Introduction
Most cancer deaths are not due to primary tumors,
but instead due to metastasis (1).
Metastasis arises from biological and physical changes of cancer
cells in the primary tumor based on the following process: i)
detachment from primary tumor, ii) penetration into vasculature
(intravasation), iii) circulation in vasculature, iv) penetration
out of vasculature to surrounding organ tissue (extravasation), and
v) seeding and secondary tumor formation. In order for cells to
undergo the first step of detachment from the primary tumor, cells
undergo a process known as epithelial to mesenchymal transition
(EMT). This motility allows for the cells to move into the blood
vessels in the process known as intravasation. After intravasation,
the cancer cells are able to travel throughout the body either to
localized or distant sites throughout the circulation. They are
therefore dubbed 'circulating tumor cells' (CTCs) before undergoing
either adhesion to the blood vessel walls or entrapment into
smaller diameter blood vessels.
Detection of cancer metastasis is challenging since
most metastasizing cancers are only detected after a sizable
formation of secondary tumors. Early detection technology for
metastasis has focused on identifying CTCs obtained from blood
samples. These blood samples are tested for specific cell surface
markers to identify the CTCs. Most clinically used CTC detection
devices, such as CellSearch®, rely on antibody-based
detection of cytokeratins as well as EpCAM, a widely accepted CTC
marker due to its expression in epithelial cancers (2–5).
Despite the advances in CTC detection, detecting metastasis is
still challenging due to the low detection of CTCs, false
positives, and changing nature of the cells.
Although metastasis involves progression via blood
circulation, the effects of blood circulation on breast cancer CTCs
have not been extensively studied. CTCs inherently experience fluid
shear stress (FSS) in the blood. In the tumor, cells experience
interstitial flow with an average shear stress of 0.1
dyn/cm2, but CTCs in blood flow experience a higher FSS
range that averages from 0.5–30.0 dyn/cm2 that are
calculated by using the Hagen-Poiseuille equation (6). Survival of CTCs in blood circulation
makes them unique from other non-hematological cells. It has been
observed that cancer cells, including breast cancer cells, have a
higher resistance to FSS than non-cancerous cells (7). The resistance of CTCs in FSS is
believed to be due to these cells being of cancer stem cell (CSC)
origin (8,9).
CSCs are a subpopulation of cancer cells that have
stem cell-like properties, which allows them to self-renew and
differentiate into bulk tumor cells. The CSC population is
therefore believed to be responsible for tumor initiation and
relapse (10,11). CSCs have been identified in many
types of cancers, including breast cancer, in which they are
identified as
CD44+/CD24−/low/ALDH+ cells
(12,13). CSC population in in vitro
conditions are affected by the culture conditions (14). Typically, breast cancer cell lines
are cultured in tissue culture flasks adherently, but recent
studies show that mammosphere cultures better retain the CSC
population of breast cancer (15).
However, the characteristics of CSCs in FSS conditions are
understudied.
Therefore, in this study we mimicked the biological
FSS on normal breast and breast cancer cells with an in
vitro fluid flow model. This model allowed us to assess how FSS
affects the important phenotypes such as EMT, CTC, and CSC marker
expression that arise during metastasis. Our model provides a new
understanding of the relationship between CTC- and CSC-signatures
for breast cancer cells in FSS.
Materials and methods
Cell culture
Three breast cancer cell lines MCF7, MDA-MB-231, and
SKBR3 (ATCC, Manassas, VA, USA) were cultured in phenol-red free
Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, NY,
USA) supplemented with 1% L-glutamine (Life Technologies, Carlsbad,
CA, USA), 10% Fetal Bovine Serum (FBS) (Gibco), and 1%
penicillin/streptomycin (Corning, Manassas, VA, USA). The
non-cancerous breast epithelial cell line 184A1 (ATCC) was cultured
in mammary epithelial basal medium (MEBM) (Lonza) supplemented with
0.005 mg/ml transferrin, 1 ng/ml cholera toxin, and 1%
penicillin/streptomycin (Corning). All cell lines were cultured
with their respective recommended protocols. MCF7 and MDA-MB-231
mammospheres were formulated by culture in phenol-red free DMEM:
Nutrient Mixture F12 (DMEM/F12) (Gibco) supplemented with 1X B27
(Gibco), 5 mg/l insulin (MBL International Corp., Woburn, MA, USA),
20 µg/l bFGF (Shenandoah Inc., Warwick, PA, USA), 20
µg/l EFg (Shenandoah Inc.), 1% penicillin/streptomycin
(Corning), 0.5 mg/l hydrocortisone (Sigma Aldrich, St. Louis, Mo,
USA), and 2.5 mM L-glutamine (Life Technologies) modified from
(16).
Fluid shear stress (FSS) and flow
apparatus
A Fusion 200 Series syringe pump (Chemyx, Stafford,
TX, USA) was used in applying FSS on the cell lines in Polyether
ether-ketone (PEEK) tubing (IDEX Health & Science) with a 46 cm
length and 125 µm inner diameter. A 3 ml syringe (BD
Sciences) was attached to the PEEK tubing by a leur-lok and leur
tight fitting (IDEX Health & Science) (Fig. 1A).
