Introduction
Hepatocellular carcinoma (HCC) is the sixth most
prevalent type of cancer and the second leading cause of
cancer-related deaths worldwide (1–3).
Epidemiological studies show that the incidence of HCC is markedly
varied across geographical regions, ancestry groups and between
genders (4). HCC incidence is
highest in East Asia and Africa and rapid increases in prevalence
have occurred in Western countries (5). The typical characteristics of HCC
include fast infiltrating growth, abnormal cell differentiation,
high-grade malignancy, early metastasis and poor prognosis
(6,7). Therefore, identifying novel and
reliable biomarkers to identify, predict and treat HCC are urgently
needed.
NEK2 [NIMA (never in mitosis gene A)-related
expressed kinase 2], a serine/threonine centrosomal kinase, plays a
critical role in regulating the cell cycle and mitosis by
centrosome splitting during the cell division process (8). Uncontrolled NEK2 activity can result
in chromosome instability (CIN) and abnormal chromosome content
(9,10). NEK2 overexpression has been
demonstrated in several types of human cancers, such as breast
(11–14), prostate (11), non-Hodgkin lymphoma (15) and colorectal cancer (16). Additionally, some reports have
indicated NEK2 as a potential biomarker for pancreatic ductal
adenocarcinoma and non-small cell lung cancer prognosis (17,18),
but NEK2 has rarely been investigated with regard to HCC.
With the advancements in the next generation of
sequencing technologies, RNA-seq has become a powerful tool for
deciphering global gene expression patterns, including an
unprecedented capability to discover novel genes, alternative
transcript variants, chimeric transcripts, expressed sequence
variants and allele-specific expressions (19–22).
RNA-seq has expanded the study of cancer transcriptomics in the
areas of gene expression, chimeric events and alternative splicing
in search of novel biomarkers for the disease (23).
By integrating the RNA-seq data for the cells in the
present study and the tissues in the study by Huang et al
(24), we found that NEK2
expression was significantly upregulated and associated with a poor
prognosis in patients with HCC. Therefore, NEK2 may be a very
promising prognostic biomarker for predicting HCC. Furthermore, the
possible mechanisms responsible for NEK2 overexpression in HCC also
were investigated.
Materials and methods
Patient information
A total of 63 patients who were diagnosed with HCC
and treated with partial liver resection surgery at the Affiliated
Tumor Hospital of Guangxi Medical University from 2010 to 2013 were
enrolled in this study. These patients included 52 males and 11
females, with a mean age of 47.86 years (range, 28–71 years) at the
time of the operation. The patients were pathologically diagnosed
with HCC of histological grade II (n=28), grade III (n=20) and
grade IV (n=15) according to the modified nuclear grading scheme
outlined by the Edmondson and Steiner system. A summary of the
patient characteristics and the pathological characteristics is
presented in Table I. For
validation using quantitative reverse transcription-polymerase
chain reaction (qRT-PCR), HCC tissues and matched adjacent
non-tumorous liver tissues from 5 different HCC patients (aged
42–68 years) were provided by the First Affiliated Hospital of
Guangxi Medical University in 2014. No prior treatments (including
chemotherapy or radiotherapy) were conducted before the liver
resection surgery. This study was approved by the Ethics Committee
of the Guangxi Medical University. All patients provided written
informed consent in order to participate in this study.
 | Table IRelationships between NEK2,
phospho-AKT and MMP-2 expression and clinicopathological variables
of HCC. |
Table I
Relationships between NEK2,
phospho-AKT and MMP-2 expression and clinicopathological variables
of HCC.
|
Characteristics | n | NEK2 (lg IOD)
| P-AKT (lg IOD)
| MMP-2 (lg IOD)
|
|---|
| Mean ± SD | P-value | Mean ± SD | P-value | Mean ± SD | P-value |
|---|
| Age (years) | | | 0.321 | | 0.551 | | 0.415 |
| ≤50 | 36 | 3.64±0.86 | | 2.98±0.73 | | 4.47±0.56 | |
| >50 | 27 | 3.72±0.90 | | 3.08±0.71 | | 4.56±0.40 | |
| Gender | | | 0.285 | | 0.805 | | 0.450 |
| Male | 52 | 3.69±0.92 | | 3.03±0.74 | | 4.45±0.45 | |
| Female | 11 | 3.60±0.66 | | 2.97±0.55 | | 4.41±0.70 | |
| Serum AFP
(ng/ml) | | | 0.076 | | 0.164 | | 0.497 |
| ≤25 | 28 | 3.75±0.91 | | 3.20±0.72 | | 4.55±0.54 | |
| 25–400 | 6 | 3.77±0.65 | | 2.93±0.62 | | 4.36±0.49 | |
| >400 | 29 | 3.59 ±0.89 | | 2.86±0.73 | | 4.51±0.44 | |
| Tumor size
(cm) | | | 0.024a | | 0.041a | | 0.013a |
| ≤10 | 46 | 3.73±0.89 | | 3.13±0.75 | | 4.65±0.41 | |
| >10 | 17 | 3.53±0.84 | | 2.78±0.60 | | 4.29±0.72 | |
| Portal vein
thrombosis | | | 0.428 | | 0.282 | | 0.628 |
| Presence | 20 | 3.62±0.80 | | 3.14±0.80 | | 4.46±0.41 | |
| Absence | 43 | 3.70±0.92 | | 2.95±0.67 | | 4.52±0.53 | |
| Diolame
complete | | | 0.000c | | 0.948 | | 0.009b |
| Yes | 28 | 3.51±0.97 | | 3.03±0.75 | | 4.41±0.56 | |
| No | 35 | 3.79±0.79 | | 3.02±0.69 | | 4.72±0.38 | |
| Tumor nodule
number | | | 0.012a | | 0.046a | | 0.024a |
| Solitary | 44 | 3.59±0.91 | | 2.96±0.71 | | 4.44±0.55 | |
| Multiple (≥2) | 19 | 3.80±0.76 | | 3.34±0.74 | | 4.75±0.44 | |
| Edmondson
grade | | | 0.121 | | 0.293 | | 0.855 |
| II | 28 | 3.85±0.88 | | 3.29±0.85 | | 4.62±0.57 | |
| III | 20 | 3.97±0.53 | | 3.02±0.35 | | 4.54±0.44 | |
| IV | 15 | 3.61±0.90 | | 2.80±0.57 | | 4.50±0.39 | |
| Liver
cirrhosis | | | 0.474 | | 0.728 | | 0.192 |
| Yes | 53 | 3.68±0.84 | | 3.01±0.71 | | 4.54±0.50 | |
| No | 10 | 3.57±1.21 | | 2.93±0.71 | | 4.33±0.52 | |
| HBV-DNA | | | 0.435 | | 0.918 | | 0.315 |
| Positive | 42 | 3.67±0.90 | | 3.02±0.76 | | 4.51±0.47 | |
| Negative | 21 | 3.73±0.79 | | 3.04±0.56 | | 4.62±0.32 | |
| Recurrence | | | 0.004b | | 0.045a | | 0.992 |
| Yes | 32 | 3.98±0.77 | | 3.17±0.53 | | 4.57±0.48 | |
| No | 31 | 3.66±0.92 | | 2.66±0.86 | | 4.57±0.47 | |
Tissues samples and cell lines
All HCC tissues and matched adjacent non-tumorous
liver tissues were obtained immediately after hepatectomy and were
frozen in liquid nitrogen and stored at −80°C or collected in 10%
formalin and embedded in paraffin for histopathological analysis.
