Introduction
Gastric adenocarcinoma has one of the highest
prevalence and mortality rates among malignant tumors (1). Global incidence of primary tumor
locations and the histological types are constantly changing: in
United States and in Western Europe the incidence of Barrett's type
carcinoma and gastric cardia adenocarcinoma is increasing (2), while there has been a reduction of
incidence of distal gastric adenocarcinoma since the 1970s
(3). Although gastric
adenocarcinoma mortality has been reduced, it remains a disease
with poor prognosis and high mortality, second only to lung tumor
in the world. The prognosis of gastric adenocarcinoma depends on
stage and signature metastasis type of peritoneal dissemination
have poor prognosis and when the disease is confined to the stomach
mucosa, 5-year survival is ~95%, while the reported 5-year survival
rate for advanced gastric adenocarcinoma with peritoneal
dissemination varies from 10 to 20% (3).
Peritoneal dissemination of gastric adenocarcinoma
is particularly frequent, driving the need for therapies that
minimize the cancer spread. One model for the mechanism of
peritoneal metastasis of gastric adenocarcinoma involves growth of
tumor at the primary lesion, invasion into serous surface, expose
abdominal cavity, dissociation of cancer cells, and implantation
and growth on the peritoneum. Various genetic alterations have been
studied, and involvement of a number of genes has been
reported.
MicroRNA (miRNA) is a small non-coding RNA that
functions in RNAs silencing and post-transcriptional regulation of
gene expression (4–6). These are the fundamental gene
regulators that control proliferation, differentiation, and
apoptosis during development. They target a large number of mRNAs
and induce mRNA degradation or inhibition of translation by
targeting the 30-untranslated regions (5). Some miRNAs had controlled the
peritoneal dissemination of gastric adenocarcinoma (7–9).
miR-27 promoted epithelial-mesenchymal transition (EMT) and
metastasis of human gastric cancer cells (10). EMT is a portmanteau concept that
can be applied to the metastatic behavior of carcinoma cells at a
number of junctures, and has been associated with peritoneal
dissemination of gastric adenocarcinoma (11). Various genetic alterations
associated with EMT have been studied, and involvement of a number
of genes has been reported. Ribopholin II (RPN2) is one of the
genes that promote EMT through the stabilization of mutant p53
(12), and there is a possibility
that miRNA is controlling RPN2. However, in gastric adenocarcinoma,
no report exists on the relation between RPN2 and metastasis and
prognosis of gastric adenocarcinoma.
This study was conducted to compare the expression
of RPN2 with the invasiveness of the primary lesion in human
gastric adenocarcinoma, and the clinicopathological features and
patient prognosis, as reported below.
Materials and methods
Patients and samples
Surgical specimens and adjacent normal gastric
tissues were obtained from 242 patients with sporadic primary
gastric adenocarcinoma, and surgically resected in the Department
of First Surgery, University of Fukui, Japan, between 2002 and
2010. As histopathological findings varied within the same tumors,
the diagnosis was based upon the dominant pattern evaluated by two
pathologists. All samples were fixed in 10% paraformaldehyde (pH
6.8) for 24 h, and embedded in paraffin.
The eligibility criteria were as follows: i)
histopathologically confirmed primary gastric adenocarcinoma, ii)
resection of gastric adenocarcinoma with extended (D1+ or D2) lymph
node dissection (13), iii)
pathological diagnosis for the classification used the 7th edition
UICC TNM classification, iv) histological curative resection (stage
I–III), v) an Eastern Cooperative Oncology Group performance status
of 0 or 1, vi) no chemotherapy or radiotherapy before surgical
resection, vii) patients with stage II/III received S-1 after
surgical resection, viii) patients with stage IV received S-1 +
cisplatin after surgical resection, ix) patients with stage I
received no chemotherapy after surgical resection, and x) all
patients were followed up for recurrence at regular intervals for
five years, underwent chest X-ray, computed tomography, and testing
of gastrointestinal fiber. All the patients provided written
informed consent before the samples were collected.
Ethical approval
We attest that the research was performed in
accordance with the humane and ethical rules for human
experimentation that are stated in the Helsinki Declaration of 1964
and latest version. The procedures of our study received ethical
approval with Institutional Committee Responsible for Human
Experimentation at University of Fukui and all those who
participated in our study did so voluntary, having given their
informed consent.
Immunohistochemical study
Paraffin sections, 4-µm thick, were
de-paraffinized with xylene and dehydrate through a grade ethanol
series. Endogenous peroxidase activity was blocked by incubation
for 30 min with 1% hydrogen peroxidase methanol. These hydrate
sections were incubated in a dilution of skim milk powder for 30
min to reduce nonspecific staining, and incubated overnight with
anti-RPN2 Ab (Aviva Systems Biology, CA, USA) or anti-p53 Ab (Dako,
Denmark) at 4°C in a humidified chamber. After washing with
Tris-buffered saline (TBS) buffer, and analyzed for the expression
of RPN2 protein or p53 protein by the ChemMate method (Dako). The
sections were developed with activated
3′-diaminobenzidinetetrahydrochloride for 5 min and the reaction
was stopped in TBS. Finally, the slides were lightly counterstained
with hematoxylin. The expression was interpreted as positive when
the protein was expressed in >20% of the cancer cells using
ImageJ software (http://rsb.info.nih.gov/ij/).
Statistical analysis
Statistically significant differences in
clinicopathological findings were assessed by cross-tabulation, and
statistical evaluations were determined by the χ2 test
using SPSS software (IBM SPSS Statistics, IBM Corp., USA).
Patient survival was calculated using the
Kaplan-Meier technique. The outcomes from different groups of
patients were compared by log-rank test using SPSS software. The
Cox proportional hazards model was used in multivariate regression
analysis of survival data using SPSS software. P-values <0.05
were considered statistically significant.
Cell culture and RT-PCR analysis
Human gastric cancer cell lines MKN45, MKN74, NUGC3,
NUGC4, SNU5, KATO III and TMK1 (which were obtained from JCRB Cell
Bank) were maintained in RPMI-1640 supplemented with 10% FBS
(Gibco), 100 U/ml penicillin, and 100 µg/ml streptomycin at
37°C with 5% CO2 incubation. RNA was isolated from the
above cells using Isogen (Wako, Japan), according to the
manufacturer's instructions. cDNA was synthesized from 2 µg
of total RNA by reverse transcription, using High-Capacity cDNA
Reverse Transcription kits (Applied Biosystems). The resulting cDNA
was used for the subsequent PCR assays. RPN2 was amplified by using
the primers with following sequences: forward,
5′-GCCAGGAAGTGGTGTTTGTT-3′; and reverse,
5′-ACAGAGCGAAGAGCAGAAGC-3′.
