Introduction
Phosphoinositide 3-kinases (PI3Ks) are a family of
enzymes which are involved in cellular functions, such as cell
growth, proliferation, differentiation and intracellular
trafficking, which in turn contribute to cancer (1). Class IA PI3Ks, which
consist of a regulatory subunit (p85) and a catalytic subunit
(p110), are frequently mutated in human cancers and many of these
mutations cause the kinases to become more active (2–5).
PI3Ks can be activated by growth factor stimulation,
which results in the phosphorylation of phosphatidylinositol
4,5-bisphosphate (PIP2) to produce phosphatidylinositol
3,4,5-bisphosphate (PIP3) (6). PIP3 directly binds
phosphoinositide-dependent kinase 1 (PDK1) and protein kinase B
(AKT), causing the colocalization of activated PDK1 with AKT, which
allows AKT phosphorylation on threonine 308 (T308) by PDK1
(7,8). Complete activation of AKT occurs by
phosphorylation on serine 473 (S473) by mammalian target of
rapamycin (mTOR)-containing protein complex 2 (mTORC2) (9). PI3K/AKT signaling is closely
associated to cellular proliferation and survival through several
mechanisms, such as inhibition of pro-apoptotic B-cell lymphoma 2
(Bcl-2) family members BAD and BAX, activation of transcription
factor NF-κB, suppression of p53 by phosphorylating E3
ubiquitin-protein ligase (Mdm2) and promotion of protein synthesis
by stimulating mTORC1 (6,10,11).
Because inhibition of PI3K signaling can diminish
cell growth and promote cell death, targeting the PI3K signaling
pathway by inhibitors is being evaluated in clinical trials for
cancer therapeutics. Preclinical studies have shown that PI3K
pathway inhibitors have remarkable curative effects in several
types of cancer, such as human epidermal growth factor receptor 2
(HER2)-amplified breast cancers, cancers with PIK3CA mutations and
phosphatase and tensin homolog (PTEN)-deficient cancers (12–14).
Several reports have shown that PI3K inhibitors
could be used as adjuvant therapy for pancreatic cancer, as it
improved the anticancer drug efficacy by inhibiting the PI3K
pathway and extended the overall survival in mouse models (15–18).
In this study, however, the PI3K inhibitor LY294002 has been shown
to enhance PI3K-dependent AKT phosphorylation in pancreatic cancer
cells. Interestingly, this phosphorylation induced by LY294002 only
occurs once the cells have gained GEM-resistance. Moreover,
inhibition of PI3K by treatment with wortmannin did not induce any
apparent cytotoxicity in GEM-resistant cells. Our data indicate
that the uncertainty and risk is still high for treating PC by
using PI3K inhibitors.
Materials and methods
Cell lines
All cell lines were obtained from Cell Resource
Center for Biomedical Research Institute of Development, Aging and
Cancer, Tohoku University. Cells were cultured in Roswell Park
Memorial Institute-1640 (RPMI-1640; Gibco, 05918, Gaithersburg, MD,
USA) medium supplemented with 10% heat-inactivated fetal bovine
serum (FBS; Gibco, 26140-079) and 21 mM of L-glutamine, at 37°C, in
a humidified 5% CO2-95% air mixture. GEM-resistant cells
derived from KLM1 (KLM1-R) was established as previous descriptions
(19,20). KLM1 cells were tretaed with 10
µg/ml of GEM for 2 weeks, after which, cells were washed and
resuspended in fresh medium. This was followed by a 2-week culture
period which served as a recovery procedure. This procedure was
repeated for another 3 cycles.
Materials
LY294002 (9901) and wortmannin (9951S) were
purchased from Cell Signaling Technology (Boston, MA, USA).
AKTi-1/2 (ab142088) was purchased from Abcam Biochemicals
(Cambridge, MA, USA). Epithelial growth factor (E9644) was
purchased from Sigma (St. Louis, MO, USA). The antibodies specific
for p-ERKT204/202 (sc-7383), ERK (sc-94200), Hsp27
(sc-13132) and actin (sc-1616) were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). The antibodies specific for
Src (Src Antibody Sampler kit, 9935), p-Hsp27S78 (2405),
p-Hsp27S82 (2401), PTEN (9552S), AKT (9272) and
p-AKTS473 (4058) were purchased from Cell Signaling
Technology.
Western blotting
Total protein was extracted from the cells using
lysis buffer (1% NP-40, 1 mM sodium vanadate, 11 mM PMSF, 501 mM
Tris, 101 mM NaF, 101 mM EDTA, 1651 mM NaCl, 10 µg/ml
leupeptin, and 10 µg/ml aprotinin) (21). Equal amounts of protein (20
µg) were resolved by 5–20% SDS-polyacrylamide gel and then
transferred onto PVDF membrane (Immobilon-P; Millipore, Bedford,
MA, USA). The membrane was incubated with a primary antibody at 4°C
overnight and a horseradish peroxidase (HRP)-conjugated secondary
antibody for 1 h at room temperature. The immunoblots were
visualized with a chemiluminescent reagent (Immunostar, Wako,
Osaka, Japan) and detected by using an Image Reader LAS-1000 Pro
(Fujifilm Corp., Tokyo, Japan).