The human HCC cell line SMMC-7721 and primary human normal liver
cell line HL-7702 were purchased from the Committee on Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). All
cell lines were maintained under recommended culture conditions.
Cells were incubated in a 37°C humidified incubator containing 5%
CO2.
Transcriptome sequencing
For whole transcriptome sequencing, RNA from
1×107 SMMC-7721 and HL-7702 cells was extracted using
the TRIzol reagent kit (Invitrogen, Waltham, MA, USA) and then
quantified using NanoDrop 2000 (Thermo Fisher Scientific, Waltham,
MA, USA). The whole transcriptome RNA-seq procedure was performed
using the Ion Total RNA-Seq kit, the Ion PI™ Chip kit, the Ion PI™
Template OT2 200 kit, and the Ion PI™ Sequencing 200 kit based on
the protocols of Life Technologies Corp. (Waltham, MA, USA). In
brief, mRNA was purified using oligo-dT beads from 100 µg of
total RNAs for each sample and then fragmented. The cleaved RNA
fragments were reverse-transcribed into First-Strand cDNA, followed
by Second-Strand cDNA synthesis. Then, a single 'A' base was added
to the cDNA fragments at the 3′ end. The cDNAs were ligated to
adapters and enriched by polymerase chain reaction (PCR) to
generate the final cDNA library. After amplifying the sequencing
template, RNA-seq was performed using the Ion Proton System (Life
Technologies) with the standard protocol.
qRT-PCR
Total RNA was extracted from cell lines and liver
specimens using the TRIzol reagent kit (Invitrogen) according to
the manufacturer's instructions. To avoid any DNA contamination,
isolated RNA was treated with RNase-free DNase I (Invitrogen) and
quantified by NanoDrop 2000 (Thermo Fisher Scientific). The RNA
samples were measured using optical density at 260 nm and then
reverse-transcribed into cDNA using the M-MLV First-Strand system
for the qRT-PCR kit (Invitrogen) according to the manufacturer's
protocols. qRT-PCR was performed using the FastStart Universal
SYBR-Green Master (Roche Diagnostics, Shanghai, China) and repeated
three times in an ABI 7500 system. The primer sequences used to
detect mRNA were as follows: NEK2, forward
5′-CTTCCCGGGCTGAGGACTAT-3′ and reverse 5′-TCAGCTTCTGTCATGGAGCC-3′;
β-actin, forward 5′-GGGAAATCGTGCGTGACAT-3′ and reverse
5′-CTGGAAGGTGGACAGCGAG-3′.
The PCR cycling conditions were as follows: initial
melting at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec
and 60°C for 64 sec. Analysis of the melting curve for the primers
was conducted to confirm the specificity of the PCR product, and
the threshold cycle (Ct) value for triplicate reactions was
averaged. The relative expression of NEK2 mRNA for each sample was
calculated as follows: ΔCt = Ct (sample) − Ct (β-actin), ΔΔCt
(sample) = ΔCt (sample) − ΔCt (calibrator). The fold changes in
mRNA were calculated through relative quantification
(2−ΔΔCt).
Immunohistochemistry and
immunohistochemical assessment
Immunohistochemical studies on NEK2, phospho-AKT and
MMP-2 were performed on formalin-fixed, paraffin-embedded tissue
sections obtained from the aforementioned patients with HCC and
were performed according to the standard procedures. Sections were
cut at a thickness of 5 µm and heated in a 60°C oven.
Briefly, tissue sections were deparaffinized, rehydrated and boiled
in 0.01 mol/l sodium citrate buffer (pH 6.0) in a microwave oven
for 10 min for antigen epitope retrieval. Endogenous peroxidase was
blocked with 0.3% hydrogen peroxide for 10 min. Then, the sections
were blocked for 30 min using 10% normal goat serum and were
separately incubated with the primary antibodies directed against
NEK2 (ab55550, 1:1,000 dilution; Abcam, Cambridge, MA, USA),
phospho-AKT (Ser473) (#4060, 1:50 dilution; Cell Signaling
Technology) and MMP-2 (ab86607 1:500, dilution; Abcam) at 37°C for
3 h. After washing, the sections were incubated for 30 min with
biotinylated secondary antibody (Envision™ Detection kit; Gene
Tech, Shanghai, China) at 37°C. The staining of the tissue sections
was performed using the streptavidin-biotin-peroxidase complex for
NEK2, phospho-AKT and MMP-2. The complex was visualized with
diaminobenzidine (DAB) and counterstained with hematoxylin. The
sections were then dehydrated in a graded series of alcohol,
cleared in xylene and mounted onto glass slides.
The staining was quantified by digital image
analysis with Image-Pro Plus 6.0 software (Media Cybernetics,
Silver Spring, MD, USA) according to the method developed by Xavie
et al (25). Briefly, an
area of interest in each section was first selected at ×40
magnification, and 10 digital images at 1360×1024 pixel resolution
and ×400 magnification were captured using an AX-70 microscope
equipped with a DP70 CCD camera (Olympus, Tokyo, Japan). Identical
settings were used for each field. The measurement parameter was
integrated optical density (IOD). Optical density was calibrated,
and the area of interest was set as follows: hue, 0–30; saturation,
0–255; and intensity, 0–255. The values were then counted.
The Cancer Genome Atlas (TCGA) data
acquisition and survival analysis
The TCGA project provides multimodal data on 359 HCC
cases, which can be acquired from the TCGA website (https://tcga-data.nci.nih.gov/tcga/). The dataset
was searched for HCC cases based on the RNASeqV2 data. The
expression value of the NEK2 gene was collected for each case and
was divided into the high-expression and the low-expression groups
using the cut-off point. Kaplan-Meier survival analysis was used to
determine the survival differences between the high-expression and
low-expression subgroups, with P-values calculated using the
log-rank test.
Statistical analysis
Statistical analyses were performed with IBM SPSS
Statistics 20.0 (IBM Corp., Armonk, NY, USA). Relationships between
the expressions of NEK2, phospho-AKT and MMP-2 and the
clinicopathological parameters were determined using the two-tailed
unpaired Student's t-test. Significance among three groups was
determined by analysis of variance (ANOVA) followed by the Duncan's
new multiple range test. The correlations between the NEK2
expression with phospho-AKT and MMP-2 expressions were studied
using the Spearman's coefficient. Data are shown as the means ±
standard error of the means. P<0.05 was considered to indicate
statistically significant differences.
Results
Analysis of the cell RNA-seq data
RNA-seq of the HCC cell line SMMC-7721 and the
normal liver cell line HL-7702 was performed using the Ion Proton
System. On average, ~78.1 million 96-bp-long sequencing reads and
7.5 G of raw sequence data were obtained for samples sequenced on
one lane. The normalized gene expression was measured as fragments
per kilobase of transcript per million mapped reads (FPKM). To
evaluate differential gene expression, the absolute value of the
log2-transformed fold change (FC) ≥1 and the q-values <0.05 were
used as the criteria to determine the significance of gene
expression differences. A total of 610 differentially expressed
genes (DEGs) were revealed in the transcriptome comparison, 297 of
which were upregulated and 313 were downregulated in HCC.