Cell transfection
MKN74 and KATO III cells were seeded onto 6-well
plates (Corning) 24 h before transfection. Cells were transfected
using Lipofectamine 3000 (Life Technologies) at 80–90% confluency
according to the manufacturer's instructions. For each well of a
6-well plate, a total of 1 µg of sgRPN2 (single-guided RNA
to RPN2) + pGuide-it-ZsGreen1 plasmid or empty plasmid was used.
The CRISPR/Cas9 system used in this study was Guide-it CRISPR/Cas9
systems (Clonetch). We designed sequences targeting the RPN2
locus (Fig. 4A). The plasmid
enhanced green fluorescent protein (eGFP) and was used as a
fluorescent marker to sort transfected cells. Forty-eight hours
posttransfection, cells were pelleted in PBS + 2% FBS and sorted in
96-well plates using fluorescence-activated cell sorting (FACS)
with a FACSAria II cell sorter (BD BioSciences). Single cells from
two populations of GFP-expressing cells (high expression and medium
expression) were expanded to obtain individual clones.
Detection of nuclease-induced
mutations
Genomic DNA was extracted from cells with the
Multisource Genomic DNA Miniprep kit (Axygen), and then amplified
by PCR with forward primer 5′-GCCAGGAAGTGGTGTTTGTT-3′ and reverse
primer 5′-ACAGAGCGAAGAGCAGAAGC-3′. The PCR product was resolved in
1.5% agarose gel. Target bands were cut and purified with QIAEX II
Gel Extraction kit (Qiagen). The purified PCR products were mixed
with 2 µl 10X T7E1 nuclease buffer and nuclease-free water
to a volume of 19 µl. These products were denatured for 10
min at 95°C, annealed by gradual cooling in a thermocycler and
digested by 1 µl T7E1 nuclease (GeneCopoeia). The digestion
was performed at 37°C, in a water bath for 40 min, and followed by
analyzing in 1.5% agarose gel. Briefly, the gel was imaged and the
intensity of the bands in each lane was measured by using ImageJ
Software. For each lane, we calculated the fraction of the PCR
product cleaved by using the following formula: fcut =
(B + C)/(A + B + C), where
A is the intensity of the undigested PCR product, while
B and C are the intensities of each cleaved band. The
Indel percentage was estimated by applying the following formula:
Indel (%) = 100 × [1 − √(1−fcut)].
Transfection of small interfering
RNA
Small interfering RNA (siRNA) against RPN2 and
control non-targeting siRNA were obtained from Invitrogen, Inc.
(CA, USA). The non-silencing control siRNA, which has no sequence
homology to any known human gene sequence, was used as a control
for nonspecific effects in all experiments. Subconfluent MKN45
cells were transfected with siRNA using Lipofectamine 3000
transfection regent (Life Technologies) according to the
manufacturer's instructions. Two days after transfection, the
efficacy of siRNA knockdown was assessed using western blot
analysis.
Western blot analysis
Total cell protein was extracted using RIPA buffer.
Proteins in the lysate were resolved by SDS-PAGE using a 5–20%
SuperSep gel (Wako). The resolved proteins were transferred to
nitrocellulose membrane. Protein bands were incubated with primary
antibody overnight at 4°C. Signals were visualized by enhanced
chemiluminescence according to the manufacturer's instructions (GE
Healthcare). Anti-RPN2 Ab was from Aviva Systems and anti-GAPDH Ab
was from IMGENEX.
Cell invasion assay
This assay was performed using a cell invasion kit
from Cell Biolabs, Inc. (CA, USA). Briefly, the invasion chambers
were warmed up at room temperature for 10 min, and the basement
membrane layer was rehydrated with 300 µl of warm serum-free
media for 1 h at room temperature. After removing the rehydration
medium from the inserts, 300 µl of 1.0×106
cells/ml in serum-free media was added to the inside of each
insert, and 500 µl of media containing 10% FBS was added to
the lower well of the invasion plate. The plate was then incubated
at 5% CO2 for 24 h. The media was aspirated from the
inside of the insert and the non-invasive cells were removed. The
inserts were then transferred to clean wells containing 400
µl of cell staining solution from the kit and were incubated
for 10 min at room temperature. The stained inserts were washed
several times with water and were air-dried. Inserts were
transferred to new wells with 200 µl of extraction solution
from the kit and were incubated for 10 min with rotation. A volume
of 100 µl solution was used to measure the OD 560 nm in a
spectrometer.
Results
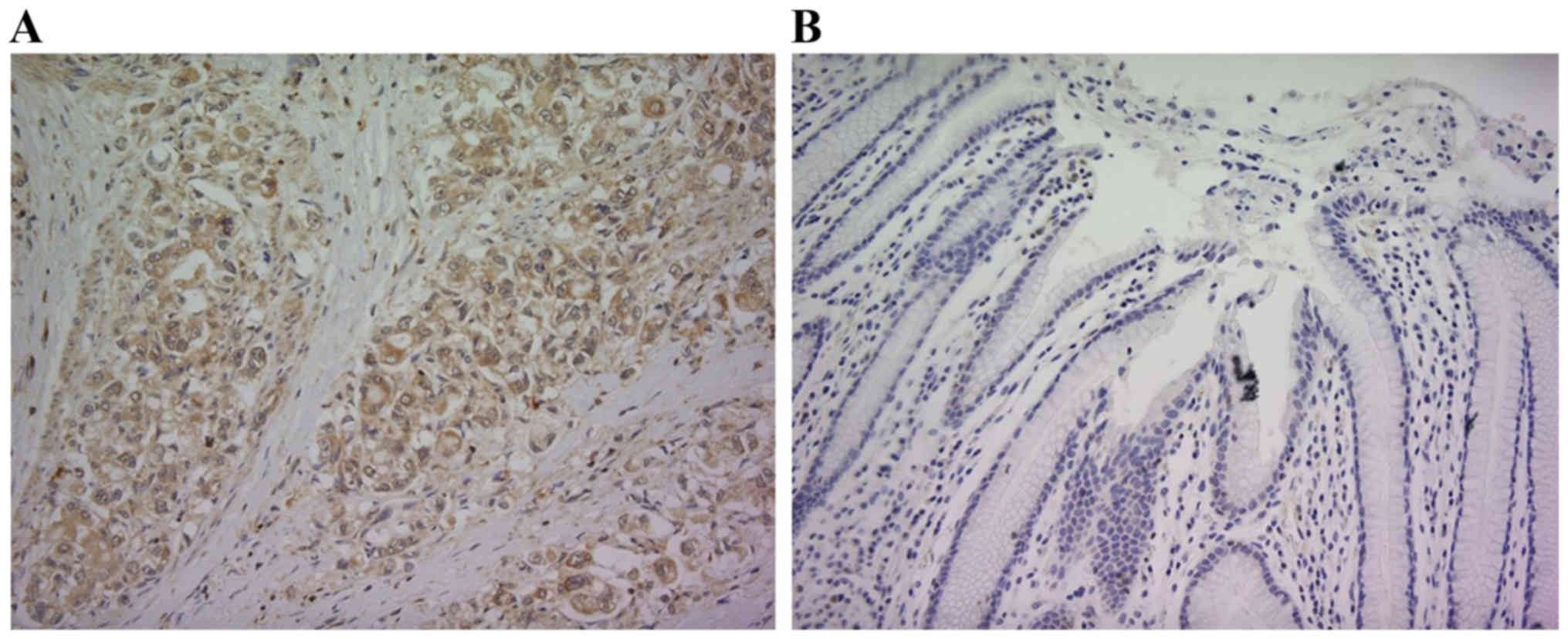
RPN2 expression in human gastric
adenocarcinoma
Although RPN2 expression was not observed in the
normal gastric mucosal membrane adjacent to human gastric
adenocarcinoma, its expression was intense in the primary lesion of
gastric adenocarcinoma. Fig. 1
shows a respective representative case. RPN2 expression was
observed in the cytoplasm. RPN2 expression was observed in 119
(49.2%) of 242 gastric adenocarcinoma patients.
