Introduction
Multidrug resistance (MDR) is considered to be a
major contributor to failure of chemotherapy in treating cancer
(1–3). Among cancer therapies, chemotherapy
can palliate symptoms and prolong life for some cancer patients.
However, cancer cells can have intrinsic or acquired resistance
after treatment with chemotherapeutic drugs leading to the
development of MDR. MDR attenuates the efficacy of anticancer drugs
and results in treatment failure for cancer (2,4). The
phenomenon of multidrug resistance (MDR) in cancer is associated
with the overexpression of the ATP-binding cassette (ABC)
transporter proteins, including multidrug resistance-associated
protein 1 (MRP1), breast cancer resistance protein (BCRP) and
P-glycoprotein (5–8). Additionally, the genetic alterations
of several resistance proteins, including caveolin-1 and HOXC6,
have been associated with one of the major processes that
characterize MDR development in many cancers (9,10).
Although a clear association has been established between MDR and
genetic alterations, the mechanism by which multi-drug resistance
develops in oral cancer has not yet been fully elucidated.
Photodynamic therapy (PDT) uses tumor-selective
photosensitizers and subsequent activation by a specific wavelength
of light to generate reactive oxygen species. PDT has been used
increasingly in the treatment of various cancers (11,12).
PDT has been considered to be an alternative for treatment of
resistant cancers because its mechanism is completely different
from that of conventional chemotherapy (11). In fact, some studies have showed
that PDT is reported to inhibit MDR cancers through different cell
death pathways and that its effectiveness is dependent on the types
of cancer cell lines and photosensitizers used, including
mesoporphyrin, phthalocyanines and photofrin (13–15).
Recently, we synthesized pheophorbide a (Pa) by removing a
magnesium ion and a phytyl group from chlorophyll-a. Our studies
reported that Pa-based PDT can effectively inhibit tumor cell
proliferation in several cancer cells, including skin and head and
neck cancer (16–18). Other research groups reported that
Pa-based PDT can effectively inhibit tumor cell proliferation in
hepatocellular carcinoma (19,20),
colon cancer (21), pigmented
melanoma (22) and breast
adenocarcinoma (23); however, its
therapeutic potential in oral cancer (especially instances of MDR)
has not been fully investigated. It is therefore, necessary to
focus on the search for strong therapeutic candidates against MDR
oral cancer that utilize PDT.
It is known that the HOX family member HOXC6 is
associated with several human carcinomas, including
gastrointestinal, breast, lung and prostate. HOXC6 is overexpressed
in osteosarcomas and medulloblastomas (24–28).
In previous studies, HOXC6 was shown to play an important role in
several cellular events through the regulation of its functional
biological targets such as bone morphogenic protein 7, fibroblast
growth factor receptor 2 and platelet-derived growth factor
receptor, as well as the PI3K/AKT, Notch and Wnt signaling pathways
(29–33). In addition, we found that HOXC6 is
an important mechanism of the multidrug resistance pathway via
regulation of Bcl-2 and MDR-1 (10,34).
Although HOXC6 is critical for various regulated cellular processes
and is correlated with the progression of multidrug resistance, the
function of HOXC6 in oral cancer is largely unknown.
The present study investigated the efficacy and
pathway of Pa-PDT-based HOXC6 sensitization to resolve multidrug
resistance. Here, our data provided novel evidence that MDR-1 was a
direct regulatory target of HOXC6. These data suggest a potential
role for Pa-PDT-mediated HOXC6 inhibition in the regulation of
chemotherapeutic drug resistance.
Materials and methods
Cell culture
FaDu human pharynx squamous cell carcinoma (SCC) was
purchased from the American Type Culture Collection (ATCC;
Rockville, MD). FaDu-PTX cells were created by exposing wild-type
FaDu cells to increasing concentrations of paclitaxel for more than
10 months. The FaDu and FaDu-PTX cell lines were incubated in
Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal
bovine serum (FBS) and 100 units/ml penicillin-streptomycin
(Invitrogen, Carlsbad, CA, USA) at 37°C in an atmosphere containing
5% CO2.
Cell proliferation assay
Cells were plated in 12-well plates at a density of
5×104 cells/well plate. A total of 100 μl
(3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT
reagent (Sigma-Aldrich) was added to each well. After a 2-h
incubation at 37°C, the supernatant was aspirated, and formazan
crystals were dissolved in 500 μl dimethyl sulfoxide (DMSO) at 37°C
for 15 min under gentle agitation. The absorbance per well was
measured at 570 nm using a multimode ELISA reader (Beckman Coulter,
Inc., Wals-Siezenheim, Austria).
Reverse transcription quantitative PCR
(RT-qPCR) analysis
Quantitative real-time PCR was performed using
SYBR-Green (Applied Biosystems, Foster City, CA, USA). PCR runs and
fluorescence detection were performed in a Rotor-Gene 6000
Real-Time PCR system (Corbett Research, Sydney, NSW, Australia). On
the basis of HOXC6 and MDR-1, two pairs of gene-specific primers
were designed. The reaction mixture contained 10 ng of cDNA diluted
in 2.5 μl DEPC-treated water, 5 μl Power SYBR-Green PCR Master Mix
(2X; Applied Biosystems) and 2 μl of gene-specific primers (final
concentration 50 nM each) in a final reaction volume of 10 μl. The
sequences of the real-time PCR primers were as follows: HOXC6,
forward 5′-CACCGCCTATGATCCAGTGAGGCA-3′ and reverse
5′-GCTGGAACTGAACACGACATTCTC-3′; MDR-1, forward
5′-GACTGTCAGCTGCTGTCTGGGCAA-3′ and reverse
5′-GCCAAGACCTCTTCAGCTACTGC-3′; glyceraldehyde 3-phosphate
dehydrogenase, forward 5′-AGCCAAAAGGGTCATCATCTCTGC-3′ and reverse
5′-GCATTGCTGATGATCTTGAGGCTG-3′. The cycling conditions were as
follows: denaturation at 95°C for 10 min followed by 40 cycles of
95°C for 20 sec, 58°C for 20 sec and 70°C for 20 sec.
Measurement of intracellular ROS
After Pa-PDT, the cells were washed twice with
phosphate-buffered saline (PBS) and incubated in a medium
containing 10 μM 2′7′-dichloro-fluorescein diacetate and
H2DCFDA (Invitrogen) at 37°C in an atmosphere containing
5% CO2 for 30 min. The fluorescence was analyzed
immediately using a multimode ELISA reader (Beckman Coulter).
Flow cytometric cell cycle analysis and
Annexin V-FITC/PI staining
For cell cycle analysis, FaDu and FaDu-PTX cells
were fixed in chilled 70% methanol for 1 h at 4°C and stained with
propidium iodine (PI) solution (100 μg/ml RNase and 10 μg/ml PI in
PBS) at 37°C for 30 min. In addition, the cells were stained using
the Vybrant apoptosis assay kit (Molecular Probes) followed by
labeling with Alexa Fluor® 488 Annexin V and PI for
apoptosis analysis. Data acquisition and analysis were performed
using the Cell Lab Quanta™ SC flow cytometer and software (Beckman
Coulter, Inc., Miami, FL, USA).
Western blot analysis
The cells were treated with Pa-PDT for 24 h. The
cells were then washed with PBS and harvested in lysis buffer.
Samples containing equal amounts of protein were loaded onto
individual lanes of an SDS-polyacrylamide gel for electrophoresis
and subsequently transferred onto a polyvinylidene difluoride
membrane. The membranes were blocked and then incubated with
antibodies. Antibodies against p-ERK1/2, p-AKT, p-p70S6K, CDKs,
cyclin B and cyclin D1 were purchased from Cell Signaling
Technology (Beverly, MA, USA), whereas HOXC6, Notch1, Notch-4,
MDR-1 and β-actin antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). For detection, an ECL kit
(Amersham Biosciences Life Sciences) was used according to the
manufacturer’s instructions.
