Introduction
Endometrial cancer occurs in the glandular lining of
the uterus and is the most common cancer of the female genital
tract and the fourth most common cancer in women (1). Type I 'endometrioid' carcinoma
accounts for approximately 85% of all cases (2). It is often preceded by endometrial
hyperplasia; a proliferative process within the endometrial glands
that leads to an increase in the glandular-stromal ratio. This
process is commonly associated with unopposed estrogen stimulation
and may also be due to specific genetic alterations (2). Type I tumours are staged according to
the guidelines of the International Federation of Gynecology and
Obstetrics (3). Tumour grade
(G1-3) is based histologically on the extent to which the cancer
forms glands that display similar morphology to normal endometrium,
and also metastatic behavior; the extent to which the cancer
invades the uterine corpus and the surrounding peritoneum (2).
Cytokines produced within the tumour
microenvironment can promote cancer cell growth, attenuate
apoptosis and facilitate invasion and metastasis, making them
attractive therapeutic targets. We identified interleukin 11 (IL11)
as a critical regulator of placental trophoblast invasion in
vitro and in vivo in the uterus during the establishment
of pregnancy (4–6) and also endometrial tumourigenesis
(7). We demonstrated that targeted
blockade of endometrial cancer epithelial cell IL11 signalling
reduced primary tumour growth and impaired metastasis in ectopic
and orthotopic endometrial tumour xenograft models in vivo.
IL11 receptor (R) α inhibition in G2-derived HEC1A cell line
xenograft tumours retained a well-differentiated, endometrial
epithelial phenotype vs. control, suggesting that it prevented
epithelial-to-mesenchymal transition (EMT) (7).
We have previously shown that IL11 upregulates
chondroitin sulfate proteoglycan (CSPG4) in primary human first
trimester villous trophoblasts (8). CSPG4 silencing promotes trophoblast
cell proliferation, but impairs invasion and migration (8). We have reported that IL11 plays
opposing roles in trophoblast cell vs. cancer cell invasion in the
endometrial lining of the uterus (4,5,9,10),
whereby IL11 blocks trophoblast cell invasion, while it facilitates
endometrial cancer cell migration/invasion.
CSPG4, also known as nerve glial antigen 2 (NG2),
melanoma chondroitin sulfate proteoglycan (MCSP) (11) and N-methylpurine-DNA glycosylase
(HMPG) (12) is a single membrane
spanning protein (13). CSPG4 is
overexpressed in several human cancers, including soft tissue
sarcomas (14), melanoma (15) and breast cancer (16). It has established functional roles
in promoting cancer cell proliferation, migration and invasion
(17–19). These functions are thought to be
mediated via CSPG4 interactions with extracellular and
intracellular binding proteins (20). However, the expression, regulation
and function of CSPG4 in endometrial cancer have not been
investigated.
We hypothesized that CSPG4 expression is
overexpressed in human endometrial cancer, as in other epithelial
malignancies and IL11 stimulates CSPG4 in endometrial cancer cells.
We aimed to determine the expression and localization of CSPG4 in
type I human endometrioid endometrial cancer across G1-3 tumours
and normal endometrium. We determined the effect of CSPG4 knockdown
on HEC1A (endometrial epithelial cancer cell line) cell
proliferation and migration using the xCELLigence real-time system,
or wound healing migration assays in vitro. Furthermore, we
investigated whether IL11 alters CSPG4 levels in human endometrial
epithelial cancer cells in vitro and in subcutaneous
xenograft tumours in vivo, in mice.
Materials and methods
Cell culture
HEC1A cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA; 2013) and cultured in
McCoy's medium with 10% fetal calf serum (FCS). Ishikawa cells were
provided by Dr M. Nishida (Tsukuba University, Tochigi, Japan) and
cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% FCS.
Monash Health Translational Precinct Medical Genomics authenticated
these cell lines in June 2016.
HEC1A in vitro cytokine treatments
Confluent HEC1A cells were serum starved for 24 h
and treated with recombinant human IL11 (10 or 100 ng/ml) or
phosphate-buffered saline (PBS) (4). Cell lysates were collected after 6 h
of treatment. RNA was extracted and quantitative real-time RT-PCR
performed.
Animals
Animal experiments were conducted in female, 5–7
week old, athymic, BALB/c nude mice purchased from the Animal
Resources Centre; Western Australia, housed in specific
pathogen-free conditions, with food and water available ad
libitum and held in a 12-h light and dark cycle. Use of all
animals was in accordance with the guidelines of the Monash Medical
Centre Animal Ethics Committee under Ethics Approval number
MMCB/2012/07.