FSS was calculated using Hagen-Poiseuille's equation
for blood flow. A FSS of 60 dyn/cm2 was used in this
experiment which correlates to the higher FSS cells experience
during blood flow. The time of FSS ranged to approximately 30 min
for a 2 ml volume. The fluid viscosity of the cell media,
1.0×10−3 kg/m/sec, was used for µ. The inner
diameter of the tubing was used for d. The volumetric flow rate was
calculated to be 68.9 µl/min for a FSS of 60
dyn/cm2.
All experiments were performed at ambient conditions
in a biological hood for sterile conditions. Cells were collected
in PBS after passaging at 5×105 cells/ml and drawn up
manually into the syringe. The leur-lok, tight fitting, and PEEK
tubing were attached to the syringe. The syringe was placed in the
syringe pump and set at the calculated flow rate. Dispelled cells
were collected in open 1.5 ml centrifuge tubes. The cells were
centrifuged down and were either evaluated immediately for flow
cytometry (within 5 min of collection) or flash frozen as qRT-PCR
samples for later RNA isolation.
Fluid flow modeling
COMSOL Multiphysics 4.3 with the computational fluid
dynamics (CFD) module (Burlington, MA, USA) was used to model the
experimental set-up with flow in the syringe and tubing. Water
fluidic settings was used and the following parameters were
specified: 8.66 mm for the syringe diameter, 6.5 cm for the syringe
length, 1 cm for nozzle of syringe, 125 µm for the diameter
of the tubing, 45 cm for the length of the tubing, and non-slip
boundary conditions. These parameters match the experimental setup
described above. The velocity profile and FSS profiles were
obtained for the given set-up.
Cell viability experiment
Cell viability of the four cell lines were tested
immediately (within 5 min) after applying 20 dyn/cm2 and
60 dyn/cm2 FSS. Viability of non-sheared cells (i.e., 0
dyn/cm2) was measured as control. Cells were inserted
into a 3 ml syringe at a density of 3×105 cells/ml and
placed in the fluid flow set-up as previously mentioned. Cells were
collected at 10 min time intervals for three time periods. Cell
viability was tested using trypan blue (Amresco, Solon, OH, USA) to
stain for a live/dead cell count in the T20 automated cell counter
(Bio-Rad).
qRT-PCR
Primers were designed by retrieving nucleotide
sequences from NCBI gene database as follows: CDH1 forward:
CCT CCT TGG CTC AAA CGA CA, reverse: TCC ACC ACA GTG TTC AGT CG;
CDH2 forward: TGC CTC TGG TGA TGA TAC GC, reverse: GTA CCA
AGA AGT GCC AGC CT; EpCAM forward: GTG GTT GTG GTG ATA GCA
GTT G, reverse: ACT TGT TCT GTA CTC ACC AGC; VIM forward:
AGG TTC AGG TTT CAT TCA TGC C, reverse: AGT TGG CTG TGT GTA CTG CT;
SLUG forward: TCT GGT TGT GGT ATG ACA GGC, reverse: TGT GTG
TAT ACT TGC GTG TGG A; SNAIL forward: CTG GGC TTG CTG TTC
TCA TTC, reverse: CTG CTT TGC CCA TCT GCT TAG; NANOG
forward: AAT ACC TCA GCC TCC AGC AGA TG, reverse: TGC GTC ACA CCA
TTG CTA TTC TTC; and OCT4 forward: GAG AAC CGA GTG AGA GGC
AAC C, reverse: CAT AGT CGC TGC TTG ATC GCT TG. ACTB was used as a
housekeeping gene with the primer sequences for ACTB forward: TCG
TCG CCC ACA TAG GAA and reverse: AGG GCT TCT TGT CCT TTC CTTC.
GAPDH was used as a housekeeping gene with the primer
sequences for GAPDH forward: AGA GCA CAA GAG GAA GAG AGA GAC
and reverse: AGC ACA GGG TAC TTT ATT GAT GGT. Primers were
synthesized by Eurofins Genomics (Huntsville, AL, USA).
RNA isolation was performed using GeneJet RNA
Purification kit (Thermo Fisher Scientific, Pittsburg, PA, USA)
using the manufacturer's protocol for mammalian cultured cells. RNA
quantification was performed using Qubit RNA HS assay kit and Qubit
2.0 Fluorometer (Life Technologies-Invitrogen, Carlsbad, CA, USA)
and by using NanoDrop 2000 according to manufacturer's protocol
(Thermo Scientific, Wilmington, DE, USA). Complementary DNA (cDNA)
was synthesized using qScript cDNA SuperMix (Quanta Biosciences,
Gaithersburg, MD, USA) and Mastercycler Nexus Gradient (Eppendorf,
Hauppauge, NY, USA) according to the manufacturer's protocol.
Real-time PCR was performed using PerfeCTa SYBR Green Fast Mix
(Quanta Biosciences) according to the manufacturer's protocol. Eco
Real-time PCR System (Illumina) and StepOnePlus Real-time PCR
System (Thermo Fisher Scientific) were used as the qRT-PCR
instrument. EcoStudy software (v4.0) and StepOne Software (v2.3)
were used for the data analysis using the ΔΔCt method (17).
Flow cytometry
Flow cytometry was performed using BD Accuri C6 Flow
Cytometer (BD Biosciences, San Jose, CA, USA). EpCAM mouse
anti-human IgG1 FITC conjugated antibody was used for epithelial
marker expression (BD Bioscience, cat. # 347197). Antibodies for
breast CSC marker expression included primary antibody CD24 mouse
IgG1 biotin anti-human, secondary antibody anti-biotin PE mouse
IgG1 (Miltenyi Biotec, cat. # 130-098-902 and 130-090-756,
respectively), and CD44 mouse anti-human IgG2b APC conjugated
antibody (BD Biosciences, cat. # 559942) according to
manufacturer's protocol.
