Introduction
Pancreatic cancer (PC) is one of the most common
causes of cancer mortality in the United States (1). Because of the aggressive growth and
metastatic tendencies of PC, PC patients usually enter advanced
stage when diagnosed. Therapeutic methods, including surgery and
medical interventions, were mainly unsuccessful, and the prognosis
was often not optimistic (1,2).
Previous studies have illuminated multiple potential pathogenicity
mechanisms and risk factors for PC (3); however, the recurrent or metastatic
molecular mechanism of PC is still unknown. As a member of the
small GTPase family, Rab11 has a significant influence on vesicular
trafficking, especially translocation of proteins from the
trans-Golgi network to the plasma membrane and recycling of
membrane protein, as well as cytokinesis (4–6). The
C-termini of Rab11-family interacting proteins (Rab11-FIPs) possess
a highly conserved short motif, called the Rab11-binding domain,
that interacts strongly with Rab11-GTP (7). Rab11-FIPs have three main functions:
recycling of cargo to the membrane surface, transport of membrane
to the cleavage furrow or midbody during cell division and linking
Rab11 to molecular motor proteins (8). It is different from other Rab11-FIPs
in that the N-terminus of Rab11-FIP4 possesses an EF-hand
calcium-binding motif (8,9). Rab11-FIP4, as a downstream effector
of Rab11, plays an important role in the membrane trafficking in
association with cytokinesis (10). The proliferation and
differentiation of retinal progenitor cells is regulated by
Rab11-FIP4 in the process of mouse and zebrafish retinal
development (11,12). Hypoxia increases tumor cell
invasion by modulating Rab11 (13). The invasion and metastasis
potential of hepatocellular carcinoma (HCC) were significantly
promoted by hypoxia-induced Rab11-FIP4. The level of Rab11-FIP4 was
overexpressed in HCC and contributed to an unfavourable clinical
outcome in HCC patients (14).
Hypoxia is significantly correlated with tumor growth, metastasis
and poor clinical outcome in PC (15,16).
However, at present, the underlying effect of Rab11-FIP4 on PC
progression remains unknown.
In this study, we investigate the expression level
of Rab11-FIP4 in PC tissues and the relationship with
clinicopathological features of PC. Furthermore, whether Rab11-FIP4
plays critical roles in PC progression were also studied.
Materials and methods
Patients and specimens
Sixty pairs of pancreatic tumor specimens and 60
corresponding para-carcinoma tissue samples were acquired from the
Second Affiliated Hospital and the First Affiliated Hospital of
Wenzhou Medical University, China. Tumor grade and stage were on
the basis of the American Joint Committee on Cancer/International
Union Against Cancer staging manual (2009). Patient clinical
information and tumor characteristics are summarized in Table I. All patients were followed-up
22–60 months (mean, 48.2 months) after the surgery. All PC tissue
specimens were acquired using protocols approved by the Ethics
Committee of the Second Affiliated Hospital of Wenzhou Medical
University, and all patients signed a written informed consent.
 | Table IThe relationship between Rab11-FIP4
expression and clinicopathological features. |
Table I
The relationship between Rab11-FIP4
expression and clinicopathological features.
| Clinicopathological
feature | Parameter | Rab11-FIP4
| P-value |
|---|
| Low | High |
|---|
| Age | ≥60 | 20 | 16 | 0.832 |
| <60 | 14 | 10 | |
| Gender | Male | 21 | 12 | 0.228 |
| Female | 13 | 14 | |
| Tumor site | Head | 19 | 14 | 0.875 |
| Body, tail | 15 | 12 | |
| Size | >3 cm | 7 | 19 | 0.0001 |
| ≤3 cm | 27 | 7 | |
| Histologic
grade | Well to
moderate | 24 | 11 | 0.028 |
| Poor | 10 | 15 | |
| Metastasis | Yes | 10 | 19 | 0.001 |
| No | 24 | 7 | |
| TNM stage | 0-IIa | 22 | 7 | 0.004 |
| IIb-IV | 12 | 19 | |
| Survival | Mean (months) | 28.5 | 16.4 | 0.0036 |
Immunohistochemistry analysis
The detection of Rab11-FIP4 expression in pancreatic
cancer specimens followed a standard immunohistochemistry protocol.
Ordinarily, to retrieve the antigen, the deparaffinized and
rehydrated slides were placed into boiling ethylenediamine
tetraacetic acid buffer. Then, the samples were incubated in 3%
H2O2 (10 min, room temperature) to eliminate
the activity of endogenous peroxidases. After 5% goat serum
blocking, the slides were applied with anti-Rab11-FIP4 antibody
(Santa Cruz Biotechnology, CA, USA) with a dilution of 1:100 at 4°C
overnight in a humidified chamber. All the sections were incubated
with a biotinylated secondary antibody for 2 h, followed by
incubation with a streptavidin-horseradish-peroxidase complex.
Sequentially, the sections were stained with 3,3′-diaminobenzidine
(DAB) and then counter-stained with Mayer's hematoxylin. The
Rab11-FIP4 expression in the pancreatic tumor samples was
independently assessed by two clinical pathologists.
Immunohistochemical evaluation of the Rab11-FIP4 protein was
performed according to previously described methods, taking into
account the extent (graded from 0 to 4) and intensity (0–3) of
immunopositivity (17,18). We multiplied the intensity score
with positive extent to define the final score, and a final score
range was 0–12. Final scores of ≥4 were considered as high
expression, and scores of <4 were considered as low
expression.
Construction
According to the Rab11-FIP4 gene sequence (forward,
5′-GAGAATGACAGCCTGACCAAT-3′), three single-guide RNAs (sgRNAs) were
constructed on a lentiviral backbone: sgRNA-1, forward,
5′-TGTCGGGAGTCCTGCCGAGA-3′; sgRNA-2, forward,
5′-CTGATGGCGAGCTCATCCCC-3′; sgRNA-3, forward,
5′-AGCCCGACTGAAAAACCTGA-3′, and a negative control (NC) sgRNA
(5′-CGCTCGCGGCCCGTTCAA-3′) duplex was chemically synthesized. Then,
the corresponding Rab11-FIP4-sgRNA oligos were synthesized
(Rab11-FIP4-sgRNA-1, forward, 5′-caccgTGTC GGGAGTCCTGCCGAGA-3′;
Rab11-FIP4-sgRNA-2, forward, 5′-caccgCTGATGGCGAGCTCATCCCC-3′;
Rab11-FIP4-sgRNA-3, forward, 5′-caccgAGCCCGACTGAAAAACCTGA-3′). The
resulting Rab11-FIP4-sgRNAs for the target site were cloned into
the vector GV371 (GeneChem Co. Ltd. Shanghai, China), which
contained the EGFP reporter, to produce recombinant plasmid
GV371-Rab11-FIP4-sgRNA. The other plasmid vector, Lenti-CAS9-puro,
was purchased from GeneChem Co. Ltd. Virus packaging was completed
in 293T cells after the co-transfection of the
GV371-Rab11-FIP4-sgRNA plasmid or the Lenti-CAS9-puro plasmid with
the packaging plasmid.