Subcutaneous tumour inoculation
HEC1A cells were resuspended in serum-free medium at
a concentration of 20×106 cells/ml. Both flanks of each
animal were inoculated with 100 µl (2×106 cells)
(n=3/group). Once palpable, tumours were measured with digital
calipers (Hare & Forbes Machineryhouse) and tumour volume
calculated using the following formula: (length ×
width2)/2 (mm3) (7).
Animal treatments and tissue
collection
Once subcutaneous tumours measured 80–100
mm3, mice were randomized into groups and administered
by intraperitoneal injection with recombinant human IL11 (500
µg/mg/kg) (4) or saline
vehicle control three times weekly for two weeks. At the completion
of the study, tumours were fixed in 4% paraformaldehyde for 24 h
and paraffin embedded for immunohistochemistry.
Participants and patient samples
Endometrial cancer tissue biopsies (n=10/grade), or
benign endometrium (n=4) were collected from postmenopausal women
undergoing total abdominal hysterectomy at the Monash Medical
Centre Melbourne, Australia. The Human Ethics Committee approved
the research project and informed consent was obtained from each
patient. Tumors were graded histologically by a specialist
gynecological pathologist according to the guidelines of FIGO, 2009
as previously described (21).
Proliferative phase endometrium (n=6) was collected at curettage
from women between day 7 and 13 of their menstrual cycle that were
scheduled for tubal ligation, as a non-tumour control group. A
pathologist declared no obvious endometrial pathology. Women had no
steroid treatment or other medication for at least 2 months before
tissue collection. Written informed consent was obtained from each
patient and the study was approved by the Southern Health Human
Research and Ethics committee. Biopsies were fixed in 10% neutral
buffered formalin overnight, prior to paraffin embedding.
Immunohistochemistry
Formalin-fixed human endometrial cancer,
postmenopausal, or proliferative phase endometrial tissue, or
subcutaneous HEC1A xenograft tumour tissue sections (4 µm)
were dewaxed in histosol (2×10 min), rehydrated in ethanol and
antigen retrieval performed in 0.01 M sodium citrate (pH 6.0)
before endogenous peroxidase activity was quenched with 3% hydrogen
peroxide in methanol for 10 min. Non-specific binding was blocked
with 10% normal goat serum and 2% normal human serum, in
Tris-buffered saline (TBS) for 30 min. The CSPG4 antibody (#4235; 4
µg/ml; Cell Signaling Technology, Danvers, MA, USA) was
applied overnight at 4°C. Equal concentration of negative control
isotype rabbit IgG (Dako) was included for every tissue section.
Antibody localization was detected by sequential application of
biotinylated goat anti-rabbit IgG (7.5 µg/ml; Vector
Laboratories, Burlingame, CA, USA) for 30 min followed by
Vectastain ABC Elite kit (Vector Laboratories) for 30 min.
Peroxidase activity was visualized by the application of
diaminobenzidine substrate (DakoCytomation). Tissues were
counterstained with Harris hematoxylin (Sigma-Aldrich) and mounted.
Staining intensity in the epithelial and stromal compartements were
scored from 0. no staining to 3, intense staining by two
independent, blinded assessors.
RNA preparation and quantitative
real-time RT-PCR
RNA from human endometrial cancer or benign
endometrium whole tissue was obtained from the Victorian Cancer
Biobank (Project #13018). Total RNA was isolated from endometrial
epithelial cancer cell lines, or subcutaneous HEC1A xenograft
tumour tissue using TRI reagent (Sigma-Aldrich). Genomic DNA was
digested using the DNA-free kit (Ambion) according to the
manufacturer's instructions. To test the RNA yield, purity and
concentration, 2 µl was analyzed using the NanoDrop
spectrophotometer (Thermo Fisher Scientific) at an absorbance ratio
of A260/280 nm. cDNA was synthesized from total RNA (250 ng) using
SuperScript III reverse transcriptase (Invitrogen). Real-time
RT-PCR analyses were performed on the ABI 7500HT fast block
real-time PCR system (Applied Biosystems) in triplicate (final
reaction volume, 10 µl) in Optical 384-well microplates
(Applied Biosystems). For each sample, 25 ng of cDNA was added to a
PCR mix made with the 2X FastStart SyBR-Green Master Mix containing
ROX passive reference dye (Applied Biosystems) and 10 nM primers.