Immunofluorescence
Cells were cultured on coverslips in 12-well plates
at a density of 1×105 cells per well. Cells were fixed
using fresh 3.7% paraformaldehyde in phosphate-buffered saline
(PBS) and permeabilized with 0.1% Triton X-100. After washing with
PBS they were incubated in blocking solution (1% goat serum or 1%
donkey serum in PBS with 0.1% Tween-20) for 1 h at room temperature
(22). Cells were treated with a
primary antibody in blocking solution overnight at 4°C and a
secondary antibody for 1 h at room temperature. Cell nuclei were
counter-stained with 1.43 µM DAPI
(4,6′-diamidino-2-phenylindole). Confocal images were obtained by
using Laser Scan Confocal Microscope (LSM 510 META; Carl Zeiss,
Mobicity, Australia).
Cell proliferation assay
Cells were cultured in 96-well plates. After
treatment, 20 µl of the
3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) dye (Promega, Madison, WI, USA) was added to each well of the
plate and incubated for 2 h at 37°C, in a humidified 5%
CO2-95% air mixture. Optical density (OD) was read
directly at 492 nm using the iMark™ microplate absorbance reader
(Bio-Rad, Hercules, CA, USA). Each experiment was repeated three
times.
Results
The PI3K inhibitor LY294002 (but not
wortmannin) enhances AKT phosphorylation in PK59 cells
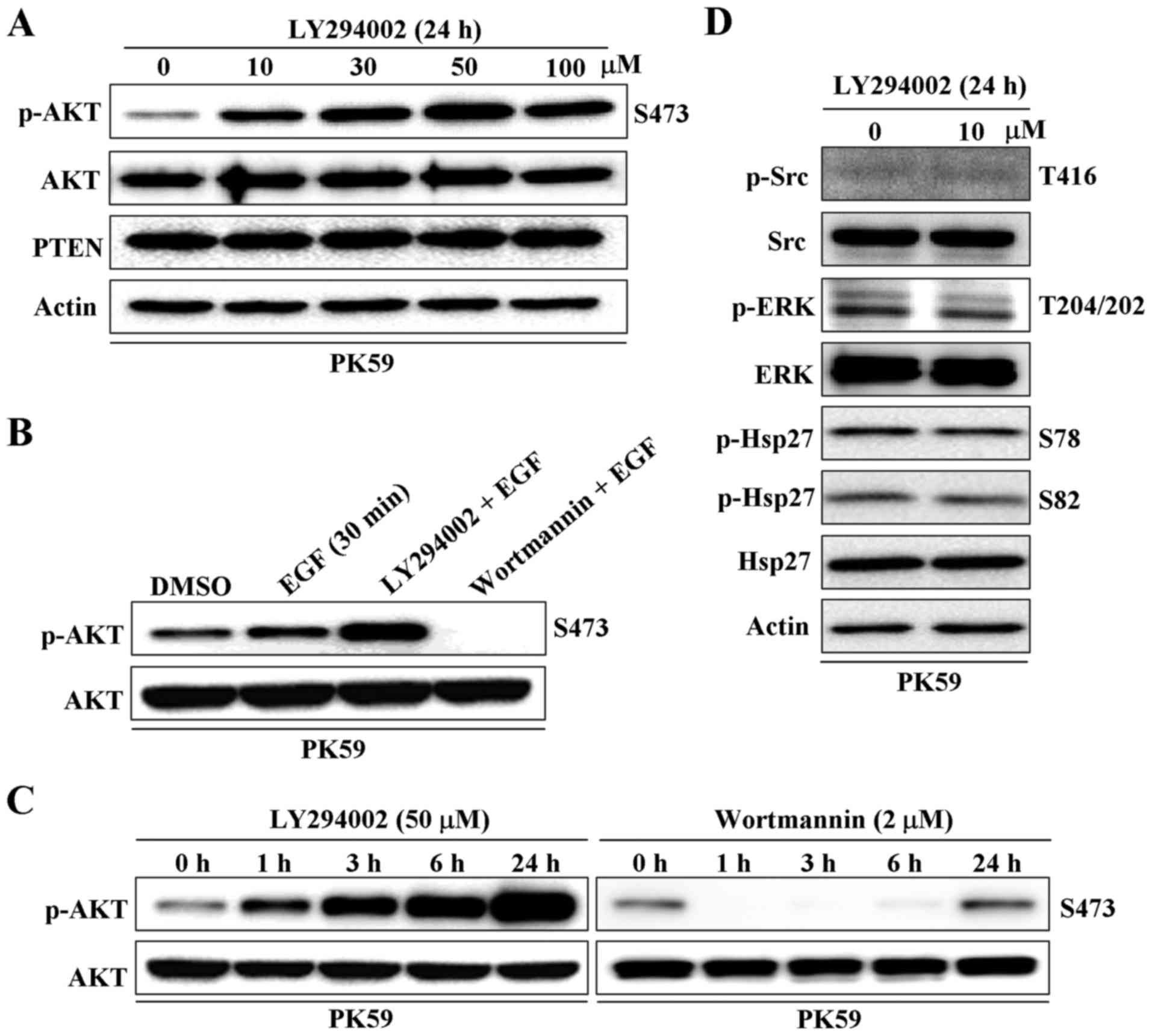
The effect of the PI3K inhibitor, LY294002, on AKT
phosphorylation at serine 473 in PC PK59 cells was detected by
western blot analysis. Unexpectedly, p-AKT was significantly
increased rather than decreased by treatment with LY294002 for 24 h
in a dose-dependent manner and over a time course (Fig. 1A and C). LY294002 was also shown to
enhance epithelial growth factor (EGF)-triggered AKT
phosphorylation (Fig. 1B).
