Introduction
Neuropilin (NRP), first identified by Takagi et
al in 1987 (1), is a 130–140
kDa multifunctional single-spanning transmembrane glycoprotein that
plays a central role in neuronal and blood vessel development as
receptors for members of the class-3 semaphorin family (SEMAs) of
axonal guidance modulators, and also for members of the vascular
endothelial growth factor (VEGF) family of angiogenesis stimulators
(2–4). Two neuropilin genes, neuropilin-1
(NRP-1) and NRP-2, have been identified. NRP-1 is predominantly
expressed in arterial endothelial cells, whereas NRP-2 is
preferentially expressed in vein and lymphatic endothelial cells
(5). Both NRP-1 and NRP-2 have
~44% amino acid sequence identity and share a similar domain
structure, which contains a large extracellular region and a short
cytoplasmic tail of ~40 amino acids, lacking any enzymatic activity
(3). Their extracellular regions
contain three domains: the a1a2 domain is involved in
SEMA3-binding, the b1b2 domain is involved in both SEMA3- and
VEGF-binding, and the c domain is involved in dimerization
(6,7). The binding site for VEGF ligands has
been localized to the b1b2 domains of NRP-1 and NRP-2, whereas the
binding of semaphorins requires both the a1a2 and b1b2 repeats
(8). NRP-1 and NRP-2 interact
selectively with different members of the VEGF and semaphorin
families and have non-overlapping expression patterns (8). NRP-1 binds VEGF-A165, VEGF-B, VEGF-E,
SEMA3A, SEMA3B and SEMA3C, whereas NRP-2 binds VEGF-A165,
VEGF-A145, VEGF-C, VEGF-D, SEMA3B, SEMA3C and SEMA3F (4,8).
However, VEGF-A165 binds 50-fold more strongly to NRP-1 than NRP-2
(9). NRP-1 was found to interact
with VEGF-A165, and to act as a VEGF co-receptor that specifically
enhances VEGFR-2 signaling to promote VEGF biological activity,
including endothelial cell migration, sprouting and angiogenesis
(6,7). Nevertheless, NRP-2 has different
binding preferences for VEGF family members, and is a co-receptor
for VEGFR-3 that is involved in lymphatic endothelial cell function
(10). Knockout of the NRP-2 gene
results in abnormal lymphatic development (11), including an abnormal patterning and
marked reduction in small lymphatic vessel and capillaries,
supporting a role for NRP-2 in VEGF-C mediated VEGFR-3 signaling
and developmental lymphangiogenesis.
Previous studies demonstrated that NRP-1 is
expressed on several types of human tumor cells and its expression
has been correlated with tumor progression and/or poorer prognosis
(3,6,12).
Mounting data of evidence suggest that NRP-2 not only plays a
central role in lymphangiogenesis, but also in angiogenesis due to
a co-receptor for members of VEGF family (13,14).
Furthermore, NRP-2 is found to be overexpressed in a variety of
human cancers and its upregulation is associated with tumor grade,
progression and metastasis, such as lung cancer (15), renal carcinoma (16), pancreatic cancer (17), bladder cancer (18) and colorectal carcinoma (19). Interestingly, the overexpression of
NRP-2 is positively correlated with tumor therapy resistance, poor
prognosis, and short survival (20,21).
Therapeutically targeting NRP-2 expressed in tumor cells using a
blocking antibody inhibited the tumor growth and prevented the
tumor metastases by blocking the formation of tumor angiogenesis
and lymphangiogenesis (19,22).
Therefore, NRP-2 has great value as a molecular target for
therapeutic intervention, a prognostic indicator of patient
survival, and a predictive marker of the response to antineoplastic
therapy. Accordingly, NRP-2 inhibition using monoclonal antibodies
is considered as a promising strategy for cancer therapy.
In vivo imaging of tumor-receptor offers a
more accurate and real-time assay of receptor expression both for
patient stratification and monitoring expression-level changes in
response to therapy, without such biopsy-associated pitfalls and
the need of repetitive invasive biopsies (23). Our previous studies showed that a
novel monoclonal antibody against NRP-2 b1b2 domain (anti-NRP-2
mAb), generated by our laboratory (24), can inhibit tumor proliferation,
growth, and migration, such as bladder and colorectal cancer
(unpublished data), suggesting that anti-NRP-2 mAb may be effective
agents for NRP-2-targeted imaging and therapy. Anti-NRP-2 mAb
specifically binds to NRP-2 b1b2 domain, but not NRP-1 b1b2 domain,
consistent with a previous study (22). Therefore, anti-NRP-2 mAb may be
valuable to specifically exploit NRP-2 expression, eliminating any
possible undesirable effects mediated by NRP-2. In the present
study, we aimed to perform the chloramine-T strategy to label the
anti-NRP-2 monoclonal antibody (anti-NRP-2 mAb) with iodine-131,
and further determine whether the resulting SPECT (single-photon
emission computed tomography) probe 131I-anti-NRP-2 mAb
is a suitable agent for imaging mice bearing NRP-2 expression
tumors with human lung adenocarcinoma A549 cells.
Materials and methods
General
Chloramine-T was purchased from J&K Scientific,
Ltd. (Shanghai, China). The recombinant human peptide sequence of
NRP-2 b1b2 was kindly provided by Professor Craig W. Vander Kooi
(Department of Molecular and Cellular Biochemistry and Center for
Structural Biology, University of Kentucky, Kentucky, USA).
Na131I was obtained from the China Institute of Atomic
Energy (Beijing, China). Goat anti-mouse IgG antibody was purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). A PD-10
Sephadex G-25 column from GE Healthcare Bioscience, Ltd. (Diegem,
Belgium). rProtein A Sepharose columns were purchased from GE
Healthcare Bioscience, Ltd. (Uppsala, Sweden). DMF-96 gamma counter
and FCY radioactive chromatography scanner from Hefei Zhongcheng
Electromechanical Technology Development Co., Ltd. (Hefei, China).
CRC-25R Dose Calibrator from Capintec, Inc. (Ramsey, NJ, USA).
SPX-6 SPECT from GE Healthcare Medical Systems, Inc. (Waukesha, WI,
USA). Human lung adenocarcinoma A549 cell line, human non-small
cell lung carcinoma H1299 cell line, and human normal bronchial
epithelial cell line 16HBE were obtained from the Cell Culture
Center of Institute of Basic Medical Sciences of Chinese Academy of
Medical Sciences (Beijing, China). Female nude mice, 6 and 8 weeks
of age, and Balb/c mice were purchased from the Experimental Animal
Center of Xiamen University (Xiamen, China).
Cell culture
Two human non-small cell lung carcinoma cell lines
(A549, H1299) and a human normal bronchial epithelial cell line
16HBE were cultured in DMEM (Gibco, Grand Island, NY, USA)
supplemented with 10% FBS (Gemini, Woodland, CA, USA) and 1%
penicillin-streptomycin (Gibco). The cells were maintained in a
humidified atmosphere of 5% CO2 at 37°C, with the medium
changed every two days. A 70–80% confluent monolayer was detached
by 0.1% trypsin and dissociated into a single cell suspension for
further cell culture.
Quantitative real-time PCR (qRT-PCR)
The expression of NRP-2 mRNA in A549, H1299 and
16HBE cell lines is determined by the qRT-PCR based on the method
described earlier (12,25). In short, total RNA was isolated
with TRIzol reagent (Invitrogen Life Technologies Inc., Grand
Island, NY, USA) according to the manufacturer's instructions. The
cDNA was synthesized from 2 µg of total RNA using MMLV
transcriptase (Toyobo, Shanghai, China) with random hexamers.
qRT-PCR were performed using SYBR Premix Ex Taq (Takara, Dalian,
China). The human β-actin gene was used as the endogenous control.
All cDNA samples were normalized to the β-actin endogenous control;
2−ΔΔCT method was used to calculate the relative
quantification of NRP-1 mRNA expression. Primers used for real-time
PCR are listed in Table I. All
qRT-PCRs were performed in duplicate.
 | Table IPrimers for real-time PCR. |
Table I
Primers for real-time PCR.
| Gene | Forward
primers | Reverse
primers |
|---|
| NRP-2 |
CACGACTGCAAGTATGACT |
TTTTGAGCAATCTTCAGAGC |
| β-actin |
TACCCAGGCATTGCTGACAGG |
ACTTGCGGTGCACGATGGA |
Western blot analysis
According to the previously described methods
(24), the expression of NRP-2
protein in A549, H1299 and 16HBE cell lines was determined by
western blot analysis. Briefly, A549, H1299 and 16HBE cells were
lysed in 1 ml RIPA solution (containing 1% Triton X-100, 1%
deoxycholate, and 0.1% SDS; Beyotime Biotechnology, Haimen, China)
supplemented with a protease inhibitor (PMSF; Sangon, Shanghai,
China) for 30 min at 4°C, then centrifuged at 12,000 rpm for 30 min
at 4°C to obtain the total cell extracts. Fifteen milliliters of
cell extracts was subjected to SDS-PAGE and transferred onto a PDVF
membranes by Mini-PROTEAN (Bio-Rad, Hercules, CA, USA). The PDVF
membranes were blocked with TBST (10 mM pH 7.4 Tris-HCL, 150 mM
NaCl, and 0.1% Tween-20) containing 5% skim milk for 2 h at 37°C,
and incubated overnight at 4°C with anti-NRP-2 mAb (diluted 1:100).
After washed with TBST three times, the membranes were again
incubated with goat anti-mouse IgG labeled peroxidase (diluted
1:5,000) for 1 h at 37°C. Proteins of interest were visualized with
ECL in Kodak Image Station 4000R (Carestream Health, Rochester, NY,
USA). β-actin was used as an internal control. All experiments were
performed in duplicate.
Cellular immunofluorescence staining
Cellular immunofluorescence staining was performed
as previously described (12,24).
Briefly, the cell-seeded coverslips were washed and fixed. The
anti-NRP-2 mAb (diluted 1:100) and fluorescence (TRITC)-labeled
secondary antibody (diluted 1:50; Gibco) were added restained with
Hoechst 33258 (Beyotime Biotechnology, Jiangsu, China). The slides
with cells were incubated without the anti-NRP-2 mAb as control.