NEK2 was listed as an HCC candidate
biomarker by integrated analysis
Huang et al (24) performed RNA-seq analyses of 10
matched pairs of cancerous and non-cancerous tissues from HCC
patients on the Solexa/Illumina GAII platform. The results showed
that a total of 1378 DEGs with 808 upregulation and 570
downregulation in HCC tissues compared with adjacent non-tumorous
liver tissues. An integrated analysis was then performed on the
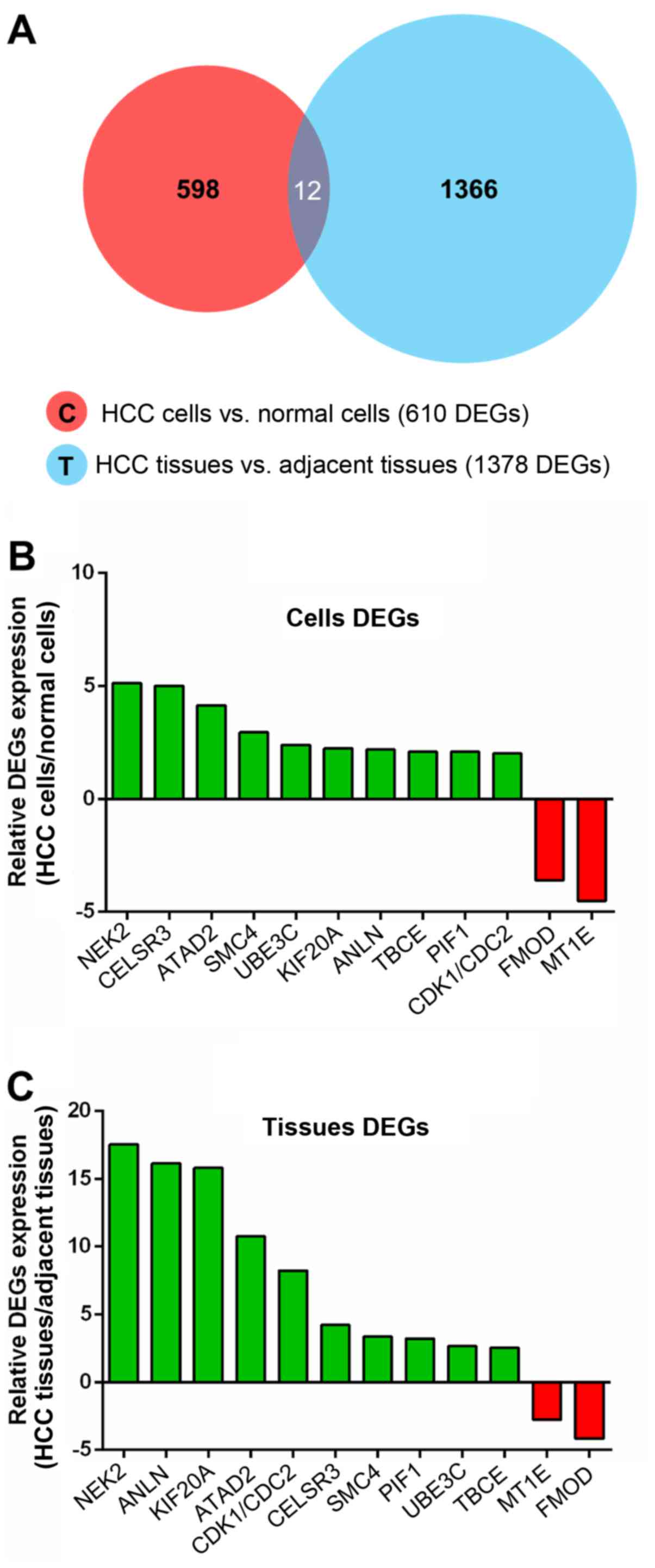
RNA-seq data of the cells and tissues. As shown in the Venn diagram
in Fig. 1A, 12 common differential
genes were found between the HCC cells and the normal cells and
between the HCC tissues and the adjacent tissues, 10 of which were
upregulated and 2 were downregulated in HCC (Table II). Excitingly, NEK2 exhibited the
most significant difference in expression of all the DEGs in the
cell and tissue RNA-seq data and was therefore listed as an HCC
candidate biomarker for further verification (Fig. 1B and C).
 | Table IIList of common differential genes
between cells DEGs and tissues DEGs. |
Table II
List of common differential genes
between cells DEGs and tissues DEGs.
| No. | Gene symbol | Regulation | Cells
(HCC/N) | Tissues
(HCC/N) | Gene
description |
|---|
| 1 | CELSR3 | Upregulation | 5.00 | 4.21 | Cadherin EGF LAG
seven-pass G-type receptor 3 precursor |
| 2 | UBE3C | Upregulation | 2.39 | 2.67 | Ubiquitin-protein
ligase E3C |
| 3 | ANLN | Upregulation | 2.18 | 18.13 | Actin-binding
protein anillin |
| 4 | KIF20A | Upregulation | 2.24 | 15.8 | Kinesin-like
protein KIF20A |
| 5 | SMC4 | Upregulation | 2.94 | 3.35 | Structural
maintenance of chromosomes protein 4 |
| 6 | TBCE | Upregulation | 2.08 | 2.53 | Tubulin-specific
chaperone E |
| 7 | NEK2 | Upregulation | 5.13 | 17.52 |
Serine/threonine-protein kinase Nek2 |
| 8 | PIF1 | Upregulation | 2.08 | 3.21 | ATP-dependent DNA
helicase PIF1 |
| 9 | ATAD2 | Upregulation | 4.14 | 10.74 | ATPase family AAA
domain-containing protein 2 |
| 10 | CDK1/CDC2 | Upregulation | 2.00 | 8.22 | Cell division
control protein 2 homolog |
| 11 | FMOD | Downregulation | 0.28 | 0.24 | Fibromodulin
precursor |
| 12 | MT1E | Downregulation | 0.22 | 0.36 |
Metallothionein-1E |