RPN2 expression and clinicopathological
factors in human gastric adenocarcinoma tissue
No relationships could be detected between RPN2
expression and the clinicopathological factors gender, histological
type, and liver metastasis. However, expression was significantly
higher in the cases with depth of wall invasion, lymph node
metastasis, lymphatic invasion, venous invasion, peritoneal
dissemination, and pathological stage (Table I).
 | Table ICorrelation of RPN2 expression and
clinicopathological findings. |
Table I
Correlation of RPN2 expression and
clinicopathological findings.
| No. of cases | RPN2-positive
| P-value |
|---|
| No. of cases (%) |
|---|
| All cases | 242 | 119 (49.2) | |
| Gender | | | 0.236 |
| Male | 162 | 84 (51.9) | |
| Female | 80 | 35 (43.8) | |
| Histological
type | | | 0.059 |
| Pap, tub | 128 | 56 (43.8) | |
| Por, sig | 111 | 60 (54.1) | |
| Muc | 3 | 3 (100) | |
| Depth of wall
invasion | | | <0.001 |
| T1 | 79 | 13 (16.5) | |
| T2 | 30 | 17 (56.7) | |
| T3 | 26 | 15 (57.7) | |
| T4 | 107 | 74 (69.2) | |
| Lymph node
metastasis | | | <0.001 |
| N0 | 95 | 24 (25.3) | |
| N1-3 | 147 | 95 (64.6) | |
| Lymphatic
invasion | | | <0.001 |
| Negative | 59 | 6 (10.2) | |
| Positive | 181 | 112 (61.9) | |
| Venous
invasion | | | <0.001 |
| Negative | 81 | 13 (16.0) | |
| Positive | 159 | 105 (66.0) | |
| Liver
metastasis | | | 0.178 |
| Negative | 232 | 112 (48.2) | |
| Positive | 10 | 7 (7.0) | |
| Peritoneal
dissemination | | | <0.001 |
| Negative | 229 | 107 (46.7) | |
| Positive | 13 | 12 (92.3) | |
| Stage | | | <0.001 |
| I | 87 | 17 (19.5) | |
| II | 41 | 22 (53.7) | |
| III | 59 | 33 (55.9) | |
| IV | 55 | 47 (85.5) | |
Relationship between RPN2 expression and
the histological stage of gastric adenocarcinoma
RPN2 expression was found in 17 (19.5%) of 87 stage
IA and IB gastric adenocarcinoma patients, 22 (53.7%) of 41 stage
IIA and IIB patients, 33 (55.9%) of 59 stage IIIA, IIIB, and IIIC
patients, and 47 (85.5%) of 55 stage IV patients, indicating that
the expression rate goes up with the advancement of stage (Table I).
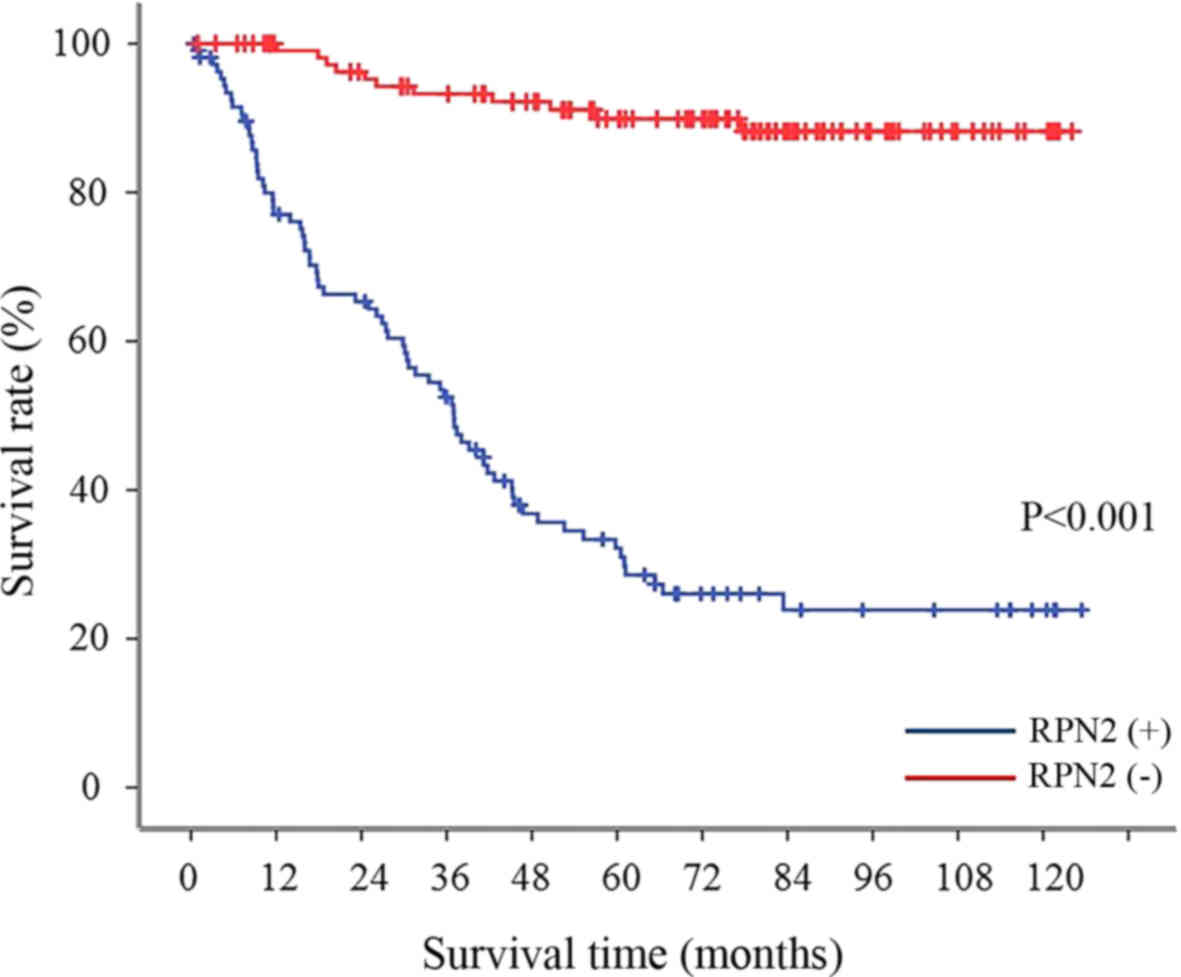
Relationship between RPN2 expression and
survival rate in all gastric adenocarcinoma patients
The 5-year survival rate was 89.9% in the gastric
adenocarcinoma with no RPN2 expression in the primary lesion;
whereas the 5-year survival rate of patients with RPN2 expression
was significantly lower (29.8%) (Fig.