The fluorescence images were captured at excitation laser of 360 nm
and emission laser of 460 nm for Hoechst 33258, and at excitation
laser of 488 nm and emission laser of 530 nm for FITC by using
Olympus FV 1000 Inverted Confocal Fluorescence Microscope (Olympus,
Columbia, SC, USA). All experiments were performed in
duplicate.
Immunohistochemical staining (IHC)
The expression of NRP-2 in A549 tumor tissues and
normal lung tissues was examined by IHC as described previously
(12). In brief, the tissue chips
were dewaxed and hydrated. Endogenous peroxidase was inactivated
with 0.3% H2O2 for 10 min. The sections were
microwaved for antigen retrieval in 0.01 M citrate buffer (pH 6.0)
for 20 min and were incubated with a primary anti-NRP-2 mAb
(diluted 1:100) overnight at 4°C. Subsequently, the sections were
incubated with a biotinylated goat anti-rabbit secondary antibody
for 30 min at room temperature. Then applying the following steps:
color-developing, redyeing, dehydration, making transparent and
sealing. The findings of immunostaining were visualized with a
Motic AE2000 inverted microscope [Motic (Xiamen) Medical Diagnostic
Systems Co., Ltd., Xiamen, Fujian, China].
Production and purification of anti-NRP-2
mAb
Anti-NRP-2 mAb was produced by hybridomas derived
from mice immunized with a recombinant human NRP-2 b1b2 in our
laboratory according to a method described earlier (24). Briefly, Six-week-old Balb/c mice
were injected with hybridoma cells
(2×105–106). After 7–10 days, ascites (5–10
ml/mouse) with anti-NRP-2 mAb were collected and centrifuged at
10,000 × g for 15 min to acquire the supernatant. The supernatant
was purified by rProtein A sepharose column chromatography
according to the manufacturer's protocol to obtain purified
anti-NRP-2 mAb. To assess the purity, the purified NRP-2 mAb was
subjected to sodium salt-polyacrylamide gel electrophoresis
(SDS-PAGE) and was analyzed by Quantity One software (Bio-Rad). The
animal procedures were performed according to a protocol approved
by the Institutional Animal Care and Use Committee of Zhongshan
Hospital Xiamen University.
Analyses for anti-NRP-2-specific antibody
and its titer
Indirect ELISA was performed to test
anti-NRP-2-specific antibody and its titer according to previously
described methods (22,24,26).
Briefly, 96-well plates were coated with 10 µg/ml of NRP-2
b1b2 in carbonate buffer (pH 9.6) and incubated overnight at 4°C.
Non-specific binding was blocked with 5% non-fat dry milk in PBS
(pH 7.5) for 2 h at 37°C followed by washing three times with
washing buffer (0.05% Tween-20 in PBS). Then the plates were
incubated with supernatant of hybridoma cells (ascites) or
anti-NRP-2 mAb or anti-NRP-1 mAb (A6-11-26) or antiserum IgG of
mice for 2 h at 37°C, respectively. After washing, the plates were
incubated with goat anti-mouse IgG-HRP conjugate for 1 h at 37°C.
Finally, the plates were washed as before and O-phenylenediamine
(OPD) was added to develop color. The optical density (OD) was
determined at 450 nm by a microplate ELISA reader (Bio-Rad, Tokyo,
Japan) after the reaction was stopped with 2 M
H2SO4. Data were analyzed and graphed with
OriginPro 8.1 software (OriginLab, Northampton, MA, USA). All
experiments were performed in duplicate.
Labeling anti-NRP-2 mAb with
iodine-131
Anti-NRP-2 mAb was labeled with Na131I by
the chloramine-T method according to a previous study (27). Briefly, in a 1-ml vial, 100
µg anti-NRP-2 mAb was dissolved in 100 µl 0.01 M
phosphate-buffered saline (PBS, pH 7.4), followed by the addition
of Na131I (5.5 MBq 25 µl). Then 50 µl
chloramine-T (1 mg/ml), freshly prepared in water, was added. The
reaction mixture was allowed to stand for 3 min at room
temperature. Then reaction was terminated by adding 50 µl of
Na2S2O5 (2 mg/ml, freshly prepared
in water). Radiolabeled antibodies were then purified by
size-exclusion chromatography using a PD-10 Sephadex G-25 column.
For routine quality control of labeling, the labeling efficiency
and radiochemical purity of radiolabeled anti-NRP-2 mAb probes were
calculated with TLC method (polyamide film/saline).
In vitro stability analysis
In vitro stability in PBS was determined with
TLC method (polyamide film/saline) as described with minor
modifications (26). Briefly,
131I-anti-NRP-2 mAb (3.11 MBq, 100 µl) was added
to 2.0 ml 0.01 M PBS (pH 7.4) and was incubated at 37°C for 6, 24,
48 and 72 h. At each time-point, 2 µl of the mixture was
placed 2 cm above the lower edge and was allowed to evaporate
spontaneously, one strip was developed with the mixture developed
with saline. After complete development, the paper sheet was
removed, dried, and counted in radioactive chromatography scanner.
All experiments were performed in duplicate.
Cell assays
Cell uptake and binding affinity assay were
performed as previously described with minor modifications
(28–30). Briefly, the A549 cells lines were
cultured in DMEM supplemented with 10% FBS and 1%
penicillin-streptomycin. The cells were maintained in a humidified
atmosphere of 5% CO2 at 37°C, with the medium changed
every two days. A 70–80% confluent monolayer was detached by 0.1%
trypsin and dissociated into a single cell suspension for further
cell culture.
Cell uptake assays
The A549 cells were washed three times with 0.01 M
PBS (pH 7.4) and dissociated with 0.25% trypsin-EDTA. DMEM medium
was then added to neutralize trypsin-EDTA. Cells were spun down and
re-suspended with serum free DMEM. Cells (0.5×106) were
incubated at 37°C for 0.5–4 h with 1.11×10−2 MBq 100
µl 131I-anti-NRP-2 mAb in 0.5 ml serum-free DMEM
medium. The non-specific binding of the probes with A549 cells was
determined by co-incubation with 10.0 µg unlabeled
anti-NRP-2 mAb. At each time-point, the cells were washed three
times with chilled PBS and spun down at a speed of 7,000–8,000 rpm.
The cell pellets at the bottom of the tube were spliced, and the
radioactivity of the pellets was measured using a gamma counter.
The uptake (counts/min) was normalized to the percentage of binding
for analysis using Excel (Microsoft Software Inc., Redmond, WA,
USA). All experiments were performed in duplicate.
Binding affinity assay
The A549 cells (0.5×106) were plated on
6-well plates one day before the experiment. Cells were washed with
PBS three times and incubated at 37°C for 3 h with
1.11×10−2 MBq 100 µl 131I-anti-NRP-2
mAb in 0.5 ml serum-free DMEM medium. Non-specific binding of the
probes with A549 cells was determined by co-incubation with
unlabeled anti-NRP-2 mAb (0.01–10,000 nM final concentration).
After incubation, the cells were washed with cold PBS three times
and detached with 1 M 0.5 ml NaOH for 5 min. The radioactivity in
the cells was measured using a gamma counter and were corrected for
physical decay. The data were analyzed using GraphPad Prism
(GraphPad Software, Inc., San Diego, CA, USA), and the half maximal
inhibitory concentration (IC50 value) of 131
I-anti-NRP-2 mAb was measured using a least squares fitting
routine. All experiments were performed in duplicate.
Biodistribution study
The animal procedures were performed according to a
protocol approved by the Institutional Animal Care and Use
Committee of Zhongshan Hospital Xiamen University. Cultured A549
cells (~5×106) suspended in PBS were implanted
subcutaneously in the right hind limbs of nude mice. Tumors were
allowed to grow to around 0.8–1.0 cm in diameter (7–10 days) and
then the tumor-bearing mice were subject to in vivo
biodistribution and imaging studies.
For biodistribution studies, A549 tumor-bearing mice
(n=4 for each group) were injected with 131I-anti-NRP-2
mAb (0.37 MBq, 200 µl) through the tail vein. At 6, 24, 48
and 72 h after injection, the mice were sacrificed, and tumors and
normal tissues of interest were removed and weighed, and their
radioactivity was measured in a gamma counter. The radioactivity
uptake in the tumor and normal tissues was expressed as a
percentage of the injected radioactivity per gram of tissue
(%ID/g). In order to study the in vivo NRP-2 targeting
specificity of 131I-anti-NRP-2 mAb, based on the
previous studies (44,49), unlabeled anti-NRP-2 mAb (100 µg)
was co-injected with 131I-anti-NRP-2 mAb in nude mice
bearing A549 tumors (n=4 for each group) via a tail vein, and
biodistribution studies were conducted at 48 h after injection.
SPECT imaging
SPECT imaging of tumor-bearing mice was performed on
a single-head SPECT scanner with a pinhole collimator. The mice
bearing A549 tumor (n=4 for each group) were injected with
131I-anti-NRP-2 mAb (3.7 MBq, 200 µl) with or
without co-injection of unlabeled anti-NRP-2 mAb (100 µg)
through the tail vein. At 6, 24, 48 and 72 h after injection, the
mice were anesthetized with 2% isoflurane and placed on SPECT bed
(ventral side down). Whole body static images (200,000 counts) were
acquired with a matric of 218×218, and zoom of 2.0. Regions of
interest (ROIs) were drawn over the tumor and contralateral muscle,
and then the ratio of tumor to contralateral muscle (T/NT) were
calculated.
