Introduction
Hepatocellular carcinoma (HCC) is a highly malignant
disease with extremely poor prognosis (1). Due to its difficult early diagnosis,
high malignancy, and most importantly the ineffectiveness of
treatments using radiotherapy and chemotherapy, HCC is the third
leading cause of cancer deaths worldwide, with more than 700,000
deaths each year (2,3). Conventional surgical resection is
still the major treatment strategy for HCC (4). However, the overall survival rate
after hepatic resection remains low. Finding new therapeutic
strategies and understanding the molecular mechanisms underlying
liver tumor formation, cancer progression, recurrence and
metastasis may contribute to discover more effective methods and
liver cancer treatment targets.
Quercetin (3,3′,4′,5,7-pentahydroxy-flavone), a
flavonoid found in a wide variety of plants and present in human
diet (5). It is a
naturally-occurring flavone that is found at high concentrations in
different berries, onions, apples, and red wine (6,7).
Quercetin exhibits beneficial effects on human health with its
broad pharmacological properties, including anti-inflammation and
anti-oxidation (8). Quercetin has
selective anti-proliferative and antitumor effects via apoptotic
mechanisms on different human cancer cell lines. Quercetin
treatment resulted in cell cycle arrest during G0/G1 in leukemia, S
phase in colorectal carcinoma and G2/M phases of the cell cycle in
leukemia, breast carcinoma, as well as esophageal adenocarcinoma
cells (9–11).
Currently, the application of polymeric drug
delivery systems, including polymeric nanoparticles is regarded as
one promising strategy for disease prevention or treatment
(12–15). Nanoparticles have advantages in
comparison to the traditional chemotherapeutic drugs. Nanoparticles
may further enhance the tumor-targeted delivery through regulation
of the nanoparticle surface with specific tumor or cancer cell
targeting ligands, including biotin, folic acid, and antibodies
(16). In addition, nanoparticles
could transport numerous types of agents via different antitumor
mechanisms, such as a chemotherapeutic drug combined with a
chemosensitizer, displaying synergistic anticancer effects
(17). Further, nanoparticles help
to accumulate higher tumor encapsulated drugs through the promoted
effect of permeability and retention (18). However, the precise molecular
mechanism of quercetin nanoparticle action against liver cancer has
not been elucidated, which prompted us to evaluate the effects of
quercetin nanoparticles on liver cancer through apoptosis induction
and proliferation inhibition in liver cancer cells.
Here, quercetin nanoparticles were used to achieve
the promoted effects on liver cancer suppression through multiple
cell signaling pathways. The role of quercetin nanoparticles in
cell viability, cell morphology, apoptosis, colony formation, as
well as cell migration was investigated in our study to reveal the
underlying molecular mechanisms. It was the first time that
quercetin nanoparticles inhibited liver cancer progression via
regulation of signaling pathways, including caspase/Cyto-c,
NF-κB/COX-2, AP-2β/hTERT, as well as Akt/ERK1/2, which possible
provide therapeutic strategies for liver cancer suppression.
Materials and methods
Quercetin nanoparticle preparation
High performance liquid chromatography (HPLC)-grade
quercetin was purchased (>98%, Sigma-Aldrich, USA) in an
anhydrous powdered form. Gold nanoparticles (AuNPs) were
synthesized by reducing 1 mM gold chloride with a freshly prepared
quercetin solution in absolute alcohol. The pale-yellow solution
turned to deep red as the quercetin nanoparticles were formed. PLGA
(50 mg) was added to an aqueous dispersion of AuNPs. Next, we added
this mixture drop-wise to 20 ml of an aqueous solution with a
stabilizer (1% polyoxyethylene-polyoxypropylene; F68). The mixture
was stirred at 400 rpm and 4°C until the organic solvent had
evaporated completely. The redundant stabilizer was removed by
repeated washing and centrifugation (25,000 x g and 4°C for 30
min), and the pellet was then resuspended in Milli-Q water. The
quercetin nanoparticles were stored at 4°C for further study.
Fluorescent dye was conjugated to the gold surface by adding FITC
dye to the PLGA and quercetin nanoparticle mixture, which were
performed in the dark. In addition, scanning transmission electron
microscopy (SEM) (FEI Quanta650, USA) was used to determine the
size of naked quercetin nanoparticles. Further, dynamic light
scattering (DLS) with LB-550 DLS particle size analyzer (Horiba
Scientific, Edison, NJ, USA) was used to examine the average size
of quercetin nanoparticles. We analyzed the data in the automatic
mode. Size is presented as the mean value of 20 runs, with
triplicate measurements for each run. We measured the zeta
potential of the quercetin nanoparticles in the same instrument
using the same procedure.
Cell culture
The human liver cancer cell lines of MHCC97H, Hep3B,
HCCLM3 and Bel7402 were obtained from American Type Culture
Collection (ATCC, VA, USA). Cells were cultured as monolayers in
RPMI-1640 culture media supplemented with 10% heat-inactivated
fetal bovine serum, 1×105 U/l streptomycin sulfate, pH
7.2 (Gibco Corp., Gaithersburg, MD, USA) with a concentration of
1×106/ml at 37°C, 5% CO2 at 37°C.
Cell viability assay
Liver cancer cell viability was assessed using MTT
assay (Roche Diagnosis, IN, USA). In brief, liver cancer cell lines
were planted at 4×103 cells/well in 96-well plates.
Cells were then cultured overnight, and next the cells were changed
into fresh medium with various doses of quercetin nanoparticles
dissolved in DMSO with final concentration of 0.1%. After
incubation for 48 h, the cell growth was measured. The cell
viability was assessed as the percent cell viability compared to
the vehicle-treated control cells without quercetin nanoparticles
administration, which were determined arbitrarily as 100%
viability.
Colony formation assays
One hundred liver cancer cells per well in 60-mm
plates were cultured in 10% FBS DMEM. Cells were treated with
quercetin nanoparticles of the indicated concentrations for 24 h.
After another 7 days of incubation, the cell colonies were washed
twice with PBS, fixed with 4% paraformaldehyde for 15 min and then
stained by Giemsa for 30 min. Each clone with >50 cells were
evaluated. Clone forming efficiency for cells was calculated based
on: Plate colony formation inhibitory ratio = (number of colonies
treated with quercetin nanoparticles / number of cells inoculated)
× 100%.
Wound-healing assay
Wound-healing assays were carried out using
migration culture dish inserts. Liver cancer cells were seeded in
the chambers of the culture dish insert and transfected.
Forty-eight hours after transfection, the insert was removed and
fresh culture medium was added to start the migration process.
Cells were treated with indicated doses of quercetin nanoparticles
in full medium and kept in a CO2 incubator. After 48 h,
medium was replaced with PBS, and images were acquired using a
Zeiss Axiovert 24 light microscope and an Axiocam MRc camera.
Flow cytometry assays
Flow cytometric assay was used to clarify cell
apoptosis. The cells were collected with trypsinisation and then
washed twice with PBS, and fixed in cold 80% ethanol, and finally
stored at 4°C overnight. The cells were washed with PBS twice and
RNase A (10 mg/ml) was administered for analysis. Propidium iodide
was then added to tubes at a concentration of 0.05 mg/ml and then
incubated for 20 min at 4°C in the dark. FITC-labeled Annexin V/PI
staining was applied according to the manufacturer's instructions
(Keygen, Nanjing, China). In brief, 1×106 cells in each
well were suspended with buffer containing FITC-conjugated Annexin
V/PI. Samples were then analyzed via flow cytometry.
Western blot analysis
For western blot analysis, sample tissues and cells
were homogenized into 10% (wt/vol) hypotonic buffer (25 mM
Tris-HCl, pH 8.0, 1 mM EDTA, 5 µg/ml leupeptin, 1 mM
Pefabloc SC, 50 µg/ml aprotinin, 5 µg/ml soybean
trypsin inhibitor, 4 mM benzamidine) to yield a homogenate.