2).
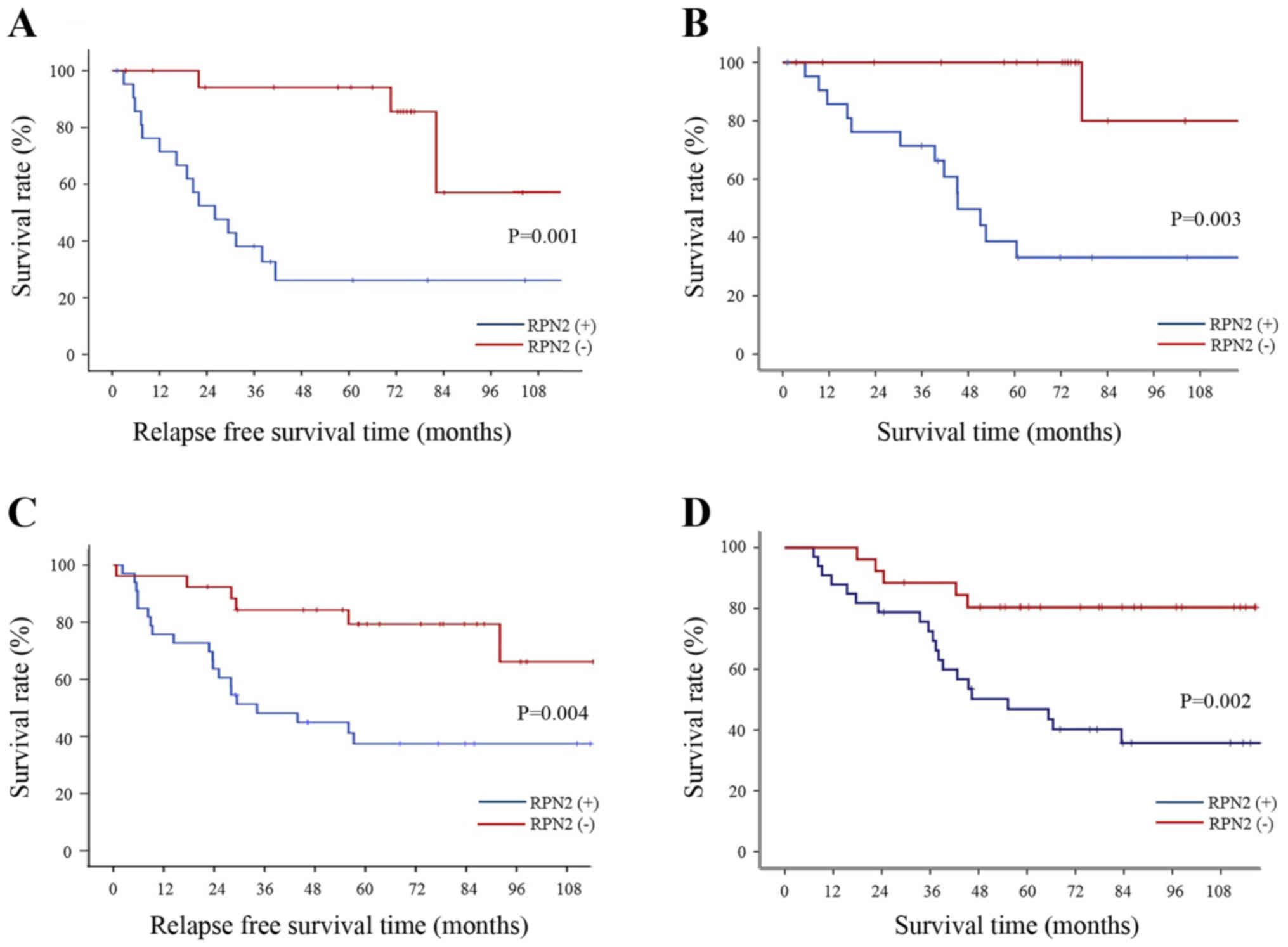
Relationship between RPN2 expression and
recurrence rate by stage of gastric adenocarcinoma
The tumor recurrence rate among patients with stage
II and III gastric adenocarcinoma was significantly higher for
patients with RPN2 expression in the primary lesion compared to
patients who did not express RPN2 (Fig. 3A and C). Also, the 5-year survival
rate for stage II and III gastric adenocarcinoma patients with RPN2
expression-negative primary tumor was 100 and 80.4%, whereas it was
38.7 and 46.9% for patients with RPN2 expression-positive tumors
(Fig. 3B and D). Among patients
with stage I gastric adenocarcinoma, no significant difference was
observed in the recurrence rate between patients who expressed RPN2
in the primary lesion and those who did not (data not shown).