The primer sequence details are listed in Table I. A template-free negative control
in the presence of primers and RNase-free water and, RNase-free
water only negative controls were added for each run. The PCR
protocol was as follows: 95°C for 10 min and 40 cycles of 95°C for
15 sec followed by 60°C for 1 min. Relative expression levels were
calculated by the comparative cycle threshold method (ΔΔCt) as
outlined in the manufacturer's user manual, with 18s ribosomal RNA
serving as the endogenous control for normalization.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
| CSPG4 |
AGCTAGCCAGGACTGATGGA |
CAGCCTAACCTGCTCCAAAG |
| ECAD |
ACACCATCTGTGCCCACTTT |
CAGGTCTCCTCTTGGCTCTG |
| TWIST |
GGAGTCCGCAGTCTTACGAG |
TGGAGGACCTGGTAGAGGAA |
| SNAIL |
AGATGCATATTCGGACCCAC |
CCTCATGTTTGTGCAGGAGA |
| ICAM-1 |
GGCCGGCCAGCTTATACAC |
TAGACACTTGAGCTCGGGCA |
| 18s |
GATCCATTGGAGGGCAAGTCT |
CCAAGATCCAACTACGAGCTT |
PCR and gel electrophoresis
RNA and cDNA were prepared as above. PCR reactions
were performed using PCR express machine (Thermo Fisher Scientific)
and GoTaq Master Mix (Promega) according to the manufacturer's
instructions. cDNA was analyzed for CSPG4 and 18s (Table I) using reaction conditions of
denaturation at 95°C for 3 min, followed by 30 cycles of:
denaturation, 95°C for 30 sec; annealing, 60°C for 30 sec;
extension, 72°C for 1 min; with a final extension at 72°C for 10
min. PCR products were run on a 1.5% agarose gel with 1000 bp DNA
ladder (Invitrogen).
Small interfering RNA (siRNA)
transfections
HEC1A cells were cultured to 70% confluence and
transfected with commercially generated and validated ON-TARGETplus
SMARTpool siRNA (Dharmacon, Lafayette, CO, USA) targeting CSPG4
(CSPG4 siRNA) or no specific sequence as a scrambled control (scr).
Delivery was performed using the Lipofectamine RNAiMAX
(Invitrogen-Life Technologies) according to the manufacturer's
instructions. Cells were transfected for 72 h prior to RNA
collection to test for transfection efficiency or prior beginning
functional experiments (8).
xCELLigence real-time cell proliferation
assay
Experiments were carried out using the RTCA DP
xCELLigence instrument (Roche), which was placed in a humidified
incubator maintained at 37°C with 95% air/5% CO2. Cells
were seeded in E-plate 96 at 10,000 cells/well in 5% FCS DMEM
medium and the plate was monitored once every hour for a total of
48 h (22). Data was calculated
using RTCA Software 1.2, supplied with the instrument (ACEA) and
exported for statistical analysis.
Wound healing migration assay
HEC1A cells were seeded at 100,000 cells/well in a
12-well plate and grown to sub-confluence. Cells were transfected
with siRNA or control as above, before wounding by using vacuum
suction through a protein electrophoresis pipette tip (Bio-Rad
Laboratories). On the day of wounding, designated time 0 (0 h),
cell wounds were photographed using a Motic AE31 inverted
microscope and camera and Motic Images Plus 2.0 software (Motic
microscopy; Motic). To assess differences in wound repair, the area
of each wound was manually outlined and they are quantitated using
ImageJ (v10.2 NIH) software, at 0 and at 48 h. The 48-h time-point
was chosen since a clear wound boundary was distinguishable, as the
wound was not fully repaired. Data was expressed as percent repair
at 48 h vs. the 0 h time-point. For each experiment, wounds were
performed and assessed in triplicate and repeated in three separate
experiments.
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism (GraphPad Software 6.0) and data assessed by the Student's
t-test for two groups. Multiple groups were compared using one-way
ANOVA, with the Tukey's post-hoc test. Results of P<0.05 were
considered statistically significant.
Results
CSPG4 mRNA and protein are stimulated by
IL11 in HEC1A endometrial epithelial cancer cells and xenograft
tumours in mice
To investigate the stimulation of CSPG4 in human
endometrial epithelial cancer cells, HEC1A cells were treated with
IL11 in vitro. There was a significant 3-fold increase in
CSPG4 gene expression following treatment with IL11 (100 ng/ml)
compared to control or IL11 at (10 ng/ml) after 6 h (P<0.05,
n=3/gp) (Fig. 1A). To confirm this
effect in vivo, subcutaneous HEC1A xenograft tumours were
established in mice, and mice were administered with saline vehicle
control, or recombinant IL11 (500 µg/mg/kg). IL11
significantly increased subcutaneous tumour growth vs. saline
control (P<0.01, n=3/gp) (Fig.