Photodynamic therapy
The PDT irradiation light source was a
light-emitting diode (LED; 613–645 nm; Philips Luxeon Lumileds, San
Jose, CA, USA). The cells (1×105/well) were
pre-incubated with Pa (0.2 μM) in complete growth medium in the
dark for 2 h. For the subsequent experiments, the cells were
irradiated at 1.2 J/cm2. For the control group, the
cells were incubated in the same medium with neither Pa nor
light.
siRNA interference assay
siRNA constructs for HOXC6 were obtained in the form
of Silencer® Select Validated siRNA (Applied
Biosystems). The sense sequence of the HOXC6 siRNA was 5′-CUC GUU
CUC GGC UUG UCU A (dTdT)-3′ and the antisense sequence was 5′-UAG
ACA AGC CGA GAA CGA G (dTdT)-3′. Cells were transfected with siRNA
(20 nM) using Lipofectamine 2000 RNAiMAX transfection reagent
(Invitrogen) according to the manufacturer’s instructions. The
cells were harvested 48 h after transfection. Total cell lysates
were separated by SDS-PAGE and analyzed by western blot analysis as
described above.
In vivo study in C3H mice
FaDu-PTX cells were transfected with either HOXC6
siRNA (20 nM) for 48 h or Pa-PDT (0.2 μM, 1.2 J/cm2) for
24 h. Cells (3×106 per mouse) were injected
subcutaneously into the left flank of 4-week-old male
immunocompetent C3H mice (Samtaco Bio, Sungnam, Korea) in each
group (n=10). Two weeks later, the tumor volume was measured by
caliper and calculated by the formula V = (ab2)/2, in
which a is the longest diameter and b is the shortest diameter of
the tumor. All mice were euthanized on day 14, and the tumors were
removed, scaled, and subjected to further analysis.
Immunohistochemistry of tumors and H&E staining were performed
as previously described (18).
Statistical analysis
Statistical analysis was performed with the data
obtained from three independent experiments. The data are presented
as the mean ± SEM. A P<0.05 was considered statistically
significant.
Results
Antitumor effect of Pa-PDT on human oral
cancer cells
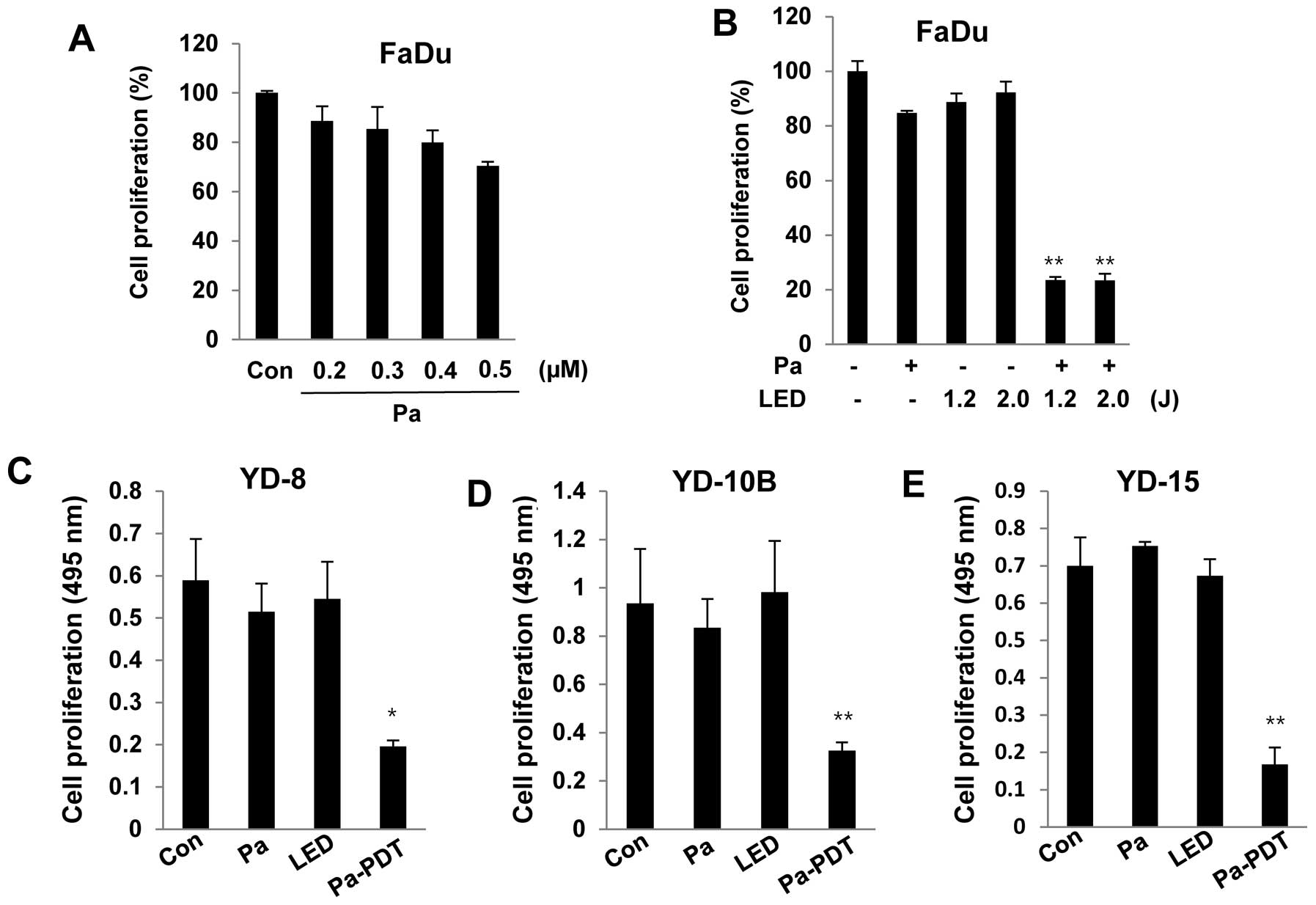
To examine the cytotoxicity of Pa, FaDu cells were
incubated with various concentrations (0.2 to 0.5 μM) of Pa for 24
h in the dark. Cell viability was measured using an MTT assay. As
shown in Fig. 1A, significant
cytotoxicity was not observed in FaDu cells at 0.2 μM Pa. FaDu
cells were pre-treated with 0.2 μM Pa for 2 h, followed by
photoactivation with 1.2 or 2.0 J/cm2 of LED. Cell
proliferation was strongly decreased after the Pa-PDT treatment. In
addition, Pa-PDT also induced significant cytotoxicity in other
human YD-8, YD-10B and YD-15 oral cancer cell lines under the same
conditions (Fig. 1C–E). These
results demonstrated that Pa-PDT exerts an antiproliferative effect
on human oral cancer cells.
Pa-PDT inhibits the growth of
paclitaxel-resistant FaDu-PTX cells
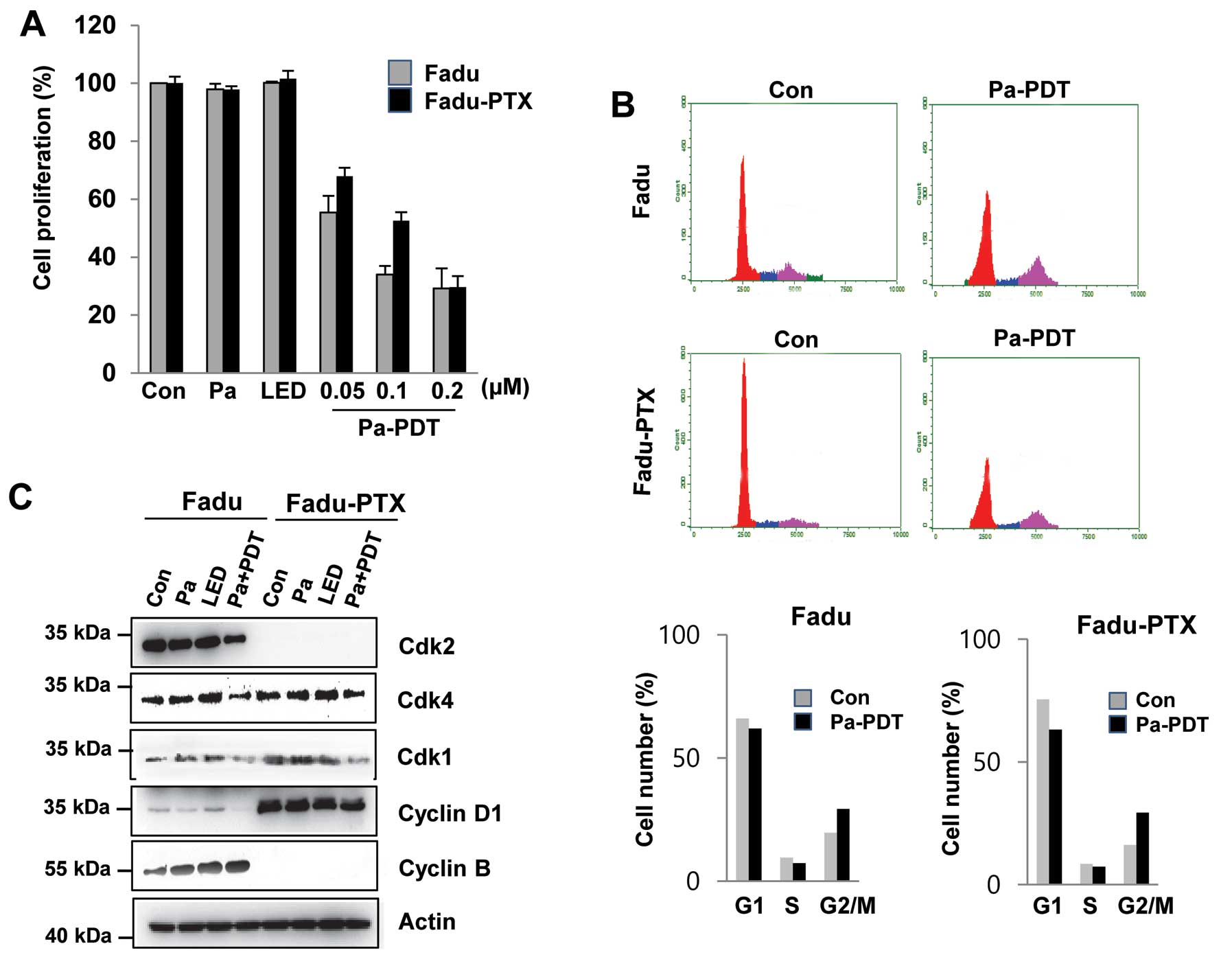
To examine the effects of Pa-PDT on MDR, FaDu-PTX
cells were pre-incubated with various concentrations of Pa for 2 h
followed by photoactivation with 1.2 J/cm2 of light.