Limiting dilution assay
Limiting dilution assay (LDA) was performed on MCF7
and MDA-MB-231 suspension cells under static and FSS conditions.
LDAs were seeded in 96-well plates at concentrations of 50, 20, 10,
5, and 2 cells per well (n=20 for each seeding concentration).
Cells were cultured for 10 days with feeding every 4 days. Wells
were analyzed at day 10 for sphere formation. Sphere formation was
determined as spheres with diameters ≥50 µm. Significant
statistical difference of samples were determined by a 95%
confidence interval (CI). Clonogenicity index was defined as slope
± SE of the LDA linear regression (18).
Western blotting
Adherent, suspension static, and suspension FSS MCF7
and MDA-MB-231 cells were lysed in NP40 buffer (Amresco) with 0.5 M
EDTA (G-Bioscience, St. Louis, MO, USA), protease inhibitor
(G-Bioscience), phosphatase inhibitor (Sigma), and nuclease (Thermo
Scientific). Protein concentrations were measured by BSA
(G-Bioscience). Protein was loaded for western blotting. Skim milk
(5%) (Criterion) was used for membrane blocking and then stained
with following antibodies: ACTB (GeneTex, cat. # GTX26276), NANOG
(Thermo Scientific, cat. # MA1-017), and OCT4 (Thermo Scientific,
cat. # MA5-15736). Western blots were developed in a digital imager
(Bio-Rad ChemiDoc MP) and normalized to ACTB. Protein expression
was quantified using Image lab software (Bio-Rad).
Statistical analysis
Results were analyzed using Student's two tailed
t-test and ANOVA with equal or unequal variance in Minitab 17
(Minitab Inc., State College, PA, USA). F-test and Levene's test
were used to determine equal or unequal variance prior to t-test
and ANOVA. Differences with p-values <0.05 were considered
significant.
Results
Breast epithelial and cancer cells have
varying tolerance to FSS
Computational modeling of the fluid flow was
generated based on the experimental set-up of an in vitro
model that mimics flow in the blood vessels (Fig. 1A). COMSOL modeling of the
experimental set-up showed that fluid flow becomes fully developed
laminar flow at 0.05 mm past the entrance into the tubing, which
correlates to 0.01% of the length of the tubing used in our set-up
(Fig. 1B). Therefore, entry
effects in the tubing were considered insignificant. A fluid flow
rate of 23.0 µl/min and 68.9 µl/min was calculated
using the Hagen-Poiseuille equation for 20 and 60
dyn/cm2 of FSS, respectively. COMSOL modeling further
demonstrated that the FSS in the syringe is negligible compared to
the FSS in the tubing (Fig. 1C).
COMSOL modeling therefore confirmed that our set-up could be used
to apply FSS in a controlled manner.
FSS has the potential to detrimentally affect cells
and even cause cell death. Therefore, cell viability was evaluated
in our experimental set-up under conditions of FSS. Physiological
FSS values of 20 and 60 dyn/cm2 were tested on the
184A1, MCF7, SKBR3, and MDA-MB-231 cell lines. Cell viability under
FSS was normalized to the control conditions of the cell lines at 0
