters at baseline indicated shorter
progression-free survival and overall survival (11). An additional prognostic value of
CTC clusters in patients with elevated CTCs were reported in two
prospective cohorts of advanced stage or metastatic breast cancer
(78,79). Furthermore, information regarding
CTC clusters were indicative of the failure of chemotherapy
treatments in colorectal cancer (60). Similarly, CTC clusters also had
predictive values in NSCLC patients (38). It was demonstrated that the
prevalence of CTC clusters increased in NSCLC of higher stages
(10/23 in stage IV, 5/12 in stage IIIB, and 0/5 in stage IIIA)
(38). Unfortunately, there was no
association found between histological subtypes and the presence of
CTC clusters (38). Likewise, CTC
clusters can be detected in more than half of 28 consecutive
patients with stage III–IV lung cancer, but no correlation between
the number of CTC clusters and tumor type or stage was observed
(80). Moreover, the association
between abundance of CTC clusters and disease stage also failed to
be detected in colorectal cancer, though a trend of CTC cluster
elevation was noted in patients with higher stage (33).
 | Table IIAssociation between CTC cluster and
clinical characteristics. |
Table II
Association between CTC cluster and
clinical characteristics.
| Type of cancer | Clinical
relevance | Refs. |
|---|
| Prostate
cancer | Antioxidant gene
expression level in CTC clusters could be used for tumor diagnosis
and recurrence prediction | (77) |
| Breast cancer | The presence of CTC
cluster was associated with worse PFS | (78) |
| The presence of CTC
cluster impaired prognosis | (79 |
| Renal cancer | The presence of CTC
cluster was correlated with larger primary tumor, with higher
frequency of lung metastasis, and with micronodular phenotype in
the primary tumor | (85) |
| SCLC | The presence of CTC
cluster was associated with shorter PFS and OS | (10) |
| NSCLC | The presence of CTC
cluster was not associated with histological subtype | (38) |
| The presence of CTC
cluster was not associated with tumor type or stage | (80) |
| No significant
difference between numbers of CTC cluster and disease stage | (83) |
| Liver cancer | The presence of CTC
cluster was association with tumor diffusion, portal tumor
thrombosis, and shorter survival | (41) |
| Colorectal
cancer | Elevation of CTC
cluster number was correlated with macroscopic progression, and the
numbers of cancer cells in the CTC clusters were significantly
higher in non-responder after chemotherapy | (60) |
| Pancreatic
cancer | PFS and OS were
significantly shorter in unfavorable group (>30/2 ml) compared
to those of favorable group (<30/2 ml). CTC cluster was an
independent prognostic factor for PFS and OS | (87) |
| Melanoma | OS was
significantly decreased in patients with CTC clusters, independent
of treatment | (88) |
Gene expression and changes of CTC clusters during
treatment were also studied. In patients with prostate cancer, the
antioxidant gene expression level had excellent prognostic and
predictive value, and could be used for evaluation of therapy and
monitoring of tumor recurrence (81). Although treatments may influence
the stability of CTC clusters, chemotherapy was incapable of
destroying all CTC clusters. A higher reproducible rate of CTC
clusters than that of single CTCs was found during the follow-up of
patients after adjuvant chemotherapy (33). Similarly, the reduction of the
proportion of CTC clusters was not significant even though the
total number of CTCs was dramatically reduced (75), suggesting that CTC clusters may
have different clinical relevance with single CTCs.
Although the importance of CTC clusters is gradually
accepted, few effects have been made in cancer treatment by
targeting CTC clusters. Recently, a Korean group succeeded in
prolonging survival in a mouse model of breast cancer by
dissociating CTC clusters using urokinase (82), making a first step in CTC
cluster-relevant treatment. However, because the dissociated CTCs
can still form metastases, the effects of such strategy may be mild
and needs further evaluation.
In conclusion, the clinical value of CTC clusters
currently remains elusive. Further diligent study is necessary in
order to exploit the full potential of CTC clusters in clinical
applications.