Cell proliferation was determined using an MTT assay. As shown in
Fig. 2A, we found that these cell
lines exhibited no significant decrease in cell proliferation due
to either Pa or light alone. However, significant cytotoxicity was
observed in FaDu-PTX cells after Pa treatment (0.05–0.2 μM) in a
dose-dependent manner, with IC50 values of ~ 0.05 and
0.1 μM in the FaDu and FaDu-PTX cells, respectively. In FaDu-PTX
cells, Pa doses of 0.2 μM with LED (1.2 J/cm2) resulted
in cell growth inhibition rates of 70.7% at 24 h (Fig. 1A). The effects of Pa-PDT on cell
cycle distribution were investigated by flow cytometry. Compared
with the untreated control, Pa-PDT treatment caused a reduction of
the G1/S phase population and an increase of the G2/M population in
both FaDu and FaDu-PTX cells (Fig.
2B). Western blot analysis was then used to determine whether
G2/M arrest was associated with CDKs. The expression level of CDK1
protein was found to be down-regulated after Pa-PDT treatment in
FaDu and FaDu-PTX cells whereas that of CDK2 was shown to be
downregulated in FaDu cells and undetectable in FaDu-PTX cells
(Fig. 2C).
Pa-PDT induces apoptosis in FaDu-PTX
cells
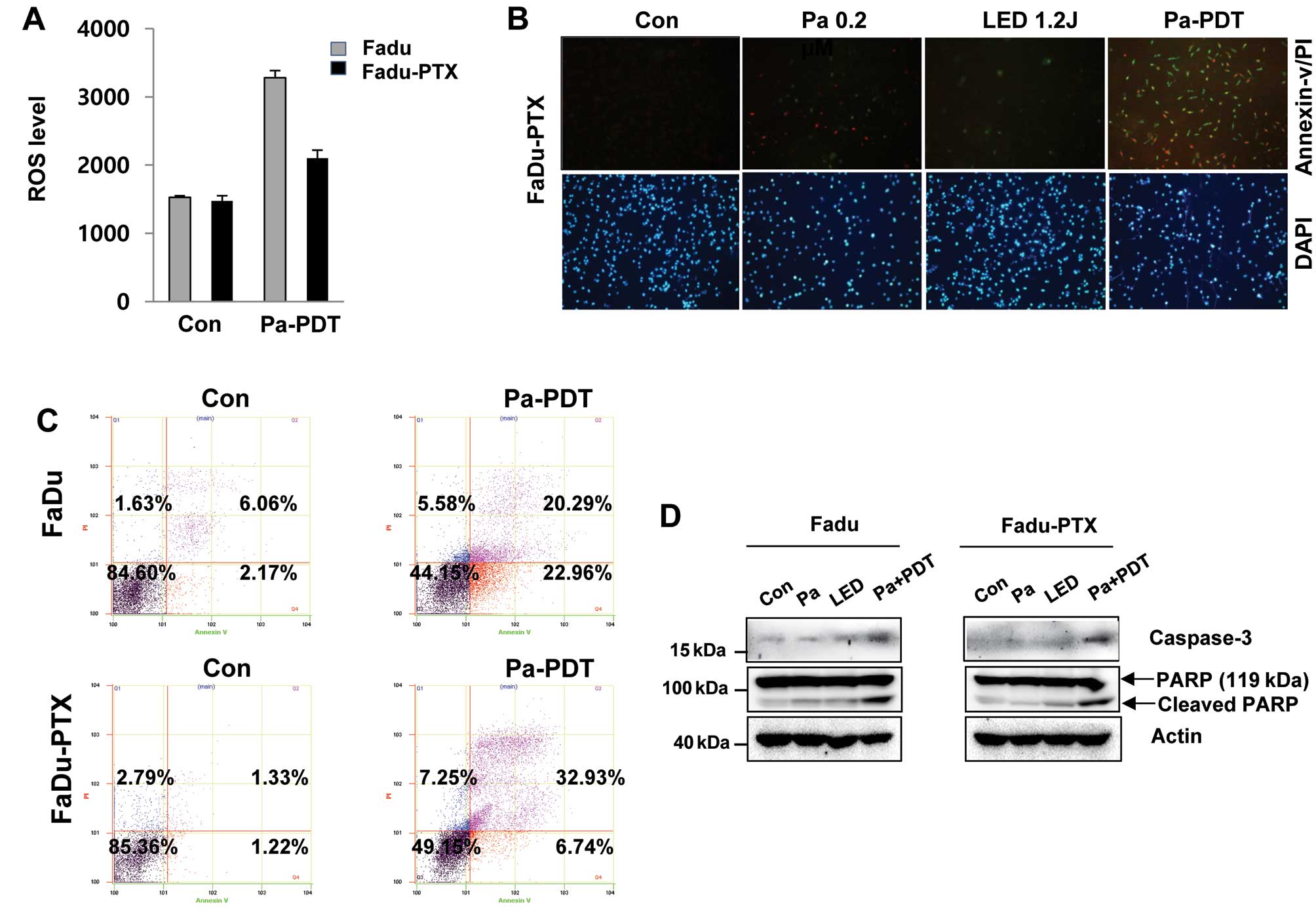
We examined that ROS production can be correlated
with the Pa-PDT mediated cell proliferation. The levels of ROS were
lower following Pa-PDT in FaDu-PTX cells than in FaDu cells
(Fig. 3A). We quantified apoptosis
by fluorescence microscopy and flow cytometry using the Annexin
V-FITC/PI double staining assay. As shown in Fig. 3B, the incubation of cells with
Annexin V-FITC/PI showed an increase in green fluorescence after
Pa-PDT treatment in FaDu-PTX cells. Similarly, a significant number
of apoptotic cells (39.8%) was detected in FaDu-PTX cells after
Pa-PDT treatment, whereas 43.3% apoptotic-positive cells were
detected in FaDu cells (Fig. 3C).
Consistent with this observation, caspase-3 activity were also
detected in FaDu and FaDu-PTX cells treated with Pa-PDT. In
addition, the cleaved form of PARP was increased by Pa-PDT
treatment in both cells (Fig.
3D).