dyn/cm2. The breast epithelial cell line 184A1
experienced a significant decrease in cell viability with only half
of the 184A1 cells surviving under FSS conditions. The MCF7 and
MDA-MB-231 cell line viability continued to remain high under FSS
conditions (Fig. 1D).
Breast cancer cell lines have different
baseline EMT gene expressions
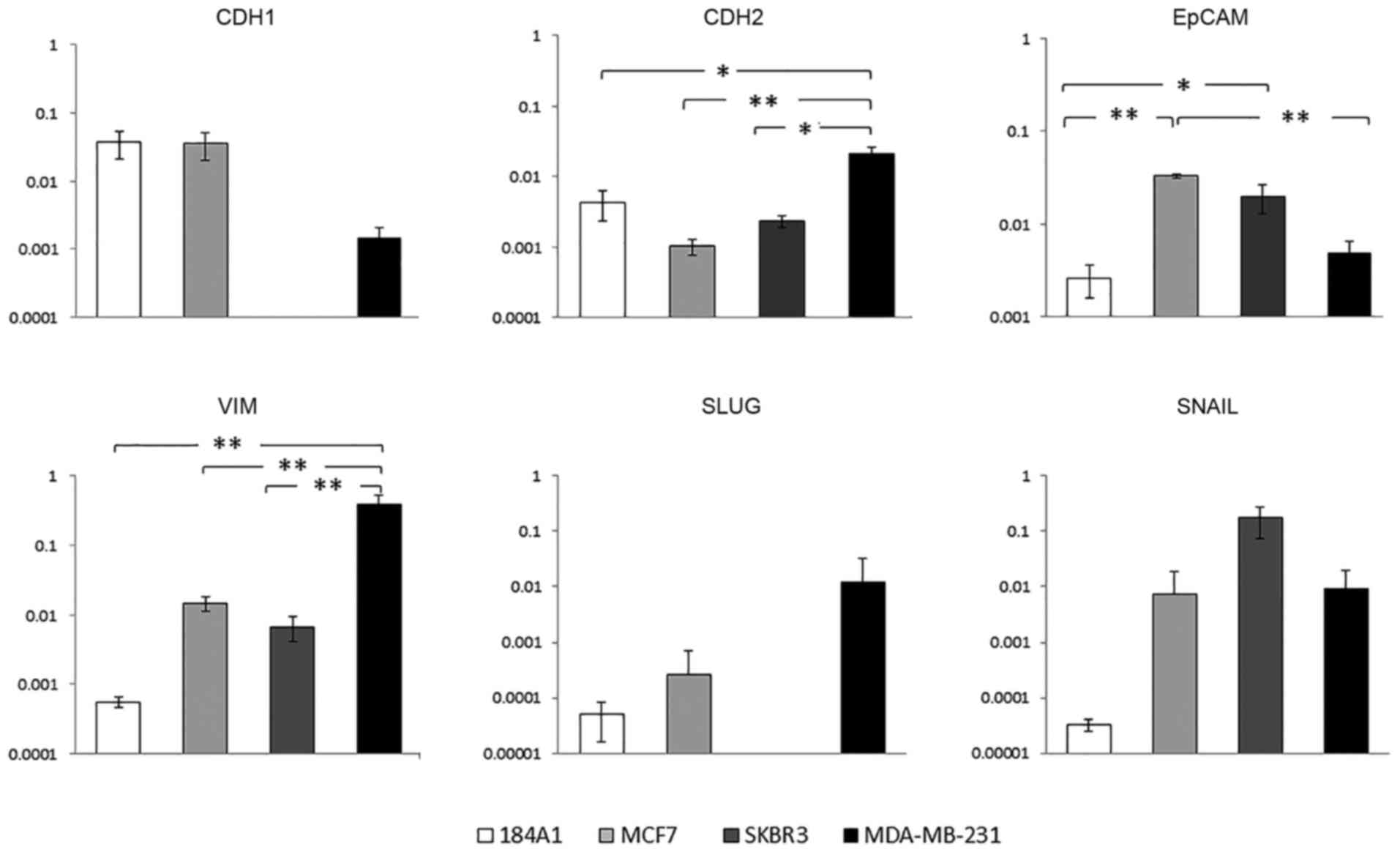
EpCAM (CTC marker), CDH1 (E-cadherin; epithelial
marker), CDH2 (N-cadherin; mesenchymal marker), VIM, SLUG, and
SNAIL (mesenchymal markers), expression in 184A1, MCF7, SKBR3, and
MDA-MB-231 were examined (Fig. 2)
(19–21). One of the most noted features of
metastasis has been the transition of cells from an epithelial
phenotype to a mesenchymal phenotype through EMT. The CDH1
epithelial marker, which is involved in epithelial cell adhesion,
had higher expression in the MCF7 and 184A1 cell lines (Fig. 2). The more invasive MDA-MB-231 cell
line had significantly higher gene expression of CDH2 and VIM
(p<0.05 and p<0.01, respectively). This correlates to CDH2
and VIM being mesenchymal markers involved in invasion and
migration (22). EpCAM was
expressed significantly higher in the MCF7 and SKBR3 breast cancer
cell lines (p<0.05 and p<0.01, respectively). Moreover, 184A1
breast epithelial cell line had low EpCAM expression. EpCAM
expression is not solely used as an EMT marker, but instead is
commonly used as a CTC marker (23). As a CTC marker, EpCAM is seen to be
highly expressed in cancer cells compared to non-cancerous
epithelial cells allowing for epithelial based cancer detection.
Therefore, variable gene expression of the cell lines was due to
their different cellular characteristics. The MCF7 and MDA-MB-231
cell lines were used for further studies due to their difference in
gene expression and higher cell viability under FSS.
Suspension culture induces EMT gene
expression in MCF7
Since cells typically undergo EMT as the first steps
towards metastasis, EMT gene expression was examined with the
expectation of an increase in mesenchymal gene expression and a
decrease in epithelial gene expression. MCF7 experienced no
significant expression change under FSS conditions in comparison to
suspension static culture (Fig. 3;
p>0.05). Furthermore, MDA-MB-231 had no significant changes in
gene expression between the adherent and suspension cultures
(p>0.05). On the other hand, MCF7 suspension culture cells
showed a significant increase in gene expression of EpCAM, CDH1,
and CDH2 compared to the adherent culture cells (p<0.001,
p<0.1, and p<0.5, respectively). The largest increase in gene
expression from the adherent to suspension cultures was seen with
CDH2 (20- to 30-fold increase; Fig.
3). EpCAM and CDH1 expression change was more modest
(approximately 8- and 4-fold increase, respectively). These results
suggest that there is a strong EMT induction when culturing MCF7 in
suspension culture media and that FSS does not negatively impact
this induction in MCF7.