Cell culture and transfection
The human PC cell lines, CFPAC-1, PANC-1 and AsPC-1,
were acquired from the Shanghai Cell Bank of the Chinese Academy of
Sciences. All cells were cultured in Roswell Park Memorial
Institute-1640 (RPMI-1640) medium containing 10% fetal bovine serum
(Mediatech, VA, USA) and incubated in a 37°C chamber with 5%
CO2.
We used the CRISPR/Cas9 (19–22)
system to construct a double vector lentiviral vector. First, the
Lenti-CAS9 lentivirus transfected PANC-1 cells. After 72 h, the
PANC-1 cells that stably expressed Cas9 were selected by puromycin
(Clontech, San Francisco, CA, USA). Then, the stably expressing
Cas9-PANC-1 cells were transfected with the sgRNA lentivirus. A
fluorescent microscope (Olympus, Tokyo, Japan) was used to detect
EGFP expression. When the lentiviral transfection efficiency was
greater than 80%, the cells were harvested for the experiment.
Detection of introduced mutations in
genomic DNA
The Cruiser nuclease digestion assay was conducted
to detect introduced mutations in the genomic DNA using the
Knockout and Mutation Detection kit (GeneChem Co. Ltd.). The cells
were harvested 7 days after transfection, and DNA was extracted
using a Genomic DNA extraction kit (Tiangen, Beijing, China)
according to the manufacturer's instructions. Polymerase chain
reaction (PCR) amplification of the region containing the target
site was carried out using the following primers: sgRNA-1, forward,
5′-TGTCGGGAGTCCTGCCGAGA-3′; sgRNA-2, forward,
5′-CTGATGGCGAGCTCATCCCC-3′; and sgRNA-3, forward,
5′-AGCCCGACTGAAAAACCTGA-3′; then, the PCR products were annealed.
The annealed PCR products were naturally cooled below 40°C at room
temperature. Then, the PCR products (3 µl) were examined by
electrophoresis. Lastly, in a sterile PCR tube, the reaction
solution containing 3 µl PCR products, 2 µl detecase
buffer, 1 µl detecase and 4 µl ddH2O were
combined. After a nuclease digestion reaction lasting 20 min at
45°C, 2 µl of stop buffer was added to the reaction
solution. Subsequently, all the reaction solutions were detected by
2% agarose gel electrophoresis.
MTT assay
To measure the cell proliferation after transfection
with Rab11-FIP4, the MTT assay was performed. The cells
(2×103 cells/well) were seeded into 96-well culture
plates (Corning, CA, USA). Then, 20 µl of MTT reagent (5
mg/ml; Genview, FL, USA) was added after 24, 48, 72, 96 and 120 h.
After 4-h incubation at 37°C, the medium was replaced with 100
µl of DMSO (Shanghai Shiyi Chemical Reagent Co. Ltd.,
Shanghai, China), and the plate was rotated for 2–5 min at room
temperature. The spectrometric absorbance was measured at 490 nm
with a microplate reader (Tecan Infinite, Mannedorf,
Switzerland).
Apoptosis assay
Seven days after transfection, apoptosis of
transfected PANC-1 cells was detected by Annexin V-APC
(eBioscience, CA, USA) single staining. In brief, these cells were
washed two times with complete culture medium and were incubated in
200 µl binding buffer and 10 µl Annexin V-APC at room
temperature for 15 min in the dark. Then, the stained cells were
analysed by flow cytometry (Millipore, MA, USA).
Cell cycle analysis
Flow cytometry was used to detect the cell cycle
distribution. In brief, the cells were washed and resuspended in
staining buffer containing 10 µg/ml propidium iodide (PI)
and 25 µg/ml RNase. The cell cycle was analysed by the Guava
easyCyte HT flow cytometer (Millipore) following staining.
Histograms were used to stand for the proportion of cells in phase
G1, S and G2/M.
Migration and invasion assay
Migration and invasion experiments were performed
with PC cells using a 24-well Transwell plate (Corning). For the
migration assay, stably transfected cells (PANC-1) in 100 µl
of serum-free DMEM were plated in the upper chamber. Then, 600
µl of DMEM supplemented with 30% FBS was placed in the lower
chamber. After the cells were incubated at 37°C for 24 h, the cells
that could not migrate were removed from the upper surface of the
membrane with a cotton swab. The cells that had passed through the
membranes were stained with 0.1% rystal violet for 10 min. The
migrating cells were viewed under an inverted microscope (Olympus,
Tokyo, Japan), and nine random fields (magnification, ×200) were
photographed and counted.
For the invasion assay, stably transfected cells
(PANC-1) in 500 µl of serum-free DMEM were plated in the
upper chamber of Matrigel-precoated (Becton-Dickinson Co., NJ, USA)
Transwell plates. Then, 750 µl of DMEM supplemented with 30%
FBS was placed in the lower chamber. After the cells were incubated
at 37°C for 40 h, the non-invading cells were removed from the
upper surface of the membrane, and the cells on the lower surface
of the insert were stained and counted as the migration assay. The
experiment was performed three times.
Real-time PCR
TRIzol reagent (Pu Fei, Shanghai, China) was used to
extract total RNA from cultured PC cells following the kit
instructions. Reverse transcription was performed using the M-MLV
Reverse Transcription kit (Promega, WI, USA) according to the
manufacturer's instructions. Real-time PCR (RT-PCR) was
subsequently performed with the SYBR Premix Ex Taq (Takara, Dalian,
China). The PCR reaction conditions were as follows: 95°C for 30
sec followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec.
The sequences of the primers used were as follows: Rab11-FIP4,
forward, 5′-GAGAATGACAGCCTGACCAAT-3′; reverse,
5′-GCTCTGTATTTTCTTCCTCCAAC-3′. GAPDH, forward,
5′-TGACTTCAACAGCGACACCCA-3′; reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′.
The mRNA relative expression levels were quantified using the
2−ΔΔCt method.
Statistical analysis
IBM SPSS Statistics software program version 22.0
(IBM Corp., NY, USA) was used to process the data. Data are
presented as the means ± SEM of values from three independent
experiments. Statistical significance among different groups was
analysed by one-way analysis of variance (ANOVA). The correlation
between Rab11-FIP4 and the clini-copathological features were
assessed by χ2 test and Fisher's exact test. The
Kaplan-Meier method with the log-rank test or Cox regression method
was used to evaluate overall survival. A P-value <0.05 was
considered statistically significant.