Additionally, the final supernatants were obtained by
centrifugation at 12,000 rpm for 20 min. Protein concentration was
determined by BCA protein assay kit (Thermo, USA) with bovine serum
albumin as a standard. The total protein extract will be used for
western blot analysis. Equal amounts of total protein of tissues
were subjected to 10 or 12% SDS-PAGE followed by immunoblotting
using the primary polyclonal antibodies (Table I). Immunoreactive bands were
visualized by ECL Immunoblot Detection system (Pierce
Biotechnology, Inc., Rockford, IL, USA) and exposed to Kodak
(Eastman Kodak Co., USA) X-ray film. Each protein expression level
was defined as grey value (Version 1.4.2b, Mac OS X, ImageJ,
National Institutes of Health, USA) and standardized to
housekeeping genes (GAPDH) and expressed as a fold of control.
 | Table IPrimary antibodies for western blot
analysis. |
Table I
Primary antibodies for western blot
analysis.
| Primary
antibodies | Dilution ratio | Corporation |
|---|
| Rabbit
anti-P27 | 1:1,000 | Abcam |
| Rabbit
anti-c-Myc | 1:1,000 | Abcam |
| Rabbit
anti-cyclin-D1 | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-MMP7 | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-CDK1 | 1:1,000 | Abcam |
| Rabbit
anti-β-catenin | 1:1,000 | Abcam |
| Mouse
anti-caspase-9 | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-caspase-3 | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-Cyto-c | 1:200 | Santa Cruz
Biotechnology |
| Rabbit
anti-AP-2β | 1:1,000 | Abcam |
| Mouse
anti-hTERT | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-COX2 | 1:1,000 | Abcam |
| Rabbit
anti-IKKα | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-p-IKKα | 1:1,000 | Cell Signaling
Technology |
| Mouse
anti-IκBα | 1:1,000 | Abcam |
| Mouse
anti-p-IκBα | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-NF-κB | 1:1,000 | Abcam |
| Rabbit
anti-p-NF-κB | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-P50 | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-Akt | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-p-Akt | 1:1,000 | Cell Signaling
Technology |
| Mouse anti-Raf | 1:1,000 | Abcam |
| Rabbit
anti-ERK1/2 | 1:1,000 | Cell Signaling
Technology |
| Rabbit
anti-p-ERK1/2 | 1:1,000 | Cell Signaling
Technology |
| GAPDH | 1:200 | Santa Cruz
Biotechnology |
Real-time quantitative PCR
Total RNA from the cultured cancer cells was
obtained by the miRNA Isolation kit (Sigma, USA) according to the
manufacturer's instructions. Then the cDNA was synthesized from
RNA. Real-time PCR was conducted with the Applied Biosystems 7500
Sequence Detection system by the use of iQ™ SYBR Green Supermix
(Bio-Rad Laboratories, USA) with 5 ng cDNA and 10 pM related
primer. The cycling condition was conducted at 94°C for 60 sec;
followed by 45 cycles at 95°C for 30 sec, 58°C for 30 sec and 72°C
for 30 sec; followed by 95°C for 10 sec, 65°C for 45 sec, and 40°C
for 60 sec. The data were normalized to the housekeeping gene GAPDH
and U6 small nuclear RNA expression and calculated as
2−ΔΔCT expression. The primers used are shown in
Table II.
 | Table IIPrimer sequences used for real-time
PCR (5′→3′). |
Table II
Primer sequences used for real-time
PCR (5′→3′).
| Gene | Forward primer | Reverse primer |
|---|
| AP-2β |
GGAAGCGATCTGAGAAGTGCA |
CACTGGGAACGGTATACTGATT |
| hTERT |
CCAATCCCGCCATGATCC |
GAGAACGGATCTGCCATCACA |
| GAPDH |
GACTCATGACAGTCCATGACCC |
AGCGGAGAATGAGGTTCTTGG |
Immunofluorescence analysis
The cells grown on chamber slides were washed in PBS
and fixed for 15 min at room temperature with 4% paraformaldehyde,
followed by 20 and 30% sucrose dehydration for 24 h each. Then, the
samples were incubated with primary antibodies (Cyto-c, P50 and
NF-κB, Cell Signaling Technology, USA) at 4°C overnight after
deparaffinized and rehydrated. Fluorophore-conjugated secondary
antibodies were treated for 1 h at 25°C thermostat. The Alexa Fluor
488 labeled anti-rabbit secondary antibodies (Invitrogen, CA, USA)
and DAPI (Sigma-Aldrich) were used in this part. Samples were then
subjected to immunofluorescence staining via epifluorescence
microscopy (Sunny Co.). Leica TCS SP5 confocal microscope (Leica,
Richmond Hill, ON, Canada) was used to obtain images and carried on
blinded with respect to treatment groups. ImageJ 'measure' tool
analyzed fluorescence intensity through examining mean intensity of
each selected areas (a minimum of 10 rectangles).
Cell transfection
The transfection of targeted siRNAs or expression
vectors were conducted by Lipofectamine 2000 reagent based on the
manufacturer's protocol (Invitrogen).
DNA-protein binding by
streptavidin-agarose pull-down assay
Streptavidin-agarose pull-down assay was used to
determine the binding of AP-2β, p50 to hTERT or COX-2 core promoter
probes. A biotin-labeled double-stranded probe corresponding to
hTERT and COX-2 promoter sequence was synthesized. The binding
assay was applied by mixing 4 µg biotinylated DNA probe, 400
µg nuclear extract proteins and 40 µl 4%
streptavidin-conjugated agarose beads at room temperature for an
hour in a rotating shaker. Beads were then pelleted via
centrifugation in order to pull down the DNA-protein complex. After
washing, proteins in complex were evaluated through immunoblotting
with antibodies (1 µg/ml of each sample) specific for AP-2β
and p50. The mixture was then incubated at room temperature for 1 h
with shaking, and then centrifuged to pull down the DNA-protein
complex. DNA-bound AP-2β and p50 protein was dissociated and
analyzed by western blotting. Non-immune rabbit IgG (1
µg/ml) was used as negative controls.
Establishment of xenograft tumor
models
The mouse experiments were conducted in the Animal
Laboratory Center. MHCC97H cells (1×107 cells) treated
with or without quercetin nanoparticles were suspended in
100-µl serum-free medium and injected subcutaneously into
the left flank of 4- to 6-week old male BALB/c nu/nu nude mice.
After two weeks, when the tumor diameters reached 3×4 mm, the tumor
cell-inoculated mice were divided into four treatment groups
randomly, the control (Con) treated with PBS; the quercetin
nanoparticle groups treated with 30, 40 and 50 mg/kg, respectively,
by intraperitoneal injection every day. Tumor size was measured
with digital caliper and calculated as V = LS2/2 (L is the longest
diameter and S is the shortest diameter). Tumor volume and animal
weight were measured twice every seven days, and at ~5 weeks after
treatment, mice were sacrificed. Body weights were also recorded.
Tumors were excised, weighted, fixed in 10% neutral formalin, and
embedded in paraffin for histological analysis.
Immunohistochemical assays
The xenograft tumors were performed for hematoxylin
and eosin staining. In brief, fresh tissues were fixed in paraffin,
and for immunohistochemistry, the fresh tumor tissues were fixed in
formalin for 48 h. Then the tissue block was put in paraffin and
next cut into the desired thickness with a microtome, and was then
fixed into a slide. After washing, the sections were prepared for
blocking and incubating with antibodies, including AP-2β, TUNEL and
COX2, which were diluted 1:100 in 5% horse serum with PBS at 4°C
overnight. Sections were then incubated with diluted
streptavidin-peroxidase HRP conjugates at room temperature by a
staining kit, according to the manufacturer's instructions. The
sections were then stained with hematoxylin for 3 min and mounted
and analyzed under a phase-contrast microscope.
Statistical analysis
Data were expressed as mean ± standard error of the
mean (SEM). Statistical analyses were performed using GraphPad
PRISM (version 6.0; Graph Pad Software) by ANOVA with Dunnet's
least significant difference post hoc tests. A p-value <0.05 was
considered statistically significant.
Results
Quercetin nanoparticle is toxic to liver
cancer cells
The quercetin nanoparticle surface morphology was
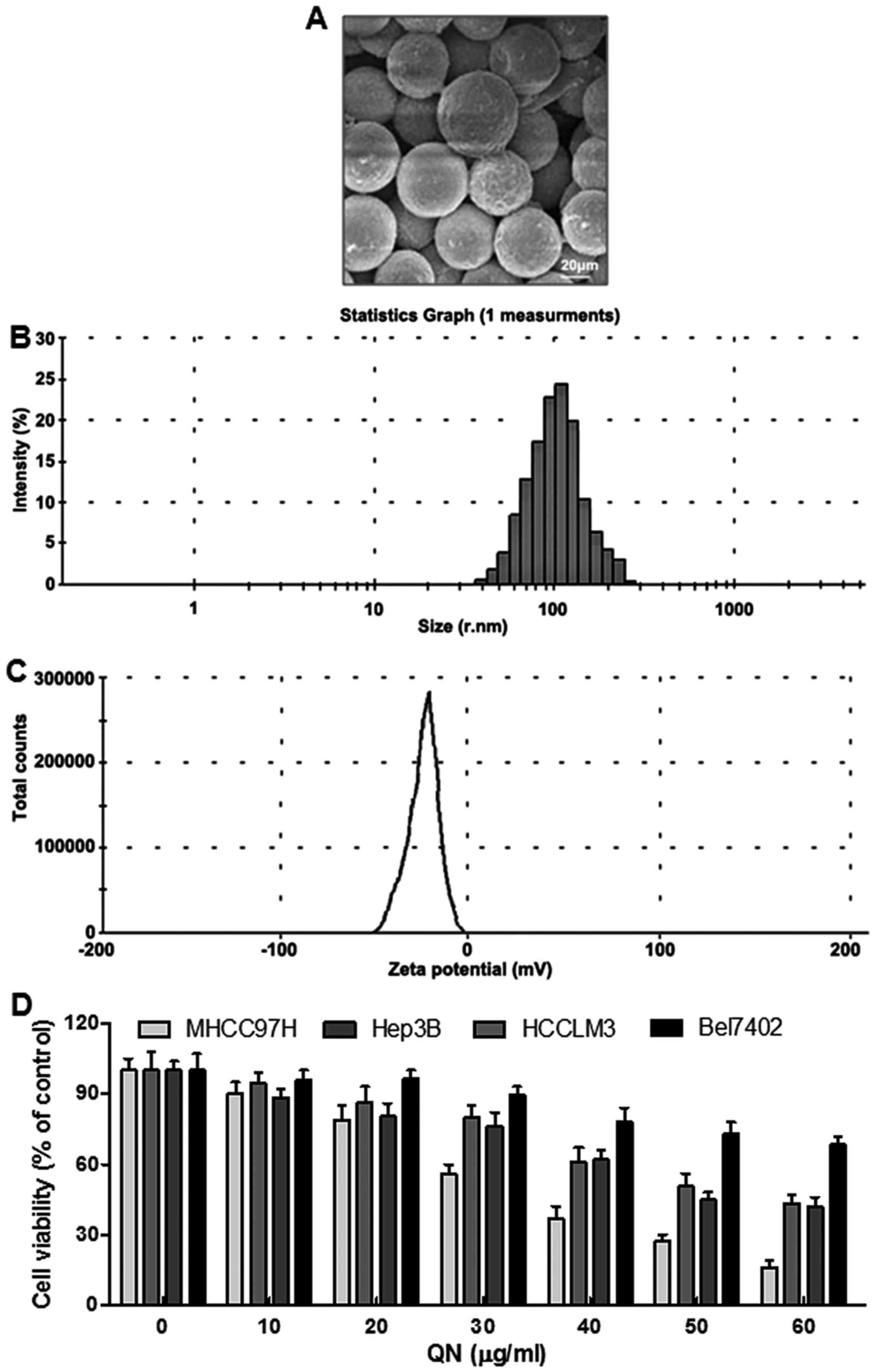
explored by scanning electron microscopy (SEM) (Fig. 1A). The images displayed
spherically-shaped quercetin nanoparticles with a smooth surface
and without pinholes or cracks. The dynamic light scattering (DLS)
data indicated that the mean quercetin nanoparticle diameter was
106.7 nm (Fig. 1B). Additionally,
the zeta potential was −19.1 mV (Fig.