MCF7 is more susceptible to changes in
CSC expression
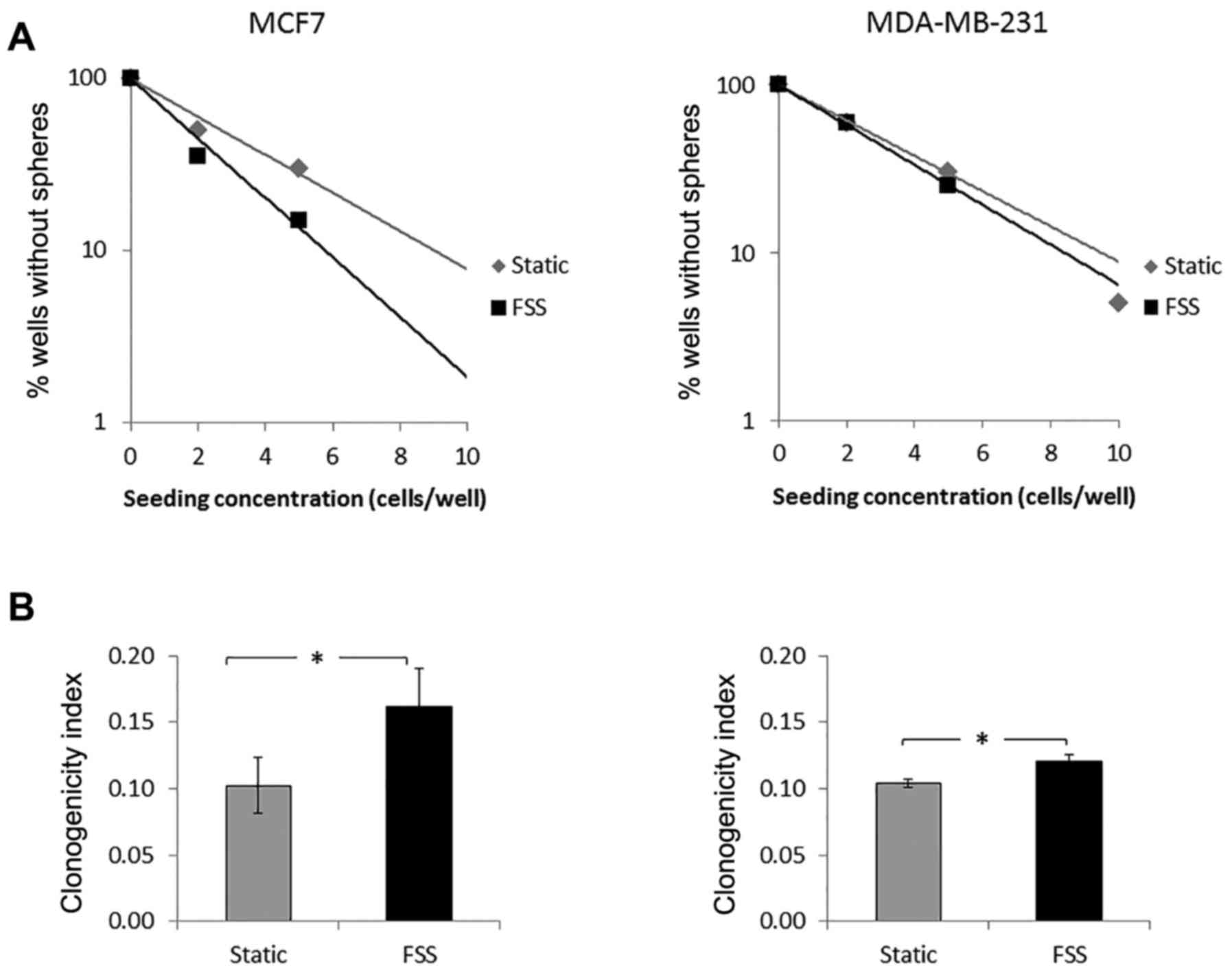
To assess clonogenicity, limiting dilution assays
(LDAs) were performed on MCF7 and MDA-MB-231 suspension static and
suspension FSS cells (Fig. 4). We
observed a statistically significant increase in clonogenicity upon
applying FSS to both cells, but there was a higher increase of
clonogenicity with MCF7 cells at 1.6-fold (vs. 1.2 for MDA-MB-231).
Changes in stem cell marker expression upon FSS were further
explored using the stemness markers NANOG and OCT4. A higher
stemness expression was observed in MCF7 suspension culture than in
adherent cultured cells (Fig. 5A and
B). There was a further increase in the stemness marker
expression upon FSS at both protein and transcript levels,
corroborating the data seen with the LDAs. No increase of the
stemness marker expression was seen in the MDA-MB-231 western blot
data, though there was a significant increase observed for the OCT4
transcript (Fig. 5B). However, the
fold change for OCT4 was much greater in MCF7 than in
MDA-MB-231.
As a more specific measure of breast CSC expression,
surface protein expression of the CD24 and CD44 markers was tested
with MCF7 and MDA-MB-231 in adherent static, suspension static, and
suspension FSS conditions. MCF7 CD44+/CD24−
expression increased significantly from the adherent culture to
suspension static culture with approximately a 25% increase in
expression (from 7% expression to 31% expression, respectively)
(Fig. 5C). This was expected as
serum-free culture is known to concentrate CSCs (15,24).
CD44+/CD24− expression also increased in MCF7
suspension static to suspension FSS conditions from 31 to 46%,
respectively. This showed that MCF7 cells can be induced to a
higher CSC population by changes in culture conditions from
adherent to suspension and under FSS conditions. The MDA-MB-231
cell line showed no changes in CD44+/CD24−
expression and remained consistently high at over 80% expression in
all three samples.
Since EpCAM is a clinically used surface protein
marker for CTCs, EpCAM expression was tested using flow cytometry.
