Introduction
Cervical cancer is one of the most common cancers in
women with an estimated 528,000 new cases each year (1), and approximately 266,000 mortalities
caused by cervical cancer in the world, which accounts for 7.5% of
all cancer mortalities related to females (2). Approximately 87% of the mortalities
resulted from cervical cancer in relatively not developed countries
and regions. The women, aged from 30 to 50, are more sensitive to
cervical cancer due to factors such as gene mutation, or
environment, including working stress and emotion (3). Studies related to the molecular
mechanisms revealing cervical tumor invasion, metastasis and the
novel therapeutic strategies are necessary to suppress cancer
progression (4,5).
Pentacyclic triterpenoids show various
pharmacological activities, including anti-oxidant,
anti-inflammatory and anticancer properties. Pentacyclic
triterpenoids have many pharmacological functions, including
anti-inflammation effect, antioxidant activity, hepatoprotective
and antitumor activity (6–8). In addition, the plant-derived
chemicals ameliorating diseases progression have gained increasing
attention. Pentacyclic triterpenoids, such as imberbic acid,
betulinic acid, zeylasteral and ursolic acid have been reported to
have anticancer effect (9,10). As a pentacyclic triterpene acid,
ursolic acid has a number of pharmacological effects, such as
anti-oxidative (11), antifungal,
antibacterial, anti-inflammatory, antiangiogenic, anti-mutagenic,
anticarcinogenic, anti-viral, anti-atherosclerotic, antitumor,
hepatoprotective, as well as anti-hyperlipidemic activity (12–16).
Ursolic acid is also known to enhance apoptotic response in various
human cancer cell lines (17).
However, there are limited reports involving the key role of
ursolic acid in regulating cervical cancer progression.
Use of nanoparticles in cancer treatment has been
widely investigated. The special features of nanoparticles can
improve the defects in the use of small molecules as therapeutic
agents in biomedical applications (18,19).
For instance, the uptake of medicine may be promoted by the
'enhanced permeability and retention effect', enhancing the
medicine accumulation in the target cancer part and decrease the
efflux pump-regulated drug resistance (20). In addition, capsuled by different
nanoparticles, the solubility and the half-life of small molecular
drugs could be improved with the controllable releasing behavior
(21). To increase ursolic acid
biocompatibility and water solubility, drug nanocarriers are
developed according to nanotechnology. Additionally, the slow and
controlled release of the drug helps to provide a sustainable dose
at the therapeutic level, which is helpful to improve efficiency
and safety (22–24). In the present study, we developed
PLGA nanoparticles as biodegradable and biocompatible carriers for
loading and delivery of the binary drug ursolic acid, which
displayed an excellent anticancer performance in vitro and
in vivo.
Materials and methods
Cell treatment
Human cervical cancer cell lines, including CaSki,
HeLa, C4–1 and SiHa, were obtained from the American Type Culture
Collection (ATCC; Rockville, MD, USA). The cell lines of 293T and
L02 were also obtained from ATCC. All the cells were maintained in
RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (Gibco-BRL/Life Technologies) at 37°C with
a humidified incubator in 5% CO2 atmosphere. The cells
were treated with different concentrations of ursolic acid
nanoparticles for different time as shown in the figures.
Ursolic acid nanoparticle
preparation
High performance liquid chromatography (HPLC)-grade
ursolic acid was obtained (>98%; NanoBiotech, Co., Shanghai,
China) in anhydrous powder form. AuNPs were obtained by
downregulated 1 mM gold chloride with a freshly ready-made ursolic
acid solution in alcohol. The yellow color solution changed to deep
red as the ursolic acid nanoparticles were finished. A total of 50
mg poly(lactic-co-glycolic acid) (PLGA) was added to an AuNPs water
dispersion. Next, the mixture drops were added to 20 ml of an
aqueous solution with a stabilizer (1%
polyoxyethylene-polyoxypropylene; F68). The mixture drops were
mixed at 500 rpm and 4°C until the alcohol had evaporated
completely. Washing and centrifugation were performed again (25,000
× g, 4°C for 30 min), in order to remove redundant stabilizer
completely. The pellet was then resuspended in Milli-Q water. The
ursolic acid nanoparticles were stored at 4°C for further study.
Fluorescent dye was conjugated to the gold surface by adding FITC
dye to the PLGA and ursolic acid nanoparticles mixture, which were
performed in the dark. In addition, scanning transmission electron
microscopy (SEM) (FEI Quanta 650; FEI Co., Hillsboro, OR, USA) was
used to determine naked quercetin nanoparticles size. Furthermore,
dynamic light scattering (DLS) with LB-550 DLS particle size
analyzer (Horiba Scientific, Edison, NJ, USA) was used to examine
the average size of ursolic acid nanoparticles. We analyzed the
data in the automatic mode. Twenty runs of size were determined,
with triplicate measurements for each run. We measured the zeta
potential of the ursolic acid nanoparticles in the same instrument
using the same procedure.
MTT assay
MTT method was used to analyze the cell viability.
The culture medium was totally removed and 5 µl MTT
(Sigma-Aldrich, St. Louis, MO, USA) solution (10 mg/ml) was added
to 100 µl of phenol red-free growth medium and plates were
incubated at 37°C, 5% CO2 cell culture environment.
Mitochondrial decrease of MTT develops formazan crystals after
dissolving in dimethyl sulfoxide (DMSO). Afterwards, microplate
reader (Bio-Rad Laboratories, Hercules, CA, USA) was used to
measure absorbance of each well at 540 nm.
Colony-forming assay
Cervical cancer cells were suspended in 0.9%
methylcellulose-based semisolid medium MethoCult H4100 (Stem Cell,
Beijing, China). Individual primary clones (450 cells) after 15
days were trypsinized and re-plated in the same conditions to test
the secondary colony forming ability for self-renewal.
Migration and invasion assays
Matrigel (2 µg/well; BD Biosciences, San
Jose, CA, USA) was used to pre-coat 24-well plates with 8-µm
pore size polycarbonate membrane (Millipore, Bedford, MA, USA) for
invasion assays. Cells under different conditions (exposure to
different concentrations of drugs for 48 h). Cells
(1.0×105) were seeded on the upper chamber with
serum-free DMEM (200 µl). DMEM (1 ml) with 30% serum was
added to the lower chamber. After 16 h, cell migration or invasion
(plates coated with Matrigel) was terminated and the membranes were
fixed with 4% paraformaldehyde and stained with crystal violet.
Five visual fields were randomly selected from each membrane, and
the cell numbers were counted via a light microscope. All
experiments were performed in triplicate.
Establishment of xenograft tumor
models
Forty male, 6–8-weeks old athymic nude mice were
purchased from the Shanghai Experimental Animal Center (Shanghai,
China) and were kept in a temperature and humidity-controlled
environment (25±2°C, 50±10% humidity) with a standard 12-h light
and dark cycle with food and water in cages under the germ-free
conditions. CaSki, SiHa and HeLa cells (1×107) were
suspended in 100 µl serum-free medium and injected
subcutaneously into the left flank of nude mice. Then, the animal
models were treated with ursolic acid (10 and 20 mg/kg). The mice
were divided into two groups. Animals in the control group were
treated with sterile DMSO via intraperitoneal injection for five
days. Animals in ursolic acid-treated group were intraperitoneally
administered with 20 mg/kg ursolic acid nanoparticles for five
days, respectively. Tumor size was measured with digital caliper
and calculated twice a week for 5 weeks. Then, the mice were
sacrificed. Tumors were excised, weighted, fixed in 10% neutral
formalin, and embedded in paraffin for histological analysis.
Ethics approval was obtained from the Department of Gynecology, The
Affiliated Yantai Yuhuangding Hospital of Qingdao University.