RT-qPCR validation of selected MDR
related genes
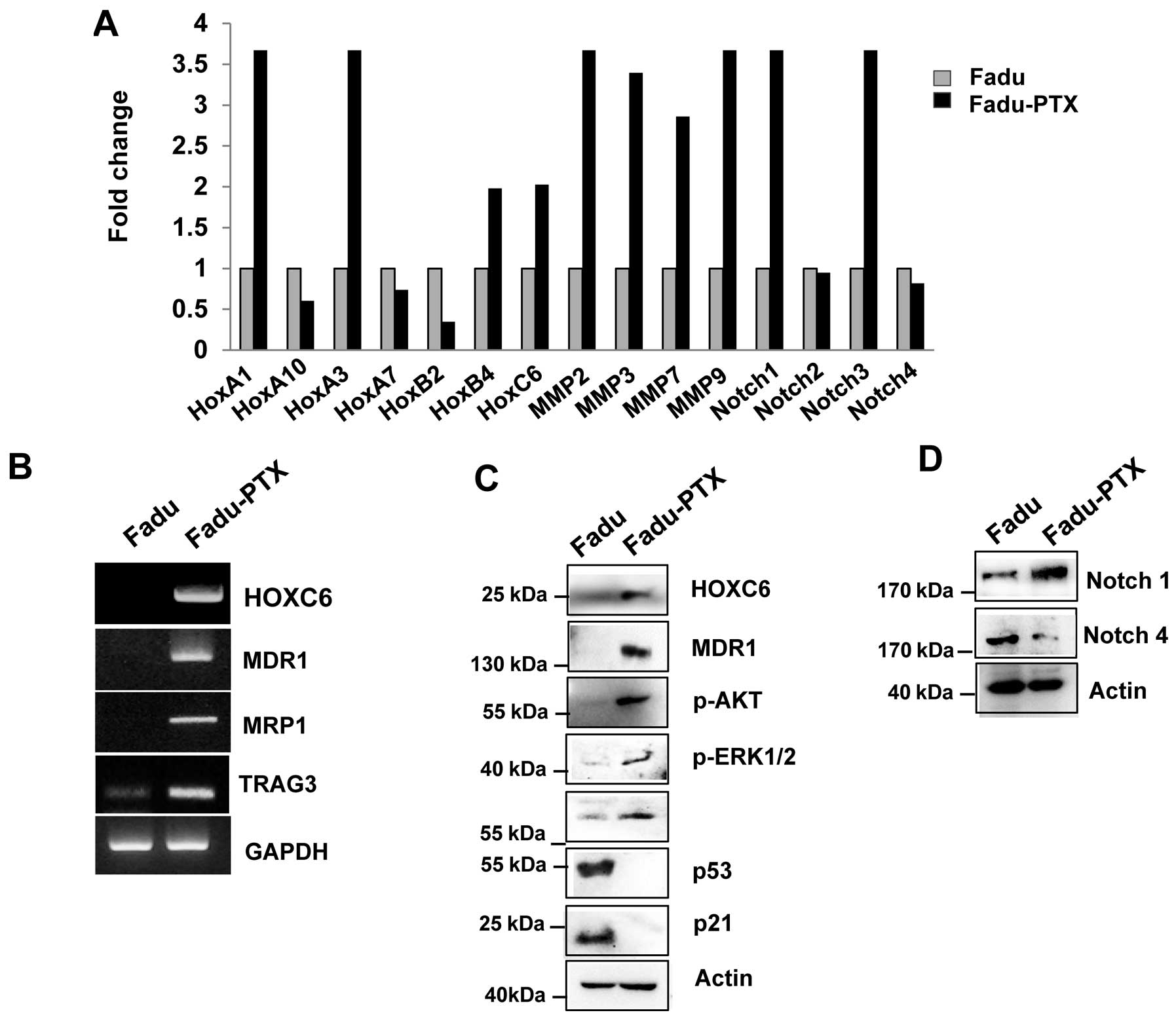
To validate the MDR results by reverse transcription
quantitative PCR (RT-qPCR), we primarily assayed the expression of
HOXA1, HOXA10, HOXA3, HOXA7, HOXB4, HOXC6, MMP-2, MMP-3, MMP-7,
MMP-9, Notch-1, Notch-2, Notch-3 and Notch-4 in FaDu and FaDu-PTX
cells. Ten of the 15 genes examined were upregulated and 3 were
downregulated (Fig. 4A). Of these,
10 genes were altered by >2-fold, including 7 that were altered
by >3-fold. As we previously confirmed the aberrant expression
of one of the candidates (HOXC6) in multidrug resistance cancer
(10), we evaluated the expression
of the other HOX genes, HOXA1, HOXA10 and HOXB4, using
AccuPower® qPCR assays in FaDu-PTX cells and comparing
the values with those from FaDu cells. Significant upregulation of
MMP-2, -3, -7, -9, Notch-1 and Notch-3 was observed in FaDu-PTX
cells compared to FaDu cells. RT-qPCR analysis demonstrated that
the transcription levels of drug resistance-associated genes was
increased in FaDu-PTX cells.
To confirm these data, we performed RT-PCR and
western blot analysis. HOXC6, MDR-1, MRP1 and taxol
resistance-associated gene 3 (TRAG3) mRNA was strongly expressed in
FaDu-PTX cells but weakly expressed in FaDu cells (Fig. 4B). Western blot results
demonstrated that, compared with the FaDu cells, the protein levels
of HOXC6 and MDR-1 were also increased. According to previous
studies, the chemotherapeutic resistance was also attributed to
PI3K/AKT/mTOR and Notch pathway activation (35–37).
Western blot analysis showed that phosphorylated p-Akt, p-p70S6K,
p-ERK and Notch-1 levels were significantly induced in FaDu-PTX
cells compared to FaDu cells (Fig. 4C
and D). In addition, a previous study showed that p53/p21 is
involved in the pathway conferring resistance to paclitaxel
(38). However, we found that p53
and p21 levels were unchanged in FaDu-PTX cells.
Expression of HOXC6 and MDR1 in
Pa-PDT-treated FaDu-PTX cells
To further elucidate the underlying mechanisms of
Pa-PDT in FaDu-PTX cells, we assessed the levels of HOXC6 and
MDR-1, both of which play a crucial role in MDR. Western blotting
revealed that Pa-PDT caused a decrease in the expression of HOXC6
in a dose-dependent manner (Fig.
5A), and the expression of HOXC6 was reduced at 6 h in both
FaDu and FaDu-PTX cells (Fig. 5B and
C). The inhibition of MDR-1 expression was clearly observed at
6 h after Pa-PDT treatment in FaDu-PTX cells (Fig. 5C). However, no significant
expression of MDR-1 proteins was observed in the FaDu cells.
To determine the effects of Pa-PDT on the
AKT/mTOR/p70S6K signaling pathway, cells were treated with Pa-PDT,
and lysates were collected at 6 h after treatment and examined by
western blot analysis. These experiments revealed that Pa-PDT
treatment inhibited AKT/mTOR/p70S6K signaling in FaDu-PTX cells, as
indicated by the reduced levels of p-AKT and p-p70S6K. p-ERK1/2 had
similar expression trends as AKT and p70S6K. However, the
expression of p53 and p21 did not affect Pa-PDT-treated FaDu-PTX
cells (Fig. 5D). Combined with the
strong inhibition of HOXC6 and MDR-1 in Pa-PDT-treated FaDu-PTX
cells, these results suggested that MDR-1 may be correlated with
HOXC6 and/or the AKT/mTOR signaling pathway in PDT-treated MDR
cancer cells.