1C). In this experiment, four liver cancer cell lines,
including MHCC97H, Hep3B, HCCLM3 and Bel7402, were chosen to
explore whether quercetin nanoparticle was effective on liver
cancer inhibition. Hence, a 24-h dose-dependent (0–60 µg/ml)
study with MHCC97H, Hep3B, HCCLM3 and Bel7402 cells was conducted.
As shown in Fig. 1D, the liver
cancer cell viability decreased. Additionally, quercetin
nanoparticle was much more toxic to MHCC97H cells than the other
cells. Therefore, the following experiments were performed with
MHCC97H cells.
Quercetin nanoparticles promote the liver
cancer cell growth inhibition and suppression of colony
formation
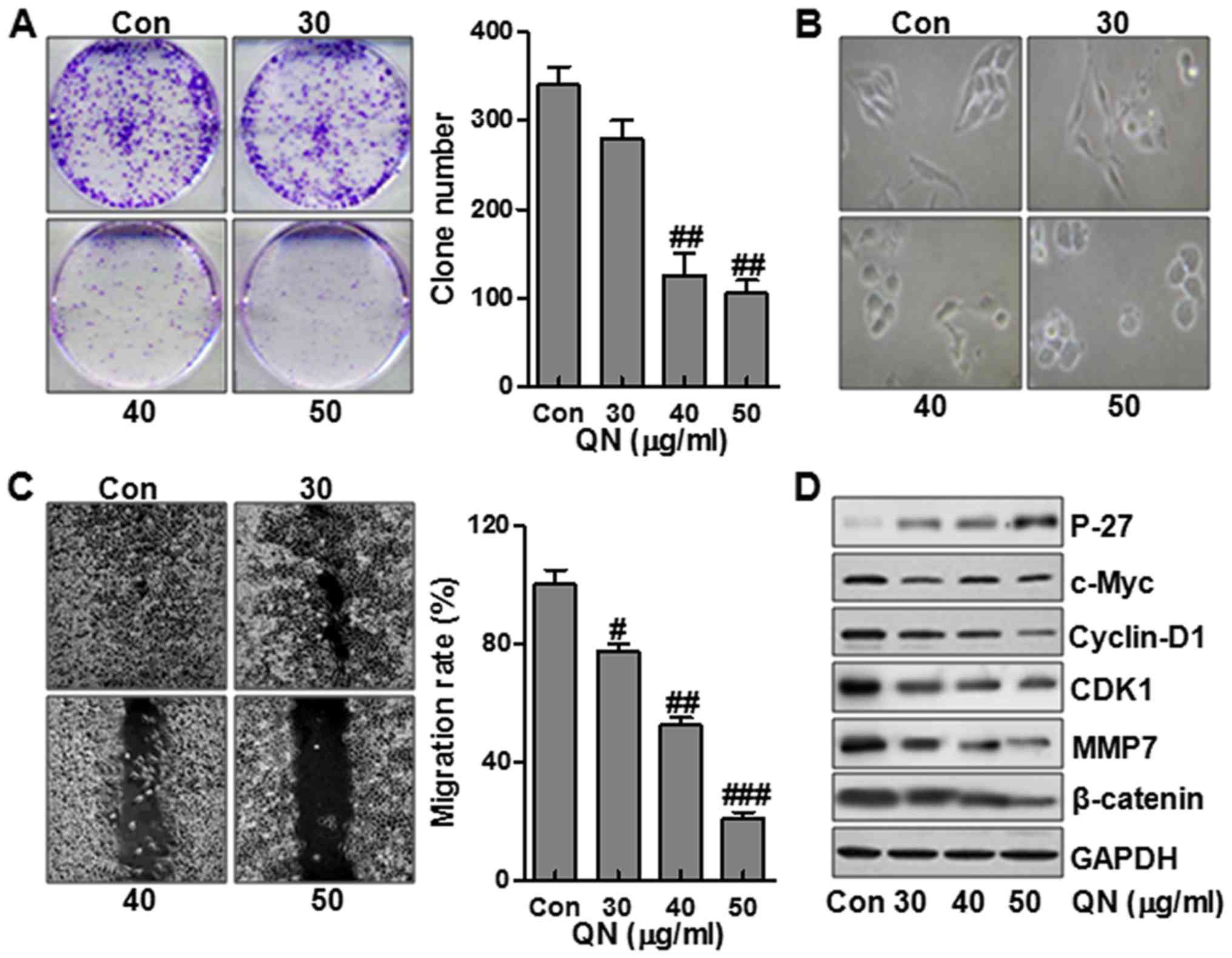
We first evaluated the effects of quercetin
nanoparticle on MHCC97H cell growth. As shown in Fig. 2A, treatment with quercetin
nanoparticle inhibited colony formation in a dose-dependent manner.
We next analyzed the effect of quercetin nanoparticle on changes in
cell morphology and spreading in MHCC97H cells. As shown in
Fig. 2B, the cells treated without
quercetin nanoparticle contributed to a cell layer. In addition,
more spread and filopodia was observed. In the contrast, treatment
with quercetin nanoparticle significantly promoted the cell-to-cell
contact and reduced the cell spreading with lower filopodia
formation compared with control group, suggesting that quercetin
nanoparticle enhanced alterations in MHCC97H cell morphology and
spreading. Further, wound-healing assay was used to determine the
role of quercetin nanoparticle in MHCC97H cell migration.
Consistently, after making a scratch the gap and wounding space
between MHCC97H cell layers was occupied partially due to the
migrating cells after 48 h in the control group. However, the empty
space was not occupied by the MHCC97H cells after quercetin
nanoparticle treatment, suggesting that the liver cancer cell
migration was inhibited markedly after quercetin nanoparticle
administration, which was dose-dependent (Fig. 2C). To further identify the possible
mechanisms related to cell migration, we assessed the P-27, c-Myc,
cyclin-D1, CDK1, MMP7 and β-catenin protein levels. The results
showed that P-27 was expressed highly after quer-cetin nanoparticle
treatment, displaying antitumor activity via P-27 upregulation.
However, c-Myc, cyclin-D1, CDK1, MMP7 and β-catenin, important
factors promoting cell cycle, were significantly downregulated
after quercetin nanoparticle treatment (Fig. 2D).