Western blot analysis
After treatments under different conditions, the
cells were harvested and the medium was removed. Then, the cells
were washed with chilled phosphate-buffered saline (PBS) three
times and lysed in ice-cold lysis buffer in the presence of fresh
protease inhibitor cocktail. Frozen lung tumor tissue samples were
obtained from xenograft nude mice after treatments. Approximately a
100 mg tumor tissue sample was lysed with 1 ml lysis buffer. The
cell lysates were centrifuged at 15,000 × g for 15 min at 4°C to
collect the supernatant. BSA protein assay kit was used to detect
the protein concentrations following the manufacturer's instruction
(Thermo Fisher Scientific, Waltham, MA, USA). A total of 40 ng of
protein extracts were separated by 10% SDS-PAGE and were then
transferred to polyvinylidene fluoride membrane (PVDF) (Millipore).
The PVDF with proteins were blocked with 5% skim fat dry milk in
0.1% Tween-20 in Tris-buffered saline (TBS) for 2 h to block the
non-specific sites on blots. The primary antibodies dissolved in
blocking buffer were used to detect the target protein blots at 4°C
overnight for incubation. The bands on PVDF were covered by
chemiluminescence with Pierce ECL Western Blotting Substrate
reagents (Thermo Fisher Scientific). All experiments were performed
in triplicate and carried out three times independently. The
primary antibodies are as follows: Bcl-2 (1:1,000; Abcam,
Cambridge, MA, USA), Bax (1:1,000; Abcam), caspase-3 (1:1,000; Cell
Signaling Technology, Beverly, MA, USA), caspase-8 (1:1,000; Cell
Signaling Technology), caspase-9 (1:1,000; Cell Signaling
Technology) and GAPDH (1:500; Cell Signaling Technology).
Immunofluorescence assays
After induction by conditioned culture medium, the
different cells were fixed in 4% paraformaldehyde, permeabilized
with 0.1% Triton X-100 in PBS containing 0.5% BSA (PBS-BSA) for 30
min. The cells were subsequently incubated with caspase-3 for 30
min, followed by labeling with Alexa Fluor 488-conjugated rabbit
anti-mouse or goat anti-rabbit IgG antibody. The cells were viewed
under a fluorescence microscope.
Histopathologic examination of
tissues
After harvesting, the xenograft tumors were fixed in
formalin and embedded in paraffin. Five micrometer sections of
these tumors were deparaffinized in xylene and then hydrated
through a series of xylene and ethanol washes. Sections were
incubated in a citrate buffer (pH 6.0) for 30 min at 95°C for
antigen retrieval. After blocking, sections were incubated
overnight with appropriate dilatation of primary antibodies (p53
and Bcl-2; Cell Signaling Technology) followed by incubation with a
secondary antibody and analyzed for staining as previously
described (25). Then the samples
were observed under a microscope.
Statistical analysis
Data are expressed as means ± SEM. Treated cells,
tissues and the corresponding controls were compared using GraphPad
Prism (version 6.0; GraphPad Software, La Jolla, CA, USA) by a
one-way ANOVA with Dunn's least significant difference tests.
Differences between groups were considered significant at
P<0.05.
Results
The effects of ursolic acid nanoparticles
on cell growth and proliferation
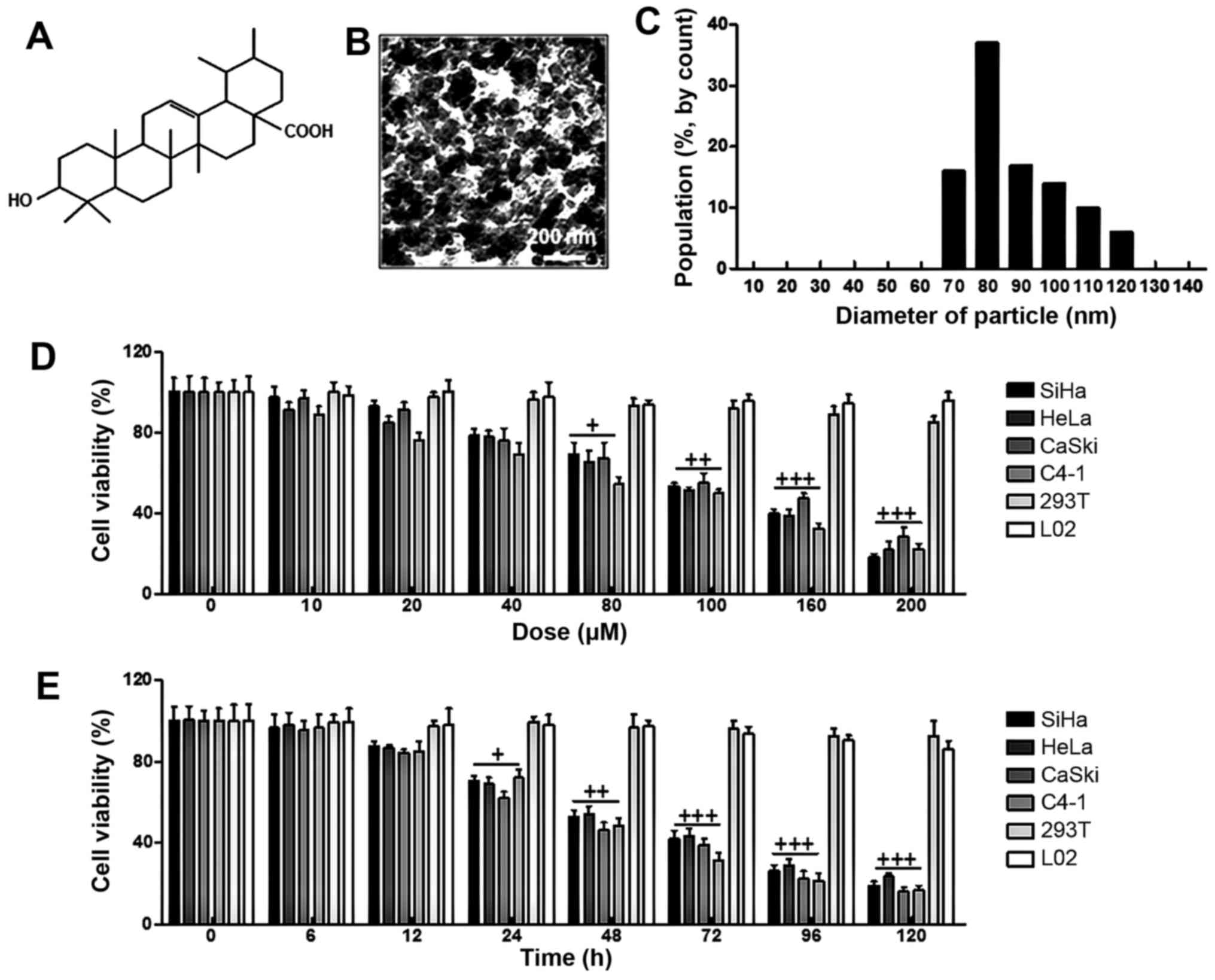
From Fig. 1B, the
surface morphology of ursolic acid nanoparticles was studied under
SEM. The image displayed spherically shaped ursolic acid
nanoparticles with a smooth surface without pinholes or cracks. In
addition, DLS data indicated that the mean ursolic acid
nanoparticle diameter was 80 nm (Fig.
1C). Furthermore, in this study, four cervical cancer cell
lines and the other two cell types from different origins were
selected to calculate whether ursolic acid nanoparticles were
specific. As shown in Fig. 1D, we
found that ursolic acid nanoparticles reduced cell viability after
48 h in various cancer cell lines, which was shown in a
dose-dependent manner, especially over 40 µM. Notably, in
cells of 293T and L02, no alterations in cell viability were
observed. Next, the cell viability was further explored at the
concentration of 160 µM ursolic acid nanoparticles from 0 to
120 h. Ursolic acid nanoparticles obviously decreased the viability
of cancer cells in a time-dependent manner. Similarly, no
significant difference was detected in normal cells of 293T and L02
(Fig. 1E). Taken together, the
data above indicated that ursolic acid nanoparticles had an
inhibitory role in cervical cancer cell proliferation, which was in
a dose- and time-dependent manner without cytotoxicity to normal
cells, suggesting that ursolic acid nanoparticles might be
effective in controlling cervical cancer progression.