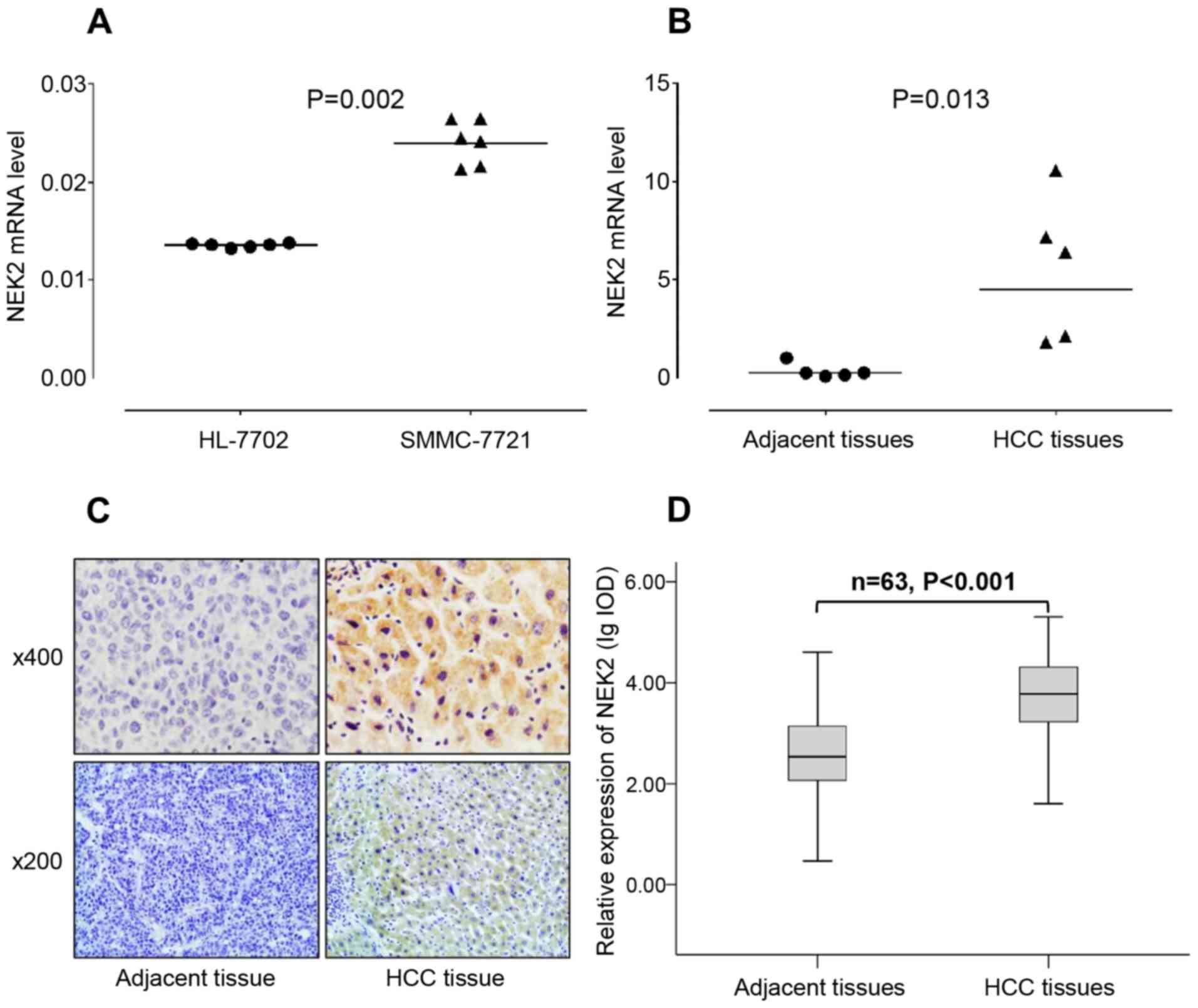
The overexpression of NEK2 in HCC cells
and tissues
NEK2 expression status in the HCC cell line
SMMC-7721 and the primary HCC tissue samples was assessed using
qRT-PCR. The NEK2 mRNA transcript level was 0.024±0.0026 in the HCC
cell line SMMC-7721, which was 1.71-fold higher than the NEK2 mRNA
level of 0.014±0.0003 in the normal liver cell line HL-7702
(P=0.002) (Fig. 2A), as evidenced
by qRT-PCR analysis using β-actin as a loading control. In 5
matched HCC and adjacent non-tumorous liver tissue samples, the
NEK2 mRNA level in the HCC tissues was 5.60±3.69, which was
16.47-fold higher than the NEK2 mRNA level of 0.34±0.38 in adjacent
non-tumorous liver tissues (P=0.013) (Fig. 2B).
The expression of NEK2 in 63 cases of HCC and
matched adjacent non-tumorous liver tissues was examined using
immunohistochemical staining. The NEK2 protein is mainly expressed
in the cytoplasm, and we quantified its expression using Image-Pro
Plus 6.0 digital image analysis software. We found that NEK2
expression presented as positive staining in HCC tissues and
negative staining in adjacent non-tumorous liver tissues (Fig. 2C). Furthermore, the expression of
NEK2 protein was significantly higher in the HCC tissues than in
the adjacent non-tumorous liver tissues (P<0.001) (Fig. 2D).
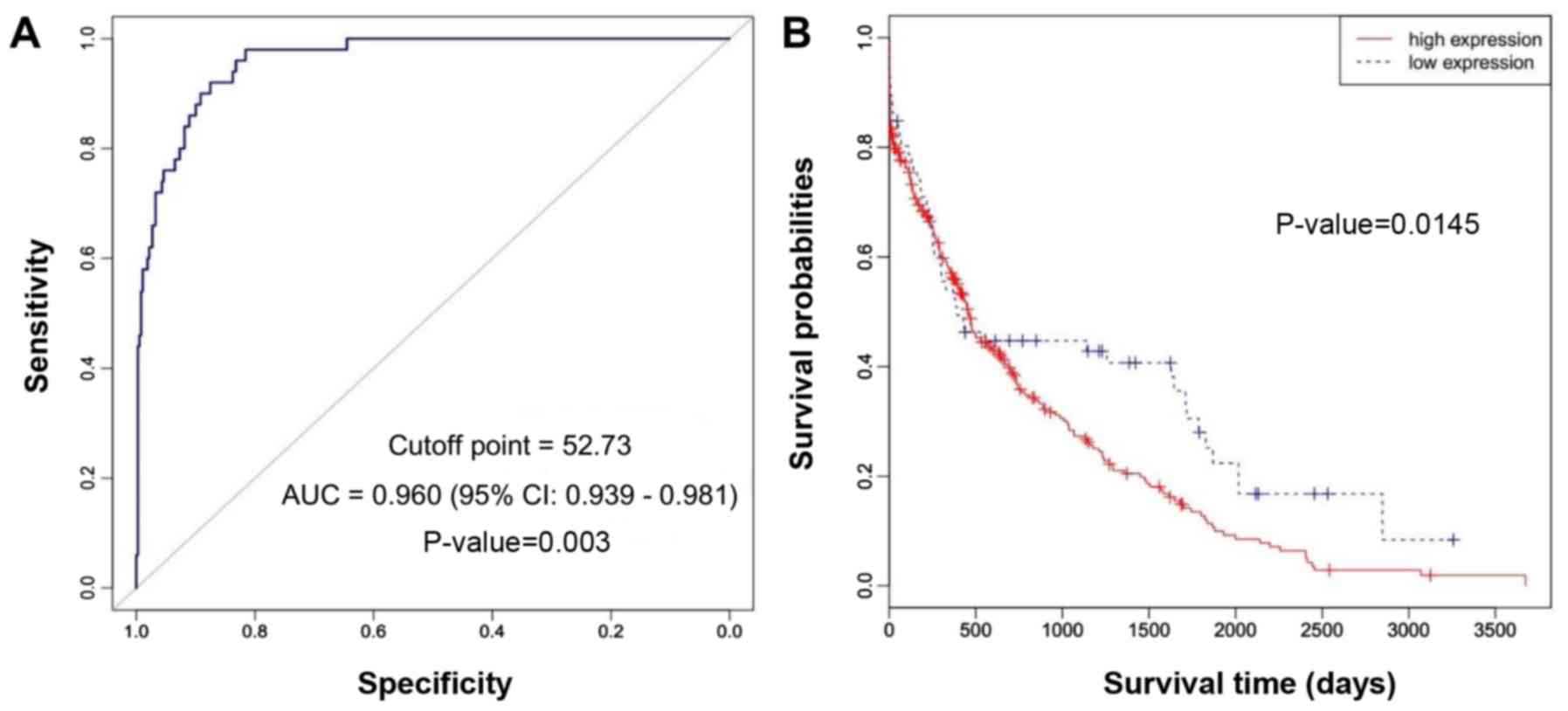
NEK2 overexpression is significantly
associated with poor prognosis in HCC
To evaluate the clinical significance of NEK2
overexpression, the correlation between the survival and the
expression of NEK2 in the cases of 359 patients with HCC was
analyzed using RNASeqV2 data available from The Cancer Genome Atlas
(TCGA) website. According to the receiver operating characteristic
(ROC) curve, we defined RPKM=52.73 as the cut-off point to
distinguish HCC patients with high and low NEK2 expression levels
(Fig. 3A). The sensitivity and
specificity of NEK2 in the diagnosis of HCC were 0.98 and 0.82,
respectively. Kaplan-Meier survival analysis of HCC patients was
performed based on the expression levels of NEK2. The results
revealed that HCC patients with a high expression of NEK2 had a
poor prognosis (log-rank test, P=0.0145) (Fig. 3B).