Quercetin nanoparticles accelarates liver
cancer cell apoptosis through enhancing the activity of
Cyto-c/caspase signaling
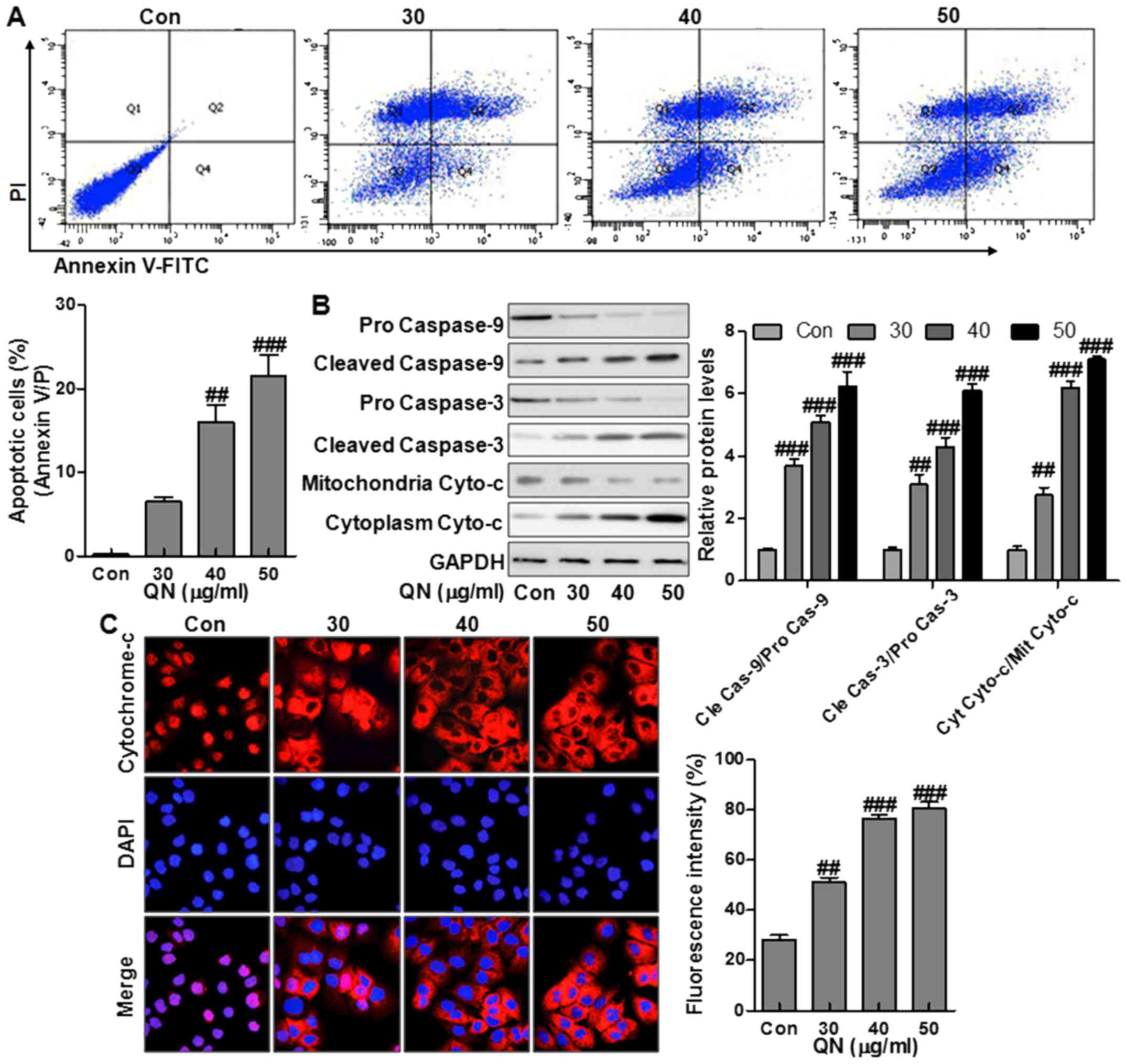
The effect of quercetin nanoparticles on apoptosis
in MHCC97H cells was studied. Treatment with quercetin nanoparticle
at different doses of 30, 40, and 50 µg/ml was performed on
the liver cancer cells (Fig. 3A).
Compared to the control group, treatment with quercetin
nanoparticle significantly upregulated the number of apoptotic
cells (Fig. 3A). Caspase cascade
activation forms the essential basis for apoptosis. Cytochrome
c releasing from the mitochondrial inter-membrane space into
the cytoplasm is the precondition of caspase-dependent apoptosis
pathway. Thus, we measured the expression of pro-apoptotic
proteins, including caspase-9, caspase-3, and Cyto-c in MHCC97H
cells via western blot analysis. Treatment with quercetin
nanoparticle promoted the upregulation of the cleaved caspase-9,
caspase-3 and cytoplasm Cyto-c effectively compared with the
control group (Fig. 3B),
indicating the effect of quercetin nanoparticle on apoptosis
induction in liver cancer cells. Immunofluorescence imaging (IFI)
was also performed to observe the changes of subcellular
localization of Cyto-c in MHCC97H cells to explore whether
quercetin nanoparticle could stimulate Cyto-c release. Treatment
with quercetin nanoparticle at different concentrations induced
Cyto-c release from the inter-mitochondrial space into the
cytoplasm (Fig. 3C). The results
above demonstrated that quercetin nanoparticle might enhance the
caspase activation via Cyto-c-dependent apoptosis in liver cancer
cells.
Quercetin nanoparticles induce liver
cancer inhibition via suppression of AP-2β/hTERT signaling
pathway
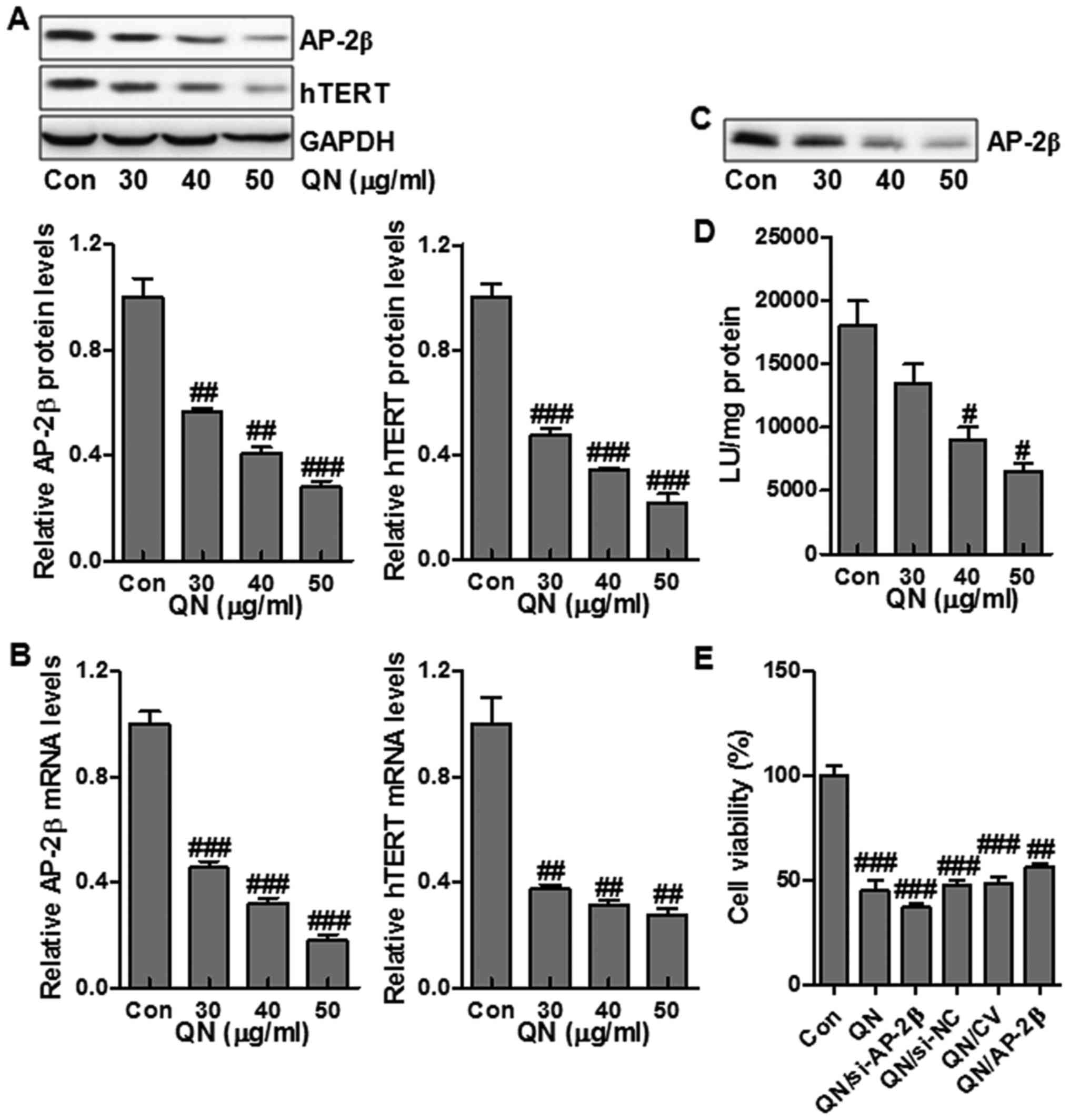
hTERT is a mark of tumorigenesis, which is highly
modulated by transcriptional factor AP-2β (19). In order to determine if quercetin
nanoparticle influenced the AP-2/hTERT signaling pathway in liver
cancer cells, MHCC97H cells were administrated with quercetin
nanoparticles, and the protein and mRNA expression of AP-2β and
hTERT were examined via western blot and RT-PCR assays. Treatment
with quercetin nanoparticles reduced the hTERT and AP-2β protein
and mRNA levels compared to the control group (Fig. 4A and B). hTERT expression is
closely related to the AP-2β binding activity on hTERT promoter.