Ursolic acid nanoparticles effectively
suppressed proliferation, migration and invasion in cervical cancer
cells
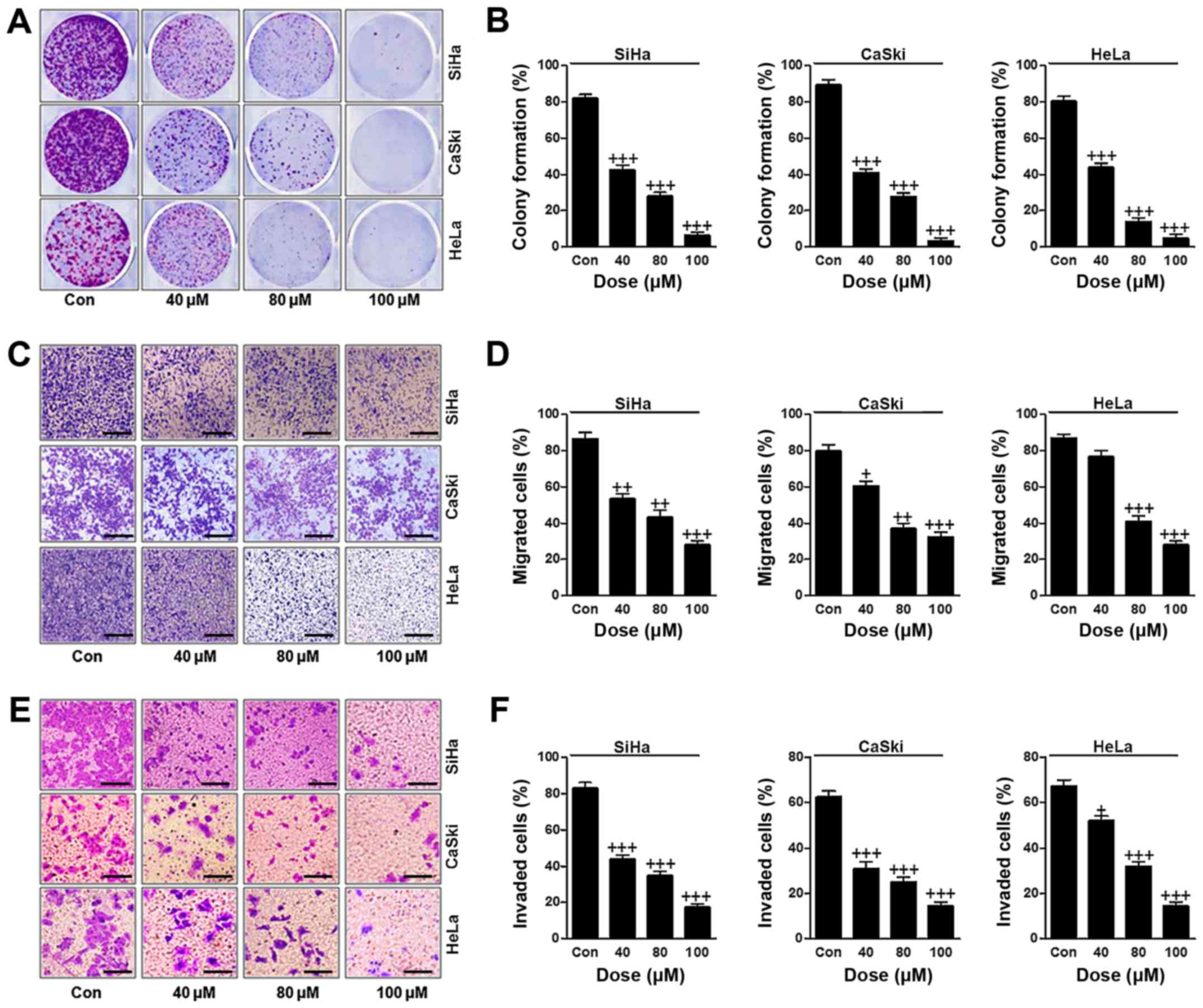
In this regard, we attempted to evaluate how ursolic
acid nanoparticles influenced cervical cancer cells proliferation,
migration and invasion. The colony formation analysis indicated
that ursolic acid nanoparticles had an inhibitory role in
suppressing colony formation in cervical cancer cells of SiHa,
CaSki and HeLa in a dose-dependent manner with significant
difference (Fig. 2A and B). In
addition, the percentage of migrated cervical cancer cells was also
reduced apparently after different concentrations of ursolic acid
nanoparticle treatment (Fig. 2C and
D). Finally, the invasion of cervical cancer cells was
evaluated, and the results showed that the number of invaded cells
was reduced significantly by the use of ursolic acid nanoparticles
(Fig. 2E and F). In conclusion,
the data above suggested that ursolic acid nanoparticles could
suppress cervical cancer progression by suppressing the
proliferation, migration and invasion of cervical cancer cells.
The effects of ursolic acid nanoparticles
on apoptosis in the cervical cancer cell lines via flow cytometry
assays
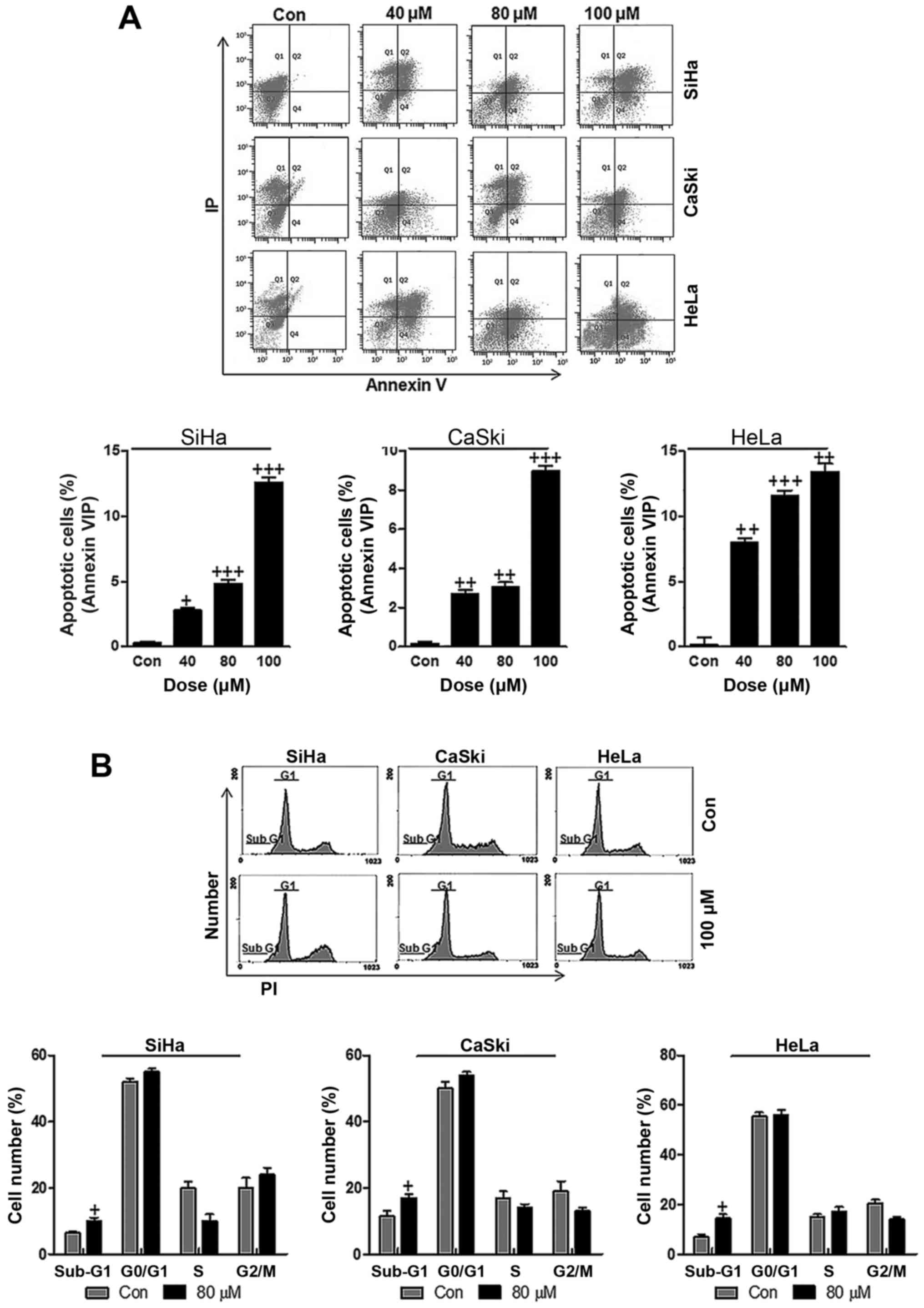
Apoptosis is well known to be of great importance in
suppressing cancer progression through inducing cell death
(26,27). In order to calculate whether
ursolic acid nanoparticles could perform its effects on cervical
cancer suppression, flow cytometric analysis was performed to
confirm our hypothesis. As shown in Fig. 3A, different concentrations of
ursolic acid nanoparticles were administered to cervical cancer
cells, SiHa, CaSki and HeLa. Consistently, ursolic acid
nanoparticle treatment significantly accelerated apoptosis in
cervical cancer cell lines, especially at the highest concentration
of 100 µM in comparison to the control groups. Also, cell
cycle arrest showed that the number of cells in the Sub-G1 phase