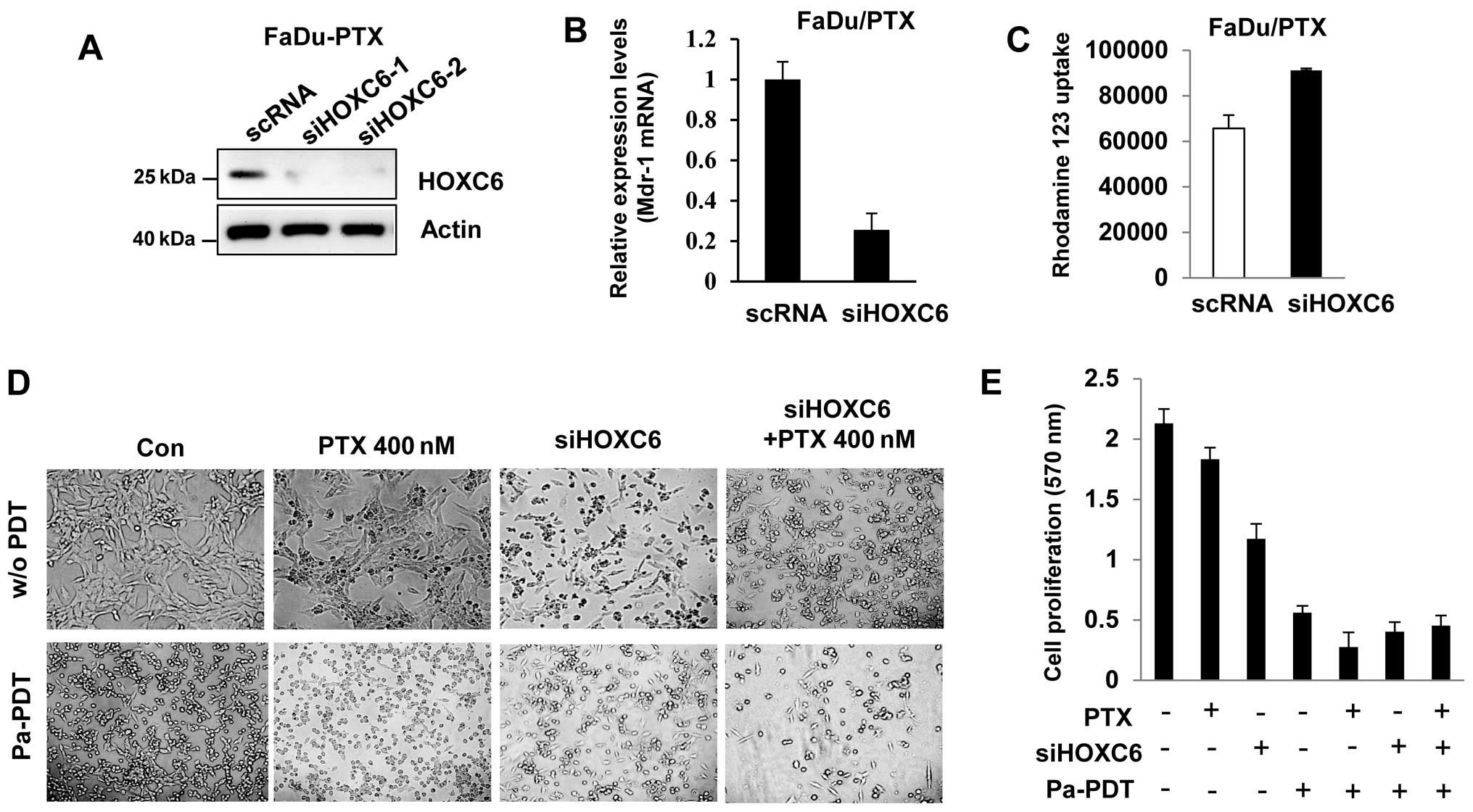
HOXC6 siRNA induces drug sensitivity and
Pa-PDT mediated apoptosis in FaDu/PTX cells
To determine the effect of HOXC6 siRNA, we
determined the drug sensitivity in FaDu-PTX cells. Western blotting
showed that HOXC6 expression was decreased in siHOXC6-transfected
FaDu-PTX cells (Fig. 6A). HOXC6
siRNA inhibited MDR-1 mRNA expression compared with scramble
siRNA-transfected cells (Fig. 6B).
Rho123 accumulation analysis confirmed that Rho123 accumulation
increased in siHOXC6-transfected cells compared with the scRNA
control (Fig. 6C).
Next, we treated FaDu-PTX cells with either a
non-toxic dose of paclitaxel (400 nM) or HOXC6 siRNA followed by
treatment either with or without Pa-PDT. We then tested Pa-PDT
sensitivity by observing changes in cell proliferation. The MTT
assay indicated that the proliferation/viability of FaDu-PTX cells
was slightly decreased by paclitaxel compared with untreated cells.
Either HOXC6 siRNA or Pa-PDT treatment inhibited FaDu-PTX cell
viability compared with the control-treated cells. Notably,
treatment with either paclitaxel or siHOXC6 combined with Pa-PDT
significantly inhibited cell proliferation compared with siHOXC6 or
paclitaxel alone. Furthermore, the FaDu-PTX cells receiving Pa-PDT
had similar results between the paclitaxel and siHOXC6 treatment
groups (Fig. 6D and 6E).
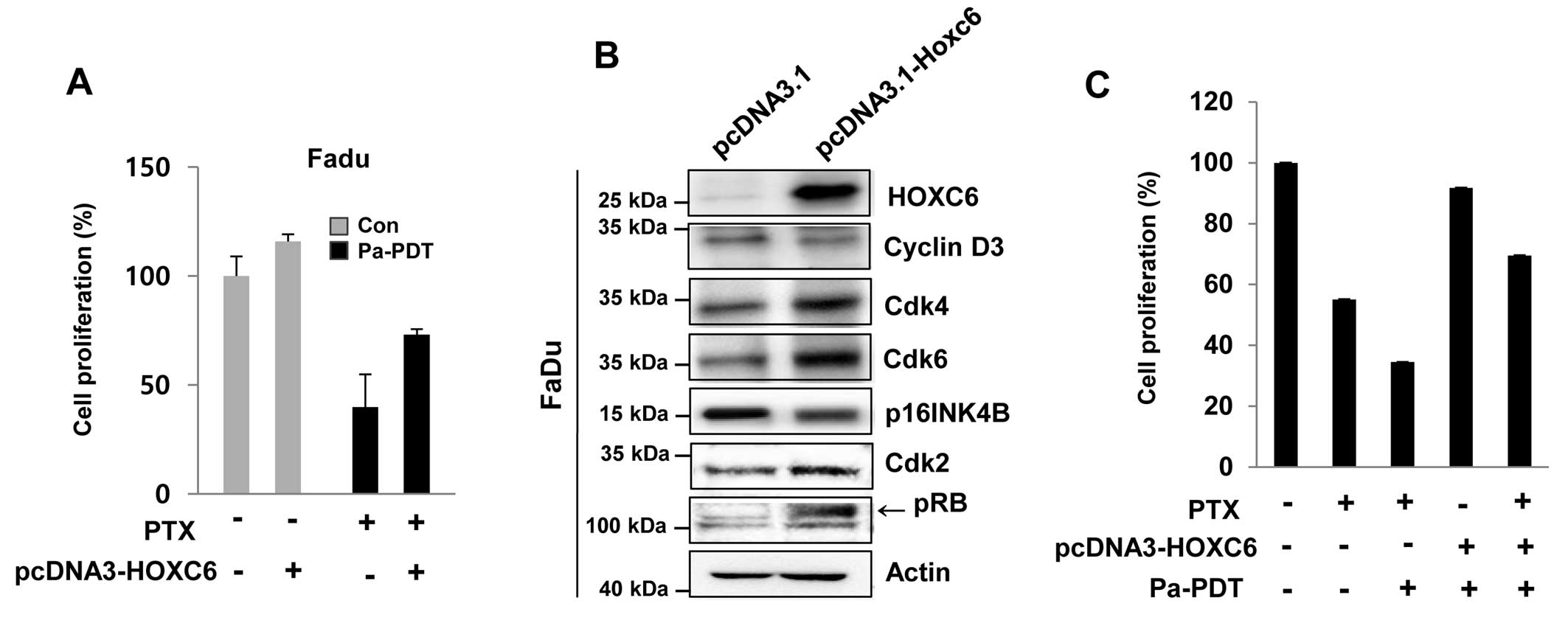
Reversal of Pa-PDT sensitivity in FaDu
cells by HOXC6 overexpression
The HOXC6 was found to be less inducible in FaDu
cells compared with to FaDu-PTX cells. Thus, we examined the effect
of HOXC6 overexpression in FaDu cells, which showed strong
sensitivity to paclitaxel due to a low induction of MDR-1. HOXC6
overexpression conferred paclitaxel resistance to FaDu cells
(Fig. 7A). HOXC6 over-expression
was confirmed by immunoblot assay (Fig. 7B). Notably, a remarkable difference
between mock-transfected cells and HOXC6-overexpressing FaDu cells
was also observed regarding the expression of cell cycle regulatory
proteins. The HOXC6-overexpressing FaDu cells revealed that HOXC6
induced the expression of CDK4, CDK6 and CDK2, whereas HOXC6
overexpression importantly inhibited the cell cycle negative
regulators p16 and phospho-RB (Fig.
