Introduction
Various pathophysiological stresses can disrupt
essential cellular signaling pathways and increase the
concentration of unfolded or misfolded proteins. A cellular defense
mechanism induces the expression of heat shock proteins (Hsps),
which serve as molecular chaperones to refold the damaged proteins
and protect native proteins (1,2). The
induction of stress-responsive genes is mainly regulated by heat
shock transcription factor 1 (HSF1). Upon exposure to stressful
stimuli, HSF1 translocates to the nucleus, where it binds to heat
shock elements (HSEs) to induce the expression of Hsps (3,4).
Bcl2-associated athanogene (BAG) 3 (also known as
CAIR-1 or Bis) is a member of the BAG co-chaperone family. BAG
family members share a conserved BAG domain in their C-terminal
region, through which this family members interact with the ATPase
domain of Hsp70 (5,6). In addition to the BAG domain, BAG3
has a WW domain, two IPV motifs and a proline-rich (PXXP) motif.
Through these motifs, BAG3 interacts with other binding partners
such as Hsp22 and phospholipase C and participates in a wide
variety of cellular processes including apoptosis, proliferation,
migration and autophagy (6–11).
Among the six BAG family members, BAG3 is the only
member induced by various cellular stresses such as oxidative
stress, heat shock, heavy metals and HIV-1 infection (12–14).
It has been reported that activated HSF1 induced the expression of
BAG3 upon heat stress (15).
Recently, we reported that BAG3 is a nuclear-cytoplasmic shuttling
protein that interacts with HSF1 under both normal and
heat-stressed conditions through its BAG domain. We also
demonstrated that BAG3 affects HSF1 nuclear translocation and
subsequent expression of target genes (16). HSF1 plays a central role in the
cellular defense mechanism and Hsp70, a major stress-inducible
protein, is elevated in many human cancers. Moreover, recent
studies have shown that BAG3 levels are also elevated in various
human cancers such as glioblastoma, leukemia, prostate and
pancreatic cancer, suggesting the possibility that BAG3 may
actively be involved in the initiation and progression of cancer
(17–20).
Cinnamon, which is obtained from the stem bark of
Cinnamomum cassia, has been widely used as flavoring and as
a traditional medicine. Cinnamaldehyde is the major compound
(45–65% of the essential oil from bark) of cinnamon (21). As a major active compound,
cinnamaldehyde has been well investigated and its diverse
biological activities against bacteria, fungi, inflammation and
tumor have been reported (22–25).
2′-Hydroxycinnamaldehyde (HCA), one of the natural derivatives of
cinnamaldehyde, was reported to exert antitumor activity by
inhibiting cell proliferation and inducing cell death in
TNF-α-treated colon cancer cells through the inactivation of NF-κB
and AP-1 (26–28). Recently, we also demonstrated that
HCA effectively induces apoptosis in human head and neck cancer
cells in a p53-independent manner (29,30).
These previous reports suggest HCA as an effective therapeutic
agent for treating cancer.
In the present study, we investigated the role of
co-chaperone BAG3 on HCA-induced apoptosis in SW480 colon cancer
cells and demonstrated the novel role of BAG3 on
chemotherapy-induced cancer cell death.
Materials and methods
Cell culture and reagents
SW480 and SW620 human colon carcinoma cell lines
were cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone
Laboratories, Inc., Logan, UT, USA) supplemented with 10% fetal
bovine serum (FBS), 100 units/ml penicillin, and 100 µg/ml
streptomycin (Gibco-BRL, Grand Island, NY, USA). Cells were
maintained at 37°C in a humidified atmosphere with 5%
CO2. HCA was purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA) and dissolved in 0.1% dimethyl sulfoxide
(DMSO).
Cell viability assay
The effect of HCA on cell viability was assessed
using the trypan blue exclusion assay. SW480 and SW620 cells were
seeded on a 12-well plate at a density of 3×105
cells/ml. The cells were cultured overnight and then treated with
various concentrations of HCA. After 24 h, the cells were
trypsinized and stained with trypan blue dye (Sigma-Aldrich, St.
Louis, MO, USA). The number of viable cells was counted using a
hemocytometer.
Western blot analysis
SW480 and SW620 cells were treated with various
concentrations of HCA for the indicated time periods. The cells
were then washed with ice-cold phosphate-buffered saline (PBS)
twice and lysed in RIPA buffer (PBS supplemented with 1% NP-40,
0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 1
µg/ml aprotinin and 1 mM sodium orthovanadate).