Next, streptavidin-agarose pull-down assay was performed to test
the effect of quercetin nanoparticle on AP-2β binding activity in
MHCC97H cells. Treatment with quercetin nanoparticle had a
potential role in suppressing AP-2β protein levels (Fig. 4A), thus suppressing the binding of
AP-2β to hTERT promoter (Fig. 4C).
Further, we also explored the role of quercetin nanoparticle in
regulating hTERT promoter activity. The results suggested that
treatment with quercetin nanoparticle effectively inhibited hTERT
promoter activity (Fig. 4D).
Finally, in order to further clarify that the AP-2β signaling is
associated with the promotion of cell growth inhibition, MHCC97H
cells were transfected with 100 nM AP-2β siRNA or AP-2β-expressing
vector and next cotreated with quercetin nanoparticle (50
µg/ml). As shown in Fig.
4E, compared with the non-specific AP-2β siRNA control (si-NC),
AP-2β knockdown (si-AP-2β) slightly reduced the cell growth
regulated by quercetin nanoparticles. Of note, AP-2β overexpression
through AP-2β transfection significantly upregulated the liver
cancer cell growth compared to transfection with control vector
(CV) (Fig. 4E). The data above
illustrated that the promotion of growth suppression by quercetin
nanoparticle was regulated, at least partly, through AP-2β/hTERT
signaling pathway inhibition in MHCC97H cells.
Quercetin nanoparticles ameliorate liver
cancer progression via p65/COX-2 signaling inhibition
COX-2 signaling is related to cancer cell growth,
invasion, migration and proliferation (20,21).
In this regard, we determined the role of quercetin nanoparticle in
regulating COX-2 protein levels in MHCC97H cells via western
blotting. Treatment with quercetin nanoparticle significantly
suppressed COX-2 protein levels (Fig.
5A and B). To prove that quercetin nanoparticles promoted COX-2
signaling inhibition, MHCC97H cells were administered with
COX-2-selective inhibitor (COX2-Inh) (20 µM), and then
treated with quercetin nanoparticle (50 µg/ml). Treatment
with COX-2-selective inhibitor inhibited MHCC97H cell viability
(Fig. 5D). However, a combination
with quercetin nanoparticle did not affect cell viability
suppression significantly regulated by COX-2 inhibitor, suggesting
that COX-2 signaling pathway was linked with quercetin
nanoparticle-regulated liver cancer inhibition. In addition, COX-2
expression was related to the p50 binding activity on the COX-2
promoter structure. Subsequently, we attempted to explore whether
quercetin nanoparticle suppressed the binding of p50 to COX-2
promoter in MHCC97H cells. Streptavidin-agarose pull-down assay
results indicated that treatment with quercetin nanoparticle
significantly suppressed p50 binding on COX-2 promoter in
comparison with the control group (Fig. 5C). The p50 protein levels were not
significantly affected by quercetin nanoparticle (Fig. 5A and B).
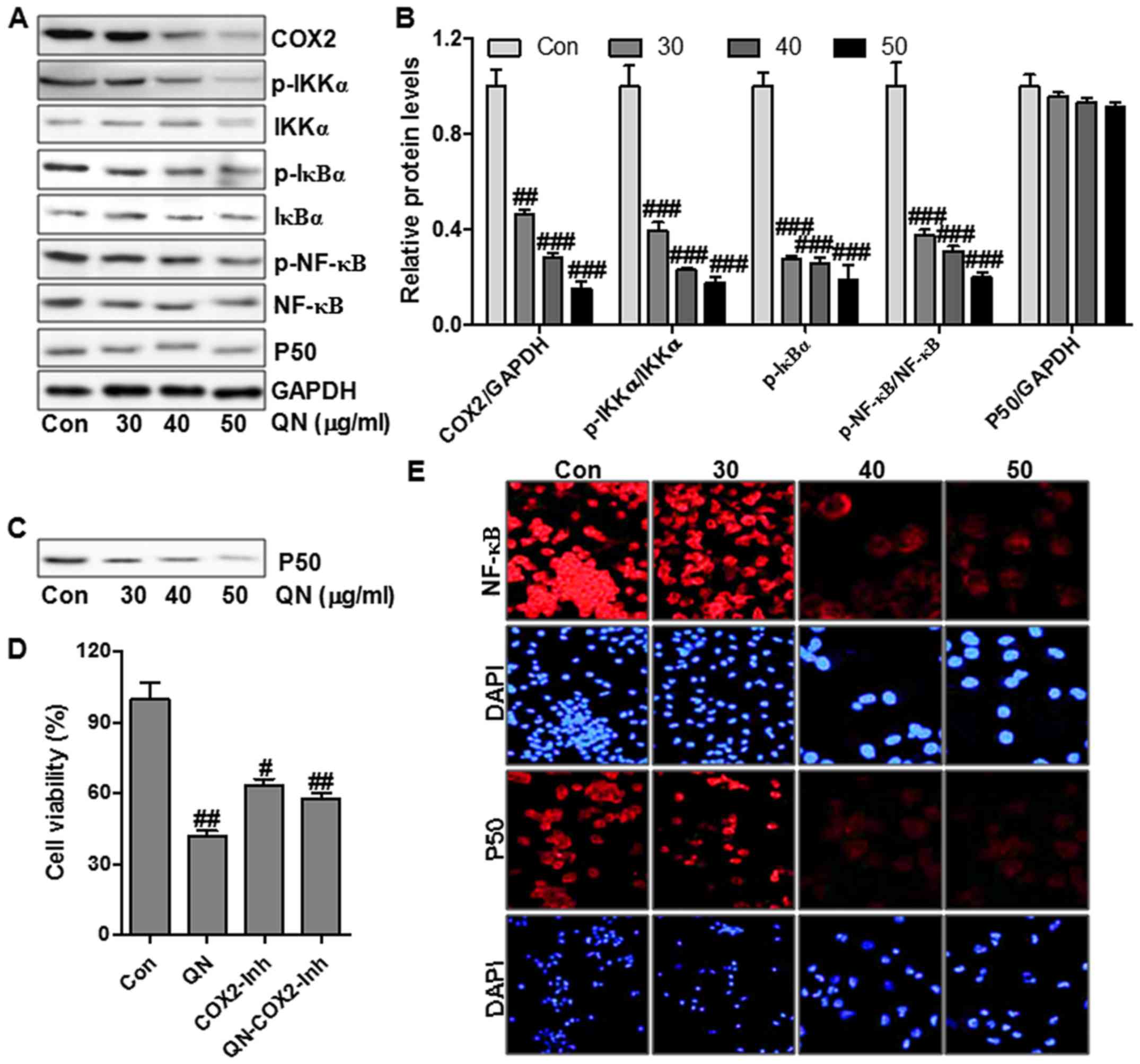
NF-κB translocation in cell nuclei and cytoplasm
plays an important role in modulating COX-2 expression (22). NF-κB activation was regulated
highly by its upstream signals, including IKKα and IκBα (23). Fig.
5A shows that quercetin nanoparticle treatment could reduce the
phosphorylated IKKα and IκBα, leading to the alteration of NF-κB
activation. Immunofluorescence assay was performed to explore the
role of quercetin nanoparticle in p50 and NF-κB translocation
through a confocal microscope. Treatment with different
concentrations of quercetin nanoparticles caused translocation of
NF-κB and p50 from the cell nuclei into cytoplasm (Fig. 5E). The results illustrated that the
enhanced suppression of liver cancer cell growth by quercetin
nanoparticle might be also regulated, at least partly, through the
p50/NF-κB/COX-2 pathway in liver cancer cells.