Results
Overexpression of Rab11-FIP4 in PC is
related to the clinicopathologic features of the PC patients
The immunohistochemical results revealed that the
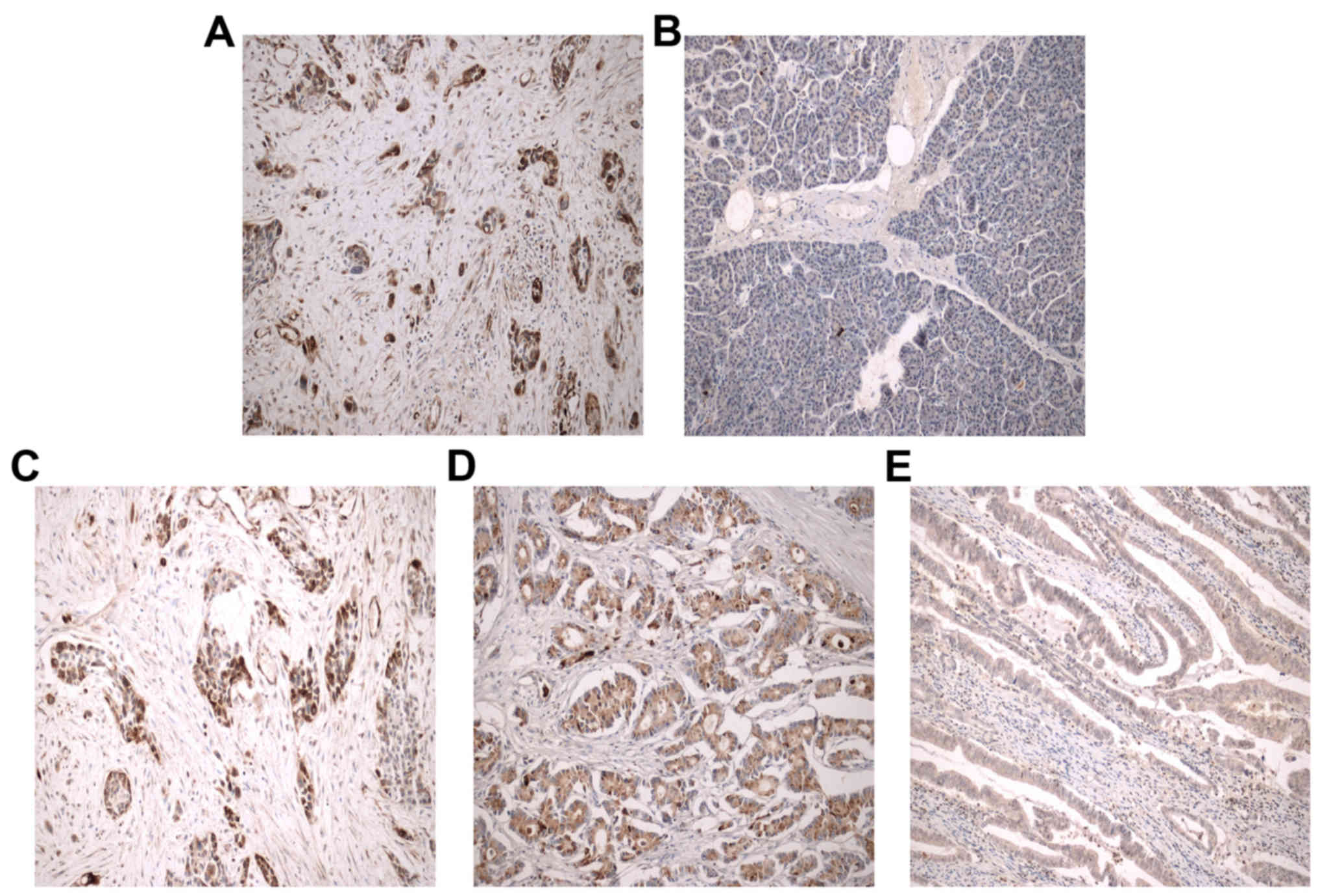
Rab11-FIP4 protein was highly expressed in 26 (26/60, 43.3%) PC
patients (Fig. 1A) and only in 5
(5/60, 8.3%) adjacent non-cancerous pancreatic tissues (P=0.0001).
Moreover, the Rab11-FIP4 protein expression in most of the adjacent
non-cancerous pancreatic tissues was negative (Fig. 1B). Interestingly, the staining
density was related to histological grade, and high Rab11-FIP4
expression was more often observed in poorly differentiated
pancreatic carcinoma tissues (Fig.
1C–E). Rab11-FIP4 overexpression was related to tumor size,
histological grade, metastasis and TNM stage, but not with age,
gender or tumor site in the 60 pancreatic tumor specimens (Table I).
Relationship between Rab11-FIP4
expression and prognosis of PC patients
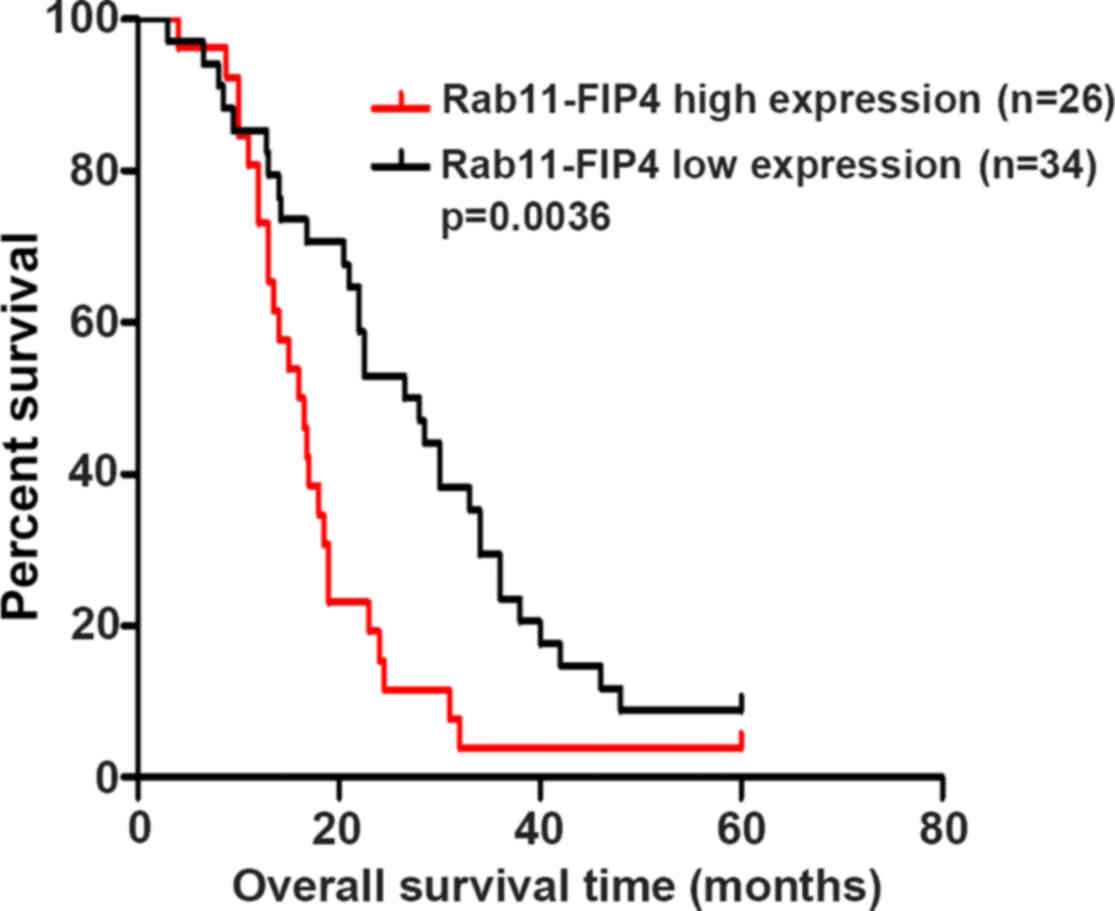
We studied whether Rab11-FIP4 overexpression was
related to the overall survival of PC patients. The sixty PC
patients were divided into two groups according to the
immunohistochemical results. The overall survival time of high
Rab11-FIP4 expression group was (16.4±5.9) months, while it was
(28.5±12.7) months in low Rab11-FIP4 expression group. The results
indicated that patients with low Rab11-FIP4 expression had much
longer OS times (P=0.0036) (Fig.