Alternatively, the cytoplasmic and nuclear fractions were obtained
according to the previously described method (16). The protein samples were resolved by
SDS-polyacrylamide gel electrophoresis and then transferred onto
nitrocellulose membranes. The membranes were blocked and then
incubated with antibodies. Antibodies against PARP (sc-7150) and
HSF1 (sc-9144) were purchased from Santa Cruz Biotechnology. The
caspase-7 (#9492), caspase-9 (#9502), p38 (#9212), ERK (#4695), JNK
(#9258), phospho-p38 (#9211), phospho-ERK (#4370) and phospho-JNK
(#4668) antibodies were from Cell Signaling Technology (Danvers,
MA, USA). Antibodies against BAG3 (ab47124) and TBP (ab818) were
purchased from Abcam (Cambridge, UK), and antibodies against actin
(A1978) were purchased from Sigma-Aldrich. Antibodies for BAG3 and
actin were diluted 1:10,000 and all other antibodies were diluted
1:5,000. The immunoreactive bands were detected using the
SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher
Scientific, Rockford, IL, USA). All the experiments were performed
at least 3 times.
RNA isolation and RT-PCR
SW480 cells were treated with 50 µM HCA for
the indicated time periods. Total RNA was isolated using
TRIzol® reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. Semi-quantitative
RT-PCR was conducted using the One Step RT-PCR PreMix kit (iNtRON
Biotechnology, Seongnam, Korea) according to the manufacturer's
instructions. The specific primers used for RT-PCR were as follows:
BAG3, forward primer (5′-GAAAGTGGAAGCCATCCTGGA-3′) and reverse
primer (5′-CCCAAGTTACTGCATACCAAG CG-3′); GADPH, forward primer
(5′-CCAAGGTCATCCATGACAACTTTG-3′) and reverse primer
(5′-GTCATACCAGGAAATGAGCTTGACA-3′). RT-PCR was performed under the
following conditions: 1 cycle of 30 min at 45°C; 1 cycle of 5 min
at 94°C; and 27 cycles of 30 sec at 94°C, 30 sec at 56°C, and 40
sec at 72°C; with a final extension at 72°C for 5 min. The PCR
products were then electrophoresed on a 1.7% agarose gel and
visualized by ethidium bromide staining.
siRNA experiment
SW480 cells were seeded in a 12-well plate at a
density of 1.5×105 cells/ml and then transfected with
1.5 µg/ml of either siGENOME BAG3 siRNA or HSF1 siRNA
(Dharmacon, Lafayette, CO, USA) using the DharmaFECT transfection
reagent (Dharmacon) according to the manufacturer's instructions. A
siGENOME non-targeting siRNA pool (Dharmacon) was used as a
control. After 24 h, the cells were treated with 50 µM HCA
and incubated for an additional 24 h. The cells were harvested, and
the expression levels of BAG3 and HSF1 were analyzed by western
blot analysis as described above.
Luciferase reporter gene assay
To assess the effects of HCA on HSF1 activity, SW480
cells were co-transfected with pGL3-Hsp70-Luc and pCH110 using
FuGENE® HD (Promega, Madison, WI, USA) according to the
manufacturer's instructions. After 8 h, the cells were treated with
various concentrations of HCA and incubated for an additional 24 h.
The cells were lysed with the reporter lysis buffer (Promega), and
luciferase activity was measured using a Luciferase activity assay
kit (Promega) according to the manufacturer's instructions.
β-galactosidase activity was determined to normalize the luciferase
activities.
Annexin V staining
SW480 cells were seeded on eight-chamber slides at a
density of 1.5×105 cells/ml. Cells were cultured
overnight and transfected with 1.5 µg/ml of either siGENOME
BAG3 siRNA or a non-targeting siRNA pool as described above. Twelve
hours after transfection, the cells were treated with 50 µM
HCA. After 24 h, the cells were washed with PBS and stained using
the Annexin V-Fluos staining kit (Roche Molecular Biochemicals,
Indianapolis, IN, USA) according to the manufacturer's
instructions. Cell nuclei were stained with DAPI (Molecular Probes,
Eugene, OR, USA). The apoptotic cells were analyzed by conventional
fluorescence microscopy (Axio observer D1; Carl Zeiss, Oberkochen,
Germany).
Statistical analysis
The data are expressed as the means ± SD. ANOVA and
the Student's t-test were applied to determine the statistical
significance. P<0.01 were considered to be significant.
Results
HCA induces the apoptotic cell death on
SW480 and SW620 colon cancer cells
We first evaluated the effect of HCA on the
viability of SW480 and SW620 colon cancer cells. The cells were
treated with various concentrations of HCA for 24 h and the trypan
blue exclusion assay was performed. As shown in Fig. 1A, cell viability was decreased
depending on the HCA concentration in both SW480 and SW620 cells
with IC50 values 18.7 and 16.5 µM, respectively.