1B). Following IL11 treatment, immunohistochemical staining of
tumour tissue identified that CSPG4 predominantly localized to the
cell membrane and to a lesser extent to the cytoplasm increased in
IL11-treated tumours vs. control (Fig.
1C). Semi-quantitation of the immunostaining intensity
confirmed a significant increase in CSPG4 staining intensity in the
cell membrane following IL11 treatment compared to control
(P<0.05, n=3/gp) (Fig. 1D).
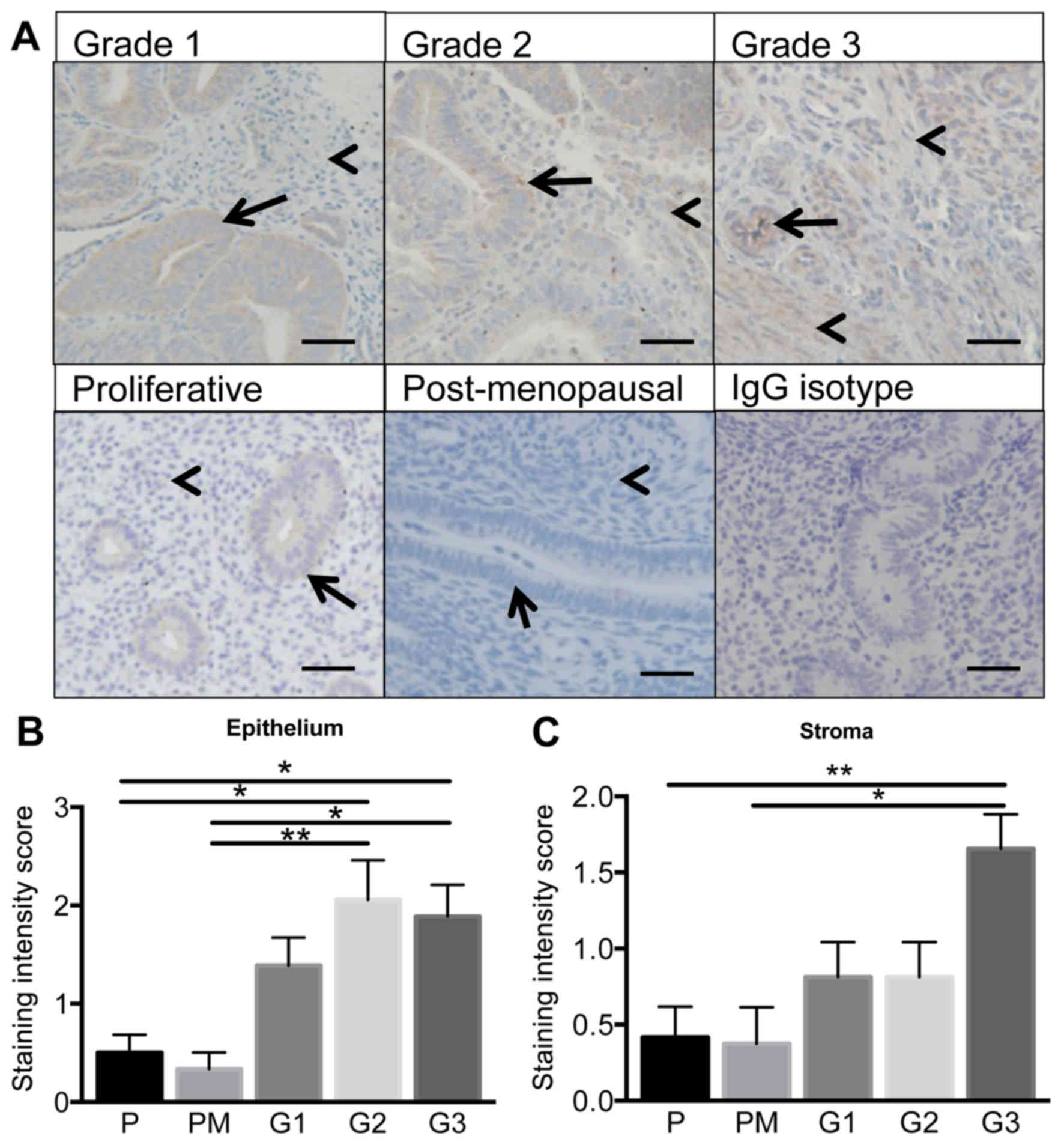
CSPG4 protein levels are elevated in type
I endometrioid cancer with increaing tumour grade
CSPG4 protein was immunolocalized in human G1-3
endometrial tumour or proliferative phase or post-menopausal
endometrial tissue. CSPG4 predominantly localized to the epithelial
compartment of G1-3 endometrial tumours (Fig. 2A) and staining intensity levels
were significantly elevated in the epithelium of G2 and G3 tumour
tissue compared to proliferative phase or postmenopausal
endometrium (P<0.05, P<0.01; n= 6–10/group) (Fig. 2B). Very minimal staining was
evident in the stroma of proliferative phase or postmenopausal
endometrium (Fig. 2A). Staining
intesity levels were significantly elevated in the stromal
compartment in G3 malignant tumour tissue vs. nonmalignant tissue
(P<0.05, P<0.01; n=6–10/group) (Fig. 2C).