As seen with qRT-PCR, MCF7 had a higher overall EpCAM expression
than MDA-MB-231 (Fig. 5D). There
was an increase in EpCAM for MCF7 suspension FSS cells from the
static conditions. However, only 7% of the MDA-MB-231 expressed
EpCAM in the adherent static conditions. This dropped to 1.57 and
1.13% in the suspension static and suspension FSS cells,
respectively.
Discussion
The changes in cancer cell characteristics are
important in understanding the metastatic process. However, cancer
metastasis is difficult to model in experimental research. Most
research relies on animal models to study tumor formation and
distant colonization (25,26). These studies provide an ultimate
outcome when metastasis occurs, but provide little insight on the
processes. Although this study did not focus on the direct
mechanism of FSS on metastatic potential, there have been previous
studies of the effects of FSS in the biological pathway Tie2
(9,27,28).
One study focused on the Tie2 pathway where FSS as high as 20
dyne/cm2 was found to activate Tie2 and PI3k/Akt
phosphorylation in endothelial cells (28). Furthermore, PI3k/Akt activation has
been seen in ovarian carcinoma under low FSS condition (9) and most recently has been reported
that Tie2 regulates stemness and metastatic properties in prostate
cancer (27). However,
metastasizing cancer cell properties have not been thoroughly
studied under the effects of fluid shear stress (FSS). Blood flow
and its components can have both a negative and positive effect on
circulating tumor cells (CTCs). Studies have shown CTC-platelet
adhesion aids in CTC survival by protecting them from FSS (29,30).
CTCs have also been seen to have higher viability under FSS than
their non-cancerous counterparts (Fig.
1) (7,31). Therefore, these previous reports
and our data motivated us to examine EMT, CTC, and CSC marker
expression of cells under FSS as a model for metastasis.
Since EMT is an important first step of metastasis,
it was hypothesized that FSS can induce EMT expression in breast
cancer cell lines. An increase in mesenchymal gene expression and a
decrease in epithelial gene expression were originally expected in
MCF7 and MDA-MB-231. In this study, however, we did not observe a
decrease in epithelial gene expression. Nevertheless, EMT gene
expression (i.e. mesenchymal) was high in non-adherent cells. Other
models have shown that cells do not need to undergo complete EMT in
order to metastasize to secondary tumors (32,33).
Previous studies have shown that CDH2 can promote motility in
certain breast cell lines regardless of high CDH1 expression
(34). It is possible that only a
partial EMT is occurring where cells maintain their epithelial
characteristics while still undergoing upregulation of mesenchymal
markers.
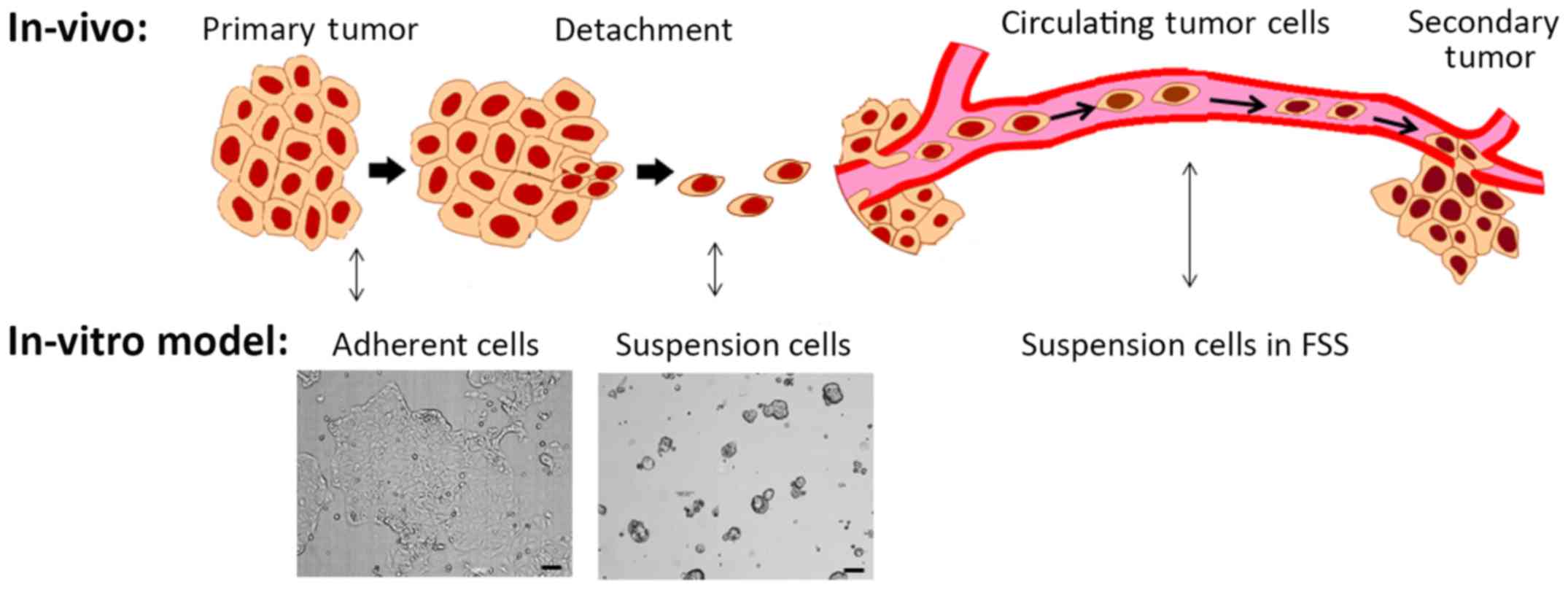
Based on the above results, we proposed an in
vitro model of metastasis that incorporated different culture
conditions and FSS (Fig. 6).