was higher after 100 µM ursolic acid nanoparticle treatment
compared to the control group in the three cervical cancer cell
lines, suggesting that apoptosis was induced for ursolic acid
nanoparticles, which was in agreement with the above apoptotic
results (Fig. 3B). In summary, the
data above indicated that ursolic acid nanoparticles could, at
least partly, suppress cervical cancer progression and development
through inducing apoptosis.
Effect of ursolic acid nanoparticles on
apoptosis induction through caspase regulation
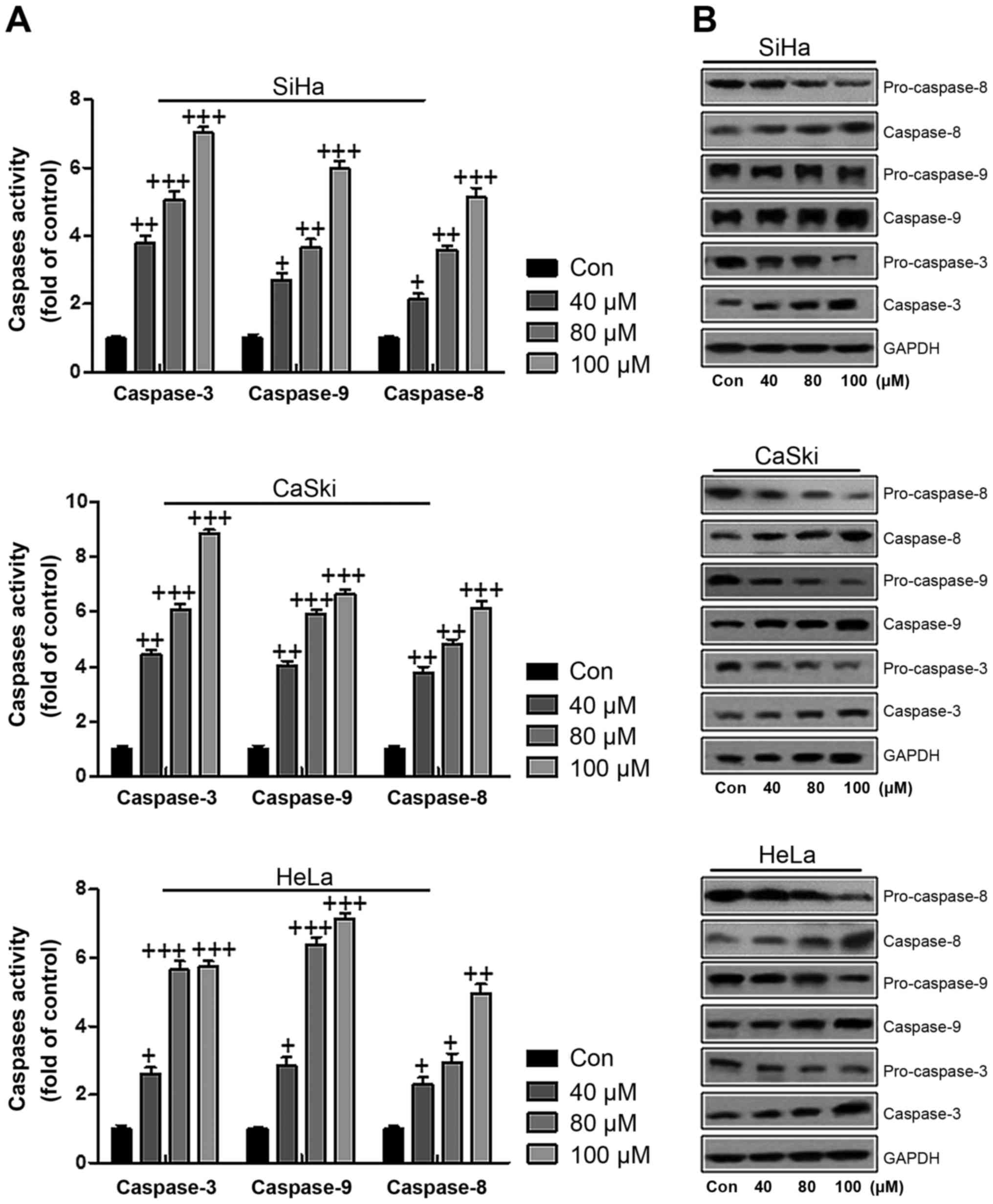
As mentioned above, apoptosis has been investigated
in connection to cervical cancer cell death. According to previous
reports, caspases play an essential role in modulating apoptosis
(28). Therefore, western blot
analysis was used to explore whether caspase activity participated
in apoptosis in the present study. As shown in Fig. 4A, caspase-3, caspase-9 and
caspase-8 were analyzed through ELISA. Compared to the control
group, we found that caspase-3, caspase-9 and caspase-8 were highly
expressed in SiHa cells after ursolic acid nanoparticle
administration, further indicating that apoptosis was induced
(Fig. 4A). In line with the
results in SiHa cells, caspase-3, caspase-9 and caspase-8 were
overexpressed in CaSki and HeLa cells treated with ursolic acid
nanoparticles (Fig. 4A). To
further prove that caspases were activated in cervical cancer cells
after ursolic acid nanoparticles treatment, western blot assays
were performed to show how caspase-3 and caspase-9 changed. In
Fig. 4B, we found that the
caspase-8, caspase-3 and caspase-9 were expressed highly, while the
pro-caspase-8, pro-caspase-9 and pro-caspase-3 were reduced after
ursolic acid nanoparticles treatment in a dose-dependent manner,
contributing to apoptosis in cervical cancer cells. The results
here indicated that ursolic acid nanoparticle treatment induced
apoptosis in cervical cancer cells.