To address whether HCA induces apoptotic cell death in both cell
lines, the cells were treated with HCA and examined for the levels
of cleaved PARP. As shown in Fig. 1B
and C, the levels of cleaved PARP were largely induced by HCA
in a dose- and time-dependent manner. Consistent with the changes
in the cleaved PARP levels, caspase-7 and caspase-9 were also
increased depending on the HCA concentration (Fig. 1D). By staining SW480 cells with
Annexin V-FITC, we confirm the role of HCA in caspase-mediated
apoptosis (Fig. 1E).
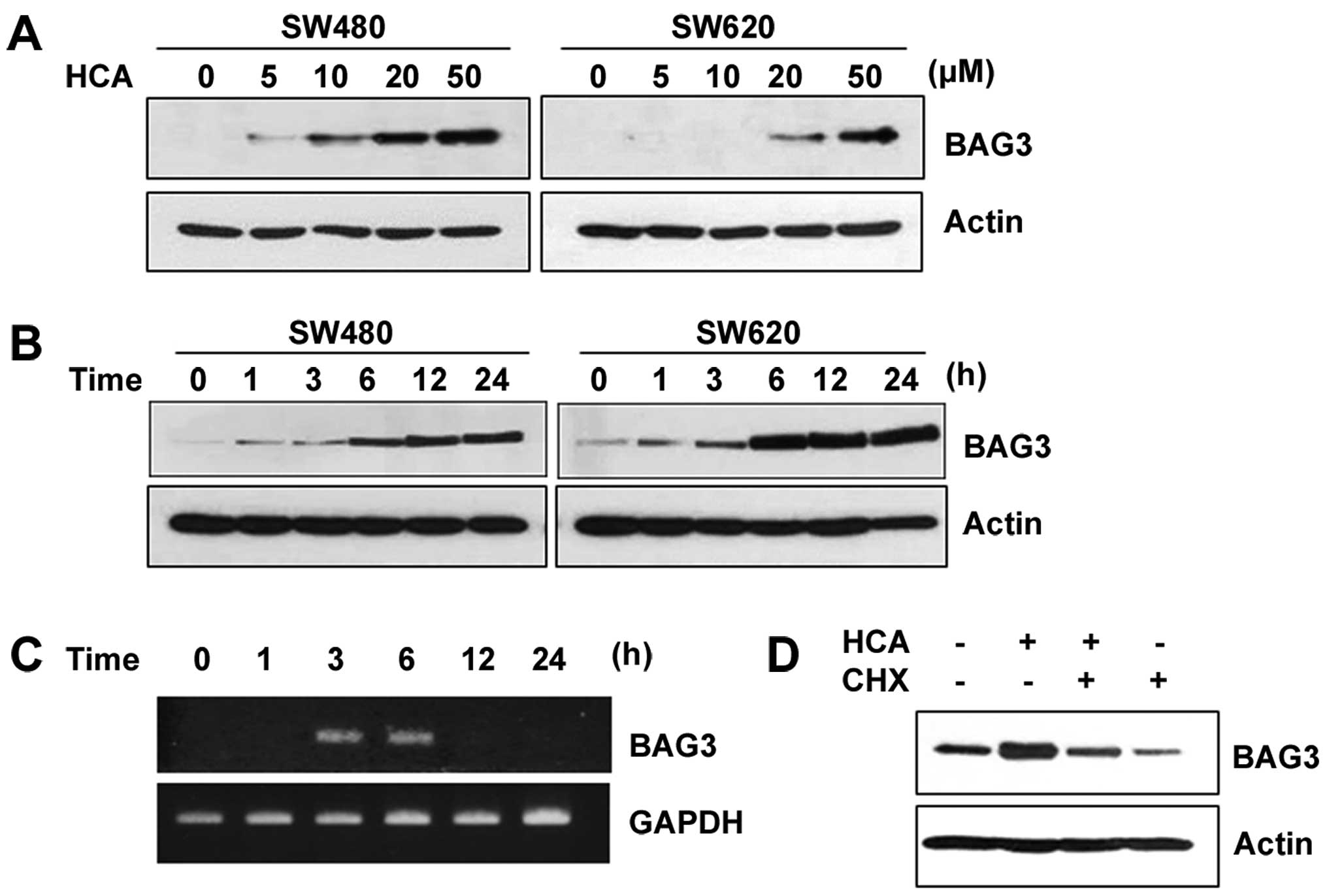
HCA induces the expression of BAG3
Previous studies have shown that the BAG3 expression
can be induced by cellular insults such as heat shock, heavy metals
and oxidative stress (12,13). Therefore, we investigated whether
HCA, a chemotherapeutic agent, can induce BAG3 expression. Notably,
HCA largely increased the protein level of BAG3 in a dose- and
time-dependent manner in both SW480 and SW620 cells (Fig. 2A and B). To investigate whether the
increased protein levels of BAG3 are caused by increased BAG3 gene
transcription, SW480 cells were treated with 50 µM HCA for
various times, and semi-quantitative RT-PCR was performed. As shown
in Fig. 2C, HCA largely increased
the level of BAG3 mRNA between 3 and 6 h of treatment. By showing
that cycloheximide, an inhibitor of protein synthesis, effectively
inhibited the HCA-induced levels of BAG3 protein expression, we
confirmed that HCA promotes BAG3 gene expression (Fig. 2D).