However, the other PI3K inhibitor, wortmannin, showed the expected
performance in inhibiting the PI3K/AKT pathway (Fig. 1B and C). Interestingly, the effect
of wortmannin on inhibiting AKT phosphorylation was weakened after
6 h of treatment and completely disappeared after 24 h (Fig. 1C). In addition, the phosphorylation
of tyrosine-protein kinase CSK (Src), extracellular regulated
protein kinases (ERK) and heat shock protein 27 (Hsp27), which have
been shown to be involved in AKT activation (23–26),
were not altered by treatment with LY294002 (Fig. 1D). These results indicate that
LY294002, but not wortmannin, displays the opposite function to
that expected in regulating the PI3K/AKT signaling pathway in PK59
cells.
LY294002-induced AKT phosphorylation
specifically occurs in GEM-resistant PC cells
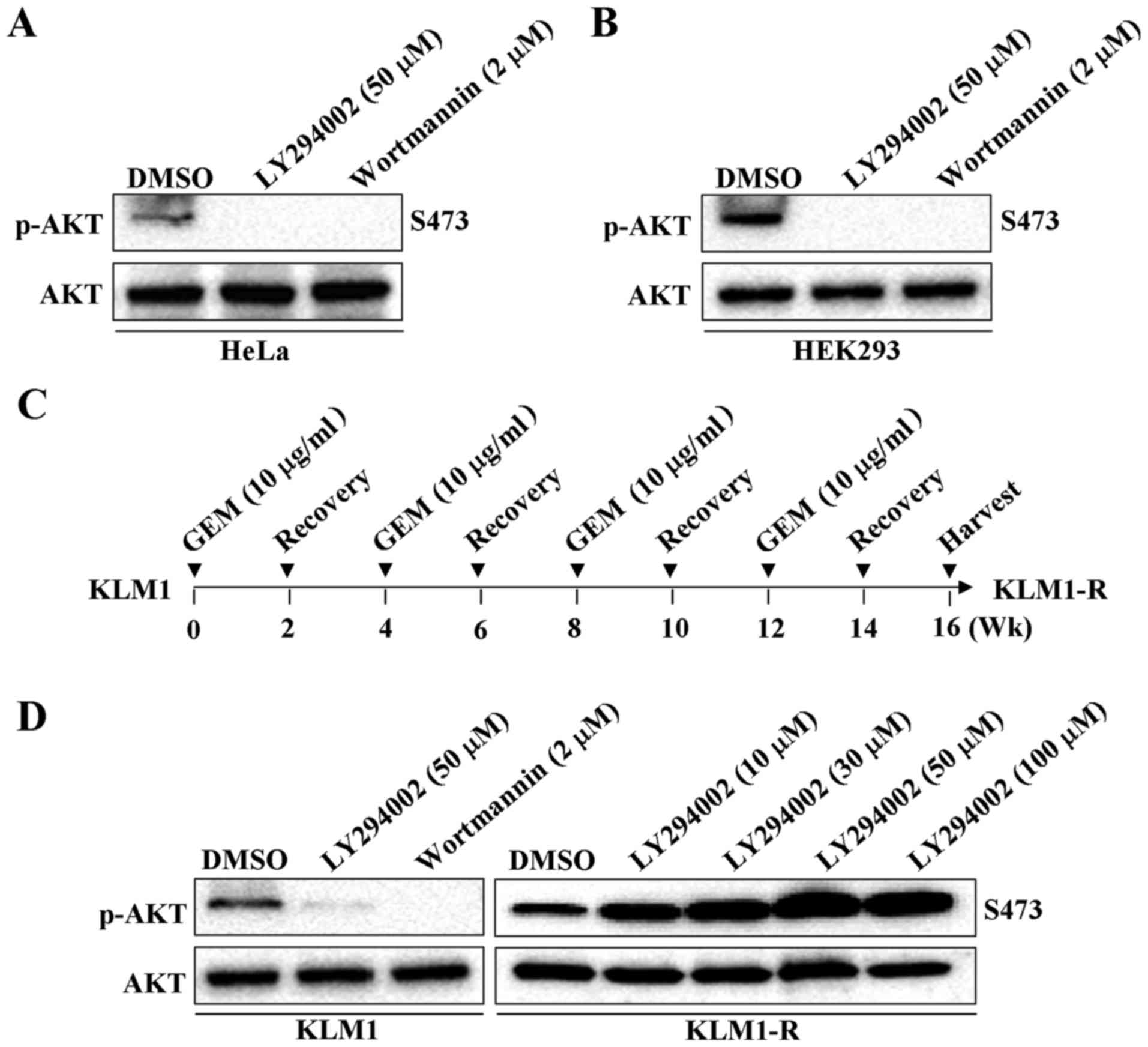
LY294002, as well as wortmannin, have shown their
ability to inhibit PI3K/AKT activity in HeLa and HEK293 cells
(Fig. 2A and B). LY294002 could
also inhibit AKT phosphorylation in various types of PC cancer cell
lines, such as in Panc-1, MIA-PaCa-2, AsPC-1 and BxPC-3 cells
(27–29). We previously reported that PK59 is
much less sensitive to GEM (IC50, 294.72 µg/ml)
than Panc-1 (IC50, 8.07 µg/ml), MIA-PaCa-2
(IC50, 6.81 µg/ml), AsPC-1 (IC50, 1.05
µg/ml) or BxPC-3 (IC50, 6.67 µg/ml) cells
(30). We thus tested whether
LY294002-induced AKT phosphorylation specifically occurs in
GEM-resistant PC cells. A GEM-resistant PC cell line KLM1-R has
been established by culturing the gemcitabine-sensitive KLM1 cells
with 10 µg/ml of GEM (19,20),
as shown (Fig. 2C). As
hypothesized, upregulation of p-AKT instead of downregulation was
observed by treatment with LY294002 in GEM-resistant KLM1-R
compared to KLM1 cells in a dose-dependent manner (Fig. 2D). These results suggest that the
inhibiting ability of LY294002 on PI3K could be altered in PC, once
cells acquire GEM-resistance. PI3K activity is required for the
LY294002-induced AKT phosphorylation and associated intracellular
membrane translocation.