Quercetin nanoparticles suppress liver
cancer cell growth through Akt/ERK1/2 signaling inactivation
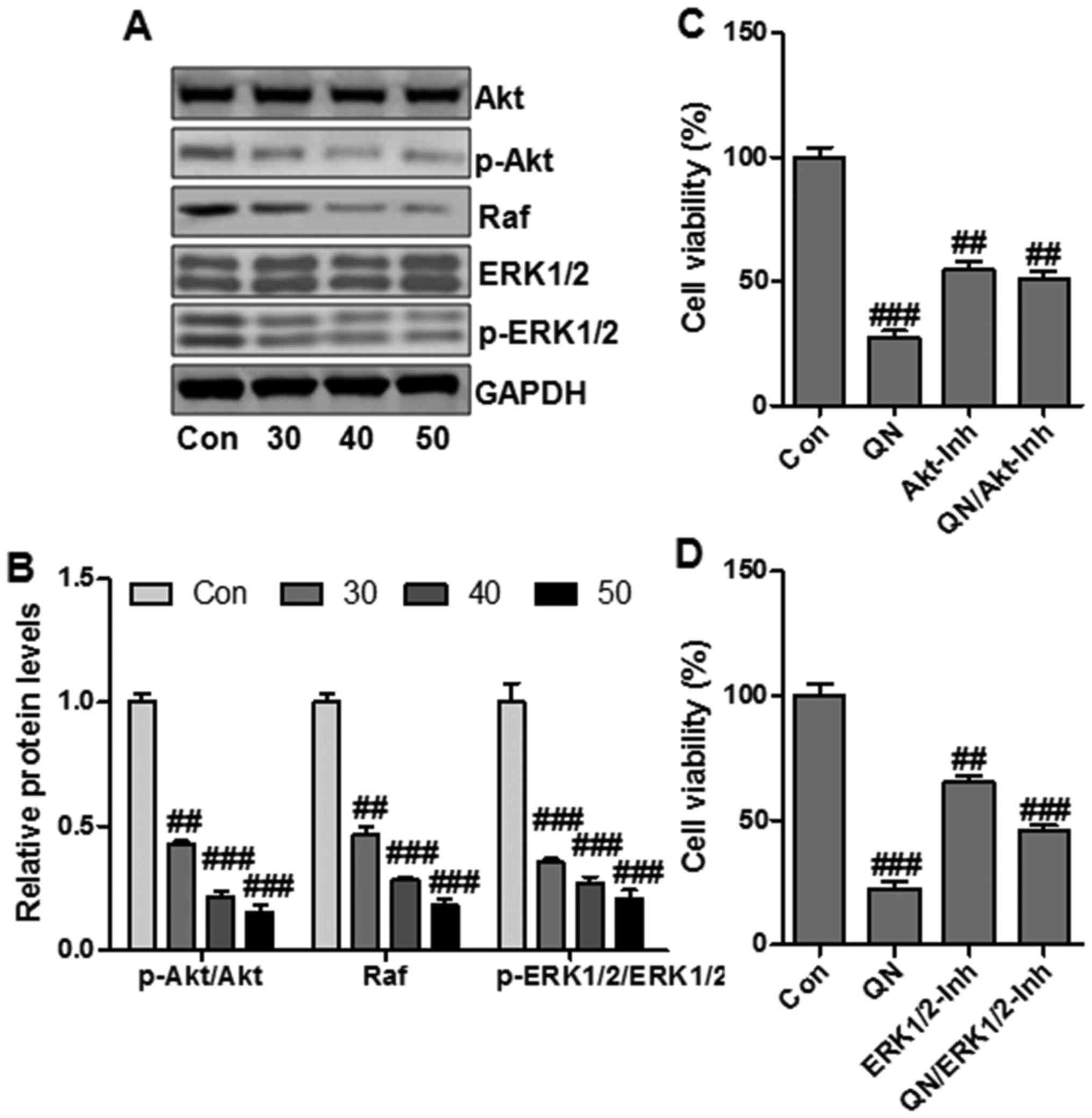
PI3K/Akt and Raf/ERK1/2 signaling pathways play
essential roles in cancer progression and are involved in the
cancer-related gene regulation, including hTERT and COX-2 (24). To determine whether PI3K/Akt and
Raf/ERK1/2 signaling pathways were related to quercetin
nanoparticle-regulated liver cancer cell growth suppression, we
then explored the effect of quercetin nanoparticle on Akt and ERK
activity in MHCC97H cells through western blotting. Quercetin
nanoparticle treatment downregulated the phosphorylated Akt and
ERK1/2 (Fig. 6A and B).
Additionally, the total Akt and ERK1/2 protein levels were not
significantly affected by quercetin nanoparticle.
In order to further confirm that quercetin
nanoparticle could regulate Akt and ERK pathways to suppress
MHCC97H cell growth, the effect of Akt or ERK1/2-selective
inhibitor on quercetin nanoparticle-modulated suppression of cell
viability in MHCC97H cells was calculated. Treatment with Akt
inhibitor (Akt-Inh) and ERK1/2 inhibitor (ERK1/2-Inh) downregulated
cell viability effectively (Fig. 6C
and D). The data above revealed that Akt/ERK1/2 signaling
pathways were important targets for quercetin nanoparticles in
suppressing MHCC97H cell growth.
Quercetin nanoparticles inhibit liver
cancer growth and progression in xenograft tumor model in vivo
To confirm the role of quercetin nanoparticle in
liver cancer growth inhibition, we explored the effects of
quercetin nanoparticle on tumorigenicity using an MHCC97H xenograft
mouse model in vivo. After administration with quercetin
nanoparticles for 35 days, the tumor volumes (Fig. 7A and B) and tumor weights (Fig. 7C) were suppressed significantly by
treatment with quercetin nanoparticles. The quercetin nanoparticle
treatment did not influence the body weight significantly of the
mice (Fig. 7D). These results
above supported that quercetin nanoparticle could suppress the
xenografted human liver cancer cell growth and proliferation
without remarkable adverse effects.
In addition, the IHC staining further illustrated
that the tumors in the quercetin nanoparticle-treated groups
expressed much lower AP-2β and COX2 levels in comparison to the
control group (Fig. 8A and B). In
contrast, TUNEL levels were upregulated significantly after
quercetin nanoparticle administration, indicating apoptosis was
induced by quercetin nanoparticle (Fig. 8A and B). The molecular mechnism by
which quercetin nanoparticles inhibited liver cancer progression
was explored. As shown in Fig. 8C and
D, the Cyto-c/caspase, P50/NF-κB, AP-2β/Htert and Akt/ERK1/2
signaling pathways were inhibited by quercetin nanoparticle
administration. As shown in Fig.
8C, the cleaved caspase-9, cleaved caspase-3 and cytoplasm
Cyto-c were downregulated significantly after quercetin
nanoparticle treatment. Further, the phosphorylated IKKα, IκBα and
NF-κB were reduced in the quercetin nanoparticle-treated groups. In
addition, P50 was not altered significantly by quercetin
nanoparticle treatment, which was consistent with previous results
in vitro (Fig. 8D). Also,
AP-2β and Htert were decreased after quercetin nanoparticle
administration (Fig. 8E). Finally,
the reduced p-Akt, Raf and p-ERK1/2 further clarified that
quercetin nanoparticle had an inhibitory role in liver cancer
progression in vivo (Fig.
8F). Our data above indicated that quercetin nanoparticle could
suppress liver cancer development via inhibition of Cyto-c/caspase,
P50/NF-κB, AP-2β/Htert and Akt/ERK1/2 signaling pathways.
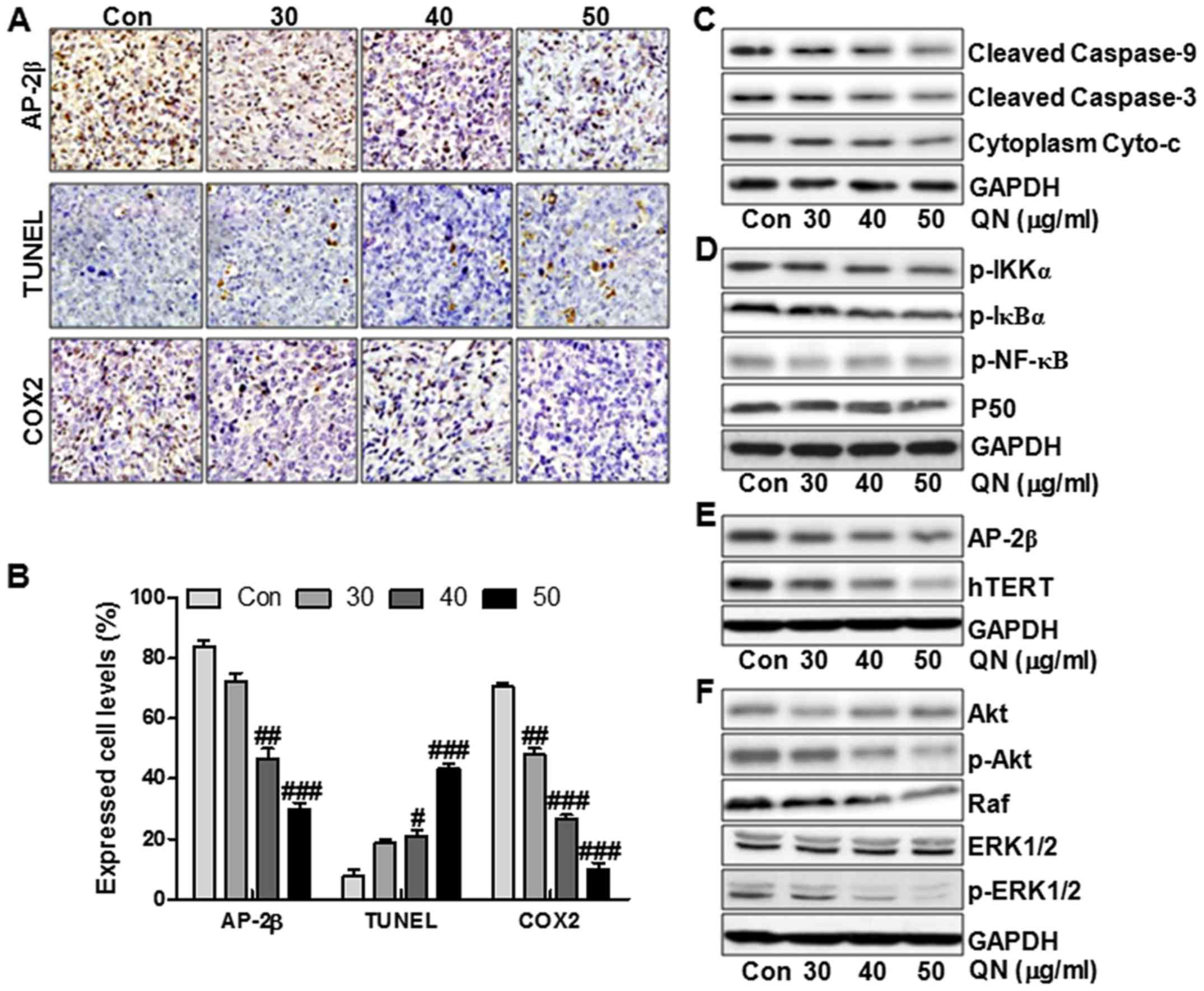 | Figure 8Quercetin nanoparticles inhibite
liver cancer development via apoptosis induction and proliferation
inhibition in vivo. (A) Histopathology of xenograft tumors.