HCA induces HSF1 transcriptional activity
and subsequent BAG3 expression
Our new finding that HCA induces BAG3 expression
leads us to investigate the signaling pathway involved in BAG3
expression. Previously, we and others have shown that the
stress-responsive induction of BAG3 occurs mainly through the
activation of HSF1 (15,16). To address the signaling mechanism
responsible for HCA-induced BAG3 expression, we performed a
reporter gene assay to evaluate the effect of HCA on the
transcriptional activity of HSF1. SW480 cells were transfected with
a pGL3-Hsp70-Luc luciferase reporter vector and then treated with
various concentrations of HCA. Interestingly, HCA markedly promoted
HSF1 transcriptional activity in a dose-dependent manner (Fig. 3A). As shown in Fig. 3B, HCA treatment resulted in rapid
nuclear translocation of HSF1, suggesting that HCA effectively
induced the transcriptional activity of HSF1. To further confirm
the HSF1-mediated expression of BAG3 by HCA, the cells were
transfected with HSF1-specific siRNA. As shown in Fig. 3C, the level of HSF1 was reduced by
HSF1 siRNA. Consistent with the result shown in Fig. 2, HCA increased the level of BAG3.
However, knockdown of HSF1 effectively inhibited the induction of
BAG3 by HCA, confirming that HCA activates the transcription factor
HSF1 and subsequent BAG3 expression.
Role of BAG3 on HCA-induced cell
death
To investigate the role of BAG3 on the HCA-induced
apoptotic cell death, SW480 cells were transfected with
BAG3-specific siRNA. As shown in Fig.
4A, HCA-induced BAG3 levels were dose-dependently decreased by
the BAG3 siRNA. Interestingly, the increased level of cleaved PARP
by HCA was also decreased in BAG3 siRNA-transfected cells,
suggesting that BAG3 is actively involved in the HCA-induced
apoptosis (Fig. 4A). To further
confirm this role of BAG3, cells were transfected with BAG3 siRNA
and stained with Annexin V-FITC. As shown in Fig. 4B, HCA largely increased the number
of apoptotic cells. However, BAG3 knockdown effectively decreased
apoptotic cells, suggesting that BAG3 is actively involved in
HCA-induced apoptosis.
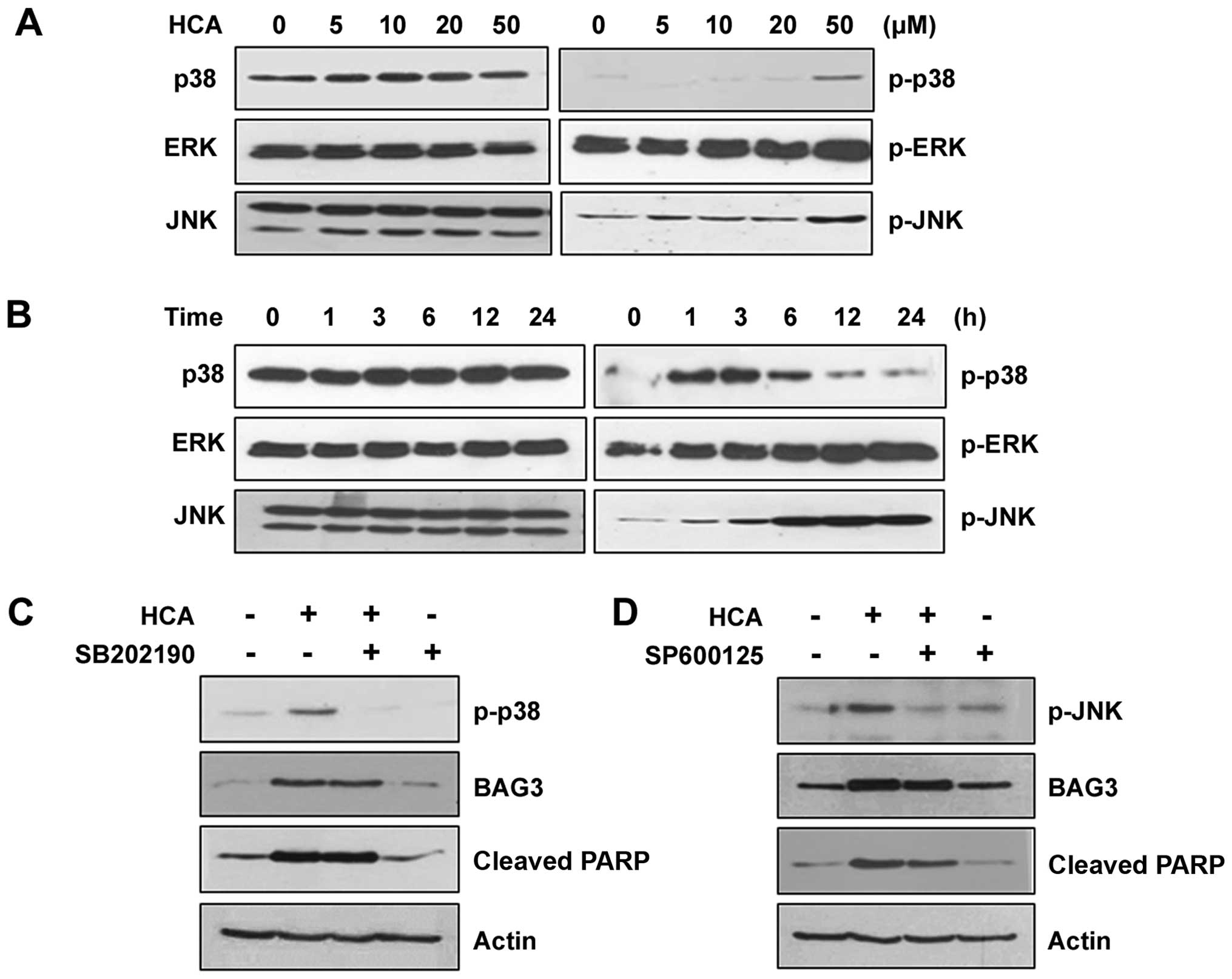
MAPK signaling pathways are not relevant
to the induction of BAG3 expression
We next examined the MAPK signaling pathways in the
HCA-treated SW480 cells to verify the involvement of MAPKs in
HCA-induced BAG3 expression and/or apoptosis. HCA treatment had no
detectable effect on the level of phosphorylated ERK. However, the
levels of phosphorylated p38 and JNK were increased depending on
the HCA concentration (Fig. 5A).
Similarly, phosphorylated p38 and JNK were increased in a
time-dependent manner, suggesting that p38 and/or JNK signaling
pathways may be involved in HCA-induced BAG3 expression and/or
apoptosis (Fig. 5B). To confirm