Statistical methods
The experimental data were analyzed by SPSS 18.0
(SPSS Co., Chicago, IL, USA). Statistical analysis was performed
using two tailed Student's t-test for unpaired data. Data are
expressed as mean ± standard deviation and P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of NRP-1 in A549 cell lines
and tumor tissue
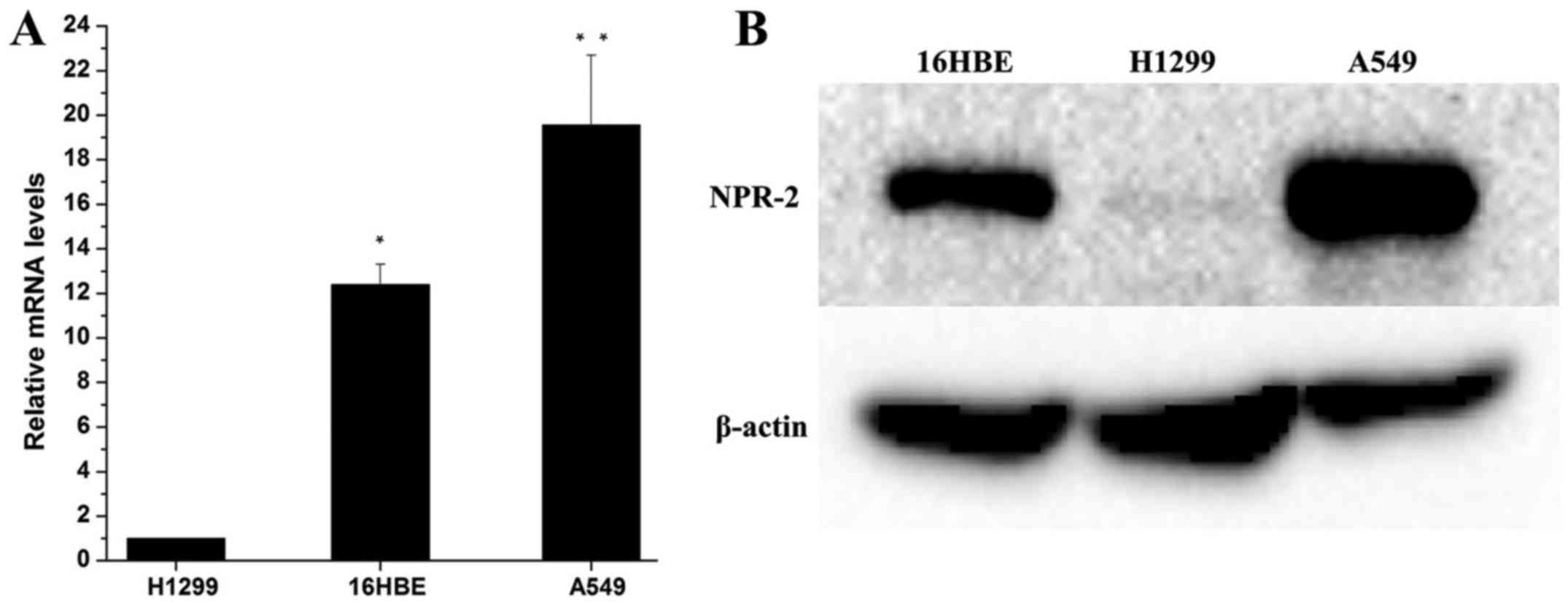
As shown in Fig.
1A, qRT-PCR results indicated that the expression of NRP-2 mRNA
in A549 cells was significantly higher than that in H1299 and 16HBE
cells (P<0.01). Consistent with qRT-PCR. Western blot analysis
(Fig. 2B) demonstrated that A549
cells exhibited higher levels of NRP-2 protein expression
(P<0.05). Interesting, the expression of NRP-2 in H1299 cells
was significantly lower than that in 16HBE cells (P<0.05). IHC
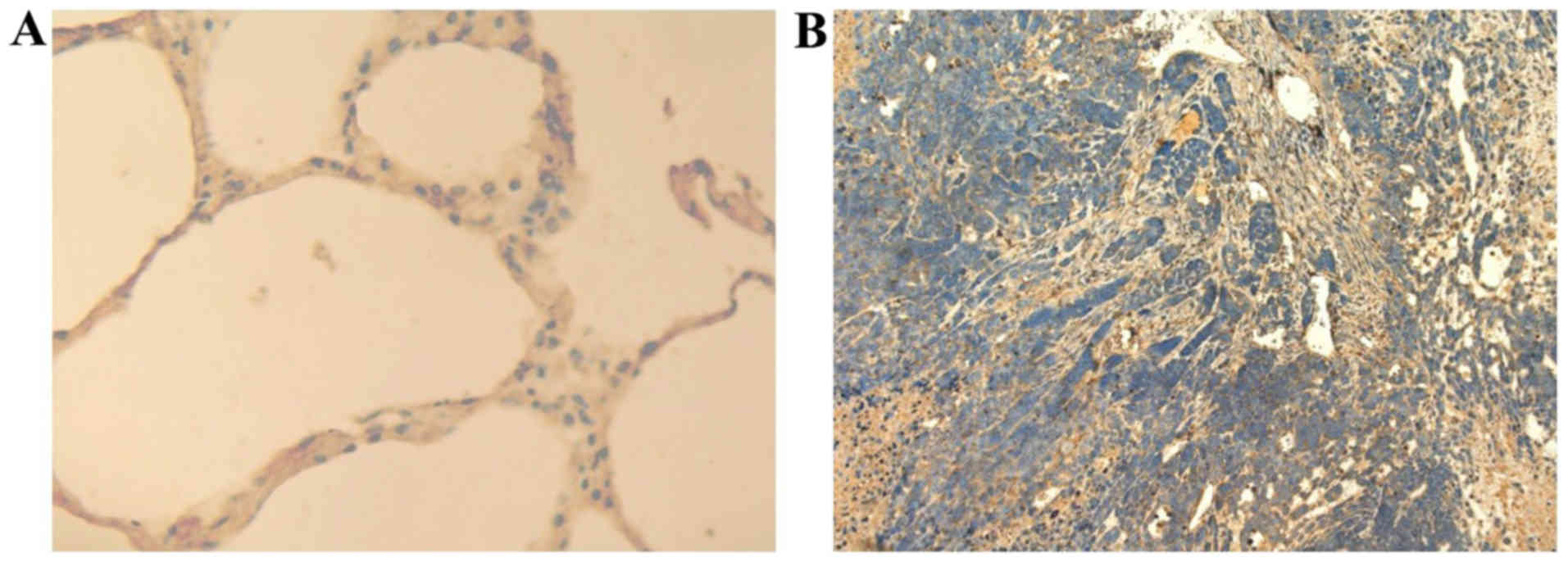
(Fig. 2) also showed that the
expression of NRP-2 is markedly increased in tumor tissue bearing
A549, compared to normal mouse lung tissues. Immunofluorescence
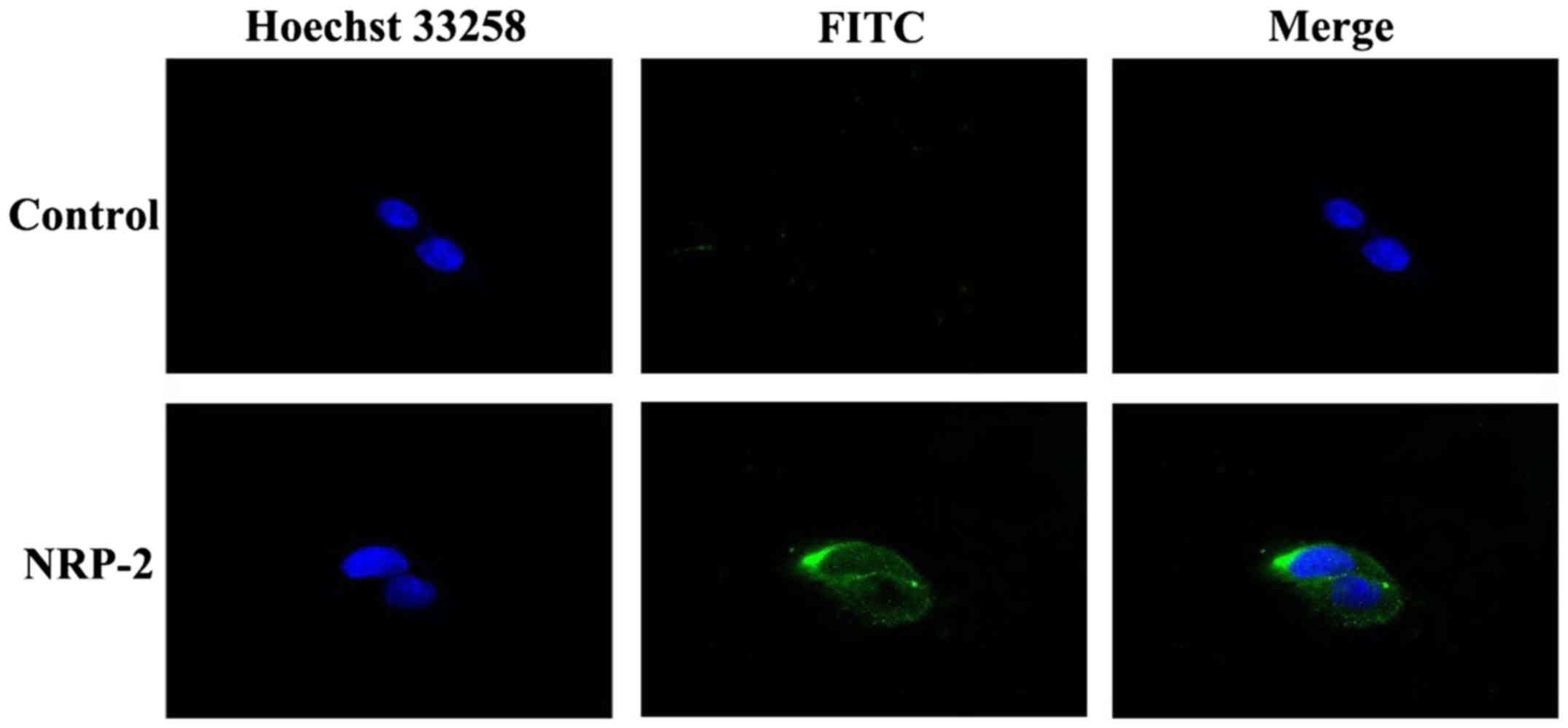
analysis showed (Fig. 3) that
anti-NRP-2 mAb could bind well with NRP-2 receptor on the surface
of the A549 cells.
Based on the results we concluded that NRP-2 was
overexpressed in the human lung adenocarcinoma A549 cells.