Effect of ursolic acid nanoparticles on
apoptosis formationrelated gene and protein expression in SiHa,
CaSki and HeLa cancer cell lines
p53 is known to be closely related to cell
proliferation and apoptosis (29).
In this regard, immunofluorescence analysis was used first to
calculate p53 alteration in SiHa, CaSki and HeLa cells after 100
µM ursolic acid nanoparticle treatment. As shown in Fig. 5A, higher expression of p53 was
observed in cervical cancer cells in comparison to the control
ones, suggesting that ursolic acid nanoparticles upregulated p53
levels. The protein analysis by western blot showed that the p53
and other four apoptosis-related proteins were changed in SiHa,
CaSki and HeLa cells treated with ursolic acid nanoparticles for 48
h. The tested compound caused an increase in the level of p53, Bax
and Fas proteins significantly, which was similar to a decrease in
Bcl-2 and cIAP-1 protein expression in SiHa, CaSki and HeLa cells
(Fig. 5B). Together, the data
above illustrated that ursolic acid nanoparticles suppressed
cervical cancer cell development and progression via p53 and
apoptosis-related signals modulation.
Ursolic acid nanoparticles suppressed
cervical cancer growth and progression in a mouse xenograft
model
In order to further confirm the inhibitory role of
ursolic acid nanoparticles in cervical cancer progression, a mouse
xenograft model was used in in vivo experiments. From
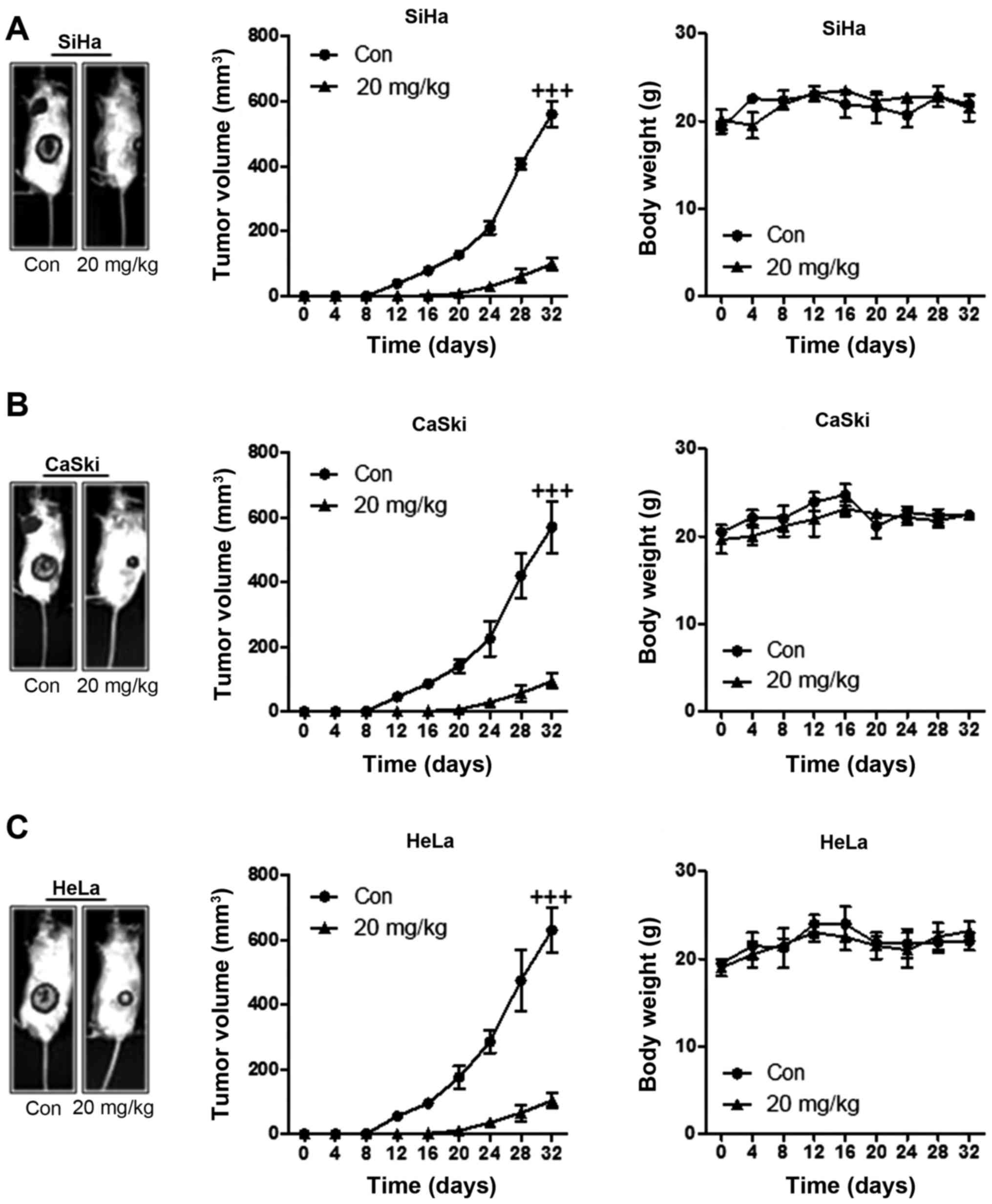
Fig. 6A, we found that the tumor
volume in SiHa-induced xenograft mice was downregulated
significantly compared to the control group after ursolic acid
nanoparticles administration. However, no remarkable alteration of
the mouse body weight was observed here, suggesting that ursolic
acid nanoparticles might be of little toxicity to animals.
Consistent with the results above, the volume of tumor in CaSki-
and HeLa-induced mice was also reduced significantly in comparison
to the control ones. Also, there was no remarkable alteration in
the weight of animals (Fig. 6).
The data here suggested that ursolic acid nanoparticles, at least
partly, had an effective role in suppressing cervical tumor
progression and development in vivo.