Clinicopathologic prognostic factors
based on multivariate analysis
The factors found to differ significantly between
RPN2-expressing and non-expressing patients by univariate analysis
were examined by multivariate analysis. Histological type,
peritoneal dissemination and RPN2 expression were determined to be
clinicopathologic prognostic factors. The risk rate for RPN2
expression is shown in Table
II.
 | Table IIPathological findings and RPN2 as
prognostic factor for gastric cancer patients. |
Table II
Pathological findings and RPN2 as
prognostic factor for gastric cancer patients.
| Univariate analysis
| Multivariate
analysis
|
|---|
| Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Gender | 1.155
(0.746–1.788) | 0.519 | | |
| RPN2 | 10.540
(5.849–18.991) | <0.001 | 5.509
(2.959–10.258) | <0.001 |
| Histological
type | 1.862
(1.236–2.804) | 0.03 | 1.935
(1.236–3.030) | 0.004 |
| Serosal
invasion | 3.442
(2.237–5.295) | <0.001 | 1.234
(0.759–2.007) | 0.397 |
| Lymphatic
invasion | 20.478
(5.040–83.200) | <0.001 | 2.118
(0.369–12.173) | 0.400 |
| Venous
invasion | 10.590
(4.623–24.258) | <0.001 | 2.328
(0.826–6.547) | 0.109 |
| Lymph node
invasion | 5.332
(2.963–9.594) | <0.001 | 1.908
(1.021–3.567) | 0.043 |
| Peritoneal
dissemination | 5.309
(2.645–10.656) | <0.001 | 4.957
(2.366–10.382) | <0.001 |
| Liver
metastasis | 5.316
(2.876–9.825) | <0.001 | 1.520
(0.772–2.993) | <0.225 |
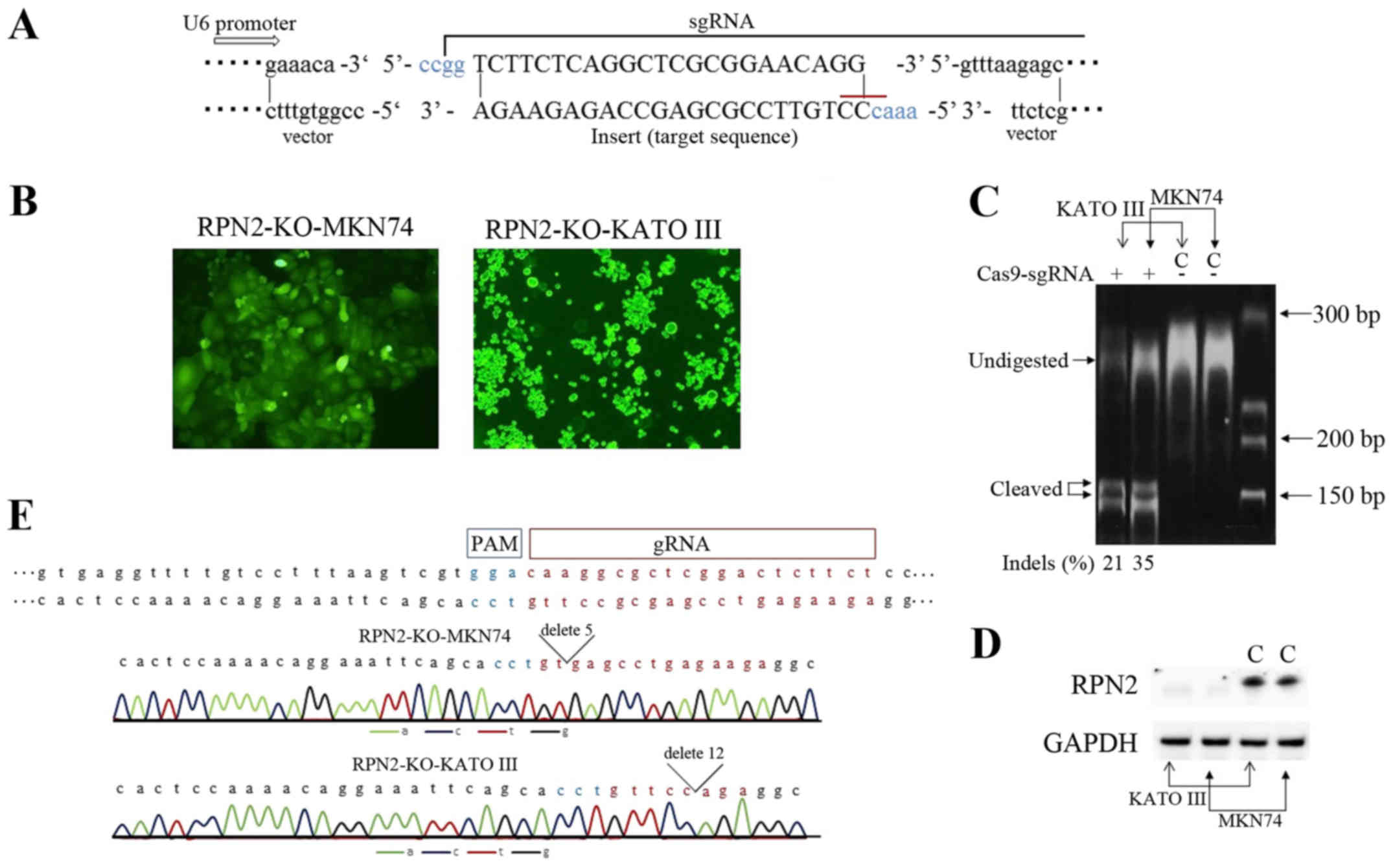
Generation of RPN2 knockout cell lines by
CRISPR/Cas-mediated genome editing
In human gastric cancer cell lines, MKN45, MKN 74,
KATO III and NUGC3 cells RPN2 expression was detected by RT-PCR
methods (data not shown). For human gastric adenocarcinoma cell
lines, MKN74 and KATO III, deficiency for RPN2 gene developed. We
used the CRISPR/Cas9 technology to knockout RPN2 gene. Transformed
and control cells for RPN2-KO-MKN74 (i.e., MKN74 transfected with a
sgRPN2-expressing vector) RPN2-KO-KATO III (i.e., KATO III
transfected with a sgRPN2-expressing vector) and control MKN74 and
KATO III (empty vector transfected cells) were sorted based on the
positive expression of the GFP reporter gene (Fig. 4B). RPN2 knockout was validated
using the T7E1 assay. One guide used was efficient in knocking-out
RPN2 gene in gastric adenocarcinoma cell lines. The Indel
percentage from T7E1 assay was estimated at 21 and 35%, for RPN2-KO
KATO III and RPN2-KO MKN74 cells (Fig.
4C). Moreover, protein extracts were analyzed for RPN2
expression. The significant expression of RPN2 was undetected both
in RPN2-KO MKN74 and KATO III cells, compared to respective intense
bands in empty vector-transfected cells (Fig. 4D). We showed the Sanger-sequence
data of RPN2-KO-MKN74 and RPN2-KO-KATO III cells to prove
CRISPR/Cas9 worked correctly (Fig.
4E). Chromatogram traces of RPN2-KO-MKN74 cells and
RPN2-KO-KATO III cells showed the sequence of deletion
mutations.