this possibility, cells were treated with either SB202190 or
SP600125, which are specific inhibitors against p38 and JNK,
respectively. As shown in Fig. 5C,
SB202190 effectively inhibited phosphorylated p38; however, it did
not affect HCA-induced BAG3 expression, suggesting that the p38
signaling pathway is not relevant to the HCA-induced BAG3
expression. Consistent with the level of BAG3, HCA-induced
increases in cleaved PARP was not affected by SB202190, confirming
that BAG3 is a major player in HCA-induced apoptosis. Similarly,
although SP600125 effectively inhibited JNK phosphorylation, BAG3
induction was unaffected (Fig.
5D). The levels of HCA-induced cleaved PARP was also largely
unaffected by SP600125, which supported the role of BAG3 on
HCA-induced apoptosis.
Discussion
Heat shock response is one of the most evolutionary
conserved cell protective mechanisms. Environmental insults induce
various responses to help cells adapt to stressful conditions. HSF1
is placed in a center of the control of cellular responses to
stress. In stressful conditions, HSF1 induces expression of Hsps,
which are molecular chaperones that prevent protein aggregation and
promote the refolding of misfolded proteins. If the cellular stress
is too severe and misfolding exceeds a certain threshold, a signal
that leads to apoptosis is activated thereby providing a balance
between survival and death (1–4,31).
Due to high levels of proteotoxic stress, the stress
responsive pathway is important for cancer cell survival and
proliferation. In this regard, it is not surprising that elevated
levels of Hsps are commonly observed in a wide range of human
tumors and that heat shock response is considered as a potential
target for anticancer therapies (31,32).
However, the multifaceted outcomes of the HSF1-mediated stress
response hinders the understanding of which stress-related
signaling pathways are activated under certain circumstances, with
regard to whether HSF1 plays a supportive or inhibitory role in
cancer progression.
We demonstrated that HCA strongly induces apoptotic
cell death in both SW480 and SW620 colon cancer cells.
Interestingly, HCA largely increased both protein and mRNA levels
of BAG3 in a dose- and time-dependent manner. This induction of
BAG3 expression led us to investigate the involvement of the
HSF1-mediated signaling pathway. Our data showed that HCA increased
target gene promoter activity and nuclear translocation of HSF1.
Furthermore, by showing that knockdown of HSF1 inhibited
HCA-induced BAG3 expression, we verified that HCA strongly induced
HSF1-mediated BAG3 expression. Considering the role of HSF1 in
cellular protection and the elevated level of Hsp70 in many human
cancers, our results suggest that BAG3 may be actively involved in
cancer progression.