2).
Rab11-FIP4 knockout in PANC-1 cells using
the CRISPR/Cas9 system
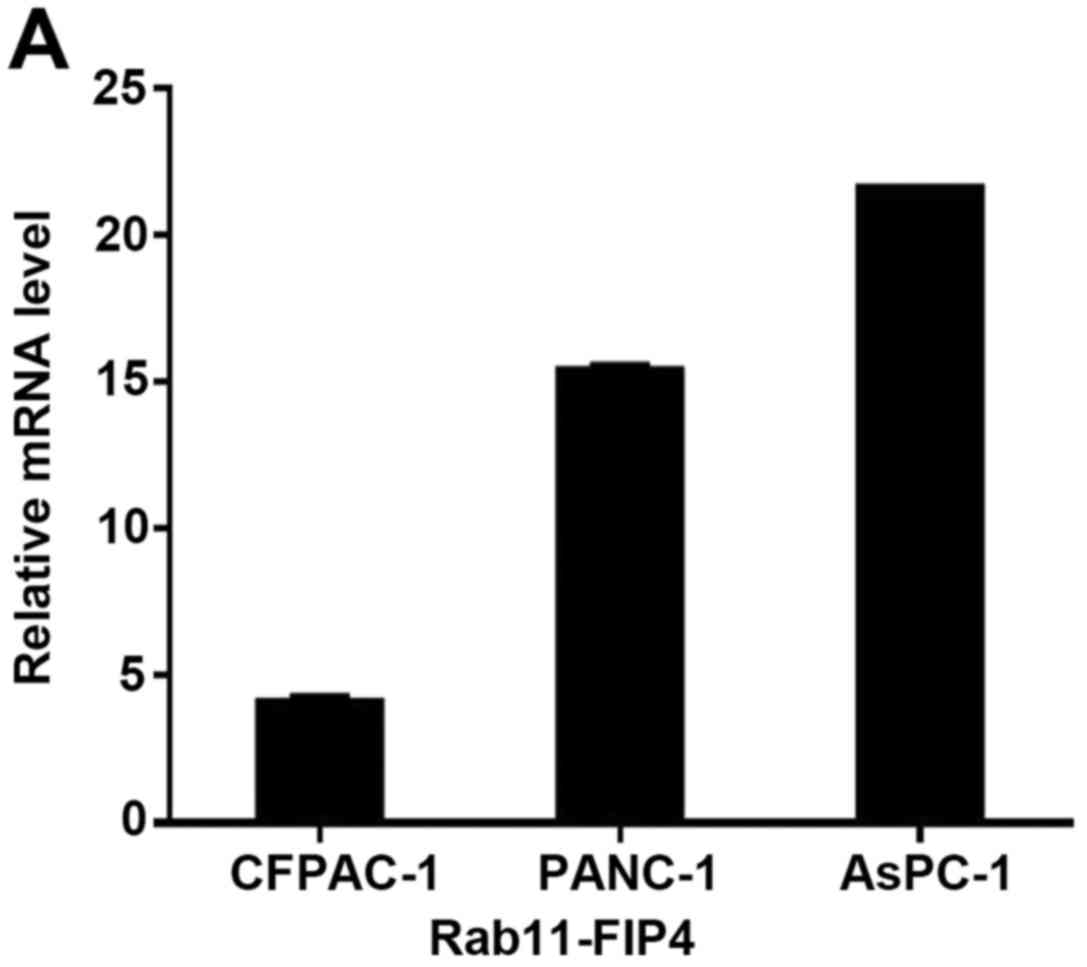
Rab11-FIP4 expression was analysed in several PC
cell lines using RT-PCR. The relative Rab11-FIP4 expression level
is shown in Fig. 3A. The cell line
PANC-1, moderately expressed Rab11-FIP4, exhibiting a relative
expression level of 15.368 ± 0.132 (Fig. 3A). Therefore, PANC-1 cells were
selected for the subsequent cellular function test of Rab11-FIP4 in
PC.
To verify whether the three sgRNAs contained the
positions corresponding to the loci of single nucleotide
polymorphisms (SNPs), the sgRNA fragments were amplified by PCR,
and the PCR products were sequenced. As shown in Fig. 3B, the sequencing results showed
that the three sgRNAs had no SNP loci. The corresponding positions
of sgRNA-1 and sgRNA-2 were very close, so their PCR amplification
primers were the same.
We used the CRISPR/Cas9 genome-editing technique,
which has been reported to efficiently knock out genes in various
organisms (22). After
GV371-Rab11FIP4-sgRNA Lentivirus efficiently transfected the stably
expressed Cas9-PANC-1 cells, Cruiser-nuclease digestion assay was
used to detect sgRNA knockout activity. As shown in Fig. 3C, compared to the negative control
group, the cutting strip appeared in the expected position in the
experimental groups (KO1, KO2 and KO3). This result illustrated
that the sgRNA has efficient knockout activity. The knockout
activity of KO1 was more efficient than KO2 and KO3, so KO1 was
selected for functional experiments. The result also showed that
the mutation was successfully induced at the Rab11-FIP4 gene target
locus. Therefore, Rab11-FIP4 was efficiently knocked out by the
CRISPR/CAS9 double vector lentiviral system (Fig. 3D and E).