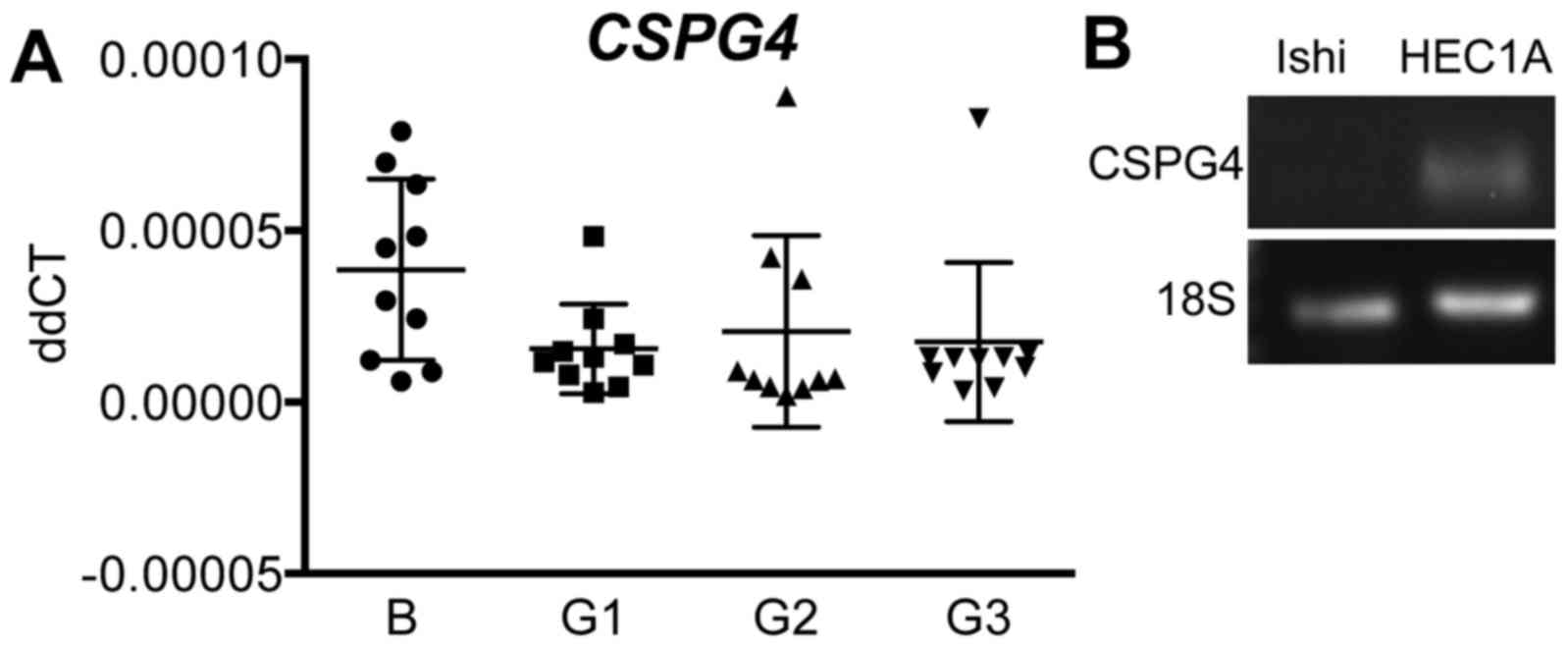
CSPG4 mRNA expression in primary human
endometrial cancer and cancer cell lines
Quantitative real-time RT-PCR was performed to
measure CSPG4 mRNA levels in whole tissue from G1-3 endometrial
tumours vs. benign endometrium. There were no differences in gene
expression across all endometrial cancer grades compared to the
benign group (n=10/group) (Fig.