6. Perspectives
The molecular characterization of CTC clusters may
revolutionize our interpretation of cancer metastasis. However,
many questions need to be answered before CTC clusters can begin
making sense for biological understanding and clinical use. First,
methods optimized for specific isolation of CTC clusters preserving
their original status are required due to the lack of an efficient
or widely approved detection platform that does not hinder in-depth
study of CTC clusters. Second, further identification is needed for
the adhesion molecules responsible for CTC cluster formation, as
well as the genetic and biological specialty of the tumor cells
harboring these molecules. Third, the biological details of
creation, travelling in the circulation, and metastases formation
in distant organs of CTC clusters also demand further
investigation. Finally, the clinical significance of CTC clusters
is awaiting confirmation, and treatments based on CTC clusters may
be promising to reduce metastasis events. Finding viable solutions
to these problems will open up further fields of study for CTC
clusters, and affirm its value in clinical practice spanning from
early diagnosis of cancer to relapse monitoring.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81401954) and the Medical Science and
Technology Program of Zhejiang Province, China (2015KYA114).
References
|
1
|
Krebs MG, Metcalf RL, Carter L, Brady G,
Blackhall FH and Dive C: Molecular analysis of circulating tumour
cells - biology and biomarkers. Nat Rev Clin Oncol. 11:129–144.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lianidou ES: Circulating tumor cell
isolation: A marathon race worth running. Clin Chem. 60:287–289.
2014. View Article : Google Scholar
|
|
4
|
Plaks V, Koopman CD and Werb Z: Cancer.
Circulating tumor cells. Science. 341:1186–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW, et al: Circulating tumor cells, disease progression,
and survival in metastatic breast cancer. N Engl J Med.
351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cristofanilli M: Circulating tumor cells,
disease progression, and survival in metastatic breast cancer.
Semin Oncol. 33(Suppl 9): S9–S14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou JM, Krebs MG, Lancashire L, Sloane R,
Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, et
al: Clinical significance and molecular characteristics of
circulating tumor cells and circulating tumor microemboli in
patients with small-cell lung cancer. J Clin Oncol. 30:525–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fidler IJ: The relationship of embolic
homogeneity, number, size and viability to the incidence of
experimental metastasis. Eur J Cancer. 9:223–227. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lione A and Bosmann HB: Quantitative
relationship between volume of tumour cell units and their
intravascular survival. Br J Cancer. 37:248–253. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liotta LA, Kleinerman J and Saidel GM:
Quantitative relationships of intravascular tumor cells, tumor
vessels, and pulmonary metastases following tumor implantation.
Cancer Res. 34:997–1004. 1974.PubMed/NCBI
|
|
14
|
Liotta LA, Saidel MG and Kleinerman J: The
significance of hematogenous tumor cell clumps in the metastatic
process. Cancer Res. 36:889–894. 1976.PubMed/NCBI
|
|
15
|
Thompson SC: The colony forming efficiency
of single cells and cell aggregates from a spontaneous mouse
mammary tumour using the lung colony assay. Br J Cancer.
30:332–336. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aceto N, Bardia A, Miyamoto DT, Donaldson
MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al:
Circulating tumor cell clusters are oligoclonal precursors of
breast cancer metastasis. Cell. 158:1110–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JF, Ho H, Lichterman J, Lu YT, Zhang
Y, Garcia MA, Chen SF, Liang AJ, Hodara E, Zhau HE, et al:
Subclassification of prostate cancer circulating tumor cells by
nuclear size reveals very small nuclear circulating tumor cells in
patients with visceral metastases. Cancer. 121:3240–3251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Wit S, van Dalum G, Lenferink AT, Tibbe
AG, Hiltermann TJ, Groen HJ, van Rijn CJ and Terstappen LW: The
detection of EpCAM(+) and EpCAM(−) circulating tumor cells. Sci
Rep. 5:122702015. View Article : Google Scholar
|
|
19
|
Meng S, Tripathy D, Frenkel EP, Shete S,
Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, et
al: Circulating tumor cells in patients with breast cancer
dormancy. Clin Cancer Res. 10:8152–8162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe S: The metastasizability of tumor
cells. Cancer. 7:215–223. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garvie WH and Matheson AB: The effect of
intravenous fluids on the development on experimental tumour
metastases: Their effect on tumour cell aggregation. Br J Cancer.
20:838–846. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Topal B, Roskams T, Fevery J and Penninckx
F: Aggregated colon cancer cells have a higher metastatic
efficiency in the liver compared with nonaggregated cells: An
experimental study. J Surg Res. 112:31–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knisely WH and Mahaley MS Jr: Relationship
between size and distribution of spontaneous metastases and three
sizes of intravenously injected particles of VX2 carcinoma. Cancer
Res. 18:900–905. 1958.PubMed/NCBI
|
|
24
|
Peeters DJ, Brouwer A, Van den Eynden GG,
Rutten A, Onstenk W, Sieuwerts AM, Van Laere SJ, Huget P, Pauwels
P, Peeters M, et al: Circulating tumour cells and lung
microvascular tumour cell retention in patients with metastatic
breast and cervical cancer. Cancer Lett. 356B:872–879. 2015.