Effect of Rab11-FIP4 knockout on PANC-1
cell proliferation
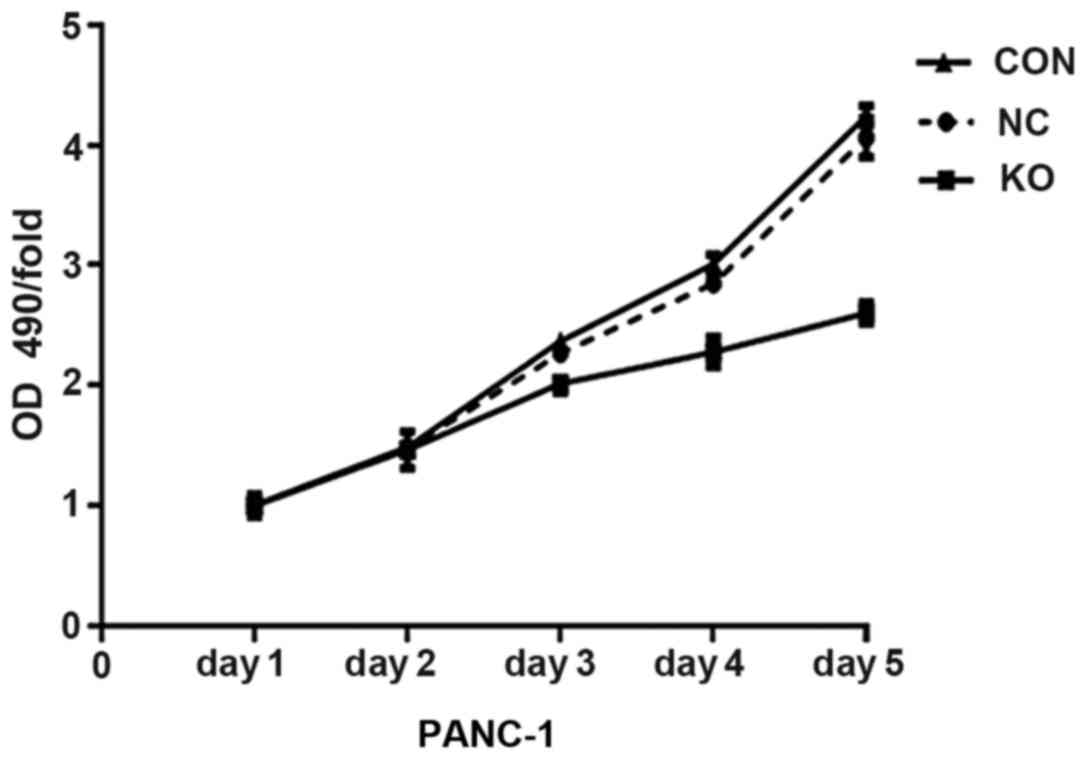
The effect of Rab11-FIP4 on the growth ability of PC
cells was assessed by MTT assay. As shown in Fig. 4, the EGFP-expressing transfected
cells were counted once a day for 5 days. The proliferation rates
of PANC-1 cells markedly decreased following Rab11-FIP4 knockout on
days 4 and 5 (P<0.05).
Effect of Rab11-FIP4 knockout on PANC-1
apoptosis
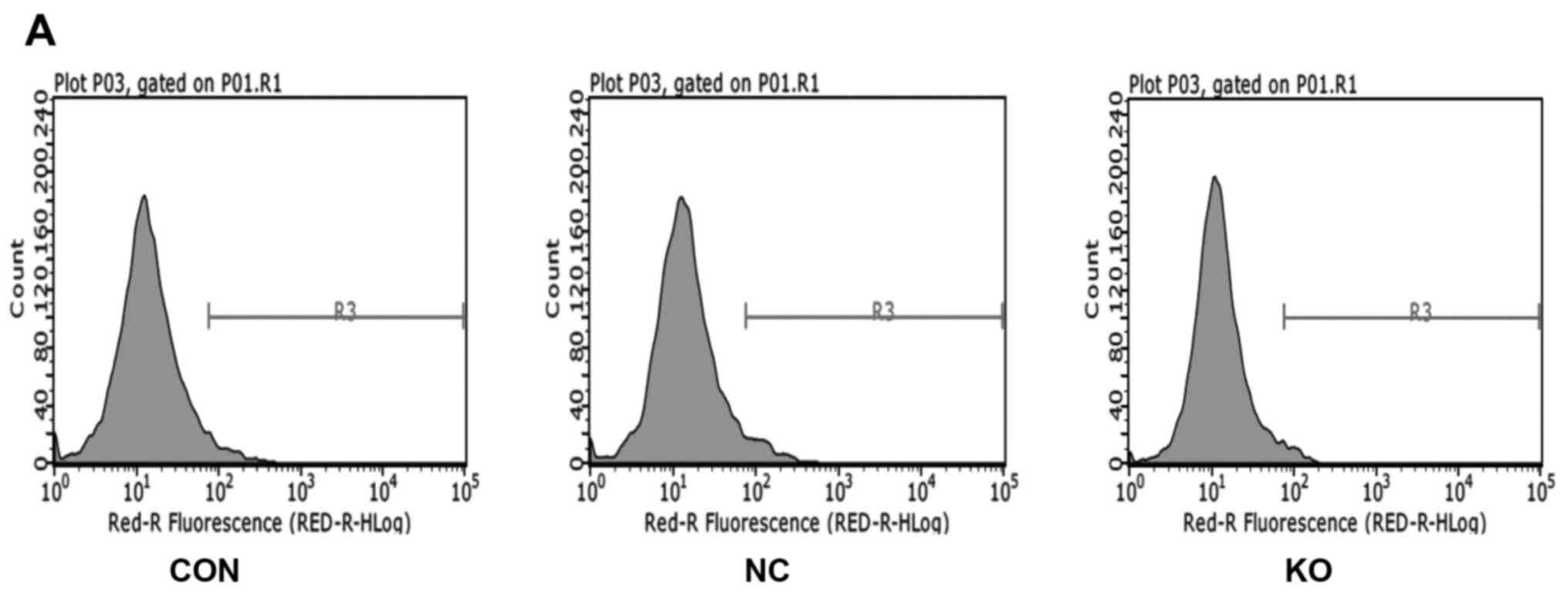
In order to study the effect of Rab11-FIP4 knockout
on PANC-1 apoptosis, we used flow cytometry to identify the
apoptosis rate. As shown in Fig.
5, the percentage of apoptosis was 4.73±0.82% in the negative
control group (NC) cells and 2.55±0.20% in knockout group (KO)
cells (P<0.05). Because the apoptosis rate of each group was
<5%, the result showed that the cells were not undergoing
apoptosis.