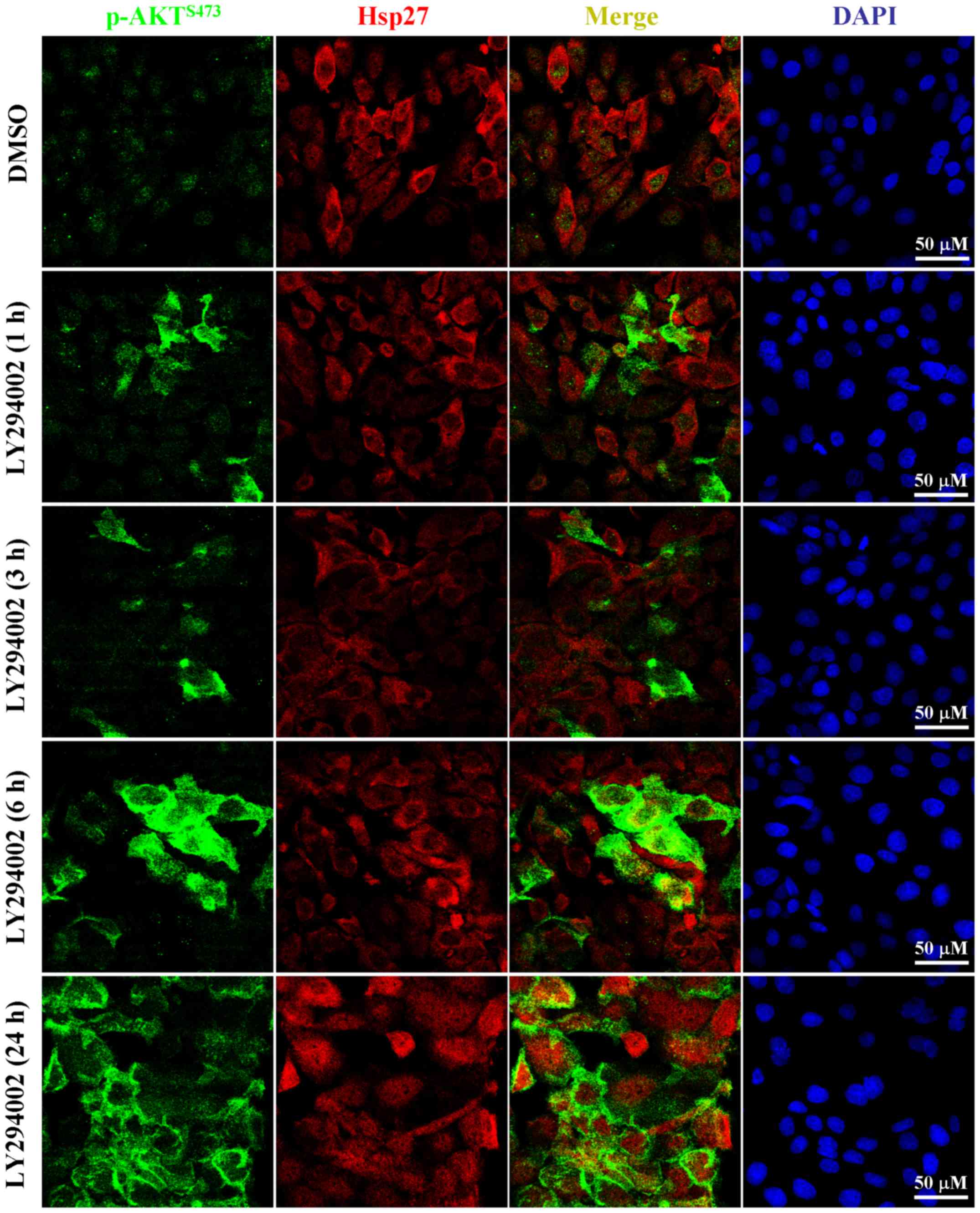
Using immunofluorescent analysis, p-AKT could only
be observed in the nuclei of untreated PK59 cells (Fig. 3). However, AKT phosphorylation was
initiated in the cytoplasm of LY294002-treated cells and the p-AKT
eventually translocated onto the intracellular membrane after
treatment for 24 h (Fig. 3),
indicating that the LY294002-induced p-AKT may have functions
associated with this specific localization. This kind of
phosphorylation and intracellular membrane translocation of AKT
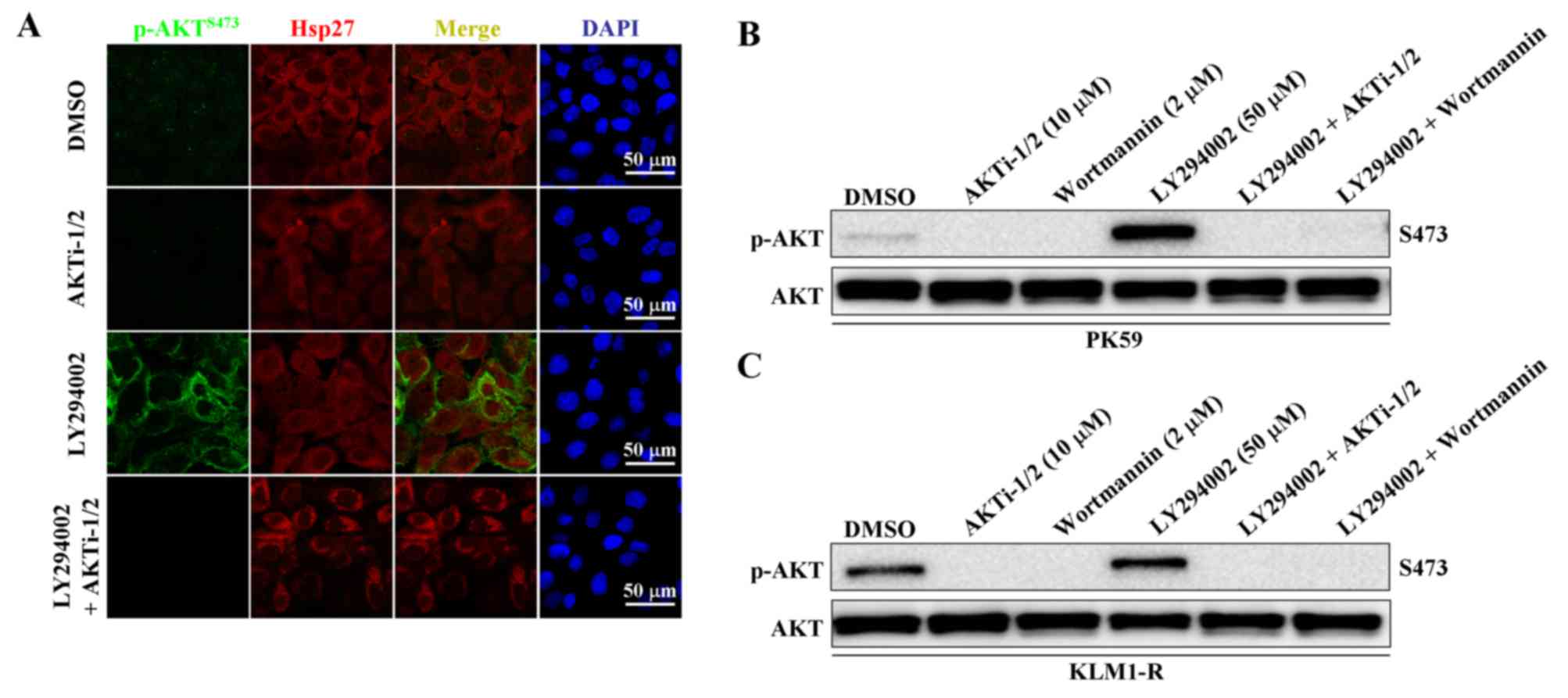
could be abolished by combined treatment with an AKT specific
inhibitor, AKTi-1/2 (Fig. 4A). We
next examined whether LY294002-induced AKT phosphorylation is
mediated by PI3K. Following treatment with LY294002 alone, p-AKT
was significantly upregulated, but following combined treatment
with LY294002 and either AKTi-1/2 or wortmannin, p-AKT was
completely inhibited in both PK59 and KLM1-R cells (Fig. 4B and C). These data reveal that
LY294002-induced AKT phosphorylation and intracellular membrane
translocation are still dependent on PI3K.
LY294002-induced AKT phosphorylation
contributes to cell survival
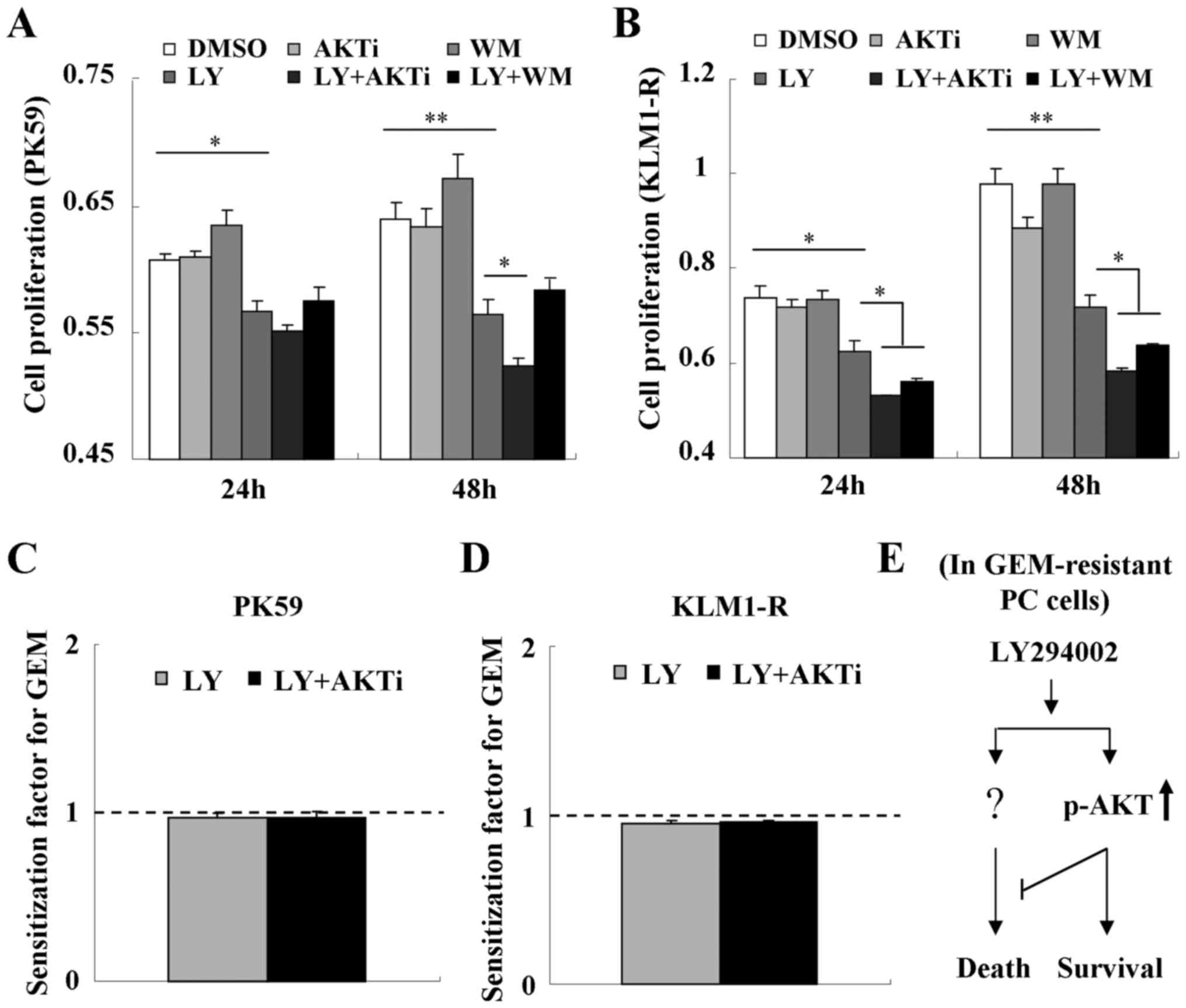
We next assessed the role of LY294002-induced p-AKT
in cell proliferation. Treatment with wortmannin was shown to
increase or have no effect on cell proliferation in PK59 and KLM1-R
cells, respectively (Fig. 5A and
B), indicating that GEM-resistant PC cells may also be
resistant to the inhibitor of PI3K. Treatment with LY294002 alone
significantly inhibited cell proliferation in PK59 and KLM1-R cells
and this inhibition could be further enhanced by combined treatment
with either AKTi-1/2 or wortmannin (Fig. 5A and B). These results indicate