7B).
We also investigated the effect of either paclitaxel
or Pa-PDT on the overexpression of HOXC6 in FaDu cells. As
expected, we detected remarkable inhibition of cell proliferation
with paclitaxel and/or Pa-PDT cell treatment (Fig. 7B). However, Pa-PDT- and/or
paclitaxel-induced cell growth inhibition was restrained by the
overexpression of HOXC6, which conferred resistance to paclitaxel
and/or Pa-PDT (Fig. 7C).
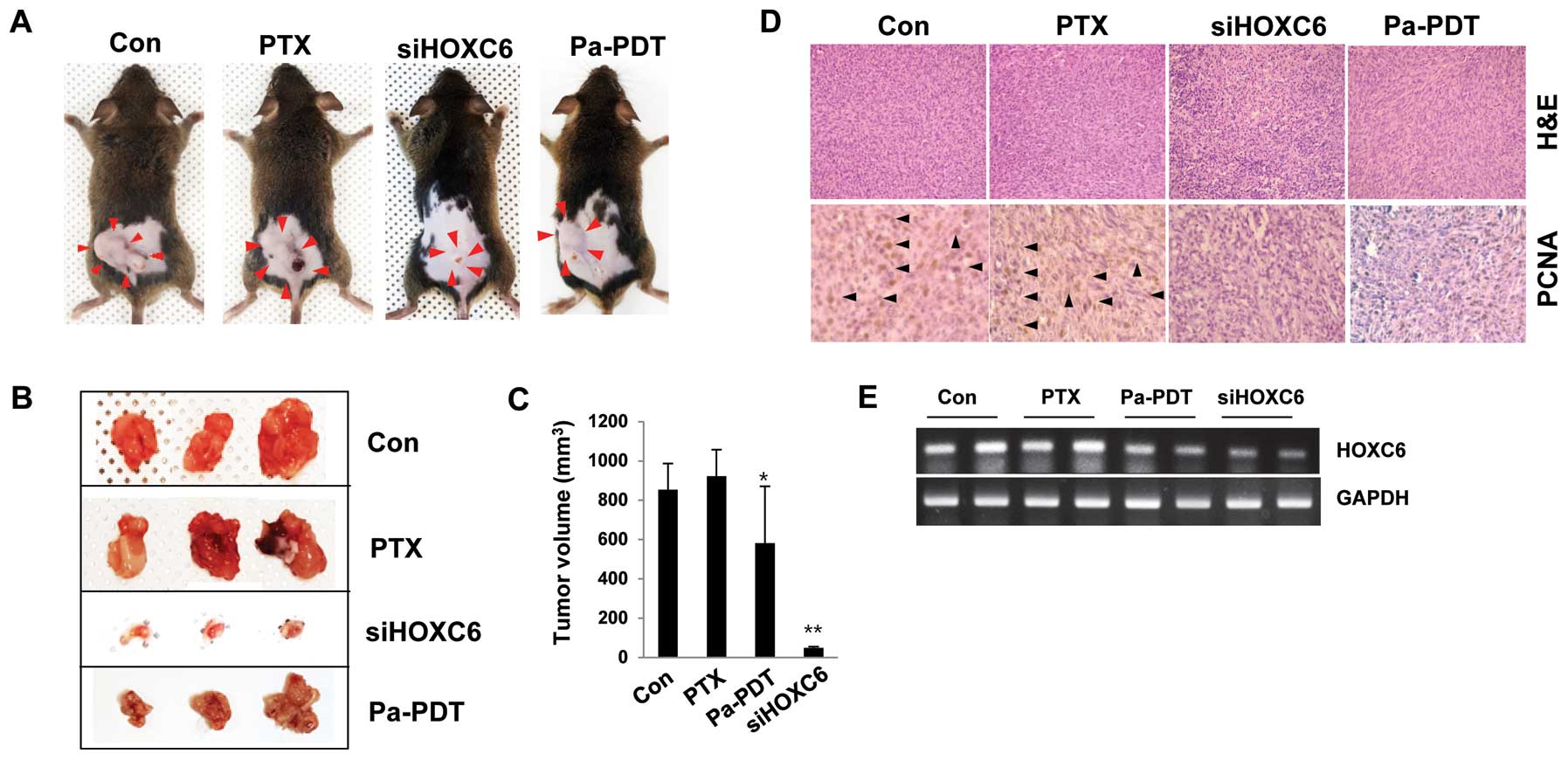
The effect of HOXC6 depletion and Pa-PDT
in a FaDu-PTX xenograft mouse model
In support of the identified HOXC6-dependent effects
on multidrug resistance in vitro, a tumor xenograft model
was used to examine the alterations in the pathological
characteristics in vivo by modifying HOXC6 transcription.
C3H mice were randomized and subcutaneously engrafted with FaDu-PTX
cells treated in vitro with either HOXC6 siRNA or Pa-PDT.
The xenograft mice injected with HOXC6-deficient cells strongly
showed a condensed tumor mass and induced susceptibility to
apoptosis. Pa-PDT also reduced the tumor size and increased cell
death in the xenograft tumor (Fig.
8A–C). No systemic toxicity (including the body weight changes
or other apparent adverse effects) was observed in the animals
throughout the study period (data not shown). Specifically, PCNA
expression, a marker of cell proliferation, was decreased in
implanted tumors derived from cells treated with either HOXC6 siRNA
or Pa-PDT compared with the control or PTX-treated groups (Fig. 8D). We next assessed the levels of
HOXC6 mRNA in tumor tissue. As expected, HOXC6 mRNA was strongly
expressed in the control and paclitaxel-treated groups but weakly
expressed in the siHOXC6- and Pa-PDT-treated groups (Fig. 8E). These data confirmed that the
described genetic and other substantial factors affecting HOXC6
transcription directly affect the tumor progression/proliferation
of multidrug-resistant cancer cells, specifically PTX-resistant
tumors.
Discussion
In this study, the cytotoxicity of Pa-PDT was
measured in the human oral cancer cell lines YD-5, YD10B, YD-15 and
FaDu, as well as the MDR line FaDu-PTX. Pa-PDT significantly
inhibited cell proliferation of human oral cancer cells; however,
there was no cytotoxicity in either Pa- or light-treated cells.
This result is consistent with our previous finding in skin and
lung cancer cells. We first observed that Pa-PDT predominantly
inhibited cell growth and induced apoptosis in FaDu-PTX cells as
indicated by multiple independent approaches that revealed either
the expression of apoptosis-specific proteins or the formation of
pro-apoptotic morphology.
Because ROS is critical for cell death induced by
PDT, we examined ROS production by Pa-PDT in FaDu and FaDu-PTX
cells. Fluorescent scanning analysis showed that Pa-PDT led to
intracellular ROS production in both cell lines. However, ROS
production was highly induced after Pa-PDT treatment in the FaDu
cells compared with the FaDu-PTX cells. From these data, we
speculated that FaDu-PTX cells had lower ROS levels compared to its
parental cell line due to MDR-1 overexpression. However, our
results proved that FaDu and FaDu-PTX cell lines exhibited similar
cell death by Pa-PDT treatment. These findings indicated that the
cell death of FaDu and FaDu-PTX cells due to Pa-PDT-induced
photocytotoxicity was not attributed to the difference in
intracellular ROS levels. One possible explanation for this might
be the altered genetic factors in MDR cells (5–10).
The mechanisms underlying the MDR response are less
clear in oral cancer than in other tumor types (39). We used quantitative real-time PCR
to investigate changes in the expression profile of MDR signaling
pathway-related genes between FaDu and FaDu-PTX cells. Compared
with the parental FaDu cells, 10 genes that were upregulated
>2-fold (HOXA1, HOXA10, HOXB4, HOXC6, MMP-2, -3, -7, -9, Notch-1
and Notch-3) and 3 genes that were more down-regulated (HOXA10,
HOXA7 and HOXB2) were found in the FaDu-PTX cells. Moreover, we
previously demonstrated that HOXC6 plays an important role in the
process of MDR. Although the expression of HOXA1, HOXA10, HOXB4,
HOXC6, MMP-2, -3, -7, -9, Notch-1 and Notch-3 was beyond our
expectations, they may be involved in complicated regulatory
mechanisms with regard to MDR-related genes. Several studies have
demonstrated that activation of Notch signaling contributes to the
MDR regulatory mechanism (35,40).