Ursolic acid nanoparticles suppressed
cervical cancer growth through apoptosis-related genes
The results mentioned above (Fig. 5) in vitro showed that p53
and Bcl-2 were associated with cervical tumor growth, which could
be regulated by ursolic acid nanoparticle administration. In in
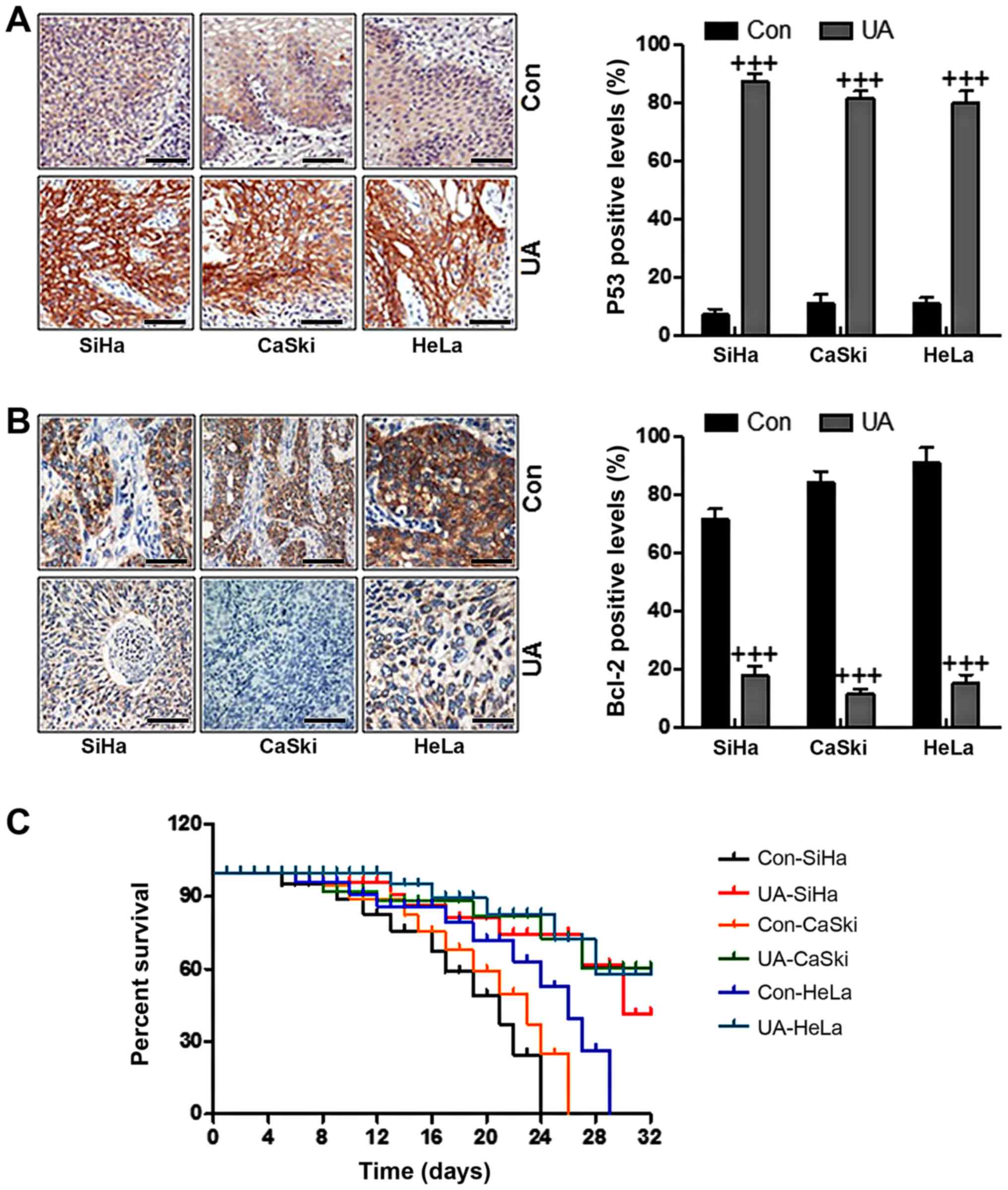
vivo studies, immunohistochemical analysis elucidated that
ursolic acid nanoparticle treatment significantly upregulated p53
expression in cervical cancer cells compared to the control ones
(Fig. 7A). By contrast, Bcl-2, as
mentioned above, is an anti-apoptotic factor, which was reduced
after administration of ursolic acid nanoparticles, suggesting that
ursolic acid nanoparticles could induce cervical cancer cell death
through inhibiting the expression of anti-apoptotic factors
(Fig. 7B). Finally, we also
determined how the survival rate changed in animal models with
cervical cancer after ursolic acid nanoparticles treatment. As
shown in Fig. 7C, the survival
rate was higher in mice after the treatment of ursolic acid
nanoparticles. The data indicated that the survival rate of SiHa-,
CaSki- and HeLa-induced animal models with cervical tumor was
increased.
Discussion
Cervical cancer is one of the leading causes for
gynecologic cancer death among women worldwide and approximately
528,000 new cervical cancer patients are deduced, resulting in
266,000 deaths every year (30).
Over 80% of cervical cancer cases are diagnosed in developing
countries (31,32). In addition, new cases suffering
from cervical cancer are up 150,000 in China each year, accounting
for approximately 30% of new cases across the world. Although a
large number of cervical cancer patients benefit from neoadjuvant
chemotherapy with concurrent chemotherapy and radiotherapy,
currently the survival rate still remains poor among cervical
cancer patients (33). Resistance
to chemotherapy has been reported as a common reason of treatment
failure in patients with cervical cancer (34).
Ursolic acid, a pentacyclic terpenoid, exhibits a
wide and powerful pharmaceutical activities (35). Ursolic acid is reported as a
secondary plant metabolite, often found in the leaves, stem bark or
fruit peel (36). The promoting
activities for health of this compound have been used for
centuries, which is an ingredient of herb extracts involved in folk
medicine. Finding natural biologically active substances, has come
back to this source of knowledge acquired over generations
(37,38). Furthermore, ursolic acid is one of
the most promising substances of biological origin when it is used
for the prevention and therapy of tumors (36). New pharmacological strategies not
only rely on the termination of cancer cells, but also regulate
their metabolism to restrain angiogenesis and metastasis, promote
cancer cell differentiation and protect normal and healthy tissue
from inflammation and oxidative stress (39,40).
The anticancer activity of ursolic acid is related to its ability
to influence the activity of several enzymes. Thus, it is able to
regulate processes inside tumor cells, leading to cell death often
by apoptosis and inhibition resulting in proliferation, growth and
migration of tumors (41,42). The effective and successful
application of liposome nanocarriers has been catalyzed through
targeted delivery and subsequent preferential intracellular uptake,
enhancing permeability and retention effect with enhanced
selectivity, efficacy, as well as overall safety (43–45).
In this study, we found that ursolic acid
nanoparticles showed inhibitory role in cervical cancer
progression, while no toxicity was observed to cells 293T and L02,
suggesting that ursolic acid nanoparticles indeed inhibit cervical
cancer development, which could be a potential therapeutic strategy
for patients in future. Furthermore, the colony formation assay
indicated that ursolic acid nanoparticles suppressed cervical
cancer cell proliferation significantly. In addition, the migration
and invasion analysis also suggested that ursolic acid
nanoparticles showed inhibitory role in cervical cancer cell
lines.
Apoptosis normally occurs during the development to
homeostasis in normal cells and tissues or occurs as a defense
mechanism to eliminate the defective or unwanted cells selectively
damaged by disease (46–48). It has been reported as a necessary
complementary to inhibit cell proliferation and plays an important
role in the development and regulation of immune system, the
removal of damaged cells, and furthermore, the apoptosis disruption
is involved in tumor development (49). In the present study, flow
cytometric analysis indicated that ursolic acid nanoparticles
induced apoptosis, leading to cell death during cervical cancer
progression. Additionally, the number of cells in sub-G1 was also
higher in cervical cancer cells treated by ursolic acid
nanoparticles, which further suggested that ursolic acid
nanoparticles induced apoptosis in cervical cancer to suppress
cervical cancer development. Caspases were further examined for
their activity to regulate signal transduction by positively or
reversely regulating kinases, phosphatases and other signal
molecules in cells (50).
Considering the well-characterized effect of caspase-3, an
important apoptosis regulator in both extrinsic and intrinsic
pathways, attention on its pro-apoptotic ability has increased
(51–54). In this study, we found that
caspase-3, caspase-9 and caspase-8 were upregulated in ursolic acid
nanoparticle-treated cervical cancer cells, suggesting that ursolic
acid nanoparticles induced cervical cancer cell death via apoptosis
induction through activating caspases.
Cell signal pathways when they are de-regulated,
relate to apoptosis and cell cycle, and have a significant role in
the development and progression of diseases, including cancer. The
members of p53 family are known as significant players responding
to the cellular stress in cancer and, thus, these genes may act as
therapeutic targets (55).
Cellular stress, such as DNA damage, ribosomal and endoplasmic
reticulum stress, stimulates p53 activity while it is strictly
maintained at low levels under normal conditions (56). The cell cycle arrest and apoptosis
are two main molecular mechanisms, regulating response of p53 to
the DNA damage (57). Here, we
found that p53 was upregulated in cervical cancer cells treated by
ursolic acid nanoparticles, helping to promote apoptosis, which was
in agreement with previous studies, further indicating that ursolic
acid nanoparticle could suppress cervical cancer progression in
vitro (55–57). In line with a previous study, our
immunofluorescence results suggested that p53 was involved in
ursolic acid nanoparticle-inhibited cervical cancer.