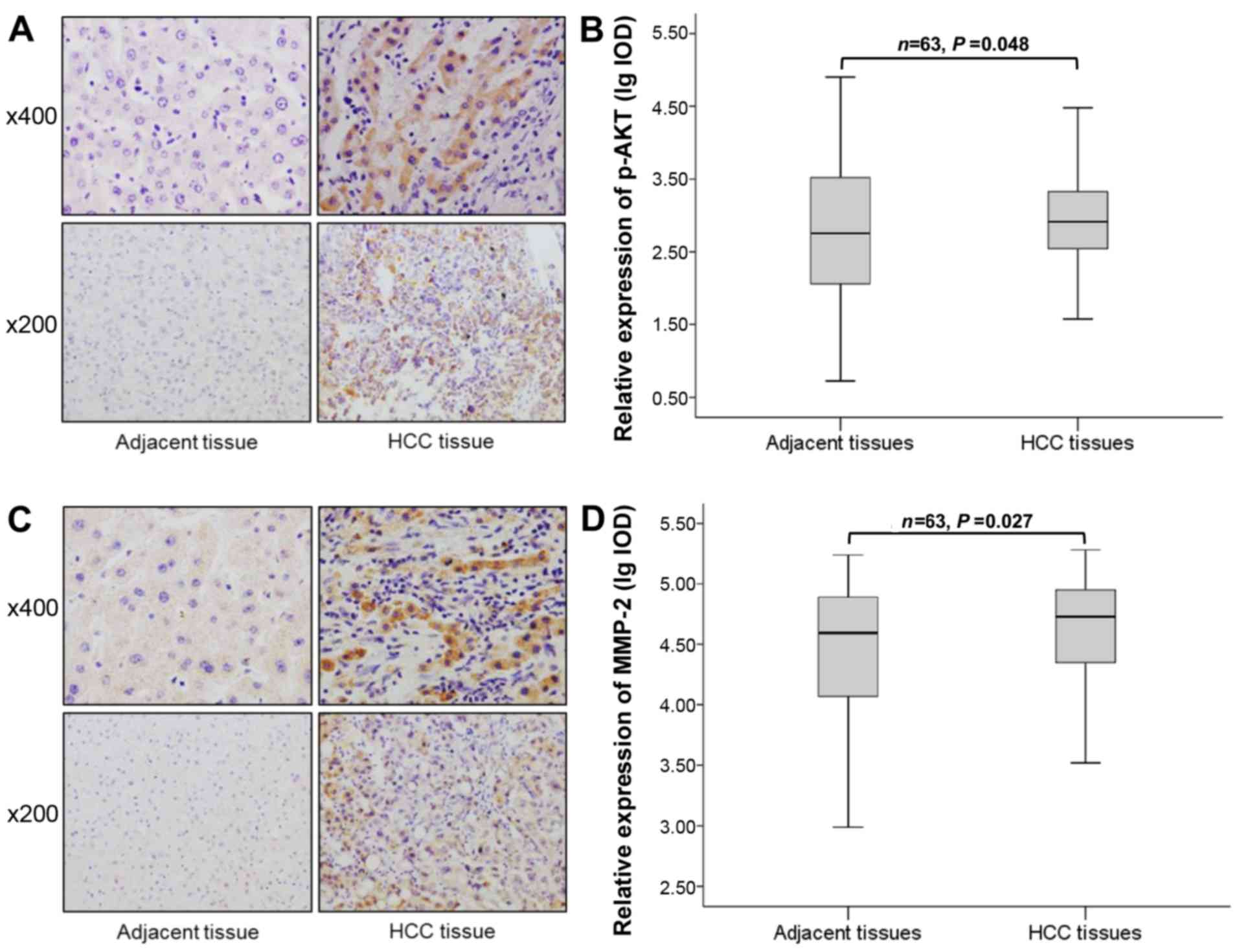
Expressions of phospho-AKT and MMP-2 were
increased in HCC clinical samples
Previous studies suggested that the overexpression
of NEK2 promotes activation of AKT, a potent and critical oncogene
for a variety of malignancies (26,27).
Moreover, the PI3K/AKT signaling pathway plays an important role in
upregulating MMP expression (28).
Therefore, to further investigate the relationship between the NEK2
expression and the expression of phospho-AKT and MMP-2 in HCC, we
assessed the expression of phospho-AKT and MMP-2 in 63 cases of HCC
and matched adjacent non-tumorous liver tissues using
immunohistochemical staining. The staining for phospho-AKT and
MMP-2 was mostly positive in the cytoplasm of the tumor cells
(Fig. 4A and C). We found that the
expression of phospho-AKT and MMP-2 was increased in HCC tissues
compared with matched adjacent non-tumorous liver tissues (P=0.048
and 0.027) (Fig. 4B and D).
Relationships between the expression of
NEK2, phospho-AKT, and MMP-2 and clinicopathological
parameters
Table I summarizes
the relationships between the expression of NEK2, phospho-AKT and
MMP-2 and the clinicopathological parameters of patients with HCC,
including patient age, gender, AFP level, tumor size, portal vein
thrombosis, diolame complete, tumor nodule number, Edmondson grade,
cirrhosis, HBV DNA and recurrence. The results showed that NEK2,
phospho-AKT and MMP-2 expression in the HCC tumor size ≤10 cm group
was 1.58-fold (P=0.024), 2.24-fold (P=0.041), and 2.29-fold
(P=0.013) higher, respectively, than that in the HCC tumor size
>10 cm group (Fig. 5A). NEK2
and MMP-2 expression in the HCC diolame incomplete group was
1.91-fold (P<0.001) and 2.04-fold (P=0.009) higher,
respectively, than that in the HCC diolame complete group, but no
obvious change was observed in phospho-AKT expression in the HCC
diolame incomplete group (P=0.948) (Fig. 5B). NEK2, p-AKT and MMP-2 expression
in the HCC multinodular group was 1.62-fold (P=0.012), 2.40-fold
(P=0.046), and 2.04-fold (P=0.024) higher, respectively, than that
in the HCC uninodular group (Fig.
5C). NEK2 and p-AKT expression in the HCC recurrence group was
2.09-fold (P=0.004) and 3.24-fold (P=0.045) higher, respectively,
than that in the HCC non-recurrence group, but no obvious change
was observed in MMP-2 expression in the HCC non-recurrence group
(P=0.992) (Fig. 5D).