Adherent static cultured cells can be thought to represent cancer
cells in the solid primary tumor due to the solid tumor stiff
epithelial morphology. Suspension cultured cells are likely models
of cells detaching from the primary tumor. CTCs were then modeled
as suspension cultured cells under FSS. Testing the EpCAM, CDH1,
and CDH2 markers showed that our 'detached' cells were the most
prone to EMT expression and that FSS did not attenuate this
response.
Cancer stem cells (CSCs) have been suggested as an
important factor contributing to metastasis. As a test of stemness
and proliferation, clonogenicity via limiting dilution assay was
used on suspension static and suspension FSS cells. A significant
increase in clonogenicity was seen in both MCF7 and MDA-MB-231
suspension FSS compared to suspension static culture (Fig. 4). MCF7 had a higher increase in
clonogenicity of suspension FSS from static conditions in
comparison to MDA-MB-231. This was further corroborated with NANOG
and OCT4 stemness marker expression where MCF7 increased in
suspension FSS compared to suspension static culture (Fig. 5A). Similar to the LDAs, OCT4
significantly increased in suspension FSS culture for both cell
lines but, a larger fold increase was seen in MCF7 than MDA-MB-231
(Fig. 5B).
Breast CSC markers were tested under FSS. The
individual marker expression for CD24 and CD44 was initially tested
from cells under FSS. As expected and seen in other reports
(35), the less invasive MCF7 had
an overall lower CD44+ population and higher
CD24+. In contrast, the highly invasive MDA-MB-231 cell
line had over 90% CD44+ expression (data not shown).
Furthermore, the effect of FSS on CSC-like
CD44+/CD24− population was tested on adherent
and suspension cultured cells. Overall, MDA-MB-231 had a
consistently high CSC signature that did not change with FSS or
culture conditions. However, the CSC signature increased in the
suspension culture and in FSS conditions for MCF7 (Fig. 5D), which corroborates the
correlation between CTCs and CSCs previously seen in cells
undergoing EMT (36,37). Furthermore, our data also showed
that FSS induced CSC-like expression in the MCF7 cell line.
Our data demonstrated that the MCF7 cell line was
overall more susceptible to changes in EMT and CSC expression.
Although FSS induced an increase in CSC expression, the culture
conditions of the cell line had a larger impact on expression
changes. However, the MDA-MB-231 cell line was more resistant to
changes in both gene and protein expression. This is most likely
due to differences in the cell line characteristics. Breast cancer
can be divided into the following four subtypes in order of
increasing invasiveness and severity: luminal A, luminal B, human
epidermal growth factor receptor 2 (HER2)-positive and
triple-negative (TNBC) (38,39).
Dividing breast cancer into these subtypes has allowed for better
characterization according to their genetic makeup. These subtypes
can also be characterized by the expression status of three key
cell surface hormone receptor proteins: estrogen receptor (ER),
progesterone receptor (PR), and HER2. Luminal A breast cancers are
ER+/PR+/HER2−, and triple-negative
are ER−/PR−/HER2−. MCF7, a
non-invasive breast cancer, belongs to the luminal A subtype, while
MDA-MB-231, an invasive breast cancer, belongs to the TNBC subtype
(40). MDA-MB-231, which is
already highly invasive and more aggressive as seen in both EMT and
CSC gene expression, is therefore more resistant to changes in
culture conditions and FSS.
In conclusion, the importance of cancer metastasis
has resulted in a variety of new detection devices and treatment
options; however, the effects of FSS on cancer cells have not been
exclusively studied. Our study addresses these issues by using an
in vitro model of FSS on breast cancer cells. Herein, we
found that the effect of FSS is dependent on breast cancer subtype.
Culture conditions also had a large effect on EMT/CTC and CSC
expression which was used as a model for metastasis (Fig. 6).
Acknowledgments
We are grateful to Dr John W. Van Zee and Dr Matthew
Jenny for providing equipment support. We are also grateful to Ria
Corder and Brittney Sunday for providing technical assistance, and
to Dr Dong Woon Kim for his critical reading and input to our
manuscript. This material is based upon work supported by the
National Science Foundation (NSF) under grant no. 1342388 and
1604677. N.L.K. was a participant of the Research Experience for
Undergraduates (REU) at the University of Alabama, supported
through the NSF REU Site grant no. 1358750.
Glossary
Abbreviations
Abbreviations:
|
FSS
|
fluid shear stress
|
|
EMT
|
epithelial to mesenchymal
transition
|
|
CSC
|
cancer stem cell
|
|
CTC
|
circulating tumor cell
|
References
|
1
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong Y, Skelley AM, Merdek KD, Sprott KM,
Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP and Smirnov DA:
Microfluidics and circulating tumor cells. J Mol Diagn. 15:149–157.
2013. View Article : Google Scholar
|
|
3
|
Li P, Stratton ZS, Dao M, Ritz J and Huang
TJ: Probing circulating tumor cells in microfluidics. Lab Chip.
13:602–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedlander TW, Premasekharan G and Paris
PL: Looking back, to the future of circulating tumor cells.