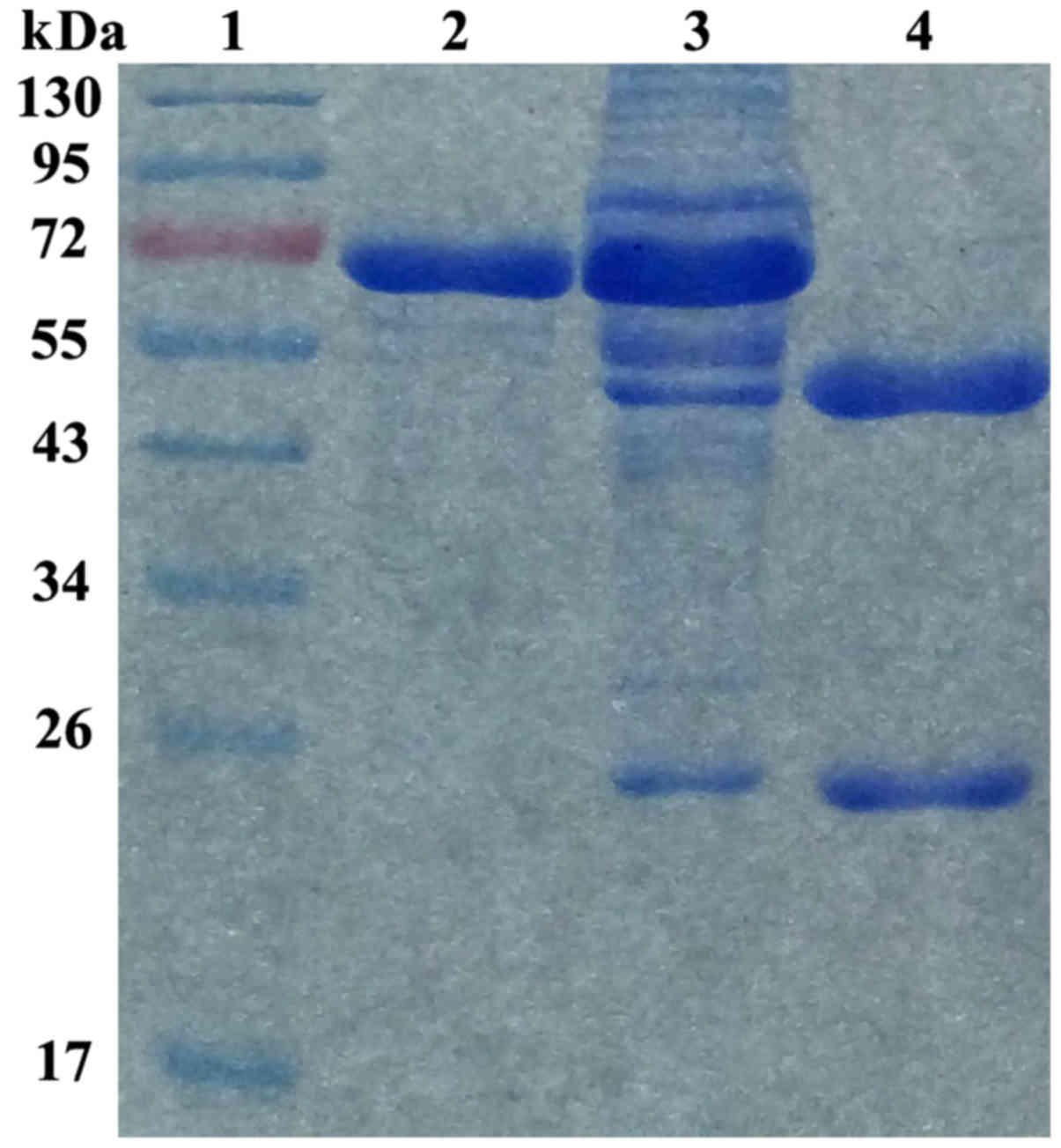
Characterization of anti-NRP-2 mAb
Our previous western blot results showed that
anti-NRP-2 mAb was specifically combined with both NRP-2 b1b2
recombinant protein and whole NPR-2 (24). To identify the purity of the
current anti-NRP-2 mAb obtained from ascites, the purified
anti-NRP-2 mAb was resolved by an 8% SDS-PAGE gel (Fig. 4). The purity of anti-NRP-2 mAb was
determined to be >95%, as detected by Gray analysis of Quantity
One 1D-analysis software (GE Healthcare), at a concentration of 2.0
mg/ml. Moreover, the results showed that anti-NRP-2 mAb was IgG1
isotype.
The purified anti-NRP-2 mAb was diluted to measure
the titers and specificity against NRP-2 b1b2 by indirect ELISA. As
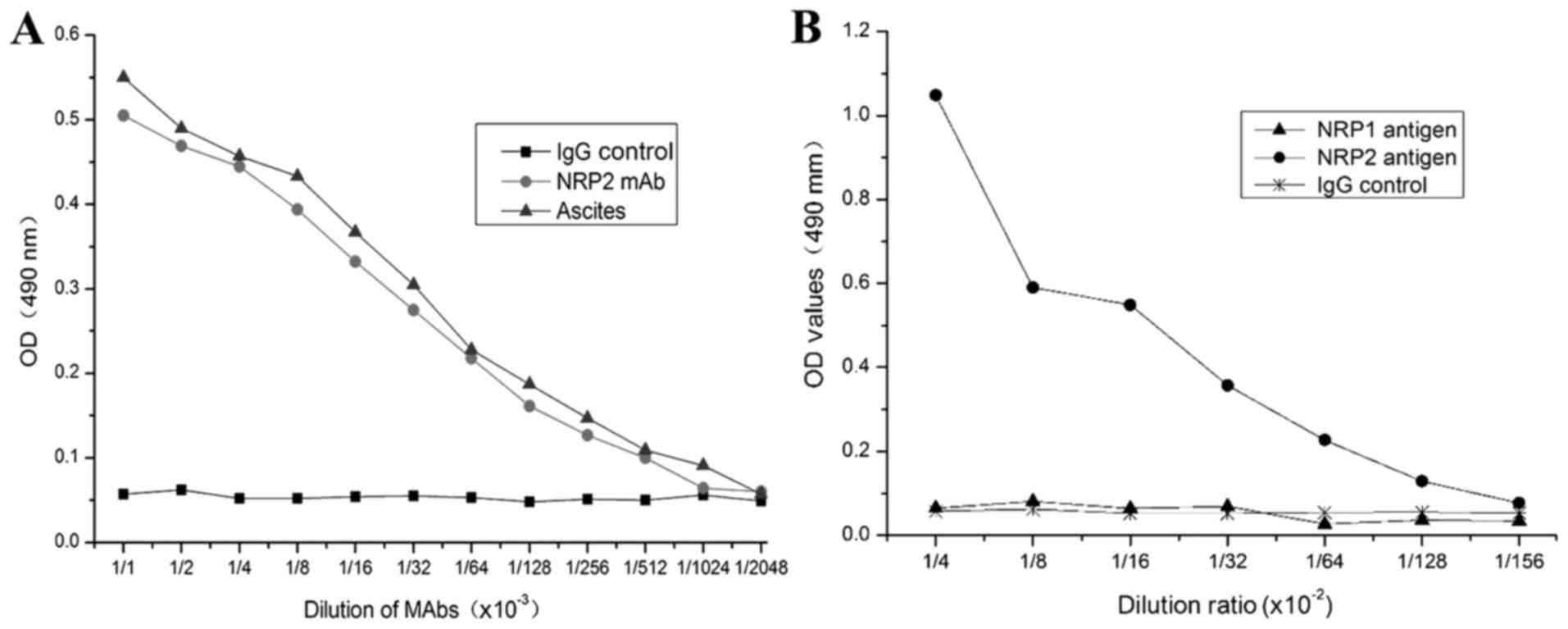
shown in Fig. 5, the purified
anti-NRP-2 mAb can bind to synthetic immunogenic peptides with a
titer of 2.28×105, and specifically binds to NRP-2 b1b2
domain, but not NRP-1 b1b2 domain.
Radioiodination of anti-NRP-2 mAb
131I-anti-NRP-2 mAb was successfully
radioiodinated. The radiolabeling efficiency, radiochemical purity
and specific activity of 131I-A6-11-26 was 94.69±3.63,
98.56 ±0.48 and 162.35±19.6 MBq/µg, respectively.