View Article : Google Scholar
|
|
25
|
Glaves D: Correlation between circulating
cancer cells and incidence of metastases. Br J Cancer. 48:665–673.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stott SL, Hsu CH, Tsukrov DI, Yu M,
Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK,
et al: Isolation of circulating tumor cells using a
microvortex-generating herringbone-chip. Proc Natl Acad Sci USA.
107:18392–18397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duda DG, Duyverman AM, Kohno M, Snuderl M,
Steller EJ, Fukumura D and Jain RK: Malignant cells facilitate lung
metastasis by bringing their own soil. Proc Natl Acad Sci USA.
107:21677–21682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Upreti M, Jamshidi-Parsian A, Koonce NA,
Webber JS, Sharma SK, Asea AA, Mader MJ and Griffin RJ:
Tumor-endothelial cell three-dimensional spheroids: New aspects to
enhance radiation and drug therapeutics. Transl Oncol. 4:365–376.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou JM, Krebs M, Ward T, Sloane R, Priest
L, Hughes A, Clack G, Ranson M, Blackhall F and Dive C: Circulating
tumor cells as a window on metastasis biology in lung cancer. Am J
Pathol. 178:989–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Friedlander TW, Ngo VT, Dong H,
Premasekharan G, Weinberg V, Doty S, Zhao Q, Gilbert EG, Ryan CJ,
Chen WT, et al: Detection and characterization of invasive
circulating tumor cells derived from men with metastatic
castration-resistant prostate cancer. Int J Cancer. 134:2284–2293.
2014. View Article : Google Scholar
|
|
31
|
Balasubramanian P, Lang JC, Jatana KR,
Miller B, Ozer E, Old M, Schuller DE, Agrawal A, Teknos TN, Summers
TA Jr, et al: Multiparameter analysis, including EMT markers, on
negatively enriched blood samples from patients with squamous cell
carcinoma of the head and neck. PLoS One. 7:e420482012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brandt B, Junker R, Griwatz C, Heidl S,
Brinkmann O, Semjonow A, Assmann G and Zänker KS: Isolation of
prostate-derived single cells and cell clusters from human
peripheral blood. Cancer Res. 56:4556–4561. 1996.PubMed/NCBI
|
|
33
|
Molnar B, Ladanyi A, Tanko L, Sréter L and
Tulassay Z: Circulating tumor cell clusters in the peripheral blood
of colorectal cancer patients. Clin Cancer Res. 7:4080–4085.
2001.PubMed/NCBI
|
|
34
|
Wang ZP, Eisenberger MA, Carducci MA,
Partin AW, Scher HI and Ts'o PO: Identification and
characterization of circulating prostate carcinoma cells. Cancer.
88:2787–2795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sarioglu AF, Aceto N, Kojic N, Donaldson
MC, Zeinali M, Hamza B, Engstrom A, Zhu H, Sundaresan TK, Miyamoto
DT, et al: A microfluidic device for label-free, physical capture
of circulating tumor cell clusters. Nat Methods. 12:685–691. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vona G, Sabile A, Louha M, Sitruk V,
Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et
al: Isolation by size of epithelial tumor cells: A new method for
the immunomorphological and molecular characterization of
circulating tumor cells. Am J Pathol. 156:57–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vona G, Estepa L, Béroud C, Damotte D,
Capron F, Nalpas B, Mineur A, Franco D, Lacour B, Pol S, et al:
Impact of cytomorphological detection of circulating tumor cells in
patients with liver cancer. Hepatology. 39:792–797. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krebs MG, Hou JM, Sloane R, Lancashire L,
Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, et al:
Analysis of circulating tumor cells in patients with non-small cell
lung cancer using epithelial marker-dependent and -independent
approaches. J Thorac Oncol. 7:306–315. 2012. View Article : Google Scholar
|
|
39
|
Adams AA, Okagbare PI, Feng J, Hupert ML,
Patterson D, Göttert J, McCarley RL, Nikitopoulos D, Murphy MC and
Soper SA: Highly efficient circulating tumor cell isolation from
whole blood and label-free enumeration using polymer-based
microfluidics with an integrated conductivity sensor. J Am Chem
Soc. 130:8633–8641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gleghorn JP, Pratt ED, Denning D, Liu H,
Bander NH, Tagawa ST, Nanus DM, Giannakakou PA and Kirby BJ:
Capture of circulating tumor cells from whole blood of prostate
cancer patients using geometrically enhanced differential
immunocapture (GEDI) and a prostate-specific antibody. Lab Chip.