The tumor sections were under IHC staining using antibody against
AP-2β, TUNEL and COX2. (B) The percentage of AP-2β, TUNEL and COX2
positive cells was calculated. Western blot assays were used to
evaluate protein levels of cleaved caspase-9, cleaved caspase-3 and
Cyto-c (C), p-IKKα, p-IκBα, p-NF-κB and P50 (D), AP-2β and hTERT
(E), and (F) p-Akt, Raf and p-ERK1/2. Data are expressed as the
mean ± SEM (n=6–10). #p<0.05, ##p<0.01
and ###p<0.001 versus the control (Con) group. |
Discussion
Development of effective, novel and safe drugs with
lesser side effects and less toxicity is necessary for cancer
therapeutic. Phytochemicals are used extensively for their
properties in cancer therapeutic (25). Quercetin is a compound investigated
for its antiproliferative and anticancerous properties (26,27).
The natural flavonoid is considered to be an antioxidant and has
inhibitory role in several pathologies. Recently, lipid-based
nanocarriers and liposomes have displayed efficacy in drug and gene
therapy (14,28,29).
The successful and effective application of liposome nanocarriers
has been catalyzed through targeted delivery and subsequent
preferential intracellular uptake, enhancing permeability and
retention effect with improved selectivity, efficacy, and overall
safety (30). Here, quercetin
nanoparticles were used to achieve the promoting effects on liver
cancer suppression through multiple cell signaling pathways. The
role of quercetin nanoparticles in cell viability, cell morphology,
apoptosis, colony formation, as well as cell migration was
investigated in our study to reveal the underlying molecular
mechanisms. This is the first report that quercetin nanoparticles
suppressed liver cancer progression and development via
modification of different signaling pathways, including
cyclin-D/p27, caspase/Cyto-c, NF-κB/COX-2, AP-2β/hTERT, as well as
Akt/ERK1/2, providing possible therapeutic strategies for liver
cancer suppression.
We found that quercetin nanoparticles indeed had a
potential role in liver cancer cell growth suppression and
apoptosis induction. All the results here might serve as a basis
for providing the possible treatment of natural anticancer
compounds in developing new therapy for liver cancer in future.
Dysregulation of cell cycle is a key feature of tumor cells and
hence targeting the cell cycle is an important approach in cancer
therapy (31,32). Cell cycle machinery is controlled
by cyclin-dependent kinase (CDK), cyclins and CDK inhibitory
proteins. CDK inhibitory protein, p27, plays an essential role in
signaling molecule through regulating the cell cycle progression by
interacting with CDK/cyclin complexes directly (33). The expression of p21 has been
investigated in the development of chemotherapeutic drugs,
disrupting tumorigenesis via suppressing cell cycle in cancer cells
(34). Also, an increased
expression of MMPs has been shown to be associated with an invasive
phenotype of cancer cells. It is also of paramount importance to
note that expressions of MMP-7 are associated with cancer
development and progression (35).
The inhibition of MMP-7 expression suppresses the tumor invasion
and metastatic potential of cancer (36). These results indicated quercetin
nanoparticles upregulated p27 in liver cancer cells. Also we found
that in the cancer cells treated with quercetin nanoparticles,
c-Myc, cyclin-D1, CDK1, MMP7 and β-catenin were inhibited
significantly in a dose-dependent manner, contributing to apoptosis
in liver cancer cells. Caspase cascade activation forms the
important basis for apoptosis. Cyto-c releasing from inter-membrane
space of mitochondria into the cytoplasm is known as the
precondition of caspase-dependent apoptosis pathway. In our study,
the results suggested that quercetin nanoparticles promoted
activity of caspases markedly and enhanced the release of Cyto-c
from mitochondrial to cytoplasm.
Human telomerase reverse transcriptase (hTERT) is
the main subunit of the core enzyme telomerase, which consists of
three subunits (19,37). Telomeres are essential for
chromosomal stability and integrity, protecting the ends of
chromosomes from degradation and preventing chromosomal end fusions
and recombination (38). A loss of
telomere function is a major mechanism for the generation of
chromosomal abnormalities. It is known to express highly in tumors,
such as lung cancer. Additionally, hTERT inhibition was found to be
effective in proliferation prevention and apoptosis induction
(39). hTERT activity is regulated
by activating enhancer-binding protein-2β (AP-2β), which could bind
hTERT in the corresponding sites, exerting biological effects via a
number of cancer-related genes activation and signaling pathway
activity, such as PI3K/Akt, and Raf/ERK1/2 (40). However, no research is available on
AP-2β/hTERT signaling pathway by quercetin nanoparticles in human
liver cancer cells. In this study, we found that quercetin
nanoparticles decreased AP-2β and hTERT expression. Consequently,
the liver cancer cell proliferation was inhibited, demonstrating
that quercetin nanoparticles had a potential role in suppressing
liver cancer via AP-2β and hTERT modulation.
Cyclooxygenase-2 (COX2) is an important effector
molecule of inflammation and was reported to be involved in tumor
angiogenesis (20). COX2
expression is closely correlated with the malignant transformation
and that COX2 may be used as a molecular marker for the early
malignant transformation of cancer progression via inducing cell
proliferation, invasion, angiogenesis, and metastasis (41). This study also demonstrated that
quercetin nanoparticles displayed an important role in inhibition
of COX-2 expression in liver cancer cells. COX2 expressed levels
are transcriptionally regulated by multiple transactivators binding
and by coactivators on the corresponding sites, which are located
in the promoter. NF-κB binding site is known as an essential site
for COX2 promoter activation (42,43).
Due to the enhancement of quercetin nanoparticles on COX2
suppression, we then explored the NF-κB alteration in the liver
cancer cells. In this study, the promoted suppression of COX2
expression by quercetin nanoparticles is at least partly regulated
by P50 stimulation of the translocation from the nuclear to
cytoplasm of liver cancer cells. In addition, our data revealed the
increased inhibitory role of quercetin nanoparticles in liver
cancer cells by impeding the P50 binding to COX2 promoter. Also,
Akt/ERK1/2 signaling pathway, performs a crucial role in cell
apoptosis, proliferation and autology (44). In our study, we found that
quercetin nanoparticles downregulated the phosphorylated Akt and
ERK1/2 activity, which was important for liver cancer
inhibition.
In conclusion, quercetin nanoparticles enhanced the
inhibitory role in liver cancer progression through multiple routes
of action, including caspase/Cyto-c activation, AP-2β/hTERT
inhibition, NF-κB/COX-2 and Akt/ERK1/2 suppression. Therefore, our
results suggest that quercetin nanoparticle is a promising
candidate in liver cancer therapeutics in future. However, the
molecular mechanism of the anti-proliferative and apoptotic effects
of quercetin nanoparticles remains to be determined.
References
|
1
|
Marquardt JU, Andersen JB and Thorgeirsson
SS: Functional and genetic deconstruction of the cellular origin in
liver cancer. Nat Rev Cancer. 15:653–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chatterjee R and Mitra A: An overview of
effective therapies and recent advances in biomarkers for chronic
liver diseases and associated liver cancer. Int Immunopharmacol.
24:335–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruix J, Raoul JL, Sherman M, Mazzaferro
V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M,
Sangiovanni A, et al: Efficacy and safety of sorafenib in patients
with advanced hepatocellular carcinoma: Subanalyses of a phase III
trial. J Hepatol. 57:821–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis JM, Murphy EA and Carmichael MD:
Effects of the dietary flavonoid quercetin upon performance and
health. Curr Sports Med Rep. 8:206–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiczkowski W, Romaszko J, Bucinski A,
Szawara-Nowak D, Honke J, Zielinski H and Piskula MK: Quercetin
from shallots (Allium cepa L. var. aggregatum) is more bioavailable
than its glucosides. J Nutr. 138:885–888. 2008.PubMed/NCBI
|
|
7
|
Guo Y, Mah E, Davis CG, Jalili T, Ferruzzi
MG, Chun OK and Bruno RS: Dietary fat increases quercetin
bioavailability in overweight adults. Mol Nutr Food Res.