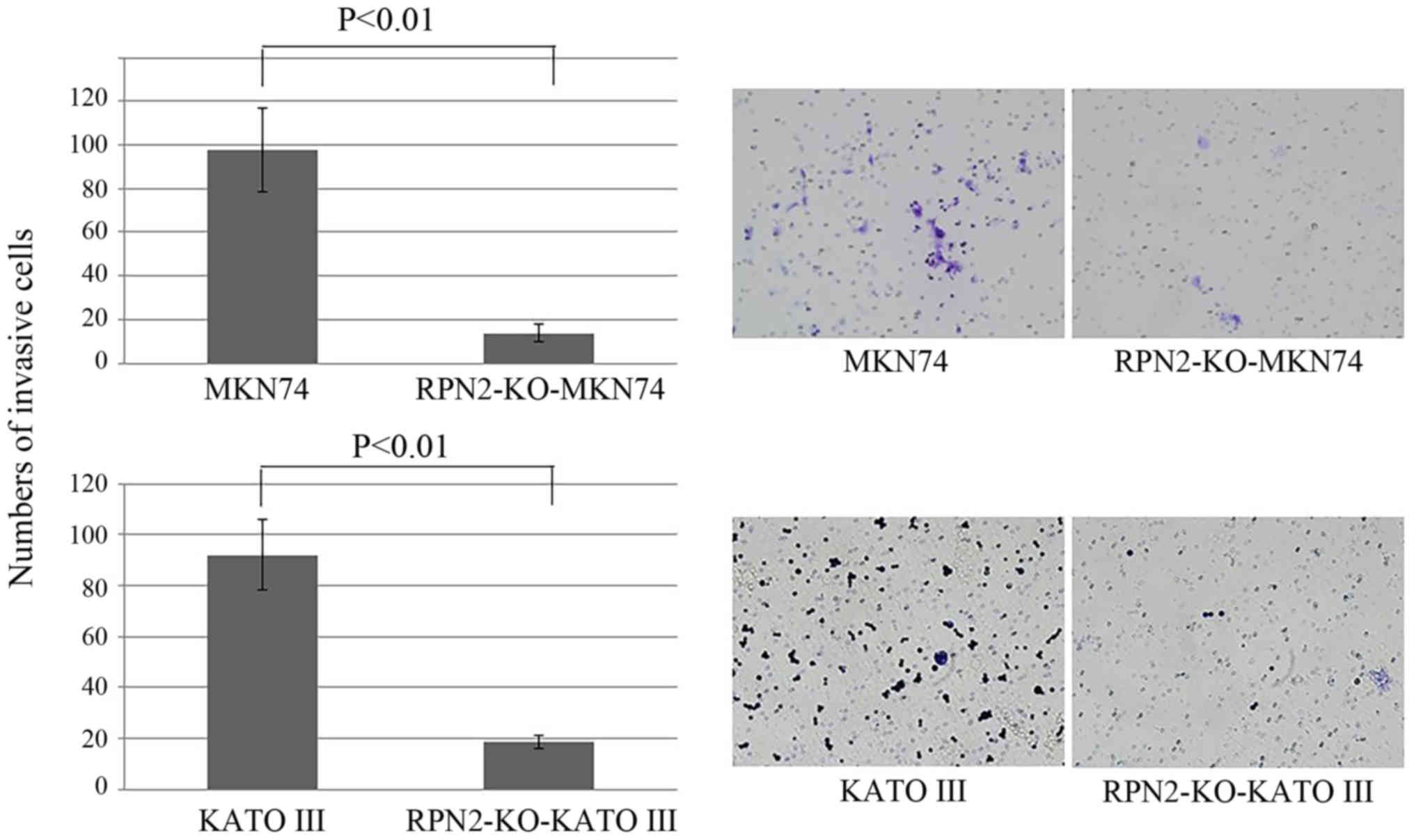
Knockout of RPN2 expression reduced the
invasion ability of gastric adenocarcinoma cells
Fig. 5 demonstrates
the cell invasion. While the number of invasion of MKN74 cells was
97 on average, the number of invasive RPN2-KO MKN74 cells was 13 on
average. Similarly, while the mean number of invasive KATO III
cells was 87, the mean number of invasive RPN2-KO KATO III cells
was 18.
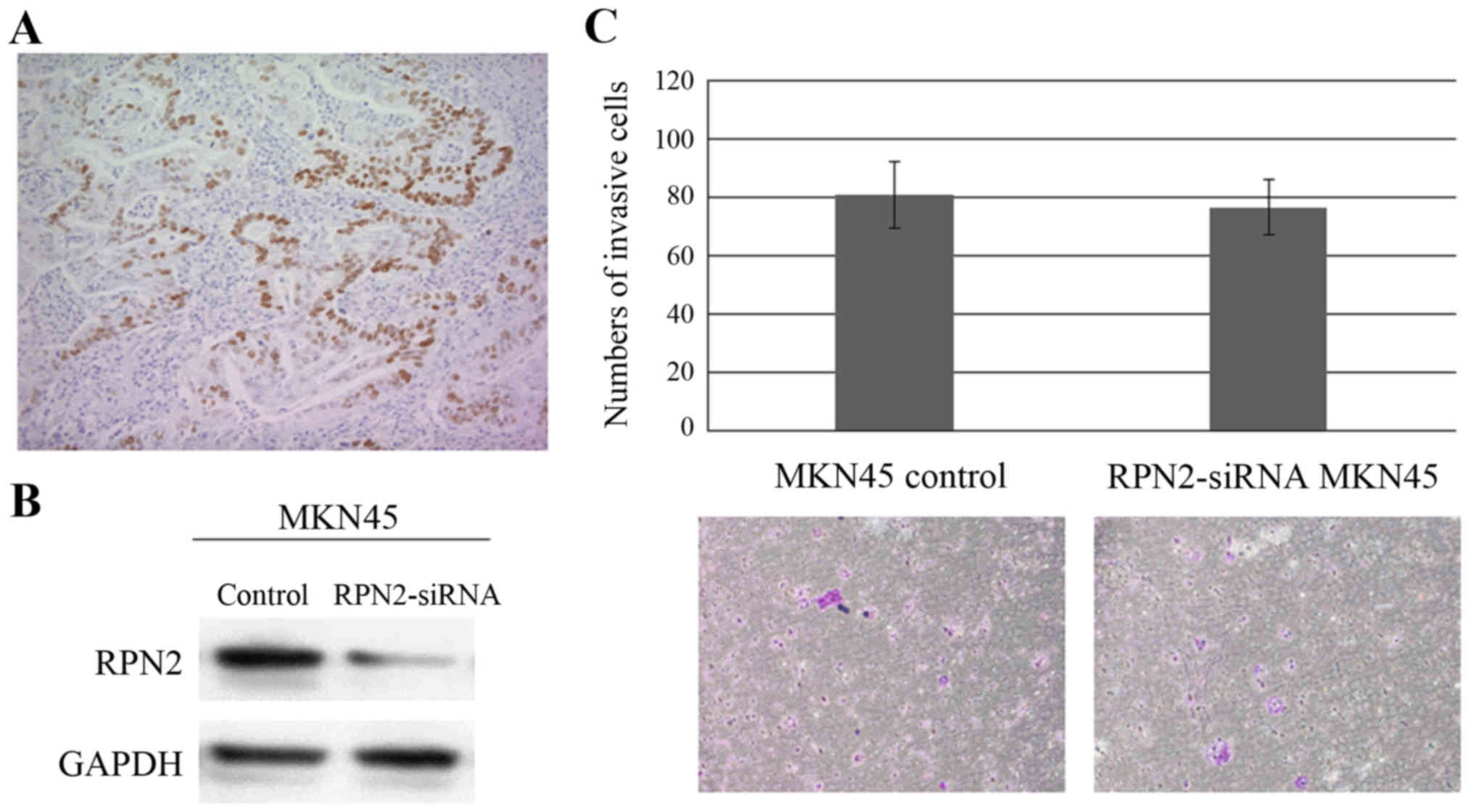
Relationship between RPN2 and mutant
p53
The expression of p53 was analyzed in the same
samples of gastric adenocarcinoma. Eighty of 242 primary tumors
showed positive immunoreactivity for p53; immunoreactivity was
detected in the nuclei of carcinoma cells (Fig. 6A). Significant association was
found between RPN2 immunostaining and p53 immunostaining
(p<0.001) (Table III). The
patients with tumors expressing both RPN2 and p53 concomitantly was
associated with higher depth of wall invasion, lymph node
metastasis, lymphatic invasion, venous invasion, and pathological
stage (Table IV). We show the
number of invasion of MKN45 cells and MKN45 cells which were
suppressed by RPN2 siRNA (Fig.