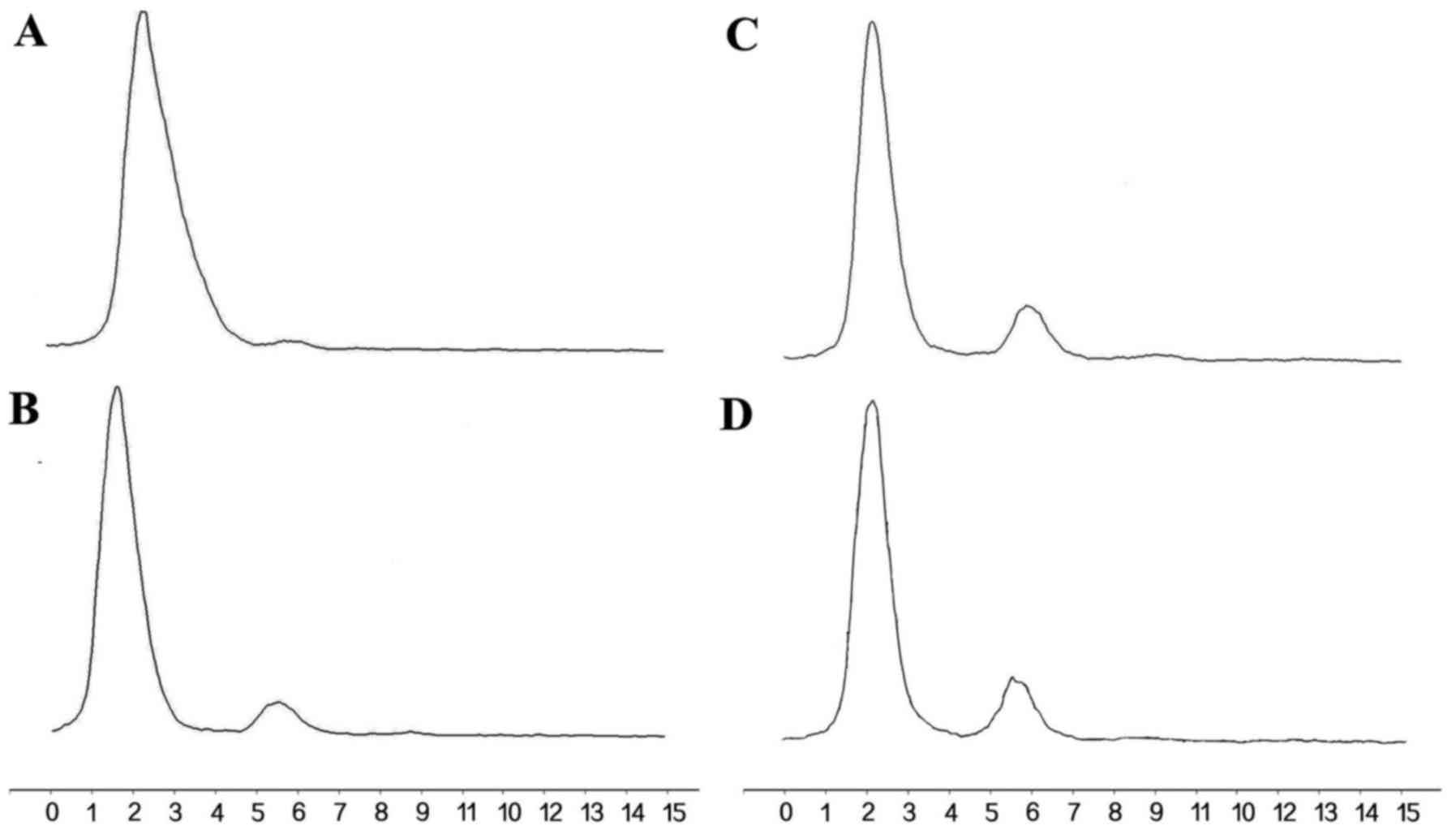
In vitro stability analysis
In vitro stability studies (Fig. 6) showed that >85% of
131I-anti-NRP-2 mAb remained intact during 6–72 h of
incubation in PBS, indicating that 131I-anti-NRP-2 mAb
maintained excellently stable in PBS.
Cell assays
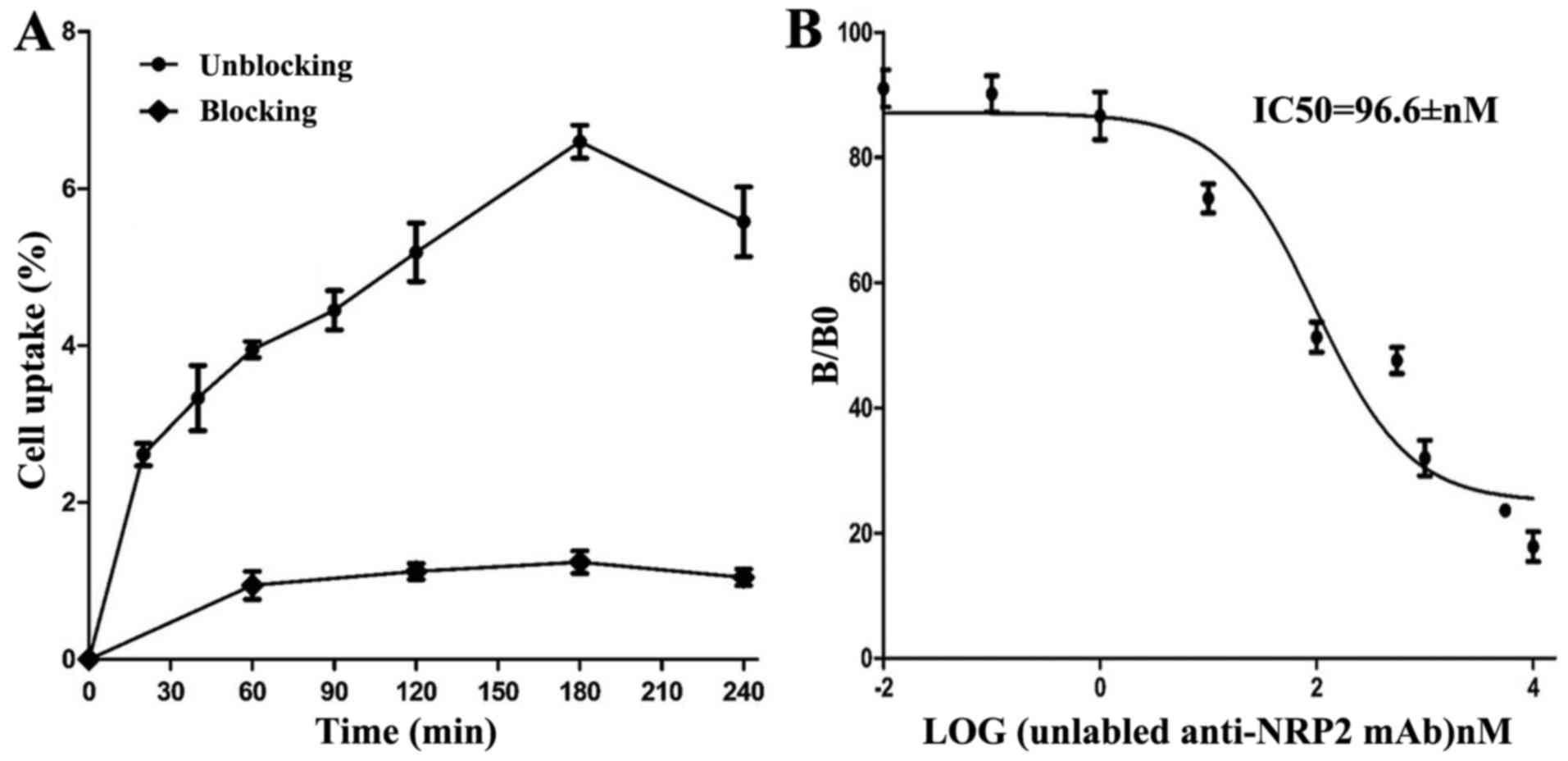
Cell uptake ratios of 131I-anti-NRP-2 mAb
are shown in Fig. 7A.
131I-anti-NRP-2 mAb accumulated in A549 cells and
reached a highest value of 6.60±0.36% of applied activity at 180
min. When the probe was incubated with large excess of
non-radioactive anti-NRP-2 mAb, its uptake levels in A549 cells was
significantly inhibited (P<0.05) at all incubation
time-points.
The binding affinity of 131I-anti-NRP-2
mAb to NRP-2 was determined through competition binding assays. As
shown in Fig. 7B, the
IC50 value of 131I-anti-NRP-2 mAb was
96.6±1.44 nM.
Overall, these results strongly suggested that
131I-anti-NRP-2 mAb had high NRP-2 binding specificity,
affinity in A549 cells, which warranted their further evaluation
in vivo.
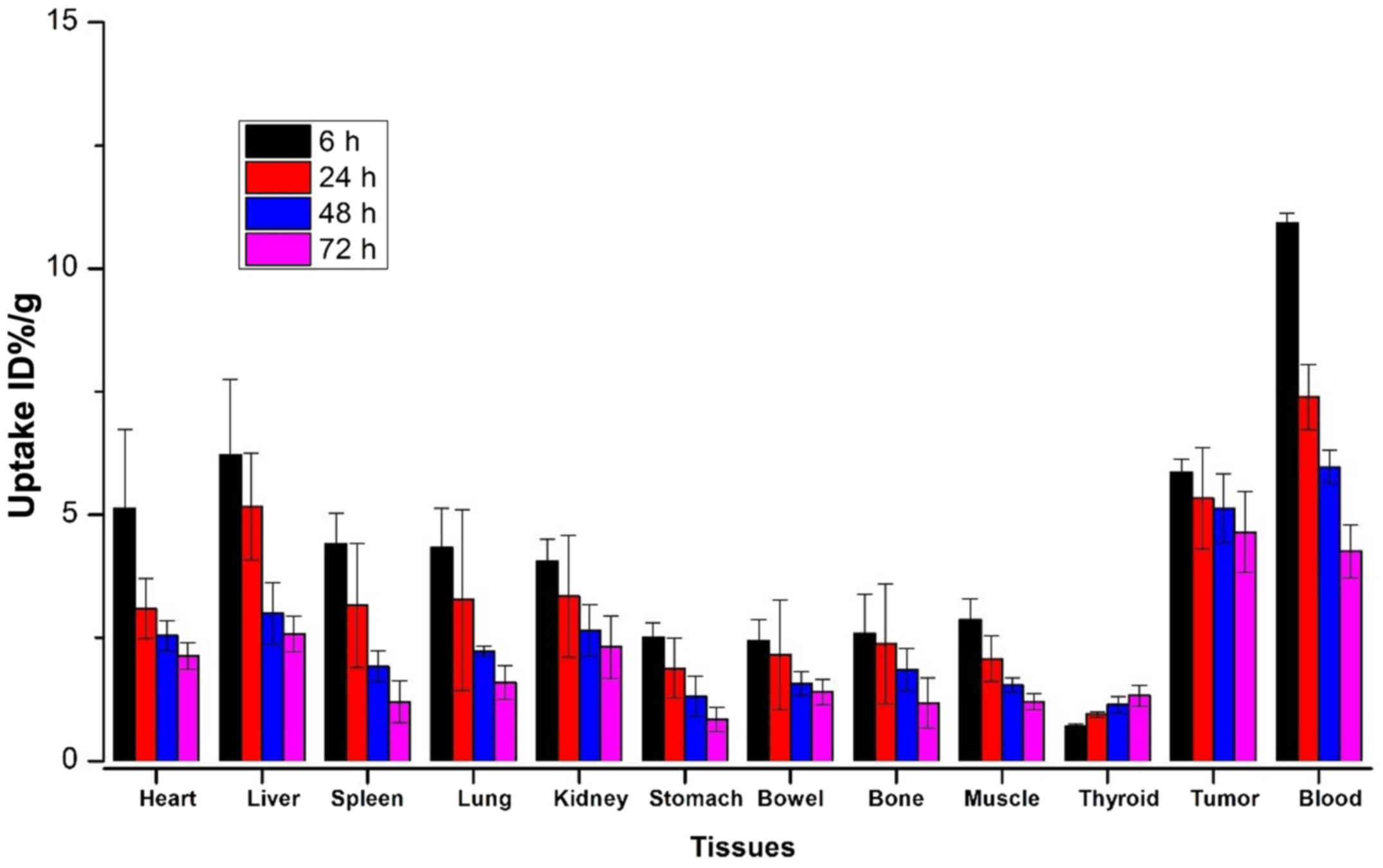
Biodistribution study
At 6, 24, 48 and 72 h after administration, the
biodistribution profiles of 131I-anti-NRP-2 mAb are
presented in Fig. 8 and Table II, 131I-anti-NRP-2 mAb
exhibited high levels of radioactivity accumulation in A549 tumors.