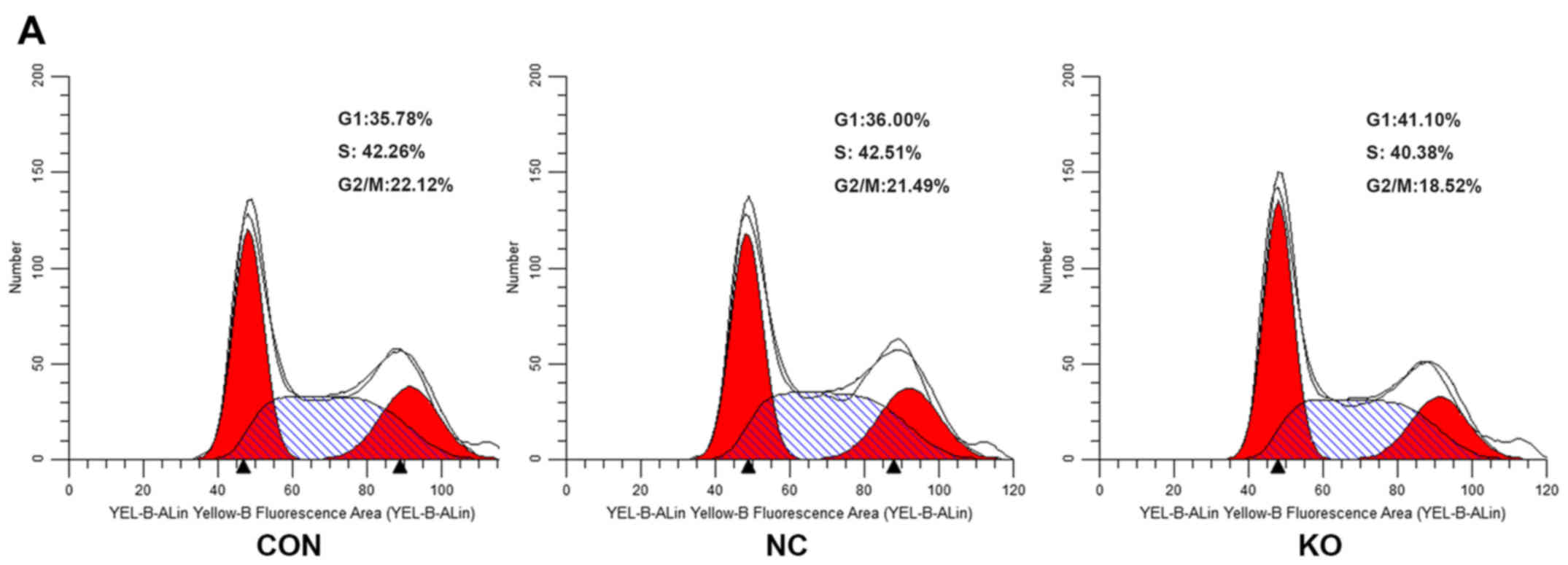
Effect of Rab11-FIP4 knockout on the
PANC-1 cell cycle
PANC-1 cells were transfected with the CRISPR/Cas9
double vector lentivirus for 7 days, and cell cycle distribution
was assessed by flow cytometry. There were significant differences
in cell cycle progression, including G1, S and G2/M phases, between
the Rab11-FIP4 KO and NC. As shown in Fig. 6, the Rab11-FIP4 knockout
significantly reduced the S stage fraction (KO 42.51%, NC 40.38%;
P=0.0073) and G2/M stage fraction (KO 21.49%, NC 18.52; P=0.0002)
while increasing the G1 stage fraction (KO 36%, NC 41.1%;
P=0.0001). These data indicate that Rab11-FIP4 knockout suppressed
PANC-1 cell cycle progression.
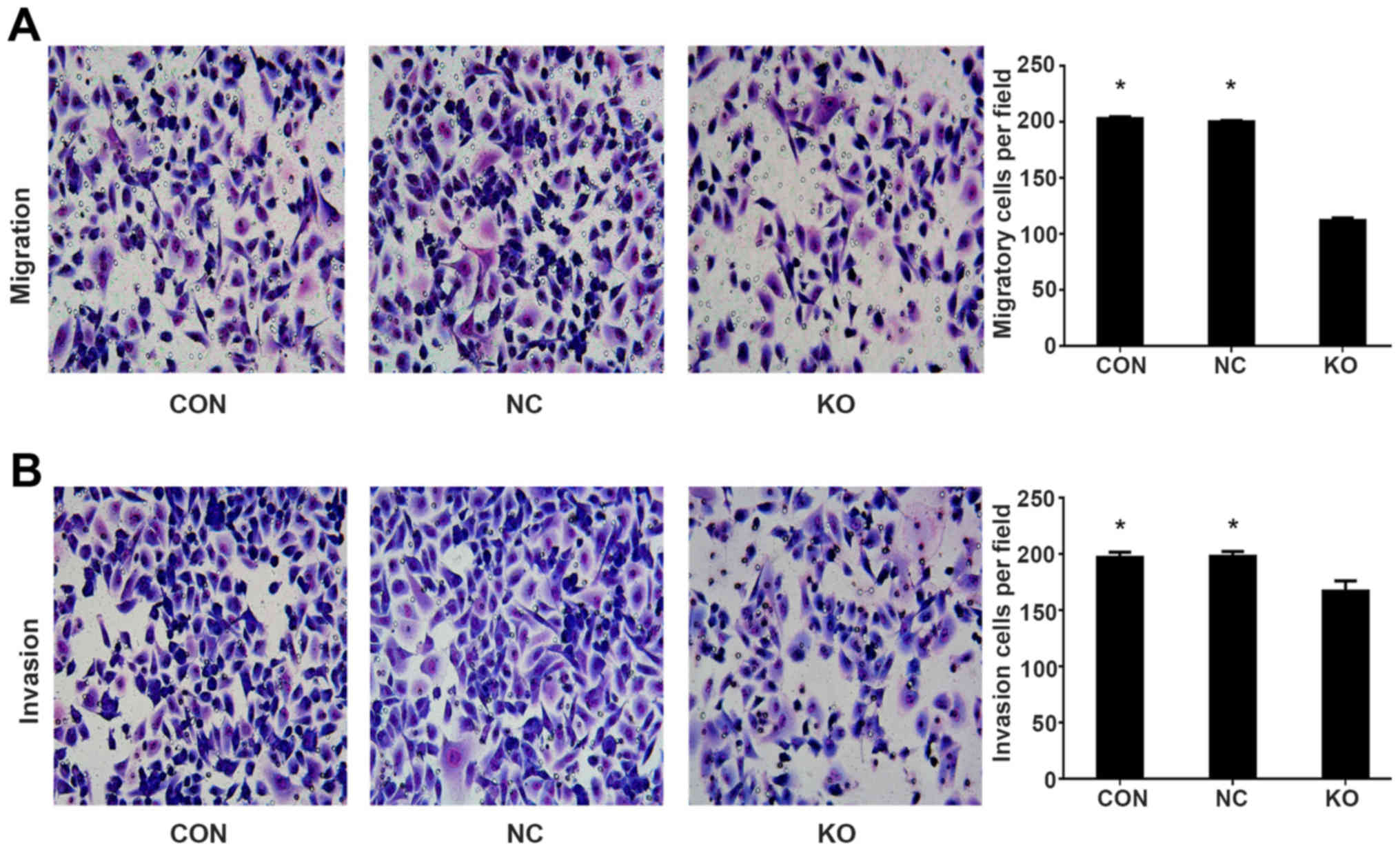
Effect of Rab11-FIP4 knockout on PANC-1
cell migration and invasion
To study the role of Rab11-FIP4 in PANC-1 cell
migration and invasion, we stably knocked out Rab11-FIP4 in PANC-1
cells using CRISPR/CAS9 double vector lentiviral system. Transwell
assays and Matrigel invasion assays were performed to detect the
migratory and invasive capacities of PANC-1 cells. As shown in
Fig. 7, the Rab11-FIP4 knockout
group had markedly fewer migrating and invading cells than the
parental control group. These results suggest that knockout of
Rab11-FIP4 remarkably inhibited the migratory and invasive ability
of PANC-1 cells.