6B). There was no significant difference in invasive ability
between MKN45 cells and MKN45 cells, which were suppressed by siRNA
(Fig. 6C).
 | Table IIICorrelation between RPN2 and p53
expression in gastric adenocarcinoma. |
Table III
Correlation between RPN2 and p53
expression in gastric adenocarcinoma.
| p53 expression | RPN2 expression
| P-value |
|---|
| Negative | Positive |
|---|
| Negative | 92 | 39 | |
| Positive | 31 | 80 | <0.001 |
 | Table IVCorrelation of the expression of RPN2
and p53 concomitantly and clinicopathological findings. |
Table IV
Correlation of the expression of RPN2
and p53 concomitantly and clinicopathological findings.
| No. of cases | RPN2-positive
| P-value |
|---|
| No. of cases
(%) |
|---|
| All cases | 242 | 80 (33.1) | |
| Gender | | | 0.384 |
| Male | 162 | 57 (35.2) | |
| Female | 80 | 23 (28.8) | |
| Histological
type | | | 0.785 |
| Pap, tub | 128 | 41 (32.0) | |
| Por, sig | 111 | 37 (33.3) | |
| Muc | 3 | 2 (66.7) | |
| Depth of wall
invasion | | | 0.001 |
| T1 | 79 | 7 (8.9) | |
| T2 | 30 | 14 (46.7) | |
| T3 | 26 | 14 (53.8) | |
| T4 | 107 | 45 (42.1) | |
| Lymph node
metastasis | | | 0.001 |
| N0 | 95 | 15 (15.8) | |
| N1-3 | 147 | 65 (44.2) | |
| Lymphatic
invasion | | | 0.001 |
| Negative | 59 | 4 (6.8) | |
| Positive | 181 | 76 (42.0) | |
| Venous
invasion | | | 0.001 |
| Negative | 81 | 10 (12.3) | |
| Positive | 159 | 70 (44.0) | |
| Liver
metastasis | | | 0.734 |
| Negative | 232 | 76 (32.8) | |
| Positive | 10 | 4 (4.0) | |
| Peritoneal
dissemination | | | 0.365 |
| Negative | 229 | 74 (32.3) | |
| Positive | 13 | 6 (46.2) | |
| Stage | | | 0.001 |
| I | 87 | 11 (12.6) | |
| II | 41 | 17 (41.5) | |
| III | 59 | 25 (42.4) | |
| IV | 55 | 27 (49.1) | |
Discussion
In East Asia, gastric adenocarcinoma is one of the
most common malignancies. In spite of the improvement in surgical
treatment and chemotherapy, gastric adenocarcinoma of an advanced
stage is still subject to a poor prognosis, although cases of early
stage are successfully controlled. The risk factors for mortality
among tumors are metastasis and drug resistance, and several
reports describe the factors related to the invasiveness of
malignant tumors and patient prognosis (14,15).
The human RPN2 gene examined in this study is positioned on
chromosome 20q12–13.1, a region that is frequently deleted in
patients with myeloid malignancies (16,17).
The RPN2 protein is a component of an N-oligosaccharyl transferase
complex that conjugates high mannose oligosaccharides to asparagine
residues in the N-X-S/T consensus motif of nascent polypeptide
chains (18,19). RPN2 was reported as a key player of
drug resistance by modulating glycosylation of multidrug resistance
protein and, in vivo delivery of siRNA specific for
RPN2 markedly reduced tumor growth in breast cancer
(20). Also, downregulation of
RPN2 efficiently induced apoptosis in the presence of docetaxel,
and RPN2 promoted EMT through stabilization of mutant p53 in breast
cancer (12,20). Recent studies suggested that the
acquisition of drug resistance by cancer cells might be modulated
via the changes in miRNA levels and miRNAs play critical role in
EMT of cancer cells (21–23).
In this study, RPN2 expression was found in the
primary lesion of 49.2% of human gastric adenocarcinoma resection
cases. In the RPN2 expression cases, the prevalence of
clinicopathologic events was high, such as depth of wall invasion,
lymph node metastasis and peritoneal dissemination, and associated
with poor patient prognosis. RPN2 expression was also identified as
an independent prognosis factor. Furthermore, we showed correlation
between RPN2 expression and mutant-type p53 expression in the
primary lesion, and patients with tumors expressing both RPN2 and
p53 concomitantly were associated with depth of wall invasion,
lymph node metastasis, lymphatic invasion, venous invasion, and
pathological stage. The knockout of RPN2 expression reduced the
ability of invasion in gastric adenocarcinoma cell lines MKN74 and
KATO III. However, the knockout of RPN2 expression in MKN45 cell
line did not reduce the ability of invasion. MKN74 and KATO III
cell lines have mutant-type p53, and MKN45 cell line has wild-type
p53 (24,25). RPN2 stabilized mutant-type p53 by
suppressing glycogen synthase kinase 3β in breast cancer cells
(12). Additionally, RPN2 also
regulated Bax and Bcl-2 in intrinsic apoptosis control (26). We believe that the invasive
potential of gastric adenocarcinoma cells greatly affected the
stabilization of mutant-type p53 by RPN2. Furthermore, the fact
that the prognosis of RPN2-expressing gastric adenocarcinoma
patients was poor was considered to involve not only the stabilized
mutant-type p53 by RPN2 but also the control of p53-independent
apoptosis pathway by RPN2.