At 24 h, the tumor uptake was 5.86±0.27 %ID/g, higher than that in
the other organs except for liver (6.22±1.53 %ID/g) and blood
(10.93±0.25% ID/g). Moreover, at 72 h, 131I-anti-NRP-2
mAb in the tumor still remained at high level (4.64±0.82% ID/g),
significantly higher than that in the other organs including the
liver and blood. Lower levels of radioactivity were observed in
muscle and bone (1.21±0.16 and 1.18±0.51% ID/g, respectively). The
thyroid uptake increased slightly from 0.70±0.05 to 1.33± 0.97%
ID/g during 6–72 h post-injection. Furthermore,
131I-anti-NRP-2 mAb provided significantly higher tumor
to contralateral muscle ratio (T/NT) and lower tumor-to-blood and
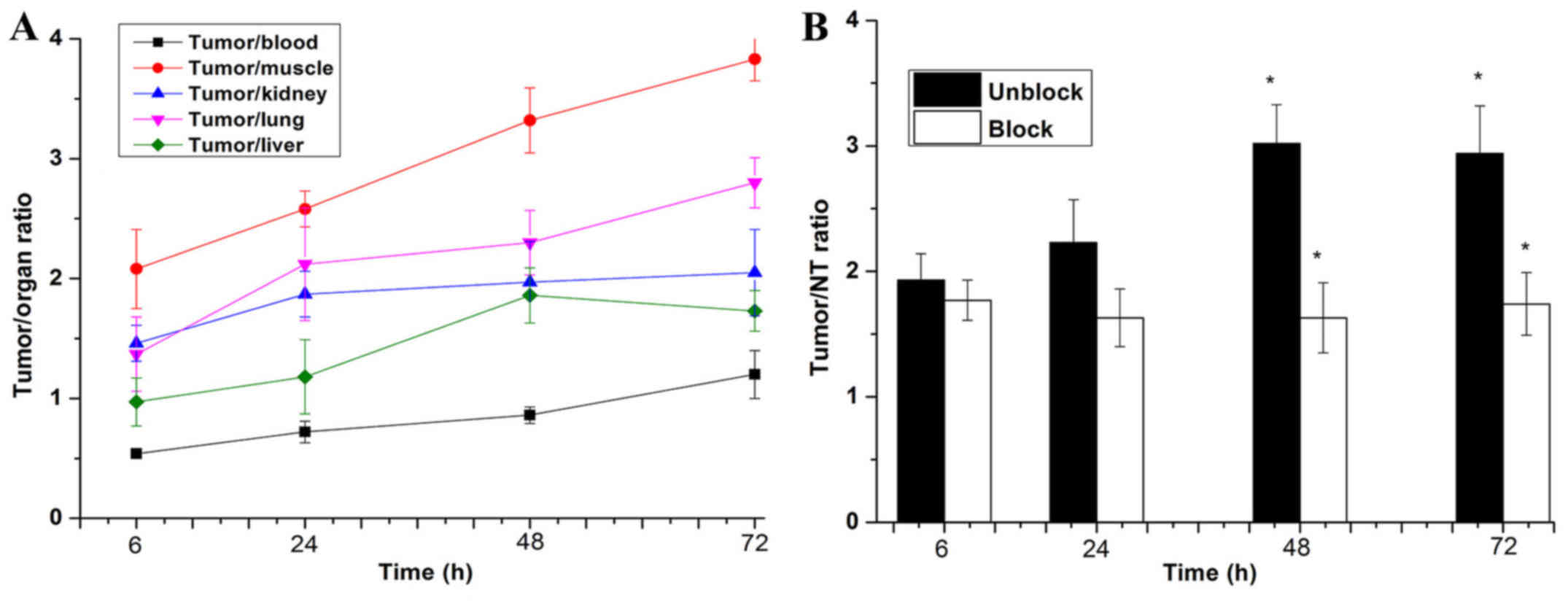
tumor to liver ratios (Fig. 9A).
At 6 h, the ratio of tumor to contralateral muscle ratio
(T/NT=2.08±0.33) was the highest among the tumor to blood
(T/B=0.54±0.03), tumor to liver (T/L=0.97±0.22), tumor to kidney
(T/K=1.46±0.15), and tumor to lung (T/L=1.37±0.31). Moreover,
during 24–72 h, the T/NT ratio increased gradually over time.
 | Table IIComparison of biodistribution for
131I-anti-NRP-2 mAb in A549 xenogratfs between 0
µg (unblock) and 100 µg (block). |
Table II
Comparison of biodistribution for
131I-anti-NRP-2 mAb in A549 xenogratfs between 0
µg (unblock) and 100 µg (block).
Organ
(%ID/g)
(Spiked dose) |
131I-anti-NRP-2 mAb (48 h)
|
|---|
0
µg
(unblock) | 100
µg
(block) |
|---|
| Tumor | 5.12±0.71a | 2.13±0.09a |
| Heart | 2.54±0.31 | 2.33±0.40 |
| Liver | 2.90±0.73 | 2.10±0.03 |
| Spleen | 1.92±0.31 | 1.63±0.01 |
| Lung | 2.22±0.08a | 1.28±0.03a |
| Kidney | 2.65±0.53 | 2.37±0.04 |
| Stomach | 1.31±0.41 | 1.21±0.06 |
| Bowel | 1.58±0.24 | 1.41±0.18 |
| Bone | 1.85±0.43 | 1.79±0.01 |
| Muscle | 1.54±0.15 | 1.29±0.05 |
| Thyroid | 1.15±0.17 | 1.14±0.05 |
| Blood | 5.97±0.34 | 4.68±0.45 |
| Uptake ratio | | |
| Tumor to blood | 0.86±0.07a | 0.43±0.02a |
| Tumor to
Muscle | 3.32±0.27a | 1.66±0.01a |
For in vivo blocking study (Table II), 131I-anti-NRP-2 mAb
was coinjected with a large excess (100 µg) of the unlabeled
anti-NRP-2 mAb to saturate endogenous and overexpressed NRP-2. The
co-injection of anti-NRP-2 mAb reduced the uptake in several
tissues including liver, kidneys, lung, heart and tumor.
SPECT imaging
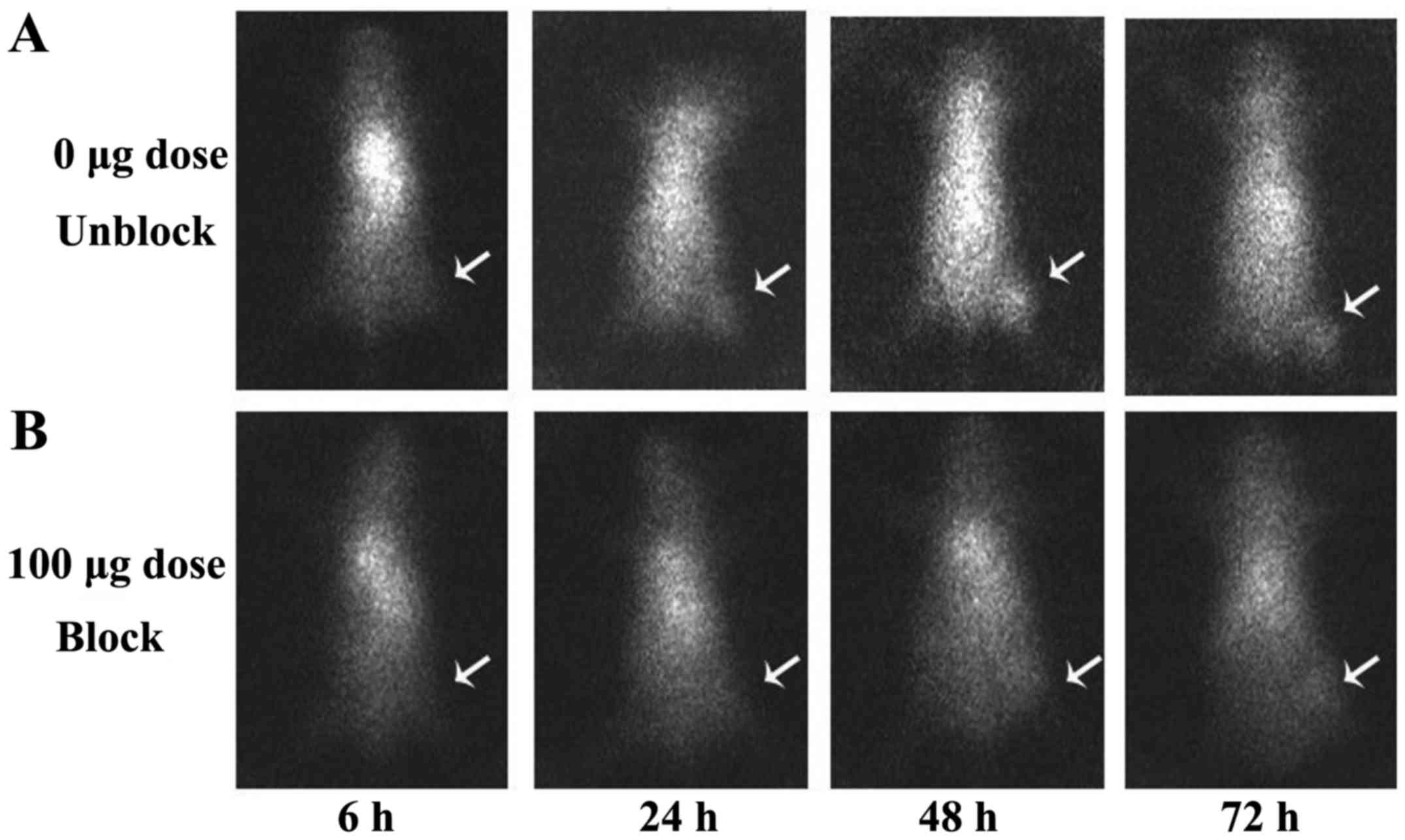
SPECT images acquired at 6, 24, 48 and 72 h after
injection of 131I-anti-NRP-2 mAb are shown in Fig. 10A. 131I-anti-NRP-2 mAb
accumulated in the A549 tumor at 6 h and then showed a gradual
increase of uptake. During 24–72 h after injection, A549 tumors
were clearly visible, with good tumor to background contrast. When
co-injected with unlabeled anti-NRP-2 mAb (100 µg), the
tumor was barely visible on SPECT images at 6–72 h after injection
(Fig. 10B). Regions of interest
(ROIs) analysis of SPECT showed lower ratio of tumor to
contralateral muscle (T/NT) for mice injected with 100 µg
blocking dose compared to unblocking dose at 6–72 h post-injection
(Fig. 9B), especially, at 48 and
72 h after injection (P<0.05).