Importantly, BAG3 knockdown clearly verified that
HCA promoted apoptosis via BAG3 expression. BAG3 is a
stress-inducible co-chaperone protein, and its expression level is
elevated in several human cancers (12,13,17–20).
Previously, Liu and colleagues (33) have shown that apoptosis induced by
bortezomib, a proteasome inhibitor, is greatly potentiated by BAG3
silencing in leukemic cells. Mani et al (34) also showed that BAG3 knockdown
sensitized bladder cancer cells to apoptosis induced by ABT-737, a
BH3 mimetic. These reports characterized BAG3 as an anti-apoptotic
protein. However, contrary to the above, we showed that HCA induced
apoptosis by increasing BAG3 expression.
Normally, HSF1-mediated cellular defense mechanisms
protect the cells under stressful conditions. However, if the
cellular stress is too severe to overcome, the cell undergoes
apoptotic cell death. It is difficult to conclude whether the
cellular defense mechanism plays a supportive or inhibitory role in
cancer progression because this mechanism appears to have cell
specificity. Even within the same cell type, cells can respond
differently depending on its placed circumstances. BAG3 has many
functional domains through which BAG3 interacts with other proteins
(6–11). our result showed that BAG3
critically participated in HCA-induced apoptosis and suggests the
possible role of BAG3 as a key player in orchestrating the role of
interacting protein(s) under given cell conditions.
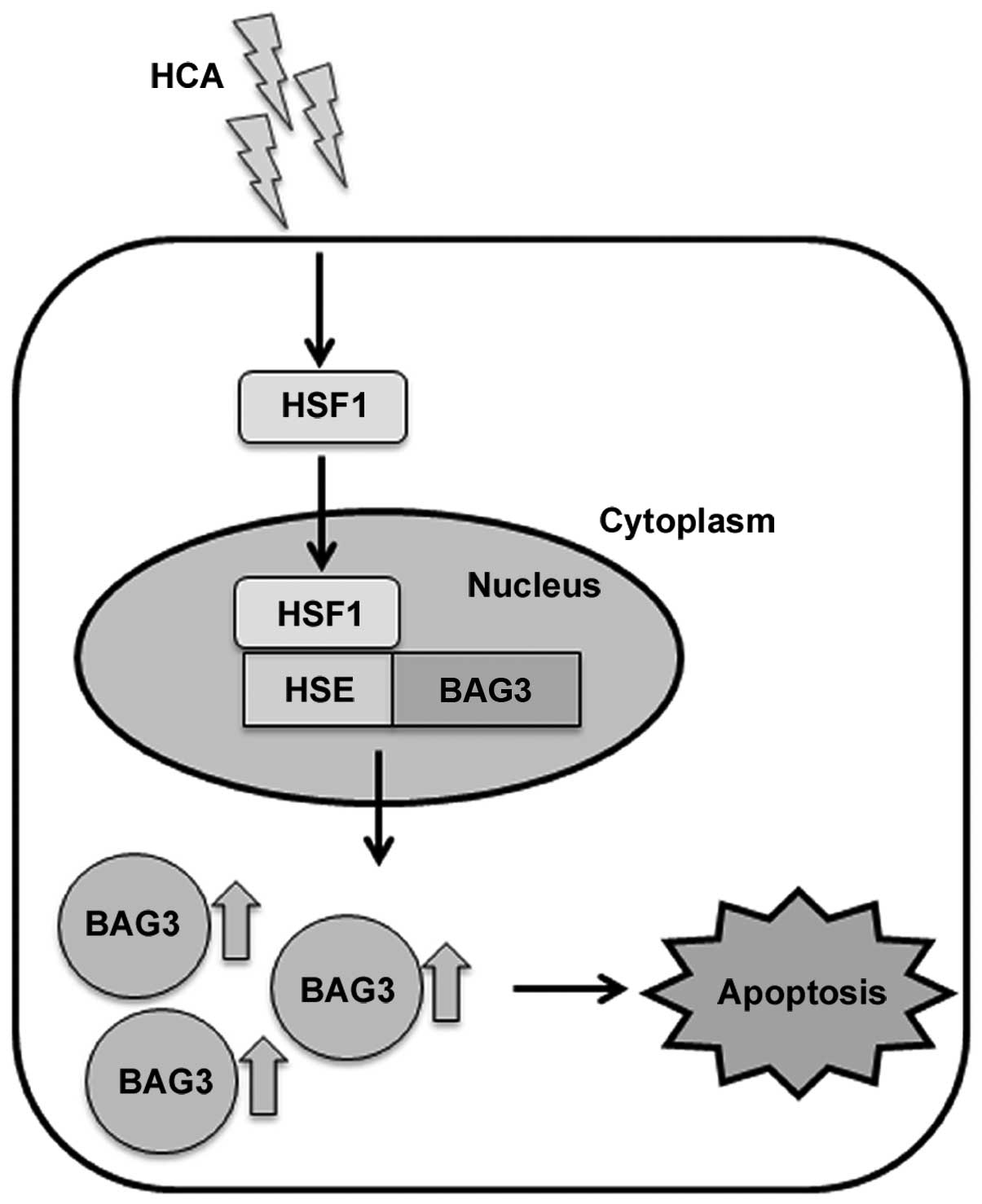
In the present study, we verified that HCA induces
apoptotic cell death in SW480 colon cancer cells. To the best of
our knowledge, this is the first report describing that HCA induces
apoptosis through the activation of HSF1 and subsequent BAG3
expression (Fig. 6). Further
studies on BAG3 and its interacting proteins under stress
conditions will reveal the role of BAG3 as a co-chaperone.