that LY294002-induced p-AKT plays a negative role in
LY294002-triggered cytotoxicity in GEM-resistant PC cells. In
addition, LY294002-induced p-AKT did not contribute to reducing the
sensitivity of PK59 or KLM1-R cells to GEM (Fig. 5C and D).
Discussion
LY294002 is a synthetic compound that was designed
as a PI3K inhibitor based on the flavonoid quercetin (31). Like most other protein kinase
inhibitors, the mode of action of LY294002 is through competition
with ATP for binding the PI3K active site (32). However, while quercetin is a
broad-spectrum protein kinase inhibitor, LY294002 acts as a
specific inhibitor for PI3K, with no inhibitory effect on other
protein kinases such as the AMP-dependent protein kinase and c-Src
(at a concentration of 50 mM) (31). Apart from PI3K, LY294002 has been
shown to inhibit several key signaling components, such as NF-κB
(33), heat shock proteins 27
(HSP27) and 72 (HSP72), AKT phosphorylation and survivin (34).
Interestingly, LY294002 binds PI3K in a different
orientation to quercetin by a 180° rotation. When binding PI3K,
LY294002 only occupies part of the active site of the catalytic
domain, filling the space occupied by the ribose of the ATP
molecule, without extending into the phosphate binding region. In
comparison, wortmannin almost completely fits the active site of
the catalytic domain of PI3K, inducing a fairly large
conformational rearrangement. Moreover, wortmannin forms a covalent
complex, which irreversibly inhibits PI3K (35). The covalently-linked complex is the
reason why in this study a decrease in wortmannin activity over
time was observed, as the irriversibly bound complex was degraded
and free PI3K was replenished.
LY294002 is a promising therapeutic compound because