Discussion
The reason that PC patients have a poor prognosis
and short survival is that PC has highly invasive and metastatic
behaviour (23,24). However, the detailed molecular
mechanisms underlying these characteristics remain barely
understood. In this study, we found that Rab11-FIP4 could promote
PC cell growth, invasion and metastasis. Rab11-FIP4 is
overexpressed in PC tissues, which is closely related to a worse
clinical outcome in PC patients.
Rab11-FIP4 has an important influence on the process
of cytokinesis (10,25,26).
The Rab11-FIP4 expression level in human cytomegalovirus
(HCMV)-infected cells has an impact on infectious virus production,
and the interaction between Rab11-FIP4 and Rab11 regulates
vesicular transport during cytokinesis (27). Recent studies have indicated that
Rab11-FIP4 acts as a Rab11 effector in HeLa cells and performs
cellular functions other than transferrin recycling (28). Rab11-FIP4 expressed abundantly in
the mouse and zebrafish nerve tissues and promoted the growth of
retinal progenitors (11,12). Rab11-FIP4 is a member of the family
of the Rab11-FIPs, which is composed of six members (7). Rab11-FIPs are likely to be related to
tumorigenesis, and they may act as tumor promoters in some cancers.
Rab11-FIPs are closely associated with the occurrence and
metastasis of HCC, gastric cancer, breast cancer and colorectal
cancer. The expression of Rab11-FIPs is increased significantly in
some tumor tissues (12,29–32).
For instance, Rab11-FIP2 overexpression enhanced gastric cancer
cell ability of invasion and metastasis (32). The studies on the relationship
between Rab11-FIP4 and malignant tumors are not extensive. The
latest study demonstrated that Rab11-FIP4, functioning as
regulatory factor, promoted hepatocellular carcinoma cell invasion
and metastasis. Hypoxia upregulated Rab11-FIP4 expression levels in
HCC cells. Also, the study revealed that the high expression of
Rab11-FIP4 in hepatocellular cancer tissues had a significant
correlation with lower survival rates (14). The results of our study are
consistent with the above.
In our study, we first observed Rab11-FIP4
expression in PC tissues. Immunohistochemistry analysis
demonstrated that the expression of Rab11-FIP4 in PC tissues was
higher than adjacent non-tumorous tissues. Clinical and
pathological data also indicated that the patients of PC with a
high expression of Rab11-FIP4 exhibited a higher lymph node
metastasis ratio (LMR). Kaplan-Meier method revealed that
overexpression of Rab11-FIP4 serves as a marker of worse prognosis
in PC patients. This suggests that high expression of Rab11-FIP4 is
a potential and novel prognostic factor of overall survival in PC
patients. The poor prognosis is attributed to the high incidence of
metastasis and the powerfully invasive ability of cancer. These
results indicate that the invasion and metastasis ability of PC may
be associated with the high Rab11-FIP4 expression.
To investigate the biological function of Rab11-FIP4
in PANC-1 cells, we knocked out Rab11-FIP4 in PANC-1 cells using
the CRISPR/Cas9 system. Then, we estimated the effects of
Rab11-FIP4 on PANC-1 cell proliferation and discovered that
Rab11-FIP4 knockout had a significant effect on PANC-1 cell
proliferation. We found that deficiency of Rab11-FIP4 in PANC-1
cells did not significantly alter apoptosis, suggesting that the
effects of Rab11-FIP4 on cell growth contribute to alterations in
cell proliferation, rather than the alterations in apoptosis. Cell
cycle analysis further manifested a prominent decrease in stage S
and G2/M in Rab11-FIP4 knockout cells, which showed that Rab11-FIP4
knockout induces cell cycle arrest in PANC-1 cells. Thus,
Rab11-FIP4 knockout can suppress PANC-1 cell growth.
To further evaluate the impact of Rab11-FIP4 on
PANC-1 cell invasion and metastasis, a Transwell assay was
performed. The results indicated that knockout of Rab11-FIP4
suppressed the migratory and invasive ability of PANC-1 cells.
Therefore, knockout of Rab11-FIP4 suppresses PANC-1 cell growth,
invasion and metastasis. The underlying mechanisms need to be
explored in further studies. The overexpression of Rab11-FIP4 may
enhance PANC-1 cell growth, invasion and metastasis, leading to
unfavourable outcomes in PC.
In conclusion, our findings suggest that Rab11-FIP4
exhibits tumor promotion and leads to a poor clinical outcome in
PC. Rab11-FIP4 may be a novel target gene for pancreatic cancer
therapy.
Acknowledgments
This study received research funding from the
Zhejiang Provincial Natural Science Foundation of China
(Y2100546).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goral V: Pancreatic cancer: Pathogenesis
and diagnosis. Asian Pac J Cancer Prev. 16:5619–5624. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuno S, Egawa S, Fukuyama S, Motoi F,
Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, et al:
Pancreatic Cancer Registry in Japan: 20 years of experience.