Considering the importance of the stress defense mechanism on
cancer progression, our results suggest that BAG3 is a potential
target for development in cancer therapy.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(2010-0023366).
References
|
1
|
Parsell DA and Lindquist S: The function
of heat-shock proteins in stress tolerance: Degradation and
reactivation of damaged proteins. Annu Rev Genet. 27:437–496. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaufman RJ: Molecular chaperones and the
heat shock response. Sponsored by Cold Spring Harbor Laboratory,
6–10 May 1998. Biochim Biophys Acta. 1423:R13–R27. 1999.PubMed/NCBI
|
|
3
|
Morimoto RI: Regulation of the heat shock
transcriptional response: Cross talk between a family of heat shock
factors, molecular chaperones, and negative regulators. Genes Dev.
12:3788–3796. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pirkkala L, Nykänen P and Sistonen L:
Roles of the heat shock transcription factors in regulation of the
heat shock response and beyond. FASEB J. 15:1118–1131. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kabbage M and Dickman MB: The BAG
proteins: A ubiquitous family of chaperone regulators. Cell Mol
Life Sci. 65:1390–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosati A, Graziano V, De Laurenzi V,
Pascale M and Turco MC: BAG3: A multifaceted protein that regulates
major cell pathways. Cell Death Dis. 2:e1412011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doong H, Price J, Kim YS, Gasbarre C,
Probst J, Liotta LA, Blanchette J, Rizzo K and Kohn E: CAIR-1/BAG-3
forms an EGF-regulated ternary complex with phospholipase C-gamma
and Hsp70/Hsc70. Oncogene. 19:4385–4395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCollum AK, Casagrande G and Kohn EC:
Caught in the middle: The role of Bag3 in disease. Biochem J.
425:e1–e3. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi H, Xu H, Li Z, Zhen Y, Wang B, Huo S,
Xiao R and Xu Z: BAG3 regulates cell proliferation, migration, and
invasion in human colorectal cancer. Tumour Biol. 37:5591–5597.
2016. View Article : Google Scholar
|
|
10
|
Suzuki M, Iwasaki M, Sugio A, Hishiya A,
Tanaka R, Endo T, Takayama S and Saito T: BAG3 (BCL2-associated
athanogene 3) interacts with MMP-2 to positively regulate invasion
by ovarian carcinoma cells. Cancer Lett. 303:65–71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Behl C: BAG3 and friends: Co-chaperones in
selective autophagy during aging and disease. Autophagy. 7:795–798.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pagliuca MG, Lerose R, Cigliano S and
Leone A: Regulation by heavy metals and temperature of the human
BAG-3 gene, a modulator of Hsp70 activity. FEBS Lett. 541:11–15.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosati A, Di Salle E, Luberto L, Quinto I,
Scala G, Turco MC and Pascale M: Identification of a Btk-BAG3
complex induced by oxidative stress. Leukemia. 23:823–824. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosati A, Leone A, Del Valle L, Amini S,
Khalili K and Turco MC: Evidence for BAG3 modulation of HIV-1 gene
transcription. J Cell Physiol. 210:676–683. 2007. View Article : Google Scholar
|
|
15
|
Franceschelli S, Rosati A, Lerose R, De
Nicola S, Turco MC and Pascale M: Bag3 gene expression is regulated
by heat shock factor 1. J Cell Physiol. 215:575–577. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin YH, Ahn SG and Kim SA: BAG3 affects
the nucleocytoplasmic shuttling of HSF1 upon heat stress. Biochem
Biophys Res Commun. 464:561–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Festa M, Del Valle L, Khalili K, Franco R,
Scognamiglio G, Graziano V, De Laurenzi V, Turco MC and Rosati A:
BAG3 protein is overexpressed in human glioblastoma and is a
potential target for therapy. Am J Pathol. 178:2504–2512. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu H, Wu W, Fu Y, Shen W, Miao K, Hong M,
Xu W, Young KH, Liu P and Li J: Overexpressed BAG3 is a potential
therapeutic target in chronic lymphocytic leukemia. Ann Hematol.