10:27–29. 2010. View Article : Google Scholar
|
|
41
|
Mohamed H, Murray M, Turner JN and Caggana
M: Isolation of tumor cells using size and deformation. J
Chromatogr A. 1216:8289–8295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tan SJ, Yobas L, Lee GY, Ong CN and Lim
CT: Microdevice for the isolation and enumeration of cancer cells
from blood. Biomed Microdevices. 11:883–892. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu Y, Phillips JA, Yan J, Li Q, Fan ZH and
Tan W: Aptamer-based microfluidic device for enrichment, sorting,
and detection of multiple cancer cells. Anal Chem. 81:7436–7442.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng S, Lin H, Liu JQ, Balic M, Datar R,
Cote RJ and Tai YC: Membrane microfilter device for selective
capture, electrolysis and genomic analysis of human circulating
tumor cells. J Chromatogr A. 1162:154–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo
RA, Tan DS, Lim WT, Han J, Bhagat AA and Lim CT: Isolation and
retrieval of circulating tumor cells using centrifugal forces. Sci
Rep. 3:12592013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Harouaka RA, Zhou MD, Yeh YT, Khan WJ, Das
A, Liu X, Christ CC, Dicker DT, Baney TS, Kaifi JT, et al: Flexible
micro spring array device for high-throughput enrichment of viable
circulating tumor cells. Clin Chem. 60:323–333. 2014. View Article : Google Scholar
|
|
47
|
Cho EH, Wendel M, Luttgen M, Yoshioka C,
Marrinucci D, Lazar D, Schram E, Nieva J, Bazhenova L, Morgan A, et
al: Characterization of circulating tumor cell aggregates
identified in patients with epithelial tumors. Phys Biol.
9:0160012012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ge F, Zhang H, Wang DD, Li L and Lin PP:
Enhanced detection and comprehensive in situ phenotypic
characterization of circulating and disseminated heteroploid
epithelial and glioma tumor cells. Oncotarget. 6:27049–27064. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Wang F, Ning N, Chen Q, Yang Z,
Guo Y, Xu D, Zhang D, Zhan T and Cui W: Patterns of circulating
tumor cells identified by CEP8, CK and CD45 in pancreatic cancer.
Int J Cancer. 136:1228–1233. 2015. View Article : Google Scholar
|
|
50
|
Zamay GS, Kolovskaya OS, Zamay TN,
Glazyrin YE, Krat AV, Zubkova O, Spivak E, Wehbe M, Gargaun A,
Muharemagic D, et al: Aptamers selected to postoperative lung
adenocarcinoma detect circulating tumor cells in human blood. Mol
Ther. 23:1486–1496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
King MR, Phillips KG, Mitrugno A, Lee TR,
de Guillebon AM, Chandrasekaran S, McGuire MJ, Carr RT,
Baker-Groberg SM, Rigg RA, et al: A physical sciences network
characterization of circulating tumor cell aggregate transport. Am
J Physiol Cell Physiol. 308:C792–C802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cheung KJ, Padmanaban V, Silvestri V,
Schipper K, Cohen JD, Fairchild AN, Gorin MA, Verdone JE, Pienta
KJ, Bader JS, et al: Polyclonal breast cancer metastases arise from
collective dissemination of keratin 14-expressing tumor cell
clusters. Proc Natl Acad Sci USA. 113:E854–E863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Glinsky VV, Glinsky GV, Glinskii OV,
Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ and Quinn
TP: Intravascular metastatic cancer cell homotypic aggregation at
the sites of primary attachment to the endothelium. Cancer Res.
63:3805–3811. 2003.PubMed/NCBI
|
|
54
|
Geng Y, Chandrasekaran S, Hsu JW, Gidwani
M, Hughes AD and King MR: Phenotypic switch in blood: Effects of
pro-inflammatory cytokines on breast cancer cell aggregation and
adhesion. PLoS One. 8:e549592013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fidler IJ: Immune stimulation-inhibition
of experimental cancer metastasis. Cancer Res. 34:491–498.
1974.PubMed/NCBI
|
|
56
|
Gasic GJ, Gasic TB, Galanti N, Johnson T
and Murphy S: Platelet-tumor-cell interactions in mice. The role of
platelets in the spread of malignant disease. Int J Cancer.