it showed growth inhibitory effects on various types of cancer
cells through the induction of apoptosis and cell cycle arrest or
autophagy (36,37). Apoptotic cell death by LY294002 is
mainly driven via activation of AMPKα1 and inactivation of AKT
(38), but can also be via NF-κB
(33). However, it can also act by
reducing transforming growth factor β (TGFβ)-mediated epithelial to
mesenchymal transition (EMT) (39), inhibiting fatty acid synthase or
suppress metastasis (40,41).
The available literature for the combinatorial
treatment of pancreatic cancer by LY294002 and gemcitabine is
plentiful. In pancreatic cancer cells, partial reversal of the EMT
was accompanied by inhibition of cell invasion and migration as
well as increased chemosensitivity to GEM, and the process was
mediated by the phosphatidylinositol 3-kinase (PTEN)/Akt signaling
pathway (42). However, no
importance appears to have been previously given to the effect of
GEM-resistance to such treatment. The same can be said for most
other flavonoids tested. One exception has been the treatment of
GEM-resistant cell lines with GEM and apigenin (an isoconformer of
the isoflavonoid genistein), which showed additive inhibition of
cell proliferation compared to the use of either agent alone, via
increased cell cycle arrest (at both the S and G2/M phases) as well
as early apoptosis (by downregulation of pAkt) (43).
In conclusion, this is the first report to show that
treatment of the GEM-resistant pancreatic cancer cell line PK59
with LY294002 (but not wortmannin) enhanced AKT phosphorylation
specifically via PI3K and resulted in an associated translocation
to the cell membrane. The overall effect of this LY294002-induced
AKT phosphorylation on PK59 cells was an increase in cell survival.