3A). To conduct functional studies, we determined CSPG4 gene
expression in human endometrial epithelial cancer cell lines.
G2-derived HEC1A cells expressed CSPG4, although G1-derived
Ishikawa cells did not (Fig.
3B).
CSPG4 siRNA knockdown decreases HEC1A
cell proliferation and migration
To determine the functional role of CSPG4 in
endometrial epithelial cancer cell proliferation and migration, we
transiently silenced CSPG4 gene expression using siRNA in HEC1A
cells. This resulted in significant knockdown of CSPG4 at the
transcript level in siRNA-treated HEC1A cells compared to scrambled
(scr) control (P<0.001; n=4/group) (Fig. 4A). We examined HEC1A cell
proliferation using the xCELLigence real-time system. After 72 h,
there was a significant decrease in proliferation in response to
CSPG4 siRNA treatment vs. scr control (P<0.05; n=4/gp) (Fig. 4B). Cell migration was assessed by
performing a wound healing assay on CSPG4 siRNA treated, or scr
control HEC1A cells (Fig. 4C).
Analysis of the area of repair between 0 and 48 h following
wounding demonstrated a significant 16±3% reduction in migration of
CSPG4 siRNA treated HEC1A cells compared to control (Fig. 4D).
CSPG4 knockdown reduces SNAIL mRNA
expression in HEC1A cells
From assessment of key regulatory transcription
factors involved in EMT, CSPG4 knockdown resulted in significantly
reduced SNAIL gene expression compared to control in HEC1A cells
(P<0.05) (Fig. 5A), although
TWIST, ECAD and ICAM-1 mRNA levels were unchanged (Fig. 5B–D).
Discussion
This study is the first to identify and determine a
functional role for CSPG4 in endometrial cancer. The findings
established that CSPG4 protein levels are elevated in G2 and G3
endometrial tumour epithelium and G3 tumour stroma compared to
normal proliferative phase or post-menopausal endometrium. IL11, a
crucial mediator of endometrial tumourigenesis and EMT (7), upregulated CSPG4 mRNA and protein in
G2-derived HEC1A endometrial epithelial cancer cells in
vitro and xenograft tumours in vivo. Functionally, CSPG4
knockdown impaired HEC1A cell proliferation and migration in
vitro, and led to the downregulation of a central EMT
transcription factor, SNAIL, suggesting that CSPG4 may promote
endometrial cancer progression in women.
These data are supported by previous studies in
other tumour types, where CSPG4 is overexpressed in human breast
carcinoma (12,16), glioblastoma (19) and melanoma (11,17).
Interestingly, CSPG4 gene expression levels were unchanged between
endometrial tumour G1-3 and benign endometrial tissue. This could
be attributed to the heterogeneity of whole endometrial tumour
tissue; therefore, immuolocalization studies were performed to
determine which cell types express CSPG4 protein. Since it is
typically a cell surface proteoglycan (14), the localization pattern of CSPG4 as
a membrane protein on the tumour epithelial cell surface was in
line with reports in other tumour cell types.
Recently, we identified CSPG4 in the human placenta,
showing that it localized to invasive and proliferative trophoblast
subtypes of the placenta and the maternal uterine decidua (8). Furthermore, it was revealed that
knockdown of CSPG4 stimulated HTR8/SVneo trophoblast cell line
proliferation, but decreased migration. The data from this study
highlights similar roles for CSPG4 in human trophoblast and
endometrial cancer cell migration, but opposing regulation of
proliferation, suggesting that the functional roles of CSPG4 in
regulating cell cycle/growth in the uterine environment are
cell-dependent.
The present study is the first to localize CSPG4 in
the non-pregnant, cycling human endometrium. In normal
proliferative phase endometrium, CSPG4 predominantly localized to
the glandular epithelium. This localization pattern, together with
its well-established role in promoting cell-cell adhesion in other
cell types, such as breast cancer cells (23), suggest that CSPG4 could enhance
non-malignant endometrial epithelial cell adhesion. It would
therefore be interesting to examine the expression of CSPG4 across
the menstrual cycle and investigate any potential role in
endometrial epithelial cell adhesive capability required for
blastocyst adhesion and implantation.