93:425–435. 2014. View Article : Google Scholar
|
|
19
|
Ammirante M, De Laurenzi V, Graziano V,
Turco MC and Rosati A: BAG3 is required for IKKα nuclear
translocation and emergence of castration resistant prostate
cancer. Cell Death Dis. 2:e1392011. View Article : Google Scholar
|
|
20
|
Liao Q, Ozawa F, Friess H, Zimmermann A,
Takayama S, Reed JC, Kleeff J and Büchler MW: The anti-apoptotic
protein BAG-3 is overexpressed in pancreatic cancer and induced by
heat stress in pancreatic cancer cell lines. FEBS Lett.
503:151–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen Y, Jia LN, Honma N, Hosono T, Ariga T
and Seki T: Beneficial effects of cinnamon on the metabolic
syndrome, inflammation, and pain, and mechanisms underlying these
effects - a review. J Tradit Complement Med. 2:27–32. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwon JA, Yu CB and Park HD: Bacteriocidal
effects and inhibition of cell separation of cinnamic aldehyde on
Bacillus cereus. Lett Appl Microbiol. 37:61–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng SS, Liu JY, Chang EH and Chang ST:
Antifungal activity of cinnamaldehyde and eugenol congeners against
wood-rot fungi. Bioresour Technol. 99:5145–5149. 2008. View Article : Google Scholar
|
|
24
|
Chao LK, Hua KF, Hsu HY, Cheng SS, Lin IF,
Chen CJ, Chen ST and Chang ST: Cinnamaldehyde inhibits
pro-inflammatory cytokines secretion from monocytes/macrophages
through suppression of intracellular signaling. Food Chem Toxicol.
46:220–231. 2008. View Article : Google Scholar
|
|
25
|
Jeong HW, Han DC, Son KH, Han MY, Lim JS,
Ha JH, Lee CW, Kim HM, Kim HC and Kwon BM: Antitumor effect of the
cinnamaldehyde derivative CB403 through the arrest of cell cycle
progression in the G2/M phase. Biochem Pharmacol. 65:1343–1350.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SH, Lee CW, Lee JW, Choi MS, Son DJ,
Chung YB, Lee CK, Oh KW, Moon DC, Kwon BM, et al: Induction of
apoptotic cell death by 2′-hydroxycinnamaldehyde is involved with
ERK-dependent inactivation of NF-kappaB in TNF-α-treated SW620
colon cancer cells. Biochem Pharmacol. 70:1147–1157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong SH, Kim J, Kim JM, Lee SY, Shin DS,
Son KH, Han DC, Sung YK and Kwon BM: Apoptosis induction of
2′-hydroxycinnamaldehyde as a proteasome inhibitor is associated
with ER stress and mitochondrial perturbation in cancer cells.
Biochem Pharmacol. 74:557–565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee CW, Lee SH, Lee JW, Ban JO, Lee SY,
Yoo HS, Jung JK, Moon DC, Oh KW and Hong JT:
2-hydroxycinnamaldehyde inhibits SW620 colon cancer cell growth
through AP-1 inactivation. J Pharmacol Sci. 104:19–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SA, Sung YK, Kwon BM, Yoon JH, Lee H,
Ahn SG and Hong SH: 2′-Hydroxycinnamaldehyde shows antitumor
activity against oral cancer in vitro and in vivo in a rat tumor
model. Anticancer Res. 30:489–494. 2010.PubMed/NCBI
|
|
30
|
Ahn SG, Jin YH, Yoon JH and Kim SA: The
anticancer mechanism of 2′-hydroxycinnamaldehyde in human head and
neck cancer cells. Int J Oncol. 47:1793–1800. 2015.PubMed/NCBI
|
|
31
|
Dai C, Whitesell L, Rogers AB and
Lindquist S: Heat shock factor 1 is a powerful multifaceted
modifier of carcinogenesis. Cell. 130:1005–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sherman MY and Gabai VL: Hsp70 in cancer:
Back to the future. Oncogene. 34:4153–4161. 2015. View Article : Google Scholar :
|
|
33
|
Liu P, Xu B, Li J and Lu H: BAG3 gene
silencing sensitizes leukemic cells to Bortezomib-induced
apoptosis. FEBS Lett. 583:401–406. 2009. View Article : Google Scholar
|
|
34
|
Mani J, Antonietti P, Rakel S, Blaheta R,
Bartsch G, Haferkamp A and Kögel D: Knockdown of BAG3 sensitizes
bladder cancer cells to treatment with the BH3 mimetic ABT-737.
World J Urol. 34:197–205. 2016. View Article : Google Scholar
|