This should be an eye-opener for future clinical trials where the
GEM-sensitivity of the tumor is not known.
Abbreviations:
|
PC
|
pancreatic cancer
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
AKT
|
protein kinase B
|
|
GEM
|
gemcitabine
|
Acknowledgments
This study was supported in part by Grants-in-Aid
from the Ministry of Education, Science, Sports, and Culture of
Japan (no. 24501352 to Yasuhiro Kuramitsu). Immunoblot detection by
LAS-1000 was done at the Gene Research Centre of Yamaguchi
University.
References
|
1
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: Variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikenoue T, Kanai F, Hikiba Y, Obata T,
Tanaka Y, Imamura J, Ohta M, Jazag A, Guleng B, Tateishi K, et al:
Functional analysis of PIK3CA gene mutations in human colorectal
cancer. Cancer Res. 65:4562–4567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizoguchi M, Nutt CL, Mohapatra G and
Louis DN: Genetic alterations of phosphoinositide 3-kinase subunit
genes in human glioblastomas. Brain Pathol. 14:372–377. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samuels Y and Velculescu VE: Oncogenic
mutations of PIK3CA in human cancers. Cell Cycle. 3:1221–1224.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Philp AJ, Campbell IG, Leet C, Vincan E,
Rockman SP, Whitehead RH, Thomas RJ and Phillips WA: The
phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in
human ovarian and colon tumors. Cancer Res. 61:7426–7429.
2001.PubMed/NCBI
|
|
6
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alessi DR, James SR, Downes CP, Holmes AB,
Gaffney PR, Reese CB and Cohen P: Characterization of a
3-phosphoinositide-dependent protein kinase which phosphorylates
and activates protein kinase Balpha. Curr Biol. 7:261–269. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Currie RA, Walker KS, Gray A, Deak M,
Casamayor A, Downes CP, Cohen P, Alessi DR and Lucocq J: Role of
phosphatidylinositol 3,4,5-trisphosphate in regulating the activity
and localization of 3-phosphoinositide-dependent protein kinase-1.
Biochem J. 337:575–583. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duronio V: The life of a cell: Apoptosis
regulation by the PI3K/PKB pathway. Biochem J. 415:333–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Engelman JA, Chen L, Tan X, Crosby K,
Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y,
et al: Effective use of PI3K and MEK inhibitors to treat mutant
Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med.
14:1351–1356. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Serra V, Markman B, Scaltriti M, Eichhorn
PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S,
et al: NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K
signaling and inhibits the growth of cancer cells with activating
PI3K mutations. Cancer Res. 68:8022–8030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
She QB, Chandarlapaty S, Ye Q, Lobo J,
Haskell KM, Leander KR, DeFeo-Jones D, Huber HE and Rosen N: Breast
tumor cells with PI3K mutation or HER2 amplification are
selectively addicted to Akt signaling. PLoS One. 3:e30652008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Junttila MR, Devasthali V, Cheng JH,
Castillo J, Metcalfe C, Clermont AC, Otter DD, Chan E, Bou-Reslan
H, Cao T, et al: Modeling targeted inhibition of MEK and PI3 kinase
in human pancreatic cancer. Mol Cancer Ther. 14:40–47. 2015.
View Article : Google Scholar
|
|
16
|
Duong HQ, Kim HJ, Kang HJ, Seong YS and
Bae I: ZSTK474, a PI3K inhibitor, suppresses proliferation and
sensitizes human pancreatic adenocarcinoma cells to gemcitabine.
Oncol Rep. 27:182–188. 2012.
|
|
17
|
Jung KH, Yan HH, Fang Z, Son MK, Lee H,
Hong S and Hong SS: HS-104, a PI3K inhibitor, enhances the
anticancer efficacy of gemcitabine in pancreatic cancer. Int J
Oncol. 45:311–321. 2014.PubMed/NCBI
|
|
18
|
Cruceru ML, Enciu AM, Popa AC, Albulescu
R, Neagu M, Tanase CP and Constantinescu SN: Signal transduction
molecule patterns indicating potential glioblastoma therapy
approaches. Onco Targets Ther. 6:1737–1749. 2013.PubMed/NCBI
|
|
19
|
Iwasaki I, Sugiyama H, Kanazawa S and
Hemmi H: Establishment of cisplatin-resistant variants of human
neuroblastoma cell lines, TGW and GOTO, and their drug
cross-resistance profiles. Cancer Chemother Pharmacol. 49:438–444.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maehara S, Tanaka S, Shimada M, Shirabe K,
Saito Y, Takahashi K and Maehara Y: Selenoprotein P, as a predictor
for evaluating gemcitabine resistance in human pancreatic cancer
cells. Int J Cancer. 112:184–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Kuramitsu Y, Tokuda K, Okada F,
Baron B, Akada J, Kitagawa T and Nakamura K: Proteomic analysis
indicates that overexpression and nuclear translocation of
lactoylglutathione lyase (GLO1) is associated with tumor
progression in murine fibrosarcoma. Electrophoresis. 35:2195–2202.
2014.PubMed/NCBI
|
|
22
|
Wang Y, Kuramitsu Y, Tokuda K, Baron B,
Kitagawa T, Akada J, Maehara S, Maehara Y and Nakamura K:
Gemcitabine induces poly (ADP-ribose) polymerase-1 (PARP-1)
degradation through autophagy in pancreatic cancer. PLoS One.