11:704–718. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Läubli H, Stevenson JL, Varki A, Varki NM
and Borsig L: L-selectin facilitation of metastasis involves
temporal induction of Fut7-dependent ligands at sites of tumor cell
arrest. Cancer Res. 66:1536–1542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Küsters B, Kats G, Roodink I, Verrijp K,
Wesseling P, Ruiter DJ, de Waal RM and Leenders WP: Micronodular
transformation as a novel mechanism of VEGF-A-induced metastasis.
Oncogene. 26:5808–5815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Borsig L, Wong R, Hynes RO, Varki NM and
Varki A: Synergistic effects of L- and P-selectin in facilitating
tumor metastasis can involve non-mucin ligands and implicate
leukocytes as enhancers of metastasis. Proc Natl Acad Sci USA.
99:2193–2198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Molnar B, Floro L, Sipos F, Toth B, Sreter
L and Tulassay Z: Elevation in peripheral blood circulating tumor
cell number correlates with macroscopic progression in UICC stage
IV colorectal cancer patients. Dis Markers. 24:141–150. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sugino T, Kusakabe T, Hoshi N, Yamaguchi
T, Kawaguchi T, Goodison S, Sekimata M, Homma Y and Suzuki T: An
invasion-independent pathway of blood-borne metastasis: A new
murine mammary tumor model. Am J Pathol. 160:1973–1980. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ao Z, Shah SH, Machlin LM, Parajuli R,
Miller PC, Rawal S, Williams AJ, Cote RJ, Lippman ME, Datar RH, et
al: Identification of cancer-associated fibroblasts in circulating
blood from patients with metastatic breast cancer. Cancer Res.
75:4681–4687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sharma D, Brummel-Ziedins KE, Bouchard BA
and Holmes CE: Platelets in tumor progression: A host factor that
offers multiple potential targets in the treatment of cancer. J
Cell Physiol. 229:1005–1015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lecharpentier A, Vielh P, Perez-Moreno P,
Planchard D, Soria JC and Farace F: Detection of circulating tumour
cells with a hybrid (epithelial/mesenchymal) phenotype in patients
with metastatic non-small cell lung cancer. Br J Cancer.
105:1338–1341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Maddipati R and Stanger BZ: Pancreatic
cancer metastases harbor evidence of polyclonality. Cancer Discov.
5:1086–1097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Au SH, Storey BD, Moore JC, Tang Q, Chen
YL, Javaid S, Sarioglu AF, Sullivan R, Madden MW, O'Keefe R, et al:
Clusters of circulating tumor cells traverse capillary-sized
vessels. Proc Natl Acad Sci USA. 113:4947–4952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fidler IJ and Talmadge JE: Evidence that
intravenously derived murine pulmonary melanoma metastases can
originate from the expansion of a single tumor cell. Cancer Res.
46:5167–5171. 1986.PubMed/NCBI
|
|
69
|
Zhao Q, Barclay M, Hilkens J, Guo X,
Barrow H, Rhodes JM and Yu LG: Interaction between circulating
galectin-3 and cancer-associated MUC1 enhances tumour cell
homotypic aggregation and prevents anoikis. Mol Cancer. 9:1542010.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhao Q, Guo X, Nash GB, Stone PC, Hilkens
J, Rhodes JM and Yu LG: Circulating galectin-3 promotes metastasis
by modifying MUC1 localization on cancer cell surface. Cancer Res.
69:6799–6806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yu LG, Andrews N, Zhao Q, McKean D,
Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J,
Kasai K, et al: Galectin-3 interaction with Thomsen-Friedenreich
disaccharide on cancer-associated MUC1 causes increased cancer cell
endothelial adhesion. J Biol Chem. 282:773–781. 2007. View Article : Google Scholar
|
|
72
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wels J, Kaplan RN, Rafii S and Lyden D:
Migratory neighbors and distant invaders: Tumor-associated niche
cells. Genes Dev. 22:559–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Psaila B and Lyden D: The metastatic
niche: Adapting the foreign soil. Nat Rev Cancer. 9:285–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Phillips KG, Lee AM, Tormoen GW, Rigg RA,
Kolatkar A, Luttgen M, Bethel K, Bazhenova L, Kuhn P, Newton P, et
al: The thrombotic potential of circulating tumor microemboli:
Computational modeling of circulating tumor cell-induced
coagulation. Am J Physiol Cell Physiol. 308:C229–C236. 2015.