Two of the most important members associated with
apoptosis in Bcl-2 family include the pro-apoptotic protein Bax and
the anti-apoptotic protein Bcl-2 (58). Overexpression of Bcl-2
proto-oncogene damages the therapeutic action of present cancer
treatment regimes through apoptosis induction of tumor cells
(59). The broad expression of
Bcl-2 in different tumors, together with its function in resisting
chemotherapy-induced apoptosis, makes bcl-2 a rational target for
anticancer therapy (60,61). In addition, inhibitor of apoptosis
(IAP) protein families suppress apoptosis within tumor cells
(62). The cellular inhibitor of
apoptosis protein 1 (cIAP1) has been investigated in regulation of
tumor response (63). In the
present study, the findings from western blot and
immunohistochemistry analyses in vitro and in vivo
clearly revealed that the expression of Bcl-2 and cAIP1 were
significantly suppressed, but the expression of Bax was highly
upregulated, illustrating that ursolic acid nanoparticles showed
anti-apoptotic properties during the cervical cancer
progression.
In summary, our results indicated that ursolic acid
nanoparticles targeted caspases and p53 with downregulation of
Bcl-2 and cIAP, inducing apoptosis and leading to cervical cancer
cell death. Thus, ursolic acid nanoparticles can be used as a class
of antitumor drug development and treatment of cervical cancer for
its potential value.
Acknowledgments
The present study was funded by the Natural Sciences
Fund of Shandong, China (ZR2014HL072).
References
|
1
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Phongsavan K, Phengsavanh A, Wahlström R
and Marions L: Women's perception of cervical cancer and its
prevention in rural Laos. Int J Gynecol Cancer. 20:821–826. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agarwal SM, Raghav D, Singh H and Raghava
GP: CCDB: A curated database of genes involved in cervix cancer.
Nucleic Acids Res. 39(Database): D975–D979. 2011. View Article : Google Scholar :
|
|
4
|
Kawase R, Ishiwata T, Matsuda Y, Onda M,
Kudo M, Takeshita T and Naito Z: Expression of fibroblast growth
factor receptor 2 IIIc in human uterine cervical intraepithelial
neoplasia and cervical cancer. Int J Oncol. 36:331–340.
2010.PubMed/NCBI
|
|
5
|
World Health Organization: International
Agency for Research on Cancer Cervical Cancer - Estimated
Incidence. (Mortality and Prevalence Worldwide in 2012)
|
|
6
|
Pollier J and Goossens A: Oleanolic acid.
Phytochemistry. 77:10–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang EJ, Lee W, Ku SK, Song KS and Bae JS:
Anti-inflammatory activities of oleanolic acid on HMGB1 activated
HUVECs. Food Chem Toxicol. 50:1288–1294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reisman SA, Aleksunes LM and Klaassen CD:
Oleanolic acid activates Nrf2 and protects from acetaminophen
hepatotoxicity via Nrf2-dependent and Nrf2-independent processes.
Biochem Pharmacol. 77:1273–1282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu HY, Yang JJ and Lin CC: Effects of
oleanolic acid and ursolic acid on inhibiting tumor growth and
enhancing the recovery of hematopoietic system postirradiation in
mice. Cancer Lett. 111:7–13. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamai H, Sawada N, Yoshida T, Seike J,
Takizawa H, Kenzaki K, Miyoshi T, Kondo K, Bando Y, Ohnishi Y, et
al: Triterpenes augment the inhibitory effects of anticancer drugs
on growth of human esophageal carcinoma cells in vitro and suppress
experimental metastasis in vivo. Int J Cancer. 125:952–960. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ngo SN, Williams DB and Head RJ: Rosemary
and cancer prevention: Preclinical perspectives. Crit Rev Food Sci
Nutr. 51:946–954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shishodia S, Majumdar S, Banerjee S and
Aggarwal BB: Ursolic acid inhibits nuclear factor-kappaB activation
induced by carcinogenic agents through suppression of IkappaBalpha
kinase and p65 phosphorylation: Correlation with down-regulation of
cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer
Res. 63:4375–4383. 2003.PubMed/NCBI
|
|
13
|
Wang X, Zhang F, Yang L, Mei Y, Long H,
Zhang X, Zhang J, Qimuge-Suyila and Su X: Ursolic acid inhibits
proliferation and induces apoptosis of cancer cells in vitro and in
vivo. J Biomed Biotechnol. 2011:4193432011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin CC, Huang CY, Mong MC, Chan CY and Yin
MC: Antiangiogenic potential of three triterpenic acids in human
liver cancer cells. J Agric Food Chem. 59:755–762. 2011. View Article : Google Scholar
|
|
15
|
Huang CY, Lin CY, Tsai CW and Yin MC:
Inhibition of cell proliferation, invasion and migration by ursolic
acid in human lung cancer cell lines. Toxicol In Vitro.
25:1274–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KH, Seo HS, Choi HS, Choi I, Shin YC
and Ko SG: Induction of apoptotic cell death by ursolic acid
through mitochondrial death pathway and extrinsic death receptor
pathway in MDA-MB-231 cells. Arch Pharm Res. 34:1363–1372. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong YY, Liu YY, Yu S, Zhu XN, Cao XP and
Xiao HP: Ursolic acid suppresses growth and adrenocorticotrophic
hormone secretion in AtT20 cells as a potential agent targeting
adrenocorticotrophic hormone-producing pituitary adenoma. Mol Med
Rep. 9:2533–2539. 2014.PubMed/NCBI
|
|
18
|
Wang X, Wang Y, Chen ZG and Shin DM:
Advances of cancer therapy by nanotechnology. Cancer Res Treat.
41:1–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kreuter J: Nanoparticles - a historical
perspective. Int J Pharm. 331:1–10. 2007. View Article : Google Scholar
|
|
20
|
Byrne JD, Betancourt T and Brannon-Peppas
L: Active targeting schemes for nanoparticle systems in cancer
therapeutics. Adv Drug Deliv Rev. 60:1615–1626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho K, Wang X, Nie S, Chen ZG and Shin DM:
Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer
Res. 14:1310–1316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iyer AK, Khaled G, Fang J and Maeda H:
Exploiting the enhanced permeability and retention effect for tumor
targeting. Drug Discov Today. 11:812–818. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Talekar M, Kendall J, Denny W and Garg S:
Targeting of nanoparticles in cancer: Drug delivery and
diagnostics. Anticancer Drugs. 22:949–962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maeda H: Nitroglycerin enhances vascular
blood flow and drug delivery in hypoxic tumor tissues: Analogy
between angina pectoris and solid tumors and enhancement of the EPR
effect. J Control Release. 142:296–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng X, Tao W, Mei L, Huang L, Tan C and
Feng SS: Cholic acid-functionalized nanoparticles of star-shaped
PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical
cancer. Biomaterials. 34:6058–6067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayer B and Oberbauer R: Mitochondrial
regulation of apoptosis. News Physiol Sci. 18:89–94.
2003.PubMed/NCBI
|
|
27
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC
and Takahashi R: X-linked inhibitor of apoptosis protein (XIAP)
inhibits caspase-3 and -7 in distinct modes. J Biol Chem.
276:27058–27063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaul R, Mukherjee S, Ahmed F, Bhat MK,
Chhipa R, Galande S and Chattopadhyay S: Direct interaction with
and activation of p53 by SMAR1 retards cell-cycle progression at
G2/M phase and delays tumor growth in mice. Int J Cancer.
103:606–615. 2003. View Article : Google Scholar
|
|
30
|
Brake T and Lambert PF: Estrogen
contributes to the onset, persistence, and malignant progression of
cervical cancer in a human papillomavirus-transgenic mouse model.
Proc Natl Acad Sci USA. 102:2490–2495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smith JS, Green J, Berrington de Gonzalez
A, Appleby P, Peto J, Plummer M, Franceschi S and Beral V: Cervical
cancer and use of hormonal contraceptives: A systematic review.