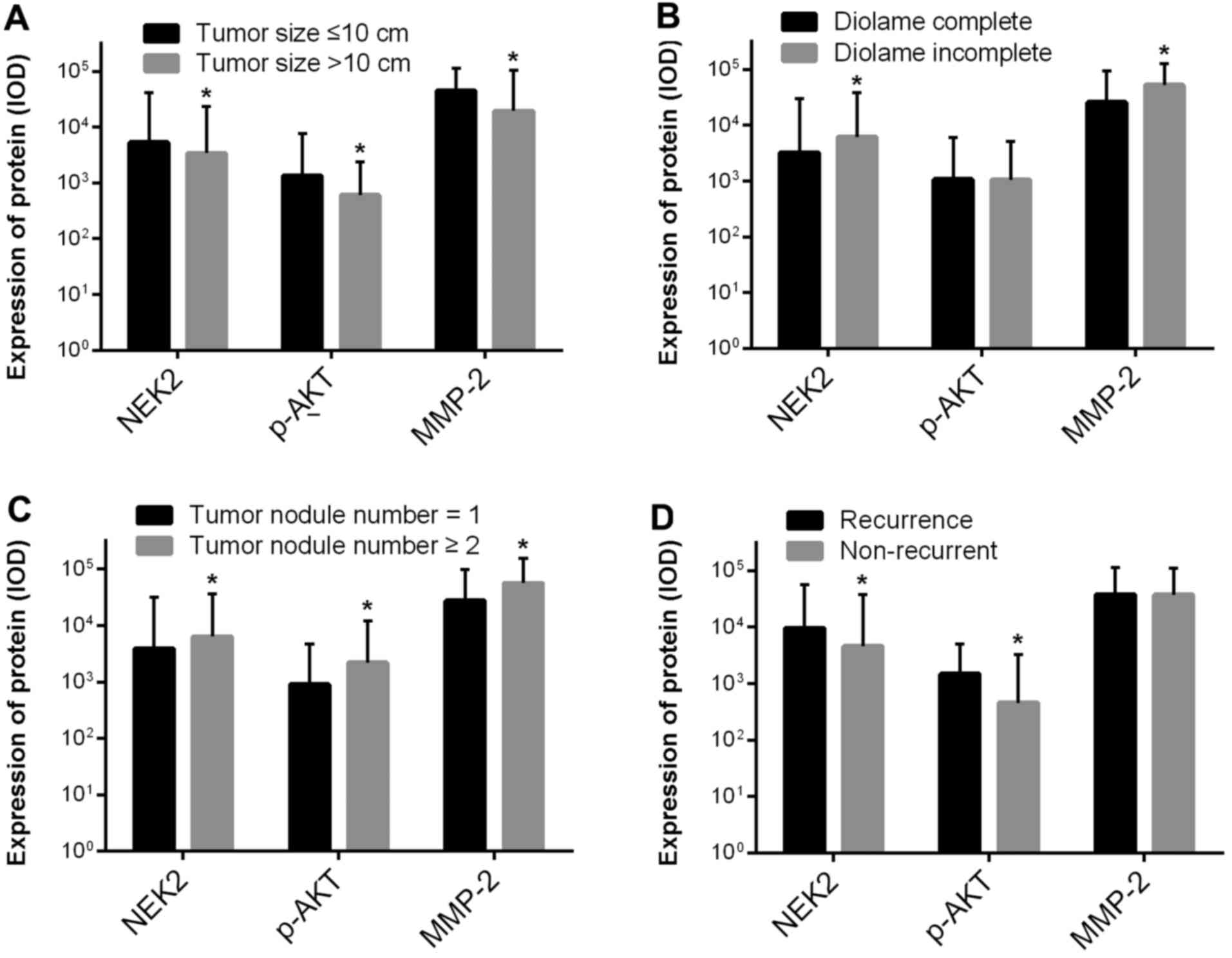 | Figure 5Relationships between NEK2,
phospho-AKT, and MMP-2 expression and clinicopathological features
in HCC. (A) NEK2, phospho-AKT and MMP-2 expression in the HCC tumor
size ≤10 cm group was 1.58-fold (P=0.024), 2.24-fold (P=0.041) and
2.29-fold (P=0.013) higher, respectively, than that in the HCC
tumor size >10 cm group. (B) NEK2 and MMP-2 expression in the
HCC diolame incomplete group was 1.91-fold (P<0.001) and
2.04-fold (P=0.009) higher, respectively, than that in the HCC
diolame complete group, but no obvious change was observed in
phospho-AKT expression in the HCC diolame incomplete group
(P=0.948). (C) NEK2, p-AKT and MMP-2 expression in the HCC
multinodular group was 1.62-fold (P=0.012), 2.40-fold (P=0.046),
and 2.04-fold (P=0.024) higher, respectively, than that in the HCC
uninodular group. (D) NEK2 and p-AKT expression in the HCC
recurrence group was 2.09-fold (P=0.004) and 3.24-fold (P=0.045)
higher, respectively, than that in the HCC non-recurrence group,
but no obvious change was observed in MMP-2 expression in the HCC
non-recurrence group (P=0.992). |
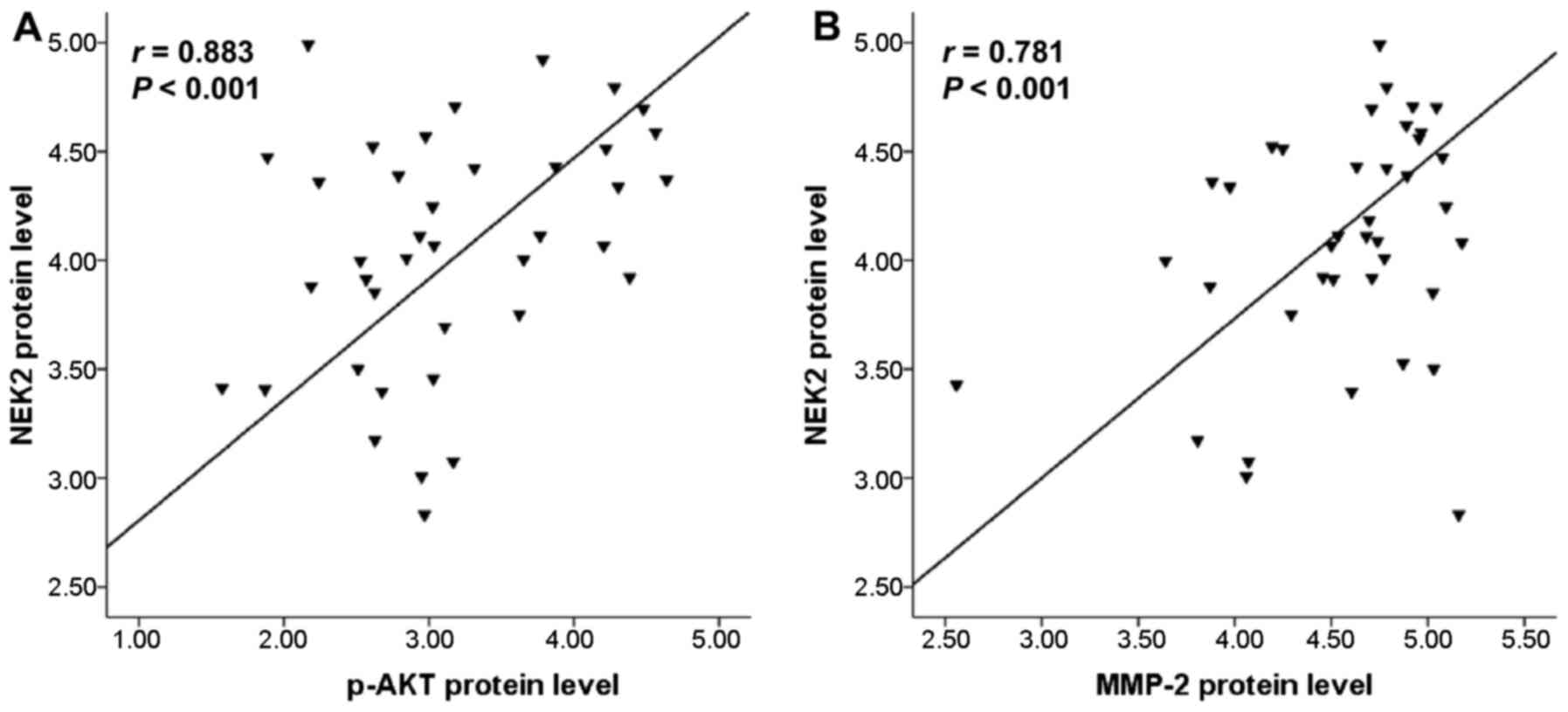
Positive correlation between NEK2
expression with phospho-AKT and MMP-2 expression
Additionally, to explore whether the NEK2 expression
level was correlated with phospho-AKT and MMP-2 expression, the
protein expressions of phospho-AKT and MMP-2 were examined in 63
cases of HCC tissues using immunohistochemical staining. Therefore,
the correlations between NEK2 expression with phospho-AKT and MMP-2
expression were analyzed. The results showed that there was indeed
evident positive correlation between the protein expression level
of NEK2 and phospho-AKT (r=0.883, P<0.01) (Fig. 6A). Notably, a significant positive
correlation was observed between the protein expression levels of
NEK2 and MMP-2 (r=0.781, P<0.01) (Fig. 6B).