Minimal CSPG4 production was evident in the stroma
of normal proliferative phase endometrial tissue and postmenopausal
endometrium. In contrast, CSPG4 localization in endometrial cancer
tissue was significantly increased in the stromal compartment in G3
tumour tissue. In women, G2 endometrial cancers display myometrial
invasion within the uterus, as well as spread to nearby pelvic and
para-aortic lymph nodes, while G3 tumours are highly metastatic
(3). Both G2 and G3 endometrial
cancers have poorer prognosis, compared to G1, with metastatic
behavior being most closely linked with clinical outcome and cause
of death (2). EMT is a process in
cancer metastasis, during which epithelial cells acquire phenotypes
of motile fibroblasts. While the functional role of CSPG4 in
endometrial cancer stromal cells remains to be determined, the
elevated staining intensity in the tumour stroma with increasing
tumour grade suggests it may act to facilitate tumour progression
by acting on the local tumour environment to promote EMT (24).
Central to EMT is the activation of TWIST and SNAIL
induced transcription, eventually causing degradation of the
basement membrane by induction of matrix metalloproteinases, loss
of epithelial markers such as E-cadherin and gain of mesenchymal
markers such as vimentin (24). We
found that CSPG4 knockdown reduced HEC1A cell migration in
vitro. Assessment of pathways involved in EMT showed that CSPG4
silencing significantly downregulated SNAIL gene expression. These
findings suggest that CSPG4 inhibition can prevent cancer cell
migration, potentially attributed to inhibition of EMT. In line
with these findings, we previously demonstrated that IL11 induces
SNAIL in HEC1A xenograft tumours in vivo (7). Here we reported that IL11 also
upregulates CSPG4, highlighting a new mechanism by which IL11 may
promote endometrial tumourigenesis.
Preclinical monoclonal antibody treatment studies
have shown promising results in breast cancer models (23,25).
Further investigation of the role of CSPG4 in endometrial cancer
pre-clinical cancer models in vivo animal models, such as
ortotopic xenograft tumour models (7), could support also our findings and
determine the potential of targeting CSPG4 for clinical translation
as a new treatment option for women with endometrial cancer.
In summary, in the present study we have
demonstrated that CSPG4 is a cell surface proteoglycan regulated by
IL11 in human endometrial epithelial cancer cells which is
upregulated in the epithelial compartment in human type I G2 and G3
endometriod endometrial cancer. Functionally, loss of CSPG4 gene
expression in endometrial epithelial cancer cells reduced
proliferation and migration of HEC1A cells in vitro,
potentially mediated by suppression of the EMT factor, SNAIL,
suggesting that elevations of CSPG4 expression/function could be
pro-tumourigenic in women.
Acknowledgments
We acknowledge the support of the Victorian
Government's Operational Infrastructure Support Program and the
Australian Government NHMRC IRIISS. E.D. was supported by the NHMRC
Fellowship (#550905). A.W. was supported by an Cancer Council
Victoria Postdoctoral Fellowship. We are grateful to all the women
who donated samples and to research nurse, Judi Hocking.
References
|
1
|
Hong B, Le Gallo M and Bell DW: The
mutational landscape of endometrial cancer. Curr Opin Genet Dev.
30:25–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Cristofano A and Ellenson LH:
Endometrial carcinoma. Annu Rev Pathol. 2:57–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winship AL, Koga K, Menkhorst E, Van
Sinderen M, Rainczuk K, Nagai M, Cuman C, Yap J, Zhang JG, Simmons
D, et al: Interleukin-11 alters placentation and causes
preeclampsia features in mice. Proc Natl Acad Sci USA.
112:15928–15933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paiva P, Salamonsen LA, Manuelpillai U and
Dimitriadis E: Interleukin 11 inhibits human trophoblast invasion
indicating a likely role in the decidual restraint of trophoblast
invasion during placentation. Biol Reprod. 80:302–310. 2009.
View Article : Google Scholar
|
|
6
|
Paiva P, Salamonsen LA, Manuelpillai U,
Walker C, Tapia A, Wallace EM and Dimitriadis E: Interleukin-11
promotes migration, but not proliferation, of human trophoblast
cells, implying a role in placentation. Endocrinology.
148:5566–5572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winship AL, Van Sinderen M, Donoghue J,
Rainczuk K and Dimitriadis E: Targeting interleukin-11 receptor-α
impairs human endometrial cancer cell proliferation and invasion in
vitro and reduces tumor growth and metastasis in vivo. Mol Cancer
Ther. 15:720–730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Sinderen M, Cuman C, Winship A,
Menkhorst E and Dimitriadis E: The chrondroitin sulfate
proteoglycan (CSPG4) regulates human trophoblast function.