Pharmacol Ther. 142:271–280. 2014. View Article : Google Scholar
|
|
5
|
Broersen LH, van Pelt GW, Tollenaar RA and
Mesker WE: Clinical application of circulating tumor cells in
breast cancer. Cell Oncol (Dordr). 37:9–15. 2014. View Article : Google Scholar
|
|
6
|
Mitchell MJ and King MR: Computational and
experimental models of cancer cell response to fluid shear stress.
Front Oncol. 3:442013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barnes JM, Nauseef JT and Henry MD:
Resistance to fluid shear stress is a conserved biophysical
property of malignant cells. PloS One. 7:e509732012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ip CK, Li SS, Tang MY, Sy SK, Ren Y, Shum
HC and Wong AS: Stemness and chemoresistance in epithelial ovarian
carcinoma cells under shear stress. Sci Rep. 6:267882016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nguyen LV, Vanner R, Dirks P and Eaves CJ:
Cancer stem cells: An evolving concept. Nat Rev Cancer. 12:133–143.
2012.PubMed/NCBI
|
|
11
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
14
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liao M-J, Zhang CC, Zhou B, Zimonjic DB,
Mani SA, Kaba M, Gifford A, Reinhardt F, Popescu NC, Guo W, et al:
Enrichment of a population of mammary gland cells that form
mammospheres and have in vivo repopulating activity. Cancer Res.
67:8131–8138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
18
|
Haley EM, Tilson SG, Triantafillu UL,
Magrath JW and Kim Y: Acidic pH with coordinated reduction of basic
fibroblast growth factor maintains the glioblastoma stem cell-like
phenotype in vitro. J Biosci Bioeng. Jan 4–2017.Epub ahead of
print. View Article : Google Scholar
|
|
19
|
Mallini P, Lennard T, Kirby J and Meeson
A: Epithelial-to-mesenchymal transition: What is the impact on
breast cancer stem cells and drug resistance. Cancer Treat Rev.
40:341–348. 2014. View Article : Google Scholar
|
|
20
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Roy F: Beyond E-cadherin: Roles of
other cadherin super-family members in cancer. Nat Rev Cancer.
14:121–134. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schnell U, Cirulli V and Giepmans BN:
EpCAM: Structure and function in health and disease. Biochim
Biophys Acta. 1828:1989–2001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan X, Curtin J, Xiong Y, Liu G,
Waschsmann-Hogiu S, Farkas DL, Black KL and Yu JS: Isolation of
cancer stem cells from adult glioblastoma multiforme. Oncogene.
23:9392–9400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bos PD, Nguyen DX and Massagué J: Modeling
metastasis in the mouse. Curr Opin Pharmacol. 10:571–577. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saxena M and Christofori G: Rebuilding
cancer metastasis in the mouse. Mol Oncol. 7:283–296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang KD, Holzapfel BM, Liu J, Lee TK, Ma
S, Jovanovic L, An J, Russell PJ, Clements JA, Hutmacher DW, et al:
Tie-2 regulates the stemness and metastatic properties of prostate
cancer cells. Oncotarget. 7:2572–2584. 2016.
|
|
28
|
Lee HJ and Koh GY: Shear stress activates
Tie2 receptor tyrosine kinase in human endothelial cells. Biochem
Biophys Res Commun. 304:399–404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Green DL and Karpatkin S: Effect of cancer
on platelets. Coagulation in Cancer. Kwaan CH and Green D:
Springer; Boston, MA: pp. 17–30. 2009, View Article : Google Scholar
|
|
30
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mitchell MJ, Denais C, Chan MF, Wang Z,
Lammerding J and King MR: Lamin A/C deficiency reduces circulating
tumor cell resistance to fluid shear stress. Am J Physiol Cell
Physiol. 309:C736–C746. 2015.PubMed/NCBI
|
|
32
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reymond N, d'Água BB and Ridley AJ:
Crossing the endothelial barrier during metastasis. Nat Rev Cancer.
13:858–870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nieman MT, Prudoff RS, Johnson KR and
Wheelock MJ: N-cadherin promotes motility in human breast cancer
cells regardless of their E-cadherin expression. J Cell Biol.
147:631–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ricardo S, Vieira AF, Gerhard R, Leitão D,
Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes
J: Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression
distribution within intrinsic molecular subtype. J Clin Pathol.
64:937–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mani SA, Guo W, Liao M-J, Eaton EN,
Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et
al: The epithelial-mesenchymal transition generates cells with
properties of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu
Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD, et al:
Breast cancer stem cells transition between epithelial and
mesenchymal states reflective of their normal counterparts. Stem
Cell Rep. 2:78–91. 2013. View Article : Google Scholar
|
|
38
|
Sanpaolo P, Barbieri V and Genovesi D:
Prognostic value of breast cancer subtypes on breast cancer
specific survival, distant metastases and local relapse rates in
conservatively managed early stage breast cancer: A retrospective
clinical study. Eur J Surg Oncol. 37:876–882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Paquet ER and Hallett MT: Absolute
assignment of breast cancer intrinsic molecular subtype. J Natl
Cancer Inst. 107:dju3572015. View Article : Google Scholar
|
|
40
|
Holliday DL and Speirs V: Choosing the
right cell line for breast cancer research. Breast Cancer Res.
13:2152011. View Article : Google Scholar : PubMed/NCBI
|