Discussion
NEK2 is a serine-threonine protein kinase of the
NIMA-related kinase family that localizes to the centrosomes, which
are the microtubule-organizing centers of a cell that regulate its
separation (8). The NIMA-related
kinase (Nek) family consists of eleven members (NEKs 1–11)
(29). In humans, NEK2 exhibits
the greatest sequence identity to NIMA (8). In the process of cell division, NEK2
promotes centrosome splitting at the beginning of mitosis by the
phosphorylation of multiple linker components (30). In addition to centrosome
separation, NEK2 also regulates the microtubule organization
capacity of the centrosome (31,32).
Recent studies have shown that an elevated expression of NEK2
induces abnormal tumor proliferation and drug resistance in breast
and ovarian cancers (13,33,34).
Furthermore, the significant upregulation of NEK2 has been
demonstrated to be associated with the progression and poor
prognosis of a series of malignant tumors originating in different
organs and tissues, such as colorectal carcinoma (35), breast carcinoma (36) and myeloma (27).
However, whether NEK2 expression is elevated and
associated with the clinicopathological features and prognosis of
HCC remains unclear. In this study, we first assessed the
expression of NEK2 in cells and tissues and found that NEK2
expression was significantly upregulated in HCC cells and tissues.
Additionally, we examined the expression of NEK2 in a relatively
large population of patients diagnosed with HCC and correlated it
with the clinicopathological parameters and prognosis to verify
whether this biomarker could predict HCC outcomes. The present
study revealed that the overexpression of NEK2 was significantly
correlated with diolame complete, tumor nodule number and
recurrence. Thus, these results strongly confirm the intriguing
possibility that the alteration of NEK2 protein levels may
contribute to the invasion and metastasis of HCC.
Furthermore, Kaplan-Meier survival curve analysis
demonstrated that HCC patients with a high expression of NEK2 had a
poor prognosis, suggesting that NEK2 may be a potential prognostic
factor for HCC patients. Therefore, NEK2 can be used as a novel
prognostic biomarker to identify, distinguish, and predict HCC. The
main limitation of this analysis is that, due to clinical
covariates on HCC cases on TCGA website are not available, the
multivariate Cox's regression survival model cannot be performed to
assess the relative contribution of the risk group when assessed
after adjusting for clinical variables.
Despite the important role of NEK2 in centrosome
regulation and spindle formation, the mechanism of the abnormal
expression and regulation of NEK2 remains unclear. Further studies
are needed to clarify the mechanism that underlies the role of
NEK2. Previous studies suggested that NEK2 may be involved in tumor
progression through the influence of other tumor pathways. The
elevation of NEK2 contributes to the activation of the PI3K/AKT
signaling pathway, a potent and critical oncogene for a variety of
malignancies (26,27). Moreover, the PI3K/AKT signaling
pathway plays an important role in upregulating MMP expression
(28), and aberrant AKT signaling
could promote cell proliferation in HCC cells (37). Therefore, we sought to verify
whether such a mechanism may contribute to HCC progression induced
by NEK2. In this study, we found that the expression level of
phospho-AKT and MMP-2 proteins was increased in HCC and positively
correlated with NEK2, which indicated that overexpression of NEK2
may result in the high expression of phospho-AKT and MMP-2 proteins
in HCC.
Furthermore, the AKT signaling pathway has been
reported to play a key role in HCC cell invasion and metastasis by
promoting the expression of MMP-2 (37), which is a key factor in HCC
invasion and metastasis (38). The
expression level of MMP-2 significantly reflects the aggressiveness
of malignant tumor cells and is associated with poor prognosis in
multiple tumor types (37).
Combining the above findings with our results, we suggest that the
invasion and metastasis effect of NEK2 in HCC may occur through the
activation of AKT signaling and promotion of MMP-2 expression.
In conclusion, our data revealed that high
expression of the NEK2 protein was common in HCC tissue samples and
cultured hepatoma cell lines and was significantly associated with
poor prognosis and unfavorable clinicopathological factors in HCC.
Moreover, the NEK2 expression level was positively correlated with
phospho-AKT and MMP-2 expression. Our results suggest that NEK2 is
important for the progression, migration and invasion of HCC and
may be a novel prognostic biomarker for HCC.
Acknowledgments
The present study was supported by the Guangxi
Natural Science Foundation (grant nos. 2015GXNSFBA139162 and
AB16380351), the National Natural Science Foundation of China
(grant nos. 81260445 and 30960332), the Program of Key Laboratory
of High-Incidence-Tumor Prevention and Treatment (Guangxi Medical
University), the Ministry of Education, China (grant nos.
GK2014-ZZ04 and GK2015-ZZ01), the Science and Technology Department
of Nanning (grant no. 20151266), and the Innovation Project of
Guangxi Graduate Education.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HY and Park JW: Clinical trials of
combined molecular targeted therapy and locoregional therapy in
hepatocellular carcinoma: Past, present, and future. Liver Cancer.
3:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Njei B, Rotman Y, Ditah I and Lim JK:
Emerging trends in hepatocellular carcinoma incidence and
mortality. Hepatology. 61:191–199. 2015. View Article : Google Scholar
|
|
4
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lazarevich NL, Cheremnova OA, Varga EV,
Ovchinnikov DA, Kudrjavtseva EI, Morozova OV, Fleishman DI,
Engelhardt NV and Duncan SA: Progression of HCC in mice is
associated with a downregulation in the expression of hepatocyte
nuclear factors. Hepatology. 39:1038–1047. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka S and Arii S: Molecular targeted
therapies in hepatocellular carcinoma. Semin Oncol. 39:486–492.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fry AM: The Nek2 protein kinase: A novel
regulator of centrosome structure. Oncogene. 21:6184–6194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Faragher AJ and Fry AM: Nek2A kinase
stimulates centrosome disjunction and is required for formation of
bipolar mitotic spindles. Mol Biol Cell. 14:2876–2889. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bahe S, Stierhof YD, Wilkinson CJ, Leiss F
and Nigg EA: Rootletin forms centriole-associated filaments and
functions in centrosome cohesion. J Cell Biol. 171:27–33. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayward DG, Clarke RB, Faragher AJ, Pillai
MR, Hagan IM and Fry AM: The centrosomal kinase Nek2 displays
elevated levels of protein expression in human breast cancer.