57:896–905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aguirre L, Arias N, Macarulla MT, Gracia A
and Portillo MP: Beneficial effects of quercetin on obesity and
diabetes. Open Nutraceuticals J. 4:189–198. 2011. View Article : Google Scholar
|
|
9
|
Bhattacharyya SS, Paul S, De A, Das D,
Samadder A, Boujedaini N and Khuda-Bukhsh AR: Poly
(lactide-co-glycolide) acid nanoencapsulation of a synthetic
coumarin: Cytotoxicity and bio-distribution in mice, in cancer cell
line and interaction with calf thymus DNA as target. Toxicol Appl
Pharmacol. 253:270–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee KM, Hwang MK, Lee DE, Lee KW and Lee
HJ: Protective effect of quercetin against arsenite-induced COX-2
expression by targeting PI3K in rat liver epithelial cells. J Agric
Food Chem. 58:5815–5820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan J, Wang S, Yang J and Liu Y: Coupled
particulate and continuum model for nanoparticle targeted delivery.
Comput Struc. 122:128–134. 2013. View Article : Google Scholar
|
|
12
|
Wang S, Zhou Y, Tan J, Xu J, Yang J and
Liu Y: Computational modeling of magnetic nanoparticle targeting to
stent surface under high gradient field. Comput Mech. 53:403–412.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puhl AC, Fagundes M, dos Santos KC,
Polikarpov I, das Gracas MF, da Silva F, Fernandes JB, Vieira PC
and Forim MR: Preparation and characterization of polymeric
nanoparticles loaded with the flavonoid luteolin, by using
factorial design. Int J Drug Deliv. 3:683–698. 2011.
|
|
14
|
Pinzón-Daza ML, Campia I, Kopecka J,
Garzón R, Ghigo D and Riganti C: Nanoparticle- and liposome-carried
drugs: New strategies for active targeting and drug delivery across
blood-brain barrier. Curr Drug Metab. 14:625–640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alvarez-Erviti L, Seow Y, Yin H, Betts C,
Lakhal S and Wood MJ: Delivery of siRNA to the mouse brain by
systemic injection of targeted exosomes. Nat Biotechnol.
29:341–345. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Y, Ye L, Zhang X, Cui W, Lou J, Nagai
T and Hou X: Transport of nerve growth factor encapsulated into
liposomes across the blood-brain barrier: In vitro and in vivo
studies. J Control Release. 105:106–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosta-Rigau L, Schattling P, Teo BM, Lynge
ME and Städler B: Recent progress of liposomes in nanomedicine. J
Mater Chem B Mater Biol Med. 2:6686–6691. 2014. View Article : Google Scholar
|
|
18
|
Sapra P and Allen TM: Ligand-targeted
liposomal anticancer drugs. Prog Lipid Res. 42:439–462. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng YL, Zhang F, Sun B, Du J, Sun C,
Yuan J, Wang Y, Tao L, Kota K, Liu X, et al: Telomerase enzymatic
component hTERT shortens long telomeres in human cells. Cell Cycle.
13:1765–1776. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuźbicki Ł, Lange D, Stanek-Widera A and
Chwirot BW: Different expression of cyclooxygenase-2 (COX-2) in
selected nonmelanocytic human cutaneous lesions. Folia Histochem
Cytobiol. 49:381–388. 2011. View Article : Google Scholar
|
|
21
|
Müller-Decker K: Cyclooxygenase-dependent
signaling is causally linked to non-melanoma skin carcinogenesis:
Pharmacological, genetic, and clinical evidence. Cancer Metastasis
Rev. 30:343–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han S, Lee JH, Kim C, Nam D, Chung WS, Lee
SG and Ahn KS, Cho SK, Cho M and Ahn KS: Capillarisin inhibits
iNOS, COX-2 expression, and proinflammatory cytokines in
LPS-induced RAW 264.7 macrophages via the suppression of ERK, JNK,
and NF-κB activation. Immunopharmacol Immunotoxicol. 35:34–42.
2013. View Article : Google Scholar
|
|
23
|
Hsiang CY, Lo HY, Huang HC, Li CC, Wu SL
and Ho TY: Ginger extract and zingerone ameliorated trinitrobenzene
sulphonic acid-induced colitis in mice via modulation of nuclear
factor-κB activity and interleukin-1β signalling pathway. Food
Chem. 136:170–177. 2013. View Article : Google Scholar
|
|
24
|
Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu
L, Liu L, Xie F, Kang T, Huang W, et al: Simultaneous modulation of
COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit
proliferation and induce apoptosis in breast cancer cells. J Pineal
Res. 53:77–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsao R: Chemistry and biochemistry of
dietary polyphenols. Nutrients. 2:1231–1246. 2010. View Article : Google Scholar
|
|
26
|
Dong YS, Wang JL, Feng DY, Qin HZ, Wen H,
Yin ZM, Gao GD and Li C: Protective effect of quercetin against
oxidative stress and brain edema in an experimental rat model of
subarachnoid hemorrhage. Int J Med Sci. 11:282–290. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobori M, Takahashi Y, Sakurai M, Akimoto
Y, Tsushida T, Oike H and Ippoushi K: Quercetin suppresses immune
cell accumulation and improves mitochondrial gene expression in
adipose tissue of diet-induced obese mice. Mol Nutr Food Res.
60:300–312. 2016. View Article : Google Scholar
|
|
28
|
Iyer AK, Khaled G, Fang J and Maeda H:
Exploiting the enhanced permeability and retention effect for tumor
targeting. Drug Discov Today. 11:812–818. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho HJ, Yoon HY, Koo H, Ko SH, Shim JS,
Lee JH, Kim K, Kwon IC and Kim DD: Self-assembled nanoparticles
based on hyaluronic acid-ceramide (HA-CE) and Pluronic®
for tumor-targeted delivery of docetaxel. Biomaterials.
32:7181–7190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma VK, Mishra D, Sharma A and
Srivastava B: Liposomes: Present prospective and future challenges.
Int J Current Pharm Rev Res. 1:6–16. 2010.
|
|
31
|
Barré B and Perkins ND: A cell cycle
regulatory network controlling NF-kappaB subunit activity and
function. EMBO J. 26:4841–4855. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu Y, Kadioglu O, Wiench B, Wei Z, Gao C,
Luo M, Gu C, Zu Y and Efferth T: Cell cycle arrest and induction of
apoptosis by cajanin stilbene acid from Cajanus cajan in breast
cancer cells. Phytomedicine. 22:462–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Satyanarayana A, Hilton MB and Kaldis P:
p21 Inhibits Cdk1 in the absence of Cdk2 to maintain the G1/S phase
DNA damage checkpoint. Mol Biol Cell. 19:65–77. 2008. View Article : Google Scholar :
|
|
34
|
Campomenosi P, Monti P, Aprile A,
Abbondandolo A, Frebourg T, Gold B, Crook T, Inga A, Resnick MA,
Iggo R, et al: p53 mutants can often transactivate promoters
containing a p21 but not Bax or PIG3 responsive elements. Oncogene.
20:3573–3579. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chien CS, Shen KH, Huang JS, Ko SC and
Shih YW: Antimetastatic potential of fisetin involves inactivation
of the PI3K/Akt and JNK signaling pathways with downregulation of
MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell
Biochem. 333:169–180. 2010. View Article : Google Scholar
|
|
36
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J, et al: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi SH, Im E, Kang HK, Lee JH, Kwak HS,
Bae YT, Park HJ and Kim ND: Inhibitory effects of costunolide on
the telomerase activity in human breast carcinoma cells. Cancer
Lett. 227:153–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim MO, Moon DO, Kang SH, Heo MS, Choi YH,
Jung JH, Lee JD and Kim GY: Pectenotoxin-2 represses telomerase
activity in human leukemia cells through suppression of hTERT gene
expression and Akt-dependent hTERT phosphorylation. FEBS Lett.
582:3263–3269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Di Stefano AL, Enciso-Mora V, Marie Y,
Desestret V, Labussière M, Boisselier B, Mokhtari K, Idbaih A,
Hoang-Xuan K, Delattre JY, et al: Association between glioma
susceptibility loci and tumour pathology defines specific molecular
etiologies. Neurooncol. 15:542–547. 2013.
|
|
40
|
Deng WG, Jayachandran G, Wu G, Xu K, Roth
JA and Ji L: Tumor-specific activation of human telomerase reverses
transcriptase promoter activity by activating enhancer-binding
protein-2beta in human lung cancer cells. J Biol Chem.
282:26460–26470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kajita S, Ruebel KH, Casey MB, Nakamura N
and Lloyd RV: Role of COX-2, thromboxane A2 synthase, and
prostaglandin I2 synthase in papillary thyroid carcinoma growth.
Mod Pathol. 18:221–227. 2005. View Article : Google Scholar
|
|
42
|
Wang D and Dubois RN: The role of COX-2 in
intestinal inflammation and colorectal cancer. Oncogene.
29:781–788. 2010. View Article : Google Scholar
|
|
43
|
Deng WG, Zhu Y and Wu KK: Role of p300 and
PCAF in regulating cyclooxygenase-2 promoter activation by
inflammatory mediators. Blood. 103:2135–2142. 2004. View Article : Google Scholar
|
|
44
|
Fu L, Chen W, Guo W, Wang J, Tian Y, Shi
D, Zhang X, Qiu H, Xiao X, Kang T, et al: Berberine Targets
AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and Cytochrome-c/caspase
signaling to suppress human cancer cell growth. PLoS One.
8:e692402013. View Article : Google Scholar
|