Discussion
Neuropilin-2 (NRP-2), originally recognized by Chen
et al (31) in 1997, is a
receptor for the secreted semaphorin Sema IV and acts selectively
to mediate repulsive guidance events in discrete populations of
neurons. Subsequently, NRP-2 was found to be expressed in venous
and lymphatic endothelial cells and, upon ligand stimulation (such
as semaphorin 3F, VEGF-C and VEGF-A), induces neural development
and the growth of newly formed blood and lymphatic vessels, and
increases survival and migration of vascular and lymphatic
endothelial cells (12,14). Interestingly, NRP-2 is also
expressed in several human tumor cells and tissues, and is involved
in tumor progression and metastasis (15–19).
Recent evidence suggests that NRP-2 is important for therapy
resistance by activating autophagy in different cancer types,
including bladder cancer (21),
prostate cancer (32) and
pancreatic cancer (17),
indicating that NRP-2 expression is a predictive marker for the
outcome of cancer patients. Furthermore, inhibition of NRP-2
expression thus appears to be a promising approach for cancer
therapy. Several NRP-2 targeting strategies, such as small
interfering RNA (siRNA) (19),
monoclonal antibodies (22) and
small-molecule peptides (33),
have shown some advantages, including inhibition of tumor
lymphangiogenesis and angiogenesis, thereby restricting tumor
metastasis and progression. Patients with cancer lesions that
express NRP-2 may benefit from NRP-2 targeted therapy. Clinical
trials have shown that there is an urgent unmet clinical need for
the development of predictive biomarkers permitting patient
selection for such therapy. Non-invasive molecular imaging,
including SPECT imaging, is an ideal method, since it can offer a
more accurate and real-time assay of NRP-2 expression, without such
biopsy-associated pitfalls and the need of repetitive invasive
biopsies. Therefore, to develop an imaging agent for NRP-2
expression is significantly important since our goal is to
ultimately apply antibody-based SPECT probes for imaging
patients.
The present study investigated the expression of
NRP-2 in the human lung cancer cells. Consistent with previous
studies (15,34), it was found that human lung
adenocarcinoma A549 cells and tumor xenografts of A549 cells
exhibited higher levels of NRP-2 expression than normal lung
tissue. Nasarre et al (15)
reported that in lung cancer cells, NRP-2 is upregulated and
contributes significantly to TGF-β-mediated epithelial-mesenchymal
transition (EMT), which is fundamental process involved in tumor
cell invasion and metastasis. In addition, in agreement with the
report by Tomizawa et al (35), NRP-2 expression in H1299 cells is
lower than that in 16HBE cells (human normal bronchial epithelial
cells) since H1299 cells, derived from a lymph node metastasis of
the lung from a patient who had received prior radiation therapy,
have a homozygous partial deletion of the p53 gene, and lack
expression of p53 protein, but can produce neuromedin B. These
results demonstrated that NRP-2 may be a novel molecular target for
lung adenocarcinoma diagnosis and therapy.
Due to their highly specific targeting ability,
monoclonal antibodies (mAbs) have been considered attractive
candidates for targeted therapy and diagnostics in a broad range of
medical indications, but especially in oncology (36). Our previous studies showed that a
novel anti-NRP-2 mAb, developed by our laboratory, can inhibit
tumor proliferation, growth, and migration (unpublished data),
indicating that anti-NRP-2 mAb may be an effective agent for
NRP-2-targeted imaging and therapy. To further study anti-NRP-2 mAb
imaging performance in targeting NRP-2, herein, we re-generated
anti-NRP-2 mAb by hybridoma. This anti-NRP-2 mAb is confirmed to
specifically bind to NRP-2 b1b2 domain and NRP-2 receptor on the
surface of the A549 cells, but not NRP-1 b1b2 domain (Figs. 2, 3 and 5B), consistent with previous studies
(22). SDS-PAGE (Fig. 4) indicated the successful
production and purification (>95%) of anti-NRP-2 mAb sufficient
for in vitro and in vivo cancer research.
Anti-NRP-2 mAb was labeled with 131I by
the chloramine-T method, and then measured the binding specificity
and affinity to NRP-2. The probe 131I-anti-NRP-2 mAb
showed high binding affinity to the A549 cell NRP-2 with an
IC50 of 96.6±1.44 nM (Fig.
7B), in agreement with the study by Parker and Vander Kooi
(33). In vitro cell uptake
experiments showed that 131I-anti-NRP-2 had rapid
accumulation in the A549 cells, and reached the highest value of
6.60±0.36% of applied activity at 180 min (Fig. 7A). This accumulation is NRP-2
specific receptor binding since the rapid cellular uptake of the
tracer could be effectively blocked by cold anti-NRP-2 mAb
(Fig. 7A), suggesting that
labeling has not influenced the ability of anti-NRP-2 mAb to bind
specifically to NRP-2. These results warranted further evaluation
of the probe for in vivo NRP-2-targeted tumor imaging.
131I-anti-NRP-2 mAb was also evaluated as
a specific targeted radiotracer in A549 xenograft-bearing mouse
models. The biodistribution data showed that
131I-anti-NRP-2 mAb had a high tumor uptake, retention,
and tumor to contralateral muscle ratio (T/NT) (Figs. 8 and 9). In addition, SPECT showed that the
radioactive accumu lation in the tumor site became visible from 6 h
post-injection, and increased continually. Evaluation of the probe
in mice demonstrated that 131I-anti-NRP-2 mAb is a
promising agent for NRP-2 imaging.
In this study, the liver, lung, spleen, blood and
kidney showed high uptake at 6 h after administration.
131I-anti-NRP-2 mAb was enriched more in the lung, liver
and kidney, because of the moderate natural expression of NRP-1 in
the lung and liver (37) and mAb
metabolism through the liver and kidney. The high level of
131I-anti-NRP-2 mAb in the blood and spleen is also
possibly due to long circulating mAbs. Whereas, at 48 and 72 h
after injection of 131I-anti-NRP-2 mAb, with
131I-anti-NRP-2 mAb clearance from blood, the level of
the tumor uptake still remained higher than that in the other
organs including the liver and blood. A moderate expression of the
target in normal organ may appreciable influence the imaging
results, especially when the target level in the tumor is low.
After optimization of spiking doses to saturate the target
expression in normal organ, increased tumor-normal ratio could be
achieved (28,29). In the study, the in vivo
NRP-2 binding specificity of 131I-anti-NRP-2 mAb was
also verified. When 100 µg of unlabeled anti-NRP-2 mAb was
co-injected, uptakes in NRP-2 expression organs/tissues, such as
the tumor, lung, and liver, were obviously reduced (Fig. 10B).
However, the characteristics of
131I-monoclonal antibody, low tumor accumulation, slow
clearance from the circulation, and high energy iodine-131, may
hamper its clinical applications. We have currently undertaken
studies to improve these parameters. For example, the antibody
fragments or anti-NRP-2 Affibody molecules and the way of labeling
with low energy 99mTc or 111In could increase
rapidly NRP-2-positive tumor targeting ability and gain high
imaging contrast within a short period after injection.
Imaging of NRP-2 expression in vivo may be
not only be of value for treatment optimization of cancer patients,
but also useful for identifying the sensitivity to chemotherapy in
the patients with pancreatic cancer, bladder cancer, prostate
cancer and lung cancer, that are resistant to chemotherapy,
radio-chemotherapy, or radiotherapy through this mechanism, because
NRP-2 overexpression increases VEGF-C/NRP-2 axis and
β-catenin-dependent signaling. VEGF-C/NRP-2 axis induces autophagy
to cancer cells from chemotherapeutic stress, and is also important
to maintain an anti-apoptotic program in cancer cells during
oxidative stress (21,32). Samuel et al (14) reported that knockdown of NRP-2 by
an anti-NRP-2 antibody sensitized gastrointestinal cancer cells in
5-fluorouracil (5-FU) toxicity via β-catenin-dependent signaling.
Thus, further research for imaging of NRP-2 expression has high
clinical translational ability and will likely find broad
application in patient therapy and management for targeting the
expression of NRP-2 and cross-talk between Wnt/β-catenin and NRP-2
signaling.
In conclusion, an anti-NRP-2 monoclonal antibody was
easily and successfully radiolabeled with iodine-131. The in
vitro and in vivo study showed the potential of
131I-anti-NRP-2 mAb as a promising SPECT probe for
imaging NRP-2-positive tumors and encouraged further investigation.
Nevertheless, since anti-NRP-2 mAb has a large molecular weight and
an immunoge nicity that may hinder its application in the clinic,
it remains a great challenge to explore a novel small fragment of
mAbs or Affibody molecules to improve imaging of
NRP-2-expression.
Acknowledgments
This study was supported by grants from the
National Natural Science Foundation of China (NSFC) (no. 81571707),
program for the Training Young Talents of Fujian Health (no.