Placenta. 34:907–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Winship A, Menkhorst E, Van Sinderen M and
Dimitriadis E: Interleukin 11: Similar or opposite roles in female
reproduction and reproductive cancer? Reprod Fertil Dev.
28:395–405. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lay V, Yap J, Sonderegger S and
Dimitriadis E: Interleukin 11 regulates endometrial cancer cell
adhesion and migration via STAT3. Int J Oncol. 41:759–764.
2012.PubMed/NCBI
|
|
11
|
Price MA, Colvin Wanshura LE, Yang J,
Carlson J, Xiang B, Li G, Ferrone S, Dudek AZ, Turley EA and
McCarthy JB: CSPG4, a potential therapeutic target, facilitates
malignant progression of melanoma. Pigment Cell Melanoma Res.
24:1148–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gibby K, You WK, Kadoya K, Helgadottir H,
Young LJ, Ellies LG, Chang Y, Cardiff RD and Stallcup WB: Early
vascular deficits are correlated with delayed mammary tumorigenesis
in the MMTV-PyMT transgenic mouse following genetic ablation of the
NG2 proteoglycan. Breast Cancer Res. 14:R672012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishiyama A, Dahlin KJ, Prince JT,
Johnstone SR and Stallcup WB: The primary structure of NG2, a novel
membrane-spanning proteoglycan. J Cell Biol. 114:359–371. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cattaruzza S, Nicolosi PA, Braghetta P,
Pazzaglia L, Benassi MS, Picci P, Lacrima K, Zanocco D, Rizzo E,
Stallcup WB, et al: NG2/CSPG4-collagen type VI interplays
putatively involved in the microenvironmental control of tumour
engraftment and local expansion. J Mol Cell Biol. 5:176–193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Price MA, Li GY, Bar-Eli M, Salgia
R, Jagedeeswaran R, Carlson JH, Ferrone S, Turley EA and McCarthy
JB: Melanoma proteoglycan modifies gene expression to stimulate
tumor cell motility, growth, and epithelial-to-mesenchymal
transition. Cancer Res. 69:7538–7547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooney CA, Jousheghany F, Yao-Borengasser
A, Phanavanh B, Gomes T, Kieber-Emmons AM, Siegel ER, Suva LJ,
Ferrone S, Kieber-Emmons T, et al: Chondroitin sulfates play a
major role in breast cancer metastasis: A role for CSPG4 and CHST11
gene expression in forming surface P-selectin ligands in aggressive
breast cancer cells. Breast Cancer Res. 13:R582011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burg MA, Grako KA and Stallcup WB:
Expression of the NG2 proteoglycan enhances the growth and
metastatic properties of melanoma cells. J Cell Physiol.
177:299–312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garusi E, Rossi S and Perris R: Antithetic
roles of proteoglycans in cancer. Cell Mol Life Sci. 69:553–579.
2012. View Article : Google Scholar
|
|
19
|
Stallcup WB and Huang FJ: A role for the
NG2 proteoglycan in glioma progression. Cell Adhes Migr. 2:192–201.
2008. View Article : Google Scholar
|
|
20
|
Burg MA, Tillet E, Timpl R and Stallcup
WB: Binding of the NG2 proteoglycan to type VI collagen and other
extracellular matrix molecules. J Biol Chem. 271:26110–26116. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yap J, Salamonsen LA, Jobling T, Nicholls
PK and Dimitriadis E: Interleukin 11 is upregulated in uterine
lavage and endometrial cancer cells in women with endometrial
carcinoma. Reprod Biol Endocrinol. 8:63–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Winship A, Cuman C, Rainczuk K and
Dimitriadis E: Fibulin-5 is upregulated in decidualized human
endometrial stromal cells and promotes primary human extravillous
trophoblast outgrowth. Placenta. 36:1405–1411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Osada T, Wang Y, Yu L, Sakakura K,
Katayama A, McCarthy JB, Brufsky A, Chivukula M, Khoury T, et al:
CSPG4 protein as a new target for the antibody-based immunotherapy
of triple-negative breast cancer. J Natl Cancer Inst.
102:1496–1512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Wang Y, Yu L, Sakakura K, Visus C,
Schwab JH, Ferrone CR, Favoino E, Koya Y, Campoli MR, et al: CSPG4
in cancer: Multiple roles. Curr Mol Med. 10:419–429. 2010.
View Article : Google Scholar : PubMed/NCBI
|