It has been demonstrated that uPAR, a serine proteinase receptor,
is also correlated with the drug resistance-related gene multidrug
resistance-1 (MDR-1). In addition, matrix metalloproteinase genes
(including MMP-2 and MMP-9) are reported to be involved in MDR-1
expression (41,42).
As multiple factors may contribute to
chemoresistance, the cell survival and apoptotic signaling pathways
determining the susceptibility of cells to chemotherapy appear to
be important in MDR. However, detailed mechanisms on how cancer
cells upregulate drug resistance-related genes and evolve the
ability to resist apoptotic stimuli by anticancer drugs remain
poorly understood. This requires further studies.
The PI3K/Akt/mTOR pathway plays a major role in cell
survival, proliferation and angiogenesis in human cancer. Recently,
the role of the PI3K/Akt/mTOR pathway in chemo-resistance has been
discussed (36,37). It has been reported that inhibition
of the PI3K/Akt/mTOR pathway results in autophagy and induction of
apoptosis as well as the restoration of drug sensitivity in
chemoresistant cancers (36).
In this study, we found that Pa-PDT induces FaDu-PTX
cell apoptosis by inhibiting AKT and mTOR target proteins such as
p70S6K phosphorylation, which was detected by western blotting. In
addition, we found that Pa-PDT decreases p-ERK1/2 levels, which
play an important role in antiproliferative and pro-apoptotic
activities. The ERK1/2 MAPK also controls various cell responses,
such as proliferation, migration, and differentiation depending on
the cell type and stimulus (43).
Thus, Pa-PDT-selective targeting of the Akt/mTOR/ERK signaling
pathway to induce apoptosis may be beneficial in the overcoming
chemoresistance of oral cancer.
p53/p21 is a key tumor suppressor pathway that is
trigged by various cell responses, including DNA damage and has
been implicated in tumor suppression (38). In this study, we examined whether
the p53/p21 pathway is involved in the tumor suppression induced by
Pa-PDT in FaDu and FaDu-PTX cells. We found that Pa-PDT in FaDu
cells resulted in decreased p53 and p21 expression. In the FaDu-PTX
cells, no detectable expression of p53 and p21 was seen. These data
suggest that the cytotoxicity of Pa-PDT is linked to changes in the
p53/p21 pathway in FaDu cells but not in FaDu-PTX cells. The reason
for the difference of the molecular mechanism involved in the
cellular Pa-PDT response between FaDu and FaDu-PTX cells is
unclear. However, the mechanism of action of Pa-PDT might depend on
the subcellular localization and molecular targets of the
photosensitizer, the metabolic potential and the genotype of the
tumor cell type.
The present study correlated chemoresistance in oral
cancer cell lines with the HOX family, HOXC6 in particular. The
expression of HOXC6 was upregulated in MDR cell lines, including
FaDu-PTX, MCF-7/ADR and SNU601/CIS cells (10). Moreover, using gene transfection
and RNA interference techniques, we demonstrated that the in
vitro drug sensitivity to paclitaxel and chemotherapeutic
drug-induced apoptosis in FaDu-PTX cells were increased in cells
transfected with siHOXC6. This strongly indicates that HOXC6 is
involved in the regulation of MDR in MDR cancer cells. In addition,
we demonstrated that the expression of MDR-1 was regulated by
HOXC6. As mentioned above, the HOXC6 signaling pathway regulates a
variety of cellular processes, including cell maintenance, cellular
differentiation, proliferation, and apoptosis, as a versatile
signaling orchestrator; HOXC6 expression has been implicated in the
development of various cancers (including oral cancer) (24–33).
We presumed that HOXC6 could be a candidate molecule for
determining MDR sensitivity to Pa-PDT. In this study, HOXC6 and
MDR-1 expression were increased more in FaDu-PTX cells compared to
FaDu cells. HOXC6 and MDR-1 were downregulated after Pa-PDT
treatment in FaDu-PTX cells. In addition, we demonstrated that
siRNA targeting HOXC6 led to the efficient and specific inhibition
of endogenous MDR-1 mRNA and Rho123 accumulation in FaDu-PTX cells.
These findings suggest a possible inhibition of the HOXC6/MDR-1
signaling pathway after treatment with Pa-PDT.
We also found that downregulation of HOXC6 reversed
chemoresistance and inhibited cell proliferation of FaDu-PTX cells
in vitro. More importantly, HOXC6 overexpression inhibited
Pa-PDT-mediated cell growth inhibition as well as the upregulation
of the expression of cell cycle-related proteins, including Cdk4,
Cdk6 and Cdk2. This provided new evidence that Pa-PDT affected
HOXC6, which could become a promising gene target. In support of
the identified HOXC6-dependent effects on multidrug resistance
in vitro, a tumor xenograft model was used to examine the
probable physiological alteration of drug-resistant tumors by
modifying HOXC6 transcription. Both HOXC6-deficient and Pa-PDT
treated tumors showed a less condensed tumor mass and increased
susceptibility to apoptosis. Additionally, although the HOXC6 siRNA
and Pa-PDT treatment inhibited the growth of FaDu-PTX cells in
vitro and in vivo, the level of cell growth inhibition
was different. The differential cytotoxicity between siHOXC6 and
Pa-PDT might be due to the different inhibition levels of either
HOXC6 or its targets. Western blot analysis showed that siHOXC6 had
a greater effect in reducing the levels of HOXC6 compared to the
Pa-PDT treatment. The inhibition levels of HOXC6 may be a result of
the alternations in the tumor growth or an MDR mechanism that
renders the cells more sensitive to either Pa-PDT or anticancer
drug. The results suggest that HOXC6 could be a new target in
treating MDR oral cancer.
In conclusion, our findings provide the first
evidence that Pa-PDT could decrease tumor growth via inhibition of
the HOXC6/MDR-1-mediated pathway. This inhibition may increase
susceptibility to cell death by enhancing the effects of
intracellular paclitaxel or other anticancer drug levels in MDR
human oral tumor cells, which would overcome the difficulty in
treating this disease in patients.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(no. 2015005588) and funded by the Korean government MSIP (no.
2008-0062283).
References
|
1
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baguley BC: Multidrug resistance in
cancer. Methods Mol Biol. 596:1–14. 2010. View Article : Google Scholar
|
|
3
|
Haimeur A, Conseil G, Deeley RG and Cole
SP: The MRP-related and BCRP/ABCG2 multidrug resistance proteins:
Biology, substrate specificity and regulation. Curr Drug Metab.
5:21–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Redmond KM, Wilson TR, Johnston PG and
Longley DB: Resistance mechanisms to cancer chemotherapy. Front
Biosci. 13:5138–5154. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szakács G, Annereau JP, Lababidi S,
Shankavaram U, Arciello A, Bussey KJ, Reinhold W, Guo Y, Kruh GD,
Reimers M, et al: Predicting drug sensitivity and resistance:
Profiling ABC transporter genes in cancer cells. Cancer Cell.
6:129–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deeley RG and Cole SP: Substrate
recognition and transport by multidrug resistance protein 1
(ABCC1). FEBS Lett. 580:1103–1111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Litman T, Druley TE, Stein WD and Bates
SE: From MDR to MXR: New understanding of multidrug resistance
systems, their properties and clinical significance. Cell Mol Life
Sci. 58:931–959. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baguley BC: Multiple drug resistance
mechanisms in cancer. Mol Biotechnol. 46:308–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pang A, Au WY and Kwong YL: Caveolin-1
gene is coordinately regulated with the multidrug resistance 1 gene
in normal and leukemic bone marrow. Leuk Res. 28:973–977. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KJ, Moon SM, Kim SA, Kang KW, Yoon JH
and Ahn SG: Transcriptional regulation of MDR-1 by HOXC6 in
multidrug-resistant cells. Oncogene. 32:3339–3349. 2013. View Article : Google Scholar
|
|
11
|
Chen J, Keltner L, Christophersen J, Zheng
F, Krouse M, Singhal A and Wang SS: New technology for deep light
distribution in tissue for phototherapy. Cancer J. 8:154–163. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kessel D and Erickson C: Porphyrin
photosensitization of multi-drug resistant cell types. Photochem
Photobiol. 55:397–399. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu HL, Syu WJ, Nishiyama N, Kataoka K and
Lai PS: Dendrimer phthalocyanine-encapsulated polymeric
micelle-mediated photochemical internalization extends the efficacy
of photodynamic therapy and overcomes drug-resistance in vivo. J
Control Release. 155:458–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gulati SC, Lemoli RM, Igarashi T and
Atzpodien J: Newer options for treating drug-resistant (MDR+)
cancer cells using photoradiation therapy. Leuk Lymphoma.