Cancer Res. 64:7370–7376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu G, Qiu XL, Zhou L, Zhu J, Chamberlin R,
Lau J, Chen PL and Lee WH: Small molecule targeting the Hec1/Nek2
mitotic pathway suppresses tumor cell growth in culture and in
animal. Cancer Res. 68:8393–8399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsunoda N, Kokuryo T, Oda K, Senga T,
Yokoyama Y, Nagino M, Nimura Y and Hamaguchi M: Nek2 as a novel
molecular target for the treatment of breast carcinoma. Cancer Sci.
100:111–116. 2009. View Article : Google Scholar
|
|
14
|
Wang S, Li W, Lv S, Wang Y, Liu Z, Zhang
J, Liu T and Niu Y: Abnormal expression of Nek2 and β-catenin in
breast carcinoma: Clinicopathological correlations. Histopathology.
59:631–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Vos S, Hofmann WK, Grogan TM, Krug U,
Schrage M, Miller TP, Braun JG, Wachsman W, Koeffler HP and Said
JW: Gene expression profile of serial samples of transformed B-cell
lymphomas. Lab Invest. 83:271–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neal CP, Fry AM, Moreman C, McGregor A,
Garcea G, Berry DP and Manson MM: Overexpression of the Nek2 kinase
in colorectal cancer correlates with beta-catenin relocalization
and shortened cancer-specific survival. J Surg Oncol. 110:828–838.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ning Z, Wang A, Liang J, Liu J, Zhou T,
Yan Q and Wang Z: Abnormal expression of Nek2 in pancreatic ductal
adenocarcinoma: A novel marker for prognosis. Int J Clin Exp
Pathol. 7:2462–2469. 2014.PubMed/NCBI
|
|
18
|
Zhong X, Guan X, Dong Q, Yang S, Liu W and
Zhang L: Examining Nek2 as a better proliferation marker in
non-small cell lung cancer prognosis. Tumour Biol. 35:7155–7162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ozsolak F and Milos PM: RNA sequencing:
Advances, challenges and opportunities. Nat Rev Genet. 12:87–98.
2011. View
Article : Google Scholar :
|
|
21
|
Sultan M, Schulz MH, Richard H, Magen A,
Klingenhoff A, Scherf M, Seifert M, Borodina T, Soldatov A,
Parkhomchuk D, et al: A global view of gene activity and
alternative splicing by deep sequencing of the human transcriptome.
Science. 321:956–960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferreira PG, Jares P, Rico D, Gómez-López
G, Martínez-Trillos A, Villamor N, Ecker S, González-Pérez A,
Knowles DG, Monlong J, et al: Transcriptome characterization by RNA
sequencing identifies a major molecular and clinical subdivision in
chronic lymphocytic leukemia. Genome Res. 24:212–226. 2014.
View Article : Google Scholar :
|
|
23
|
Rezaeian I, Tavakoli A, Cavallo-Medved D,
Porter LA and Rueda L: A novel model used to detect differential
splice junctions as biomarkers in prostate cancer from RNA-Seq
data. J Biomed Inform. 60:422–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang Q, Lin B, Liu H, Ma X, Mo F, Yu W,
Li L, Li H, Tian T and Wu D: RNA-Seq analyses generate
comprehensive transcriptomic landscape and reveal complex
transcript patterns in hepatocellular carcinoma. PLoS One.
6:e261682011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xavier LL, Viola GG, Ferraz AC, Da Cunha
C, Deonizio JM, Netto CA and Achaval M: A simple and fast
densitometric method for the analysis of tyrosine hydroxylase
immunoreactivity in the substantia nigra pars compacta and in the
ventral tegmental area. Brain Res Brain Res Protoc. 16:58–64. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Das TK, Dana D, Paroly SS, Perumal SK,
Singh S, Jhun H, Pendse J, Cagan RL, Talele TT and Kumar S:
Centrosomal kinase Nek2 cooperates with oncogenic pathways to
promote metastasis. Oncogenesis. 2:e692013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou W, Yang Y, Xia J, Wang H, Salama ME,
Xiong W, Xu H, Shetty S, Chen T and Zeng Z: NEK2 induces drug
resistance mainly through activation of efflux drug pumps and is
associated with poor prognosis in myeloma and other cancers. Cancer
Cell. 23:48–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chien CS, Shen KH, Huang JS, Ko SC and
Shih YW: Antimetastatic potential of fisetin involves inactivation
of the PI3K/Akt and JNK signaling pathways with downregulation of
MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell
Biochem. 333:169–180. 2010. View Article : Google Scholar
|
|
29
|
O'Connell MJ, Krien MJ and Hunter T: Never
say never. The NIMA-related protein kinases in mitotic control.
Trends Cell Biol. 13:221–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Q, Hirohashi Y, Du X, Greene MI and
Wang Q: Nek2 targets the mitotic checkpoint proteins Mad2 and
Cdc20: A mechanism for aneuploidy in cancer. Exp Mol Pathol.
88:225–233. 2010. View Article : Google Scholar :
|
|
31
|
Gräf R: DdNek2, the first non-vertebrate
homologue of human Nek2, is involved in the formation of
microtubule-organizing centers. J Cell Sci. 115:1919–1929.
2002.PubMed/NCBI
|
|
32
|
Prigent C, Glover DM and Giet R:
Drosophila Nek2 protein kinase knockdown leads to centrosome
maturation defects while overexpression causes centrosome
fragmentation and cytokinesis failure. Exp Cell Res. 303:1–13.
2005.
|
|
33
|
Liu X, Gao Y, Lu Y, Zhang J, Li L and Yin
F: Upregulation of NEK2 is associated with drug resistance in
ovarian cancer. Oncol Rep. 31:745–754. 2014.
|
|
34
|
Naro C, Barbagallo F, Chieffi P, Bourgeois
CF, Paronetto MP and Sette C: The centrosomal kinase NEK2 is a
novel splicing factor kinase involved in cell survival. Nucleic
Acids Res. 42:3218–3227. 2014. View Article : Google Scholar :
|
|
35
|
Takahashi Y, Iwaya T, Sawada G, Kurashige
J, Matsumura T, Uchi R, Ueo H, Takano Y, Eguchi H, Sudo T, et al:
Up-regulation of NEK2 by microRNA-128 methylation is associated
with poor prognosis in colorectal cancer. Ann Surg Oncol.
21:205–212. 2014. View Article : Google Scholar
|
|
36
|
Marina M and Saavedra HI: Nek2 and Plk4:
Prognostic markers, drivers of breast tumorigenesis and drug
resistance. Front Biosci (Landmark Ed). 19:352–365. 2014.
View Article : Google Scholar
|
|
37
|
Gao J, Ding F, Liu Q and Yao Y: Knockdown
of MACC1 expression suppressed hepatocellular carcinoma cell
migration and invasion and inhibited expression of MMP2 and MMP9.
Mol Cell Biochem. 376:21–32. 2013. View Article : Google Scholar
|
|
38
|
Li J, Lau GK, Chen L, Dong SS, Lan HY,
Huang XR, Li Y, Luk JM, Yuan YF and Guan XY: Interleukin 17A
promotes hepatocellular carcinoma metastasis via NF-κB induced
matrix metalloproteinases 2 and 9 expression. PLoS One.
6:e218162011. View Article : Google Scholar
|