Lancet. 361:1159–1167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasieni P: Cervical cancer and hormonal
contraceptives: Collaborative reanalysis of individual data for 16
573 women with cervical cancer and 35 509 women without cervical
cancer from 24 epidemiological studies. Lancet. 370:1609–1621.
2007. View Article : Google Scholar
|
|
33
|
Gavrilescu MM, Todosi AM, Aniţei MG, Filip
B and Scripcariu V: Expression of bmi-1 protein in cervical, breast
and ovarian cancer. Rev Med Chir Soc Med Nat Iasi. 116:1112–1117.
2012.
|
|
34
|
McCredie MR, Sharples KJ, Paul C, Baranyai
J, Medley G, Jones RW and Skegg DC: Natural history of cervical
neoplasia and risk of invasive cancer in women with cervical
intraepithelial neoplasia 3: A retrospective cohort study. Lancet
Oncol. 9:425–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ikeda Y, Murakami A and Ohigashi H:
Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol Nutr
Food Res. 52:26–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Andersson D, Liu JJ, Nilsson A and Duan
RD: Ursolic acid inhibits proliferation and stimulates apoptosis in
HT29 cells following activation of alkaline sphingomyelinase.
Anticancer Res. 23:3317–3322. 2003.PubMed/NCBI
|
|
37
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. Can thousands of years of ancient
medical knowledge lead us to new and powerful drug combinations in
the fight against cancer and dementia? EMBO Rep. 10:194–200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xavier CP, Lima CF, Preto A, Seruca R,
Fernandes-Ferreira M and Pereira-Wilson C: Luteolin, quercetin and
ursolic acid are potent inhibitors of proliferation and inducers of
apoptosis in both KRAS and BRAF mutated human colorectal cancer
cells. Cancer Lett. 281:162–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsu YL, Kuo PL and Lin CC: Proliferative
inhibition, cell-cycle dysregulation, and induction of apoptosis by
ursolic acid in human non-small cell lung cancer A549 cells. Life
Sci. 75:2303–2316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi BM, Park R, Pae HO, Yoo JC, Kim YC,
Jun CD, Jung BH, Oh GS, So HS, Kim YM, et al: Cyclic adenosine
monophosphate inhibits ursolic acid-induced apoptosis via
activation of protein kinase A in human leukaemic HL-60 cells.
Pharmacol Toxicol. 86:53–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cha HJ, Bae SK, Lee HY, Lee OH, Sato H,
Seiki M, Park BC and Kim KW: Anti-invasive activity of ursolic acid
correlates with the reduced expression of matrix
metalloproteinase-9 (MMP-9) in HT1080 human fibrosarcoma cells.
Cancer Res. 56:2281–2284. 1996.PubMed/NCBI
|
|
42
|
Liu L, Wu J, Zhang J, Li Z, Wang C, Chen
M, Wang Y, Sun Y, Wang L and Luo C: A compatibility assay of
ursolic acid and foodborne microbial exopolysaccharides by
antioxidant power and anti-proliferative properties in
hepatocarcinoma cells. J Food Agric Environ. 10:111–114. 2012.
|
|
43
|
Wang Y, Cui H, Li K, Sun C, Du W, Cui J,
Zhao X and Chen W: A magnetic nanoparticle-based multiple-gene
delivery system for transfection of porcine kidney cells. PLoS One.
9:e1028862014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Delyagina E, Schade A, Scharfenberg D,
Skorska A, Lux C, Li W and Steinhoff G: Improved transfection in
human mesenchymal stem cells: Effective intracellular release of
pDNA by magnetic polyplexes. Nanomedicine (Lond). 9:999–1017. 2014.
View Article : Google Scholar
|
|
45
|
Wang Y, Cui H, Sun C, Du W, Cui J and Zhao
X: Study on performance of magnetic fluorescent nanoparticles as
gene carrier and location in pig kidney cells. Nanoscale Res Lett.
8:1272013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Underbrink MP, Howie HL, Bedard KM, Koop
JI and Galloway DA: E6 proteins from multiple human
betapapillomavirus types degrade Bak and protect keratinocytes from
apoptosis after UVB irradiation. J Virol. 82:10408–10417. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kabsch K and Alonso A: The human
papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated
apoptosis in HaCaT cells by different mechanisms. J Virol.
76:12162–12172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Shi Q, Xu K, Gao C, Chen C, Li XL,
Wang GR, Tian C, Han J and Dong XP: Familial CJD associated PrP
mutants within transmembrane region induced Ctm-PrP retention in ER
and triggered apoptosis by ER stress in SH-SY5Y cells. PLoS One.
6:e146022011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu K, Wang X, Shi Q, Chen C, Tian C, Li
XL, Zhou RM, Chu YL and Dong XP: Human prion protein mutants with
deleted and inserted octarepeats undergo different pathways to
trigger cell apoptosis. J Mol Neurosci. 43:225–234. 2011.
View Article : Google Scholar
|
|
50
|
Virkajärvi N, Pääkkö P and Soini Y:
Apoptotic index and apoptosis influencing proteins bcl-2, mcl-1,
bax and caspases 3, 6 and 8 in pancreatic carcinoma.
Histopathology. 33:432–439. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sträter J, Herter I, Merkel G, Hinz U,
Weitz J and Möller P: Expression and prognostic significance of
APAF-1, caspase-8 and caspase-9 in stage II/III colon carcinoma:
Caspase-8 and caspase-9 is associated with poor prognosis. Int J
Cancer. 127:873–880. 2010.
|
|
52
|
Satoh K, Kaneko K, Hirota M, Toyota T and
Shimosegawa T: The pattern of CPP32/caspase-3 expression reflects
the biological behavior of the human pancreatic duct cell tumors.
Pancreas. 21:352–357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Meggiato T, Calabrese F, De Cesare CM,
Baliello E, Valente M and Del Favero G: C-JUN and CPP32 (CASPASE 3)
in human pancreatic cancer: Relation to cell proliferation and
death. Pancreas. 26:65–70. 2003. View Article : Google Scholar
|
|
54
|
Noble P, Vyas M, Al-Attar A, Durrant S,
Scholefield J and Durrant L: High levels of cleaved caspase-3 in
colorectal tumour stroma predict good survival. Br J Cancer.
108:2097–2105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aylon Y and Oren M: p53: guardian of
ploidy. Mol Oncol. 5:315–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Murray-Zmijewski F, Slee EA and Lu X: A
complex barcode underlies the heterogeneous response of p53 to
stress. Nat Rev Mol Cell Biol. 9:702–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Blanchette P and Branton PE: Manipulation
of the ubiquitin-proteasome pathway by small DNA tumor viruses.
Virology. 384:317–323. 2009. View Article : Google Scholar
|
|
58
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
59
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
60
|
Zhang YX, Kong CZ, Wang HQ, Wang LH, Xu CL
and Sun YH: Phosphorylation of Bcl-2 and activation of caspase-3
via the c-Jun N-terminal kinase pathway in ursolic acid-induced
DU145 cells apoptosis. Biochimie. 91:1173–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wensveen FM, Alves NL, Derks IA, Reedquist
KA and Eldering E: Apoptosis induced by overall metabolic stress
converges on the Bcl-2 family proteins Noxa and Mcl-1. Apoptosis.
16:708–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Choi YH, Baek JH, Yoo MA, Chung HY, Kim ND
and Kim KW: Induction of apoptosis by ursolic acid through
activation of caspases and down-regulation of c-IAPs in human
prostate epithelial cells. Int J Oncol. 17:565–571. 2000.PubMed/NCBI
|
|
63
|
Dubrez-Daloz L, Dupoux A and Cartier J:
IAPs: More than just inhibitors of apoptosis proteins. Cell Cycle.
7:1036–1046. 2008. View Article : Google Scholar : PubMed/NCBI
|