This study has some limitations that require
consideration. We showed that the expression of RPN2 may be
involved in gastric adenocarcinoma cell invasion. However, we did
not yet demonstrate the relationship between the RPN2 expression
and EMT, which allows to increase motility and invasiveness of
cancer cells (27). We intend to
intensively explore the relationship of the RPN2 expression and
oncogenic signal pathways including p53, which induce EMT. In
addition, we think also there is a possibility that miRNAs are
involved in the relation between EMT and RPN2 expression, because
the expression of miRNAs including miR-200 family which repressed
ZEB1/2 was controlled by p53 (28). In future, we will examine more
deeply the intrinsic roles of RPN2 on the metastasis of gastric
adenocarcinoma cells, and we want to make clear that RPN2 has the
potential to be a new therapeutic target factor.
Acknowledgments
The technical assistance of Ms.M. Saito with this
research is appreciated.
References
|
1
|
Dicken BJ, Bigam DL, Cass C, Mackey JR,
Joy AA and Hamilton SM: Gastric adenocarcinoma: Review and
considerations for future directions. Ann Surg. 241:27–39.
2005.
|
|
2
|
Nissan A, Garofalo A and Esquivel J:
Cytoreductive surgery and hyperthermic intra-peritoneal
chemotherapy (HIPEC) for gastric adenocarcinoma: Why haven't we
reached the promised land? J Surg Oncol. 102:359–360. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glehen O, Mohamed F and Gilly FN:
Peritoneal carcinomatosis from digestive tract cancer: New
management by cytoreductive surgery and intraperitoneal
chemohyperthermia. Lancet Oncol. 5:219–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takei Y, Takigahira M, Mihara K, Tarumi Y
and Yanagihara K: The metastasis-associated microRNA miR-516a-3p is
a novel therapeutic target for inhibiting peritoneal dissemination
of human scirrhous gastric cancer. Cancer Res. 71:1442–1453. 2011.
View Article : Google Scholar
|
|
8
|
Motoyama K, Inoue H, Mimori K, Tanaka F,
Kojima K, Uetake H, Sugihara K and Mori M: Clinicopathological and
prognostic significance of PDCD4 and microRNA-21 in human gastric
cancer. Int J Oncol. 36:1089–1095. 2010.PubMed/NCBI
|
|
9
|
Ueda T, Volinia S, Okumura H, Shimizu M,
Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: A microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar
|
|
10
|
Zhang Z, Liu S, Shi R and Zhao G: miR-27
promotes human gastric cancer cell metastasis by inducing
epithelial-to-mesenchymal transition. Cancer Genet. 204:486–491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okugawa Y, Inoue Y, Tanaka K, Kawamura M,
Saigusa S, Toiyama Y, Ohi M, Uchida K, Mohri Y and Kusunoki M: Smad
interacting protein 1 (SIP1) is associated with peritoneal
carcinomatosis in intestinal type gastric cancer. Clin Exp
Metastasis. 30:417–429. 2013. View Article : Google Scholar
|
|
12
|
Takahashi RU, Takeshita F, Honma K, Ono M,
Kato K and Ochiya T: Ribophorin II regulates breast tumor
initiation and metastasis through the functional suppression of
GSK3β. Sci Rep. 3:24742013. View Article : Google Scholar
|
|
13
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Löffler C, Rao VV and Hansmann I: Mapping
of the ribophorin II (RPN II) gene to human chromosome 20q12–q13.1
by in-situ hybridization. Hum Genet. 87:221–222. 1991. View Article : Google Scholar
|
|
17
|
Testa JR, Kinnealey A, Rowley JD, Golde DW
and Potter D: Deletion of the long arm of chromosome 20
[del(20)(q11)] in myeloid disorders. Blood. 52:868–877.
1978.PubMed/NCBI
|
|
18
|
Kelleher DJ, Kreibich G and Gilmore R:
Oligosaccharyltransferase activity is associated with a protein
complex composed of ribophorins I and II and a 48 kd protein. Cell.
69:55–65. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kelleher DJ and Gilmore R: An evolving
view of the eukaryotic oligosaccharyltransferase. Glycobiology.
16:47R–62R. 2006. View Article : Google Scholar
|
|
20
|
Honma K, Iwao-Koizumi K, Takeshita F,
Yamamoto Y, Yoshida T, Nishio K, Nagahara S, Kato K and Ochiya T:
RPN2 gene confers docetaxel resistance in breast cancer. Nat Med.
14:939–948. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen QY, Jiao DM, Wang J, Hu H, Tang X,
Chen J, Mou H and Lu W: miR-206 regulates cisplatin resistance and
EMT in human lung adenocarcinoma cells partly by targeting MET.
Oncotarget. 7:24510–24526. 2016.PubMed/NCBI
|
|
22
|
Zhao JJ, Chu ZB, Hu Y, Lin J, Wang Z,
Jiang M, Chen M, Wang X, Kang Y, Zhou Y, et al: Targeting the
miR-221-222/PUMA/BAK/BAX pathway abrogates dexamethasone resistance
in multiple myeloma. Cancer Res. 75:4384–4397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng
L, Shi Y, Wang H, Yin B, Xia J, et al: Down-regulation of miR-223
reverses epithelial-mesenchymal transition in gemcitabine-resistant
pancreatic cancer cells. Oncotarget. 6:1740–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yukimoto K, Nakata B, Muguruma K, Yashiro
M, Ohira M, Ishikawa T, Hino M and Hirakawa K: Apoptosis and
thymidylate synthase inductions by 5-fluorouracil in gastric cancer
cells with or without p53 mutation. Int J Oncol. 19:373–378.
2001.PubMed/NCBI
|
|
25
|
Endo F, Nishizuka SS, Kume K, Ishida K,
Katagiri H, Ishida K, Sato K, Iwaya T, Koeda K and Wakabayashi G: A
compensatory role of NF-κB to p53 in response to 5-FU-based
chemotherapy for gastric cancer cell lines. PLoS One. 9:e901552014.
View Article : Google Scholar
|
|
26
|
Fujita Y, Yagishita S, Takeshita F,
Yamamoto Y, Kuwano K and Ochiya T: Prognostic and therapeutic
impact of RPN2-mediated tumor malignancy in non-small-cell lung
cancer. Oncotarget. 6:3335–3345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boyer B, Vallés AM and Edme N: Induction
and regulation of epithelial-mesenchymal transitions. Biochem
Pharmacol. 60:1091–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim T, Veronese A, Pichiorri F, Lee TJ,
Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, et al:
p53 regulates epithelial-mesenchymal transition through microRNAs
targeting ZEB1 and ZEB2. J Exp Med. 208:875–883. 2011. View Article : Google Scholar : PubMed/NCBI
|