2014-ZQN-ZD-35), the Natural Science Foundation of Fujian (no.
2015J01519), the Technology Foundation for Selected Overseas
Chinese Scholar, Ministry of Human Resources and Social Security of
China (no. 2014-240) and the Scientific Research Foundation for the
Returned Overseas Chinese Scholars, Ministry of Education of China
(no. 2014-1685).
References
|
1
|
Takagi S, Tsuji T, Amagai T, Takamatsu T
and Fujisawa H: Specific cell surface labels in the visual centers
of Xenopus laevis tadpole identified using monoclonal antibodies.
Dev Biol. 122:90–100. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raimondi C and Ruhrberg C: Neuropilin
signalling in vessels, neurons and tumours. Semin Cell Dev Biol.
24:172–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prud'homme GJ and Glinka Y: Neuropilins
are multifunctional coreceptors involved in tumor initiation,
growth, metastasis and immunity. Oncotarget. 3:921–939. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geretti E, Shimizu A and Klagsbrun M:
Neuropilin structure governs VEGF and semaphorin binding and
regulates angiogenesis. Angiogenesis. 11:31–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geretti E and Klagsbrun M: Neuropilins:
Novel targets for antiangiogenesis therapies. Cell Adhes Migr.
1:56–61. 2007. View Article : Google Scholar
|
|
6
|
Chaudhary B, Khaled YS, Ammori BJ and
Elkord E: Neuropilin 1: Function and therapeutic potential in
cancer. Cancer Immunol Immunother. 63:81–99. 2014. View Article : Google Scholar
|
|
7
|
Plein A, Fantin A and Ruhrberg C:
Neuropilin regulation of angiogenesis, arteriogenesis, and vascular
permeability. Microcirculation. 21:315–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sulpice E, Plouët J, Bergé M, Allanic D,
Tobelem G and Merkulova-Rainon T: Neuropilin-1 and neuropilin-2 act
as coreceptors, potentiating proangiogenic activity. Blood.
111:2036–2045. 2008. View Article : Google Scholar
|
|
9
|
Parker MW, Xu P, Li X and Vander Kooi CW:
Structural basis for selective vascular endothelial growth factor-A
(VEGF-A) binding toneuropilin-1. J Biol Chem. 287:11082–11089.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kärpänen T, Heckman CA, Keskitalo S,
Jeltsch M, Ollila H, Neufeld G, Tamagnone L and Alitalo K:
Functional interaction of VEGF-C and VEGF-D with neuropilin
receptors. FASEB J. 20:1462–1472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan L, Moyon D, Pardanaud L, Bréant C,
Karkkainen MJ, Alitalo K and Eichmann A: Abnormal lymphatic vessel
development in neuropilin 2 mutant mice. Development.
129:4797–4806. 2002.PubMed/NCBI
|
|
12
|
Zhang Y, Liu P, Jiang Y, Dou X, Yan J, Ma
C, Fan Q, Wang W, Su F, Tang H, et al: High expression of
neuropilin-1 associates with unfavorable clinicopathological
features in hepatocellular carcinoma. Pathol Oncol Res. 22:367–375.
2016. View Article : Google Scholar
|
|
13
|
Hey-Cunningham AJ, Markham R, Fraser IS
and Berbic M: Dysregulation of vascular endothelial growth factors
and their neuropilin receptors in the eutopic endometrium of women
with endometriosis. Reprod Sci. 20:1382–1389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samuel S, Gaur P, Fan F, Xia L, Gray MJ,
Dallas NA, Bose D, Rodriguez-Aguayo C, Lopez-Berestein G, Plowman
G, et al: Neuropilin-2 mediated β-catenin signaling and survival in
human gastro-intestinal cancer cell lines. PLoS One. 6:e232082011.
View Article : Google Scholar
|
|
15
|
Nasarre P, Gemmill RM, Potiron VA, Roche
J, Lu X, Barón AE, Korch C, Garrett-Mayer E, Lagana A, Howe PH, et
al: Neuropilin-2 Is upregulated in lung cancer cells during
TGF-β1-induced epithelial-mesenchymal transition. Cancer Res.
73:7111–7121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao Y, Hoeppner LH, Bach S, E G, Guo Y,
Wang E, Wu J, Cowley MJ, Chang DK, Waddell N, et al: Neuropilin-2
promotes extravasation and metastasis by interacting with
endothelial α5 integrin. Cancer Res. 73:4579–4590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dallas NA, Gray MJ, Xia L, Fan F, van
Buren G II, Gaur P, Samuel S, Lim SJ, Arumugam T, Ramachandran V,
et al: Neuropilin-2-mediated tumor growth and angiogenesis in
pancreatic adenocarcinoma. Clin Cancer Res. 14:8052–8060. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dutta S, Roy S, Polavaram NS, Baretton GB,
Muders MH, Batra S and Datta K: NRP2 transcriptionally regulates
its downstream effector WDFY1. Sci Rep. 6:235882016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gray MJ, Van Buren G, Dallas NA, Xia L,
Wang X, Yang AD, Somcio RJ, Lin YG, Lim S, Fan F, et al:
Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells
implanted in the murine liver. J Natl Cancer Inst. 100:109–120.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dutta S, Roy S, Polavaram NS, Stanton MJ,
Zhang H, Bhola T, Hönscheid P, Donohue TM Jr, Band H, Batra SK, et
al: Neuropilin-2 regulates endosome maturation and EGFR trafficking
to support cancer cell pathobiology. Cancer Res. 76:418–428. 2016.
View Article : Google Scholar :
|
|
21
|
Keck B, Wach S, Taubert H, Zeiler S, Ott
OJ, Kunath F, Hartmann A, Bertz S, Weiss C, Hönscheid P, et al:
Neuropilin-2 and its ligand VEGF-C predict treatment response after
transurethral resection and radiochemotherapy in bladder cancer
patients. Int J Cancer. 136:443–451. 2015. View Article : Google Scholar
|
|
22
|
Caunt M, Mak J, Liang WC, Stawicki S, Pan
Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, et al: Blocking
neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell.
13:331–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun H, England CG, Hernandez R, Graves SA,
Majewski RL, Kamkaew A, Jiang D, Barnhart TE, Yang Y and Cai W:
ImmunoPET for assessing the differential uptake of a CD146-specific
monoclonal antibody in lung cancer. Eur J Nucl Med Mol Imaging.
43:2169–2179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Chen N, Li Z, Wang XJ, Wang SY,
Tingwu, Luo FH and Yan JH: Preparation, purification, and
identification of a monoclonal antibody against NRP2 b1b2 domain.
Monoclon Antib Immunodiagn Immunother. 34:354–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su X, Chen Q, Chen W, Chen T, Li W, Li Y,
Dou X, Zhang Y, Shen Y, Wu H, et al: Mycoepoxydiene inhibits
activation of BV2 microglia stimulated by lipopolysaccharide
through suppressing NF-κB, ERK 1/2 and toll-like receptor pathways.
Int Immunopharmacol. 19:88–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dou X, Yan J, Zhang Y, Liu P, Jiang Y, Lv
S, Zeng F, Chen X, Wang S, Zhang H, et al: SPECT imaging of
neuropilin receptor type-1 expression with 131I-labeled
monoclonal antibody. Int J Oncol. 49:961–970. 2016.PubMed/NCBI
|
|
27
|
Guo Z, Gao M, Zhang D, Li Y, Song M,
Zhuang R, Su X, Chen G, Liu T, Liu P, et al: Simultaneous SPECT
imaging of multi-targets to assist in identifying hepatic lesions.
Sci Rep. 6:288122016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su X, Cheng K, Liu Y, Hu X, Meng S and
Cheng Z: PET imaging of insulin-like growth factor type 1 receptor
expression with a 64Cu-labeled Affibody molecule. Amino Acids.
47:1409–1419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su X, Cheng K, Jeon J, Shen B, Venturin
GT, Hu X, Rao J, Chin FT, Wu H and Cheng Z: Comparison of two
site-specifically (18)F-labeled affibodies for PET imaging of EGFR
positive tumors. Mol Pharm. 11:3947–3956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo Z, Gao M, Song M, Shi C, Zhang P, Xu
D, You L, Zhuang R, Su X, Liu T, et al: Synthesis and evaluation of
(99m)Tc-labeled dimeric folic acid for FR-targeting. Molecules.
21:E8172016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen H, Chédotal A, He Z, Goodman CS and
Tessier-Lavigne M: Neuropilin-2, a novel member of the neuropilin
family, is a high affinity receptor for the semaphorins Sema E and
Sema IV but not Sema III. Neuron. 19:547–559. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stanton MJ, Dutta S, Zhang H, Polavaram
NS, Leontovich AA, Hönscheid P, Sinicrope FA, Tindall DJ, Muders MH
and Datta K: Autophagy control by the VEGF-C/NRP-2 axis in cancer
and its implication for treatment resistance. Cancer Res.
73:160–171. 2013. View Article : Google Scholar :
|
|
33
|
Parker MW and Vander Kooi CW:
Microplate-based screening for small molecule inhibitors of
neuropilin-2/vascular endothelial growth factor-C interactions.
Anal Biochem. 453:4–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jubb AM, Sa SM, Ratti N, Strickland LA,
Schmidt M, Callahan CA and Koeppen H: Neuropilin-2 expression in
cancer. Histopathology. 61:340–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tomizawa Y, Sekido Y, Kondo M, Gao B,
Yokota J, Roche J, Drabkin H, Lerman MI, Gazdar AF and Minna JD:
Inhibition of lung cancer cell growth and induction of apoptosis
after reexpression of 3p21.3 candidate tumor suppressor gene
SEMA3B. Proc Natl Acad Sci USA. 98:13954–13959. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khan AH and Sadroddiny E: Licensed
monoclonal antibodies and associated challenges. Hum Antibodies.
23:63–72. 2015. View Article : Google Scholar
|
|
37
|
Aung NY, Ohe R, Meng H, Kabasawa T, Yang
S, Kato T and Yamakawa M: Specific neuropilins expression in
alveolar macrophages among tissue-specific macrophages. PLoS One.
11:e01473582016. View Article : Google Scholar : PubMed/NCBI
|