Pancreas. 28:219–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ullrich O, Reinsch S, Urbé S, Zerial M and
Parton RG: Rab11 regulates recycling through the pericentriolar
recycling endosome. J Cell Biol. 135:913–924. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilcke M, Johannes L, Galli T, Mayau V,
Goud B and Salamero J: Rab11 regulates the compartmentalization of
early endosomes required for efficient transport from early
endosomes to the trans-golgi network. J Cell Biol. 151:1207–1220.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riggs B, Rothwell W, Mische S, Hickson GR,
Matheson J, Hays TS, Gould GW and Sullivan W: Actin cytoskeleton
remodeling during early Drosophila furrow formation requires
recycling endosomal components Nuclear-fallout and Rab11. J Cell
Biol. 163:143–154. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Junutula JR, Schonteich E, Wilson GM,
Peden AA, Scheller RH and Prekeris R: Molecular characterization of
Rab11 interactions with members of the family of Rab11-interacting
proteins. J Biol Chem. 279:33430–33437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horgan CP and McCaffrey MW: The dynamic
Rab11-FIPs. Biochem Soc Trans. 37:1032–1036. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hales CM, Griner R, Hobdy-Henderson KC,
Dorn MC, Hardy D, Kumar R, Navarre J, Chan EK, Lapierre LA and
Goldenring JR: Identification and characterization of a family of
Rab11-interacting proteins. J Biol Chem. 276:39067–39075. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fielding AB, Schonteich E, Matheson J,
Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R
and Gould GW: Rab11-FIP3 and FIP4 interact with Arf6 and the
exocyst to control membrane traffic in cytokinesis. EMBO J.
24:3389–3399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muto A, Aoki Y and Watanabe S: Mouse
Rab11-FIP4 regulates proliferation and differentiation of retinal
progenitors in a Rab11-independent manner. Dev Dyn. 236:214–225.
2007. View Article : Google Scholar
|
|
12
|
Muto A, Arai K and Watanabe S: Rab11-FIP4
is predominantly expressed in neural tissues and involved in
proliferation as well as in differentiation during zebrafish
retinal development. Dev Biol. 292:90–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon SO, Shin S and Mercurio AM: Hypoxia
stimulates carcinoma invasion by stabilizing microtubules and
promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer
Res. 65:2761–2769. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu F, Deng X, Yang X, Jin H, Gu D, Lv X,
Wang C, Zhang Y, Huo X, Shen Q, et al: Hypoxia upregulates
Rab11-family interacting protein 4 through HIF-1α to promote the
metastasis of hepatocellular carcinoma. Oncogene. 34:6007–6017.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaika NV, Gebregiworgis T, Lewallen ME,
Purohit V, Radhakrishnan P, Liu X, Zhang B, Mehla K, Brown RB,
Caffrey T, et al: MUC1 mucin stabilizes and activates
hypoxia-inducible factor 1 alpha to regulate metabolism in
pancreatic cancer. Proc Natl Acad Sci USA. 109:13787–13792. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye LY, Zhang Q, Bai XL, Pankaj P, Hu QD
and Liang TB: Hypoxia-inducible factor 1α expression and its
clinical significance in pancreatic cancer: A meta-analysis.
Pancreatology. 14:391–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao XP, Willis JE, Pretlow TG, Rao JS,
MacLennan GT, Talbot IC and Pretlow TP: Loss of fragile histidine
triad expression in colorectal carcinomas and premalignant lesions.
Cancer Res. 60:18–21. 2000.PubMed/NCBI
|
|
18
|
Xue Z, Zhou Y, Wang C, Zheng J, Zhang P,
Zhou L, Wu L, Shan Y, Ye M, He Y, et al: Latexin exhibits
tumor-suppressor potential in pancreatic ductal adenocarcinoma.
Oncol Rep. 35:50–58. 2016.
|
|
19
|
Cong L and Zhang F: Genome engineering
using CRISPR-Cas9 system. Methods Mol Biol. 1239:197–217. 2015.
View Article : Google Scholar
|
|
20
|
Zhou Y, Zhu S, Cai C, Yuan P, Li C, Huang
Y and Wei W: High-throughput screening of a CRISPR/Cas9 library for
functional genomics in human cells. Nature. 509:487–491. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shalem O, Sanjana NE, Hartenian E, Shi X,
Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, et
al: Genome-scale CRISPR-Cas9 knockout screening in human cells.
Science. 343:84–87. 2014. View Article : Google Scholar :
|
|
22
|
Hsu PD, Lander ES and Zhang F: Development
and applications of CRISPR-Cas9 for genome engineering. Cell.
157:1262–1278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rachagani S, Macha MA, Heimann N,
Seshacharyulu P, Haridas D, Chugh S and Batra SK: Clinical
implications of miRNAs in the pathogenesis, diagnosis and therapy
of pancreatic cancer. Adv Drug Deliv Rev. 81:16–33. 2015.
View Article : Google Scholar :
|
|
24
|
Shahrokni A and Saif MW: Metastatic
pancreatic cancer: The dilemma of quality vs. quantity of life.
JOP. 14:391–394. 2013.PubMed/NCBI
|
|
25
|
Horgan CP, Hanscom SR, Kelly EE and
McCaffrey MW: Tumor susceptibility gene 101 (TSG101) is a novel
binding-partner for the class II Rab11-FIPs. PLoS One.
7:e320302012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng H, Sugiura R, Wu W, Fujita M, Lu Y,
Sio SO, Kawai R, Takegawa K, Shuntoh H and Kuno T: Role of the Rab
GTP-binding protein Ypt3 in the fission yeast exocytic pathway and
its connection to calcineurin function. Mol Biol Cell.
13:2963–2976. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krzyzaniak MA, Mach M and Britt WJ:
HCMV-encoded glycoprotein M (UL100) interacts with Rab11 effector
protein FIP4. Traffic. 10:1439–1457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wallace DM, Lindsay AJ, Hendrick AG and
McCaffrey MW: Rab11-FIP4 interacts with Rab11 in a GTP-dependent
manner and its overexpression condenses the Rab11 positive
compartment in HeLa cells. Biochem Biophys Res Commun. 299:770–779.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu CL, Wang JZ, Xia XP, Pan CW, Shao XX,
Xia SL, Yang SX and Zheng B: Rab11-FIP2 promotes colorectal cancer
migration and invasion by regulating PI3K/AKT/MMP7 signaling
pathway. Biochem Biophys Res Commun. 470:397–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Liu X, Datta A, Govindarajan K,
Tam WL, Han J, George J, Wong C, Ramnarayanan K, Phua TY, et al:
RCP is a human breast cancer-promoting gene with Ras-activating
function. J Clin Invest. 119:2171–2183. 2009.PubMed/NCBI
|
|
31
|
Jing J, Tarbutton E, Wilson G and Prekeris
R: Rab11-FIP3 is a Rab11-binding protein that regulates breast
cancer cell motility by modulating the actin cytoskeleton. Eur J
Cell Biol. 88:325–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong W, Qin G and Shen R: Rab11-FIP2
promotes the metastasis of gastric cancer cells. Int J Cancer.
138:1680–1688. 2016. View Article : Google Scholar
|