View Article : Google Scholar :
|
|
76
|
Denes V, Lakk M, Makarovskiy A, Jakso P,
Szappanos S, Graf L, Mandel L, Karadi I and Geck P: Metastasis
blood test by flow cytometry: In vivo cancer spheroids and the role
of hypoxia. Int J Cancer. 136:1528–1536. 2015. View Article : Google Scholar
|
|
77
|
Al-Mehdi AB, Tozawa K, Fisher AB, Shientag
L, Lee A and Muschel RJ: Intravascular origin of metastasis from
the proliferation of endothelium-attached tumor cells: A new model
for metastasis. Nat Med. 6:100–102. 2000. View Article : Google Scholar
|
|
78
|
Mu Z, Wang C, Ye Z, Austin L, Civan J,
Hyslop T, Palazzo JP, Jaslow R, Li B, Myers RE, et al: Prospective
assessment of the prognostic value of circulating tumor cells and
their clusters in patients with advanced-stage breast cancer.
Breast Cancer Res Treat. 154:563–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jansson S, Bendahl PO, Larsson AM,
Aaltonen KE and Rydén L: Prognostic impact of circulating tumor
cell apoptosis and clusters in serial blood samples from patients
with metastatic breast cancer in a prospective observational
cohort. BMC Cancer. 16:4332016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mascalchi M, Falchini M, Maddau C,
Salvianti F, Nistri M, Bertelli E, Sali L, Zuccherelli S, Vella A,
Matucci M, et al: Prevalence and number of circulating tumour cells
and microemboli at diagnosis of advanced NSCLC. J Cancer Res Clin
Oncol. 142:195–200. 2016. View Article : Google Scholar
|
|
81
|
Giesing M, Suchy B, Driesel G and Molitor
D: Clinical utility of antioxidant gene expression levels in
circulating cancer cell clusters for the detection of prostate
cancer in patients with prostate-specific antigen levels of 4–10
ng/ml and disease prognostication after radical prostatectomy. BJU
Int. 105:1000–1010. 2010. View Article : Google Scholar
|
|
82
|
Choi JW, Kim JK, Yang YJ, Kim P, Yoon KH
and Yun SH: Urokinase exerts antimetastatic effects by dissociating
clusters of circulating tumor cells. Cancer Res. 75:4474–4482.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wendel M, Bazhenova L, Boshuizen R,
Kolatkar A, Honnatti M, Cho EH, Marrinucci D, Sandhu A, Perricone
A, Thistlethwaite P, et al: Fluid biopsy for circulating tumor cell
identification in patients with early-and late-stage non-small cell
lung cancer: A glimpse into lung cancer biology. Phys Biol.
9:0160052012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Reddy RM, Murlidhar V, Zhao L,
Grabauskiene S, Zhang Z, Ramnath N, Lin J, Chang AC, Carrott P,
Lynch W, et al: Pulmonary venous blood sampling significantly
increases the yield of circulating tumor cells in early-stage lung
cancer. J Thorac Cardiovasc Surg. 151:852–857. 2016. View Article : Google Scholar
|
|
85
|
Kats-Ugurlu G, Roodink I, de Weijert M,
Tiemessen D, Maass C, Verrijp K, van der Laak J, de Waal R, Mulders
P, Oosterwijk E, et al: Circulating tumour tissue fragments in
patients with pulmonary metastasis of clear cell renal cell
carcinoma. J Pathol. 219:287–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Loh J, Jovanovic L, Lehman M, Capp A,
Pryor D, Harris M, Nelson C and Martin J: Circulating tumor cell
detection in high-risk non-metastatic prostate cancer. J Cancer Res
Clin Oncol. 140:2157–2162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chang MC, Chang YT, Chen JY, Jeng YM, Yang
CY, Tien YW, Yang SH, Chen HL, Liang TY, Wang CF, et al: Clinical
significance of circulating tumor microemboli as a prognostic
marker in patients with pancreatic ductal adenocarcinoma. Clin
Chem. 62:505–513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Long E, Ilie M, Bence C, Butori C, Selva
E, Lalvée S, Bonnetaud C, Poissonnet G, Lacour JP, Bahadoran P, et
al: High expression of TRF2, SOX10, and CD10 in circulating tumor
microemboli detected in metastatic melanoma patients. A potential
impact for the assessment of disease aggressiveness. Cancer Med.
5:1022–1030. 2016. View Article : Google Scholar : PubMed/NCBI
|