9:e1090762014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choudhury GG, Mahimainathan L, Das F,
Venkatesan B and Ghosh-Choudhury N: c-Src couples PI 3 kinase/Akt
and MAPK signaling to PDGF-induced DNA synthesis in mesangial
cells. Cell Signal. 18:1854–1864. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moelling K, Schad K, Bosse M, Zimmermann S
and Schweneker M: Regulation of Raf-Akt cross-talk. J Biol Chem.
277:31099–31106. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi S, Xin Y, Qi Z, Xu Y, Diao Y, Lan L,
Luo L and Yin Z: HSP27 phosphorylation modulates TRAIL-induced
activation of Src-Akt/ERK signaling through interaction with
β-arrestin2. Cell Signal. 26:594–602. 2014. View Article : Google Scholar
|
|
26
|
Zheng C, Lin Z, Zhao ZJ, Yang Y, Niu H and
Shen X: MAPK-activated protein kinase-2 (MK2)-mediated formation
and phosphorylation-regulated dissociation of the signal complex
consisting of p38, MK2, Akt, and Hsp27. J Biol Chem.
281:37215–37226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh S, Srivastava SK, Bhardwaj A, Owen
LB and Singh AP: CXCL12-CXCR4 signalling axis confers gemcitabine
resistance to pancreatic cancer cells: A novel target for therapy.
Br J Cancer. 103:1671–1679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu
Q, Duan W, Yu S, Wang F, et al: Resveratrol inhibits the
epithelial-mesenchymal transition of pancreatic cancer cells via
suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013. View Article : Google Scholar
|
|
29
|
Fujiwara M, Izuishi K, Sano T, Hossain MA,
Kimura S, Masaki T and Suzuki Y: Modulating effect of the
PI3-kinase inhibitor LY294002 on cisplatin in human pancreatic
cancer cells. J Exp Clin Cancer Res. 27:762008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mori-Iwamoto S, Kuramitsu Y, Ryozawa S,
Taba K, Fujimoto M, Okita K, Nakamura K and Sakaida I: A proteomic
profiling of gemcitabine resistance in pancreatic cancer cell
lines. Mol Med Rep. 1:429–434. 2008.PubMed/NCBI
|
|
31
|
Vlahos CJ, Matter WF, Hui KY and Brown RF:
A specific inhibitor of phosphatidylinositol 3-kinase,
2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol
Chem. 269:5241–5248. 1994.PubMed/NCBI
|
|
32
|
Toledo LM, Lydon NB and Elbaum D: The
structure-based design of ATP-site directed protein kinase
inhibitors. Curr Med Chem. 6:775–805. 1999.PubMed/NCBI
|
|
33
|
Grandage VL, Gale RE, Linch DC and Khwaja
A: PI3-kinase/Akt is constitutively active in primary acute myeloid
leukaemia cells and regulates survival and chemoresistance via
NF-kappaB, Mapkinase and p53 pathways. Leukemia. 19:586–594.
2005.PubMed/NCBI
|
|
34
|
Ohnishi K, Yasumoto J, Takahashi A and
Ohnishi T: LY294002, an inhibitor of PI-3K, enhances heat
sensitivity independently of p53 status in human lung cancer cells.
Int J Oncol. 29:249–253. 2006.PubMed/NCBI
|
|
35
|
Walker EH, Pacold ME, Perisic O, Stephens
L, Hawkins PT, Wymann MP and Williams RL: Structural determinants
of phosphoinositide 3-kinase inhibition by wortmannin, LY294002,
quercetin, myricetin, and staurosporine. Mol Cell. 6:909–919. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shtivelman E, Sussman J and Stokoe D: A
role for PI 3-kinase and PKB activity in the G2/M phase of the cell
cycle. Curr Biol. 12:919–924. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xing C, Zhu B, Liu H, Yao H and Zhang L:
Class I phosphatidylinositol 3-kinase inhibitor LY294002 activates
autophagy and induces apoptosis through p53 pathway in gastric
cancer cell line SGC7901. Acta Biochim Biophys Sin (Shanghai).
40:194–201. 2008. View Article : Google Scholar
|
|
38
|
Lee YK and Park OJ: Regulation of mutual
inhibitory activities between AMPK and Akt with quercetin in MCF-7
breast cancer cells. Oncol Rep. 24:1493–1497. 2010.PubMed/NCBI
|
|
39
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Van de Sande T, De Schrijver E, Heyns W,
Verhoeven G and Swinnen JV: Role of the phosphatidylinositol
3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty
acid synthase in LNCaP prostate cancer cells. Cancer Res.
62:642–646. 2002.PubMed/NCBI
|
|
41
|
Cole GW Jr, Alleva AM, Zuo JT, Sehgal SS,
Yeow WS, Schrump DS and Nguyen DM: Suppression of pro-metastasis
phenotypes expression in malignant pleural mesothelioma by the PI3K
inhibitor LY294002 or the MEK inhibitor UO126. Anticancer Res.
26A:809–821. 2006.
|
|
42
|
Yi XP, Han T, Li YX, Long XY and Li WZ:
Simultaneous silencing of XIAP and survivin causes partial
mesenchymal-epithelial transition of human pancreatic cancer cells
via the PTEN/PI3K/Akt pathway. Mol Med Rep. 12:601–608.
2015.PubMed/NCBI
|
|
43
|
Strouch MJ, Milam BM, Melstrom LG, McGill
JJ, Salabat MR, Ujiki MB, Ding XZ and Bentrem DJ: The flavonoid
apigenin potentiates the growth inhibitory effects of gemcitabine
and abrogates gemcitabine resistance in human pancreatic cancer
cells. Pancreas. 38:409–415. 2009. View Article : Google Scholar : PubMed/NCBI
|