12:427–433. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon HE, Oh SH, Kim SA, Yoon JH and Ahn
SG: Pheophorbide a-mediated photodynamic therapy induces autophagy
and apoptosis via the activation of MAPKs in human skin cancer
cells. Oncol Rep. 31:137–144. 2014.
|
|
17
|
Ahn MY, Yoon HE, Kwon SM, Lee J, Min SK,
Kim YC, Ahn SG and Yoon JH: Synthesized pheophorbide a-mediated
photo-dynamic therapy induced apoptosis and autophagy in human oral
squamous carcinoma cells. J Oral Pathol Med. 42:17–25. 2013.
View Article : Google Scholar
|
|
18
|
Ahn MY, Kwon SM, Kim YC, Ahn SG and Yoon
JH: Pheophorbide a-mediated photodynamic therapy induces apoptotic
cell death in murine oral squamous cell carcinoma in vitro and in
vivo. Oncol Rep. 27:1772–1778. 2012.PubMed/NCBI
|
|
19
|
Tang PM, Chan JY, Au SW, Kong SK, Tsui SK,
Waye MM, Mak TC, Fong WP and Fung KP: Pheophorbide a, an active
compound isolated from Scutellaria barbata, possesses photodynamic
activities by inducing apoptosis in human hepatocellular carcinoma.
Cancer Biol Ther. 5:1111–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang PM, Zhang DM, Xuan NH, Tsui SK, Waye
MM, Kong SK, Fong WP and Fung KP: Photodynamic therapy inhibits
P-glycoprotein mediated multidrug resistance via JNK activation in
human hepatocellular carcinoma using the photosensitizer
pheophorbide a. Mol Cancer. 8:56–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hajri A, Wack S, Meyer C, Smith MK,
Leberquier C, Kedinger M and Aprahamian M: In vitro and in vivo
efficacy of photofrin and pheophorbide a, a bacteriochlorin, in
photodynamic therapy of colonic cancer cells. Photochem Photobiol.
75:140–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin ZH, Miyoshi N, Ishiguro K, Umemura S,
Kawabata K, Yumita N, Sakata I, Takaoka K, Udagawa T, Nakajima S,
et al: Combination effect of photodynamic and sonodynamic therapy
on experimental skin squamous cell carcinoma in C3H/HeN mice. J
Dermatol. 27:294–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bui-Xuan NH, Tang PM, Wong CK and Fung KP:
Photo-activated pheophorbide-a, an active component of Scutellaria
barbata, enhances apoptosis via the suppression of ERK-mediated
autophagy in the estrogen receptor-negative human breast
adeno-carcinoma cells MDA-MB-231. J Ethnopharmacol. 131:95–103.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bodey B, Bodey B Jr, Siegel SE and Kaiser
HE: Immunocytochemical detection of the homeobox B3, B4, and C6
gene products in breast carcinomas. Anticancer Res. 20(5A):
3281–3286. 2000.PubMed/NCBI
|
|
25
|
Castronovo V, Kusaka M, Chariot A, Gielen
J and Sobel M: Homeobox genes: Potential candidates for the
transcriptional control of the transformed and invasive phenotype.
Biochem Pharmacol. 47:137–143. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujiki K, Duerr EM, Kikuchi H, Ng A,
Xavier RJ, Mizukami Y, Imamura T, Kulke MH and Chung DC: Hoxc6 is
overexpressed in gastrointestinal carcinoids and interacts with
JunD to regulate tumor growth. Gastroenterology. 135:907–916.
916.e1–916.e2. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bodey B, Bodey B Jr, Siegel SE, Luck JV
and Kaiser HE: Homeobox B3, B4, and C6 gene product expression in
osteo-sarcomas as detected by immunocytochemistry. Anticancer Res.
20:2717–2721. 2000.PubMed/NCBI
|
|
28
|
Miller GJ, Miller HL, van Bokhoven A,
Lambert JR, Werahera PN, Schirripa O, Lucia MS and Nordeen SK:
Aberrant HOXC expression accompanies the malignant phenotype in
human prostate. Cancer Res. 63:5879–5888. 2003.PubMed/NCBI
|
|
29
|
McCabe CD, Spyropoulos DD, Martin D and
Moreno CS: Genome-wide analysis of the homeobox C6 transcriptional
network in prostate cancer. Cancer Res. 68:1988–1996. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grishina IB, Kim SY, Ferrara C,
Makarenkova HP and Walden PD: BMP7 inhibits branching morphogenesis
in the prostate gland and interferes with Notch signaling. Dev
Biol. 288:334–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ricort JM and Binoux M: Insulin-like
growth factor-binding protein-3 activates a phosphotyrosine
phosphatase. Effects on the insulin-like growth factor signaling
pathway. J Biol Chem. 277:19448–19454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van der Geer P, Hunter T and Lindberg RA:
Receptor protein-tyrosine kinases and their signal transduction
pathways. Annu Rev Cell Biol. 10:251–337. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin Y, Liu G, Zhang Y, Hu YP, Yu K, Lin C,
McKeehan K, Xuan JW, Ornitz DM, Shen MM, et al: Fibroblast growth
factor receptor 2 tyrosine kinase is required for prostatic
morphogenesis and the acquisition of strict androgen dependency for
adult tissue homeostasis. Development. 134:723–734. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moon SM, Kim SA, Yoon JH and Ahn SG: HOXC6
is deregulated in human head and neck squamous cell carcinoma and
modulates Bcl-2 expression. J Biol Chem. 287:35678–35688. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho S, Lu M, He X, Ee PL, Bhat U,
Schneider E, Miele L and Beck WT: Notch1 regulates the expression
of the multidrug resistance gene ABCC1/MRP1 in cultured cancer
cells. Proc Natl Acad Sci USA. 108:20778–20783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiu LY, Hu ME, Yang TY, Hsin IL, Ko JL,
Tsai KJ and Sheu GT: Immunomodulatory protein from ganoderma
microsporum induces pro-death autophagy through Akt-mTOR-p70S6K
pathway inhibition in multidrug resistant lung cancer cells. PLoS
One. 10:e01257742015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galoian K, Temple HT and Galoyan A: mTORC1
inhibition and ECM-cell adhesion-independent drug resistance via
PI3K-AKT and PI3K-RAS-MAPK feedback loops. Tumour Biol. 33:885–890.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ye S, Shen J, Choy E, Yang C, Mankin H,
Hornicek F and Duan Z: p53 overexpression increases
chemosensitivity in multidrug-resistant osteosarcoma cell lines.
Cancer Chemother Pharmacol. 77:349–356. 2016. View Article : Google Scholar
|
|
39
|
Yajima T, Ochiai H, Uchiyama T, Takano N,
Shibahara T and Azuma T: Resistance to cytotoxic
chemotherapy-induced apoptosis in side population cells of human
oral squamous cell carcinoma cell line Ho-1-N-1. Int J Oncol.
35:273–280. 2009.PubMed/NCBI
|
|
40
|
Yan S, Ma D, Ji M, Guo D, Dai J, Zhao P
and Ji C: Expression profile of Notch-related genes in multidrug
resistant K562/A02 cells compared with parental K562 cells. Int J
Lab Hematol. 32:150–158. 2010. View Article : Google Scholar
|
|
41
|
Zhou H, Tang Y, Liang X, Yang X, Yang J,
Zhu G, Zheng M and Zhang C: RNAi targeting urokinase-type
plasminogen activator receptor inhibits metastasis and progression
of oral squamous cell carcinoma in vivo. Int J Cancer. 125:453–462.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang JM, Xu Z, Wu H, Zhu H, Wu X and Hait
WN: Overexpression of extracellular matrix metalloproteinase
inducer in multidrug resistant cancer cells. Mol Cancer Res.
1:420–427. 2003.PubMed/NCBI
|
|
43
|
Liu QH, Shi ML, Sun C, Bai J and Zheng JN:
Role of the ERK1/2 pathway in tumor chemoresistance and tumor
therapy. Bioorg Med Chem Lett. 25:192–197. 2015. View Article : Google Scholar
|